Abstract
Many sandy soil foundations need to be solidified during traffic construction in Guangxi, China. Because it has a similar chemical composition as cement, municipal solid waste incineration fly ash (MSWIFA) can strengthen sandy soil. However, the chloride ions and heavy metals in MSWIFA may have a negative influence on the solidification of sandy soil. Thus, FA resource use faces great challenges. This study evaluates the feasibility of using MSWIFA to solidify sandy soil. The acetic acid buffer solution method was used in the leaching test to simulate the weak acid groundwater environment in the Guangxi karst landform. The effects of the treatment methods (washing with ferrous sulphate solution, pre-treatment of organics via chelation, and adding sugarcane ash) on the strength and environmental characteristics of fly ash cement-stabilised soil (FACS) are discussed in detail. The results indicate that the FACS unconfined compressive strength (UCS) decreased by 24.82–46.64% when 5% cement was replaced with FA. Sugarcane ash effectively improved the strength of FACS by more than 10%. The leaching concentrations of Zn and Cu in the FACS meet the concentration limit set by GB 16889-2008. The leaching concentrations of Cr and Pb after washing with 6% ferrous sulphate solution were reduced by more than 30%. Meanwhile, the FACS strength developed faster. Organic chelating agents solidified most heavy metals.
1. Introduction
Due to the advantages of its power generation capacity and quantitative reduction, municipal solid waste incineration (MSWI) has gradually replaced interment in landfills, and has become one of the most important methods of domestic waste treatment [1]. Over 500 thousand tonnes of domestic waste required harmless treatment every day in China in 2021, and the daily output of MSWI fly ash (FA) was as high as 20 thousand tonnes. MSWIFA contains heavy metals such as Cu, Zn, Pb, and Cd, and is high in carcinogens such as dioxins, which pollute the surrounding ecological environment and eventually harm human health [2,3,4,5]. As MSWIFA production increases, hazardous solid waste landfills and their surroundings are under increasing pressure. Use of MSWIFA has become one of the most important choices for realising safe disposal.
MSWIFA has high concentrations of chloride and heavy metal ions, which can cause electrochemical corrosion and environmental pollution [6,7,8,9,10]. Therefore, it is not suitable for direct use. Pre-treatment by water or acid washing and organic chelation can effectively reduce the negative impact of both chloride ions and heavy metal leaching [11,12,13,14,15,16,17]. The addition of water-washed MSWIFA effectively improves the setting characteristics, volume stability, wear resistance, and compressive strength of cement [18,19]. Prepared concrete strength is improved with water-washed MSWIFA instead of sand, and the heavy metal leaching toxicity meets the Class V standard of the Environmental Quality Standards for Surface Water (GB 3838-2002) [20,21,22,23]. The leaching concentrations of chromium and lead in FA are decreased significantly after washing with a ferrous sulphate and phosphate solution [24,25]. Moreover, organic chelating agents carbonise calcium hydroxide in MSWIFA by absorbing CO2 into the air, generating calcite (CaCO3), which has high strength and good stability and adsorb heavy metal ions on the surface through physical adsorption [26,27]. However, most of the MSWIFA selected in existing research has been derived from the fluidised bed incineration process. As incinerators are upgraded and optimised, fluidised bed incineration is gradually being replaced by grate furnace incineration. This change leads to variations in the chemical composition of the resulting MSWIFA [28,29,30], meaning that the previous research results are no longer as applicable. Moreover, the productivity development level in Guangxi, China is not high, and the implementation of waste classification is inadequate. Domestic waste is often mixed with old batteries and other pollution sources, as there are many electric vehicles in Guangxi. Thus, MSWIFA in Guangxi often contains high concentrations of Pb and Cd.
At present, Guangxi is rapidly constructing a traffic network. A large number of expressways and urban roads are under construction. Many sections have foundations that consist of sandy soil. Sandy soil has a loose structure and is prone to liquefaction due to external disturbance, and needs to be solidified to improve its bearing capacity. The chemical composition of MSWIFA is similar to that of cement, meaning that it can be used as a curing agent in foundation treatments [24,30]. However, the existing research on MSWIFA foundation reinforcement has mainly focussed on soft soil [31,32,33], and the reinforcement of sandy soil has rarely been studied. In addition, Guangxi has ubiquitous karst landforms, and its groundwater and soil are usually weakly acidic. In most research, the sulfuric and nitric acid methods have been used to detect the leaching toxicity of FA cement soil, and few studies have been conducted on the leaching toxicity of FA cement soil in weakly acidic environments. Moreover, sandy soil has weak adsorption performance and strong heavy metal leaching toxicity. The strength and heavy metal-related properties of FA cement sandy soil need to be further studied.
