Quantification of Nutrient Fluxes from Sediments of Lake Hulun, China: Implications for Plateau Lake Management
Abstract
1. Introduction
2. Materials and Methods
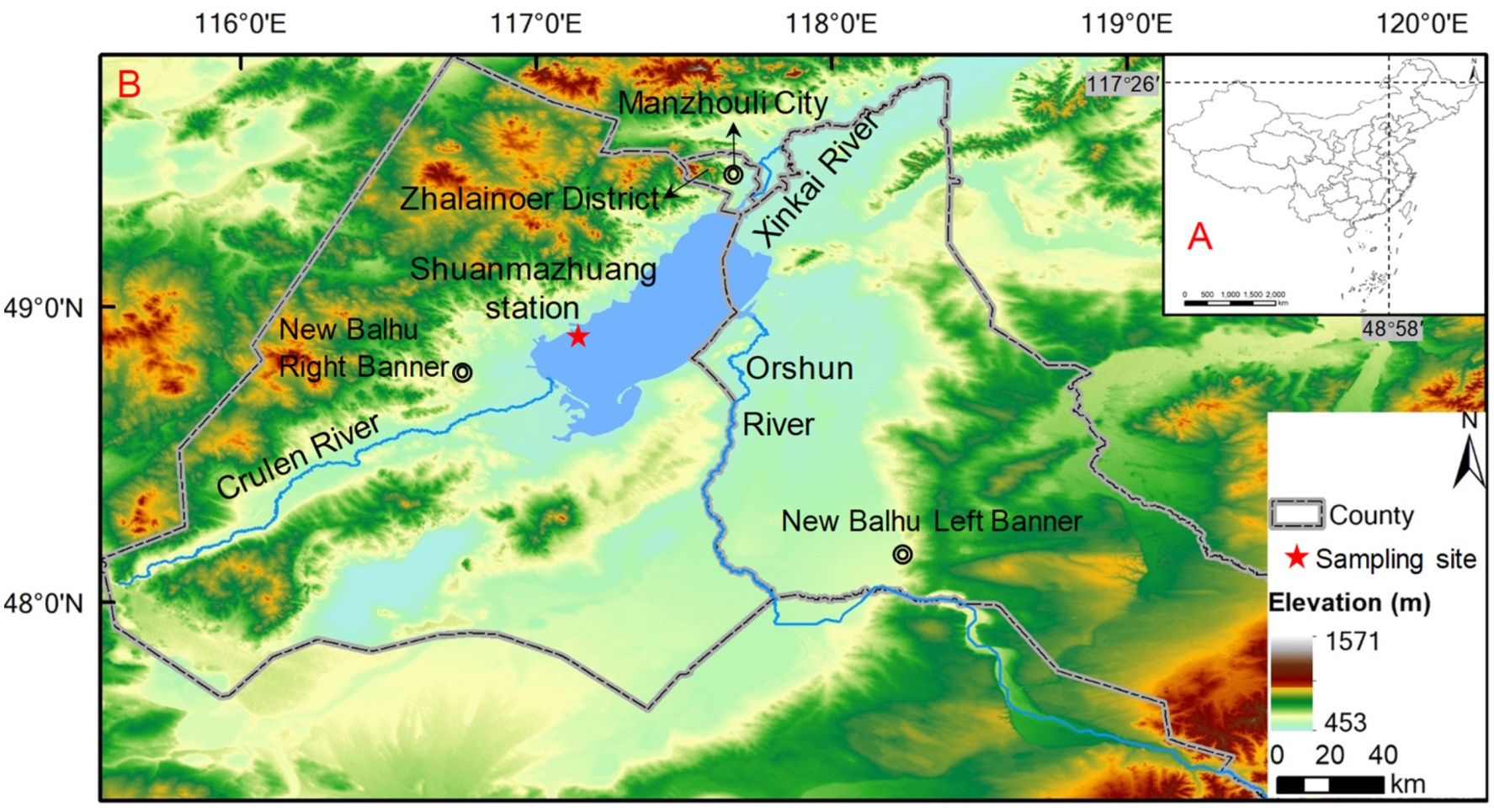
2.1. Geological Background
2.2. Sediment and Water Collection and Incubation
2.3. Sediment Analyses
2.4. Statistical Analysis
3. Results
3.1. Water and Sediment Quality
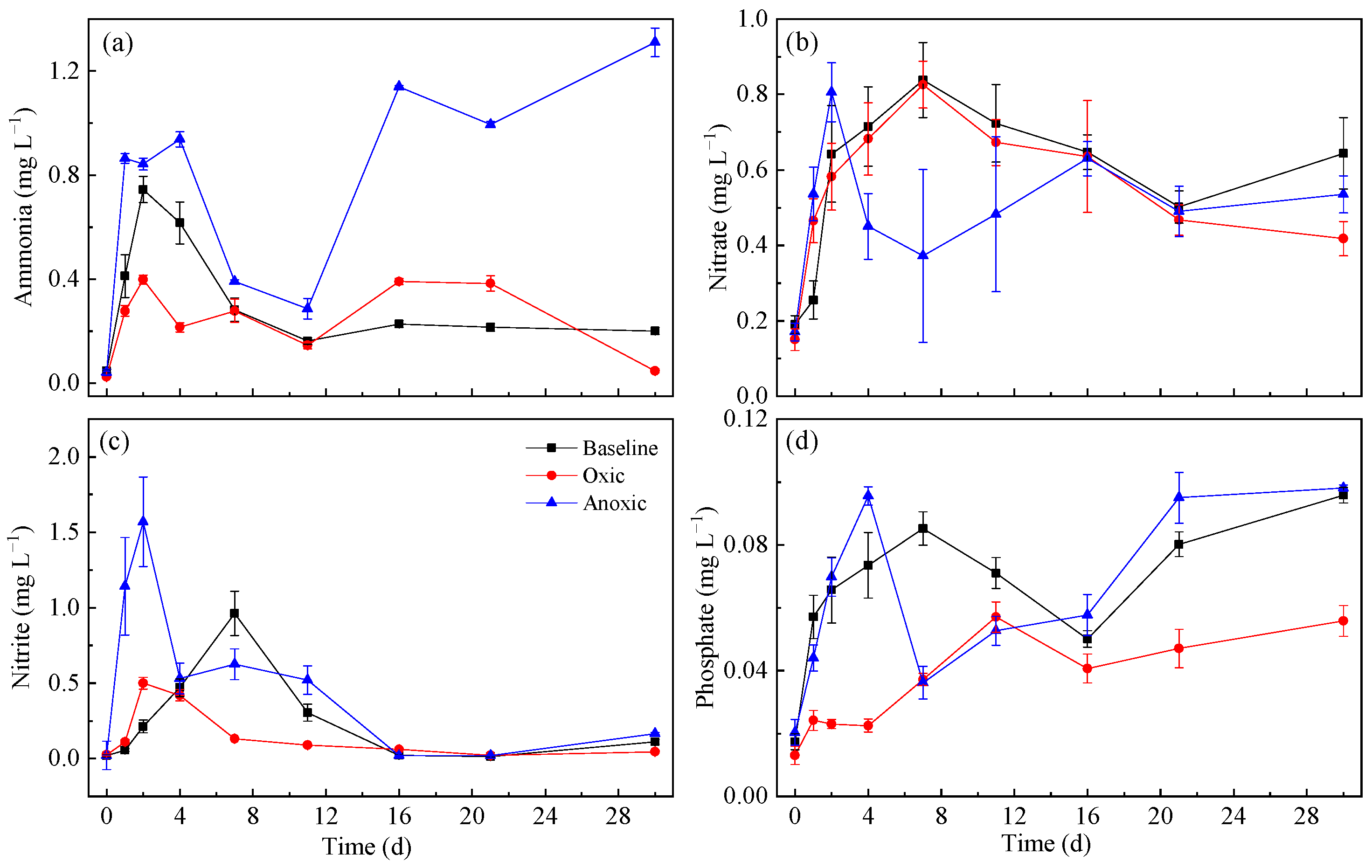
3.2. Effects of Oxygen on Sediment N and P Release
3.3. Effects of Temperature on Sediment N and P Release
3.4. Effects of pH on Sediment N and P Release
3.5. Overview of Nutrient Fluxes in Shallow Lakes
| Location | Incubation Temperature (°C) | Oxic P Release Rate (mg m−2 d−1) | Anoxic P Release Rate (mg m−2 d−1) | Oxic N Release Rate (mg m−2 d−1) | Anoxic N Release Rate (mg m−2 d−1) | References |
|---|---|---|---|---|---|---|
| Lake Taihu | 5, 15, 25 | 1.1 | 11.7 | [38] | ||
| Eastern Lake Taihu | 8–30 | 2.1 ± 1.7 | 44.9 ± 21.9 | [39] | ||
| Northern Lake Taihu (Meiliang Bay) | 8–30 | 0.5 ± 0.5 | 16.2 ± 12.0 | [39] | ||
| Western Lake Chaohu | 15–30 | 13.1–32.9 | [44] | |||
| Eastern Lake Chaohu | 15–30 | 4.5–17.4 | [44] | |||
| Northwestern Lake Chaohu | 25 | 0.1–13.0 | 14.3–128.2 | [45] | ||
| Lake Dianchi | 5, 15, 25 | 12.7–59.7 (30.2) | [46] | |||
| Lake Dianchi (Fubao Bay) | 14–16 | 0.9–4.9 (2.7) | 22.9–163.1 (111.7) | [47] | ||
| Lake Dongting | 2.0–147.0 (16.2) | [48] | ||||
| Lake Dongting | 12 | 0.04–0.3 (0.2) | [49] | |||
| Lake Hongfeng | 5, 15, 25 | 0.4, 0.6, 0.9 | [40] | |||
| Lake Nansi | 0.3–2.7 (1.1) | 3.1–10.3 (7.0) | [50] | |||
| Lake Hulun | 23 | 0.3–2.2 (0.8) (DO = 8) | 0.5–5.4 (2.1) | 0.2–50.8 (14.4) | 4.8–164.1 (40.0) | This study |
| Lake Hulun | 2, 7, 15, 23 | 0.1–4.3 (0.7) (DO = 4–6.8) | −4.0–39.6 (14.4) | This study | ||
| Western Lake Erie (America) | 20 | 0.4 ± 0.3 | 9.3 ± 6.5 | [42] | ||
| Lake Pontchartrain (America) | 25 | 0.4 ± 0.1 | 0.9 ± 0.2 | [41] | ||
| Lake Rotorua (Zew Zealand) | 20.8 | 10.6–30.7 (16.1) | 75.1–484.5 (244.3) | [51] | ||
| Lower Havel (Germany) | 3.5–36.0 | 20.0–124.0 | [52] | |||
| Swarzędzkie (Poland) | 2.0–20 | −2.4–59.5 | 2.8–26.9 | [53] |
4. Discussion
4.1. Mechanisms of Nutrient Release from Lake Sediments
4.2. Implications for Public Health and Lake Management
4.3. Research Limitation and Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welch, E.; Jacoby, J. On determining the principal source of phosphorus causing summer algal blooms in Western Washington Lakes. Lake Reserv. Manag. 2001, 17, 55–65. [Google Scholar] [CrossRef]
