Study on the Effect of External Air Supply and Temperature Control on Coal Spontaneous Combustion Characteristics
Abstract
:1. Introduction
2. Experiment and Methodology
2.1. Coal Sample
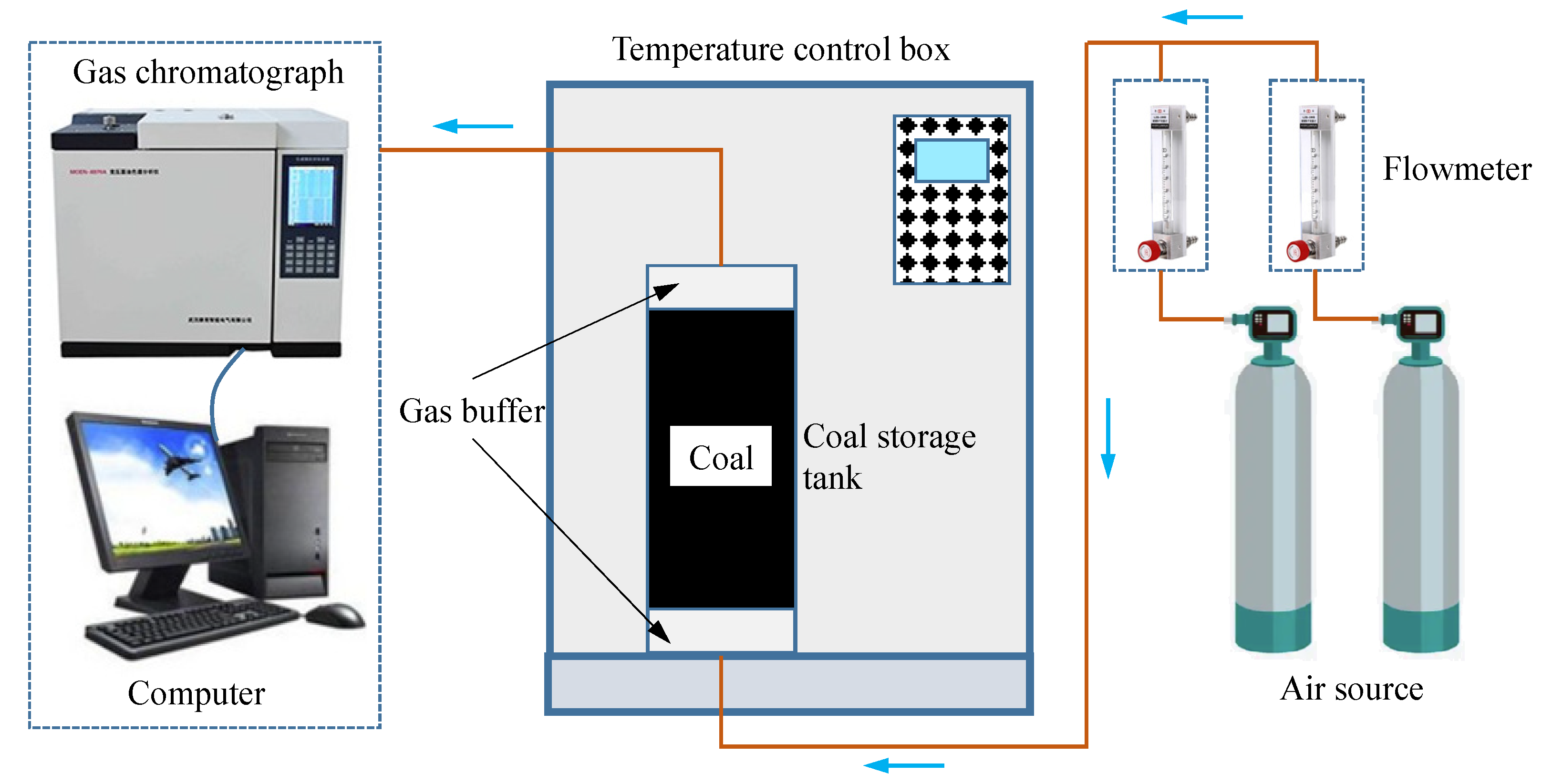
2.2. Experiment
2.3. Numerical Model