In this study, MSWIFA was chosen from a grate furnace in Hezhou, China. Unconfined compressive strength (UCS) and leaching tests were conducted on the FA cement-stabilised soil (FACS). The FACS morphology was examined by an electron microscope to explain the change law of the cement soil strength. Washing, organic chelation, and sugarcane ash were used to improve the strength and environmental characteristics of the cement soil. The purpose of this research is to explore the feasibility of MSWIFA application in sandy cement soil. The Conclusions Section of this paper summarises a number of improvement methods and makes several suggestions.
2. Material and MSWIFA Characteristics
2.1. Materials
The MSWIFA used in this paper was collected from Environmental Technology Co., Ltd. in Hezhou City, Guangxi Zhuang Autonomous Region, China. The MSWIFA was dark grey and had a certain agglomeration. All the FA was dried at 105 °C for 24 h and sieved with a 0.5-mm square hole sieve. Then, analyses of the physical characteristics and chemical composition were conducted. The sandy soil was collected from the construction site of an expressway in Wuzhou City, Guangxi Autonomous Region, China. A series of treatments was conducted, including air-drying, crushing, and 0.5-mm screening. The basic physical properties of the soil were tested as suggested by the Standard for Geotechnical Testing Method (GB/T 50123-2019). PO42.5 ordinary Portland cement (OPC) was used as the curing agent, which was produced in Anhui, China. The chemical composition of the MSWIFA is shown in Table 1.

Table 1.
Physical characteristics of MSWIFA from different countries and regions [31,32,33,34,35].
2.2. MSWIFA Characteristics
2.2.1. Physical Characteristics
The physical characteristics of the FA and sandy soil are listed in Table 1. The FA’s specific gravity and specific surface area were 2.1 g/cm3 and 4.862 m2/g, respectively. The specific gravity and plasticity index of the FA were 2.1 g/cm² and 9.29, respectively, very close to that of the sandy soil (2.61 g/cm² and 9.0, respectively). The FA’s natural moisture content (6.57%) was approximately half that of the sandy soil (14.5%). The FA was alkaline in nature, with a pH of 12.09, while the pH value of the sandy soil was 5.89. The particle size of the FA was mainly in the range of 0.075–2 mm (93.95%). Its curvature and non-uniformity coefficients were 0.781 and 4.862, respectively. The FA grade was clearly much poorer than that of the sandy soil.
The physical characteristics of FA from different countries and other Chinese cities are summarised in Table 1 for comparison. FA from different sources has similar specific gravity of around 2–3 g/cm3. American FA has a higher moisture content and Japanese FA has the lowest specific surface area. For all of the sources, FA particle size is in the 0.075–2 mm range. However, the moisture content is related to the exposure time of the FA in the air on account of its strong water absorption. The specific surface area differs greatly due to different test conditions. In general, it can be seen from the table that FA from different sources has similar physical characteristics; moreover, with Hezhou’s FA being slightly different from other MSWIFA.
2.2.2. Chemical Characteristics
The chemical composition of FA from different cities and countries is shown in Table 2. The main oxides are CaO, SiO2, Al2O3, and Na2O, which is similar to the composition of cementitious materials such as OPC, coal ash, and slag. The CaO content in most areas is typically more than 30%, mainly due to a large amount of hydrated lime mixed into the treatment of the waste incineration tail gas. Additionally, FA chemical composition varies across different regions and incineration technologies. For example, the SiO2 and Al2O3 content in FA from Japan, Hezhou, and Nanning ranges from 5 to 10%, meaning that it lacks silicon and aluminium, while the SiO2 content in Italy and Shanghai was over 20%.
The chloride ion content of Hezhou’s FA (31.5%) was significantly higher than that of most Chinese cities, as well as the United States, Japan, and Italy. This finding is because domestic waste has large amounts of plastics and salty kitchen food, which produce a large amount of chloride ions under high-temperature incineration [36,37]. Chloride ions have strong permeability, which delays OPC’s hydration process. This situation inhibits OPC’s early strength development and increases the leaching risk of heavy metal contaminants such as Pb and Zn. This phenomenon introduces difficulties and challenges in solidification/stabilisation when FA is used as a resource. The XRD results of MSWIFA from Hezhou and Nanning have also been presented in Figure 1.
