Characteristics of Tetracycline Adsorption on Commercial Biochar from Synthetic and Real Wastewater in Batch and Continuous Operations: Study of Removal Mechanisms, Isotherms, Kinetics, Thermodynamics, and Desorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Adsorbate and Adsorbent
2.2. Experiments
2.3. Analysis
2.4. Calculations
2.5. Equilibrium Studies and Kinetic Models
2.6. Adsorption Mechanism
2.6.1. Intraparticle Diffusion Model
2.6.2. Boyd Kinetic Model
2.7. Adsorption Thermodynamics Studies
3. Results and Discussion
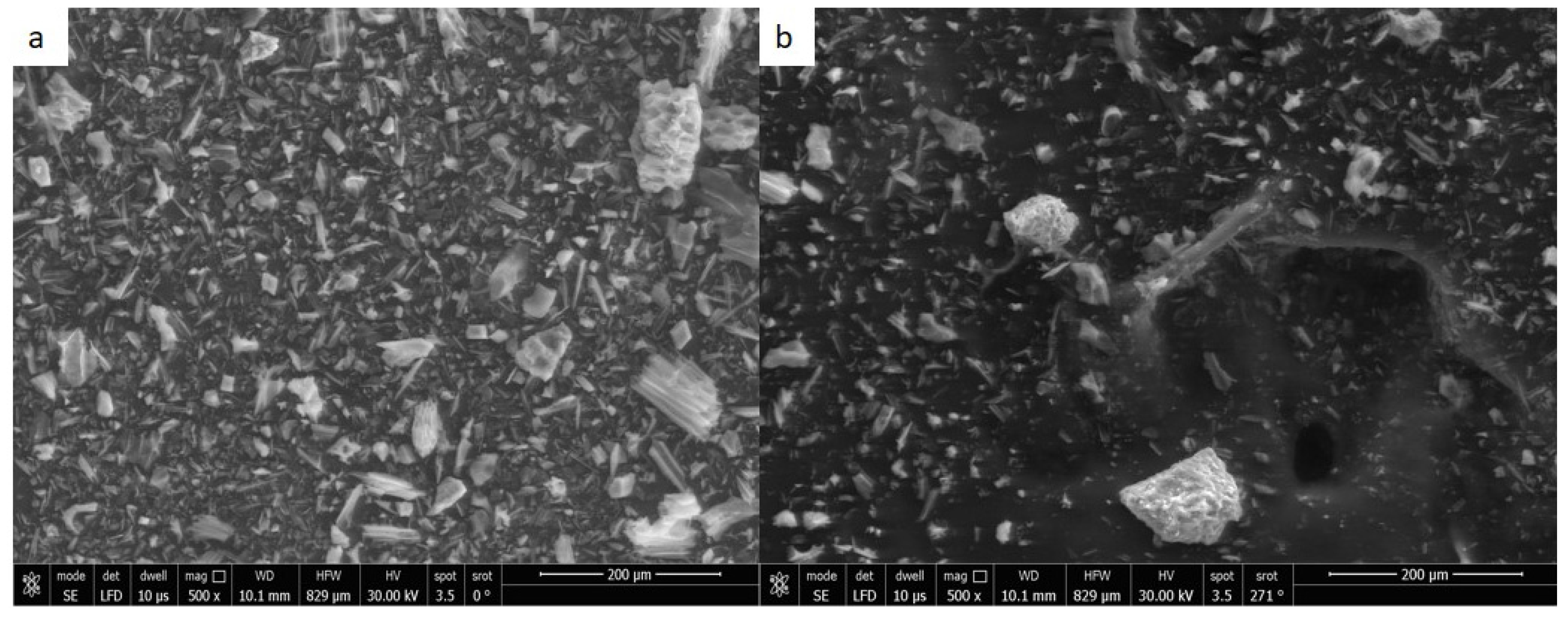
3.1. Adsorbent Characterization
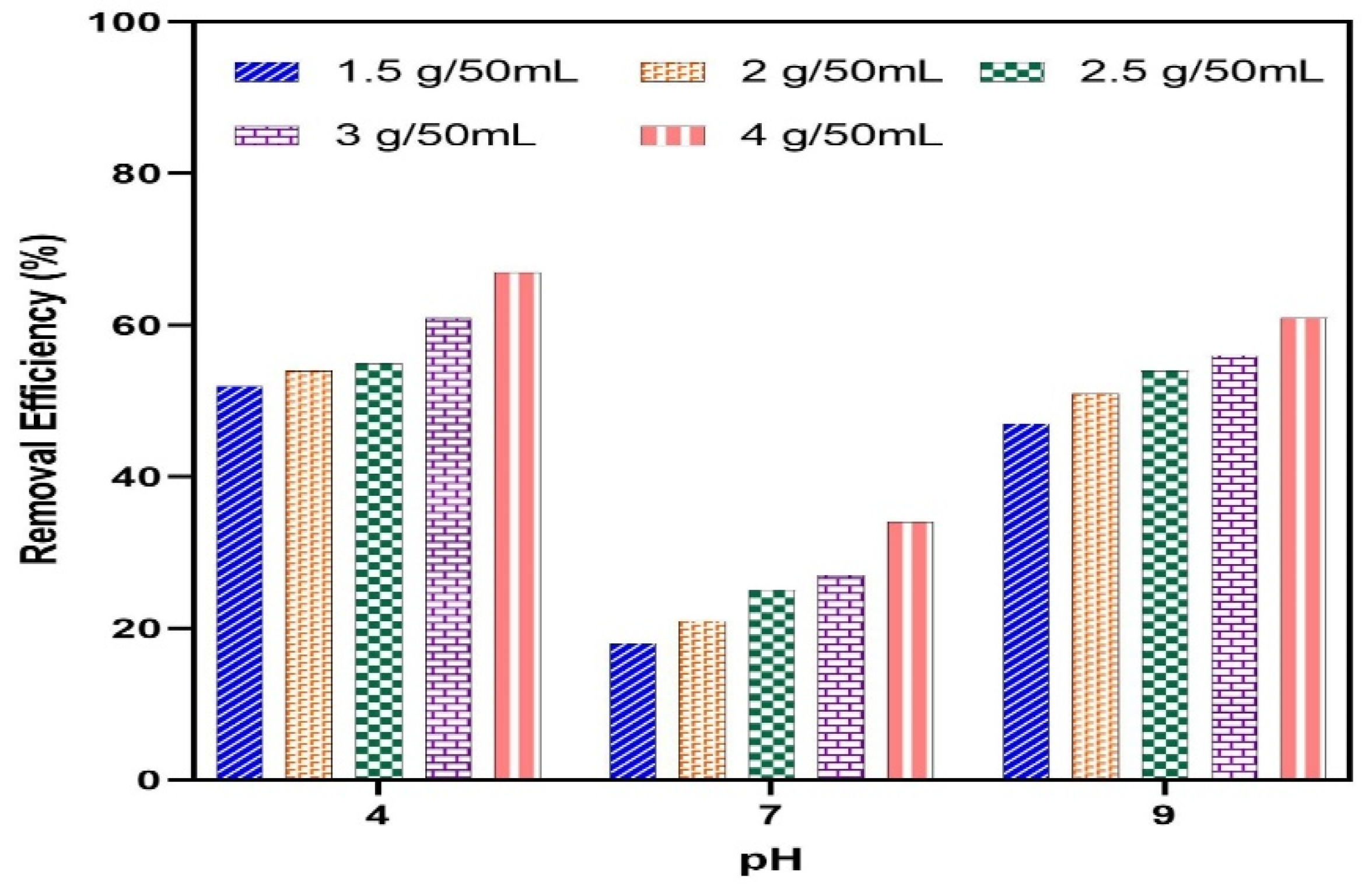
3.2. Effects of Initial pH and Adsorbent Dose
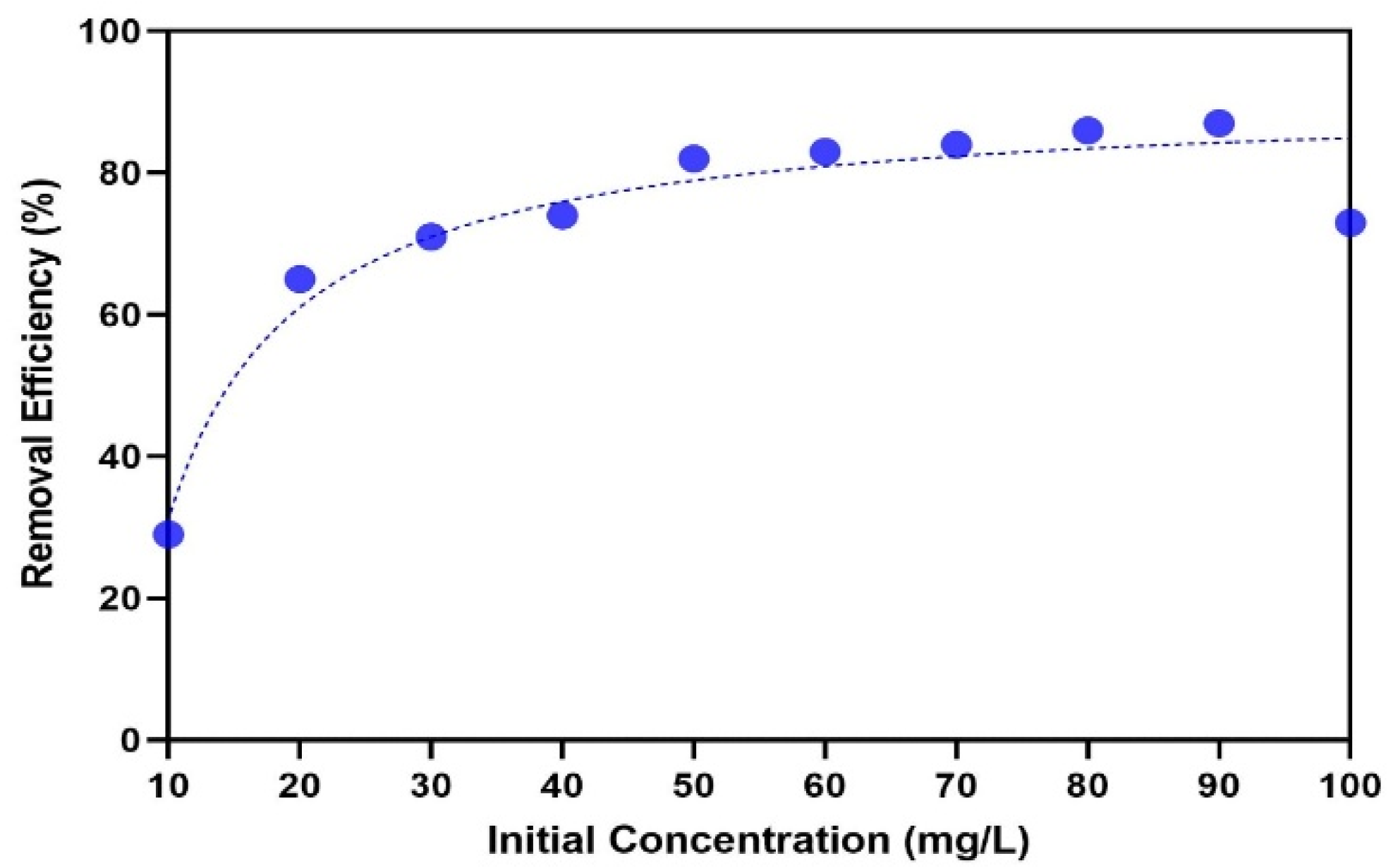
3.3. Effect of Initial Concentration
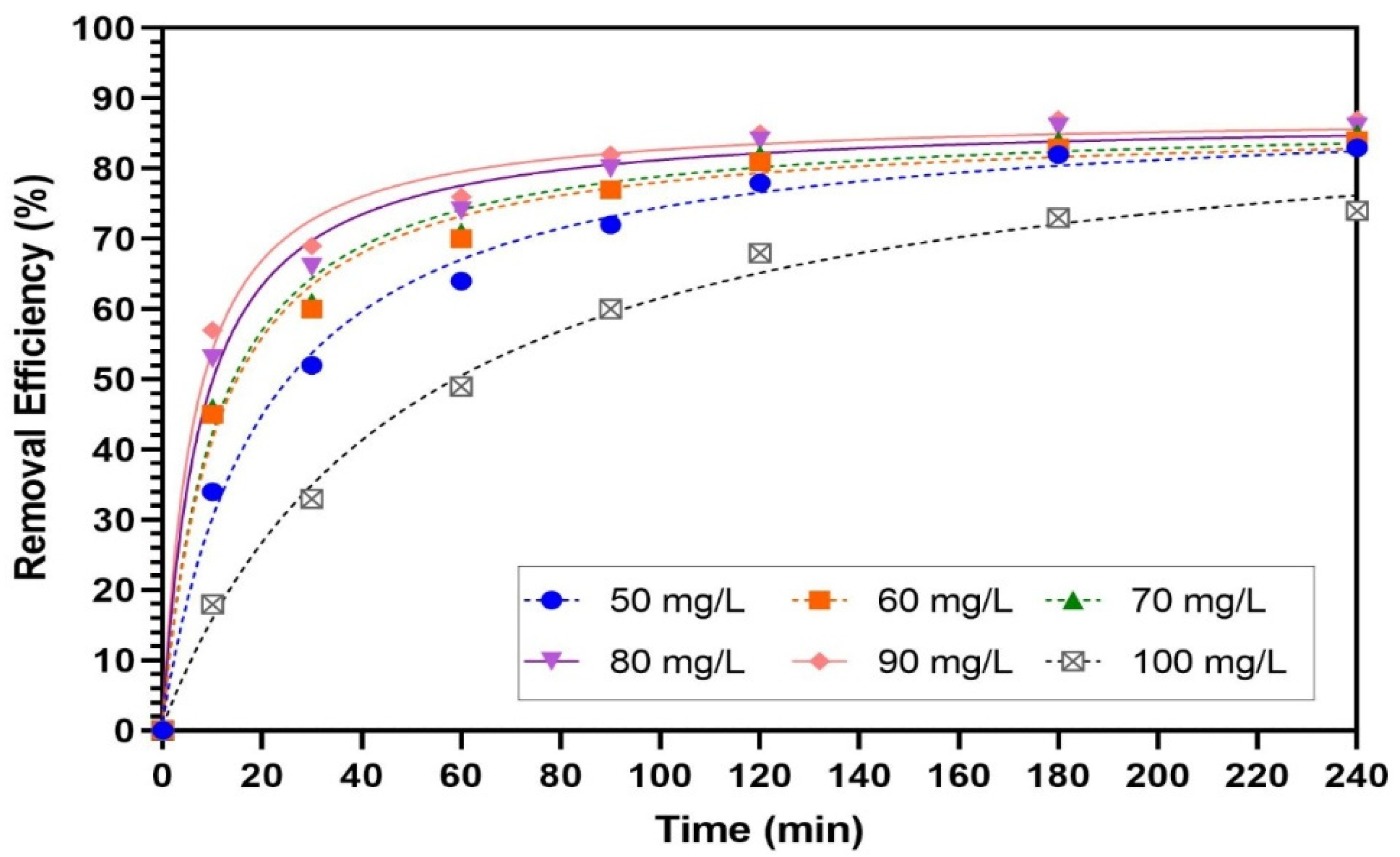
3.4. Effect of Reaction Time
3.5. Adsorption Isotherms
3.6. Adsorption Kinetics
3.7. Adsorption Mechanism
3.7.1. Intraparticle Diffusion Model
3.7.2. Boyd Model
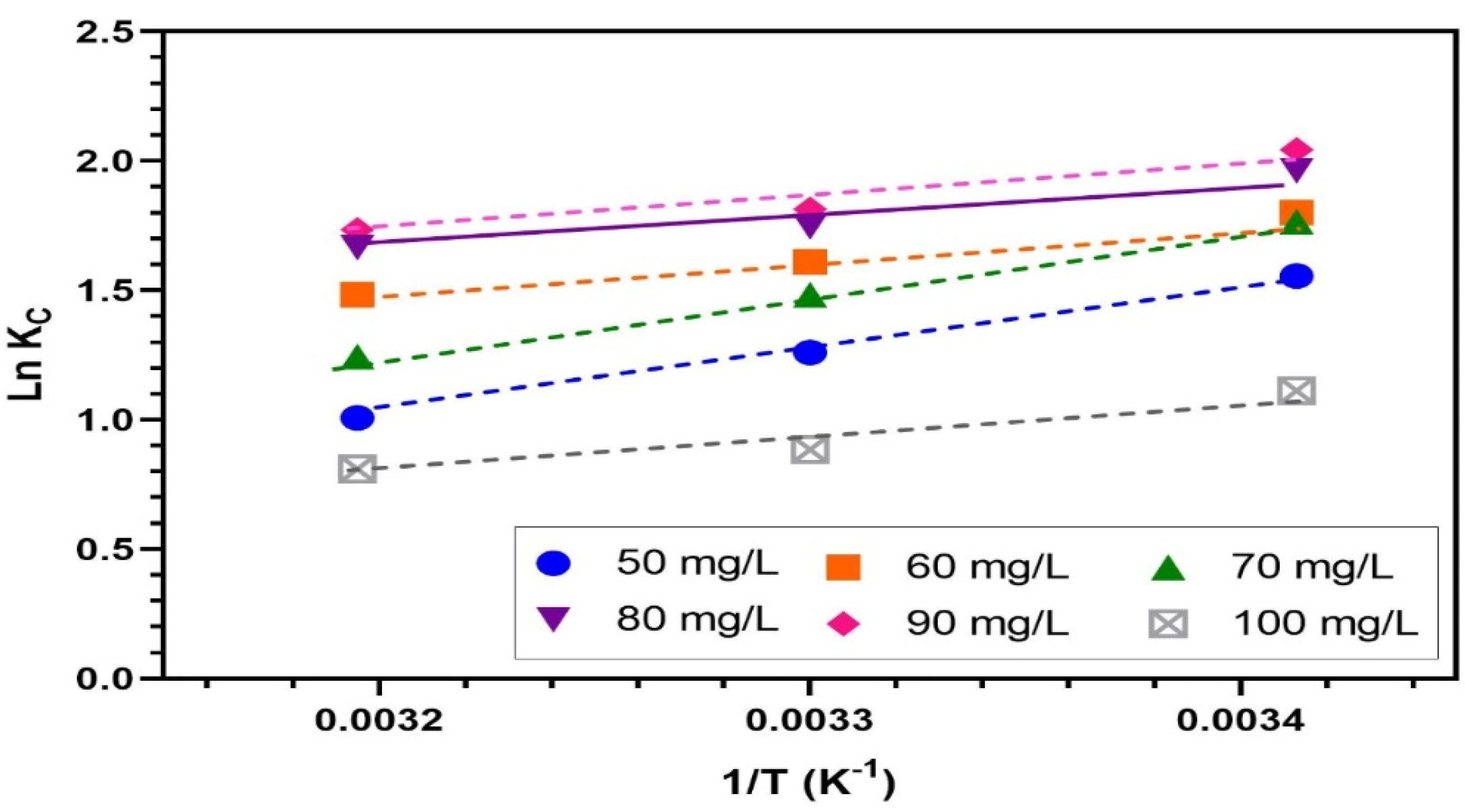
3.8. Adsorption Thermodynamics Studies
3.9. Desorption
3.10. Real Wastewater Experiments
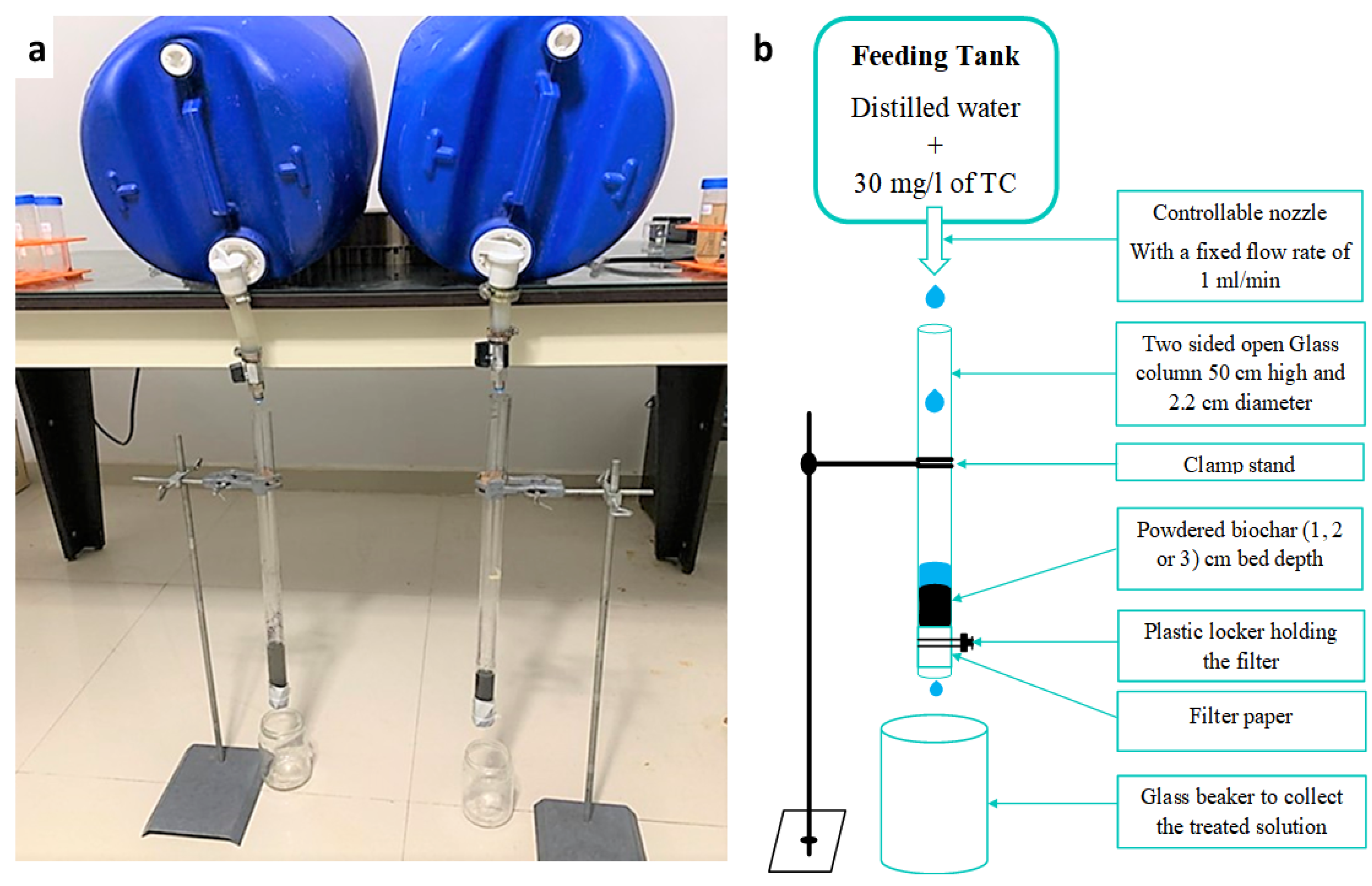
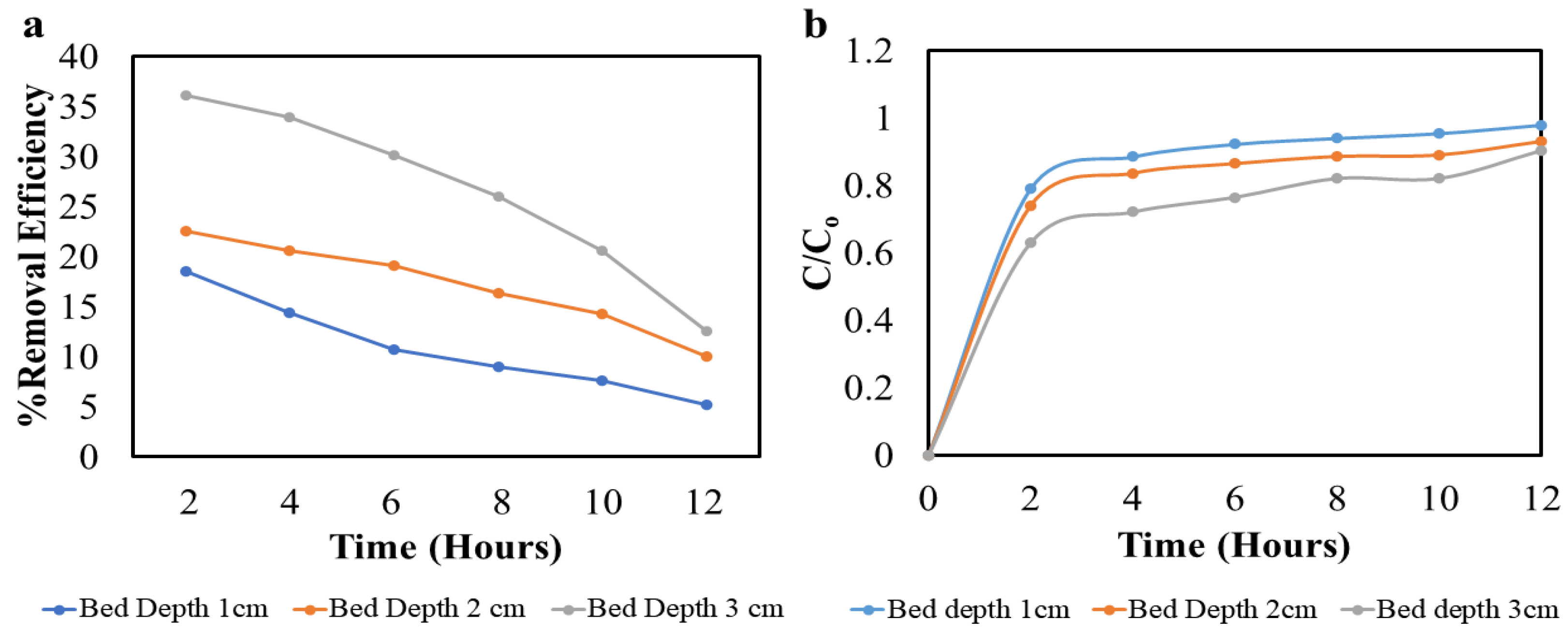
3.11. Column Test Experiment
3.12. Cost Analysis
3.13. Research Findings in Comparison with the Literature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Qi, P.S.; Liu, Y.Z. A Review on Advanced Treatment of Pharmaceutical Wastewater. IOP Conf. Ser. Earth Environ. Sci. 2017, 63, 012025. [Google Scholar] [CrossRef]
- Akter, S.; Islam, M.S.; Kabir, M.H.; Shaikh, M.A.A.; Gafur, M.A. UV/TiO2 photodegradation of metronidazole, ciprofloxacin and sulfamethoxazole in aqueous solution: An optimization and kinetic study. Arab. J. Chem. 2022, 15, 103900. [Google Scholar] [CrossRef]
- Dan, A.; Zhang, X.; Dai, Y.; Chen, C.; Yang, Y. Occurrence and removal of quinolone, tetracycline, and macrolide antibiotics from urban wastewater in constructed wetlands. J. Clean. Prod. 2020, 252, 119677. [Google Scholar]