- Welch, E.B.; Cooke, G.D. Internal phosphorus loading in shallow lakes: Importance and control. Lake Reserv. Manag. 2005, 21, 209–217. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Yu, P.; Yang, X.; Zhang, L.; Geng, Z.; He, K. Oxygenation and synchronous control of nitrogen and phosphorus release at the sediment-water interface using oxygen nano-bubble modified material. Sci. Total Environ. 2020, 725, 138258. [Google Scholar] [CrossRef]
- Wang, Y.P.; Peng, Z.L.; Liu, G.; Zhang, H.; Zhou, X.Q.; Hu, W.P. A mathematical model for phosphorus interactions and transport at the sediment-water interface in a large shallow lake. Ecol. Model. 2023, 476, 110254. [Google Scholar] [CrossRef]
- Huang, L.; Fang, H.; He, G.; Jiang, H.; Wang, C. Effects of internal loading on phosphorus distribution in the Taihu Lake driven by wind waves and lake currents. Environ. Pollut. 2016, 219, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, P.; Geng, J.; Yin, H.; Chen, K. Sediment internal nutrient loading in the most polluted area of a shallow eutrophic lake (Lake Chaohu, China) and its contribution to lake eutrophication. Environ. Pollut. 2020, 262, 114292. [Google Scholar] [CrossRef]
- Kang, M.; Peng, S.; Tian, Y.; Zhang, H. Effects of dissolved oxygen and nutrient loading on phosphorus fluxes at the sediment-water interface in the Hai River Estuary, China. Mar. Pollut. Bull. 2018, 130, 132–139. [Google Scholar] [CrossRef]
- Golterman, H. Phosphate release from anoxic sediments or ‘What did Mortimer really write?’. Hydrobiologia 2001, 450, 99–106. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Liang, L.; Li, C.; Zhao, S.; Tian, Y.; Zhang, L.; Shi, X. Analysis on the eutrophication trends and affecting factors in Lake Hulun, 2006–2015. J. Lake Sci. 2016, 28, 1265–1273. (In Chinese) [Google Scholar]
- Wang, H.; Holden, J.; Spera, K.; Xu, X.; Wang, Z.; Luan, J.; Xu, X.; Zhang, Z. Phosphorus fluxes at the sediment–water interface in subtropical wetlands subjected to experimental warming: A microcosm study. Chemosphere 2013, 90, 1794–1804. [Google Scholar] [CrossRef]
- Beutel, M.; Horne, A. Nutrient fluxes from profundal sediment of ultra-oligotrophic Lake Tahoe, California/Nevada: Implications for water quality and management in a changing climate. Water Resour. Res. 2018, 54, 1549–1559. [Google Scholar] [CrossRef]
