Assessing the Impact of Biodiversity (Species Evenness) on the Trophic Position of an Invasive Species (Apple Snails) in Native and Non-Native Habitats Using Stable Isotopes
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Collection Sites
- A.
- Maldonado, Uruguay
- B.
- China
- C.
- Oahu, Hawaii, USA
2.2. Apple Snails, Other Sympatric Animal Species, and Detritus
2.3. Species Barcoding
2.4. Polymerase Chain Reaction (PCR) Amplification
| 5 Min | 1 Min | 1 Min | 30 Sec | 20 Sec | 25 Sec | 10 Min | 50 Cycles |
| 95 °C | 45 °C | 72 °C | 95 °C | 48 °C | 72 °C | 4 °C |
| 5 Min | 1 Min | 1 Min | 30 Sec | 30 Sec | 45 Sec | 10 Min | 75 Cycles |
| 95 °C | 45 °C | 72 °C | 95 °C | 48 °C | 72 °C | 4 °C |
| 5 Min | 1 Min | 1 Min | 30 Sec | 30 Sec | 45 Sec | 10 Min | 60 Cycles |
| 95 °C | 45 °C | 72 °C | 95 °C | 48 °C | 72 °C | 4 °C |
2.5. Stable Isotope Analysis (13C and 15N)
2.6. Stable Isotope Data Analysis and Trophic-Level Determination and Evenness
1/(# of the most abundant species collected/ total # of organisms collected)
3. Results
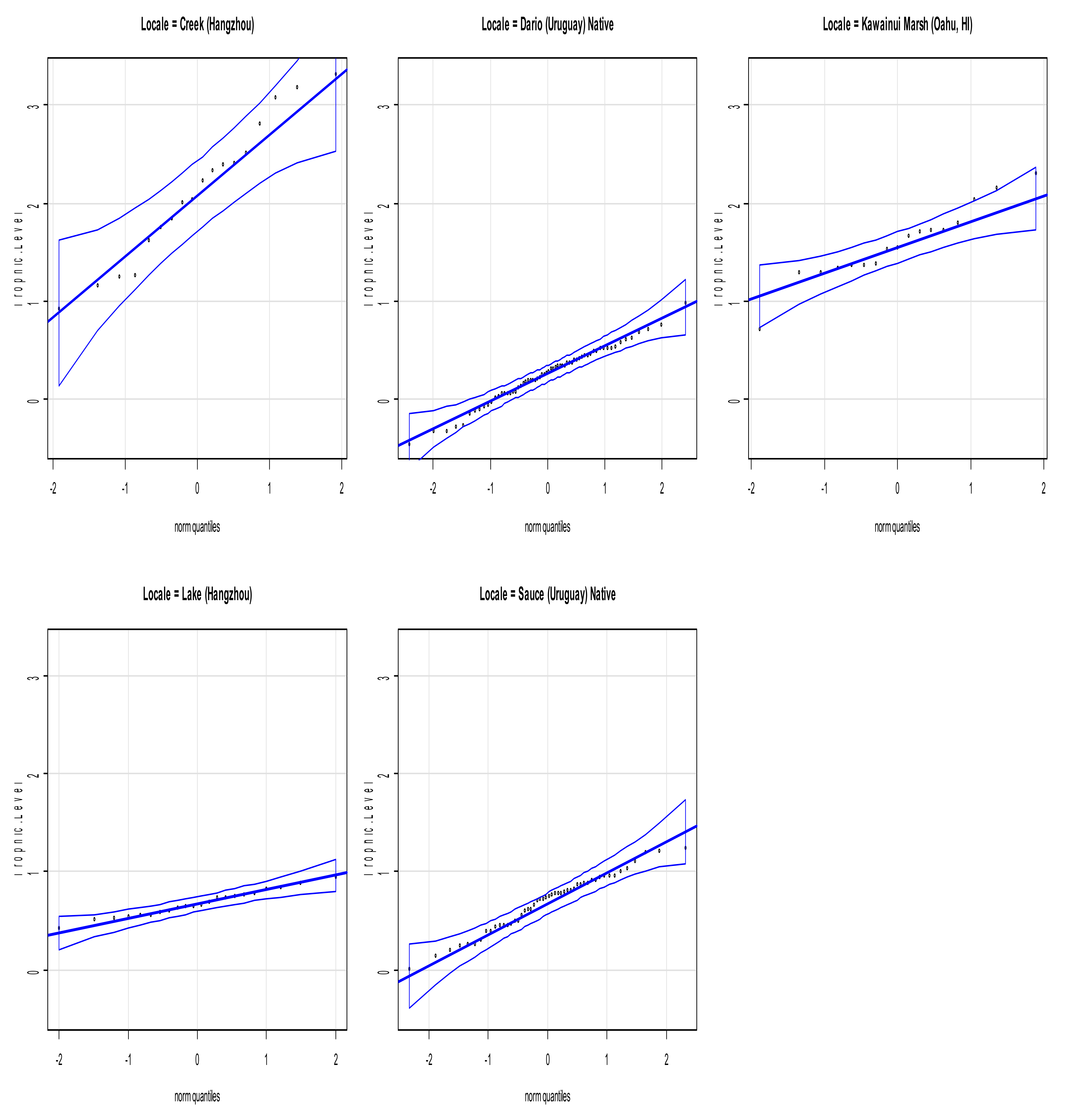
3.1. Linear Regression of Species Evenness versus Trophic Level for All Collection Sites
3.2. Interquartile Plots of Calculated Trophic Levels for P. canaliculata
4. Discussion
- An investigation of the possible relationship between the nutritional value of the plant species that comprise the apple snail diets (as previously determined using SIAR mixing models by Scriber, France, and Jackson [54])
- As well as an investigation into the differences in the variability (s2) of the trophic level occupied by these aquatic invasives to test the hypothesis that they ecological niche they occupy in aquatic habitats:
- Not only changes in the calculated trophic level of this aquatic biological invader between habitats, shown to exist by this study.
