Approaches of Egg Decontamination for Sustainable Food Safety
Abstract
1. Introduction
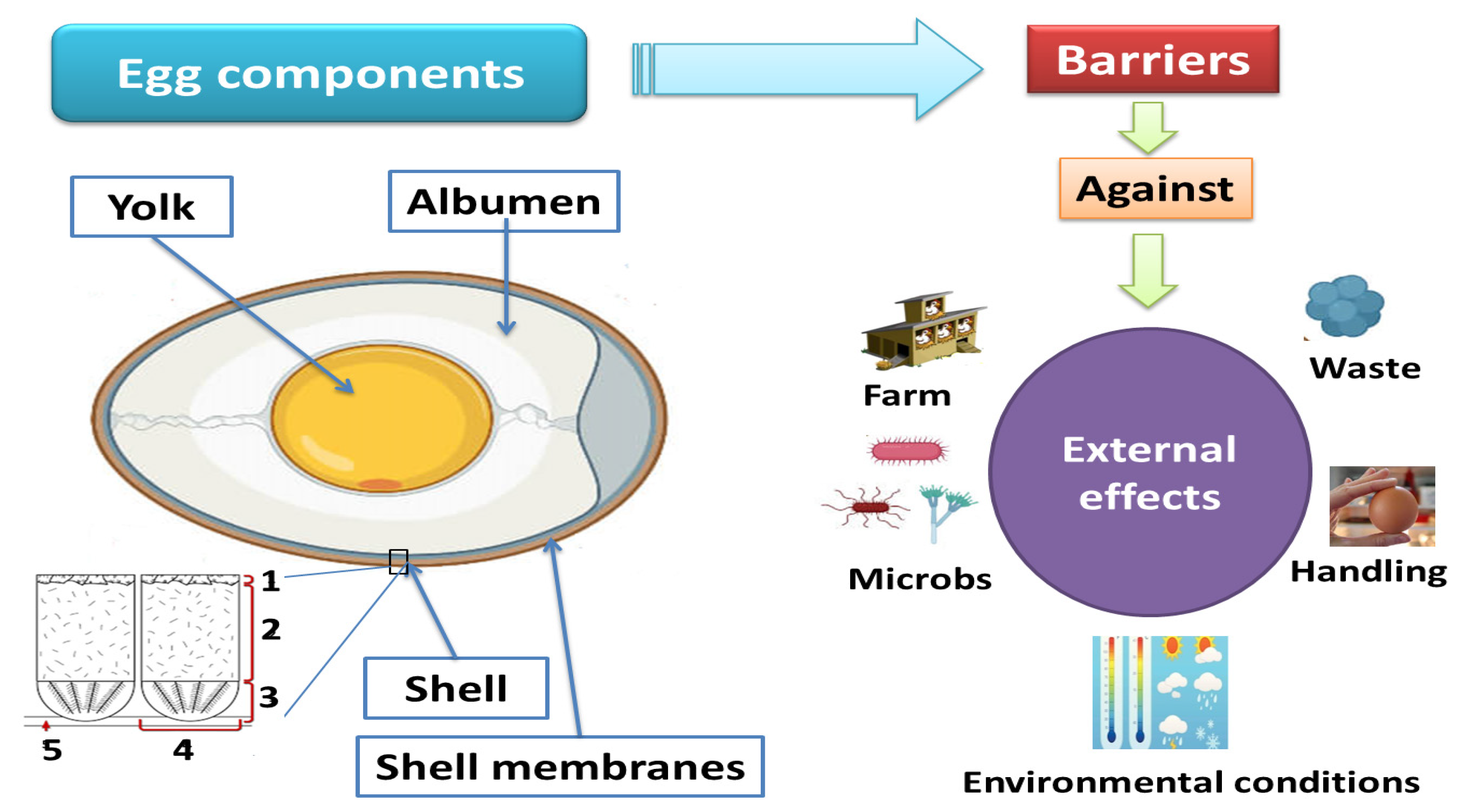
2. How the Egg Can Protect Itself
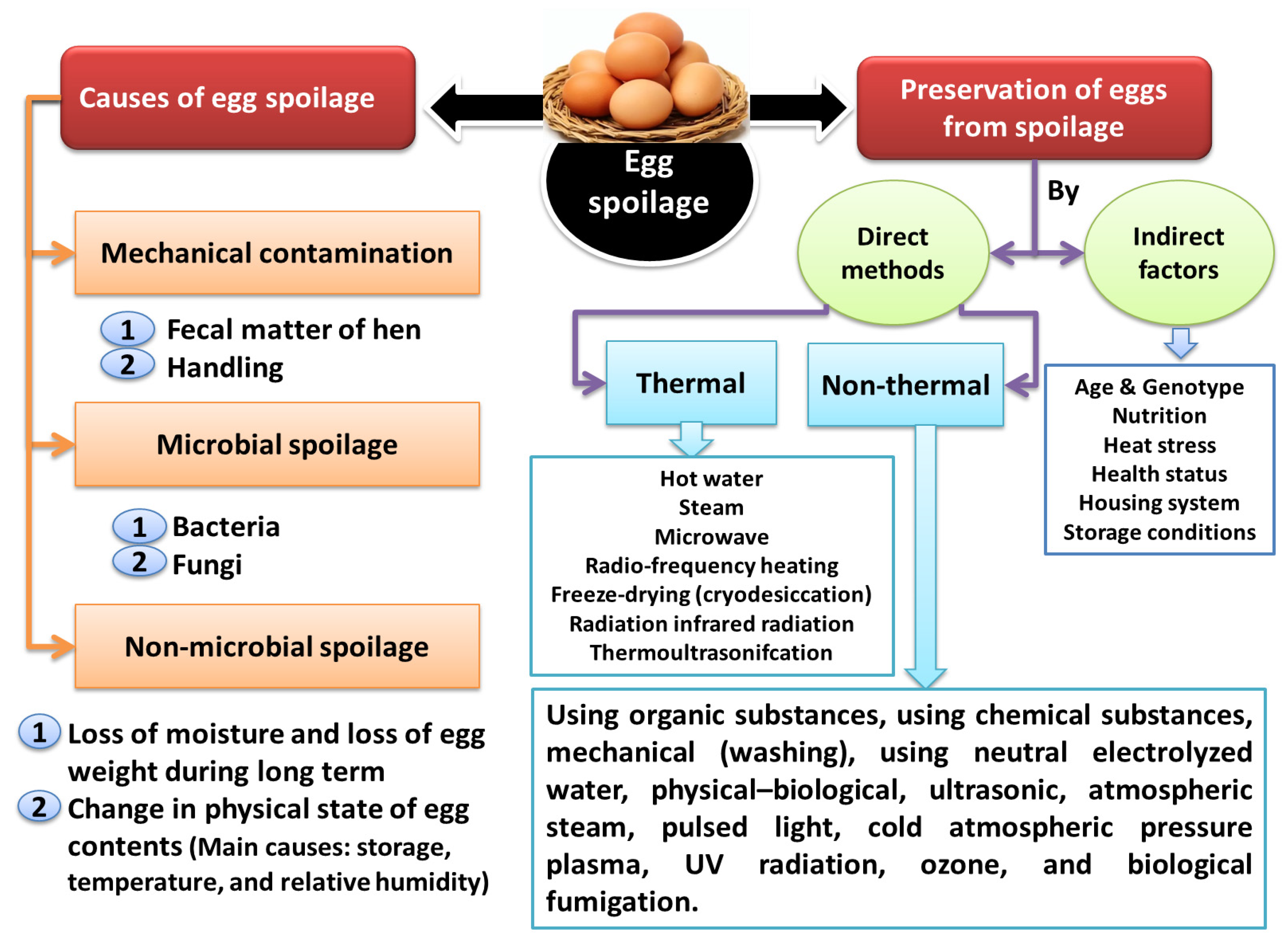
3. Causes of Egg Spoilage and Methods of Preventing It
4. Direct Methods of Preventing Egg Spoilage
5. Indirect Factors Affecting Egg Components as Barriers to External Influences and Contamination
6. Conclusions
7. Future Perspectives and Research Needs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vågsholm, I.; Arzoomand, N.S.; Boqvist, S. Food Security, Safety, and Sustainability—Getting the Trade-Offs Right. Front. Sustain. Food Syst. 2020, 4, 16. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.E.D.S.; Leite, C.E.C.; Dahlke, F.; Maiorka, A.; Miotto, M.; Scussel, V.; Lindner, J.D.D. Antifungal Action of Ozone on Chicken Eggshell Cuticles: A Preliminary Study. Ozone Sci. Eng. 2021, 44, 407–412. [Google Scholar] [CrossRef]
- Yuceer, M.; Caner, C. Antimicrobial lysozyme-chitosan coatings affect functional properties and shelf life of chicken eggs during storage. J. Sci. Food Agric. 2013, 94, 153–162. [Google Scholar] [CrossRef]
- Al-Bahry, S.N.; Mahmoud, I.Y.; Al-Musharafi, S.K.; Al-Ali, M.A. Penetration of spoilage and food poisoning bacteria into fresh chicken egg: A public health concern. Glob. J. Bio-Sci. Biotechnol. 2012, 1, 33–39. [Google Scholar]
- Nogr, H.D.; Yassin, O.E.; El Tigani, M.; Ibrahim, M.T. Marketing Activities and Egg Cracks in the Marketing Chain and Disposal Methods in Khartoum State Groceries. J. Agric. Vet. Sci. 2020, 21, 85–92. [Google Scholar]
- Keener, K.M. Shell Egg Pasteurization. In Egg Innovations and Strategies for Improvements; Academic Press: Cambridge, MA, USA, 2017; pp. 165–175. [Google Scholar] [CrossRef]
- James, C.; Lechevalier, V.; Ketteringham, L. Surface pasteurisation of shell eggs. J. Food Eng. 2002, 53, 193–197. [Google Scholar] [CrossRef]
- Kiosseoglou, V.; Paraskevopoulou, A. Molecular interactions in gels prepared with egg yolk and its fractions. Food Hydrocoll. 2005, 19, 527–532. [Google Scholar] [CrossRef]
- Allende, A.; Tomás-Barberán, F.A.; Gil, M.I. Minimal processing for healthy traditional foods. Trends Food Sci. Technol. 2006, 17, 513–519. [Google Scholar] [CrossRef]
- Debabandya, M.; Sabyasachi, M.; Saroj, G.; Abhijit, K. Application of hurdles for extending the shelf life of fresh fruits. Trends Post Harvest Technol. 2013, 1, 37–54. [Google Scholar]
- Stadelman, W.J. Quality Identification of Shell Eggs. In Egg Science and Technology, 4th ed.; Stadelman, W.J., Newkirk, D., Newby, L., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 39–66. [Google Scholar]
- Techer, C.; Baron, F.; Jan, S. Microbial spoilage of eggs and egg products. World’s Poult. Sci. J. 2013, 69, 439–445. [Google Scholar] [CrossRef]
- Sunwoo, H.H.; Gujral, N. Chemical composition of eggs and egg products. In Handbook of Food Chemistry; Cheung, P.C.K., Mehta, B.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 331–363. [Google Scholar] [CrossRef]
- Gautron, J.; Nys, Y. Eggshell matrix proteins and natural defenses of the egg. In Proceedings of the Symposium COA/INRA Scientific Cooperation in Agriculture, Tainan, Taiwan, 7–10 November 2006; Available online: https://www.angrin.tlri.gov.tw/%5C/INRA/o15.pdf (accessed on 13 November 2022).
- Legros, J.; Jan, S.; Bonnassie, S.; Gautier, M.; Croguennec, T.; Pezennec, S.; Cochet, M.-F.; Nau, F.; Andrews, S.C.; Baron, F. The Role of Ovotransferrin in Egg-White Antimicrobial Activity: A Review. Foods 2021, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- de Souza, P.M.; de Melo, R.; Santos, M.A.D.A.; Lima, F.R.; Vieira, K.H. Risk Management of Egg and Egg Products: Advanced Methods Applied. In Food Engineering; Coldea, T.E., Ed.; IntechOpen, 2019. [Google Scholar] [CrossRef]
- EFSA and ECDC. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar] [CrossRef]
- Gast, R.K.; Regmi, P.; Guraya, R.; Jones, D.R.; Anderson, K.; Karcher, D.M. Contamination of eggs by Salmonella Enteritidis in experimentally infected laying hens of four commercial genetic lines in conventional cages and enriched colony housing. Poult. Sci. 2019, 98, 5023–5027. [Google Scholar] [CrossRef] [PubMed]
- Eddin, A.S.; Ibrahim, S.A.; Tahergorabi, R. Egg quality and safety with an overview of edible coating application for egg preservation. Food Chem. 2019, 296, 29–39. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, A.R.; Chousalkar, K.K. Salmonella on Australian cage egg farms: Observations from hatching to end of lay. Food Microbiol. 2020, 87, 103384. [Google Scholar] [CrossRef] [PubMed]
- Paramithiotis, S.; Drosinos, E.H.; Skandamis, P.N. Food recalls and warnings due to the presence of foodborne pathogens—A focus on fresh fruits, vegetables, dairy and eggs. Curr. Opin. Food Sci. 2017, 18, 71–75. [Google Scholar] [CrossRef]
- Huang, X.; Hu, M.; Zhou, X.; Liu, Y.; Shi, C.; Shi, X. Role of yoaE Gene Regulated by CpxR in the Survival of Salmonella enterica Serovar Enteritidis in Antibacterial Egg White. mSphere 2020, 5, e00638-19. [Google Scholar] [CrossRef]
- Raspoet, R.; Eeckhaut, V.; Vermeulen, K.; De Smet, L.; Wen, Y.; Nishino, K.; Haesebrouck, F.; Ducatelle, R.; Devreese, B.; Van Immerseel, F. The Salmonella Enteritidis TolC outer membrane channel is essential for egg white survival. Poult. Sci. 2019, 98, 2281–2289. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Chen, T.-H.; Wu, Y.-C.; Lee, Y.-C.; Tan, F.-J. Effects of egg washing and storage temperature on the quality of eggshell cuticle and eggs. Food Chem. 2016, 211, 687–693. [Google Scholar] [CrossRef]
- Muñoz, A.; Dominguez-Gasca, N.; Jimenez-Lopez, C.; Rodriguez-Navarro, A.B. Importance of eggshell cuticle composition and maturity for avoiding trans-shell Salmonella contamination in chicken eggs. Food Control 2015, 55, 31–38. [Google Scholar] [CrossRef]