- Beutel, M.W.; Horne, A.J.; Taylor, W.D.; Losee, R.F.; Whitney, R.D. Effects of oxygen and nitrate on nutrient release from profundal sediments of a large, oligo-mesotrophic reservoir, Lake Mathews, California. Lake Reserv. Manag. 2008, 24, 18–29. [Google Scholar] [CrossRef]
- Jin, X.; Wang, S.; Pang, Y.; Wu, F. Phosphorus fractions and the effect of pH on the phosphorus release of the sediments from different trophic areas in Taihu Lake, China. Environ. Pollut. 2006, 139, 288–295. [Google Scholar] [CrossRef]
- Yang, Z.; Li, C.; Shi, X.; Sun, B.; Du, D.; Quan, D. Study on the characteristics of water nutrition status and its main influencing factors in Hulun Lake. Ecol. Environ. Sci. 2019, 28, 2273–2280. (In Chinese) [Google Scholar]
- Chuai, X.; Chen, X.; Yang, L.; Zeng, J.; Miao, A.; Zhao, H. Effects of climatic changes and anthropogenic activities on lake eutrophication in different ecoregions. Int. J. Environ. Sci. Technol. 2012, 9, 503–514. [Google Scholar] [CrossRef]
- Xue, B.; Yao, S.C.; Mao, Z.G.; Sun, Z.D.; Liu, S.T.; Dou, H.S.; Zhang, F.J. Lake Hulun; Nanjing University: Nanjing, China, 2017. (In Chinese) [Google Scholar]
- Zhang, Y.; Liang, W.; Liao, Z.; Han, Z.; Xu, X. Effects of climate change on lake area and vegetation cover over the past 55 years in Northeast Inner Mongolia grassland, China. Theor. Appl. Climatol. 2019, 138, 13–25. [Google Scholar] [CrossRef]
- Liu, Y.; Yue, H. Estimating the fluctuation of Lake Hulun, China, during 1975–2015 from satellite altimetry data. Environ. Monit. Assess. 2017, 189, 630. [Google Scholar] [CrossRef] [PubMed]
- Zhan, B.; Guo, Y.; Wang, S.; Zheng, S.; Jiang, X. Spatial-temporal changes of phosphorus and its influential factors in Lake Hulun. Res. Environ. Sci. 2021, 34, 824–830. (In Chinese) [Google Scholar]
- Zhao, H.; Wu, L.; Hao, W. Influences of climate change to ecological and environmental evolvement in the Hulun Lake wetland and its surrounding areas. Acta Ecol. Sin. 2008, 28, 1064–1071. (In Chinese) [Google Scholar]
- Mao, Z.; Gu, X.; Zeng, Q. The structure of fish community and changes of fishery resources in Lake Hulun. J. Lake Sci. 2016, 28, 387–394. (In Chinese) [Google Scholar]
- Jin, X.; Tu, Q. The Standard Methods in Lake Eutrophication Investigation, 2nd ed.; China Environmental Science Press: Beijing, China, 1990. [Google Scholar]
- Rydin, E. Potentially mobile phosphorus in Lake Erken sediment. Water Res. 2000, 34, 2037–2042. [Google Scholar] [CrossRef]
