Influence of Mineral Deposition on the Retention of Potentially Hazardous Elements in Geothermal Spring Sediments
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
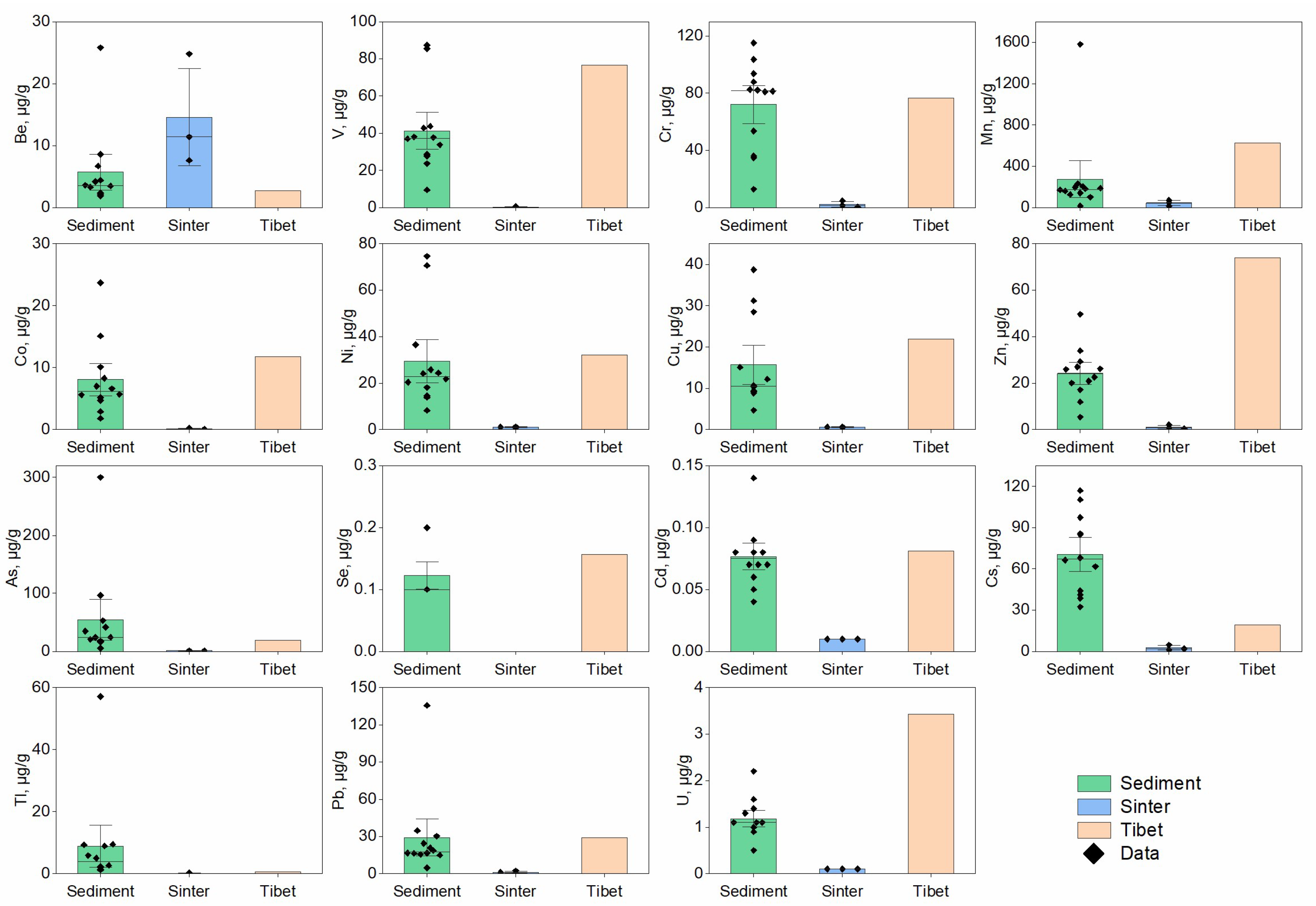
3.1. Distributions of PHEs in the Hot Spring Waters and Sediments
3.2. Mineralogical Compositions of the Hot Spring Sediments and Sinters
3.3. Immobilization of the PHEs by Mineral Precipitates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Zhou, X.; Zhuo, L.; Tao, G.; Ma, J.; Wang, Y. Structural controls of the northern Red River Fault Zone on the intensity of hydrothermal activity and distribution of hot springs in the Yunnan-Tibet geothermal belt. Geothermics 2023, 109, 102641. [Google Scholar] [CrossRef]
- Guo, Q. Hydrogeochemistry of high-temperature geothermal systems in China: A review. Appl. Geochem. 2012, 27, 1887–1898. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Tao, S. Opportunity and challenges in large-scale geothermal energy exploitation in China. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3813–3834. [Google Scholar] [CrossRef]
- Nshimyumuremyi, E.; Junqi, W. Geothermal reservoir heat transfer, temperature modelling and electrical power potential estimation: Gisenyi hot spring. IET Renew. Power Gener. 2020, 14, 1463–1470. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Tao, S. Environmental and human health impacts of geothermal exploitation in China and mitigation strategies. Crit. Rev. Environ. Sci. Technol. 2022, 53, 1173–1196. [Google Scholar] [CrossRef]
- Arslan, Ş.; Avşar, Ö. Assessment of heavy metal pollution in Köyceğiz-Dalyan coastal lagoon watershed (Muğla) SW Turkey. Arab. J. Geosci. 2020, 13, 719. [Google Scholar] [CrossRef]
- Vranovská, A.; Bodiš, D.; Sracek, O.; Ženišová, Z. Anomalous arsenic concentrations in the Ďurkov carbonate geothermal structure, eastern Slovakia. Environ. Earth Sci. 2015, 73, 7103–7114. [Google Scholar] [CrossRef]
- Bernard, R.; Taran, Y.; Pennisi, M.; Tello, E.; Ramirez, A. Chloride and Boron behavior in fluids of Los Humeros geothermal field (Mexico): A model based on the existence of deep acid brine. Appl. Geochem. 2011, 26, 2064–2073. [Google Scholar] [CrossRef]
- Baba, A.; Uzelli, T.; Sozbilir, H. Distribution of geothermal arsenic in relation to geothermal play types: A global review and case study from the Anatolian plate (Turkey). J. Hazard. Mater. 2021, 414, 125510. [Google Scholar] [CrossRef]
- Moeck, I.S. Catalog of geothermal play types based on geologic controls. Renew. Sustain. Energy Rev. 2014, 37, 867–882. [Google Scholar] [CrossRef]
- Morales-Arredondo, J.I.; Esteller-Alberich, M.V.; Armienta Hernández, M.A.; Martínez-Florentino, T.A.K. Characterizing the hydrogeochemistry of two low-temperature thermal systems in Central Mexico. J. Geochem. Explor. 2018, 185, 93–104. [Google Scholar] [CrossRef]