- But also the constriction or expansion of their ecological niche space quantified as significant differences amongst the calculated trophic-level sample variance s2 (a point estimate of σ2) between habitats.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lovejoy, T.E. The Global 2000 Report to the President; Barney, G.O., Ed.; Pergamon Press: New York, NY, USA, 1980; Volume 2, pp. 327–332. [Google Scholar]
- Fisher, R.A.; Corbet, A.S.; Williams, C.B. The relation between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 1943, 12, 42–58. [Google Scholar] [CrossRef]
- Williams, P.H.; Humphries, C.J.; Vane-Wright, R.I. Measuring biodiversity: Taxonomic relatedness for conservation priorities. Aust. Syst. Bot. 1991, 4, 665–679. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- McIntyre, P.B.; Jones, L.E.; Flecker, A.S.; Vanni, M.J. Fish extinctions alter nutrient recycling in tropical freshwaters. Proc. Natl. Acad. Sci. USA 2007, 104, 4461–4466. [Google Scholar] [CrossRef]
- Huston, M.A. Hidden treatments in ecological experiments: Re-evaluating the ecosystem function of biodiversity. Oecologia 1997, 110, 449–460. [Google Scholar] [CrossRef]
- Wardle, D.A.; Huston, M.A.; Grime, J.P.; Berendse, F.; Garnier, E.; Lauenroth, W.K.; Setälä, H.; Wilson, S.D. Biodiversity and ecosystem function: An issue in ecology. Bull. Ecol. Soc. Am. 2000, 81, 235–239. [Google Scholar]
- Lepš, J. What do the biodiversity experiments tell us about the consequences of plant species loss in the real world? Basic Appl. Ecol. 2004, 5, 529–534. [Google Scholar] [CrossRef]
- Midgley, G.F. Biodiversity and ecosystem function. Science 2012, 335, 174–175. [Google Scholar] [CrossRef]
- Duffy, J.E. Why biodiversity is important to the functioning of real-world ecosystems. Front. Ecol. Environ. 2009, 7, 437–444. [Google Scholar] [CrossRef]
- Smith, B.D.; Zeder, M.A. The onset of the Anthropocene. Anthropocene 2013, 4, 8–13. [Google Scholar] [CrossRef]
