Stoichiometric Characteristics of Abies georgei var. smithii Plants in Southeast Tibet
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Site Survey
2.3. Sample Collection and Analysis
2.4. Data Analysis
3. Results
3.1. Characteristics of Variation in C, N, P, and K Contents of Different Organs and Their Stoichiometric Ratios
3.2. Effect of Different Seasons at the Same Elevation on the C, N, P, and K Contents of Various Organs of Abies georgei var. smithii
3.3. Effect of Different Elevations on Ecological Stoichiometric Ratios of Various Organs of Abies georgei var. smithii in the Same Season
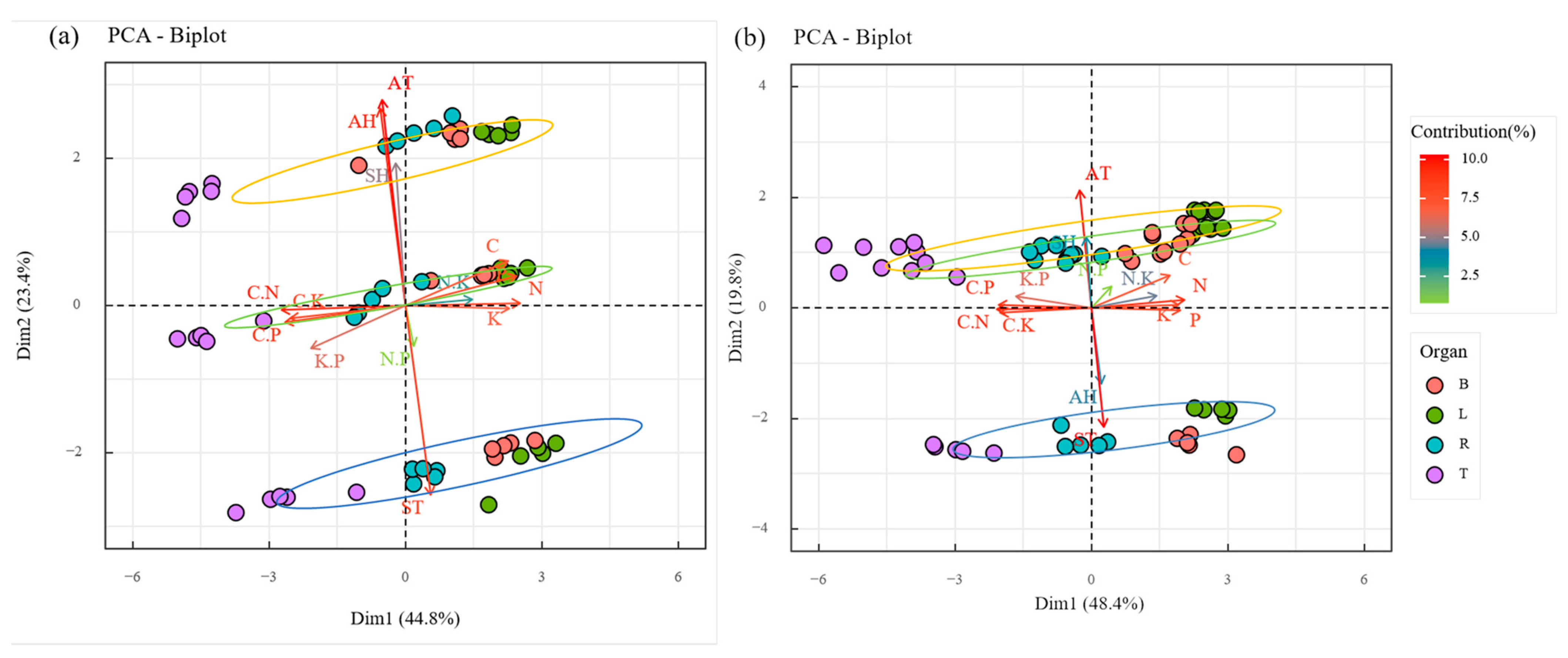
3.4. Principal Component Analysis of Environmental Factors on Plant C, N, P, K, and Stoichiometric Ratios
4. Discussion
4.1. Spatial and Temporal Dynamics of Inter-Organ Nutrient Content of Abies georgei var. Smithii
4.2. Spatial and Temporal Dynamics of Inter-Organ Stoichiometric Ratios in Abies georgei var. smithii
4.3. Relationship between Stoichiometric Ratios and Seasonal Variation in Various Organs of Abies georgei var. smithii
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, X.; Zhang, J.; Xin, Z.; Huang, Y.; Han, C.; Li, Y.; Lu, Q. Ecological Stoichiometric Characteristics in Organs of Ammopiptanthus mongolicus in Different Habitats. Plants 2023, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.J.; Suo, P.C.; Zhang, L.M.; Fu, Y.H.; Li, A.D. C, N, P stoichiometric characteristics in different organs of three constructive plants in Karst peak-cluster depressions in southern Guizhou, Southwest China. J. Guizhou Norm. Univ. 2021, 39, 36–44. [Google Scholar]
- Meng, Q.Q.; Ge, L.L.; Yang, X.M.; Wang, J.; Lin, Y.; He, Z.M.; QIU, L.Y.; Hu., H.T. Seasonal variation of C, N, and P stoichiometric characteristics in leaves of two plantations in Sanming, Fujian. Chin. J. Appl. Environ. Biol. 2019, 25, 776–782. [Google Scholar]
- Huang, K.; Cao, X.W.; Liu, J.Q.; Zhang, T.; Wang, F.; Huang, X.D. Ecological Stoichiometric Characteristics of C, N and P Contained in Different Organs of Quercus wutaishansea mary. Shaanxi For. Sci. Technol. 2021, 49, 9–13. [Google Scholar]
- Liu, S.; Luo, D.; Liu, Q.L.; Zhang, L.; Yang, H.G.; Shi, Z.M. Carbon and nitrogen storage and distribution in different forest ecosystems in the subalpine of western Sichuan. Acta Ecol. Sin. 2017, 37, 1074–1083. [Google Scholar]
- Wang, Y.H.; Cui, Y.F.; Xue, L.L. The reserve of carbon, nitrogen, phosphorus and potassium and their distribution features in youngand middle-aged Cupressus funebris forests in the Wuling MountainArea of Chongqing. J. Sichuan For. Sci. 2018, 39, 30. [Google Scholar]
- Zhao, Y.F.; Xu, F.L.; Wang, W.L.; Wang, G.X.; Chen, Q.C.; Zhao, H.Y.; Ma, Y.J. Seasonal variations of leaf C, N, P contents and stoichiometry of Larix principis-rupprechtii. J. Plant Nutr. Fertil. 2015, 21, 1328–1335. [Google Scholar]