- Knape, K.D.; Carey, J.B.; Ricke, S.C. Response of foodbornesalmonellaspp. marker strains inoculated on egg shell surfaces to disinfectants in a commercial egg washer. J. Environ. Sci. Health Part B 2001, 36, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Samiullah, S.; Chousalkar, K.; Roberts, J.; Sexton, M.; May, D.; Kiermeier, A. Effects of egg shell quality and washing on Salmonella Infantis penetration. Int. J. Food Microbiol. 2013, 165, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Al-Ajeeli, M.N.; Taylor, T.M.; Alvarado, C.Z.; Coufal, C.D. Comparison of eggshell surface sanitization technologies and impacts on consumer acceptability. Poult. Sci. 2016, 95, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Slavik, M.E. Bacterial penetration into eggs washed with various chemicals and stored at different temperatures and times. J. Food Protec. 1998, 61, 276–279. [Google Scholar] [CrossRef]
- Northcutt, J.K.; Musgrove, M.T.; Jones, D.R. Chemical Analyses of Commercial Shell Egg Wash Water. J. Appl. Poult. Res. 2005, 14, 289–295. [Google Scholar] [CrossRef]
- Soljour, G.; Assanta, M.A.; Messier, S.; Boulianne, M. Efficacy of Egg Cleaning Compounds on Eggshells Contaminated with Salmonella enterica Serovar Enteritidis. J. Food Prot. 2004, 67, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-C.; Dev, S.; Bialka, K.; Demirci, A. Electrolyzed oxidizing water for microbial decontamination of food. In Microbial Decontamination in the Food Industry; Demirci, A., Ngadi, M.O., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 563–591. [Google Scholar] [CrossRef]
- Medina-Gudiño, J.; Rivera-Garcia, A.; Santos-Ferro, L.; Ramirez-Orejel, J.C.; Agredano-Moreno, L.T.; Jimenez-Garcia, L.F.; Paez-Esquiliano, D.; Martinez-Vidal, S.; Andrade-Esquivel, E.; Cano-Buendia, J.A. Analysis of Neutral Electrolyzed Water anti-bacterial activity on contaminated eggshells with Salmonella enterica or Escherichia coli. Int. J. Food Microbiol. 2020, 320, 108538. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-R.; Hung, Y.-C.; Hsu, S.-Y.; Huang, Y.-W.; Hwang, D.-F. Application of electrolyzed water in the food industry. Food Control 2008, 19, 329–345. [Google Scholar] [CrossRef]
- Liao, L.B.; Chen, W.M.; Xiao, X.M. The generation and inactivation mechanism of oxidation–reduction potential of electrolyzed oxidizing water. J. Food Eng. 2007, 78, 1326–1332. [Google Scholar] [CrossRef]
- Len, S.-V.; Hung, Y.-C.; Chung, D.; Anderson, J.L.; Erickson, M.C.; Morita, K. Effects of Storage Conditions and pH on Chlorine Loss in Electrolyzed Oxidizing (EO) Water. J. Agric. Food Chem. 2001, 50, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Ezazi, A.; Javadi, A.; Jafarizadeh-Malmiri, H.; Mirzaei, H. Development of a chitosan-propolis extract edible coating formulation based on physico-chemical attributes of hens’ eggs: Optimization and characteristics edible coating of egg using chitosan and propolis. Food Biosci. 2021, 40, 100894. [Google Scholar] [CrossRef]
- Wardy, W.; Torrico, D.D.; Jirangrat, W.; No, H.K.; Saalia, F.K.; Prinyawiwatkul, W. Chitosan-soybean oil emulsion coating affects physico-functional and sensory quality of eggs during storage. LWT-Food Sci. Technol. 2011, 44, 2349–2355. [Google Scholar] [CrossRef]
- Dehnad, D.; Mirzaei, H.; Emam-Djomeh, Z.; Jafari, S.-M.; Dadashi, S. Thermal and antimicrobial properties of chitosan–nanocellulose films for extending shelf life of ground meat. Carbohydr. Polym. 2014, 109, 148–154. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Ye, S.-R.; Ting, C.; Yu, Y.-H. Antibacterial activity of propolins from Taiwanese green propolis. J. Food Drug Anal. 2018, 26, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality—A review. Trends Food Sci. Technol. 2018, 83, 53–62. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; dos Santos, V.M.; McManus, C. Propolis: Effects on the sanitisation of hatching eggs. World’s Poult. Sci. J. 2021, 78, 261–272. [Google Scholar] [CrossRef]
- Drabik, K.; Batkowska, J.; Próchniak, T.; Horecka, B. Citric acid as a factor limiting changes in the quality of table eggs during their storage. Poult. Sci. 2021, 100, 100995. [Google Scholar] [CrossRef]
- Soares, C.E.S.; Cartabiano-Leite, C.E.; Ferreira, W.X.; Maiorka, A.; Dahlke, F.; Scussel, V.M.; Lindner, J.D.D. Peracetic Acid: Effect on the Chicken Eggshell Cuticle and Decontaminating Action on Filamentous Fungi. Jokull J. 2021, 71, 82–96. [Google Scholar]
- Agregán, R.; Munekata, P.E.S.; Putnik, P.; Pateiro, M.; Kovačević, D.B.; Zavadlav, S.; Lorenzo, J.M. The Use of Novel Technologies in Egg Processing. Food Rev. Int. 2021; 1–21, in press. [Google Scholar] [CrossRef]
- Ragni, L.; Berardinelli, A.; Vannini, L.; Montanari, C.; Sirri, F.; Guerzoni, M.E.; Guarnieri, A. Non-thermal atmospheric gas plasma device for surface decontamination of shell eggs. J. Food Eng. 2010, 100, 125–132. [Google Scholar] [CrossRef]
- Dasan, B.G.; Yildirim, T.; Boyaci, I.H. Surface decontamination of eggshells by using non-thermal atmospheric plasma. Int. J. Food Microbiol. 2018, 266, 267–273. [Google Scholar] [CrossRef]
- Moritz, M.; Wiacek, C.; Weihe, T.; Ehlbeck, J.; Weltmann, K.; Braun, P.G. Effect of cold atmospheric pressure plasma treatment of eggshells on the total bacterial count inoculated Salmonella Enteritidis and selected quality parameters. Plasma Process. Polym. 2020, 18, 2000061. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold Plasma: A novel Non-Thermal Technology for Food Processing. Food Biophys. 2014, 10, 1–11. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.; Gilmore, B. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.J.; Yousef, A.E. Decontamination of Raw Foods Using Ozone-Based Sanitization Techniques. Annu. Rev. Food Sci. Technol. 2011, 2, 281–298. [Google Scholar] [CrossRef]
- Braun, P.; Fernández, N.; Fuhrmann, H. Investigations on the Effect of Ozone as a Disinfectant of Egg Surfaces. Ozone Sci. Eng. 2011, 33, 374–378. [Google Scholar] [CrossRef]
- Yüceer, M.; Aday, M.S.; Caner, C. Ozone treatment of shell eggs to preserve functional quality and enhance shelf life during storage. J. Sci. Food Agric. 2015, 96, 2755–2763. [Google Scholar] [CrossRef]
- Lasagabaster, A.; Arboleya, J.C.; de Marañón, I.M. Pulsed light technology for surface decontamination of eggs: Impact on Salmonella inactivation and egg quality. Innov. Food Sci. Emerg. Technol. 2011, 12, 124–128. [Google Scholar] [CrossRef]
