The Effect of Grafting on Morphological, Physiological and Molecular Changes Induced by Drought Stress in Cucumber
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.1.1. Trial Materials and Locations
2.1.2. Experiment Design
2.2. Measurement Items and Methods
2.2.1. Plant Growth Measurements
2.2.2. Chlorophyll Meter Measurements
2.2.3. Photosynthetic Activity
2.2.4. Relative Water Content (RWC)
2.2.5. Malondialdehyde (MDA) Concentration
2.2.6. Molecular Analysis
2.3. Statistical Analysis
3. Results
3.1. Plant Growth Measurements
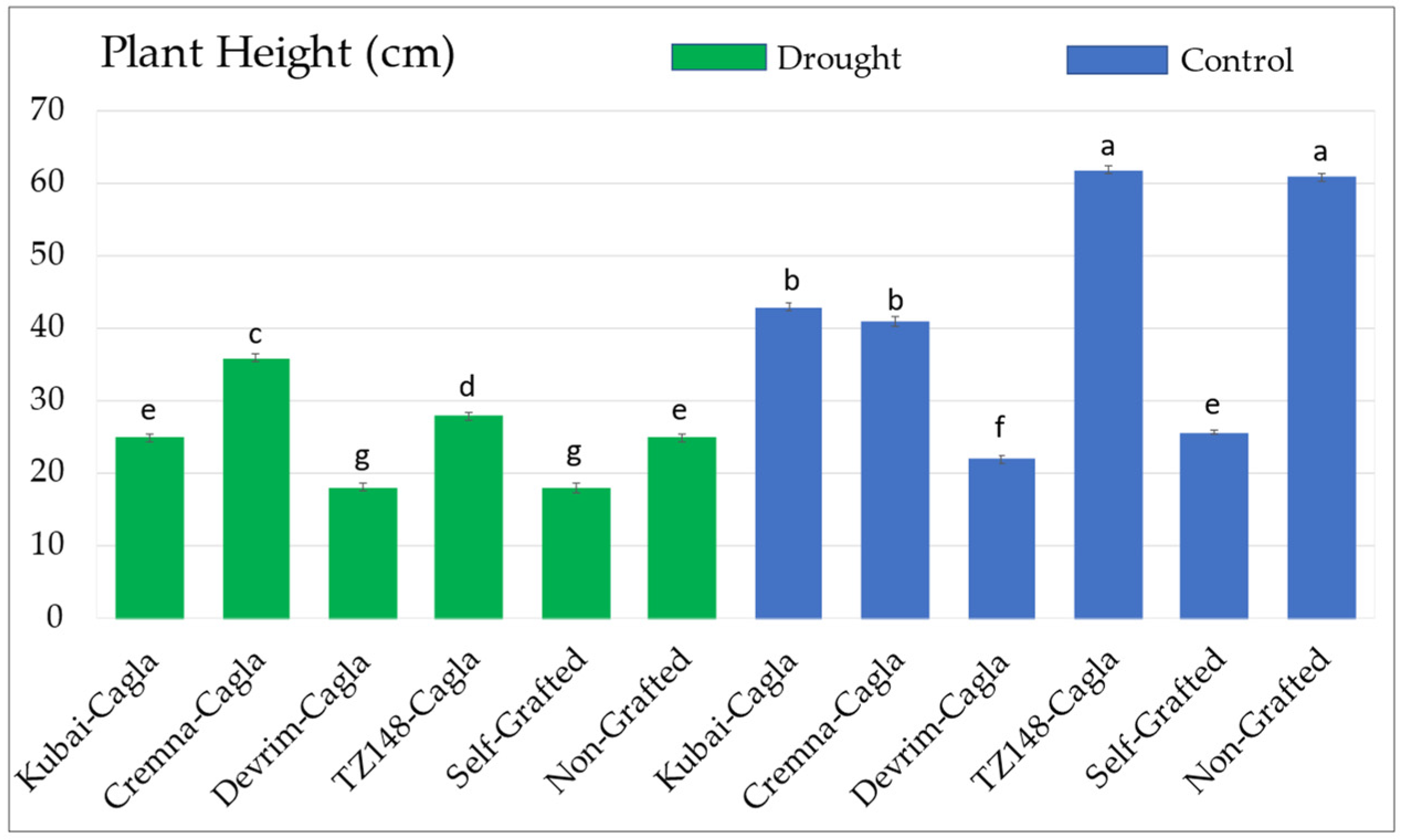
3.1.1. Plant Height Values
3.1.2. Stem Diameter Values
3.1.3. Leaf Number Values
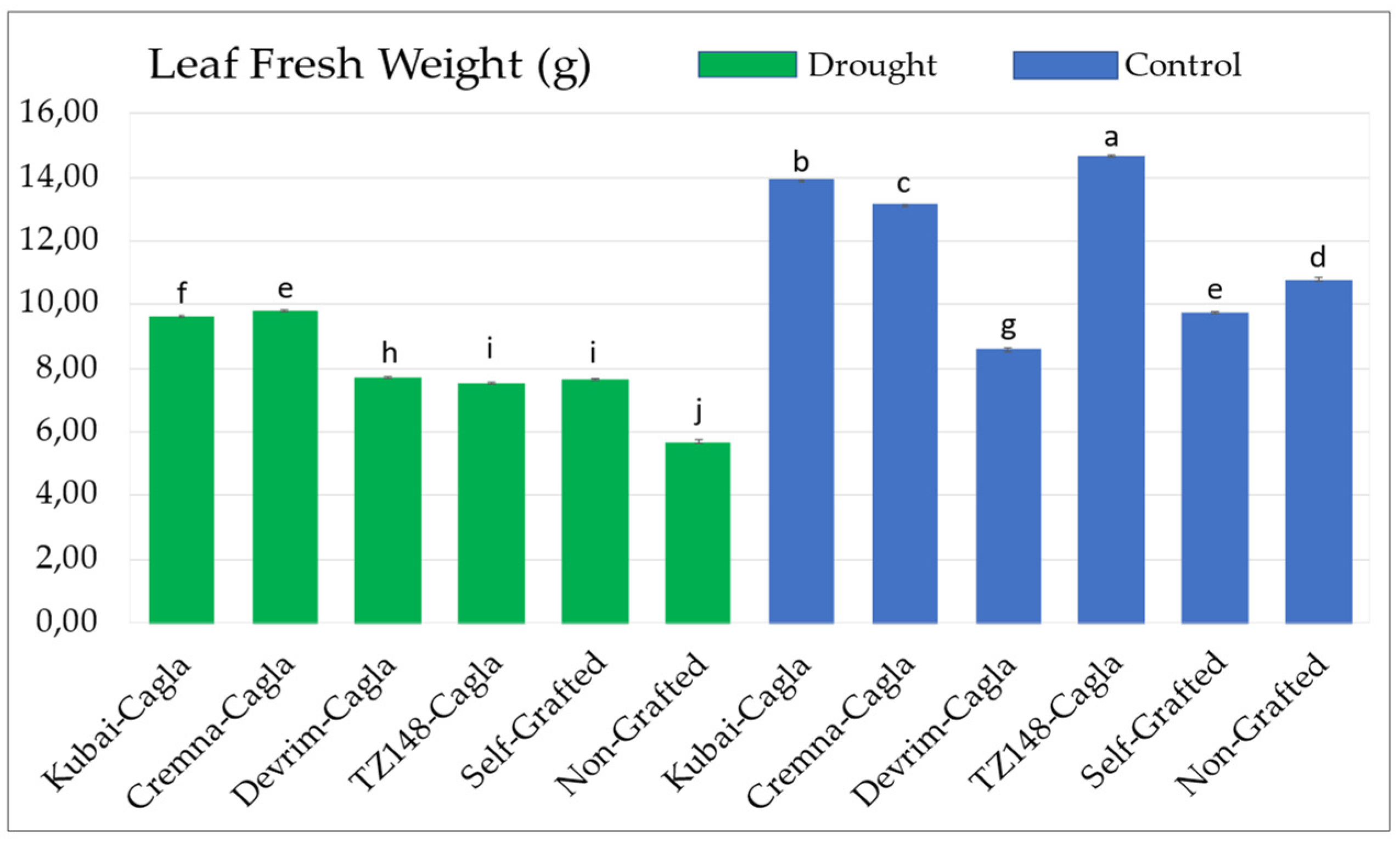
3.1.4. Leaf Fresh and Dry Weight Values
3.1.5. Stem Fresh and Dry Weight Values
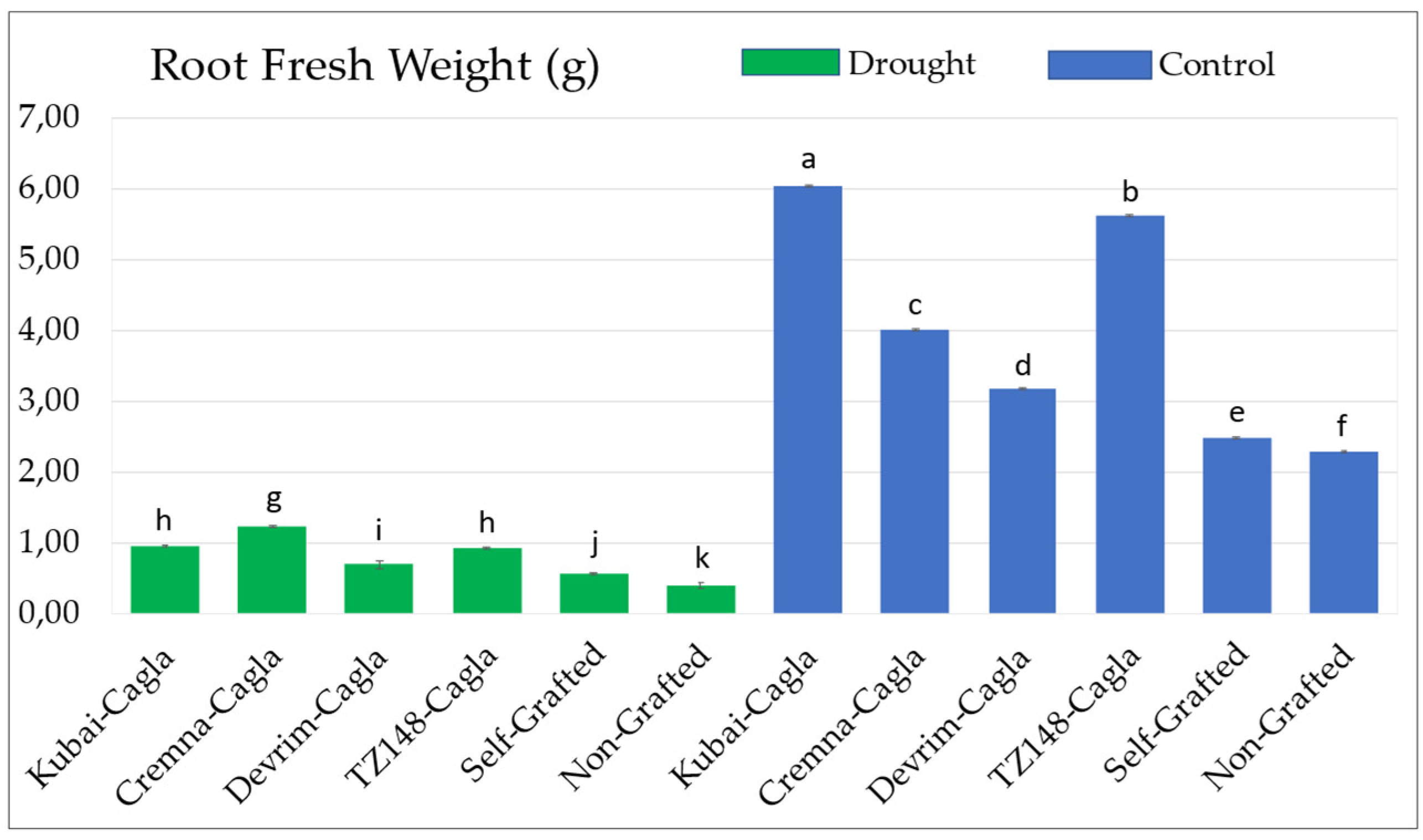
3.1.6. Root Fresh and Dry Weight Values
3.1.7. Leaf Area Values
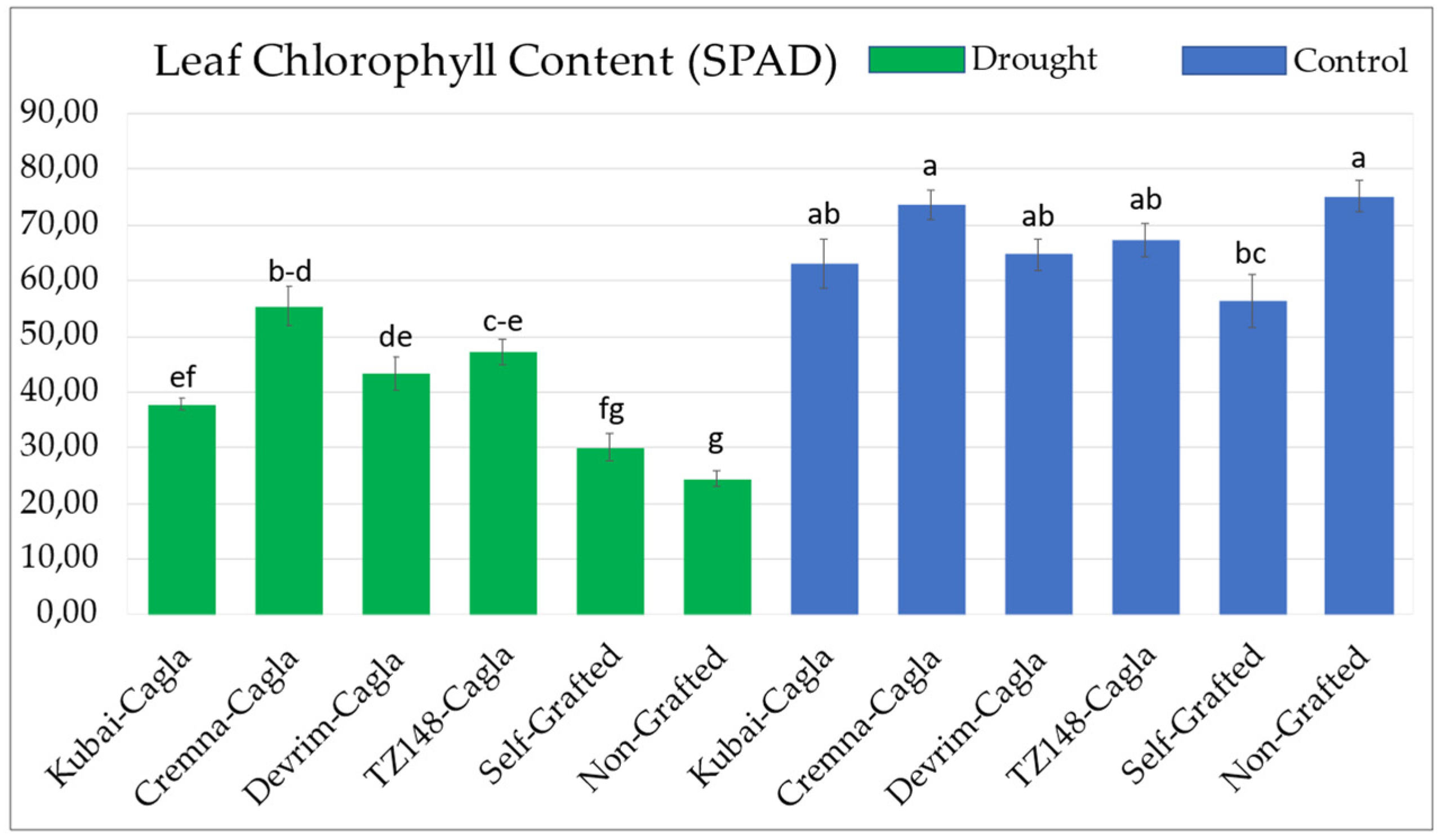
3.2. Chlorophyll Meter Measurements and Photosynthetic Activity
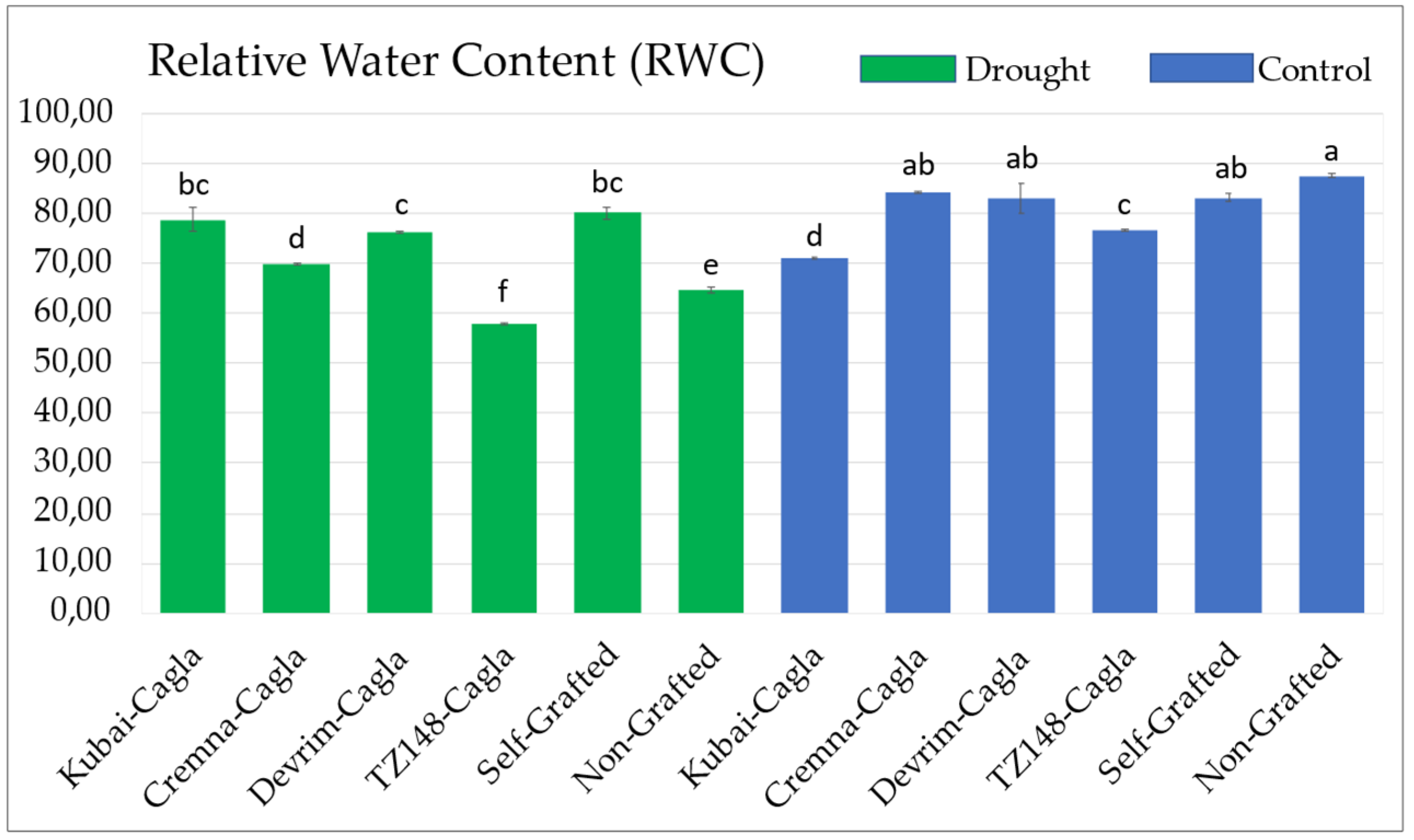
3.3. Relative Water Content (RWC)
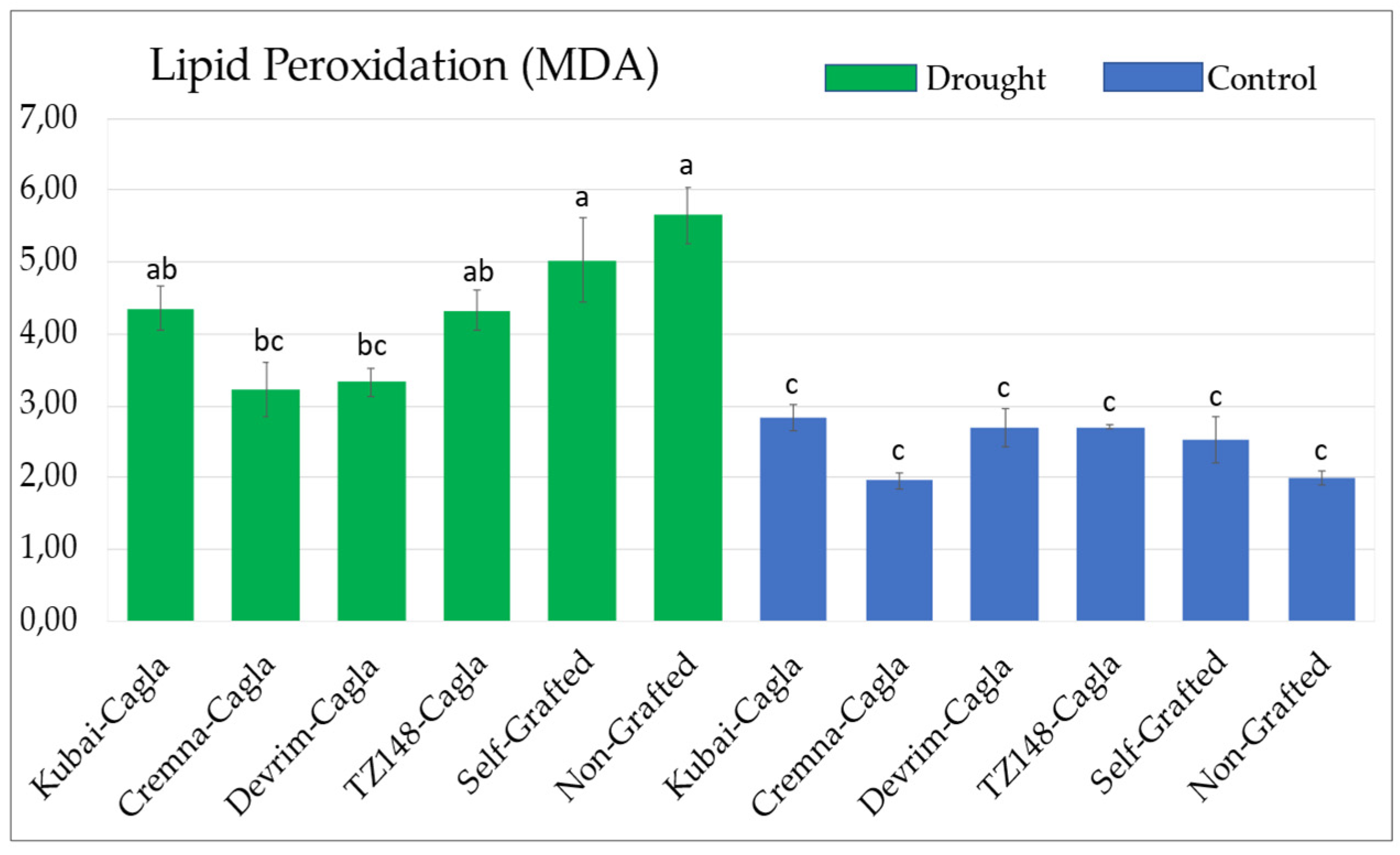
3.4. Malondialdehyde (MDA) Concentration
3.5. Correlation Analyses
3.6. Molecular Analyses
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef] [PubMed]
- Bacon, M.A. Water Use Efficiency in Plant Biology. In Water Use Efficiency in Plant Biology; Bacon, M.A., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2004; pp. 1–26. [Google Scholar]
- Rouphael, Y.; Cardarelli, M.; Schwarz, D.; Franken, P.; Colla, G. Effects of Drought on Nutrient Uptake and Assimilation in Vegetable Crops, in Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 171–195. [Google Scholar]
- Bukhari, S.A.H.; Peerzada, A.M.; Javed, M.H.; Dawood, M.; Hussain, N.; Ahmad, S. Growth and Development Dynamics in Agronomic Crops under Environmental Stress. In Agronomic Crops; Springer: Berlin/Heidelberg, Germany, 2019; pp. 83–114. [Google Scholar]
- Diatta, A.A.; Fike, J.; Battaglia, M.; Galbraith, J.; Baig, M.B. Effects of biochar on soil fertility and crop productivity in arid regions: A review. Arab. J. Geosci. 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Gholami, R.; Zahedi, S.M. Identifying superior drought-tolerant olive genotypes and their biochemical and some physiological responses to various irrigation levels. J. Plant Nutr. 2019, 42, 2057–2069. [Google Scholar] [CrossRef]