3. Results and Discussion
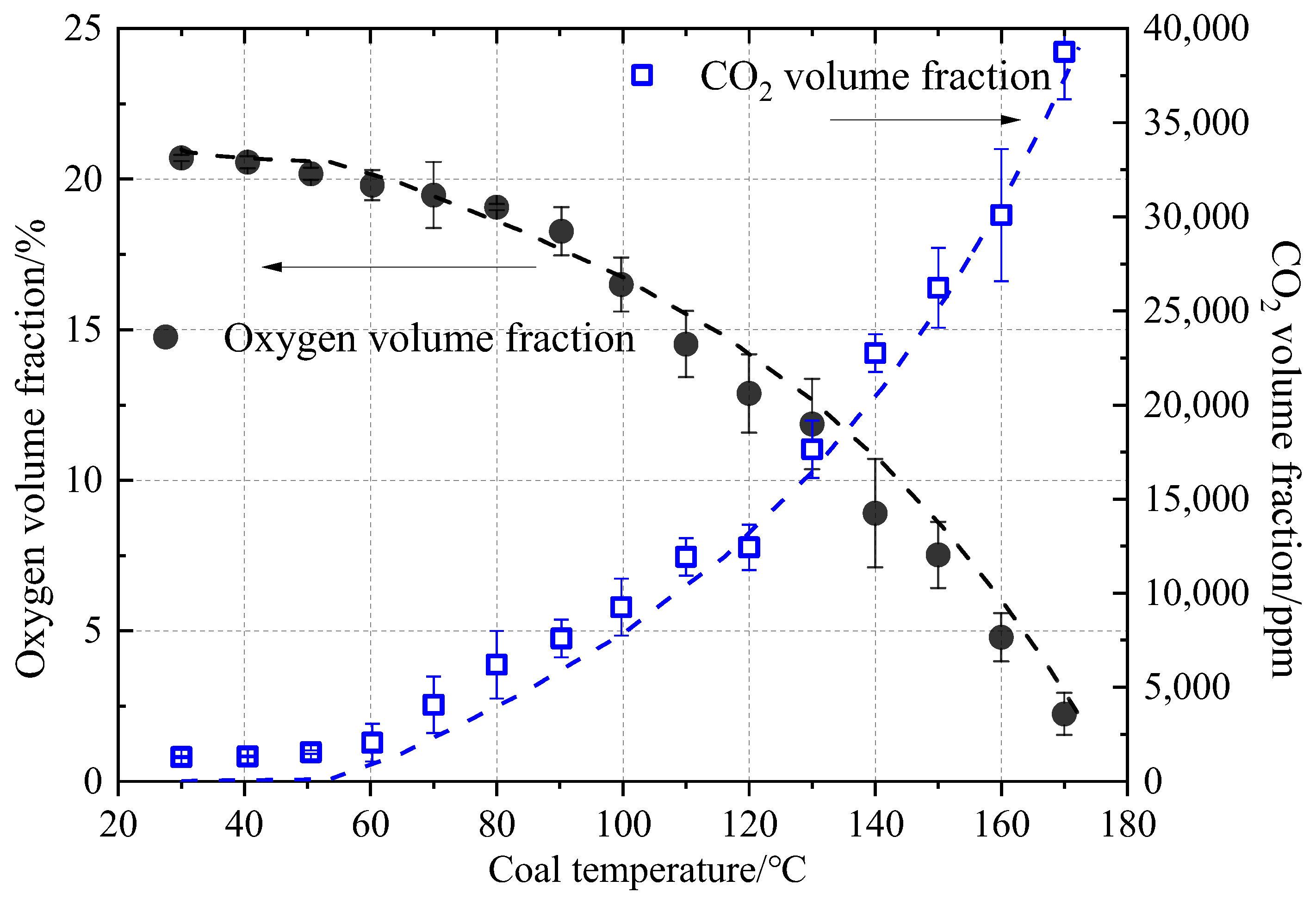
3.1. Numerical Simulation Verification
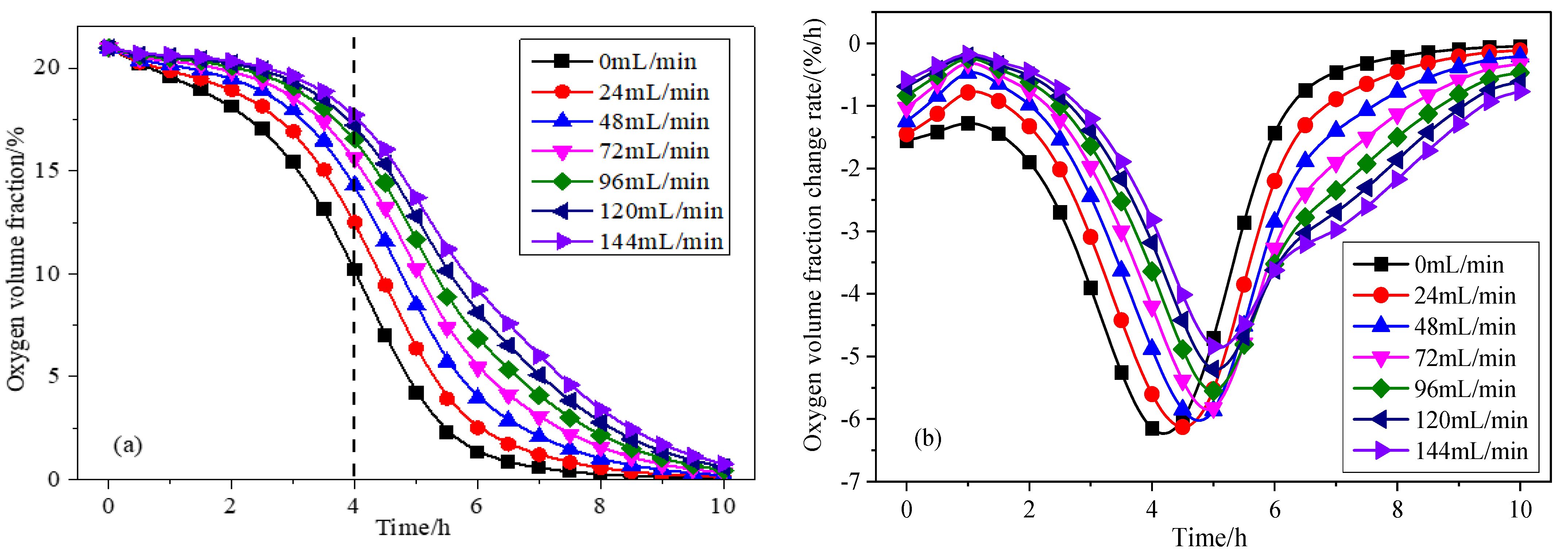
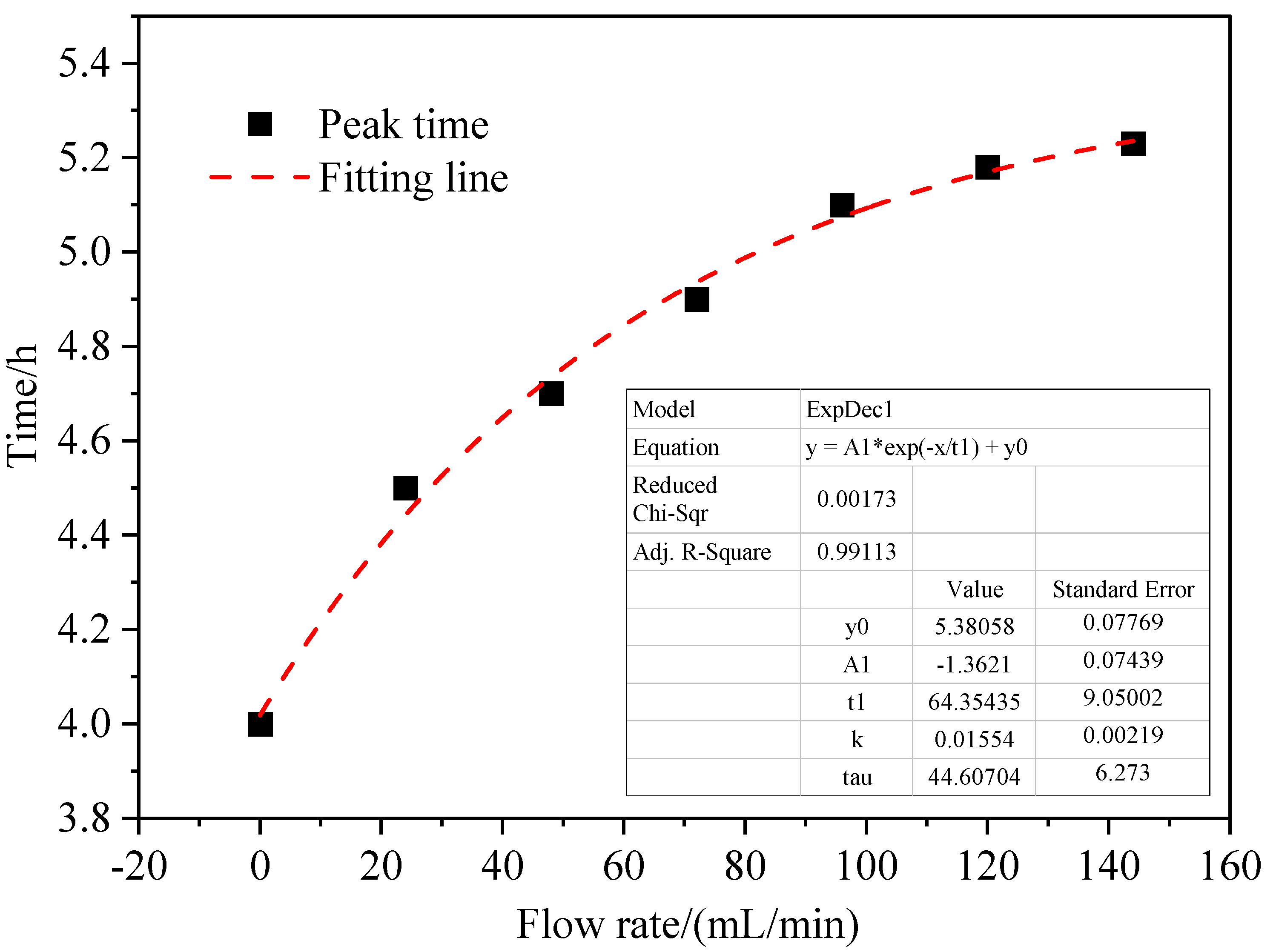
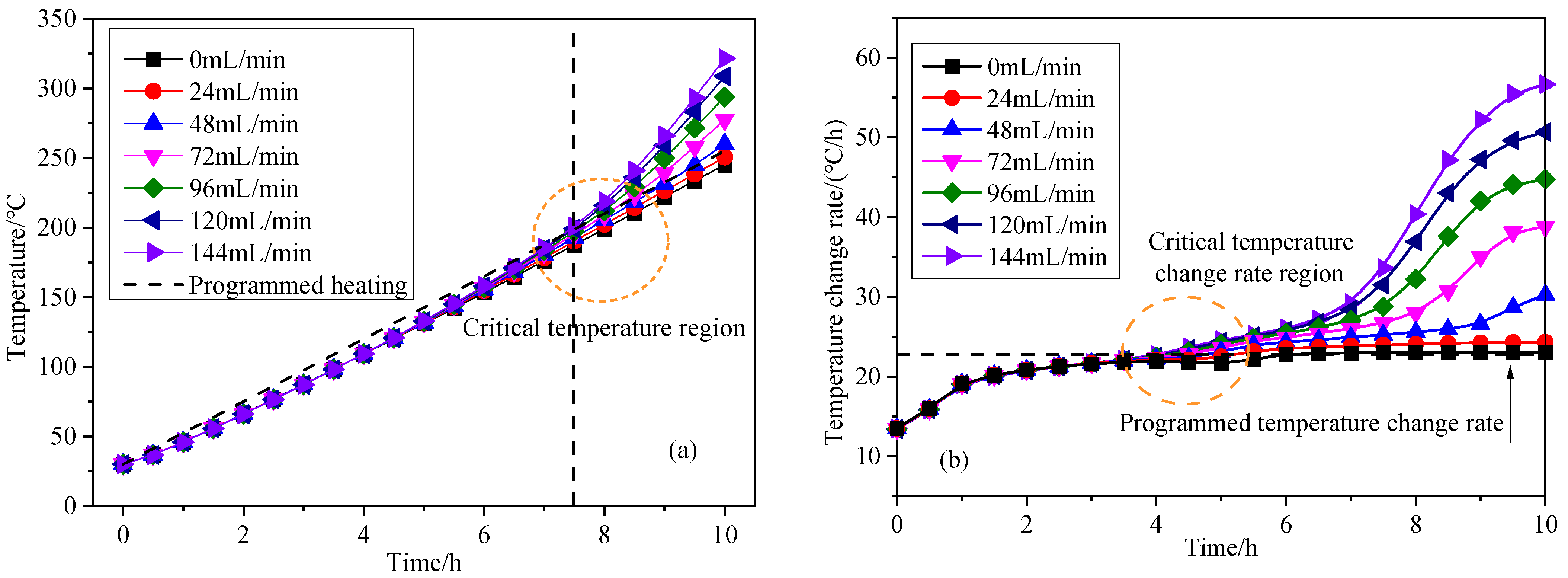
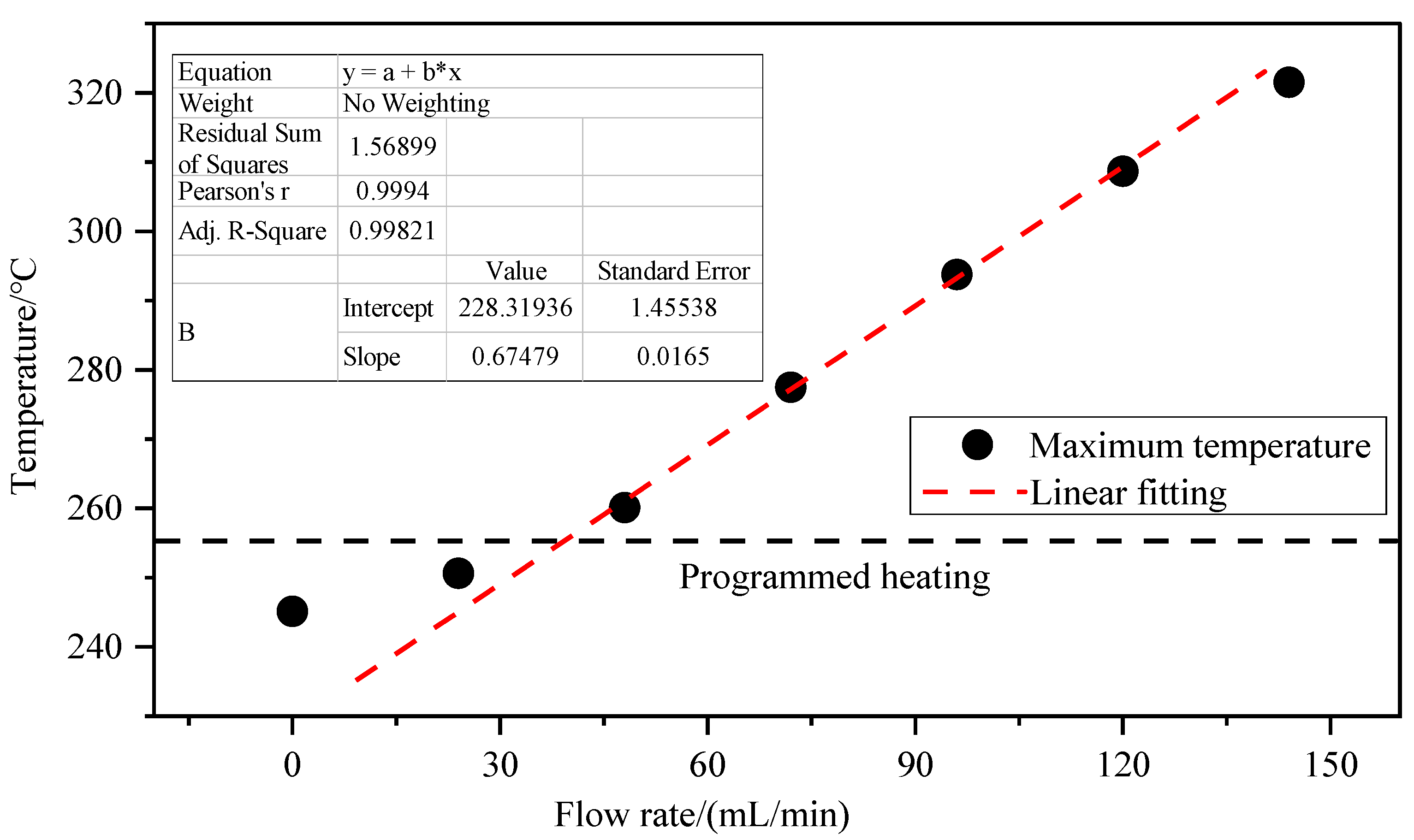