- Rosa, E.A.; York, R.; Dietz, T. Tracking the anthropogenic drivers of ecological impacts. AMBIO A J. Hum. Environ. 2004, 33, 509–513. [Google Scholar] [CrossRef]
- Foley, J.A.; Defries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Review global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Heip, C. A new index measuring evenness. J. Mar. Biol. Assoc. UK 1974, 54, 555–557. [Google Scholar] [CrossRef]
- Heip, C.H.; Herman, P.M.; Soetaert, K. Indices of diversity and evenness. Oceanis 1998, 24, 61–88. [Google Scholar]
- Stirling, G.; Wilsey, B. Empirical relationships between species richness, evenness, and proportional diversity. Am. Nat. 2001, 158, 286–299. [Google Scholar] [CrossRef]
- Jost, L. The relation between evenness and diversity. Diversity 2010, 2, 207–232. [Google Scholar] [CrossRef]
- Vaughn, C.C. Biodiversity losses and ecosystem function in freshwaters: Emerging conclusions and research directions. BioScience 2010, 60, 25–35. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Naeem, S. Ecosystem consequences of biodiversity loss: The evolution of a paradigm. Ecology 2002, 83, 1537–1552. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A sequential and selective process. In Biotic Homogenization; Springer: Boston, MA, USA, 2001; pp. 1–17. [Google Scholar]
- Trentanovi, G.; Von der Lippe, M.; Sitzia, T.; Ziechmann, U.; Kowarik, I.; Cierjacks, A. Biotic homogenization at the community scale: Disentangling the roles of urbanization and plant invasion. Divers. Distrib. 2013, 19, 738–748. [Google Scholar] [CrossRef]
- Gámez-Virués, S.; Perović, D.J.; Gossner, M.M.; Börschig, C.; Blüthgen, N.; De Jong, H.; Simons, N.K.; Klein, A.M.; Krauss, J.; Maier, G.; et al. Landscape simplification filters species traits and drives biotic homogenization. Nat. Commun. 2015, 6, 8568. [Google Scholar] [CrossRef]
- Davis, M.A. Biotic globalization: Does competition from introduced species threaten biodiversity? Bioscience 2003, 53, 481–489. [Google Scholar] [CrossRef]