- Wang, B.; Wei, W.; Aputexiakere, J.; Li, Y.; Ma, H. Surface decontamination of whole eggs using pulsed light technology and shelf life study of combined pulsed light and vaseline coating during room temperature storage. Food Control 2021, 137, 108411. [Google Scholar] [CrossRef]
- John, D.; Ramaswamy, H.S. Pulsed light technology to enhance food safety and quality: A mini-review. Curr. Opin. Food Sci. 2018, 23, 70–79. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Sert, D.; Aygun, A.; Demir, M. Effects of ultrasonic treatment and storage temperature on egg quality. Poult. Sci. 2011, 90, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Romo, L.A.; Yousef, A.E. Inactivation of Salmonella enterica serovar Enteritidis on shell eggs by ozone and UV radiation. J. Food Prot. 2005, 68, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Aygun, A.; Sert, D. Effects of vacuum packing on eggshell microbial activity and egg quality in table eggs under different storage temperatures. J. Sci. Food Agric. 2013, 93, 1626–1632. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kaewyana, C.; Yodmeeklin, A.; Kumla, J.; Matsui, K.; Lumyong, S. Evaluation of Muscodor cinnamomi as an egg biofumigant for the reduction of microorganisms on eggshell surfaces and its effect on egg quality. Int. J. Food Microbiol. 2017, 244, 52–61. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Ukuku, D.O. The role of emerging technologies to ensure the microbial safety of fresh produce, milk and eggs. Curr. Opin. Food Sci. 2018, 19, 145–154. [Google Scholar] [CrossRef]
- Keerthirathne, T.P.; Ross, K.; Fallowfield, H.; Whiley, H. Reducing Risk of Salmonellosis through Egg Decontamination Processes. Int. J. Environ. Res. Public Health 2017, 14, 335. [Google Scholar] [CrossRef]
- Lechevalier, V.; Guérin-Dubiard, C.; Anton, M.; Beaumal, V.; Briand, E.D.; Gillard, A.; Le Gouar, Y.; Musikaphun, N.; Tanguy, G.; Pasco, M.; et al. Pasteurisation of liquid whole egg: Optimal heat treatments in relation to its functional, nutritional and allergenic properties. J. Food Eng. 2016, 195, 137–149. [Google Scholar] [CrossRef]
- Uysal, R.S.; Boyacı, I.H.; Soykut, E.A.; Ertaş, N. Effects of heat treatment parameters on liquid whole egg proteins. Food Chem. 2017, 216, 201–208. [Google Scholar] [CrossRef]
- Yang, Y.; Geveke, D.J. Shell egg pasteurization using radio frequency in combination with hot air or hot water. Food Microbiol. 2019, 85, 103281. [Google Scholar] [CrossRef]
- Zion, B.; Gollop, R.; Barak, M.; Saldinger, S.S.; Arbel, A. External disinfection of shell eggs using steam in a Thermal Trap. Food Control 2021, 127, 108135. [Google Scholar] [CrossRef]
- Lakins, D.; Alvarado, C.; Thompson, L.; Brashears, M.; Brooks, J.C.; Brashears, M.M. Reduction of Salmonella Enteritidis in Shell Eggs Using Directional Microwave Technology. Poult. Sci. 2008, 87, 985–991. [Google Scholar] [CrossRef]
- Cabeza, M.C.; Garcia, M.L.; Hoz, L.D.; Cambero, I.; Ordontez, J.A. Thermoultrasonication Eliminates Salmonellae from Intact Eggshells without Changing the Functional Properties of their Components. J. Food Sci. 2006, 70, m292–m295. [Google Scholar] [CrossRef]
- de Souza Aquino, J.; da Silva, J.A.; Prado, J.P.; de Oliveira Cavalheiro, J.M. Análise dos constituintes de gema de ovo de avestruz desidratada por meio de duas metodologias de secagem. Rev. Inst. Adolfo Lutz. 2008, 67, 190–195. [Google Scholar]
- Stolz, N.; Weihe, T.; Stachowiak, J.; Braun, P.; Schluter, O.; Ehlbeck, J. Decontamination of shell eggs by using non-thermal atmospheric pressure plasma. In Proceedings of the 15th International Conference on Biomedical Engineering and Technology (ICBET 2015), Seoul, South Korea, 10–11 March 2015; Volume 81, pp. 81–84. [Google Scholar] [CrossRef]
- Georgescu, N.; Apostol, L.; Gherendi, F. Inactivation of Salmonella enterica serovar Typhimurium on egg surface, by direct and indirect treatments with cold atmospheric plasma. Food Control 2017, 76, 52–61. [Google Scholar] [CrossRef]
- Hierro, E.; Manzano, S.; Ordóñez, J.A.; de la Hoz, L.; Fernández, M. Inactivation of Salmonella enterica serovar Enteritidis on shell eggs by pulsed light technology. Int. J. Food Microbiol. 2009, 135, 125–130. [Google Scholar] [CrossRef]
- Keklik, N.M.; Demirci, A.; Puri, V.M. Decontamination of unpackaged and vacuum-packaged boneless chicken breast with pulsed UV-light. Poul. Sci. 2010, 89, 570–581. [Google Scholar] [CrossRef]
- Himathongkham, S.; Riemann, H.; Ernst, R. Efficacy of disinfection of shell eggs externally contaminated with Salmonella enteritidis: Implications for egg testing. Int. J. Food Microbiol. 1999, 49, 161–167. [Google Scholar] [CrossRef]
- Catalano, C.R.; Knabel, S.J. Destruction of Salmonella enteritidis by high pH and rapid chilling during simulated commercial egg processing. J. Food Prot. 1994, 57, 592–595. [Google Scholar] [CrossRef]
- Mudau, M.S. Functional Properties of Microwave Pasteurised and Oil Coated Whole Shell Eggs. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2007. Available online: http://hdl.handle.net/2263/26837 (accessed on 13 November 2022).
- Caner, C.; Yüceer, M. Efficacy of various protein-based coating on enhancing the shelf life of fresh eggs during storage. Poult. Sci. 2015, 94, 1665–1677. [Google Scholar] [CrossRef]
- Shin, D.; Narciso-Gaytán, C.; Regenstein, J.M.; Sánchez-Plata, M.X. Effect of various refrigeration temperatures on quality of shell eggs. J. Sci. Food Agric. 2011, 92, 1341–1345. [Google Scholar] [CrossRef]
- Hou, H.; Singh, R.; Muriana, P.; Stadelman, W. Pasteurization of intact shell eggs. Food Microbiol. 1996, 13, 93–101. [Google Scholar] [CrossRef]
- Schuman, J.; Sheldon, B.; Vandepopuliere, J.; Jr, H.B. Immersion heat treatments for inactivation of Salmonella enteritidis with intact eggs. J. Appl. Microbiol. 1997, 83, 438–444. [Google Scholar] [CrossRef]
- Geveke, D.J.; Gurtler, J.B.; Jones, D.R.; Bigley, A.B.W. Inactivation of Salmonella in Shell Eggs by Hot Water Immersion and Its Effect on Quality. J. Food Sci. 2016, 81, M709–M714. [Google Scholar] [CrossRef]
- Pasquali, F.; Fabbri, A.; Cevoli, C.; Manfreda, G.; Franchini, A. Hot air treatment for surface decontamination of table eggs. Food Control 2010, 21, 431–435. [Google Scholar] [CrossRef]