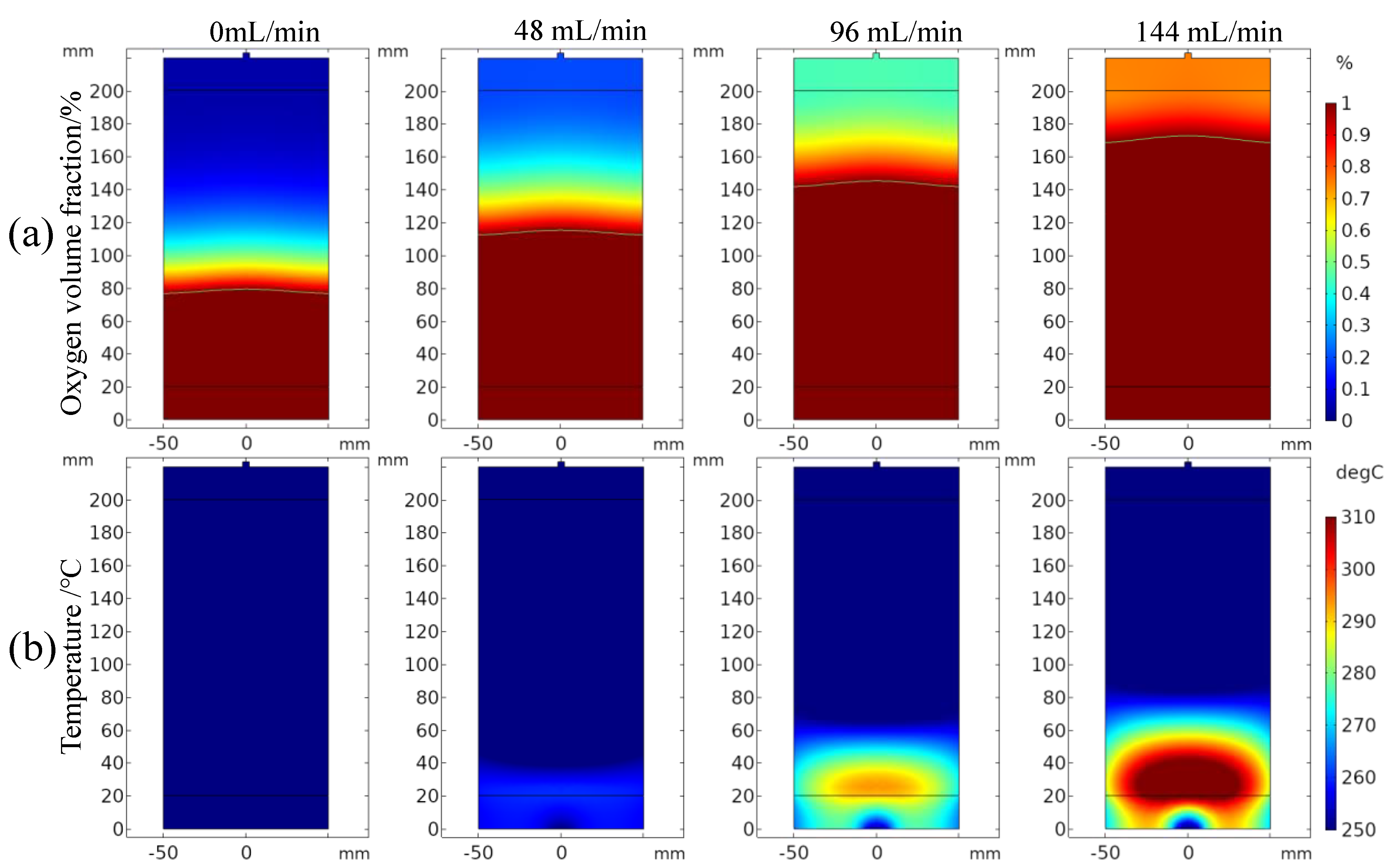
3.2. Influence of Inlet Air Volume on Coal Spontaneous Combustion Characteristics
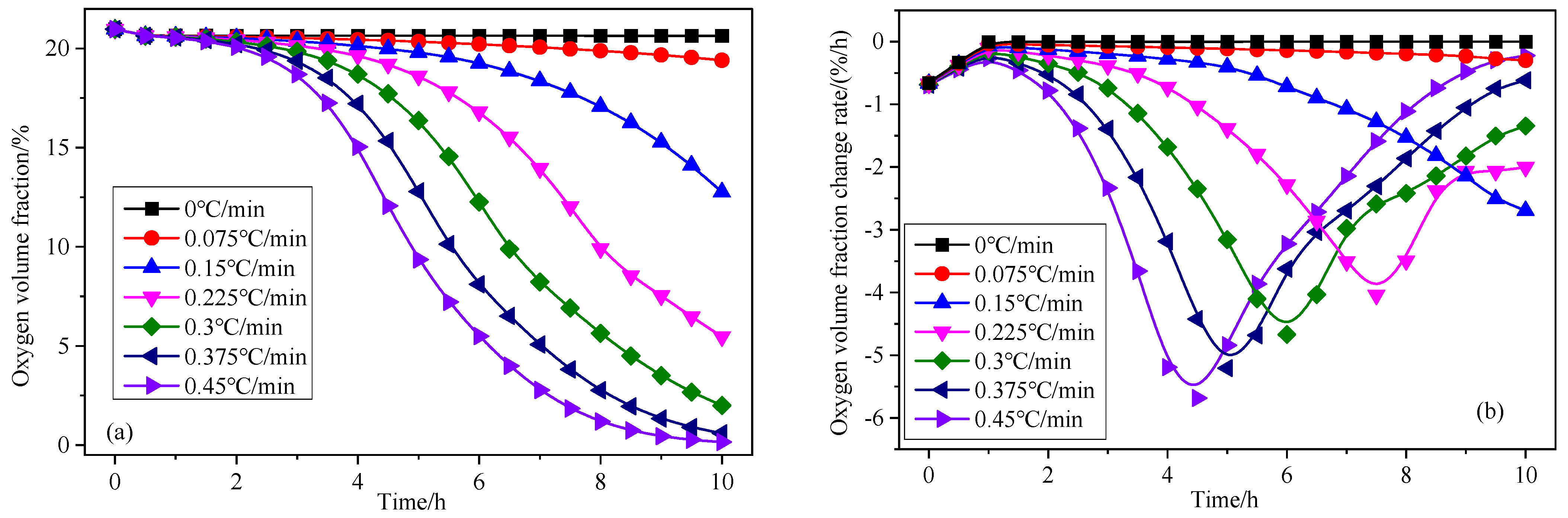
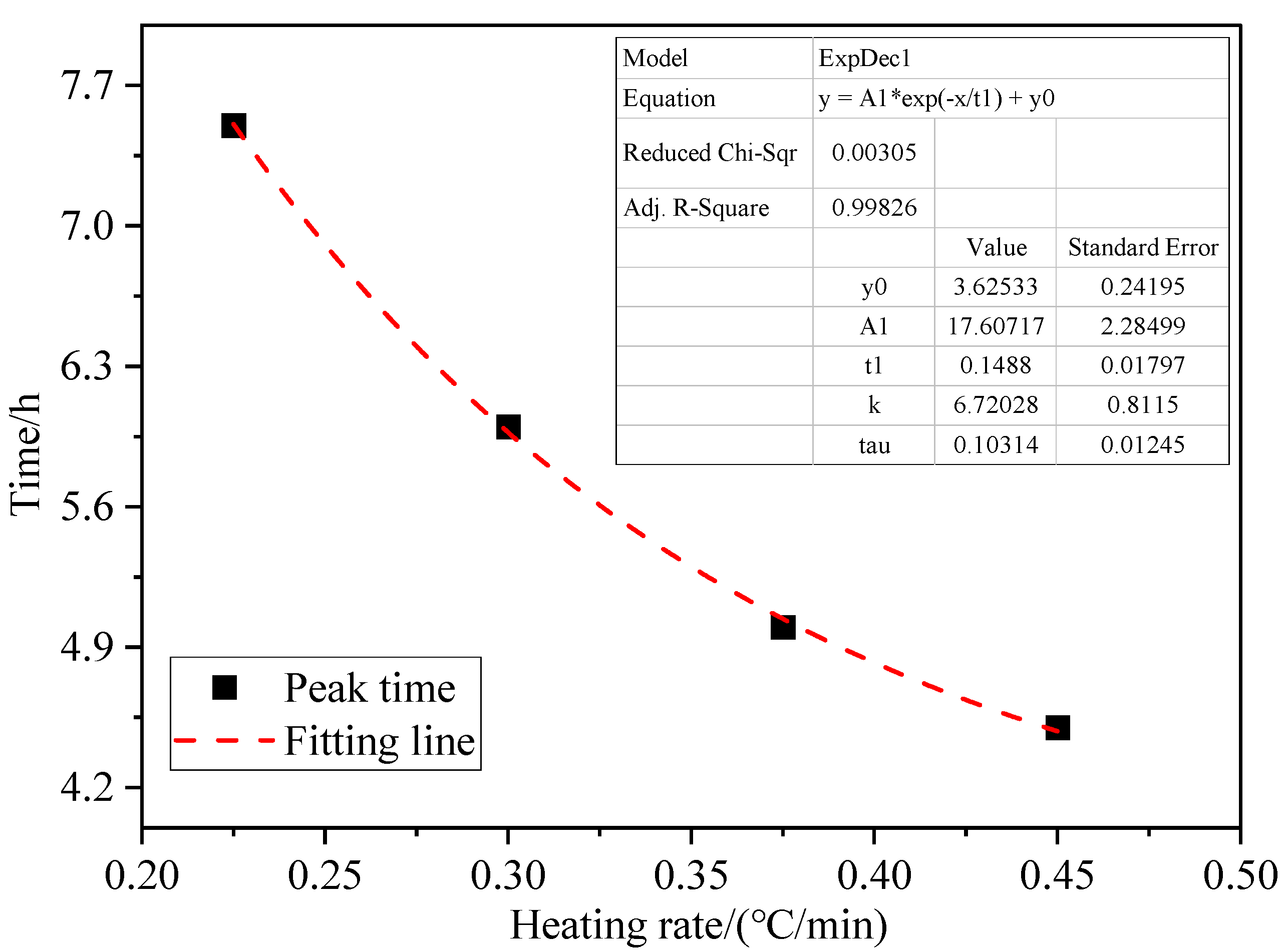
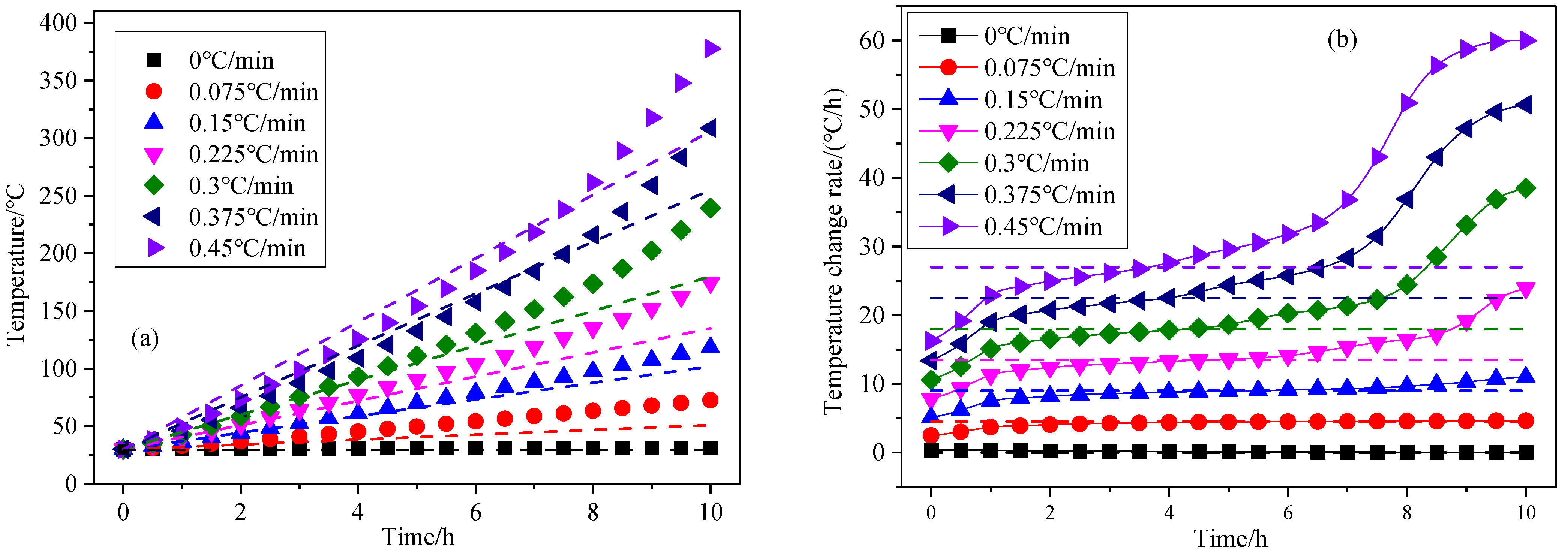
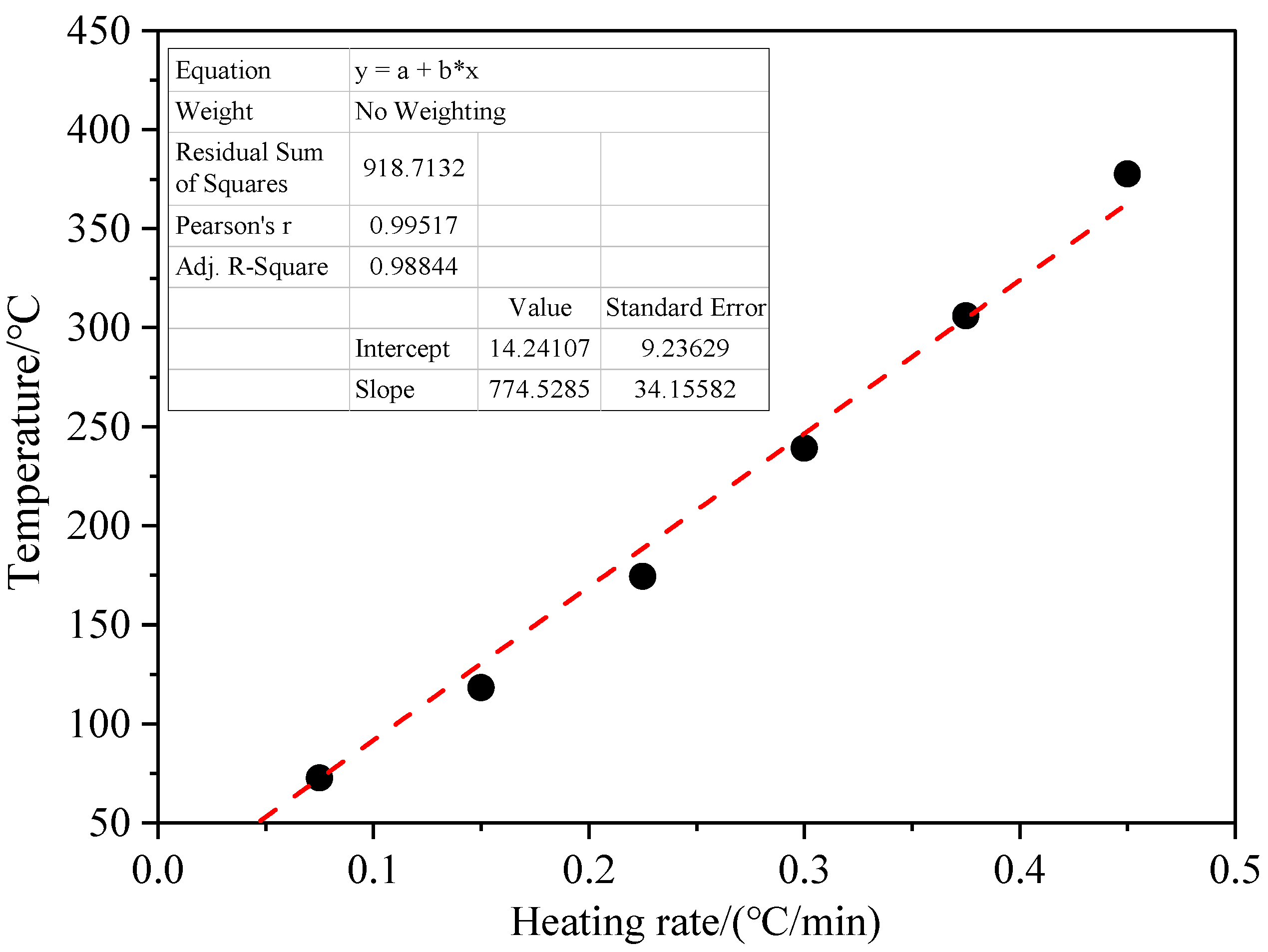