- Birkle, P.; Bundschuh, J.; Sracek, O. Mechanisms of arsenic enrichment in geothermal and petroleum reservoirs fluids in Mexico. Water Res. 2010, 44, 5605–5617. [Google Scholar] [CrossRef] [PubMed]
- Durowoju, O.S.; Odiyo, J.O.; Ekosse, G.-I.E. Variations of Heavy Metals from Geothermal Spring to Surrounding Soil and Mangifera Indica–Siloam Village, Limpopo Province. Sustainability 2016, 8, 60. [Google Scholar] [CrossRef]
- Yang, G.F.; Wang, D.L.; Li, N.; Zhuo, S.G. The Distribution and Environment Problems of Geothermal Springs in Development and Utilization in Beijing-Tianjin-Tangshan-Qinhuangdao Area, China. Appl. Mech. Mater. 2013, 295–298, 1948–1951. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y. Trace Element Hydrochemistry Indicating Water Contamination in and Around the Yangbajing Geothermal Field, Tibet, China. Bull. Environ. Contam. Toxicol. 2009, 83, 608–613. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Liu, W. Hydrogeochemistry and environmental impact of geothermal waters from Yangyi of Tibet, China. J. Volcanol. Geotherm. Res. 2009, 180, 9–20. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Liu, W. B, As, and F contamination of river water due to wastewater discharge of the Yangbajing geothermal power plant, Tibet, China. Environ. Geol. 2008, 56, 197–205. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef]
- Beckers, F.; Rinklebe, J. Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 693–794. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Varekamp, J.C. The volcanic acidification of glacial Lake Caviahue, Province of Neuquen, Argentina. J. Volcanol. Geotherm. Res. 2008, 178, 184–196. [Google Scholar] [CrossRef]
- Ng, J.C.; Wang, J.; Shraim, A. A global health problem caused by arsenic from natural sources. Chemosphere 2003, 52, 1353–1359. [Google Scholar] [CrossRef]
- Sunguti, A.E.; Kibet, J.K.; Kinyanjui, T.K. A review of the status of organic pollutants in geothermal waters. J. Nat. 2021, 4, 19–28. [Google Scholar]
- Fournier, R.O.; Rowe, J.J. Estimation of underground temperatures from the silica content of water from hot springs and wet-steam wells. Am. J. Sci. 1966, 264, 685–697. [Google Scholar] [CrossRef]
- Boudreau, A.E.; Lynne, B.Y. The growth of siliceous sinter deposits around high-temperature eruptive hot springs. J. Volcanol. Geotherm. Res. 2012, 247–248, 1–8. [Google Scholar] [CrossRef]
- Jones, B. Review of aragonite and calcite crystal morphogenesis in thermal spring systems. Sediment. Geol. 2017, 354, 9–23. [Google Scholar] [CrossRef]