- Simon, K.S.; Townsend, C.R. Impacts of freshwater invaders at different levels of ecological organisation, with emphasis on salmonids and ecosystem consequences. Freshw. Biol. 2003, 48, 982–994. [Google Scholar] [CrossRef]
- Jackson, M.C.; Wasserman, R.J.; Grey, J.; Ricciardi, A.; Dick, J.T.; Alexander, M.E. Novel and disrupted trophic links following invasion in freshwater ecosystems. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 2017; Volume 57, pp. 55–97. [Google Scholar]
- Hayes, K.A.; Burks, R.L.; Castro-Vazquez, A.; Darby, P.C.; Heras, H.; Martín, P.R.; Qiu, J.W.; Thiengo, S.C.; Vega, I.A.; Wada, T.; et al. Insights from an integrated view of the biology of apple snails (Caenogastropoda: Ampullariidae). Malacologia 2015, 58, 245–303. [Google Scholar] [CrossRef]
- Carlsson, N.O.; Brönmark, C.; Hansson, L.A. Invading herbivory: The golden apple snail alters ecosystem functioning in Asian wetlands. Ecology 2004, 85, 1575–1580. [Google Scholar] [CrossRef]
- Karraker, N.E.; Dudgeon, D. Invasive apple snails (Pomacea canaliculata) are predators of amphibians in South China. Biol. Invasions 2014, 16, 1785–1789. [Google Scholar] [CrossRef]
- López-Van Oosterom, M.V.; Ocon, C.S.; Ferreira, A.C.; Rodrigues Capítulo, A. The diet of Pomacea canaliculata (Gastropoda: Ampullariidae) in its native habitat based on gut content and stable isotopes analysis. Intropica 2016, 11, 73–83. [Google Scholar] [CrossRef]
- Yam, R.; Fan, Y.T.; Wang, T.T. Importance of macrophyte quality in determining life-history traits of the apple snails Pomacea canaliculata: Implications for bottom-up management of an invasive herbivorous pest in constructed wetlands. Int. J. Environ. Res. Public Health 2016, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Saveanu, L.; Manara, E.; Martín, P.R. Carrion consumption and its importance in a freshwater trophic generalist: The invasive apple snail Pomacea canaliculata. Mar. Freshw. Res. 2017, 68, 752–759. [Google Scholar] [CrossRef]