- Manfreda, G.; Cevoli, C.; Lucchi, A.; Pasquali, F.; Fabbri, A.; Franchini, A. Hot air treatment for surface decontamination of table eggs experimentally infected with Salmonella, Listeria, and Escherichia coli. Veter-Res. Commun. 2010, 34, 179–182. [Google Scholar] [CrossRef]
- Shenga, E.; Singh, R.P.; Yadav, A.S. Effect of pasteurization of shell egg on its quality characteristics under ambient storage. J. Food Sci. Technol. 2010, 47, 420–425. [Google Scholar] [CrossRef]
- Geveke, D.J.; Bigley, A.B.; Brunkhorst, C.D. Pasteurization of shell eggs using radio frequency heating. J. Food Eng. 2017, 193, 53–57. [Google Scholar] [CrossRef]
- Dev, S.; Raghavan, G.; Gariepy, Y. Dielectric properties of egg components and microwave heating for in-shell pasteurization of eggs. J. Food Eng. 2008, 86, 207–214. [Google Scholar] [CrossRef]
- Perry, J.; Rodriguez-Saona, L.; Yousef, A. Quality of Shell Eggs Pasteurized with Heat or Heat-Ozone Combination during Extended Storage. J. Food Sci. 2011, 76, S437–S444. [Google Scholar] [CrossRef]
- Kannan, S.; Dev, S.; Gariepy, Y.; Raghavan, G.S.V. Effect of radiofrequency heating on the dielectric and physical properties of eggs. Prog. Electromagn. Res. B 2013, 51, 201–220. [Google Scholar] [CrossRef]
- Matt, D.; Veromann, E.; Luik, A. Effect of housing systems on biochemical composition of chicken eggs. Agron. Res. 2009, 7, 662–667. [Google Scholar]
- Ketta, M.; Tumova, E. Eggshell structure, measurements, and quality-affecting factors in laying hens: A review. Czech J. Anim. Sci. 2016, 61, 299–309. [Google Scholar] [CrossRef]
- Vlčková, J.; Tůmová, E.; Ketta, M.; Englmaierová, M.; Chodová, D. Effect of housing system and age of laying hens on eggshell quality, microbial contamination, and penetration of microorganisms into eggs. Czech J. Anim. Sci. 2018, 63, 51–60. [Google Scholar] [CrossRef]
- Johnston, S.; Gous, R. Modelling the changes in the proportions of the egg components during a laying cycle. Br. Poult. Sci. 2007, 48, 347–353. [Google Scholar] [CrossRef]
- Rakib, T.M.; Akter, L.; Barua, S.R.; Azam, N.E.; Erfan, R.; Islam, M.S.; Miazi, O.F. Effects of age, rearing system and their interaction on phenotypic characteristics in hisex brown laying hens. Sci. J. Vet. Adv. 2016, 5, 87–96. [Google Scholar] [CrossRef]
- Molnár, A.; Maertens, L.; Ampe, B.; Buyse, J.; Kempen, I.; Zoons, J.; Delezie, E. Changes in egg quality traits during the last phase of production: Is there potential for an extended laying cycle? Br. Poult. Sci. 2016, 57, 842–847. [Google Scholar] [CrossRef]
- Máchal, L.; Simeonovová, J. The relationship of shortening and strength of eggshell to some egg quality indicators and egg production in hens of different initial laying lines*. Arch. Anim. Breed. 2002, 45, 287–296. [Google Scholar] [CrossRef]
- Bozkurt, Z.; Tekerli, M. The effects of hen age, genotype, period and temperature of storage on egg quality. Kafkas Univ. Vet. Fak. Derg. 2009, 15, 517–524. [Google Scholar]
- Jones, D.R.; Musgrove, M.T.; Anderson, K.E.; Thesmar, H.S. Physical quality and composition of retail shell eggs. Poult. Sci. 2010, 89, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Ledvinka, Z. The Effect of Internal and External Factors on the Quality of Eggs. Ph.D. Thesis, Czech University of Life Sciences Prague, Prague, Czechia, 2003; p. 143. (In Czech). [Google Scholar]
- Leyendecker, M.; Hamann, H.; Hartung, J.; Kamphues, J.; Ring, C.; Glunder, G.; Ahlers, C.; Sander, I.; Neumann, U.; Distl, O. Analysis of genotype–environment interactions between layer lines and housing systems for performance traits, egg quality and bone breaking strength.1st Communication: Egg quality traits. Zuchtungskunde 2001, 73, 290–307. [Google Scholar]
- Ledvinka, Z.; Tumova, E.; Arent, E.; Holoubek, J.; Klesalova, L. Egg shell quality in some white-egg and brown-egg cross combinations of dominant hens. Czech J. Anim. Sci. 2000, 45, 285–288. [Google Scholar]
- Leyendecker, M.; Hamann, H.; Hartung, J.; Kamphues, J.; Ring, C.; Gluender, G.; Ahlers, C.; Sander, I.; Neumann, U.; Distl, O. Analysis of genotype-environment interactions between layer lines and housing systems for performance traits, egg quality and bone breaking strength—2nd communication: Egg quality traits. Zuchtungskunde 2001, 73, 308–323. [Google Scholar]
- Anderson, K.; Adams, A. Effects of Floor Versus Cage Rearing and Feeder Space on Growth, Long Bone Development, and Duration of Tonic Immobility in Single Comb White Leghorn Pullets. Poult. Sci. 1994, 73, 958–964. [Google Scholar] [CrossRef]
- Singh, R.; Cheng, K.; Silversides, F. Production performance and egg quality of four strains of laying hens kept in conventional cages and floor pens. Poult. Sci. 2009, 88, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.S.; Davies, R.H.; Dewulf, J.; Gast, R.K.; Huwe, J.K.; Jones, D.R.; Waltman, D.; Willian, K.R. The impact of dif-ferent housing systems on egg safety and quality. Poult. Sci. 2011, 90, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Kontecka, H.; Nowaczewski, S.; Krystianiak, S.; Szychowiak, M.; Kupś, K. Effect of housing system on reproductive results in ring-necked pheasants (Phasianus colchicus L.). Czech J. Anim. Sci. 2014, 59, 319–326. [Google Scholar] [CrossRef]
- Mertens, K.; Bamelis, F.; Kemps, B.; Kamers, B.; Verhoelst, E.; De Ketelaere, B.; Bain, M.; Decuypere, E.; De Baerdemaeker, J. Monitoring of Eggshell Breakage and Eggshell Strength in Different Production Chains of Consumption Eggs. Poult. Sci. 2006, 85, 1670–1677. [Google Scholar] [CrossRef]
- Tumova, E.; Englmaierova, M.; Ledvinka, Z.; Charvatova, V. Interaction between housing system and genotype in rela-tion to internal and external egg quality parameters. Czech J. Anim. Sci. 2011, 56, 490–498. [Google Scholar] [CrossRef]
- Ketta, M.; Tůmová, E. Relationship between eggshell thickness and other eggshell measurements in eggs from litter and cages. Ital. J. Anim. Sci. 2017, 17, 234–239. [Google Scholar] [CrossRef]