- Ghahremani, Z.; Mikaealzadeh, M.; Barzegar, T.; Ranjbar, M.E. Foliar application of ascorbic acid and gamma aminobutyric acid can improve important properties of deficit irrigated cucumber plants (Cucumis sativus cv. Us). Gesunde Pflanz. 2021, 73, 77–84. [Google Scholar] [CrossRef]
- Ghosh, D.; Lin, Q.; Xu, J.; Hellmann, H.A. How plants deal with stress: Exploration through proteome investigation. Front. Plant Sci. 2017, 8, 1176. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Singh, A.K.; Ansari, M.I. Effect of Drought Stress on Crop Production. In New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, H., Singh, A., Singh, U., Fraceto, L., Eds.; Springer: Singapore, 2020. [Google Scholar]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Lee, J.G. Effect of drought stress on chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities in lettuce seedlings. Horticulturae 2021, 7, 238. [Google Scholar] [CrossRef]
- Nemeskéri, E.; Helyes, L. Physiological responses of selected vegetable crop species to water stress. Agronomy 2019, 9, 447. [Google Scholar] [CrossRef]
- Plazas, M.; Nguyen, H.T.; González-Orenga, S.; Fita, A.; Vicente, O.; Prohens, J.; Boscaiu, M. Comparative analysis of the responses to water stress in eggplant (Solanum melongena) cultivar. Plant Physiol. Biochem. 2019, 143, 72–82. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant. 2021, 172, 1291–1300. [Google Scholar] [CrossRef]
- Lahive, F.; Hadley, P.; Daymond, A.J. The impact of elevated CO2 and water deficit stress on growth and photosynthesis of juvenile cacao (Theobroma cacao L.). Photosynthetica 2018, 56, 911. [Google Scholar] [CrossRef]
- Kiran, S.; Baysal Furtana, G.; Ellialtioglu, S.S. Physiological and biochemical assay of drought stress responses in eggplant (Solanum melongena L.) inoculated with commercial inoculant of Azotobacter chroococum and Azotobacter vinelandii. Sci. Hortic. 2022, 305, 111394. [Google Scholar] [CrossRef]