- Rodriguez, J.B.; Self, J.R.; Soltanpour, P.N. Optimal conditions for phosphorus analysis by the ascorbic acid-molybdenum blue method. Soil Sci. Soc. Am. J. 1994, 58, 866. [Google Scholar] [CrossRef]
- Paytan, A.; Roberts, K.; Watson, S.; Peek, S.; Chuang, P.-C.; Defforey, D.; Kendall, C. Internal loading of phosphate in Lake Erie Central Basin. Sci. Total Environ. 2017, 579, 1356–1365. [Google Scholar] [CrossRef]
- Li, W.; Li, C.; Jia, K.L.; Zhang, S.; Shi, X.; Zhang, X. Distribution of nutrient elements and environmental pollution assessment in sediment of Hulun Lake, Inner Mongolia. J. Agro-Environ. Sci. 2010, 29, 339–343. (In Chinese) [Google Scholar]
- Wang, Y.P.; Xu, W.; Han, C.; Hu, W.P. Distribution of nitrogen and phosphorus in Lake Chaohu sediments and pollution evaluation. Environ. Sci. 2021, 42, 699–711. (In Chinese) [Google Scholar]
- Li, Y.; Li, X.; Huang, G.; Wang, S.; Li, D. Sedimentary organic carbon and nutrient distributions in an endorheic lake in semiarid area of the Mongolian Plateau. J. Environ. Manag. 2021, 296, 113184. [Google Scholar] [CrossRef]
- Tepe, Y.; Boyd, C.E. Sediment quality in Arkansas Bait Minnow ponds. J. World Aquac. Soc. 2007, 33, 221–232. [Google Scholar] [CrossRef]
- Lü, C.; He, J.; Wang, B. Spatial and historical distribution of organic phosphorus driven by environment conditions in lake sediments. J. Environ. Sci. 2018, 64, 32–41. [Google Scholar] [CrossRef]
- Wang, F. Geochemistry Characteristics of Nitrogen and Phosphorus of Hulin Lake. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2010. [Google Scholar]
- Fu, Y. Geochemical Characteristics of Nitrogen and Phosphorus and the Assessment for Ambient Environment in Hulun Lake. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2016. [Google Scholar]
- Li, L. Geochemical Character and Environmental Significance of iron Franctions in Lakes Sediments of West Inner Mongolia Plateau. Master’s Thesis, Inner Mongolia University, Hohhot, China, 2017. [Google Scholar]
- Beutel, M. Inhibition of ammonia release from anoxic profundal sediments in lakes using hypolimnetic oxygenation. Ecol. Eng. 2006, 28, 271–279. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Stensel, H.; Tsuchihashi, D.R.; Burton, F. Wastewater Engineering: Treatment and Resource Recovery; McGraw Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Nurnberg, G.K. Prediction of phosphorus release rates from total and reductant-soluble phosphorus in anoxic lake sediments. Can. J. Fish. Aquat. Sci. 1988, 45, 453–462. [Google Scholar] [CrossRef]