- Mabe, I.; Rapp, C.; Bain, M.; Nys, Y. Supplementation of a corn-soybean meal diet with manganese, copper, and zinc from organic or inorganic sources improves eggshell quality in aged laying hens. Poult. Sci. 2003, 82, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Leeson, S.; Summers, J.D.; Caston, L.J. Response of Layers to Low Nutrient Density Diets. J. Appl. Poult. Res. 2001, 10, 46–52. [Google Scholar] [CrossRef]
- Wu, G.; Bryant, M.M.; Voitle, R.A.; Roland, D.A. Effect of dietary energy on performance and egg composition of Bovans White and Dekalb White hens during phase I. Poult. Sci. 2005, 84, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- El-Hack, M.A.; Elnesr, S.; Alagawany, M.; Gado, A.; Noreldin, A.; Gabr, A. Impact of green tea (Camellia sinensis) and epigallocatechin gallate on poultry. World’s Poult. Sci. J. 2020, 76, 49–63. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Barkat, R.A.; Gabr, A.A.; Dhama, K. Potential role of im-portant nutraceuticals in poultry performance and health-A comprehensive review. Res. Vet. Sci. 2021, 137, 9–29. [Google Scholar] [CrossRef]
- Ghanima, M.M.A.; Alagawany, M.; El-Hack, M.E.A.; Taha, A.; Elnesr, S.S.; Ajarem, J.; Allam, A.A.; Mahmoud, A.M. Consequences of various housing systems and dietary supplementation of thymol, carvacrol, and euganol on performance, egg quality, blood chemistry, and antioxidant parameters. Poult. Sci. 2020, 99, 4384–4397. [Google Scholar] [CrossRef]
- Mashaly, M.M.; Hendricks, G.L.; Kalama, M.A.; Gehad, A.E.; Abbas, A.O.; Patterson, P.H. Effect of heat stress on pro-duction parameters and immune responses of commercial laying hens. Poult. Sci. 2004, 83, 889–894. [Google Scholar] [CrossRef]
- Etches, R.J.; John, T.M.; Gibbins, A.M.V. Behavioural, physiological, neuroendocrine and molecular responses to heat stress. In Poultry Production in Hot Climates, 2nd ed.; CABI: Oxfordshire, UK, 2008; pp. 31–66. [Google Scholar] [CrossRef]
- Samara, M.H.; Robbins, K.R.; Smith, M.O. Environmental heat stress does not reduce blood ionized calcium concentra-tion in hens acclimated to elevated temperatures. Poult. Sci. 1996, 75, 197–200. [Google Scholar] [CrossRef]
- Franco-Jimenez, D.J.; Scheideler, S.E.; Kittok, R.J.; Brown-Brandl, T.M.; Robeson, L.R.; Taira, H.; Beck, M.M. Differential Effects of Heat Stress in Three Strains of Laying Hens. J. Appl. Poult. Res. 2007, 16, 628–634. [Google Scholar] [CrossRef]
- Mahmoud, K.; Beck, M.; Scheideler, S.; Forman, M.; Anderson, K.; Kachman, S. Acute High Environmental Temperature and Calcium-Estrogen Relationships in the Hen. Poult. Sci. 1996, 75, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.R.; Souillard, R.; Bertin, J. Avian diseases which affect egg production and quality. In Improving the Safety and Quality of Eggs and Egg Products; Woodhead Publishing: Sawston, UK, 2011; pp. 376–393. [Google Scholar]
- Nasri, H.; Brand, H.V.D.; Najjar, T.; Bouzouaia, M. Egg storage and breeder age impact on egg quality and embryo development. J. Anim. Physiol. Anim. Nutr. 2019, 104, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Romão, J.; Moraes, T.; Teixeira, R.; Buxade, C.; Cardoso, W. Effect of egg storage length on hatchability and weight loss in incubation of egg and meat type Japanese quails. J. Poult. Sci. 2008, 10, 143–147. [Google Scholar] [CrossRef]
| Method/Chemical Substances | Types of Contamination | Effect on Egg Properties and Decontamination | Reference |
|---|---|---|---|
| Using organic substances | |||
| Application of peracetic acid with different concentrations. | Eggs were artificially and naturally contaminated with fungi strains | - Peracetic acid reduced the development of fungi spores and hyphae slightly compromised the cuticle structure. - Highest concentration of peracetic acid solution induced micro fragmentation and compromised egg quality. | Soares et al. [45] |
| Using an atmospheric plasma | |||
| Using an atmospheric pressure plasma jet with varying frequencies under various experimental conditions | Salmonella enteritidis (S. enteritidis) was experimentally infected in eggshells at a concentration of 7 log CFU/egg. | - No deleterious impact on egg quality. - Reduction level of S. enteritidis concentration on egg surface ranged from 2 to 5 log CFU/egg | Dasan et al. [48] |
| Using atmospheric gas plasma | Experimentally inoculated with S. typhimurium and S. enteritidis (5.5–6.5 log CFU/eggshell) | - No significant harmful impacts of the gas plasma were detected on traits of egg quality. - After 90 min S. typhimurium significantly decreased by 3.5 Log CFU/eggshell. Maximum declines for S. enteritidis ranged from 3.8 to 4.5 log CFU/eggshell | Ragni et al. [47] |
| Using atmospheric pressure plasma | artificially contaminated with S. enteritidis 8 log CFU/ml | Reduction of 99.63% of the initial S. enteritidis population | Stolz et al. [72] |
| Using indirect and direct cold atmospheric plasma under different experimental conditions | Initially contamination with 8 log CFU Salmonella cells. (Salmonella enterica serovar Typhimurium) | - No significant differences in internal egg quality. - Salmonella population on egg surface was decreased below the detection limit (2 log CFU cells/egg) after 25 min of indirect treatment and 10 min of direct treatment. - Highest inactivation of Salmonella number was more than 6 log CFU/egg for humid and about 3.90 ± 0.35 log CFU/egg for dry gas | Georgescu et al. [73] |
| Semidirect cold atmospheric pressure plasma for 300 s. | S. enteritidis on egg shell average of 4.14 log CFU (±4.04 log CFU) | - Atmospheric pressure plasma did not significantly affect internal egg. - Significant reduction in S. enteritidis was 4.1 log CFU | Moritz et al. [49] |
| Using ozone | |||
| Different ozone concentrations in combination with different exposure periods. | S. enteritidis contaminated shell eggs at two levels 2–4 log CFU and 5–6 log CFU/shell | - Inactivation of 2–4 log CFU S. enteritidis/eggshell was reached. - Salmonellae were significantly decreased up to 6 log for the high contamination level. | Braun et al. [53] |
| The eggs were exposed 60 ppm of O3 for 120 or 300 min | Eggs were contaminated with fungi | - The treatment of O3 for 120 min destroyed fungal colonies and did not injury the cuticle excessively. | Soares et al. [3] |
| Using pulsed light | |||