- Shiraishi, F.; Morikawa, A.; Kuroshima, K.; Amekawa, S.; Yu, T.-L.; Shen, C.-C.; Kakizaki, Y.; Kano, A.; Asada, J.; Bahniuk, A.M. Genesis and diagenesis of travertine, Futamata hot spring, Japan. Sediment. Geol. 2020, 405, 105706. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, X.; Zhang, Y.; Wang, M.; Tan, M.; Hai, K.; Yu, M.; Huo, D. Hydrochemical characteristics of travertine-depositing hot springs in western of Yunnan, China. Quat. Int. 2020, 547, 63–74. [Google Scholar] [CrossRef]
- Luo, L.; Capezzuoli, E.; Rogerson, M.; Vaselli, O.; Wen, H.; Lu, Z. Precipitation of carbonate minerals in travertine-depositing hot springs: Driving forces, microenvironments, and mechanisms. Sediment. Geol. 2022, 438, 106207. [Google Scholar] [CrossRef]
- Campbell, K.A.; Guido, D.M.; Gautret, P.; Foucher, F.; Ramboz, C.; Westall, F. Geyserite in hot-spring siliceous sinter: Window on Earth’s hottest terrestrial (paleo)environment and its extreme life. Earth-Sci. Rev. 2015, 148, 44–64. [Google Scholar] [CrossRef]
- Ünal Ercan, H.; Işık Ece, Ö.; Schroeder, P.A.; Gülmez, F. Characteristics and evolution of the Etili silica sinter epithermal deposits, Çanakkale—Turkey: Relation to alkali chloride vs acid-sulfate fluids. Ore Geol. Rev. 2022, 142, 104726. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, Q.; Luo, L.; Yan, K. Tungsten Accumulation in Hot Spring Sediments Resulting from Preferred Sorption of Aqueous Polytungstates to Goethite. Int. J. Environ. Res. Public Health 2021, 18, 12629. [Google Scholar] [CrossRef]
- Leal-Acosta, M.L.; Shumilin, E.; Mirlean, N.; Sapozhnikov, D.; Gordeev, V. Arsenic and Mercury Contamination of Sediments of Geothermal Springs, Mangrove Lagoon and the Santispac Bight, Bahía Concepción, Baja California Peninsula. Bull. Environ. Contam. Toxicol. 2010, 85, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, C.-Q.; Liu, H.; Jin, Z.; Han, G.; Li, L. Geochemistry of the Rehai and Ruidian geothermal waters, Yunnan Province, China. Geothermics 2008, 37, 73–83. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Y.; Luo, L. Tungsten from typical magmatic hydrothermal systems in China and its environmental transport. Sci. Total Environ. 2019, 657, 1523–1534. [Google Scholar] [CrossRef]
- Cumbal, L.; Vallejo, P.; Rodriguez, B.; Lopez, D. Arsenic in geothermal sources at the north-central Andean region of Ecuador: Concentrations and mechanisms of mobility. Environ. Earth Sci. 2010, 61, 299–310. [Google Scholar] [CrossRef]
- Guo, Q.; Planer-Friedrich, B.; Luo, L.; Liu, M.; Wu, G.; Li, Y.; Zhao, Q. Speciation of antimony in representative sulfidic hot springs in the YST Geothermal Province (China) and its immobilization by spring sediments. Environ. Pollut. 2020, 266, 115221. [Google Scholar] [CrossRef]
- Li, C.; Kang, S.; Chen, P.; Zhang, Q.; Mi, J.; Gao, S.; Sillanpää, M. Geothermal spring causes arsenic contamination in river waters of the southern Tibetan Plateau, China. Environ. Earth Sci. 2014, 71, 4143–4148. [Google Scholar] [CrossRef]