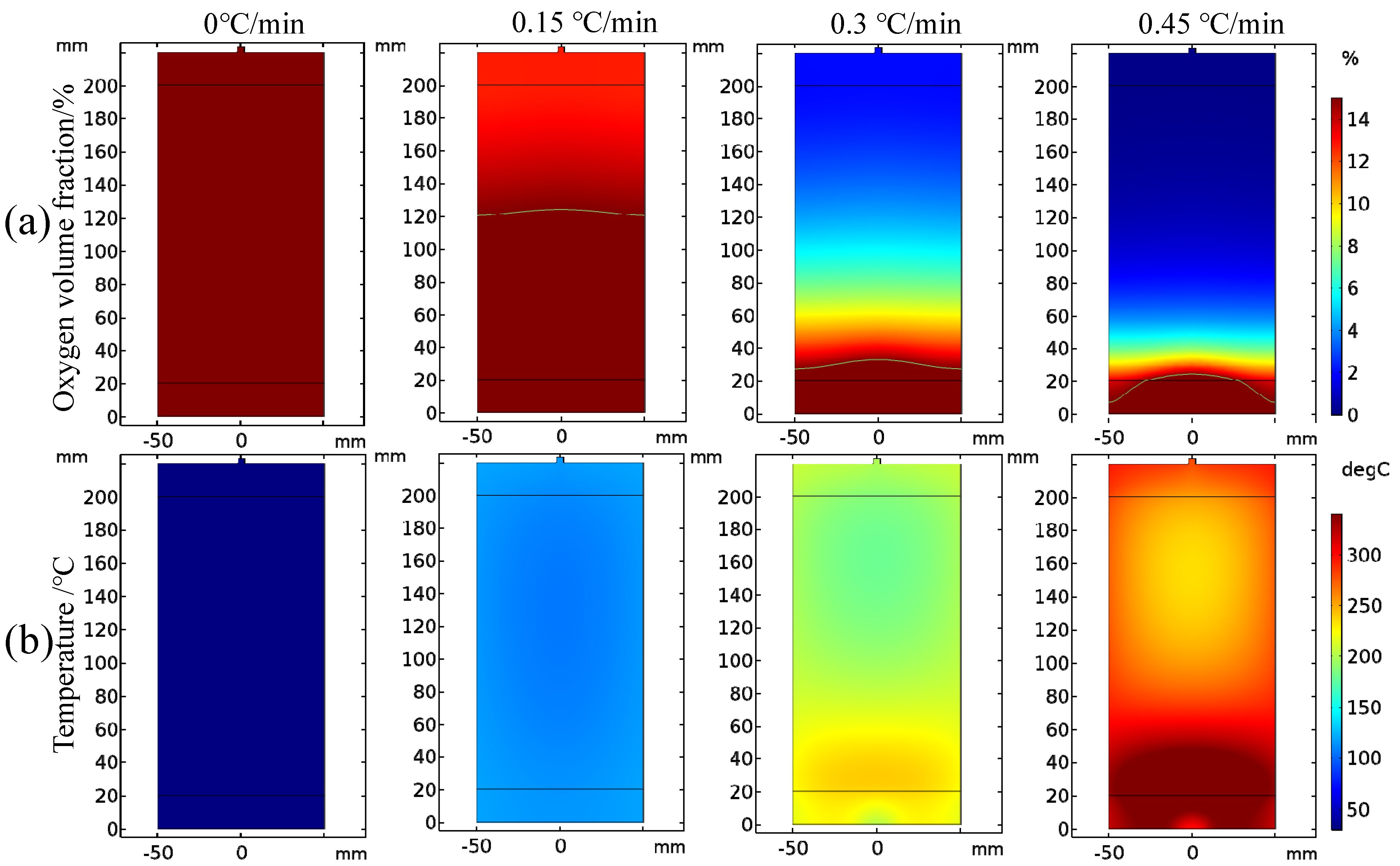
3.3. Effect of Heating Rate on Coal Spontaneous Combustion Characteristics
4. Conclusions
- (1)
- A programmed temperature rise model of coal spontaneous combustion is established using the multi-physical field coupling method to study the characteristics of coal spontaneous combustion under different external air supplies and temperature controls. The average error between simulation results of oxygen and carbon dioxide volume fractions and experimental results is only 2.3% and 4.6%, respectively. The acquisition of numerical simulation parameters and the numerical simulation method can reasonably characterize the coal oxidation process. The research results have important practical significance for the prevention and control of coal spontaneous combustion in goaf.