- Wassen, M.J.; Olde Venterink, H.G.M.; de Swart, E.O.A.M. Nutrient concentrations in mire vegetation as a measure of nutrient limitation in mire ecosystems. J. Veg. Sci. 1995, 6, 5–16. [Google Scholar] [CrossRef]
- Guo, S.J.; Xie, M.M.; Zhang, L.; Sun, H.J.; Song, Y. Temporal variation of C, N, P stoichiometric in fine roots of Castanea mollissima. J. Plant Nutr. Fertil. 2018, 24, 825–832. [Google Scholar]
- Zhang, X.L.; Qin, H.; Niu, J.J.; Zhang, Y.B.; Shi, L.H.; Zheng, Y.R. Community Diversity and C, N and P Stoichiometric Characteristics of Subalpine-Alpine Meadows in Wutai Mountain. Res. Environ. Sci. 2022, 35, 2175–2184. [Google Scholar]
- Zhang, P.; Shen, Y.; Zhang, X.J.; Die, M.H.; Wen, H.C.; Ma, H.B. Ecological stoichiometric characteristics of C, N and P in leaves of dominant plants and community stability in Ningxia desert grassland. Chin. J. Grassl. 2022, 44, 18–26. [Google Scholar]
- Zuo, W.; He, K.N.; Tian, Y.; Wang, W.L. Surface litter stoichiometry for five forest types in alpine region, Qinghai, China. Chin. J. Ecol. 2016, 35, 2271. [Google Scholar]
- Yu, L.H.; Fang, X.; Xiang, W.H.; Shi, J.; Liu, Z.D.; Li, L.D. Stoichiometry of Carbon, Nitrogen, and Phosphorus in Litter and Soil of Four Types of Subtropical Stand. Sci. Silvae Sin. 2016, 52, 10–21. [Google Scholar]
- Qui, J.; Dai, H.T.; Xing, Y.Z.; Huang, D.J.; Yi, Q.J.; Cheng, D.W. Leaves, stems and roots stoichiometry characteristics of mangrove plants at different succession stages in Shankou National Mangrove Nature Reserve, China. J. Trop. Oceanogr. 2023, 1–12. [Google Scholar]
- Yuan, C.J.; Yu, L.F.; Yan, L.B.; Pi, F.J.; Yang, R.; Wu, L.; Li, X.J. Stoichiometry Characteristics of Leaf in Plant Functional Groups at Different Community Succession Stages in Central Guizhou Karst Area. J. West China For. Sci. 2017, 46, 124–132. [Google Scholar]
- Li, Z.F.; Liu, W.S.; Zhang, B.; Zheng, Z. Response of C:N:P ecological stoichiometric ratio of young tree leavesin tropical seasonal rain forest of Xishuangbanna to change of elevation. J. Cent. South Univ. For. Technol. 2012, 32, 80–85. [Google Scholar]
- Li, A.Q.; Zhang, S.S.; Wang, H.R.; Zhang, D.D.; Zhao, X.Y.; Guan, M.R.; Xu, X.N. Fine root morphological characteristics and its functions in mature Chinese firplantations along an elevation gradient in Dabie Mountains. Acta Ecol. Sin. 2020, 40, 719–727. [Google Scholar]
- Zhu, W.T.; Liu, H.S.; He, R.; Yu, D.R.; Xia, Y.; Dang, H.S. Spatial point pattern analysis and spatio-temporal dynamics of Abies georgei var. smithii forests in southeast Tibet. Acta Ecol. Sin. 2022, 42, 8977–8984. [Google Scholar]
- Li, J.R.; Gao, T.; Zhang, W.L.; Lu, J. Soil nutrient characteristics of four forest types of Abies georgei var. smithii in southeastern Tibet. J. Cent. South Univ. For. Technol. 2021, 41, 108–119. [Google Scholar]