- Priya, S.S.; Radha, K. A Review on the Adsorption Studies of Tetracycline onto Various Types of Adsorbents. Chem. Eng. Commun. 2017, 204, 821–839. [Google Scholar] [CrossRef]
- Sayğili, H.; Güzel, F. Effective removal of tetracycline from aqueous solution using activated carbon prepared from tomato (Lycopersicon esculentum Mill.) industrial processing waste. Ecotoxicol. Environ. Saf. 2016, 131, 22–29. [Google Scholar] [CrossRef]
- Bao, J.; Zhu, Y.; Yuan, S.; Wang, F.; Tang, H.; Bao, Z.; Zhou, H.; Chen, Y. Adsorption of Tetracycline with Reduced Graphene Oxide Decorated with MnFe2O4 Nanoparticles. Nanoscale Res. Lett. 2018, 13, 396. [Google Scholar] [CrossRef]
- Ahmed, M.J. Adsorption of quinolone, tetracycline, and penicillin antibiotics from aqueous solution using activated carbons: Review. Environ. Toxicol. Pharmacol. 2017, 50, 1–10. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Varjani, S.; Liu, Y. Feasibility study on a new pomelo peel derived biochar for tetracycline antibiotics removal in swine wastewater. Sci. Total. Environ. 2020, 720, 137662. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A.; Kareem, S.L. Adsorption of tetracycline from wastewater by using Pistachio shell coated with ZnO nanoparticles: Equilibrium, kinetic and isotherm studies. Alexandria Eng. J. 2019, 58, 917–928. [Google Scholar] [CrossRef]
- Islam, S.; McPhedran, K.N.; Messele, S.A.; Liu, Y.; El-Din, M.G. Isotherm and kinetic studies on adsorption of oil sands process-affected water organic compounds using granular activated carbon. Chemosphere 2018, 202, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Ma, J.; Han, S. Adsorption of tetracycline from aqueous solutions onto multi-walled carbon nanotubes with different oxygen contents. Sci. Rep. 2014, 4, 5326. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Ghadim, E.E.; Manouchehri, F.; Soleimani, G.; Hosseini, H.; Kimiagar, S.; Nafisi, S. Adsorption properties of tetracycline onto graphene oxide: Equilibrium, kinetic and thermodynamic studies. PLoS ONE 2013, 8, e79254. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, H.; Li, Y.; Chen, J.; Zhu, M.; Zhang, X. Adsorption of tetracycline by activated carbon fibre. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar]
- Khalaf, S.M.; Al-Mahmoud, S.M. Adsorption of tetracycline antibiotic from aqueous solutions using natural Iraqi bentonite. Egypt. J. Chem. 2021, 64, 5511–5519. [Google Scholar]
- Zhou, Q.; Li, Z.; Shuang, C.; Li, A.; Zhang, M.; Wang, M. Efficient removal of tetracycline by reusable magnetic microspheres with a high surface area. Chem. Eng. J. 2012, 210, 350–356. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, T.; Liu, J.; Li, D. Adsorption of tetracycline antibiotics from an aqueous solution onto graphene oxide/calcium alginate composite fibers. RSC Adv. 2018, 8, 2616–2621. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Roy, H.; Sarkar, D.; Pervez, N.; Paul, S.; Cai, Y.; Naddeo, V.; Firoz, S.H.; Islam, S. Synthesis, Characterization and Performance Evaluation of Burmese Grape (Baccaurea ramiflora) Seed Biochar for Sustainable Wastewater Treatment. Water 2023, 15, 394. [Google Scholar] [CrossRef]
- Roy, H.; Prantika, T.R.; Riyad, M.H.; Paul, S.; Islam, M.S. Synthesis, characterizations, and RSM analysis of Citrus macroptera peel derived biochar for textile dye treatment. S. Afr. J. Chem. Eng. 2022, 41, 129–139. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Cao, Y.; Han, L. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour. Technol. 2019, 285, 121348. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Chen, Y.; Li, Y.; Sun, F.; Zhao, Y.; Yang, S. Adsorption of Congo red and tetracycline onto water treatment sludge biochar: Characterisation, kinetic, equilibrium and thermodynamic study. Water Sci. Technol. 