- (2)
- With the increase in air supply volume, the oxygen volume fraction at the outlet increases, and the peak value of the change rate of oxygen volume fraction at the outlet presents the “hysteresis” characteristic. As the air flow increases, the area with high oxygen volume fraction moves upward and the internal temperature of coal is getting higher, and linearly related to the air supply flow. After the air supply flow is greater than 24 mL/min, the maximum coal temperature exceeds the leading temperature of the programmed temperature rise after 7.5 h, its change rate exceeds the programmed temperature rise rate after 4 h, and the oxidation reaction is further intensified.
- (3)
- As the heating rate increases, the decrease in oxygen volume fraction at the outlet intensifies, and the peak value of the oxygen volume fraction change rate presents the “early appearance” characteristic. The maximum coal temperature and its change rate always change from below the programmed leading temperature (heating rate) to above the programmed leading value, and in the later stage of coal oxidation, their values increase rapidly, and the risk of coal spontaneous combustion also increases further.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, T.; Zhai, X.; Xiao, Y.; Bai, Y.; Shen, K.; Song, B.; Hao, L.; Ren, L.; Chen, X. Study on the influence of key active groups on gas products in spontaneous combustion of coal. Fuel 2023, 344, 128020. [Google Scholar] [CrossRef]
- Li, D.; Wang, E.; Kong, X.; Ali, M.; Wang, D. Mechanical behaviors and acoustic emission fractal characteristics of coal specimens with a pre-existing flaw of various inclinations under uniaxial compression. Int. J. Rock Mech. Min. 2019, 116, 38–51. [Google Scholar] [CrossRef]
- Xu, K.; Li, S.; Liu, J.; Lu, C.; Xue, G.; Xu, Z.; He, C. Evaluation cloud model of spontaneous combustion fire risk in coal mines by fusing interval gray number and DEMATEL. Sustainability 2022, 14, 15585. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Chen, X.; Zhang, Y.; Rui, L.; Guo, R.; Zhao, T.; Deng, Y. Numerical simulation on response characteristics of coal ignition under the disturbance of fluctuating heat. Combust. Flame 2022, 237, 111870. [Google Scholar] [CrossRef]
- Ren, S.; Ma, T.; Zhang, Y.; Deng, J.; Xiao, Y.; Zhai, X.; Zhang, Y.; Song, Z.; Wang, C. Sound absorption characteristics of loose bituminous coal porous media with different metamorphic degrees. Fuel 2023, 332, 126091. [Google Scholar] [CrossRef]
- Kong, X.; He, D.; Liu, X.; Wang, E.; Li, S.; Liu, T.; Ji, P.; Deng, D.; Yang, S. Strain characteristics and energy dissipation laws of gas-bearing coal during impact fracture process. Energy 2022, 242, 123028. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, J.; Wang, L.; Luo, H.; Ren, T. Forecasting spontaneous combustion of coal in underground coal mines by index gases: A review. J. Loss Prev. Proc. 2019, 57, 208–222. [Google Scholar] [CrossRef]
- Sahu, A.K.; Joshi, K.A.; Raghavan, V.; Rangwala, A.S. Comprehensive numerical modeling of ignition of coal dust layers in different configurations. Proc. Combust. Inst. 2015, 35, 2355–2362. [Google Scholar] [CrossRef]
- Zeneli, M.; Nikolopoulos, A.; Nikolopoulos, N.; Grammelis, P.; Karellas, S.; Kakaras, E. Simulation of the reacting flow within a pilot scale calciner by means of a three phase TFM model. Fuel Process. Technol. 2017, 162, 105–125. [Google Scholar] [CrossRef]
- Joshi, K.A.; Raghavan, V.; Rangwala, A.S. An experimental study of coal dust ignition in wedge shaped hot plate configurations. Combust. Flame 2012, 159, 376–384. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Li, Y.; Huang, Y.; Zhang, J.; Zhang, Y.; Li, Q. Ultrasonic extraction and oxidation characteristics of functional groups during coal spontaneous combustion. Fuel 2019, 242, 287–294. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, J.; Wang, T.; Song, J.; Zhang, Y.; Shu, C.; Zeng, Q. Assessing the effectiveness of a high-temperature-programmed experimental system for simulating the spontaneous combustion properties of bituminous coal through thermokinetic analysis of four oxidation stages. Energy 2019, 169, 587–596. [Google Scholar] [CrossRef]
- Deng, J.; Xiao, Y.; Li, Q.; Lu, J.; Wen, H. Experimental studies of spontaneous combustion and anaerobic cooling of coal. Fuel 2015, 157, 261–269. [Google Scholar] [CrossRef]
- Qi, G.; Lu, W.; Qi, X.; Zhong, X.; Cheng, W.; Liu, F. Differences in smoldering characteristics of coal piles with different smoldering propagation directions. Fire Safety J. 2018, 102, 77–82. [Google Scholar]
- Wen, H.; Wang, H.; Liu, W.; Cheng, X. Comparative study of experimental testing methods for characterization parameters of coal spontaneous combustion. Fuel 2020, 275, 117880. [Google Scholar] [CrossRef]
- Beamish, B.B.; Theiler, J. Coal spontaneous combustion: Examples of the self-heating incubation process. Int. J. Coal Geol. 2019, 215, 103297. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, X.; Deng, J.; Wen, H.; Xiao, Y.; Jia, H. Research on coal spontaneous combustion period based on pure oxygen adiabatic oxidation experiment. Fuel 2021, 288, 119651. [Google Scholar] [CrossRef]
- Jia, X.; Wu, J.; Lian, C.; Rao, J. Assessment of coal spontaneous combustion index gas under different oxygen concentration environment: An experimental study. Environ. Sci. Pollut. Res. 2022, 29, 87257–87267. [Google Scholar] [CrossRef]
- Shi, X.; Chen, X.; Zhang, Y.; Zhang, Y.; Guo, R.; Zhao, T.; Liu, R. Numerical simulation of coal dust self–ignition and combustion under inclination conditions. Energy 2022, 239, 122227. [Google Scholar] [CrossRef]
- Tang, Y.; Zhong, X.; Li, G.; Yang, Z.; Shi, G. Simulation of dynamic temperature evolution in an underground coal fire area based on an optimised Thermal-Hydraulic-Chemical model. Combust. Theory Model. 2019, 23, 127–146. [Google Scholar] [CrossRef]
- Chen, X.; Shi, X.; Zhang, Y.; Zhang, Y.; Ma, Q. Numerical simulation study on coal spontaneous combustion: Effect of porosity distribution. Combust. Sci. Technol. 2023, 195, 472–493. [Google Scholar] [CrossRef]