- Qin, B.; Zhu, G.; Zhang, L.; Luo, L.; Gao, G.; Gu, B. Estimation of internal nutrient release in large shallow Lake Taihu China. Sci. China: Ser. D Earth Sci. 2006, 19 (Suppl. I), 38–50. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, C.; Wang, J.; Zheng, C. Space-Time dependent variances of ammonia and phosphorus flux on sediment-water interface in Lake Taihu. Environ. Sci. 2006, 27, 1537–1543. (In Chinese) [Google Scholar]
- Wang, J.; Chen, J.; Luo, J.; Zhang, H.; Yu, P. Comparative study on quantitative estimations of phosphorus release flux from sediments of Lake Hongfeng, Guizhou Province, China. Earth Environ. 2018, 46, 1–6. (In Chinese) [Google Scholar]
- Roy, E.D.; Nguyen, N.T.; Bargu, S.; White, J.R. Internal loading of phosphorus from sediments of Lake Pontchartrain (Louisiana, USA) with implications for eutrophication. Hydrobiologia 2012, 684, 69–82. [Google Scholar] [CrossRef]
- Matisoff, G.; Kaltenberg, E.M.; Steely, R.L.; Hummel, S.K.; Seo, J.; Gibbons, K.J.; Bridgeman, T.B.; Seo, Y.; Behbahani, M.; James, W.F.; et al. Internal loading of phosphorus in western Lake Erie. J. Great Lakes Res. 2016, 42, 775–788. [Google Scholar] [CrossRef]
- Sondergaard, M.; Peter, K.; Jeppesen, E. Phosphorus release from resuspended sediment in the shallow and wind-exposed Lake Arresø, Denmark. Hydrobiologia 1992, 228, 91–99. [Google Scholar] [CrossRef]
- Jiang, X.; Zhong, L.; Wang, S.; Jin, X. Dissolvable nitrogen variation at water-sediment interface during alga blooming process in Chaohu Lake. China Environ. Sci. 2009, 29, 1158–1163. (In Chinese) [Google Scholar]
- Liu, C.; Shao, S.; Fan, C.; Zhou, Q.; Chen, C.; Shen, Q. Distribution and release risk of nutrients in the sediments of heavily polluted confluence bay of Chaohu Lake. Res. Environ. Sci. 2014, 27, 1258–1264. (In Chinese) [Google Scholar]
- Wang, M.; Yan, H.; Jiao, L.; Wang, S.; Liu, W.; Luo, J.; Luo, Z. Characteristics of internal nitrogen loading and influencing factors in Dianchi Lake sediment. China Environ. Sci. 2015, 35, 218–226. (In Chinese) [Google Scholar]
- Li, B.; Ding, S.; Fan, C.; Zhong, J.; Zhao, B.; Yin, H.; Zhang, L. Esitimation of releasing fluxes of sediment nitrogen and phosphorus in Fubao Bay in Dianchi Lake. Environ. Sci. 2008, 29, 114–120. (In Chinese) [Google Scholar]