- Bourne, G.R. Differential snail-size predation by snail kites and limpkins. Oikos 1993, 68, 217–223. [Google Scholar] [CrossRef]
- Reed, W.L.; Janzen, F.J. Natural selection by avian predators on size and colour of a freshwater snail (Pomacea flagellata). Biol. J. Linn. Soc. 1999, 67, 331–342. [Google Scholar] [CrossRef]
- Carlsson, N.O.; Kestrup, Å.; Mårtensson, M.; Nyström, P. Lethal and non-lethal effects of multiple indigenous predators on the invasive golden apple snail (Pomacea canaliculata). Freshw. Biol. 2004, 49, 1269–1279. [Google Scholar] [CrossRef]
- Tanaka, M.O.; Souza, A.L.; Módena, É.S. Habitat structure effects on size selection of snail kites (Rostrhamus sociabilis) and limpkins (Aramus guarauna) when feeding on apple snails (Pomacea spp.). Acta Oecologica 2006, 30, 88–96. [Google Scholar] [CrossRef]
- Platt, S.G.; Rainwater, T.R.; Finger, A.G.; Thorbjarnarson, J.B.; Anderson, T.A.; McMurry, S.T. Food habits, ontogenetic dietary partitioning and observations of foraging behaviour of Morelet’s crocodile (Crocodylus moreletii) in northern Belize. Herpetol. J. 2006, 16, 281–290. [Google Scholar]
- Yusa, Y.; Sugiura, N.; Wada, T. Predatory potential of freshwater animals on an invasive agricultural pest, the apple snail Pomacea canaliculata (Gastropoda: Ampullariidae), in southern Japan. Biol. Invasions 2006, 8, 137–147. [Google Scholar] [CrossRef]
- Francis, R.A. A Handbook of Global Freshwater Invasive Species; Routledge: London, UK, 2012; pp. 1–376. [Google Scholar]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species A selection from the Global Invasive Species Database; The Invasive Species Specialist Group (ISSG) a Specialist Group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN): Copenhagen, Denmark, 2000; pp. 1–12. [Google Scholar]
- Cowie, R.H. Apple snails (Ampullariidae) as agricultural pests: Their biology, impacts and management. In Molluscs as Crop Pests; Editor Barker, G.M., Ed.; Centre for Agriculture and Bioscience International: Wallingford, UK, 2002; pp. 145–192. [Google Scholar]