- Luo, L.; Dan, Z.; Zhu, L.P.; Zhang, H.B. Vertical Gradient Changes of Temperature and Precipitation in the Sygera Mountains, Southeastern Qinghai-Xizang Plateau. Plateau Meteorol. 2021, 40, 37–46. [Google Scholar]
- Kobierski, M.; Dlugosz, J.; Piotrowska-Dlugosz, A. Determination of spatial variability of some magnesium forms in phaeozem using geostatistical methods. J. Elem. 2014, 19, 165–176. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.N.; Li, Z.C.; Wang, B.; Zhang, Y.J.; Huang, S.Y. Ecological stoichiometry in leaves, branches and roots of Torreya grandis with different forest ages and its stoichiometric homoeostasis. J. Nanjing For. Univ. 2021, 45, 135–142. [Google Scholar]
- Zhao, Y.F.; Xu, F.L.; Wang, W.L.; Wang, L.L.; Wang, G.X.; Sun, P.Y.; Bai, X.F. Seasonal variation in carbon, nitrogen and phosphorus content and their stoichiometric characteristics in the rootstocks and leaves of larch in northern China. Chin. Bull. Bot. 2014, 49, 560–568. [Google Scholar]
- Wei, Y.W.; Zhang, T.; Liu, J.; Han, X.; Qin, S.J.; Du, T.Y.; Zhou, Y.B. Spatial Distribution and Stoichiometry Characteristics of Leaves and Soil Organic Carbon, Nitrogen, Phosphorus and Calcium of the Main Needle Tree Species-Pinus koraiensis. J. Shenyang Agric. Univ. 2021, 52, 419–427. [Google Scholar]
- Pan, L.H.; Wei, J.L.; Chen, Y.S.; Zeng, C.; Fan, H.Q. Cyperus malaccensis and the distribution characteristics and seasonal dynamics of organic carbon, total nitrogen and total phosphorus in sediments. Wetl. Sci. 2012, 10, 467–473. [Google Scholar]
- Zheng, S.X.; Shang Guan, Z.P. Spatial distribution patterns of plant leaf nutrient composition in the Loess Plateau region. Prog. Nat. Sci. 2006, 21, 965–973. [Google Scholar]
- Chen, F.J.; Li, B.; Zhang, J.; Chen, W.N.; Wu, D.; Zhang, M. Analysis of Chemical Metrological Characteristics of 13 Main Plant Leaves in the Northwestern Plateau of Sichuan. J. Nucl. Agric. Sci. 2017, 31, 1179–1184. [Google Scholar]
- Liu, W.D.; Su, J.R.; Li, S.F.; Liang, X.D.; Zhang, Z.J.; Huang, X.B. Stoichiometry study of C, N and P in plant and soil at different successional stages of monsoon evergreen broad-leaved forest in Pu’er, Yunnan Province. Acta Ecol. Sin. 2015, 39, 52–62. [Google Scholar]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Song, X.Q.; Zhang, J.W.; Zhang, Z.H.; Tang, Z.H. Ecological Stoichiometric Characteristics of C, N and P and Their Relationship with Soil Factors from Different Organs of the Halophytic Chenopodiaceae Plants in Hulunbuir. Bull. Bot. Res. 2022, 42, 910–920. [Google Scholar]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Zhang, D.Y. Evolution of Plant Life History and Reproductive Ecology; Science Press: Beijing, China, 2004. [Google Scholar]
- Shucun, S.; Lingzhi, C. Leaf nutrient dynamics and resorption efficiency of Quercus liaotungensis in the Dongling Mountain region. Acta Phytoecol. Sin. 2001, 25, 76–82. [Google Scholar]