- Xu, P.; Tan, H.; Zhang, Y.; Zhang, W. Geochemical characteristics and source mechanism of geothermal water in Tethys Himalaya belt. Geol. China 2018, 45, 13, (In Chinese with English abstract). [Google Scholar]
- Guo, Q.; Planer-Friedrich, B.; Liu, M.; Yan, K.; Wu, G. Magmatic fluid input explaining the geochemical anomaly of very high arsenic in some southern Tibetan geothermal waters. Chem. Geol. 2019, 513, 32–43. [Google Scholar] [CrossRef]
- Guo, L.; Wang, G.; Sheng, Y.; Sun, X.; Shi, Z.; Xu, Q.; Mu, W. Temperature governs the distribution of hot spring microbial community in three hydrothermal fields, Eastern Tibetan Plateau Geothermal Belt, Western China. Sci. Total Environ. 2020, 720, 137574. [Google Scholar] [CrossRef]
- Wang, X.; Yin, Y.; Yu, Z.; Shen, G.; Cheng, H.; Tao, S. Distinct distribution patterns of the abundant and rare bacteria in high plateau hot spring sediments. Sci. Total Environ. 2023, 863, 160832. [Google Scholar] [CrossRef] [PubMed]
- Mathurin, F.A.; Drake, H.; Tullborg, E.-L.; Berger, T.; Peltola, P.; Kalinowski, B.E.; Åström, M.E. High cesium concentrations in groundwater in the upper 1.2km of fractured crystalline rock—Influence of groundwater origin and secondary minerals. Geochim. Et Cosmochim. Acta 2014, 132, 187–213. [Google Scholar] [CrossRef]
- Nakaya, S.; Phan, H.M.H.; Iwai, Y.; Itoh, A.; Aoki, H.; Nakano, T. Longtime behavior of cesium (Cs) in natural spring drinking water. Sustain. Water Qual. Ecol. 2015, 6, 20–30. [Google Scholar] [CrossRef]
- CNEPA (China National Environmental Protection Administration); CNEMC (China National Envronmental Monitoring Centre). Background Values of Elements in Chinese Soil; China Environmental Science Press: Beijing, China, 1990. (In Chinese) [Google Scholar]
- Muller, G. Index of geo-accumulation in sediments of the Rhine river. GeoJournal 1969, 2, 109–118. [Google Scholar]
- Colman, D.R.; Feyhl-Buska, J.; Robinson, K.J.; Fecteau, K.M.; Xu, H.; Shock, E.L.; Boyd, E.S. Ecological differentiation in planktonic and sediment-associated chemotrophic microbial populations in Yellowstone hot springs. FEMS Microbiol. Ecol. 2016, 92, fiw137. [Google Scholar] [CrossRef]
- Pentecost, A. Travertine; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Jones, B.; Renaut, R.W. Chapter 4 Calcareous Spring Deposits in Continental Settings. In [Developments in Sedimentology] Carbonates in Continental Settings: Facies, Environments, and Processes; Alonso-Zarza, A.M., Tanner, L.H., Eds.; Elsevier: Madrid, Spain; Syracuse, NY, USA, 2010; Volume 61, pp. 177–224. [Google Scholar]
- Capezzuoli, E.; Gandin, A.; Pedley, M. Decoding tufa and travertine (fresh water carbonates) in the sedimentary record: The state of the art. Sedimentology 2014, 61, 1–21. [Google Scholar] [CrossRef]