- Levin, P. Statewide Strategic Control Plan for Apple Snail (Pomacea canaliculata) in Hawai ‘I; The Hawaii Land Restoration Institute: Wailuku, HI, USA, 2006; pp. 1–20. [Google Scholar]
- Gilal, A.A.; Muhamad, R.; Omar, D.; Aziz, N.A.A.; Gnanasegaram, M. Foes can be Friends: Laboratory Trials on Invasive Apple Snails, Pomacea sPreference to Invasive Weed, Limnocharis flava (L.) Buchenau Compared to Rice. Oryza sativa. Pak. J. Zool. 2016, 48, 673–679. [Google Scholar]
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic shifts in ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef]
- Fickbohm, S.S.; Zhu, W.X. Exotic purple loosestrife invasion of native cattail freshwater wetlands: Effects on organic matter distribution and soil nitrogen cycling. Appl. Soil Ecol. 2006, 32, 123–131. [Google Scholar] [CrossRef]
- Hobson, K.A. Tracing origins and migration of wildlife using stable isotopes: A review. Oecologia 1999, 120, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, D.R.; Hobson, K.A. From birds to butterflies: Animal movement patterns and stable isotopes. Trends Ecol. Evol. 2004, 19, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.C.; Cavelier, J.; Goldstein, G.; Meinzer, F.C.; Holbrook, N.M. Partitioning of water resources among plants of a lowland tropical forest. Oecologia 1995, 101, 197–203. [Google Scholar] [CrossRef]
- Young, H.S.; McCauley, D.J.; Dirzo, R.; Dunbar, R.B.; Shaffer, S.A. Niche partitioning among and within sympatric tropical seabirds revealed by stable isotope analysis. Mar. Ecol. Prog. Ser. 2010, 416, 285–294. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Scriber, K.E., II; France, C.A.M.; Jackson, F.L.C. Invasive Apple Snail Diets in Native vs. Non-Native Habitats Defined by SIAR (Stable Isotope Analysis in R). Sustainability 2022, 14, 7108. [Google Scholar] [CrossRef]