2022, 85, 1936–1951. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Chen, Y.; Zhang, Y.; You, N. Nanocomposites of zero-valent iron@biochar derived from agricultural wastes for adsorptive removal of tetracyclines. Chemosphere 2021, 284, 131342. [Google Scholar] [CrossRef] [PubMed]
- Hoslett, J.; Ghazal, H.; Katsou, E.; Jouhara, H. The removal of tetracycline from water using biochar produced from agricultural discarded material. Sci. Total. Environ. 2020, 751, 141755. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Kan, E. Engineered biochar from agricultural waste for removal of tetracycline in water. Bioresour. Technol. 2019, 284, 437–447. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Kirupha, S.D.; Murugesan, A.; Vidhyadevi, T.; Sivanesan, S. Adsorption behavior of nickel(II) onto cashew nut shell: Equilibrium, thermodynamics, kinetics, mechanism and process design. Chem. Eng. J. 2011, 167, 122–131. [Google Scholar] [CrossRef]
- Safwat, S.M.; Matta, M.E. Adsorption of urea onto granular activated alumina: A comparative study with granular activated carbon. J. Dispers. Sci. Technol. 2018, 39, 1699–1709. [Google Scholar] [CrossRef]
- Safwat, S.M.; Medhat, M.; Abdel-Halim, H. Phenol adsorption onto kaolin and fuller’s earth: A comparative study with bentonite. Desalination Water Treat. 2019, 155, 197–206. [Google Scholar] [CrossRef]
- Safwat, S.M.; Ali, A.; Matta, M.E. Adsorption of Copper using Fuller’s Earth: Kinetics, Equilibrium and Thermodynamics. J. Eng. Appl. Sci. 2020, 67, 1729–1746. [Google Scholar]
- Gupta, V.K.; Carrott, P.J.M.; Ribeiro Carrott, M.M.L.; Suhas. Low-Cost Adsorbents: Growing Approach to Wastewater Treatment—A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 783–842. [Google Scholar] [CrossRef]