- Al-Harbi, A.R.; Al-Omran, A.M.; Alharbi, K. Grafting improves cucumber water stress tolerance in Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, B.; Gao, S.; Xu, K. Grafting improves tomato drought tolerance through enhancing photosynthetic capacity and reducing ROS accumulation. Protoplasma 2019, 256, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Tymon, L.; Keinath, A.; Miles, C. Progress in grafting watermelon to manage Verticillium wilt. Plant Pathol. 2021, 70, 767–777. [Google Scholar] [CrossRef]
- Kiran, S.; Kusvuran, S.; Cagla, A.; Ellialtioglu, S.S. Some physiological properties and analysis of yield parameters of grafted and non-grafted eggplants under waterless conditions. Soil Water J. 2017, 6, 18–25. [Google Scholar]
- Shrestha, S.; Mattupalli, C.; Miles, C. Effect of grafting compatibility on fruit yield and quality of cantaloupe in a mediterranean-type climate. Horticulturae 2022, 8, 888. [Google Scholar] [CrossRef]
- Bie, Z.L.; Nawaz, M.A.; Huang, Y.; Lee, J.M.; Colla, G. Introduction to Vegetable Grafting. In Vegetable Grafting: Principles and Practices; Colla, G., Perez Alfocea, F., Schwarz, D., Eds.; CABI: Wallingford, UK, 2017; pp. 1–21. [Google Scholar]
- Karaman, K.; Dalda-Sekerci, A.; Yetisir, H.; Gulsen, O.; Coskun, O.F. Molecular, morphological and biochemical characterization of some Turkish bitter melon (Momordica charantia L.) genotypes. Ind. Crops Prod. 2018, 123, 93–99. [Google Scholar] [CrossRef]
- Kirac, H.; Dalda-Sekerci, A.; Coskun, O.F.; Gulsen, O. Morphological and molecular characterization of garlic (Allium sativum L.) genotypes sampled from Turkey. Gen. Res. Crop Evol. 2022, 1–9. [Google Scholar]
- Bajpai, R.; Shukla, V.; Singh, N.; Rana, T.S.; Upreti, D.K. Physiological and genetic effects of chromium (+VI) on toxitolerant lichen species, Pyxine cocoes. Environ. Sci. Pollut. Res. 2015, 22, 3727–3738. [Google Scholar] [CrossRef]
- Tawfik, R.S.; El-Mouhamady, A.B.A. Molecular genetic studies on abiotic stress resistance in sorghum entries through using half diallel analysis and inter-simple sequence repeat (ISSR) markers. Bull. Natl. Res. Cent. 2019, 43, 117. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Lv, X.; Ahammed, G.J.; Xia, X.; Zhou, J.; Shi, K.; Asami, T.; Yu, J.; Zhou, Y. Grafting cucumber onto luffa improves drought tolerance by increasing ABA biosynthesis and sensitivity. Sci. Rep. 2016, 6, 20212. [Google Scholar] [CrossRef] [PubMed]