- Wang, W.; Wang, S.; Jiang, X.; Wang, Y.; Wang, J. Occurrence characteristics and release risk of nitrogen fractions in sediments of Dongting Lake. Res. Environ. Sci. 2013, 26, 598–605. (In Chinese) [Google Scholar]
- Gao, Y.; Liang, T.; Tian, S.; Wang, L.; Holm, P.E.; Bruun Hansen, H.C. High-resolution imaging of labile phosphorus and its relationship with iron redox state in lake sediments. Environ. Pollut. 2016, 219, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, B.; Liang, Z.; Wang, L. Comparative study on endogenous release of nitrogen and phosphorus in Nansi Lake, China. Acta Sci. Circumstantiae 2013, 33, 487–493. (In Chinese) [Google Scholar]
- Burger, D.F.; Hamilton, D.P.; Pilditch, C.A.; Gibbs, M.M. Benthic nutrient fluxes in a eutrophic, polymictic lake. Hydrobiologia 2007, 584, 13–25. [Google Scholar] [CrossRef]
- Grüneberg, B.; Dadi, T.; Lindim, C.; Fischer, H. Effects of nitrogen and phosphorus load reduction on benthic phosphorus release in a riverine lake. Biogeochemistry 2015, 123, 185–202. [Google Scholar] [CrossRef]
- Kowalczewska-Madura, K.; Gołdyn, R. Internal loading of phosphorus from sediments of swarzedzkie Lake (Western Poland). Pol. J. Environ. Stud. 2009, 18, 635–643. [Google Scholar]
- Qian, Y.; Liang, X.; Yingxu, C.; Cao, R. Significance of biological effects on phosphorus transformation processes at the water–sediment interface under different environmental conditions. Ecol. Eng. 2011, 37, 816–825. [Google Scholar] [CrossRef]
- Lijklema, L. The role of iron in the exchange of phosphate between water and sediments. The role of iron in the exchange of phosphorus between water and sediments. In Interactions between Sediments and Freshwater; Golterman, H.L., Ed.; Dr W. Junk: The Hague, The Netherlands, 1977; pp. 313–317. [Google Scholar]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Internal phosphorus loading in shallow Danish lakes. Hydrobiologia 1999, 408/409, 145–152. [Google Scholar] [CrossRef]
- Orihel, D.M.; Baulch, H.M.; Casson, N.J.; North, R.L.; Parsons, C.T.; Seckar, D.C.M.; Venkiteswaran, J.J. Internal phosphorus loading in Canadian fresh waters: A critical review and data analysis. Can. J. Fish. Aquat. Sci. 2017, 74, 1–25. [Google Scholar] [CrossRef]
- Gao, L. Phosphorus release from the sediments in Rongcheng Swan Lake under different pH conditions. Procedia Environ. Sci. 2012, 13, 2077–2084. [Google Scholar] [CrossRef]