- Kwak, J.H.; Islam, M.S.; Wang, S.; Messele, S.A.; Naeth, M.A.; El-Din, M.G.; Chang, S.X. Biochar properties and lead(II) adsorption capacity depend on feedstock type, pyrolysis temperature, and steam activation. Chemosphere 2019, 231, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Meng, X.; Zhang, Y.; Huang, Y. Effects of modification and magnetization of rice straw derived biochar on adsorption of tetracycline from water. Bioresour. Technol. 2020, 311, 123455. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fang, C.; Wang, Q.; Chu, Y.; Song, Y.; Chen, Y.; Xue, X. Sorption of tetracycline on biochar derived from rice straw and swine manure. RSC Adv. 2018, 8, 16260–16268. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E. Adsorption Processes for Water; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Zhu, X.; Liu, Y.; Qian, F.; Zhou, C.; Zhang, S.; Chen, J. Preparation of magnetic porous carbon from waste hydrochar by simultaneous activation and magnetization for tetracycline removal. Bioresour. Technol. 2014, 154, 209–214. [Google Scholar] [CrossRef]
- Adak, A.; Bandyopadhyay, M.; Pal, A. Adsorption of Anionic Surfactant on Alumina and Reuse of the Surfactant-Modified Alumina for the Removal of Crystal Violet from Aquatic Environment. J. Environ. Sci. Health Part A 2005, 40, 167–182. [Google Scholar] [CrossRef]
- Korkut, F.; Saloglu, D. Synthesis, characterization, and tetracycline adsorption behavior of activated carbon dopped alginate beads: Isotherms, kinetics, thermodynamic, and adsorption mechanism. Desalin. Water Treat. 2020, 206, 315–330. [Google Scholar] [CrossRef]
- Marzbali, M.H.; Esmaieli, M. Fixed bed adsorption of tetracycline on a mesoporous activated carbon: Experimental study and neuro-fuzzy modeling. J. Appl. Res. Technol. 2017, 15, 454–463. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Yang, M.; Han, H.; Zhang, S.; Ouyang, G.; Han, R. Removal of tetracycline using modified wheat straw from solution in batch and column modes. J. Mol. Liq. 2021, 338, 116698. [Google Scholar] [CrossRef]