- Elsheery, N.I.; Helaly, M.N.; Omar, S.A.; John, S.V.S.; Zabochnicka-Swiątek, M.; Kalaji, H.M.; Rastogi, A. Physiological and molecular mechanisms of salinity tolerance in grafted cucumber. S. Afr. J. Bot. 2020, 130, 90–102. [Google Scholar] [CrossRef]
- Shehata, S.A.; Omar, H.S.; Elfaidy, A.G.S.; El-Sayed, S.S.F.; Abuarab, M.E.; Abdeldaym, E.A. Grafting enhances drought tolerance by regulating stress-responsive gene expression and antioxidant enzyme activities in cucumbers. BMC Plant Biol. 2022, 2022, 408. [Google Scholar]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Hoyos Echevarria, P.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Mashilo, J.; Shimelis, H.; Odindo, A. Drought tolerance of selected bottle gourd [Lagenaria siceraria (Molina) Standl.] landraces assessed by leaf gas exchange and photosynthetic efficiency. Plant Physiol. Biochem. 2017, 120, 75–87. [Google Scholar] [CrossRef]
- Mashilo, J.; Odindo, A.; Shimelis, H.; Musenge, P.; Tesfay, S.Z.; Magwaza, L.S. Photosynthetic response of bottle gourd [Lagenaria siceraria (Molina) Standl.] to drought stress; relationship between cucurbitacins accumulation and drought tolerance. Sci. Hortic. 2018, 231, 133–143. [Google Scholar] [CrossRef]
- Smart, R.E.; Bingham, G.E. Rapid estimates of relative water content. Plant Physiol. 1974, 53, 258–260. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 25, 189–198. [Google Scholar] [CrossRef]
- Stewart, C.N., Jr.; Via, L.E. A rapid CTAB DNA isolation technique useful for RAPD fingerprinting and other PCR applications. BioTechniques 1993, 14, 748–758. [Google Scholar]
- Dice, L.R. Measures of the amount of ecologic association between species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Rohlf, J.F. NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System; Exeter Software: New York, NY, USA, 2000. [Google Scholar]
- Wyrwicka, A.; Urbaniak, M.; Przybylski, M. The response of cucumber plants (Cucumis sativus L.) to the application of PCB-contaminated sewage sludge and urban sediment. Peer J. 2019, 7, e6743. [Google Scholar] [CrossRef] [PubMed]
- El-Mogy, M.M.; Atia, M.A.M.; Dhawi, F.; Fouad, A.S.; Bendary, E.S.A.; Khojah, E.; Samra, B.N.; Abdelgawad, K.F.; Ibrahim, M.F.M.; Abdeldaym, E.A. Towards better grafting: SCoT and CDDP analyses for prediction of the tomato rootstocks performance under drought Stress. Agronomy 2022, 12, 153. [Google Scholar] [CrossRef]