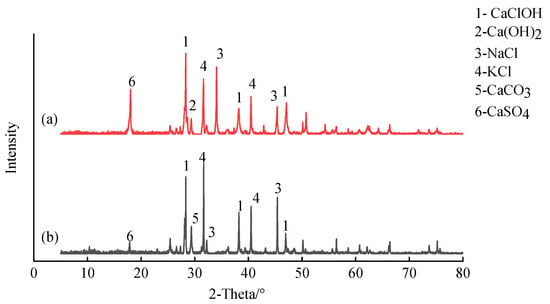
Figure 1.
XRD pattern of MSWIFA from (a) Nanning and (b) Hezhou, China.

Table 2.
Chemical composition (by wt%) of MSWIFA from different countries and regions [38,39,40,41,42,43,44].
Table 2.
Chemical composition (by wt%) of MSWIFA from different countries and regions [38,39,40,41,42,43,44].
| Country/ Region | Remarks | CaO | Na2O | SiO2 | MgO | Fe2O3 | Al2O3 | SO3 | Cl− |
|---|---|---|---|---|---|---|---|---|---|
| FA, Hezhou | China | 35.7 | 6.5 | 2.5 | 1.6 | 0.8 | 0.6 | 4.0 | 31.5 |
| FA, Nanning | 35.0 | 10.7 | 2.2 | 1.8 | 0.6 | 0.5 | 6.0 | 23.8 | |
| FA, Dalian | 45.3 | 9.9 | 2.1 | 1.2 | 0.8 | 0.4 | - | 21.5 | |
| FA, Suzhou | 39.1 | 3.4 | 16.0 | 1.6 | 1.9 | 4.4 | 7.2 | 11.9 | |
| FA, Shanghai | 23.4 | 4.0 | 24.5 | 2.7 | 4.0 | 7.4 | 12.0 | 10.0 | |
| FA, Japan | - | 13.9 | 17.2 | 12.0 | 2.6 | 1.2 | 8.1 | - | 14.9 |
| FA, Denmark | - | 13–40 | 9–21 | 4–5 | 0.7–1.3 | 0.7–1.1 | 1–4 | 7–35 | 3–22 |
| FA, American | - | 2.2 | 2.9 | 6.5 | - | 3.3 | 0.4 | 2.9 | 33.2 |
| OPC, Hezhou | China | 51.9 | 0.3 | 18.2 | 1.3 | 3 | 4.6 | 3.1 | - |
2.2.3. Environmental Characteristics
This section presents the heavy metal content of FA from different cities and countries. As shown in Table 3, Heavy metals with high contents in FA are typically Zn, Cu, Pb, Cd, and Cr. Among these heavy metals, Pb and Zn content is obviously higher, followed by Cu, Cd, and Cr. As heavy metal content is mainly affected by region and waste sources, Ni and Hg are mainly found in Shanghai, Korea, Singapore, and America, most of which are highly developed areas. Moreover, differences in heavy metal contents are greatly affected by waste incineration temperatures and the migration characteristics of heavy metals under high temperatures.

Table 3.
Heavy metal content of MSWIFA from different countries and regions [45,46,47,48,49].
During the waste incineration process, approximately 33% of lead ions (Pb) and more than 90% of cadmium ions (Cd) remain in the FA. Hezhou’s FA had the highest Cd content among all of the listed sources. This finding might be caused by the high retention rate of electric bicycles and the lower manufacturing level, as Hezhou is an undeveloped region of Western China. As a volatile heavy metal, Cd reacts with chloride ions to form metal chlorides or complexes, which leach in the presence of water. This phenomenon pollutes the surrounding environment and harms people.
In summary, Hezhou’s FA is high in chloride and cadmium ions (Cl and Cd), making it difficult to process as a resource. This situation means that new treatments are needed to ensure the strength and leaching characteristics of FA.
3. Methods
3.1. Fly Ash Pre-Treatment Methods
3.1.1. Water-Washing
Deionised water at room temperature was used for the water-washing. The solid–liquid ratio was set to 1:8, and a hand-held mixer was used for washing twice at 250 rpm. The samples were first washed for 5 min and soaked for 6 h. Then, they were washed for 10 min and soaked for 12 h, which removed as much of the chloride as possible. After water-washing, the supernatant was removed and the FA was dried at 105 °C for 24 h.