- Wen, H.; Yu, Z.; Deng, J.; Zhai, X. Spontaneous ignition characteristics of coal in a large-scale furnace: An experimental and numerical investigation. Appl. Therm. Eng. 2017, 114, 583–592. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Chen, X.; Zhang, Y. Effects of thermal boundary conditions on spontaneous combustion of coal under temperature-programmed conditions. Fuel 2021, 295, 120591. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Liu, H.; Zhai, X.; Zhao, S.; Yu, Z. Numerical analysis on the potential danger zone of compound hazard in gob under mining condition. Process. Saf. Environ. 2021, 147, 1125–1134. [Google Scholar] [CrossRef]
- Ma, L.; Guo, R.; Wu, M.; Wang, W.; Ren, L.; Wei, G. Determination on the hazard zone of spontaneous coal combustion in the adjacent gob of different mining stages. Process. Saf. Environ. 2020, 142, 370–379. [Google Scholar] [CrossRef]
- Yuan, L.; Smith, A.C. Numerical study on effects of coal properties on spontaneous heating in longwall gob areas. Fuel 2008, 87, 3409–3419. [Google Scholar] [CrossRef]
- Liu, W.; Qin, Y.; Shi, C.; Guo, D. Dynamic evolution of spontaneous combustion of coal in longwall gobs during mining-stopped period. Process. Saf. Environ. 2019, 132, 11–21. [Google Scholar] [CrossRef]
- Ren, L.; Deng, J.; Li, Q.; Ma, L.; Zou, L.; Laiwang, B.; Shu, C. Low-temperature exothermic oxidation characteristics and spontaneous combustion risk of pulverised coal. Fuel 2019, 252, 238–245. [Google Scholar] [CrossRef]
- Yan, H.; Nie, B.; Liu, P.; Chen, Z.; Yin, F.; Gong, J.; Lin, S.; Wang, X.; Kong, F.; Hou, Y. Experimental investigation and evaluation of influence of oxygen concentration on characteristic parameters of coal spontaneous combustion. Thermochim. Acta 2022, 717, 179345. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Li, Y.; Shi, X.; Che, B. Determination of ignition temperature and kinetics and thermodynamics analysis of high-volatile coal based on differential derivative thermogravimetry. Energy 2022, 240, 122493. [Google Scholar] [CrossRef]
- Sabat, G.; Gouda, N.; Panda, A.K. Effect of coal grade and heating rate on the thermal degradation behavior, kinetics, and thermodynamics of pyrolysis of low-rank coal. Int. J. Coal Prep. Util. 2022, 1–19. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, M.; Fu, X.; Cao, J.; Li, C.; Feng, M.; Li, K. Study on pyrolysis characteristics and kinetics of bituminous coal by thermogravimetric method. Combust. Sci. Technol. 2022, 194, 558–573. [Google Scholar] [CrossRef]
- Ejlali, A.; Mee, D.J.; Hooman, K.; Beamish, B.B. Numerical modelling of the self-heating process of a wet porous medium. Int. J. Heat Mass Transf. 2011, 54, 5200–5206. [Google Scholar] [CrossRef]
- Xia, T.; Zhou, F.; Wang, X.; Zhang, Y.; Li, Y.; Kang, J.; Liu, J. Controlling factors of symbiotic disaster between coal gas and spontaneous combustion in longwall mining gobs. Fuel 2016, 182, 886–896. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Zhu, P.; Li, X.; Zhang, G.; Ma, X.; Zhao, Y. Study on multi-field evolution and influencing factors of coal spontaneous combustion in goaf. Combust. Sci. Technol. 2023, 195, 247–264. [Google Scholar] [CrossRef]
- Wu, D.; Vanierschot, M.; Verplaetsen, F.; Berghmans, J.; Van den Bulck, E. Numerical study on the ignition behavior of coal dust layers in air and O2/CO2 atmospheres. Appl. Therm. Eng. 2016, 109, 709–717. [Google Scholar] [CrossRef]
- Lei, C.; Deng, J.; Cao, K.; Xiao, Y.; Ma, L.; Wang, W.; Ma, T.; Shu, C. A comparison of random forest and support vector machine approaches to predict coal spontaneous combustion in gob. Fuel 2019, 239, 297–311. [Google Scholar] [CrossRef]
- Deng, J.; Lei, C.; Xiao, Y.; Cao, K.; Ma, L.; Wang, W.; Laiwang, B. Determination and prediction on “three zones” of coal spontaneous combustion in a gob of fully mechanized caving face. Fuel 2018, 211, 458–470. [Google Scholar] [CrossRef]
- Zhang, Y.; Shu, P.; Deng, J.; Duan, Z.; Li, L.; Zhang, L. Analysis of oxidation pathways for characteristic groups in coal spontaneous combustion. Energy 2022, 254, 124211. [Google Scholar] [CrossRef]
- Wang, C.; Deng, Y.; Xiao, Y.; Deng, J.; Shu, C.; Jiang, Z. Gas-heat characteristics and oxidation kinetics of coal spontaneous combustion in heating and decaying processes. Energy 2022, 250, 123810. [Google Scholar] [CrossRef]
- Lei, C.; Jiang, L.; Bao, R.; Deng, C.; Wang, C. Study on multifield migration and evolution law of the oxidation heating process of coal spontaneous combustion in dynamic goaf. ACS Omega 2023, 8, 14197–14207. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Li, Z.; Yang, Y.; Liu, Z.; Yan, D. A review on the mechanism, risk evaluation, and prevention of coal spontaneous combustion in China. Environ. Sci. Pollut. Res. 2017, 24, 23453–23470. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Deng, J.; Cao, K.; Ma, L.; Xiao, Y.; Ren, L. A random forest approach for predicting coal spontaneous combustion. Fuel 2018, 223, 63–73. [Google Scholar] [CrossRef]
| Proximate Analysis | Ultimate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Mad/% | Vad/% | Aad/% | FCad/% | Cdaf/% | Hdaf/% | Odaf/% | Ndaf/% | Sdaf/% |
| 11.75 | 35.78 | 13.03 | 39.44 | 65.36 | 1.25 | 12.66 | 1.91 | 0.52 |
| Parameters | Description | Value |
|---|---|---|
| p0 | Pressure (atm) | 1 |
| T0 | Initial temperature (°C) | 30 |
| Rs | Specific gas constant (J/kg·K) | 287 |
| γ | Specific heat | 1.4 |
| kc | Thermal conductivity (W/m·K) | 0.21 |
| ρc | Coal density (kg/m3) | 1200 |
| Cp,c | Specific heat capacity (J/kg·K) | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, C.; Shi, X.; Jiang, L.; Deng, C.; Nian, J.; Gao, Y. Study on the Effect of External Air Supply and Temperature Control on Coal Spontaneous Combustion Characteristics. Sustainability 2023, 15, 8286. https://doi.org/10.3390/su15108286
Lei C, Shi X, Jiang L, Deng C, Nian J, Gao Y. Study on the Effect of External Air Supply and Temperature Control on Coal Spontaneous Combustion Characteristics. Sustainability. 2023; 15(10):8286. https://doi.org/10.3390/su15108286
Chicago/Turabian StyleLei, Changkui, Xueqiang Shi, Lijuan Jiang, Cunbao Deng, Jun Nian, and Yabin Gao. 2023. "Study on the Effect of External Air Supply and Temperature Control on Coal Spontaneous Combustion Characteristics" Sustainability 15, no. 10: 8286. https://doi.org/10.3390/su15108286
APA StyleLei, C., Shi, X., Jiang, L., Deng, C., Nian, J., & Gao, Y. (2023). Study on the Effect of External Air Supply and Temperature Control on Coal Spontaneous Combustion Characteristics. Sustainability, 15(10), 8286. https://doi.org/10.3390/su15108286