- Li, Z.; Han, L.; Liu, Y.H.; An, S.Q.; Leng, X. C, N and P stoichiometric characteristics in leaves of Suaeda salsa during different growth phase in coastal wetlands of China. Chin. J. Plant Ecol. 2012, 36, 1054–1061. [Google Scholar] [CrossRef]
- Yang, X.Y.; Han, Y.Z.; Zhang, Y.X.; Wu, X.G. Effects of cutting disturbance on spatial heterogeneity of fine root biomass of Larix principis-rupprechtii. Acta Ecol. Sin. 2012, 32, 64–73. [Google Scholar] [CrossRef]
- Xie, H.C.; Ge, Y.; Sui, J.W.; Li, J.H.; Yang, M.S. The variation of nutrient con tents in needles of prince rupprecht’s larch plantation. J. For. Environ. 2005, 2, 163–166. [Google Scholar]
- Zheng, X.R.; Song, Y.L.; Wang, K.Q.; Zhang, Y.J.; Pan, Y. Response of nutrient release and ecological stoichiometry of litter to simulated nitrogen deposition in evergreen broad-leaved forest in central Yunnan. Chin. J. Appl. Ecol. 2021, 32, 23–30. [Google Scholar]
- Shipley, B.; Lechowicz, M.J.; Wright, I.; Reich, P.B. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 2006, 87, 535–541. [Google Scholar] [CrossRef]
- Niu, D.C.; Li, Q.; Jiang, S.G.; Chang, P.J.; Fu, H. Seasonal variations of leaf C:N:P stoichiometry of six shrubs in desert of China’s Alxa Plateau. Chin. J. Plant Ecol. 2013, 37, 317–325. [Google Scholar] [CrossRef]
- Cai, H.Y.; Li, Y.Q. Seasonal dynamics of leaf C, N and P stoichiometry in plants of typical steppe in Nei Mongol China. Chin. J. Plant Ecol. 2020, 44, 1138–1153. [Google Scholar]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N: P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Chen, M.; Huang, L.; Li, M.; Zhang, X.; Cao, Y. Response of plant, litter, and soil C:N:P stoichiometry to growth stages in Quercus secondary forests on the Loess Plateau, China. J. For. Res. 2022, 40, 8570–8581. [Google Scholar] [CrossRef]
- Fan, Q.C.; Xie, W.X.; Wang, Z.Q.; Li, P. Seasonal Variations in C, N and P Stoichiometry of Spartina alterniflora. Environ. Sci. Technol. 2019, 42, 12–19. [Google Scholar]
- Tian, D.; Yan, Z.B.; Fang, J.Y. Review on characteristics and main hypotheses of plant ecological stoichiometry. Chin. J. Plant Ecol. 2021, 45, 682–713. [Google Scholar] [CrossRef]
- Olde Venterink, H.; Wassen, M.J.; Verkroost, A.W.M.; Ruiter, P.C. Species richness–productivity patterns differ between N-, P-, and K-limited wetlands. Ecology 2003, 84, 2191–2199. [Google Scholar] [CrossRef]
- Xing, S.; Cheng, X.; Kang, F.; Wang, J.; Yan, J.; Han, H. The patterns of N/P/K stoichiometry in the Quercus wutaishanica community among different life forms and organs and their responses to environmental factors in northern China. Ecol. Indic. 2022, 137, 108783. [Google Scholar] [CrossRef]
- Lin, T. The Ecophysiological Response of Leaves and Branches of Pinus Massoniana during 3-yr Continuous Rain Exclusion in a Red Soil Erosionarea; Fujian Normal University: Fuzhou, China, 2019. [Google Scholar]