- Rekaby, S.A.; Awad, M.; Majrashi, A.; Ali, E.F.; Eissa, M.A. Corn cob-derived biochar improves the growth of saline-irrigated quinoa in different orders of Egyptian soils. Horticulturae 2021, 7, 221. [Google Scholar] [CrossRef]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.M.; Testerink, C. Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Su, X.; Xia, Z. Grafting improves drought tolerance by regulating antioxidant enzyme activities and stress-responsive gene expression in tobacco. Environ. Exp. Bot. 2014, 107, 173–179. [Google Scholar] [CrossRef]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; García-Caparrós, P.; Ali, O.M.; Latef, A.A.H.A. Influence of glycine betaine (natural and synthetic) on growth, metabolism and yield production of drought stressed maize (Zea mays L.) plants. Plants 2021, 10, 2540. [Google Scholar] [CrossRef]
- Alam, A.; Ullaha, H.; Thuenproma, N.; Tisarumc, R.; Cha-umc, S.; Datta, A. Seed priming with salicylic acid enhances growth, physiological traits, fruit yield, and quality parameters of cantaloupe under water-deficit stress. S. Afr. J. Bot. 2022, 150, 1–12. [Google Scholar] [CrossRef]
- Eid, M.A.M.; El-hady, M.A.A.; Abdelkader, M.A.; Abd-Elkrem, Y.M.; El-Gabry, Y.A.; El-temsah, M.E.; El-Areed, S.R.M.; Rady, M.M.; Alamer, K.H.; Alqubaie, A.I. Response in physiological traits and antioxidant capacity of two cotton cultivars under water limitations. Agronomy 2022, 12, 803. [Google Scholar] [CrossRef]
- Khah, E.M.; Kakava, E.; Mavromatis, A.; Chachalis, D.; Goulas, C. Effect of grafting on growth and yield of tomato (Lycopersicon esculentum Mill.) in greenhouse and open-field. J. Appl. Hortic. 2006, 8, 3–7. [Google Scholar] [CrossRef]
- Sánchez-Rodriguez, E.; del Mar Rubio-Wilhelmi, M.; Blasco, B.; Leyva, R.; Romero, L.; Ruiz, J.M. Antioxidant response resides in the shoot in reciprocal grafts of drought-tolerant and drought-sensitive cultivars in tomato under water stress. Plant Sci. 2012, 188, 89–96. [Google Scholar] [CrossRef]
- Metwaly, E.-S.E.; Al-Yasi, H.M.; Ali, E.F.; Farouk, H.A.; Farouk, S. Deteriorating harmful effects of drought in cucumber by spraying glycinebetaine. Agriculture 2022, 12, 2166. [Google Scholar] [CrossRef]