| Parameter | Value |
|---|---|
| pH | 8.5 |
| EC (DS/M) | 2.52 |
| N (%) | 1.5 |
| P (%) | 7 |
| K (%) | 2 |
| Organic C (%) | 70 |
| O/C | 0.6 |
| H/C | 0.36 |
| Parameter | Value |
|---|---|
| pH | 7.45 |
| Total suspended solids (TSS) (mg/L) | 15.5 |
| Volatile suspended solids (VSS) (mg/L) | 11.3 |
| Biochemical oxygen demand (BOD5) (mg/L) | 17.3 |
| CaO | CoO | Cl | FeO | K2O | MnO | MoO3 | Nb2O5 | P2O5 | SO3 | SrO | SiO2 | TiO2 | ZnO | ZrO2 | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50.99 | 0.08 | 7.54 | 6.72 | 14.71 | 0.19 | 0.38 | 0.31 | 0.94 | 4.20 | 0.43 | 9.01 | 0.92 | 0.75 | 0.18 | 97.35 |
| Freundlich | Langmuir | Temkin | Dubinin–Radushkevich | ||||
|---|---|---|---|---|---|---|---|
| 1/n (g/L) | 0.35 | Qmax (mg/g) | −0.3263 | B1 (mg/L) | 1.6949 | qDR (mg/g) | 11.4146 |
| Kf (mg/g)(mg/L)n | 0.001038245 | KL (L/mg) | −0.0678 | KT (L/mg) | 0.1512 | KDR (mg2/J2) | −2.3912 × 10−6 |
| R2 | 0.9059 | R2 | 0.9245 | b (J/mol) | 1430.1327 | Ea (kJ/mol) | 0.2093 |
| R2 | 0.8788 | R2 | 0.9034 | ||||
| Kinetic Model | Parameter | Initial Concentration of TC (mg/L) | |||||
|---|---|---|---|---|---|---|---|
| 50 | 60 | 70 | 80 | 90 | 100 | ||
| PFO | K1 (min−1) | 0.0193 | 0.0198 | 0.0189 | 0.0209 | 0.0195 | 0.0215 |
| qe (exp)(mg/g) | 0.4785 | 0.5792 | 0.6860 | 0.7966 | 0.9100 | 0.8537 | |
| qe (calc)(mg/g) | 0.3480 | 0.3115 | 0.3594 | 0.3772 | 0.3862 | 0.9481 | |
| R2 | 0.9978 | 0.985 | 0.9925 | 0.9954 | 0.9960 | 0.9897 | |
| PSO | K2 (g/mg/min) | 0.0904 | 0.1247 | 0.1083 | 0.1203 | 0.1171 | 0.0185 |
| qe (exp)(mg/g) | 0.4785 | 0.5792 | 0.6860 | 0.7966 | 0.9100 | 0.8537 | |
| qe (calc)(mg/g) | 0.5192 | 0.6093 | 0.7175 | 0.8276 | 0.9382 | 1.0750 | |