- Randrianalijaona, J.A.; Ramanoelina PA, R.; Rasoarahona, J.R.E.; Gaydou, E.M. Seasonal and chemotype influences on the chemical composition of Lantana camara L.: Essential oils from Madagascar. Anal. Chim. Acta 2005, 545, 46–52. [Google Scholar] [CrossRef]
- Deng, C.H.; Wu, L.L.; Zhang, Y.T.; Qiao, H.; Liu, X.Y.; Hu, Y.J.; Chen, X.B.; Su, Y.R.; He, X.Y. The stoichiometry characteristics of soil and plant carbon. nitrogen, and phosphorus in different stand ages in Camellia oleifera plantation. Acta Ecol. Sin. 2019, 39, 9152–9161. [Google Scholar]
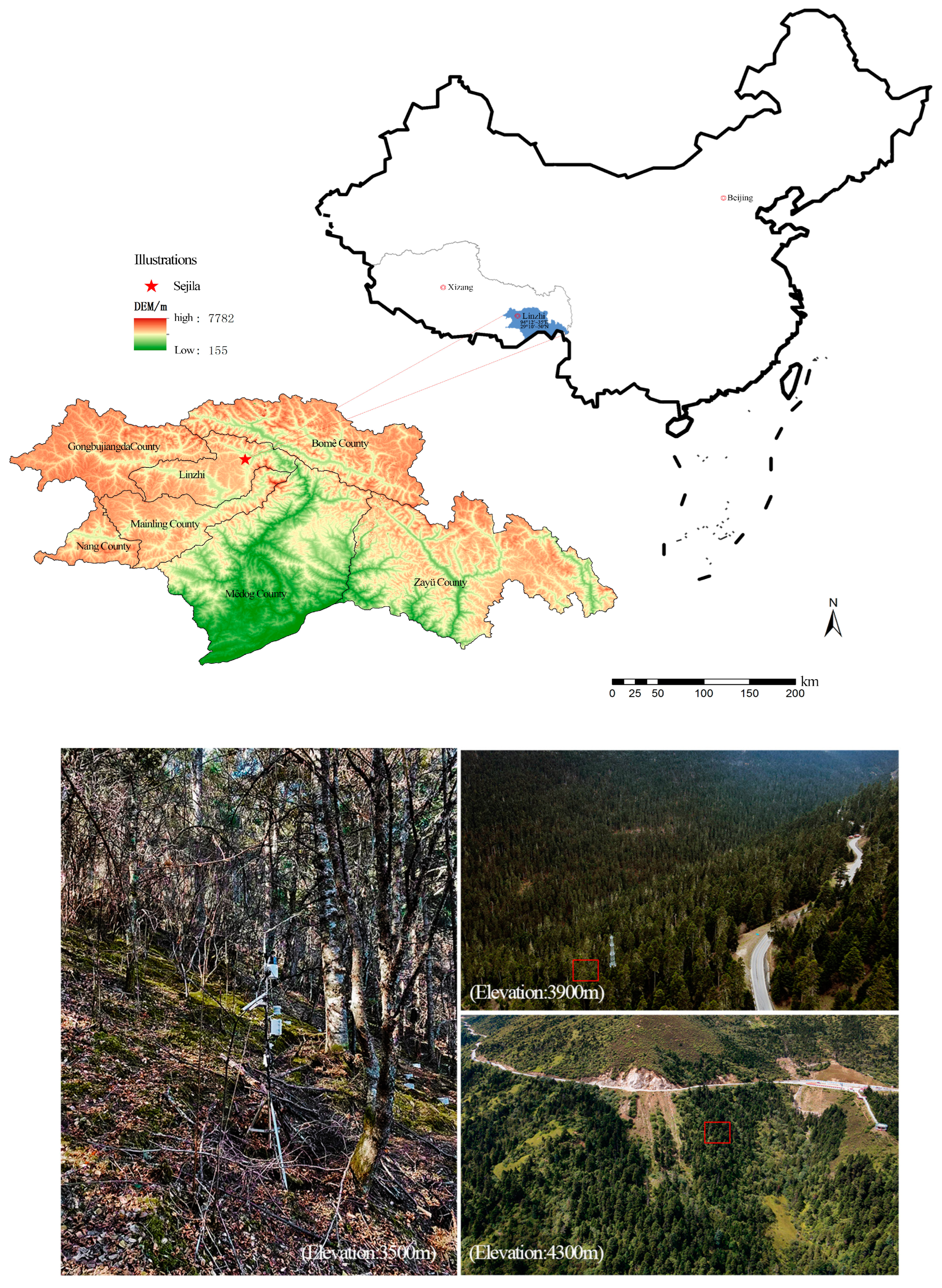
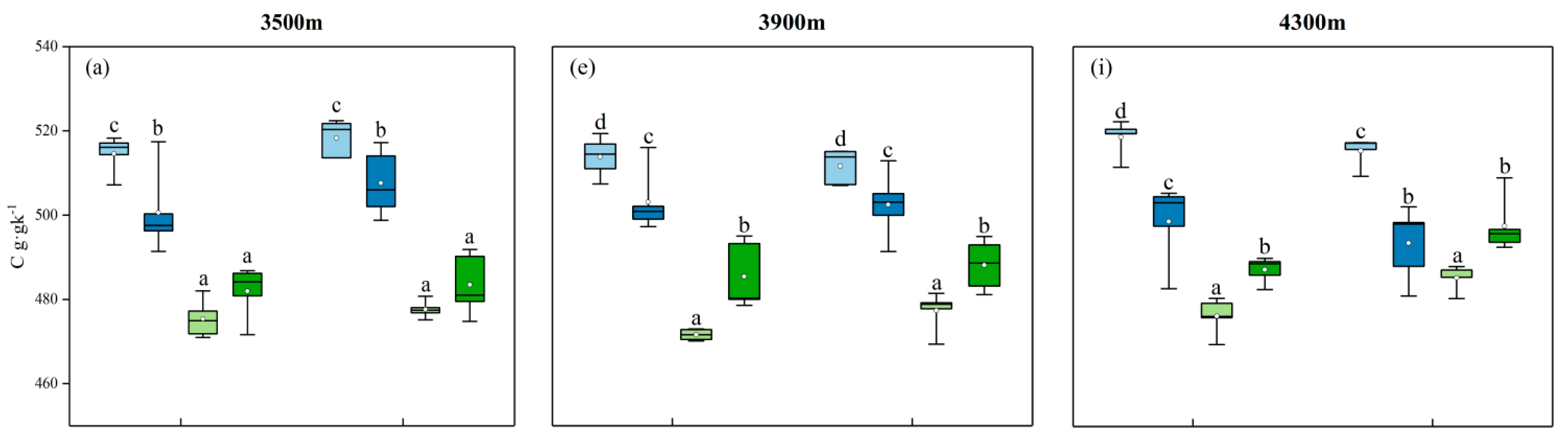

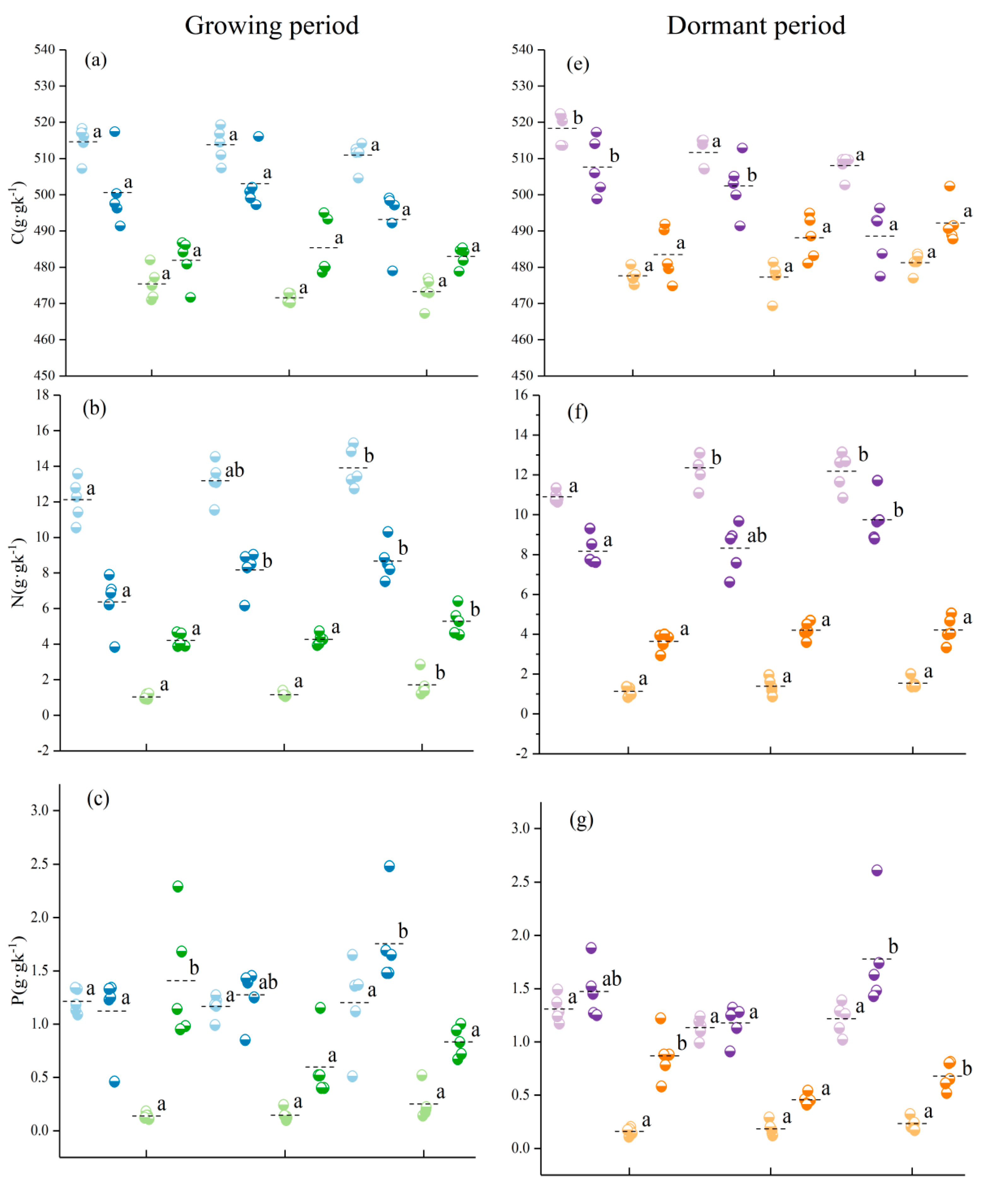
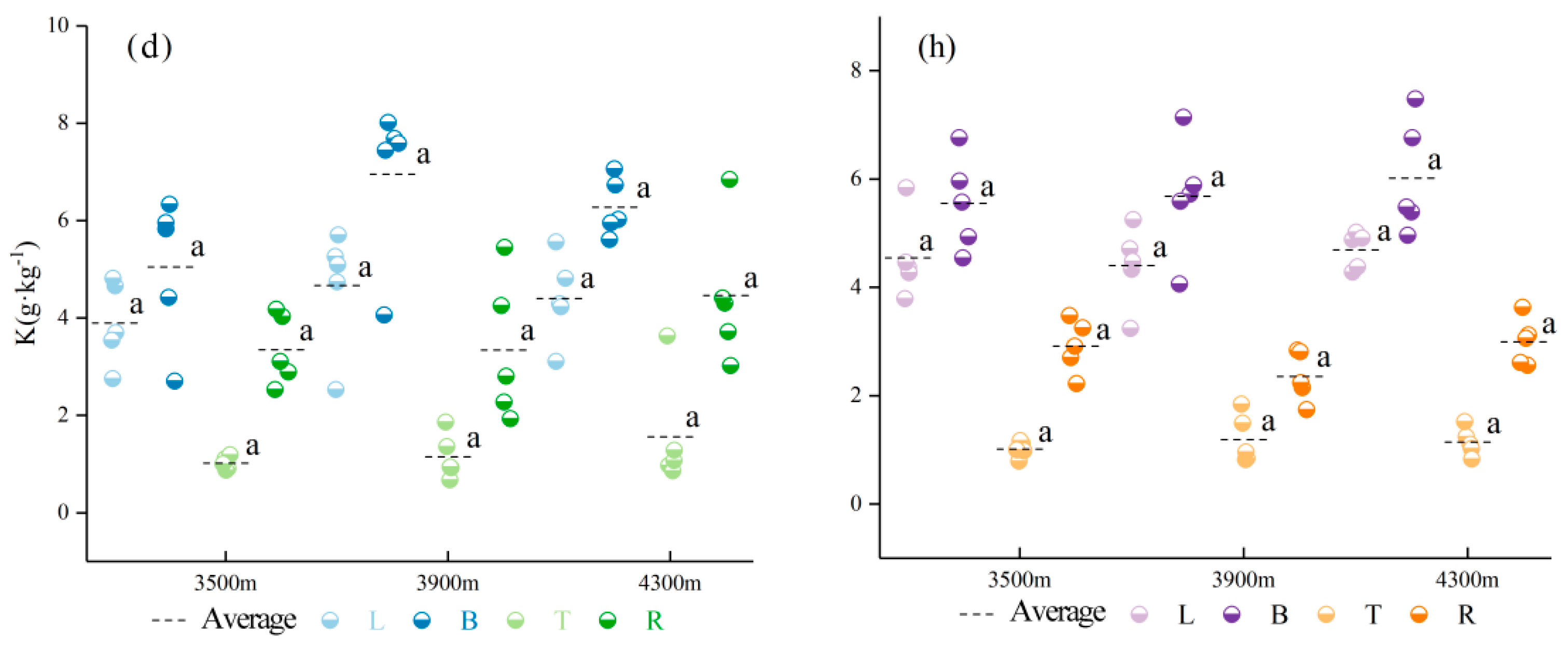
| Tree Species | Elevation (m) | Slope (°) | CD (%) | TH (m) | DBH (cm) | MAT (℃) | Type |
|---|---|---|---|---|---|---|---|
| Abies georgei var. smithii | 3510 | 22 | 70 | 21.5 ± 1.2 | 187.6 ± 6.3 | 5.78 | ENA |
| 3900 | 26 | 85 | 21.4 ± 1.4 | 177.4 ± 7.2 | 4.40 | ||
| 4300 | 20 | 75 | 13.2 ± 0.6 | 134.3 ± 4.3 | 1.23 |
| Item | Sensor Model | Range | Accuracy | Resolution | Manufacturer |
|---|---|---|---|---|---|
| Air temperature | HOBO U23-001 | −40–70 °C | ±0.18 °C | 0.02 °C | USA Onset |
| Air humidity | HOBO U23-001 | 0–100% | ±2.50% | 0.03% | USA Onset |
| Soil temperature | HOBO S-TMB-M006 | −40–100 °C | ±0.20 °C | 0.03 °C | USA Onset |
| Soil moisture | HOBO S-SMD-M005 | 0–570 m3/m3 | ±3.30% | 0.08% | USA Onset |
| Parameter | Item | Sources of Variation | ||||||
|---|---|---|---|---|---|---|---|---|
| Organ (O) | Elevation (A) | Season (S) | O × A | O × S | A × S | O × A × S | ||
| C | Type ⅢSS | 22,224.81 | 293.31 | 187.80 | 1189.99 | 181.15 | 24.78 | 341.40 |
| DF | 3.00 | 2.00 | 1.00 | 6.00 | 3.00 | 2.00 | 6.00 | |
| MS | 7741.60 | 146.65 | 187.80 | 198.33 | 60.38 | 12.39 | 56.90 | |
| F | 228.87 | 4.34 | 5.55 | 5.86 | 1.79 | 0.37 | 1.68 | |
| P | 0.00 | 0.02 | 0.02 | 0.00 | 0.16 | 0.69 | 0.13 | |
| N | Type ⅢSS | 2096.92 | 29.49 | 1.13 | 7.76 | 20.73 | 1.29 | 4.63 |
| DF | 3.00 | 2.00 | 1.00 | 6.00 | 3.00 | 2.00 | 6.00 | |
| MS | 698.97 | 14.75 | 1.13 | 1.29 | 6.91 | 0.65 | 0.77 | |
| F | 1044.43 | 22.03 | 1.69 | 1.93 | 10.32 | 0.97 | 1.15 | |
| P | 0.00 | 0.00 | 0.20 | 0.08 | 0.00 | 0.38 | 0.34 | |
| P | Type ⅢSS | 26.86 | 1.20 | 0.04 | 2.54 | 0.61 | 0.01 | 0.54 |
| DF | 3.00 | 2.00 | 1.00 | 6.00 | 3.00 | 2.00 | 6.00 | |
| MS | 8.95 | 0.60 | 0.04 | 0.42 | 0.21 | 0.00 | 0.09 | |
| F | 142.43 | 9.56 | 0.63 | 6.74 | 3.26 | 0.07 | 1.44 | |
| P | 0.00 | 0.00 | 0.43 | 0.00 | 0.03 | 0.94 | 0.21 | |
| K | Type ⅢSS | 361.21 | 5.59 | 2.72 | 5.62 | 5.61 | 3.59 | 3.10 |
| DF | 3.00 | 2.00 | 1.00 | 6.00 | 3.00 | 2.00 | 6.00 | |
| MS | 120.40 | 2.79 | 2.72 | 0.94 | 1.87 | 1.79 | 0.52 | |
| F | 144.54 | 3.35 | 3.26 | 1.13 | 2.24 | 2.15 | 0.62 | |
| P | 0.00 | 0.04 | 0.07 | 0.35 | 0.09 | 0.12 | 0.71 | |