3.1.2. Washing by Ferrous Sulphate and Phosphoric Acid
The solid–liquid ratio, washing temperature, stirring speed, and washing times were the same as for water-washing. First, a phosphoric acid (H3PO4) solution with a mass fraction of 4% was used to wash the sample for 20 min, and the solution’s pH was controlled within the range of 4–5. Second, a ferrous sulphate (FeSO4) solution with a mass fraction of 2/4/6% was used to wash the sample for 20 min, which was then soaked for 1 h. The treatments after washing were consistent with the water-washing method.
3.1.3. Organic Chelation
The organic chelating agent was taken from the same place as the FA. The chelating agent content was set to 1.25/2.5/5%. First, a certain amount of FA was weighed in a plastic box and the chelating agent was diluted by deionised water. Then, the FA and chelating agent were mixed using a glass mixing rod until the FA colour changed completely from grey white to dark yellow. Finally, the plastic box was sealed and placed in a normal temperature environment for 48 h. After that, the FA was taken out and dried at 105 °C for 24 h. Then, it was crushed, screened to 5 mm, and sealed.
3.1.4. Adding Sugarcane Ash
Sugarcane ash is a byproduct of sugarcane obtained from sugar production. It was obtained from the dust removal device in a thermal power station. Sugarcane ash was selected to improve the FA silica (SiO2) content, as SiO2 can make up as much as 78% of sugarcane ash, and the sugar industry in Guangxi is well developed. The material was taken from a sugar factory in Nanning, Guangxi Zhuang Autonomous Region, China. The silica contents in the FA and the OPC were compared, and the sugarcane ash content was calculated to be 4/8/12%. The sugarcane ash was mixed evenly with the FA before use.
3.2. Experimental Methods
3.2.1. Sample Preparation
Before preparing the FA cement soil sample, all the raw materials were dried at 105 °C for 24 h. All the preparation processes followed China’s Specification for the Mix Proportion Test of Cement-Mixed Soil (JGJ/T 233-2011). First, certain amounts of FA, cement, and sandy soil were weighed and evenly mixed according to a predetermined ratio (see Table 4). Water was added and the samples were mixed at 250 rpm for 10 min to make a cement soil slurry. Then, the slurry was poured into a cube test mould with a length, width, and height of 70.7 mm3 and placed on the shaking table for 2 min to remove bubbles. Finally, the test mould was sealed with plastic film at a normal temperature (20 ± 2 °C) and in a normal humidity environment. After 24 h, the samples were demoulded and cured for 7, 14 and 28 days for testing (see Figure 2a).

Table 4.
Design of fly ash cement-stabilized soil parameters.
Figure 2.
Photograph of sample and test instruments.
3.2.2. UCS Test
The UCS test was carried out in accordance with China’s Standard for the Test Method of Performance Building Mortar (JGJ/T70-2009). A TJW-1000 electro-hydraulic servo rock multifunctional testing machine was used as the loading machine. A TM–Ⅲ mortar deformation modulus tester and displacement meter were chosen to record the deformation of the cement soil during compression. During the test, two square cushion blocks with side lengths of 70.7 mm and thicknesses of 20 mm were used to clamp the cement soil test block in the middle, and a ball support was placed on the upper cushion block to make the cement soil stress uniform and ensure that the displacement metre was in equilibrium at the start of the pressurisation (see Figure 2b). The test was performed at a loading rate of 0.15 KN/s, and ended when the peak intensity appeared and the axial strain reached 6%.
3.2.3. Heavy Metal Leaching Test
The heavy metal leaching test was carried out in accordance with the Solid Waste-extraction Procedure for Leaching Toxicity—Acetic Acid Buffer Solution Method (HJ/T 300-2007). An extraction fluid with a pH of 2.64 was prepared with glacial acetic acid. Two grams of the sample were dried, crushed, and screened to 9.5 mm. The sample was then mixed with the extraction fluid in a centrifuge tube with an L/S ratio of 20. The centrifuge tubes were placed in a flip shaker for 18 h at 30 rpm (see Figure 2c). Then, the sample was centrifuged for 4 min at 4000 rpm speed and the supernatant was taken out, filtered, and acidified. Finally, the sample in the centrifuge tubes was analysed using an inductively coupled plasma mass spectrometer (ICP-MS, ICAP7000, Thermo Fisher Scientific, USA).