| R2 | 0.9977 | 0.9985 | 0.9985 | 0.9988 | 0.9989 | 0.9924 | |
| Elovich | α (g/mg/min) | 0.0707 | 0.2460 | 0.3352 | 1.02659 | 1.96380 | 0.0480 |
| β (g/mg) | 10.2459 | 10.5263 | 9.1996 | 9.2937 | 8.8261 | 4.3122 | |
| R2 | 0.9971 | 0.9915 | 0.9938 | 0.9937 | 0.9946 | 0.9806 | |
| T (°C) | Initial TC Concentration (mg/L) | |||||
|---|---|---|---|---|---|---|
| 50 | 60 | 70 | 80 | 90 | ||
| ∆H° (kJ/mol) | −20.45 | −19.47 | −11.88 | −11.15 | −11.61 | |
| ∆S° (J/mol/K) | −57.19 | −52.11 | −25.97 | −22.24 | −23.19 | |
| ∆G° (kJ/mol) | 20 | −3.70 | −4.20 | −4.27 | −4.63 | −4.82 |
| 30 | −3.13 | −3.68 | −4.01 | −4.41 | −4.59 | |
| 40 | −2.55 | −3.16 | −3.75 | −4.19 | −4.35 | |
| TC Initial Concentration (mg/L) | Average Removal Efficiency (%) | Percentage of Desorption (%) vs. HCl Concentrations | |||
|---|---|---|---|---|---|
| 0.2 M | 0.4 M | 0.6 M | 0.8 M | ||
| 50 | 82% | 80.50% | 83.27% | 84.62% | 87.24% |
| 60 | 83% | 84.58% | 85.48% | 86.81% | 88.04% |
| 70 | 84% | 83.99% | 85.10% | 87.18% | 88.51% |
| 80 | 86% | 88.57% | 91.31% | 91.73% | 92.95% |
| 90 | 87% | 88.27% | 88.83% | 89.52% | 91.26% |
| 100 | 74% | 88.31% | 89.20% | 91.17% | 91.71% |
| Adsorbent Used | Dose (g/L) | pH | T °C | TC Initial Conc. (mg/L) | Surf. Area (m2/g) | Isotherm | Kinetic Model | Thermodynamics | Removal Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Pistachio shell Coated with ZnO Nanoparticles | 80 | 4 | 25 | 70 | - | Freundlich | PSO | EXO. | 84.87 | [10] |
| Natural Iraqi Bentonite | 0.2 | 6–7 | 20–40 | 20 | - | Langmuir and Freundlich | PSO | EXO. | 90 | [16] |
| Cow manure biochar | 1.25 | 3 | 25 | 50 | 31.23 | Freundlich | PSO | ENDO. | 47 | [22] |
| Water treatment sludge biochar | 4 | 4 | 60 | 34.22 | Langmuir and Freundlich | PSO | ENDO. | 99 | [23] | |
| Activated carbon Dopped alginate beads | 0.2 | 7 | 25 | 20 | 850 | Langmuir and Freundlich | PSO | ENDO. | 100 | [38] |