| Using various levels of pulsed light fluencies. | Inoculated eggs with S. enterica serovar Typhimurium CECT 4156 (6.0 ± 0.2 log CFU/eggshell) | - Eggs exposure to 2.1 J/cm2 and up to 10.5 J/cm2 decreased Salmonella cells on the eggshells. - No significant harmful influence on the egg albumen quality, sensory, and functional properties was observed. | Lasagabaster et al. [55] |
| Using different levels of pulsed light fluencies. | S. enteritidis was 6.3 log CFU/washed egg and 4.5 log CFU/unwashed egg. | Maximum decontamination was 3.6 and 1.8 log CFU/egg for unwashed and washed eggs respectively. | Hierro et al. [74] |
| Using different methods: pulsed light, coating, and washing on shelf life of eggs and the interior qualities of the eggs during storage for 6 weeks at 25 °C. | (Escherichia coli ATCC 8739 strain) 9 log CFU/ml | - Increasing storage time had a negative effect on internal egg quality and weight loss - Maximum inactivation of E.coli (3.77 log CFU/egg) was achieved at pulsed light fluence of 1.32 J/cm2. - Eggshell decontamination by pulsed light ranged from 3.16 to 3.40 log CFU/egg | Wang et al. [56] |
| Using pulsed light on eggs inoculated with S. enteritidis. | S. enteritidis approximately 8 log CFU/ml | - No visual injury to the egg. - A maximum log reduction of 5.3 logs/cm2 of Salmonella was detected with the treatment of 20-s and the dose of 0.1 J/cm2. | Keklik et al. [75] |
| Using chemical substances | |||
| Using different disinfection procedures under different experimental conditions Chlorine, Chlorhexidine, and Lugol’s solution | The initial contamination of the eggs was about 7.5 log CFU (S. enteritidis) in the shell and membranes but not in the egg content. | - The reductions in log CFU caused by disinfection ranged from 0.28 to 1.86. - Disinfection with chlorhexidine, ethanol, Lugol’s solution, quarternary ammonium solutions, or flaming after dipping in ethanol failed to reach complete decontamination of the shell and membranes. | Himathongkham et al. [76] |
| Using spraying Sanitizing treatments consisted of distilled deionized water, iodine-based disinfectant, and chlorine sanitizer. | S. typhimurium and S. enteritidis inoculated on eggshell surfaces under simulated industry egg processing conditions S. typhimurium populations (3.48:5.29 log CFU/egg for no spray) S. enteritidis populations (4.95:7.85 log CFU/egg for no spray) | - All treatments significantly reduced Salmonella spp. population on the shell. - S. typhimurium populations were 1.27:2.65, 1.85:2.23, and 1.9:2.45 for deionized water, iodine-based disinfectant, and chlorine spray, respectively. - S. enteritidis populations were 3.59:3.99, 3.51:4.57, and 3.75:4.28 for deionized water, iodine-based disinfectant and chlorine spray, respectively. | Knape et al. [27] |
| Evaluation of spraying different sanitizing treatments alone or in combination with ultra violet Eggs were taken at 0, 7, and 14 days of storage at 4 °C. | S. enteritidis counts of 4.3, 3.2, and 3.2 log CFU/egg of S. enteritidis for the 3 replicate trials, respectively, | - Appling H2O2+UV treatment on shell eggs is a novel technology with significant implications for egg quality and safety preservation. - No differences in customers’ preferences for overall flavor among the four treatments tested. - All treatments lowered SE below the detection limit (2.3 log CFU/egg). | Al-Ajeeli et al. [29] |
| Washing by chemical substances | |||
| Washing eggs by a hydroxide and hypochlorite based solution followed by a compatible sanitizer at 32 °C. | Salmonella Infantis: approximately 3 and 5 log colony forming units (CFU) per ml | - Washing increased cuticle cover - Shell thickness of unwashed and washed eggs was significantly different. - Salmonella Infantis penetration did not differ significantly between cleaned and unwashed eggs. | Chousalkar et al. [28] |
| Various methods | |||
| Using neutral electrolyzed water and 0.9% NaCl solution or 2% citric acid solution for 1 min | Eggs were artificially contaminated with E. coli or Salmonella (a concentration of 6 log CFU/mL) | - Citric acid 2% solution damaged the cuticle and exposed eggshell pores. - Salmonella was reduced by 0.62 log CFU/egg, 1.45 log CFU/egg for 2% citric acid, and neutral electrolyzed water, respectively. - E. coli was reduced by 6.39 log CFU/egg for neutral electrolyzed water vs.0.06 log CFU/egg for 2% citric acid. | Medina-Gudiño et al. [34] |
| Combined method ozone (O3) and UV | |||
| Using several methods, including gaseous ozone (O3) and UV light (UV:1500 to 2500 mW/cm2) for one minute, followed by ozone at 5 lb/in2 gauge for one minute. | Eggshell externally contaminated with Salmonella (5.9 to 4.60 log CFU/g eggshell) | - Treating egg shells with ozone (O3) or UV light significantly reduced Salmonella by 5.9 and 4.6 log units, respectively. - Salmonella was inactivated on eggshell in a short period and at low temperature with the combination of ozone and UV radiation. | Rodriguez-Romo and Yousef [60] |
| At 37.7 °C, the contaminated eggs were washed with either pH 9 or pH 11 wash water. Both cleaned and unwashed eggs were chilled either quickly or slowly. | Eggshell were inoculated with a double mutant of S. enteritidis (resistant to both streptomycin and nalidixic acid) | Keeping wash water at pH 11and 37.7 °C then fast chilling of washed eggs to 7.2 °C decreased S. enteritidis on the surface of eggs. | Catalano and Knabel [77] |
| Biological fumigation | |||
| Applying biological fumigation of fungal volatile organic compounds (M. cinnamomi). | 16 strains of the tested microorganisms were inactivated after exposing of fungal volatile organic components | - Using M. cinnamomi as a biological control agent reduced microorganisms present on the eggshell surface by fumigation. - Egg quality was not affected by using fumigation. - Fumigated eggs had significantly better egg quality than non-fumigated eggs under different storage periods | Suwannarach et al. [62] |
| Range | 3–9 log CFU | 0.62–5.9 log S. enteritidis,1.27–4.9 log S. typhimurium and 0.06–6.39 log E. coli |
| Method/Chemical Substances | Effect on Egg Properties | Reference |
|---|---|---|
| Using organic substances | ||