- Aslani, Z.; Hassani, A.; Mandoulakani, B.A.; Barin, M.; Ramin Maleki, R. Effect of drought stress and inoculation treatments on nutrient uptake, essential oil and expression of genes related to monoterpenes in sage (Salvia officinalis). Sci. Hortic. 2023, 309, 111610. [Google Scholar] [CrossRef]
- Farouk, S.; Omar, M.M. Sweet basil growth, physiological and ultrastructural modification, and oxidative defense system under water deficit and silicon forms treatment. J. Plant Growth Regul. 2020, 39, 1307–1331. [Google Scholar] [CrossRef]
- Poor, R.E. Investigating the effect of grafted watermelon on tolerance to drought and salinity. J. Novel Appl. Sci. 2015, 4, 670–673. [Google Scholar]
- Zhang, Z.; Liu, Y.; Cao, B.; Chen, Z.; Xu, K. The effectiveness of grafting to improve drought tolerance in tomato. Plant Growth Regul. 2020, 91, 157–167. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, L.; Gheith, E.M.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morphophysiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef]
- Asayesh, Z.M.; Arzani, K.; Mokhtassi-Bidgoli, A.; Abdollahi, H. Enzymatic and non-enzymatic response of grafted and ungrafted young European pear (Pyrus communis L.) trees to drought stress. Sci. Hortic. 2023, 310, 111745. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Hoveizeh, N.F.; Kadkhodaei, S.; Vaculík, M. Comparative morphological, physiological and molecular analyses of drought-stressed strawberry plants affected by SiO2 and SiO2-NPs foliar spray. Sci. Hortic. 2023, 309, 111686. [Google Scholar] [CrossRef]
- Dugasa, M.T.; Cao, F.; Ibrahim, W.; Wu, F. Differences in physiological and biochemical characteristics in response to single and combined drought and salinity stresses between wheat genotypes differing in salt tolerance. Physiol. Plant. 2019, 165, 134–143. [Google Scholar] [CrossRef]
- Karimi, A.; Tabari, M.; Javanmard, Z.; Bader, M.K.F. Drought effects on morpho-physiological and biochemical traits in persian oak and black poplar seedlings. Forests 2022, 13, 399. [Google Scholar] [CrossRef]