3.3. Analytical Methods
An S-3400N electron microscope was used for the microscopic test, with the magnification set to 5000 (see Figure 2d). A series of pre-treatments was conducted before the test. First, a few samples of the fresh FA cement soil were cut with lengths and widths of 2 mm and heights of 1 mm to complete the UCS test. Then, the samples were dried, frozen in liquid nitrogen for 3 h, and vacuumed for 24 h. Finally, the samples were glued to a storage plate in sequence, connected to the plate surface with conductive adhesive, and sprayed with gold for testing.
4. Results and Discussion
4.1. MSWIFA Behaviour in Cement Stabilisation of Sandy Soil
4.1.1. UCS and Leaching Characteristics
This section shows the test results for the UCS and heavy metal leaching concentrations of the cement soil cured for 7 days, 14 days, and 28 days under different FA cement mixture ratios. Figure 3 shows the UCS and heavy metal leaching concentrations in a line and a histogram, respectively. The peak strength of the FA cement soil typically increased along with the curing age and decreased as the FA content increased. Moreover, the cement soil strength under the air-curing condition was obviously greater than that under the water-curing condition. This result indicates that underground water plays an important role in cement soil strength.
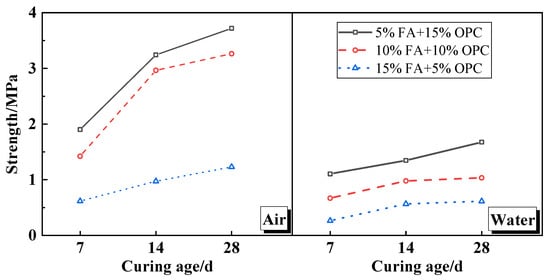
Figure 3.
UCS characteristics of cement-stabilized soil mixed with MSWIFA.
When the FA content increased by 5% (from FA5%–C15%–S80% to FA10%–C10%–S80%, cement (C); sandy soil (S)), its UCS decreased by between 24.82% and 46.64%. However, this change noticeably decreased as the curing age increased. This result implies that FA has a certain pozzolanic activity and produces the hydration product C–S–H. Moreover, FA’s pozzolanic activity was lower than was that of the cement. With another 5% increase in FA (FA15%–C5%–S80%), its UCS decreased dramatically by approximately another 50%. This result means that too much FA hinders the development and production of C–S–H in the soil skeleton, resulting in the slow growth of the cement soil strength. Generally, in this situation, part of the cement can be replaced with MSWIFA.
Figure 4 shows that the leaching ion concentrations of Cu, Zn, Pb, and Cd generally increased along with FA content. Unlike the UCS, the leaching concentrations of each ion were less affected by age. The leaching concentrations of Cu and Zn were well below the limit value of the entry standard (GB 16889-2008) for domestic landfill waste (40 and 100 mg/L, respectively) under all the mix proportions. However, the Pb and Cd concentrations did not meet this standard (0.25 and 0.15 mg/L, respectively). This result is because Pb and Cd occur more easily in a weak acid-extractable state. Acetic acid is a weak acid that continuously releases hydrogen ions in water and promotes the continuous dissolution of heavy metal ions, while Pb and Cd have relatively low limitation values. The results show that there is a risk of Pb and Cd leaching when the sand is solidified with FA and cement in an acetic acid environment. Certain heavy metals in the MSWI fly ash, such as Cr6+, Cd2+, Pb2+, and Cu2+, could dramatically accelerate C3S hydration in both the late and initial stages of hydration reaction, while Zn2+ only promotes C3S hydration during the later period of hydration.
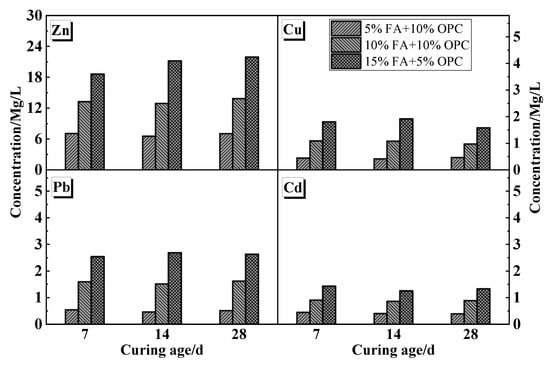
Figure 4.
Heavy metal leaching characteristics of cement-stabilized soil mixed with MSWIFA.