- García-Robledo, C.; Erickson, D.L.; Staines, C.L.; Erwin, T.L.; Kress, W.J. Tropical plant–herbivore networks: Reconstructing species interactions using DNA barcodes. PLoS ONE 2013, 8, e52967. [Google Scholar] [CrossRef]
- Brandon-Mong, G.J.; Gan, H.M.; Sing, K.W.; Lee, P.S.; Lim, P.E.; Wilson, J.J. DNA metabarcoding of insects and allies: An evaluation of primers and pipelines. Bull. Entomol. Res. 2015, 105, 717–727. [Google Scholar] [CrossRef]
- Colgan, D.J.; Hutchings, P.A.; Brown, S. Phylogenetic relationships within the Terebellomorpha. J. Mar. Biol. Assoc. UK 2001, 81, 765–773. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P.D. Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Schimmelmann, A.; Albertino, A.; Sauer, P.E.; Qi, H.; Molinie, R.; Mesnard, F. Nicotine, acetanilide and urea multi-level 2H-, 13C- and 15N-abundance reference materials for continuous-flow isotope ratio mass spectrometry. Rapid Commun. Mass Spectrom. Int. J. Devoted Rapid Dissem. Up-Minute Res. Mass Spectrom. 2009, 23, 3513–3521. [Google Scholar] [CrossRef] [PubMed]
- Vanderklift, M.A.; Ponsard, S. Sources of variation in consumer-diet δ 15 N enrichment: A meta-analysis. Oecologia 2003, 136, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Iacarella, J.C.; Dick, J.T.; Ricciardi, A. A spatio-temporal contrast of the predatory impact of an invasive freshwater crustacean. Divers. Distrib. 2015, 21, 803–812. [Google Scholar] [CrossRef]
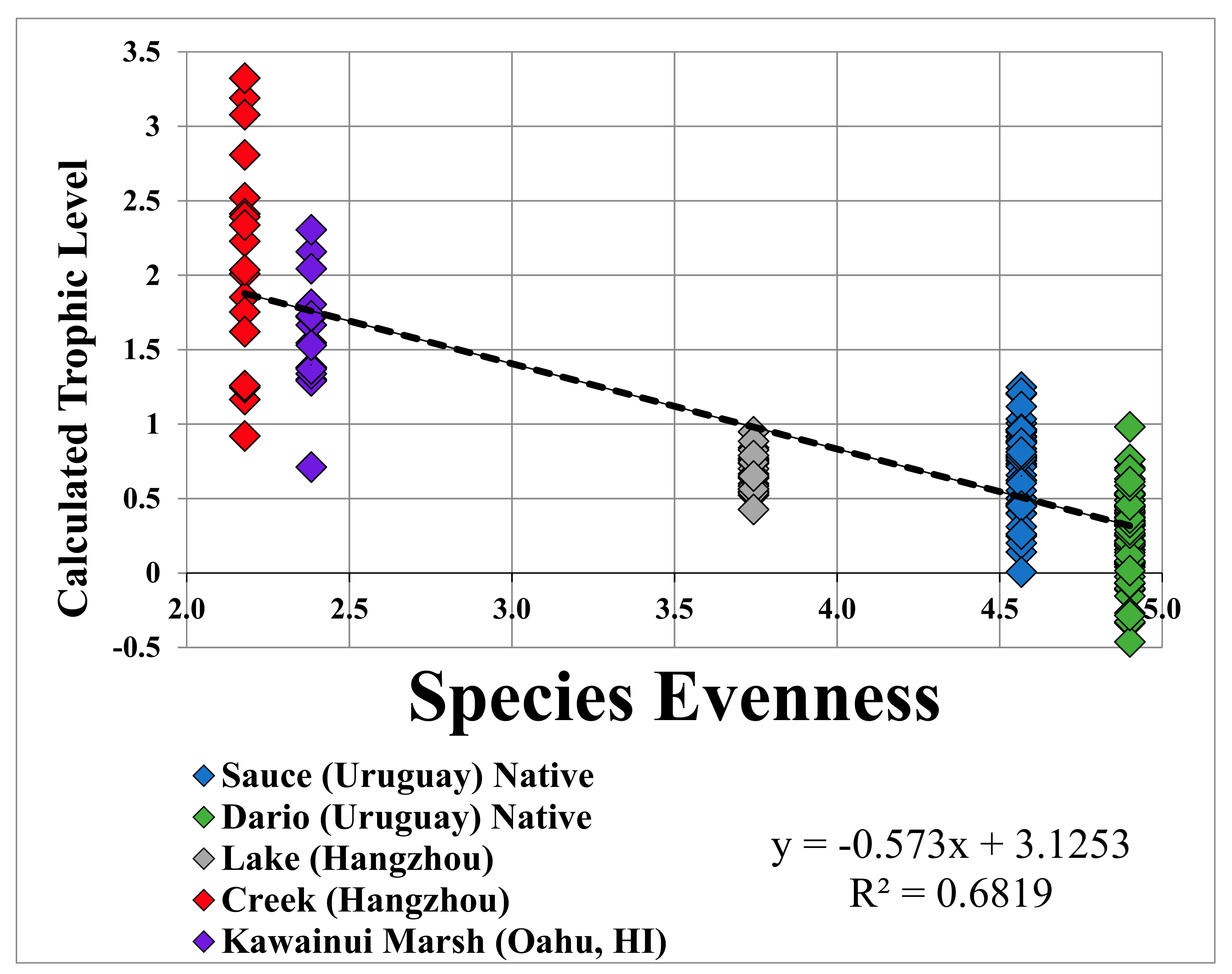
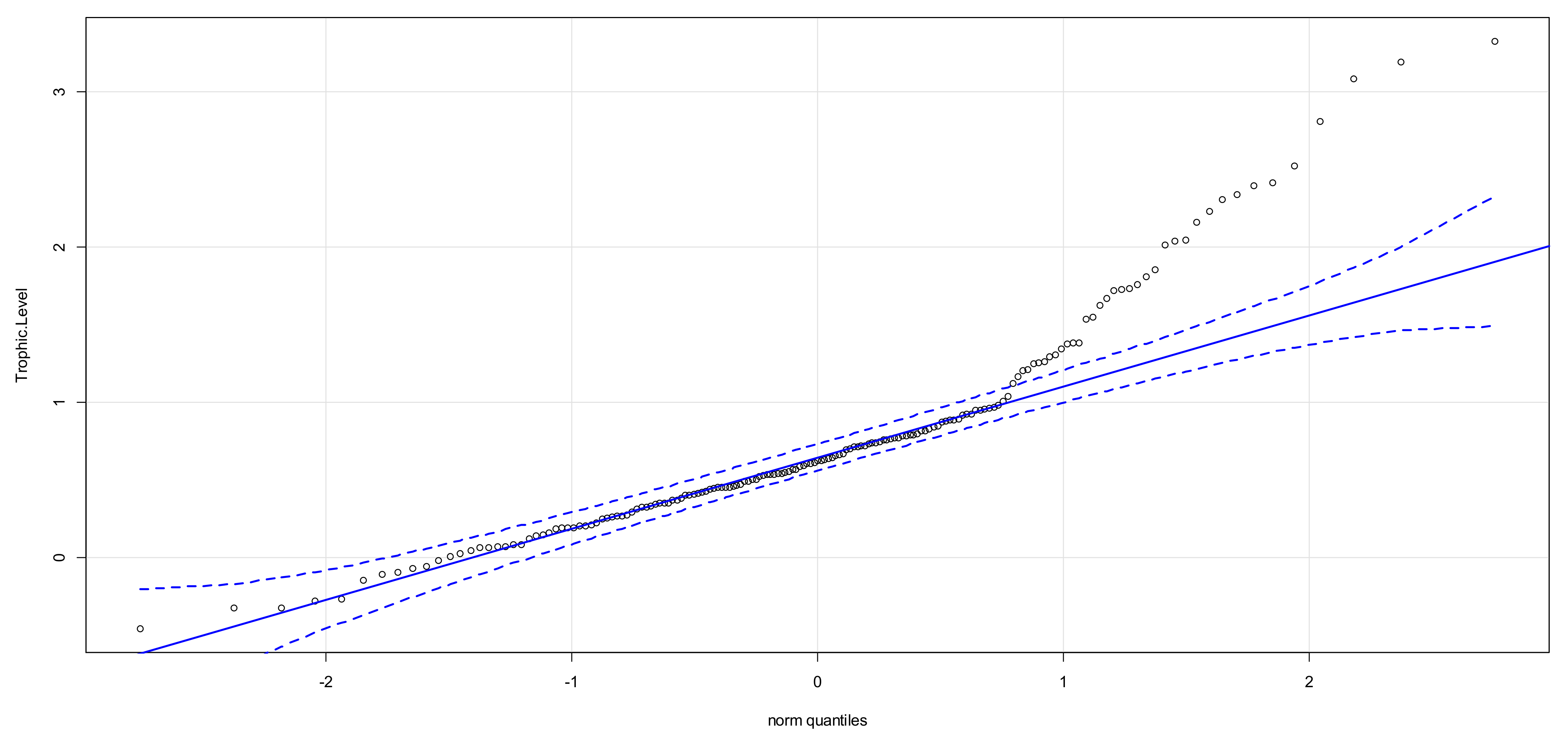
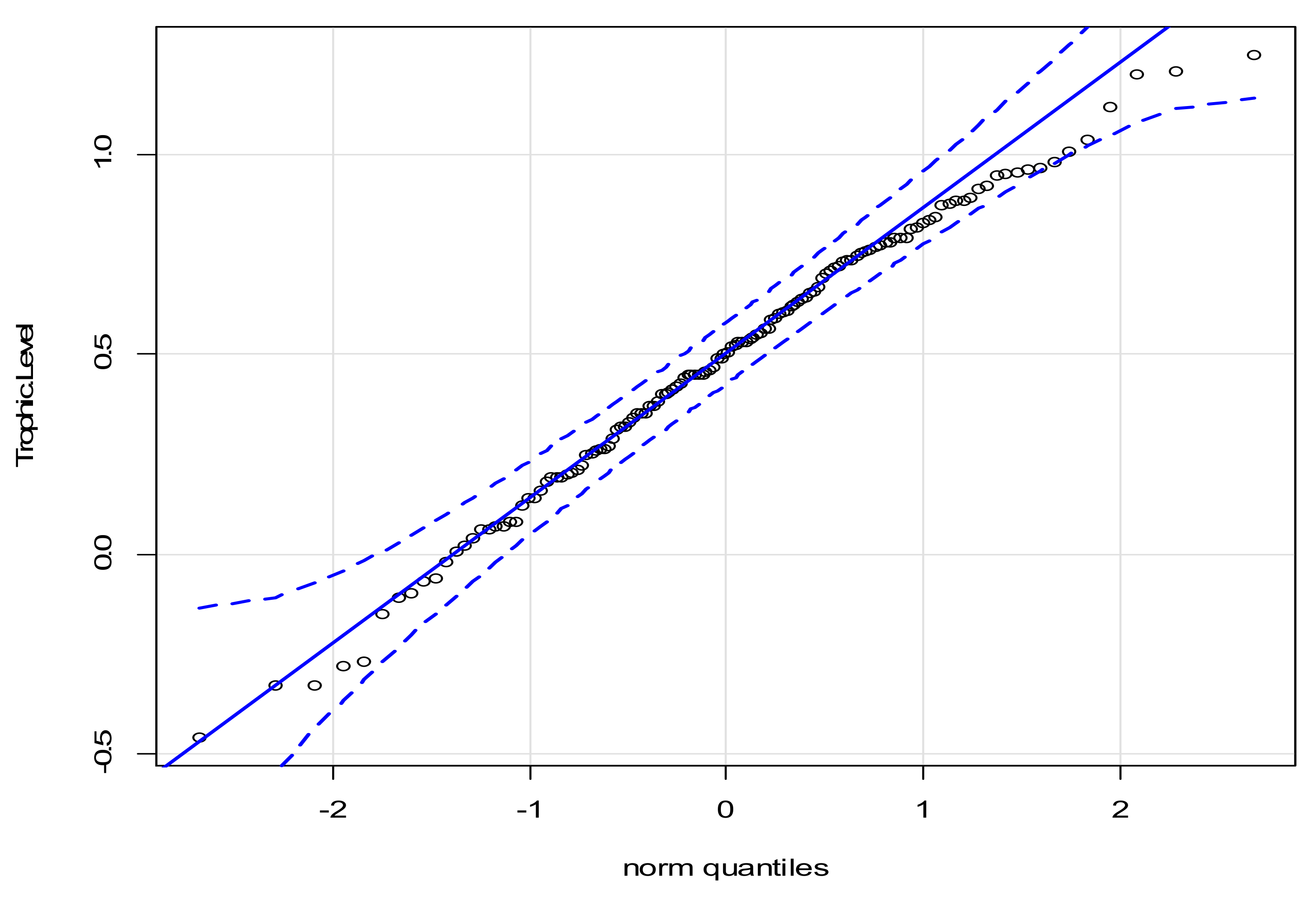
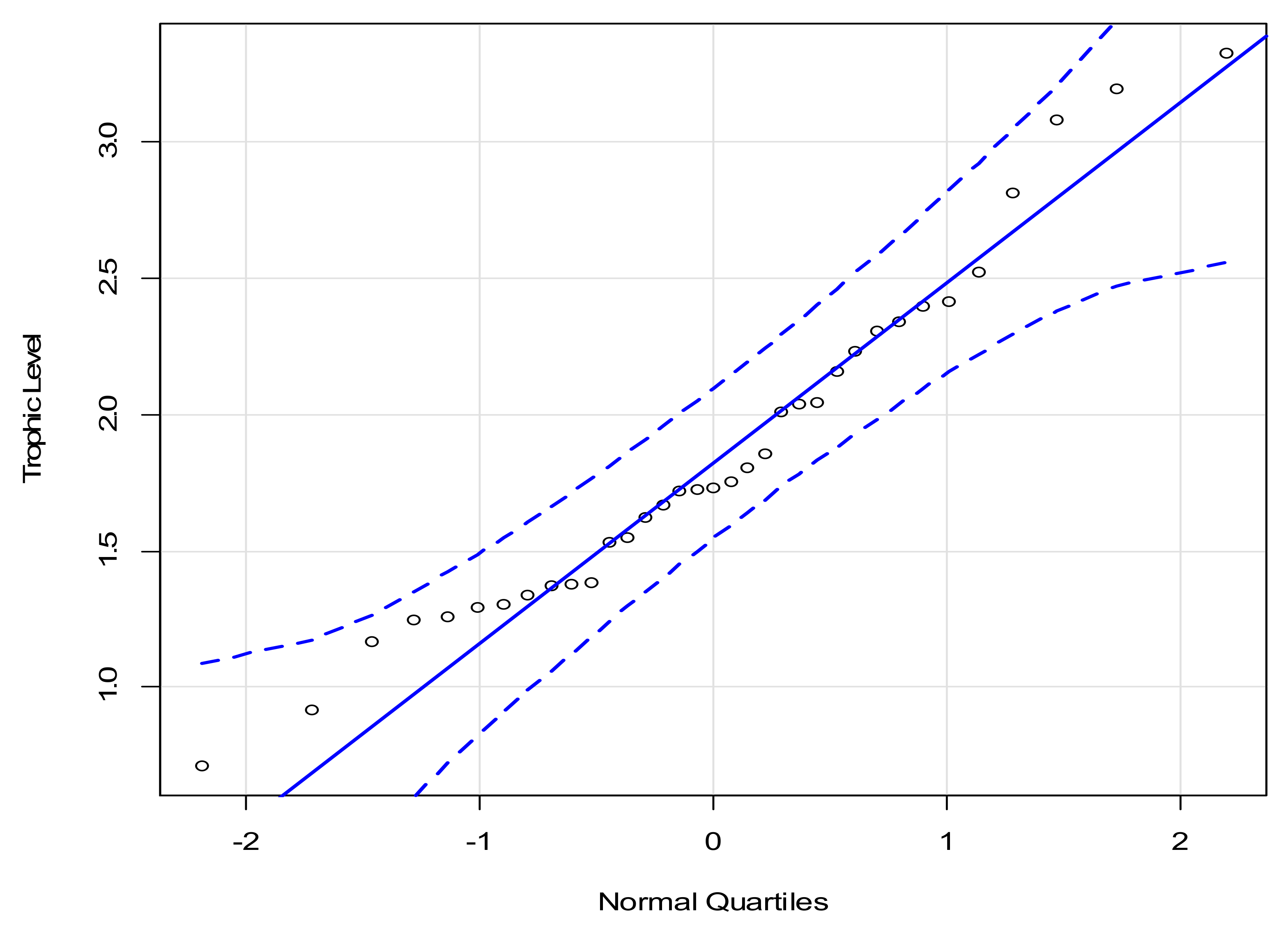
| SIA Sample | Site | n | δ15N (Mean) (‰, Air) | δ15N (stdv) (‰, Air) | Species Evenness | P. canaliculata
Trophic Level | P. canaliculata
Trophic Level (stdv) |
|---|---|---|---|---|---|---|---|
| P. canaliculata | Lake Dario | 64 | 2.0 | 0.9 | 4.9 | 0.3 | 0.3 |
| Detritus | Lake Dario | 5 | 1.3 | 0.8 | 4.9 | ||
| P. canaliculata | Lake Sauce | 50 | 4.9 | 0.9 | 4.6 | 0.7 | 0.3 |