| Using two levels of concentration aqueous solution of citric acid. The egg quality was assessed after 7, 14, 21, and 28 days (storage period) | - Enhancement of egg quality - Decrease egg weight loss, air cell and intensive water transport from albumen to yolk. - Increase structural albumen and vitelline membrane resistance. | Drabik et al. [44] |
| Coating eggs with food grade mineral oil and stored at different storage conditions of six weeks | - Coated shell eggs had a significantly lower weight loss, pH of both the yolk and albumen over time than uncoated shell eggs. - Significant differences were noted between visual sensory properties of coated and uncoated under storage conditions. - Coated shell eggs stored at the three conditions had a prolonged shelf life. | Mudau [78] |
| Coating shell egg with chitosan: The eggs were stored at ambient laboratory controlled conditions (around 25 °C with 70–75% relative humidity for 6 weeks) | - Storage duration and coating had desirable significant effects on internal egg quality. - All coated shell eggs, shell had higher pierce and strength, which resulted in longer shelf life. - The coatings of lysozyme-chitosan might be a feasible substitute for preserving the interior quality of fresh eggs during long-term storage. | Yuceer and Caner [4] |
| Using various coatings (whey protein concentrate, whey protein isolate, shellac, and zein) under different storage periods | The coatings enhanced shell strength and functional characteristics, and they might be a feasible substitute method for preserving egg interior quality during long-term storage. | Caner and Yüceer [79] |
| Using ozone | ||
| Using gaseous ozone at different concentrations with different exposure times during storage for 6 weeks at 24 °C. | - Ozone at 6 ppm negatively affected on eggshell quality. - Ozone concentrations of 2 and 4 ppm were effective in maintaining the interior quality and functional qualities of fresh eggs during storage. | Yüceer et al. [54] |
| Various methods | ||
| Using ultrasonic wave 35 kHz for 5, 15, and 30 min at 30 °C and the control (no ultrasound), and treated eggs were storage for 10 days at 5 °C, and for 10 d at 22 °C. | - The ultrasonic treatment considerably increased egg quality. -The total mesophilic aerobic bacteria values of albumen and yolk declined as the ultrasonic treatment period from 5 to 30 min. - The sensory characteristics of egg shells were enhanced by ultrasonic treatment. | Sert et al. [59] |
| Combined method ozone (O3) and UV | ||
| Using a vacuum packaging machine (1, 14, 28, 42 days). | - After 42 days at 22 °C, vacuum packed eggs had better internal egg quality. - Vacuum packed eggs decreased microbial contamination levels over the storage period. - Vacuum packing extended the egg shelf life to at least 42 days. | Aygun and Sert [61] |
| Different storage period under different storage temperature | Storage of eggs at temperatures ranging from 0.6 to 2.2 °C minimizes quality deterioration during refrigerated storage and maximizes internal egg quality retention. | Shin et al. [80] |
| Method | Types of Contamination | Effect on Egg Properties/Decontamination | Reference |
|---|---|---|---|
| Hot water immersion | |||
| Eggs pasteurized for 25 min in a 57 °C circulating water bath | Salmonella enteritidis (6 –7 log CFU) | - Pasteurization processes didn’t adversely affect the internal egg quality except albumen viscosity and turbidity. - Reductions in S. enteritidis of about 3 log cycles | Hou et al. [81] |
| Dipping eggs in hot water for three seconds | The initial contamination of eggs was about 7.5 log CFU (S. enteritidis) in the shell and membranes | Dipping eggs in hot water for three seconds completely destroyed S. enteritidis in shells and membranes, but occasionally caused the eggs to crack. | Himathongkham et al. [76] |
| Hot water immersion (at 57 °C and 58 °C for different dwell time in a water bath (0–85 min) | Six pooled strains of S. enteritidis (initially at 6·8 log CFU/g) inoculated near the center of the yolk | - Immersion heating at 57 °C or 58 °C for 35 min augmented the Haugh unit (HU) values and decreased the clarity of albumen. - Shell pasteurization processes did not harmfully affect the internal egg quality. - Immersion heating at 58 ° C for 57.5 min was effective in eliminating S. enteritidis (3.3–5.5 log cycles reduction) | Schuman et al. [82] |
| Hot water immersion at 56.7 °C for 60 min and 55.6 °C for 100 min | Eggshell was inoculated with a composite of heat resistant S. enteritidis approximately 9 log CFU/ml | - There were desirable significant effects for internal egg quality. - Hot water immersion inactivated heat-resistant S. enteritidis in eggshell by 4.5 log. | Geveke et al. [83] |
| Three thermal treatments, hot water immersion, hot water spraying and hot air, alone and in combination with different radio frequencies under different experimental conditions | The initial population of S. typhimurium in the eggshell was approximately 6.5 log CFU/ml | - Increased the length of thermal treatments had significant effect on HU scores - Combination of radio frequencies with the different thermal treatments has not significantly affected HU scores - Hot water immersion and spraying declined the Salmonella by 5: 5.2 log after 65 min and 70 min, respectively. - Combination with different radio frequencies remarkably and clearly reduced the processing time. | Yang and Geveke [67] |
| Steam method | |||
| Hot-air oven (at 55 °C and 180 min) with a forced-air circulating fan to pasteurize internally inoculated eggshell | S. enteritidis (6 –7 log CFU) | - No significant differences for HU scores, pH yolk index, and color between fresh and pasteurized eggs. - Albumen viscosity, and turbidity showed significant differences. - A 5-log reduction in S. enteritidis was achieved after 180 min of hot-air treatment. | Hou et al. [81] |
| A hot air (a treatment of 2 shots of 8 s at 600 °C) with an interval of 30 s of cold air | S. enteritidis cells contaminated eggshells ranged between 4 and 5 log CFU/eggshell | - No significant changes in the tested egg quality traits were noted (shell color, albumen turbidity, pH and the cuticle assessment). - The treatment of hot air declined the S. enteritidis load on eggshells of up to 1.9 log. | Pasquali et al. [84] |