| Parameter | Item | Sources of Variation | ||||||
|---|---|---|---|---|---|---|---|---|
| Organ(O) | Elevation(A) | Season(S) | O*A | O*S | A*S | O*A*S | ||
| C:N | Type ⅢSS | 2,261,028.42 | 42,450.98 | 91.44 | 60,853.10 | 5078.12 | 2261.94 | 3684.84 |
| DF | 3.00 | 2.00 | 1.00 | 6.00 | 3.00 | 2.00 | 6.00 | |
| MS | 753,676.14 | 21,225.49 | 91.44 | 10,142.18 | 1692.71 | 1130.97 | 614.14 | |
| F | 443.75 | 12.50 | 0.05 | 5.97 | 1.00 | 0.67 | 0.36 | |
| P | 0.00 | 0.00 | 0.82 | 0.00 | 0.40 | 0.52 | 0.90 | |
| C:P | Type ⅢSS | 130,859,648.50 | 2,634,612.57 | 251,620.63 | 5,792,439.78 | 1,492,363.42 | 68,765.44 | 377,139.26 |
| DF | 3.00 | 2.00 | 1.00 | 6.00 | 3.00 | 2.00 | 6.00 | |
| MS | 43,619,882.83 | 1,317,306.28 | 251,620.63 | 965,406.63 | 497,454.47 | 34,382.72 | 62,856.54 | |
| F | 250.14 | 7.55 | 1.44 | 5.54 | 2.85 | 0.20 | 0.36 | |
| P | 0.00 | 0.00 | 0.23 | 0.00 | 0.04 | 0.82 | 0.90 | |
| C:K | Type ⅢSS | 2,470,446.82 | 17,731.69 | 2689.39 | 16,212.26 | 10,178.25 | 2749.83 | 4697.21 |
| DF | 3.00 | 2.00 | 1.00 | 6.00 | 3.00 | 2.00 | 6.00 | |
| MS | 823,482.27 | 8865.85 | 2689.39 | 2702.04 | 3392.75 | 1374.91 | 782.87 | |
| F | 168.95 | 1.82 | 0.55 | 0.55 | 0.70 | 0.28 | 0.16 | |
| P | 0.00 | 0.17 | 0.46 | 0.77 | 0.56 | 0.76 | 0.99 | |
| N:P | Type ⅢSS | 411.46 | 89.98 | 5.43 | 82.45 | 27.04 | 5.48 | 10.06 |
| DF | 3.00 | 2.00 | 1.00 | 6.00 | 3.00 | 2.00 | 6.00 | |
| MS | 137.15 | 44.99 | 5.43 | 13.74 | 9.01 | 2.74 | 1.68 | |
| F | 41.09 | 13.48 | 1.63 | 4.12 | 2.70 | 0.82 | 0.50 | |
| P | 0.00 | 0.00 | 0.21 | 0.00 | 0.05 | 0.44 | 0.81 | |
| N:K | Type ⅢSS | 55.31 | 0.46 | 0.00 | 0.93 | 2.79 | 0.40 | 0.36 |
| DF | 3.00 | 2.00 | 1.00 | 6.00 | 3.00 | 2.00 | 6.00 | |
| MS | 18.44 | 0.23 | 0.00 | 0.16 | 0.93 | 0.20 | 0.06 | |
| F | 126.77 | 1.59 | 0.00 | 1.07 | 6.40 | 1.37 | 0.41 | |
| P | 0.00 | 0.21 | 0.95 | 0.39 | 0.00 | 0.26 | 0.87 | |
| K:P | Type ⅢSS | 134.81 | 26.78 | 6.92 | 39.14 | 7.52 | 1.05 | 4.18 |
| DF | 3.00 | 2.00 | 1.00 | 6.00 | 3.00 | 2.00 | 6.00 | |
| MS | 44.94 | 13.39 | 6.92 | 6.52 | 2.51 | 0.52 | 0.70 | |
| F | 48.74 | 14.52 | 7.51 | 7.08 | 2.72 | 0.57 | 0.76 | |
| P | 0.00 | 0.00 | 0.01 | 0.00 | 0.05 | 0.57 | 0.61 | |
| Time | Elevation | Organ | C | N | P | K | C:N | C:P | C:K | N:P | N:K | K:P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growing period | 3500 m | L | 0.76 | 8.74 | 8.50 | 19.52 | 8.40 | 8.17 | 20.25 | 4.65 | 14.50 | 18.70 |
| B | 1.78 | 21.65 | 29.77 | 26.54 | 27.93 | 51.56 | 35.04 | 19.80 | 14.15 | 15.96 | ||
| T | 0.84 | 13.04 | 16.98 | 11.63 | 12.21 | 16.57 | 11.38 | 25.86 | 16.84 | 23.71 | ||
| R | 1.15 | 8.30 | 36.43 | 19.31 | 7.72 | 30.23 | 19.20 | 29.42 | 23.21 | 20.54 | ||
| 3900 m | L | 0.83 | 7.37 | 8.15 | 23.85 | 7.57 | 8.96 | 35.11 | 3.18 | 26.93 | 19.33 | |
| B | 1.33 | 12.75 | 17.61 | 20.99 | 15.02 | 22.66 | 30.04 | 9.38 | 13.64 | 8.21 | ||
| T | 0.25 | 10.25 | 33.55 | 36.50 | 9.16 | 24.71 | 33.44 | 17.64 | 26.81 | 14.31 | ||