- Kano, A.; Okumura, T.; Takashima, C.; Shiraishi, F. Geomicrobiological Properties and Processes of Travertine; Springer: Singapore, 2019. [Google Scholar]
- Landa-Arreguín, J.F.A.; Villanueva-Estrada, R.E.; Rodríguez-Díaz, A.A.; Morales-Arredondo, J.I.; Rocha-Miller, R.; Alfonso, P. Evidence of a new geothermal prospect in the Northern-Central trans-Mexican volcanic belt: Rancho Nuevo, Guanajuato, Mexico. J. Iber. Geol. 2021, 47, 713–732. [Google Scholar] [CrossRef]
- Geptner, A.; Kristmannsdóttir, H.; Kristjansson, J.; Marteinsson, V. Biogenic saponite from an active submarine hot spring, Iceland. Clays Clay Miner. 2002, 50, 174–185. [Google Scholar] [CrossRef]
- Luan, G.; Wang, W.; Liu, D.; Liu, J. The eruptive flow sediments in Jimo warm spring of Qingdao and its depositional model. Acta Geosci. Sin. 2003, 24, 357–360, (In Chinese with English abstract). [Google Scholar]
- Pisarskii, B.I.; Konev, A.A.; Levi, K.G.; Delvaux, D. Carbon dioxide-bearingalkaline hydrotherms and strontium-bearingtravertines in the songwe river valley (Tanzania). Russ. Geol. Geophys. 1998, 39, 941–948. [Google Scholar]
- Foley, N.K.; Hofstra, A.H.; Lindsey, D.A.; Seal Ii, R.R.; Jaskula, B.W.; Piatak, N.M. Occurrence Model for Volcanogenic Beryllium Deposits; 2010-5070F; US Geological Survey Scientific Investigations Report: Reston, VA, USA, 2012; p. 52. [Google Scholar]
- Stolze, L.; Battistel, M.; Rolle, M. Oxidative Dissolution of Arsenic-Bearing Sulfide Minerals in Groundwater: Impact of Hydrochemical and Hydrodynamic Conditions on Arsenic Release and Surface Evolution. Environ. Sci. Technol. 2022, 56, 5049–5061. [Google Scholar] [CrossRef]
- Qiao, W.; Guo, H.; He, C.; Shi, Q.; Xing, S.; Gao, Z. Identification of processes mobilizing organic molecules and arsenic in geothermal confined groundwater from Pliocene aquifers. Water Res. 2021, 198, 117140. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, Y.; Luo, J.; Xu, B.; Zhao, J. Geochemical processes controlling fate and transport of arsenic in acid mine drainage (AMD) and natural systems. J. Hazard. Mater. 2009, 165, 13–26. [Google Scholar] [CrossRef]
- Garcia-Rios, M.; De Windt, L.; Luquot, L.; Casiot, C. Modeling of microbial kinetics and mass transfer in bioreactors simulating the natural attenuation of arsenic and iron in acid mine drainage. J. Hazard. Mater. 2021, 405, 124133. [Google Scholar] [CrossRef]
- Park, J.H.; Han, Y.-S.; Ahn, J.S. Comparison of arsenic co-precipitation and adsorption by iron minerals and the mechanism of arsenic natural attenuation in a mine stream. Water Res. 2016, 106, 295–303. [Google Scholar] [CrossRef]
- Rango, T.; Vengosh, A.; Dwyer, G.; Bianchini, G. Mobilization of arsenic and other naturally occurring contaminants in groundwater of the Main Ethiopian Rift aquifers. Water Res. 2013, 47, 5801–5818. [Google Scholar] [CrossRef]