4.1.2. Engineering Characteristic Mechanisms
The stress–strain curve, elastic moduli, and deformation moduli of the cement soil at different ages are shown in Figure 5. The black solid line represents cement-stabilised soil with 5% FA + 15% cement (F5C15). The blue dotted line represents cement-stabilised soil with 5% pre-treatment FA (water-washing) + 15% cement (Fw5C15). The red dashed line represents the cement-stabilised soil with 15% cement (F0C15).
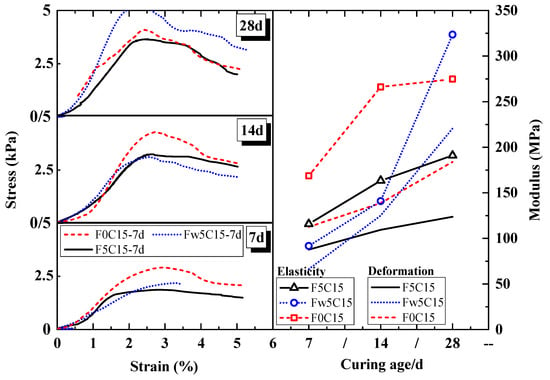
Figure 5.
Stress–strain curve, moduli and ages of cement-stabilized soil mixed with MSWIFA.
At 7–14 curing days, the peak strength, deformation modulus, and elastic modulus of F0C15 were obviously the highest, followed by Fw5C15. Typically, they differed by no more than 25%. At 28 curing days, the peak strength, deformation modulus, and elastic modulus of F0C15 were the highest. Moreover, although the F5C15 had the weakest behaviour, the gap between the F5C15 and F0C15 was increasingly small. This result is because water-washing greatly decreased the chlorine salt and free-CaO in the FA, improved its potential cementitious activity, and reduced the obstruction of impurities in cement hydration.
Additional FA delayed the strength increase of the stabilised soil within the curing time. Moreover, water-washing of the FA increased the behaviour of the stabilised soil in the later stages of curing. Thus, appropriate chlorine pre-treatment in FA enhances the resource recovery of MSWIFA. Figure 6 displays scanning electron microscope images of FA soil–cement and pure sandy soil–cement under different mixing ratios. Significant structural differences were found between the FACS and the pure cement-stabilised soil (PCS). For example, the PCS had a layered structure with obvious soil particles and with the hydration products and soil particles connected closely. Only a few pores occurred in the PCS, and they were small. This result is because the soil particles were surrounded by the cement hydration products. The growth of the C–S–H gel material between the soil particles was sufficient. Thus, its overall compactness was high. However, the FACS had small pores and obvious fracture zones, and the structure was porous and loose. This structure resulted in low adhesion among the soil particles. Thus, the FACS was weaker than the PCS.
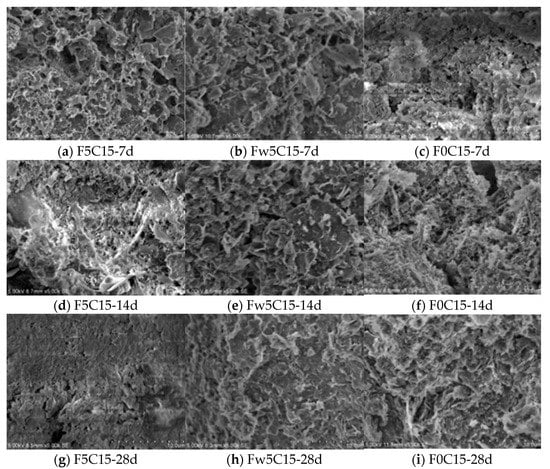
Figure 6.
SEM images of cement-stabilized soil mixed with MSWIFA.
Generally, when the mixed amount of FA was increased, its internal floccule content obviously increased, and the soil particle surface leached amorphous material. This result is due to the chlorine salt content being higher in the FA after dehydration, resulting in crystallisation in the soil particle surface. Moreover, chlorine salt led to an ettringite reaction and produced Friedel’s salt, which led to more flake and needle cement in the surface.
4.2. MSWIFA Pre-Treatment with High Chlorine
This approach aims to enhance the resource recovery of MSWIFA through appropriate chlorine pre-treatment, such as ferrous sulphate, organics, and sugarcane ash. Unless stated otherwise, the mixture ratio of the stabilised soil was 10% FA, 10% cement, and 80% soil (F10C10).