| R | 1.48 | 6.71 | 47.32 | 39.55 | 7.10 | 31.11 | 37.09 | 30.63 | 34.82 | 25.50 | ||
| 4300 m | L | 0.65 | 7.05 | 31.87 | 18.21 | 7.36 | 50.23 | 20.19 | 46.45 | 17.98 | 26.72 | |
| B | 1.52 | 10.68 | 21.15 | 8.55 | 11.26 | 17.83 | 9.49 | 10.26 | 10.54 | 18.77 | ||
| T | 0.72 | 34.22 | 55.05 | 66.85 | 25.31 | 35.79 | 37.12 | 17.77 | 26.43 | 13.96 | ||
| R | 0.50 | 13.03 | 15.24 | 28.93 | 12.27 | 15.76 | 25.27 | 20.58 | 26.28 | 20.43 | ||
| Dormant period | 3500 m | L | 0.76 | 2.28 | 8.55 | 15.11 | 2.34 | 7.68 | 13.38 | 7.36 | 11.70 | 11.07 |
| B | 1.38 | 8.05 | 15.29 | 14.03 | 6.50 | 13.31 | 13.03 | 13.47 | 12.17 | 4.21 | ||
| T | 0.38 | 17.73 | 18.73 | 12.83 | 19.46 | 21.15 | 14.11 | 7.06 | 11.69 | 9.16 | ||
| R | 1.35 | 10.84 | 23.78 | 15.07 | 13.06 | 24.63 | 16.03 | 16.08 | 20.54 | 19.33 | ||
| 3900 m | L | 0.73 | 6.09 | 7.58 | 15.01 | 6.47 | 7.94 | 17.64 | 5.20 | 13.29 | 11.62 | |
| B | 1.39 | 13.01 | 12.44 | 17.28 | 13.41 | 14.11 | 19.37 | 4.54 | 11.03 | 7.84 | ||
| T | 0.87 | 26.92 | 31.57 | 34.00 | 28.54 | 27.66 | 29.77 | 8.42 | 14.22 | 10.60 | ||
| R | 1.10 | 8.89 | 10.31 | 17.85 | 9.85 | 10.16 | 18.43 | 13.08 | 26.65 | 22.09 | ||
| 4300 m | L | 0.54 | 6.80 | 10.64 | 6.47 | 7.37 | 11.40 | 6.97 | 4.13 | 7.44 | 10.09 | |
| B | 1.42 | 10.81 | 24.20 | 15.78 | 10.89 | 20.06 | 15.99 | 12.53 | 11.03 | 17.37 | ||
| T | 0.49 | 15.63 | 21.65 | 19.92 | 13.40 | 19.86 | 20.03 | 9.23 | 12.76 | 12.71 | ||
| R | 1.07 | 14.12 | 16.57 | 13.13 | 14.64 | 17.96 | 11.97 | 11.88 | 14.12 | 26.71 |
| Season | Elevation (m) | Organ | C:N | C:P | C:K | N:P | N:K | K:P |
|---|---|---|---|---|---|---|---|---|
| Growing period | 3500 | L | 42.77 ± 3.59 Ba | 427.03 ± 34.88 Aa | 137.47 ± 27.84 Aa | 10.00 ± 0.47 Ac | 3.20 ± 0.46 Aa | 3.21 ± 0.60 Aab |
| B | 83.49 ± 23.32 Bab | 526.34 ± 271.37 Aa | 109.13 ± 38.24 Aa | 6.01 ± 1.19 ABb | 1.30 ± 0.18 Ab | 4.67 ± 0.74 ABb | ||
| T | 466.29 ± 56.95 Bc | 3527.29 ± 584.63 Ab | 474.30 ± 53.95 Ab | 7.77 ± 2.01 Ab | 1.03 ± 0.17 Ab | 7.57 ± 1.79 Ac | ||
| R | 115.15 ± 8.89 Bb | 383.73 ± 116.01 Aa | 149.34 ± 28.68 Aa | 3.35 ± 0.98 Aa | 1.31 ± 0.30 Ab | 2.54 ± 0.52 Aa | ||
| 3900 | L | 39.19 ± 2.97 ABa | 444.23 ± 39.82 Aa | 120.18 ± 42.20 Aa | 11.33 ± 0.36 Ab | 3.02 ± 0.81 Ab | 3.95 ± 0.76 Aa | |
| B | 62.71 ± 9.42 ABa | 411.54 ± 93.26 Aa | 77.21 ± 23.19 Aa | 6.51 ± 0.61 Ba | 1.21 ± 0.16 Aa | 5.42 ± 0.45 Bab | ||
| T | 411.09 ± 37.65 Bc | 3551.93 ± 877.82 Ab | 464.07 ± 155.20 Ab | 8.51 ± 1.50 Aa | 1.11 ± 0.30 Aa | 7.90 ± 1.13 c | ||
| R | 114.37 ± 8.12 Bb | 945.23 ± 294.04 Ba | 169.26 ± 62.77 Aa | 8.21 ± 2.51 Ba | 1.47 ± 0.51 Aa | 5.81 ± 1.48 Bb | ||
| 4300 | L | 36.92 ± 2.72 Aa | 501.81 ± 252.08 Aa | 120.47 ± 24.32 Aa | 13.43 ± 6.24 Ab | 3.26 ± 0.59 Ab | 3.97 ± 1.06 Aab | |
| B | 57.53 ± 6.48 Aa | 291.76 ± 52.02 Aa | 79.23 ± 7.52 Aa | 5.04 ± 0.52 Aa | 1.39 ± 0.15 Aa | 3.70 ± 0.69 Aa | ||