- Villalba, E.; Tanjal, C.; Borzi, G.; Páez, G.; Carol, E. Geogenic arsenic contamination of wet-meadows associated with a geothermal system in an arid region and its relevance for drinking water. Sci. Total Environ. 2020, 720, 137571. [Google Scholar] [CrossRef]
- Alsina, M.A.; Zanella, L.; Hoel, C.; Pizarro, G.E.; Gaillard, J.-F.; Pasten, P.A. Arsenic speciation in sinter mineralization from a hydrothermal channel of El Tatio geothermal field, Chile. J. Hydrol. 2014, 518, 434–446. [Google Scholar] [CrossRef]
- Dutta, A.; Mishra, P.; Absar, A.; Malviya, V.P.; Singh, P.K.; Srivastava, A.; Ray, B.; Kumar, A.; Nitnaware, N.V. Tracing hydrothermal mineral thenardite in geysers/hot springs of North-western Himalayan belt, Ladakh Geothermal Province, India by hydrogeochemistry, fluid-mineral equilibria and isotopic studies. Geochemistry 2023, 125973. [Google Scholar] [CrossRef]
- Awan, R.S.; Liu, C.; Yang, S.; Wu, Y.; Zang, Q.; Khan, A.; Li, G. The occurrence of vanadium in nature: Its biogeochemical cycling and relationship with organic matter—A case study of the Early Cambrian black rocks of the Niutitang Formation, western Hunan, China. Acta Geochim. 2021, 40, 973–997. [Google Scholar] [CrossRef]
- Zhu, H.; Xiao, X.; Guo, Z.; Han, X.; Liang, Y.; Zhang, Y.; Zhou, C. Adsorption of vanadium (V) on natural kaolinite and montmorillonite: Characteristics and mechanism. Appl. Clay Sci. 2018, 161, 310–316. [Google Scholar] [CrossRef]
- Taghipour, M.; Jalali, M. Effect of clay minerals and nanoparticles on chromium fractionation in soil contaminated with leather factory waste. J. Hazard. Mater. 2015, 297, 127–133. [Google Scholar] [CrossRef]
- Joe-Wong, C.; Brown, G.E., Jr.; Maher, K. Kinetics and Products of Chromium(VI) Reduction by Iron(II/III)-Bearing Clay Minerals. Environ. Sci. Technol. 2017, 51, 9817–9825. [Google Scholar] [CrossRef]







| Sample No. | pH | Temperature (°C) |
|---|---|---|
| 1 | 8.0 | 78.0 |
| 2 | 7.8 | 46.0 |
| 3 | 6.7 | 40.0 |
| 4 | 6.7 | 44.0 |
| 5 | 7.6 | 40.0 |
| 6 | 7.1 | 39.4 |
| 7 | 7.4 | 37.0 |
| 8 | 7.2 | 40.6 |
| 9 | 7.0 | 35.6 |
| 10 | 7.4 | 58.2 |
| 11 | 8.2 | 63.8 |
| 12 | 7.0 | 53.0 |
| Sample No. | CaO | SiO2 | Al2O3 | Fe2O3 | K2O | Na2O | MgO | SrO | TiO2 | BaO | SO3 | P2O5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sediment | 1 | 61.0 | 24.0 | 2.2 | 4.1 | 0.62 | 0.88 | 2.56 | 2.58 | 0.28 | 1.25 | 0.50 | 0.05 |
| 2 | 10.3 | 66.3 | 6.3 | 6.2 | 3.30 | 0.79 | 0.59 | 0.20 | 0.85 | 0.60 | 4.51 | 0.14 | |
| 3 | 8.7 | 65.3 | 7.0 | 8.0 | 3.42 | 0.77 | 0.75 | 0.16 | 1.06 | 0.51 | 4.23 | 0.09 | |
| 4 | 0.4 | 78.0 | 10.3 | 5.4 | 3.54 | 0.18 | 0.51 | 0.01 | 0.77 | 0.12 | 0.68 | 0.11 | |
| 5 | 2.5 | 75.1 | 8.1 | 5.7 | 3.77 | 0.84 | 0.64 | 0.07 | 0.91 | 0.16 | 2.03 | 0.16 | |