- Penella, C.; Nebauer, S.G.; López-Galarza, S.; San Bautista, A.; Rodríguez-Burruezo, A.; Calatayud, A. Evaluation of some pepper genotypes as rootstocks in water stress conditions. Hort. Sci. 2014, 41, 192–200. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Langaroudi, I.K.; Piri, S.; Chaeikar, S.S.; Salehi, B. Evaluating drought stress tolerance in different Camellia sinensis L. cultivars and effect of melatonin on strengthening antioxidant system. Sci Hortic. 2023, 307, 111517. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; He, X.; Li, Y.; Li, P.; Zhang, Y.; Chen, W. Growth, secondary metabolites and enzyme activity responses of two edible fern species to drought stress and rehydration in Northeast China. Agronomy 2019, 9, 137. [Google Scholar] [CrossRef]
- Li, G.; Tan, M.; Liu, X.; Mao, J.; Song, C.; Li, K.; Ma, J.; Xing, L.; Zhang, D.; Shao, J. The nutrient, hormone, and antioxidant status of scion affects the rootstock activity in apple. Sci. Hortic. 2022, 302, 111157. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef]
- Savva, D. DNA fingerprinting as a biomarker assay in ecotoxicology. Toxicol. Ecotoxicol. News 1996, 3, 110–114. [Google Scholar]
- Atienzar, F.A.; Conradi, M.; Evenden, A.J.; Jha, A.N.; Depledge, M.H. Qualitative assessment of genotoxicity using random amplified polymorphic DNA: Comparison of genomic template stability with key fitness parameters in Daphnia magna exposed to benzo[a]pyrene. Environ. Toxicol. Chem. 1999, 18, 2275–2282. [Google Scholar] [CrossRef]
- Abdelmigid, H.M. Qualitative assessment of cadmium stress using genome template stability in Hordeum vulgare. Egypt J. Genet. Cytol. 2010, 39, 291–303. [Google Scholar] [CrossRef]









| SPAD | PAR | PH | SD | NL | LFW | SFW | RFW | LDW | SDW | RDW | LA | RWC | MDA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPAD | 1 | |||||||||||||
| PAR | - | 1 | ||||||||||||
| PH | 0.63 | - | 1 | |||||||||||
| SD | - | - | - | 1 | ||||||||||
| NL | 0.68 | - | 0.46 | - | 1 | |||||||||
| LFW | 0.72 | 0.34 | 0.85 | - | 0.69 | 1 | ||||||||
| SFW | 0.69 | - | 0.79 | - | 0.57 | 0.84 | 1 | |||||||
| RFW | 0.76 | - | 0.65 | - | 0.60 | 0.76 | 0.94 | 1 | ||||||
| LDW | 0.53 | 0.43 | 0.83 | - | 0.62 | 0.88 | 0.81 | 0.69 | 1 | |||||
| SDW | 0.56 | - | 0.78 | - | 0.44 | 0.67 | 0.87 | 0.75 | 0.72 | 1 | ||||
| RDW | 0.59 | - | 0.60 | - | 0.58 | 0.77 | 0.87 | 0.91 | 0.76 | 0.64 | 1 | |||
| LA | 0.41 | - | - | - | - | 0.38 | 0.38 | 0.43 | - | - | - | 1 | ||
| RWC | 0.43 | - | - | 0.60 | 0.47 | 0.33 | - | 0.38 | - | - | - | 0.41 | 1 | |