| T | 302.03 ± 76.44 Ab | 2322.86 ± 831.35 Ab | 397.42 ± 147.54 Ab | 7.46 ± 1.33 Aa | 1.27 ± 0.34 Aa | 6.03 ± 0.84 Ac | ||
| R | 92.85 ± 11.40 Aa | 595.52 ± 93.83 Aa | 116.53 ± 29.45 Aa | 6.52 ± 1.34 Ba | 1.27 ± 0.33 Aa | 5.33 ± 1.09 Bbc | ||
| F | 0.67 | 0.55 | 0.31 | 1.55 | 0.06 | 2.67 | ||
| P | 0.52 | 0.58 | 0.74 | 0.22 | 0.94 | 0.08 | ||
| Dormant period | 3500 | L | 47.60 ± 1.11 Ba | 399.01 ± 30.64 Aa | 116.37 ± 15.57 Aab | 8.38 ± 0.62 Ad | 2.44 ± 0.29 Ac | 3.47 ± 0.38 Aa |
| B | 62.49 ± 4.06 Ba | 351.48 ± 46.78 ABa | 93.08 ± 12.13 Aa | 5.64 ± 0.76 Ab | 1.49 ± 0.18 Ab | 3.78 ± 0.16 Aa | ||
| T | 436.14 ± 84.89 Ac | 3071.08 ± 649.63 Ab | 480.83 ± 67.83 Ac | 7.04 ± 0.50 Ac | 1.12 ± 0.13 Aa | 6.34 ± 0.58 Bb | ||
| R | 134.79 ± 17.60 Ab | 590.70 ± 145.51 Aa | 170.06 ± 27.26 Ab | 4.35 ± 0.70 Aa | 1.28 ± 0.26 Aab | 3.48 ± 0.67 Aa | ||
| 3900 | L | 41.57 ± 2.69 Aa | 453.68 ± 36.04 Aa | 119.33 ± 21.05 Aab | 10.92 ± 0.57 Cc | 2.86 ± 0.38 Ac | 3.87 ± 0.45 Aa | |
| B | 61.46 ± 8.24 Ba | 433.89 ± 61.24 Ba | 91.47 ± 17.72 Aa | 7.06 ± 0.32 Ba | 1.48 ± 0.16 Aab | 4.80 ± 0.38 Bab | ||
| T | 371.05 ± 105.88 Ab | 2834.81 ± 784.12 Ac | 445.44 ± 132.62 Ac | 7.64 ± 0.64 Aa | 1.20 ± 0.17 Aa | 6.45 ± 0.68 Bc | ||
| R | 117.18 ± 11.54 Aa | 1084.65 ± 110.20 Bb | 214.15 ± 39.46 Ab | 9.33 ± 1.22 Cb | 1.87 ± 0.50 Bb | 5.25 ± 1.16 Bb | ||
| 4300 | L | 41.90 ± 3.09 Aa | 422.28 ± 48.13 Aab | 108.68 ± 7.58 Aab | 10.05 ± 0.41 Bc | 2.60 ± 0.19 Ab | 3.89 ± 0.39 Aab | |
| B | 50.72 ± 5.53 Aa | 288.74 ± 57.93 Aa | 83.37 ± 13.33 Aa | 5.64 ± 0.71 Aa | 1.64 ± 0.18 Aa | 3.48 ± 0.60 Aa | ||
| T | 319.19 ± 42.76 Ac | 2151.11 ± 427.14 Ac | 438.52 ± 87.86 Ac | 6.69 ± 0.62 Ab | 1.37 ± 0.17 Aa | 4.94 ± 0.63 Ab | ||
| R | 119.36 ± 17.47 Ab | 748.86 ± 134.51 Ab | 166.93 ± 19.98 Ab | 6.28 ± 0.75 Bab | 1.42 ± 0.20 ABa | 4.58 ± 1.22 ABab | ||
| F | 0.34 | 0.42 | 0.08 | 9.46 | 0.97 | 3.42 | ||
| P | 0.71 | 0.66 | 0.93 | 0.00 | 0.39 | 0.04 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Fu, F.; Li, J.; Chen, W.; Ding, H.; Xiao, S. Stoichiometric Characteristics of Abies georgei var. smithii Plants in Southeast Tibet. Sustainability 2023, 15, 8458. https://doi.org/10.3390/su15118458
Li Y, Fu F, Li J, Chen W, Ding H, Xiao S. Stoichiometric Characteristics of Abies georgei var. smithii Plants in Southeast Tibet. Sustainability. 2023; 15(11):8458. https://doi.org/10.3390/su15118458
Chicago/Turabian StyleLi, Yueyao, Fangwei Fu, Jiangrong Li, Wensheng Chen, Huihui Ding, and Siying Xiao. 2023. "Stoichiometric Characteristics of Abies georgei var. smithii Plants in Southeast Tibet" Sustainability 15, no. 11: 8458. https://doi.org/10.3390/su15118458
APA StyleLi, Y., Fu, F., Li, J., Chen, W., Ding, H., & Xiao, S. (2023). Stoichiometric Characteristics of Abies georgei var. smithii Plants in Southeast Tibet. Sustainability, 15(11), 8458. https://doi.org/10.3390/su15118458