| 6 | 1.8 | 76.6 | 7.9 | 5.1 | 3.36 | 0.68 | 0.48 | 0.07 | 1.19 | 0.25 | 2.49 | 0.14 | |
| 7 | 2.2 | 74.9 | 6.7 | 7.1 | 2.48 | 0.42 | 0.39 | 0.06 | 0.85 | 1.17 | 3.71 | 0.07 | |
| 8 | 1.3 | 60.0 | 6.1 | 14.0 | 2.39 | 0.49 | 0.00 | 0.06 | 0.82 | 2.88 | 11.75 | 0.11 | |
| 9 | 0.2 | 83.7 | 8.8 | 1.4 | 3.47 | 0.18 | 0.34 | 0.01 | 0.82 | 0.16 | 0.95 | 0.04 | |
| 10 | 10.2 | 65.6 | 9.1 | 6.8 | 3.20 | 1.11 | 1.00 | 0.17 | 1.05 | 1.05 | 0.53 | 0.14 | |
| 11 | 52.5 | 26.9 | 4.3 | 8.2 | 1.42 | 0.87 | 0.68 | 2.05 | 0.66 | 2.06 | 0.38 | 0.09 | |
| 12 | 33.8 | 40.2 | 8.9 | 8.4 | 2.62 | 0.95 | 1.08 | 0.93 | 1.04 | 1.36 | 0.52 | 0.19 | |
| Sinter | 1 | 91.4 | 0.9 | 0.5 | 0.9 | 0.04 | 0.37 | 1.69 | 3.44 | bdl | 0.68 | 0.10 | 0.03 |
| 2 | 92.2 | 0.7 | 0.1 | 0.9 | 0.04 | 0.62 | 0.32 | 4.10 | bdl | 0.81 | 0.08 | 0.04 | |
| 3 | 92.8 | 0.5 | 0.1 | 0.3 | bdl | 0.18 | 0.34 | 5.05 | bdl | 0.64 | 0.05 | 0.03 |
| CaO | SiO2 | Al2O3 | Fe2O3 | K2O | Na2O | MgO | SrO | TiO2 | BaO | SO3 | P2O5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | 1.00 | |||||||||||
| SiO2 | −0.96 | 1.00 | ||||||||||
| Al2O3 | −0.71 | 0.72 ** | 1.00 | |||||||||
| Fe2O3 | −0.01 | −0.26 | −0.14 | 1.00 | ||||||||
| K2O | −0.86 | 0.87 ** | 0.85 ** | −0.18 | 1.00 | |||||||
| Na2O | 0.52 * | −0.57 | −0.31 | 0.20 | −0.27 | 1.00 | ||||||
| MgO | 0.77 ** | −0.67 | −0.53 | −0.31 | −0.62 | 0.51 * | 1.00 | |||||
| SrO | 0.99 ** | −0.93 | −0.77 | −0.06 | −0.90 | 0.43 | 0.77 ** | 1.00 | ||||
| TiO2 | −0.64 | 0.57 * | 0.70 ** | 0.16 | 0.76 ** | 0.10 | −0.58 | −0.73 | 1.00 | |||
| BaO | 0.40 | −0.60 | −0.50 | 0.79 ** | −0.65 | 0.22 | −0.01 | 0.39 | −0.29 | 1.00 | ||
| SO3 | −0.40 | 0.18 | −0.15 | 0.73 ** | 0.06 | −0.21 | −0.49 | −0.37 | 0.12 | 0.52 * | 1.00 | |
| P2O5 | −0.15 | 0.05 | 0.45 | 0.32 | 0.40 | 0.49 | −0.15 | −0.27 | 0.60 * | −0.05 | 0 | 1.00 |
| Sample No. | Mineral | |||||
|---|---|---|---|---|---|---|
| Sediment | 1 | Calcite, CaCO3 | Aragonite, CaCO3 | Quartz, SiO2 | Albite, NaAlSi3O8 | / |
| 2 | Calcite, CaCO3 | / | Quartz, SiO2 | Albite, NaAlSi3O8 | / | |
| 3 | Calcite, CaCO3 | / | Quartz, SiO2 | Albite, NaAlSi3O8 | Muscovite, KAl2Si3AlO10(OH)2 | |
| 4 | / | / | Quartz, SiO2 | / | Muscovite, KAl2Si3AlO10(OH,F)2 | |
| 5 | / | / | Quartz, SiO2 | Albite, NaAlSi3O8 | Muscovite, (K0.82Na0.18)(Fe0.03Al1.97)(AlSi3)O10(OH)2 | |
| 6 | / | / | Quartz, SiO2 | Albite, NaAlSi3O8 | Muscovite, KAl2Si3AlO10(OH,F)2 | |
| 7 | Calcite, CaCO3 | / | Quartz, SiO2 | Albite, NaAlSi3O8 | Muscovite, KAl2Si3AlO10(OH,F)2 | |
| 8 | / | / | Quartz, SiO2 | Albite, NaAlSi3O8 | Orthoclase, Ba-rich, (K,Ba)(Si,Al)4O8 | |
| 9 | / | / | Quartz, SiO2 | / | Muscovite, KAl2Si3AlO10(OH)2 | |
| 10 | Calcite, CaCO3 | / | Quartz, SiO2 | Albite, NaAlSi3O8 | Muscovite, KAl2Si3AlO10(OH)2 | |
| 11 | Calcite, CaCO3 | Aragonite, CaCO3 | Quartz, SiO2 | / | Muscovite, KAl2Si3AlO10(OH,F)2 | |