| MDA | −0.82 | −0.37 | −0.48 | - | −0.63 | −0.66 | −0.60 | −0.68 | −0.46 | −0.40 | −0.50 | −0.52 | −0.54 | 1 |
| Primer Name | Primer Sequence 3′-5′ | Number of Bands | % Rate of Polymorphism | |
|---|---|---|---|---|
| Polymorphic | Total | |||
| ISSR-1 | AGA CAC ACA CAC ACA CAT | 2 | 4 | 50 |
| ISSR-11 | ACA CAC ACA CAC ACA CGG | 5 | 9 | 56 |
| ISSR-12 | AGA GAG AGA GAG AGA GCT | 5 | 7 | 71 |
| ISSR-6 | GCC TCC TCC TCC TCC TCC | 3 | 7 | 43 |
| ISSR-7 | AGA TCC TCC TCC TCC TCC | 0 | 4 | 0 |
| ISSR-9 | CAC ACA CAC ACA CAC ATG | 8 | 10 | 80 |
| UBC-808 | AGA GAG AGA GAG AGA GC | 2 | 4 | 50 |
| UBC-810 | GAG AGA GAG AGA GAG AT | 11 | 11 | 100 |
| UBC-811 | GAG AGA GAG AGA GAG AC | 8 | 10 | 80 |
| UBC-815 | CTC TCT CTC TCT CTC TG | 4 | 11 | 36 |
| UBC-818 | CAC ACA CAC ACA CAC AG | 6 | 9 | 67 |
| UBC-825 | ACA CAC ACA CAC ACA CT | 4 | 7 | 57 |
| UBC-841 | GAG AGA GAG AGA GAG ACT C | 1 | 4 | 25 |
| UBC-845 | CTC TCT CTC TCT CTC TTG | 12 | 13 | 92 |
| UBC-846 | CAC ACA CAC ACA CAC AAT | 6 | 7 | 86 |
| Total | 77 | 117 | 990 | |
| Average | 5.13 | 7.8 | 66 | |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1 | 1.000 | ||||||
| 2 | 0.738 | 1.000 | |||||
| 3 | 0.773 | 0.951 | 1.000 | ||||
| 4 | 0.881 | 0.782 | 0.794 | 1.000 | |||
| 5 | 0.850 | 0.839 | 0.836 | 0.857 | 1.000 | ||
| 6 | 0.792 | 0.815 | 0.812 | 0.806 | 0.887 | 1.000 | |
| 7 | 0.857 | 0.742 | 0.754 | 0.861 | 0.826 | 0.780 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coşkun, Ö.F. The Effect of Grafting on Morphological, Physiological and Molecular Changes Induced by Drought Stress in Cucumber. Sustainability 2023, 15, 875. https://doi.org/10.3390/su15010875
Coşkun ÖF. The Effect of Grafting on Morphological, Physiological and Molecular Changes Induced by Drought Stress in Cucumber. Sustainability. 2023; 15(1):875. https://doi.org/10.3390/su15010875
Chicago/Turabian StyleCoşkun, Ömer Faruk. 2023. "The Effect of Grafting on Morphological, Physiological and Molecular Changes Induced by Drought Stress in Cucumber" Sustainability 15, no. 1: 875. https://doi.org/10.3390/su15010875
APA StyleCoşkun, Ö. F. (2023). The Effect of Grafting on Morphological, Physiological and Molecular Changes Induced by Drought Stress in Cucumber. Sustainability, 15(1), 875. https://doi.org/10.3390/su15010875