| Hot air using steam generators with 600 °C for 8 s and followed by cold air (20–25 °C) for 32 s | E. coli (induced to possess nalidixic acidresistance), S. enteritidis (streptomycin-resistant strain), or L. monocytogenes | - No harmful influences of the hot air treatment (yolk index, albumen pH, albumen turbidity, eggshell color, and cuticle). - The reduction in S. enteritidis load on eggshells of untreated and treated eggs was 1.9 and 0.1 log CFU/eggshell, respectively. -The reduction in L. monocytogenes (1.2 log CFU/eggshell). | Manfreda et al. [85] |
| Hot air oven, 55 °C, 2 h Water bath, 57 °C, 15 min Microwave oven 9:15 s | An inoculum of S. typhimurium containing 7 log CFU was injected into the center of yolk of each egg | - No significant differences in the internal egg quality among studied groups. During 15 days of storage at ambient temperatures, the pasteurization procedure had little or no influence on the characteristics of eggs. - Dry and wet heat reduced S. typhimurium counts by 2.1 log and 2.0 log CFU/mL yolk, respectively, but microwave heating reduced S. typhimurium counts by just 1.2 log CFU/mL yolk. | Shenga et al. [86] |
| Steam is applied to the eggs as they go through a thermal trap, a partly enclosed room filled with steam | Salmonella enterica serovar Typhimurium 8 log CFU per egg | - No harmful influences on HU, yolk and albumen pH, and albumen whip were observed. - With a short treatment of a few seconds in the thermal trap prototyped completely inactivated Salmonella (>7.8 log CFU reduction). | Zion et al. [68] |
| Using the microwave technology | |||
| Using the microwave technology where frequency (from 300 MHz to 300 GHz, the wavelength (from 1 mm to 1 m) | Salmonella enteritidis in both the high (5 log CFU/g) and low (2 log CFU/g) | -There was no difference in water activities and albumen pH. - There were significant alterations in yolk pH. - A 2-log reduction in S. enteritidis in both the low (2 log CFU/g) and high (5 log CFU/g) inoculum. | Lakins et al. [69] |
| Combination or combined methods | |||
| Water-bath heating for 25 min at 57 °C followed by hot-air heating for 60 min at 55 °C | S. enteritidis (6 –7 log CFU) | - The overall functionality of pasteurized eggshell is acceptable under the studied heating conditions. - Reductions in S. enteritidis of about 7 log cycles. | Hou et al. [81] |
| The combination of Radio-frequency heating (60 MHz) for 3.5 min in water at 35 °C. | E. coli in the whole egg was measured at about 7.5 log CFU/ml | - E. coli counts were decreased (ranged from 6.5–6.6 log). - The combination of radio-frequency and hot water was faster than utilizing only hot water. | Geveke et al. [87] |
| Reduction range | 2–9 log CFU | 1.2–7.8 log S. enteritidis, 5–7.8 and 6.5–6.6 E. coli |
| Method | Effect on Egg Properties | Reference |
|---|---|---|
| Using the microwave technology | ||
| Pasteurization by using microwave energy for heating fresh in eggshell, egg white, and yolk under different frequencies | The shell membrane and eggshell exhibited very good transparency to microwaves in their dielectric properties. | Dev et al. [88] |
| Combination or combined methods | ||
| Using different methods and extended storage: (1) Immersion in hot water bath, heating followed by gaseous ozone; (2) Pasteurization; (3) Extended storage (at 4 °C, as opposed to 25 °C: 0–8 weeks. | Detrimental effects on quality markers and damaging to albumen were more severe in heat-pasteurized eggs than those treated with the ozone-based process. - Both unprocessed and processed eggs maintained superior quality when stored at 4 °C, as opposed to 25 °C. | Perry et al. [89] |
| Using different radiofrequency frequencies under different experimental conditions | - The shell membrane and eggshell exhibited very good transparency to radio waves in their dielectric properties. - Radiofrequency heating showed negative effects on foam stability, viscosity, coagulation and turbidity with increasing the heating rate. | Kannan et al. [90] |
| Hot air 180 °C for 8 s Hot water 95 °C for 10 s, Infra-red 210 °C for 30 s, Steam 100 °C for 2 s. | - The treatments displayed no visible injury to the shell or visible signs of white coagulation. -Significant reductions in Salmonella numbers (6 log without harmfully injuring the interior contents). | James et al. [8] |
| Using Pasteurization by microwave at 300 W for 40–45 min, and at 250 W for the same period. | - Microwave pasteurised eggs showed much reduced foaming ability than unpasteurized eggs, and increased yolk pH and HU values due to protein coagulation. - There was no significant variation in foam stability or albumin pH. | Mudau [78] |
| Using thermoultrasonication | ||
| Using thermoultrasonication | - No significant differences were detected in egg properties between untreated and treated eggs. - It is effective only on bacteria that are present on the egg surface and does not affect those inside. | Cabeza et al. [70] |
| Freeze-drying or cryodesiccation | Provides transportability, uniformity, ease of use, and stable microbiological quality. | de Souza Aquino et al. [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, B.Y.; Semida, D.A.; Elnesr, S.S.; Elwan, H.; El-Full, E.A. Approaches of Egg Decontamination for Sustainable Food Safety. Sustainability 2023, 15, 464. https://doi.org/10.3390/su15010464
Mahmoud BY, Semida DA, Elnesr SS, Elwan H, El-Full EA. Approaches of Egg Decontamination for Sustainable Food Safety. Sustainability. 2023; 15(1):464. https://doi.org/10.3390/su15010464
Chicago/Turabian StyleMahmoud, Bothaina Y., Doaa A. Semida, Shaaban S. Elnesr, Hamada Elwan, and Ensaf A. El-Full. 2023. "Approaches of Egg Decontamination for Sustainable Food Safety" Sustainability 15, no. 1: 464. https://doi.org/10.3390/su15010464
APA StyleMahmoud, B. Y., Semida, D. A., Elnesr, S. S., Elwan, H., & El-Full, E. A. (2023). Approaches of Egg Decontamination for Sustainable Food Safety. Sustainability, 15(1), 464. https://doi.org/10.3390/su15010464