| 12 | Calcite, CaCO3 | Aragonite, CaCO3 | Quartz, SiO2 | Albite, NaAlSi3O8 | Muscovite, (K,Na)Al2(Si,Al)4O10(OH)2 | |
| Sinter | 1 | Calcite, CaCO3 | Aragonite, CaCO3 | / | / | / |
| 2 | Calcite, CaCO3 | Aragonite, CaCO3 | / | / | / | |
| 3 | Calcite, CaCO3 | Aragonite, CaCO3 | / | / | / | |
| Oxide | CaO | SiO2 | Al2O3 | Fe2O3 | K2O | Na2O | MgO | SrO | TiO2 | BaO | SO3 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Be | 0.85 | −0.77 | −0.78 | −0.19 | −0.83 | 0.36 | 0.91 | 0.89 | −0.77 | 0.21 | −0.29 | −0.34 |
| V | −0.57 | 0.65 | 0.77 | −0.39 | 0.59 | −0.75 | −0.48 | −0.55 | 0.24 | −0.49 | −0.20 | −0.08 |
| Cr | −0.69 | 0.60 | 0.54 | 0.20 | 0.55 | −0.59 | −0.76 | −0.70 | 0.45 | −0.05 | 0.50 | 0.25 |
| Mn | −0.16 | 0.19 | 0.41 | −0.07 | 0.21 | −0.42 | −0.05 | −0.14 | −0.12 | −0.27 | −0.20 | 0.06 |
| Co | −0.47 | 0.30 | 0.27 | 0.52 | 0.25 | −0.51 | −0.57 | −0.44 | 0.06 | 0.32 | 0.70 | 0.04 |
| Ni | −0.50 | 0.39 | 0.43 | 0.33 | 0.34 | −0.63 | −0.51 | −0.46 | 0.02 | 0.10 | 0.47 | 0.01 |
| Cu | −0.54 | 0.44 | 0.35 | 0.25 | 0.25 | −0.80 | −0.62 | −0.48 | −0.01 | 0.18 | 0.54 | −0.22 |
| Zn | 0.27 | −0.32 | 0.12 | 0.18 | −0.11 | 0.12 | 0.28 | 0.23 | −0.20 | 0.04 | −0.21 | 0.36 |
| As | −0.35 | 0.12 | −0.08 | 0.75 | −0.06 | −0.35 | −0.49 | −0.30 | 0.01 | 0.65 | 0.92 | −0.10 |
| Se | 0.19 | −0.36 | −0.40 | 0.66 | −0.38 | 0.05 | −0.36 | 0.22 | −0.18 | 0.83 | 0.53 | 0.01 |
| Cd | 0.07 | −0.19 | 0.42 | 0.38 | 0.14 | 0.15 | −0.24 | −0.05 | 0.35 | 0.28 | −0.03 | 0.57 |
| Cs | 0.79 | −0.78 | −0.47 | 0.08 | −0.47 | 0.76 | 0.55 | 0.72 | −0.15 | 0.20 | −0.38 | 0.28 |
| Tl | −0.31 | 0.10 | −0.13 | 0.70 | −0.07 | −0.31 | −0.45 | −0.26 | 0 | 0.63 | 0.93 | −0.11 |
| Pb | 0.36 | −0.43 | 0.18 | 0.26 | −0.11 | 0.44 | 0.19 | 0.22 | 0.26 | 0.22 | −0.24 | 0.61 |
| U | −0.33 | 0.09 | 0.31 | 0.78 | 0.18 | 0.03 | −0.54 | −0.38 | 0.49 | 0.57 | 0.63 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cheng, H. Influence of Mineral Deposition on the Retention of Potentially Hazardous Elements in Geothermal Spring Sediments. Sustainability 2023, 15, 8040. https://doi.org/10.3390/su15108040
Wang Y, Cheng H. Influence of Mineral Deposition on the Retention of Potentially Hazardous Elements in Geothermal Spring Sediments. Sustainability. 2023; 15(10):8040. https://doi.org/10.3390/su15108040
Chicago/Turabian StyleWang, Yafeng, and Hefa Cheng. 2023. "Influence of Mineral Deposition on the Retention of Potentially Hazardous Elements in Geothermal Spring Sediments" Sustainability 15, no. 10: 8040. https://doi.org/10.3390/su15108040
APA StyleWang, Y., & Cheng, H. (2023). Influence of Mineral Deposition on the Retention of Potentially Hazardous Elements in Geothermal Spring Sediments. Sustainability, 15(10), 8040. https://doi.org/10.3390/su15108040