| Detritus | Lake Sauce | 5 | 2.8 | 0.8 | 4.6 | ||
| P. canaliculata | Chinese Lake | 50 | 4.0 | 1.0 | 3.7 | 0.4 | 0.3 |
| Detritus | Chinese Lake | 5 | 2.9 | 1.0 | 3.7 | ||
| P. canaliculata | Kawainui | 17 | 8.1 | 1.1 | 2.4 | 1.6 | 0.4 |
| Detritus | Kawainui | 5 | 3.4 | 1.4 | 2.4 | ||
| P. canaliculata | Chinese Creek | 18 | 6.2 | 2.1 | 2.2 | 2.1 | 0.7 |
| Detritus | Chinese Creek | 5 | −0.1 | 3.3 | 2.2 |
| Statistical Analysis | Results | ||
|---|---|---|---|
| Linear regression model | p-value < 0.001 | R2 = 0.6821 | Adjusted R2 = 0.8602 |
| Pearson’s product-moment correlation | p-value < 0.001 | 95% confidence interval for correlation coefficient (−0.87, −0.77) | Correlation coefficient −0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scriber, K.E., II; France, C.A.M.; Jackson, F.L.C. Assessing the Impact of Biodiversity (Species Evenness) on the Trophic Position of an Invasive Species (Apple Snails) in Native and Non-Native Habitats Using Stable Isotopes. Sustainability 2023, 15, 8560. https://doi.org/10.3390/su15118560
Scriber KE II, France CAM, Jackson FLC. Assessing the Impact of Biodiversity (Species Evenness) on the Trophic Position of an Invasive Species (Apple Snails) in Native and Non-Native Habitats Using Stable Isotopes. Sustainability. 2023; 15(11):8560. https://doi.org/10.3390/su15118560
Chicago/Turabian StyleScriber, Kevin E., II, Christine A. M. France, and Fatimah L. C. Jackson. 2023. "Assessing the Impact of Biodiversity (Species Evenness) on the Trophic Position of an Invasive Species (Apple Snails) in Native and Non-Native Habitats Using Stable Isotopes" Sustainability 15, no. 11: 8560. https://doi.org/10.3390/su15118560
APA StyleScriber, K. E., II, France, C. A. M., & Jackson, F. L. C. (2023). Assessing the Impact of Biodiversity (Species Evenness) on the Trophic Position of an Invasive Species (Apple Snails) in Native and Non-Native Habitats Using Stable Isotopes. Sustainability, 15(11), 8560. https://doi.org/10.3390/su15118560