- Søndergaard, M.; Bjerring, R.; Jeppesen, E. Persistent internal phosphorus loading during summer in shallow eutrophic lakes. Hydrobiologia 2013, 710, 95–107. [Google Scholar] [CrossRef]
- Qian, R.; Peng, F.; Xue, K.; Qi, L.; Duan, H.; Qiu, Y.; Chen, Q.; Chen, F.; Gao, J.; Huang, J. Assessing the risks of harmful algal bloom accumulation altlittoral zone of alrge lakes and reserviors: A example from Lake Chaohu. J. Lake Sci. 2022, 34, 49–60. (In Chinese) [Google Scholar]
- Zhang, Y.; Ma, X.; Guo, F.; Li, J.; Xiong, B. Community structures of phytoplankton and their relationships with environmental factors in the Jinshahe Reservoir, Hubei Province. J. Lake Sci. 2015, 27, 902–910. (In Chinese) [Google Scholar]
- Chen, C.; Kong, M.; Wang, Y.Y.; Shen, Q.S.; Zhong, J.C.; Fan, C.X. Dredging method effects on sediment resuspension and nutrient release across the sediment-water interface in Lake Taihu, China. Environ. Sci. Pollut. Res. 2020, 27, 25861–25869. [Google Scholar] [CrossRef]
- Rao, K.; Zhang, X.; Yi, X.J.; Li, Z.S.; Wang, P.; Huang, G.W.; Guo, X.X. Interactive effects of environmental factors on phytoplankton communities and benthic nutrient interactions in a shallow lake and adjoining rivers in China. Sci. Total Environ. 2018, 619–620, S0048969717328449. [Google Scholar] [CrossRef]
- Wang, Y.P.; Liu, G.; Zhu, S.L.; Hu, W.P.; Zhang, H.; Zhou, X.Q.; Peng, Z.L. Assessment of impacts of water transfer on lake flow and water quality in Lake Chaohu using a three-dimensional hydrodynamic-ecological model. J. Hydrol. Reg. Stud. 2023, 46, 101333. [Google Scholar] [CrossRef]
- Guo, Z.; Li, C.; Shi, X.; Sun, B.; Zhao, S.; Quan, D.; Hou, B. Spatial and temporal distribution characteristics of chlorophyll a content and its influencing factor analysis in Hulun Lake of cold and dry areas. Ecol. Environ. Sci. 2019, 28, 1434–1442. (In Chinese) [Google Scholar]
- Sahoo, G.; Schladow, S.; Reuter, J.; Coats, R.; Dettinger, M.; Riverson, J.; Wolfe, B.; Costa-Cabral, M. The response of Lake Tahoe to climate change. Clim. Chang. 2013, 116, 71–95. [Google Scholar] [CrossRef]
- Kononets, M.; Tengberg, A.; Nilsson, M.; Ekeroth, N.; Hyl´en, A.; Robertson, E.K.; Velde, S.v.d.; Bonaglia, S.; Rütting, T.; Blomqvist, S.; et al. In situ incubations with the Gothenburg benthic chamber landers: Applications and quality control. J. Mar. Syst. 2021, 214, 103475. [Google Scholar] [CrossRef]