| Citrus trees biochar | 70 | 4 | 20 | 90 | 364.903 | Freundlich | PSO | EXO. | 87 | Current study |
| Adsorbent Used | Bed Height (cm) | Q (mL/min) | C0 (mg/L) | pH | Removal | Ref. |
|---|---|---|---|---|---|---|
| Granular nanoporous activated carbon prepared from apricot shell | 4 | 4 | 50 | 5 | 38 | [39] |
| Modified wheat straw by diethylenetriamine, chloroacetic acid, and zirconium | 5 | 2 | 200 | 7 | 48 | [40] |
| Citrus trees biochar | 3 | 1 | 30 | 4 | 36 | Current study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizkallah, B.M.; Galal, M.M.; Matta, M.E. Characteristics of Tetracycline Adsorption on Commercial Biochar from Synthetic and Real Wastewater in Batch and Continuous Operations: Study of Removal Mechanisms, Isotherms, Kinetics, Thermodynamics, and Desorption. Sustainability 2023, 15, 8249. https://doi.org/10.3390/su15108249
Rizkallah BM, Galal MM, Matta ME. Characteristics of Tetracycline Adsorption on Commercial Biochar from Synthetic and Real Wastewater in Batch and Continuous Operations: Study of Removal Mechanisms, Isotherms, Kinetics, Thermodynamics, and Desorption. Sustainability. 2023; 15(10):8249. https://doi.org/10.3390/su15108249
Chicago/Turabian StyleRizkallah, Basem M., Mona M. Galal, and Minerva E. Matta. 2023. "Characteristics of Tetracycline Adsorption on Commercial Biochar from Synthetic and Real Wastewater in Batch and Continuous Operations: Study of Removal Mechanisms, Isotherms, Kinetics, Thermodynamics, and Desorption" Sustainability 15, no. 10: 8249. https://doi.org/10.3390/su15108249
APA StyleRizkallah, B. M., Galal, M. M., & Matta, M. E. (2023). Characteristics of Tetracycline Adsorption on Commercial Biochar from Synthetic and Real Wastewater in Batch and Continuous Operations: Study of Removal Mechanisms, Isotherms, Kinetics, Thermodynamics, and Desorption. Sustainability, 15(10), 8249. https://doi.org/10.3390/su15108249