- Han, X.; Yang, C. An analysis of the self-purification function of Hulun Lake and its effect on regional environmental conservation. J. Nat. Resour. 2002, 17, 684–690. (In Chinese) [Google Scholar]




| Parameter | Value (mg kg−1) | Percentage (%) | Reference |
|---|---|---|---|
| TN * | 2051 | [32] | |
| TN * | 2170 | [33] | |
| TP | 1079 ± 21 | 100 | this study |
| LS-P | 184 ± 8 | 17.3 | |
| Fe-P | 69 ± 4 | 6.4 | |
| Al-P | 302 ± 12 | 28.3 | |
| Org-P | 306 ± 10 | 28.7 | |
| Ca-P | 173 ± 6 | 16.2 | |
| Res-P | 37 ± 2 | 3.1 | |
| Fe * | 32,000 | [34] |
| Incubation Period | Treatments | Nutrient Fluxes (mg m−2 d−1) | ||
|---|---|---|---|---|
| Ammonia | Nitrate | SRP | ||
| DO a control | Control | 1.0–73.1 (23.1) | 3.0–45.2 (15.5) | 0.4–6.3 (2.1) |
| Oxic | 0.2–50.8 (14.4) | 1.8–63.0 (21.5) | 0.3–2.2 (0.8) | |
| Anoxic | 4.4–164.1 (40.0) | 2.6–73.2 (21.7) | 0.5–5.4 (2.2) | |
| T a control | T1 | −4.0–30.3 (9.8) | 0.7–21.4 (9.1) | 0.1–1.8 (0.5) |
| T2 | 5.9–39.6 (16.3) | −2.7–27.0 (8.1) | 0.1–1.8 (0.6) | |
| T3 | −3.1–34.9 (17.2) | −0.1–26.7 (10.5) | 0.2–4.0 (0.8) | |
| T4 | 2.6–37.3 (17.0) | 13.6–186.5 (62.9) | 0.3–4.3 (1.0) | |
| pH a control | pH1 | 1.9–54.1 (20.3) | 5.3–23.1 (13.1) | 0.3–5.5 (2.2) |
| pH2 | −1.7–61.3 (21.4) | 7.7–95.2 (36.0) | 0.5–10.3 (3.3) | |
| pH3 | −3.3–34.0 (7.4) | 11.8–129.1 (55.2) | 0.3–2.1 (0.9) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Zhang, X.; Tong, Y.; Ao, W.; Wang, Z.; Zhu, S.; Wang, Y. Quantification of Nutrient Fluxes from Sediments of Lake Hulun, China: Implications for Plateau Lake Management. Sustainability 2023, 15, 8680. https://doi.org/10.3390/su15118680
Liu B, Zhang X, Tong Y, Ao W, Wang Z, Zhu S, Wang Y. Quantification of Nutrient Fluxes from Sediments of Lake Hulun, China: Implications for Plateau Lake Management. Sustainability. 2023; 15(11):8680. https://doi.org/10.3390/su15118680
Chicago/Turabian StyleLiu, Bo, Xiaofei Zhang, Yi Tong, Wen Ao, Zenglong Wang, Senlin Zhu, and Yanping Wang. 2023. "Quantification of Nutrient Fluxes from Sediments of Lake Hulun, China: Implications for Plateau Lake Management" Sustainability 15, no. 11: 8680. https://doi.org/10.3390/su15118680
APA StyleLiu, B., Zhang, X., Tong, Y., Ao, W., Wang, Z., Zhu, S., & Wang, Y. (2023). Quantification of Nutrient Fluxes from Sediments of Lake Hulun, China: Implications for Plateau Lake Management. Sustainability, 15(11), 8680. https://doi.org/10.3390/su15118680