4.2.1. Pre-Treatment with Ferrous Sulphate
Phosphoric acid (H3PO4) and ferrous sulphate (FeSO4) solutions were adopted during the washing process, as previously summarised in Section 3.1.2. Regarding the UCS, the peak strength of the FA cement soil (the red line with square and blue line with circle) gradually increased with the washing concentration of ferrous sulphate (Figure 7) and the curing age in the air condition. This result implies that pre-treatment by ferrous sulphate washing has little influence on FACS strength. Under the water-curing condition, the increment of the stabilised soil’s UCS with the curing time was rather small (the blue line with circle). In actual engineering, stabilised soil with ferrous sulphate washing achieves its peak strength after a short curing time. This is because H3PO4 effectively removes chloride ions from the FA, reducing the reaction between chloride salt and cement hydration products (producing Friedel’s salt).
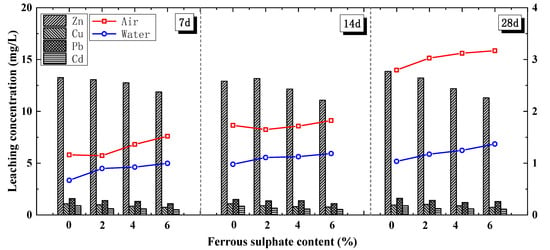
Figure 7.
UCS and leaching characteristics of FACS with ferrous sulphate.
Regarding the leaching characteristics, the leaching ion concentrations of the Cu, Zn, Pb, and Cd (Figure 7 with bar chart) decreased steadily as the FeSO4 increased. As the FeSO4 concentration increased from 2% to 6%, the decrease in the Pb concentration increased from 11.80% to 30.95%, while the decrease in the Cd concentration increased from 32.15% to 39.93% and the decreases in the Zn and Cu concentration reached peak values of 16.48% and 29.23%, respectively. Because it required the least amount, FeSO4 had a better stabilisation effect on Pb, whereas more FeSO4 was required to achieve a better effect on Cd. Generally, significant Ca quantities are needed for the skeletal structure, to replace the Fe2+ sites, and in order to form Ca4Fe9O17 in MSWIFA with ferrous sulphate. Ca4Fe9O17 on the particle surfaces in the MWSIFA prevents the dissolution of the Pb [50].
4.2.2. Pre-Treatment with Organic Chelating Agent
The organic chelating agent was added to the FA before mixing with the cement and soil using the process detailed in Section 3.1.3. As for the UCS, the peak FACS strength (the lines in Figure 8) generally increased along with the organic content and curing age. This result shows that the cement soil strength was slightly hindered by the addition of FA pre-treated with an organic, and this negative effect weakened as the amount of organic increased. This result might be because the macromolecular protein and the organic’s viscosity had a strong hindering effect on cement hydration. More specifically, the organic forms a film on the FA surface that hinders the contact between the FA and the water, resulting in greater difficulty in releasing the FA’s pozzolanic activity.
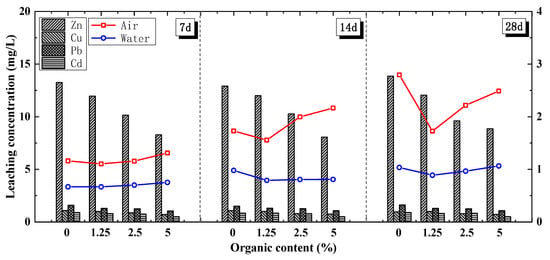
Figure 8.
UCS and leaching characteristics of FACS with organic chelating agent.
Regarding the leaching characteristics, the leaching ion concentrations of Cu, Zn, Pb, and Cd consistently decreased as organic content increased, as shown in Figure 8 (bar chart). As organic content increased from 1.25% to 5%, the leaching concentrations of Pb decreased by 19.02%, 20.90%, and 34.84%, while those of Cd decreased by 11.01%, 16.57%, and 43.27% and those of Zn and Cu decreased by more than 30%. The organic chelating agent significantly reduced the content of heavy metals.
4.2.3. Addition of Sugarcane Ash
As shown by the line in Figure 9, the 28-day UCS results of the FACS with 8% and 12% sugarcane ash were respectively 4.56% and 6.24% higher than those of the F10C10 in air-curing. In addition, the 28-day UCS results of the FACS with 4%, 8%, and 12% sugarcane ash content were 41.08%, 43.51%, and 49.07% higher, respectively, than those of the F10C10 with water curing. The FACS UCS obviously increased with the sugarcane ash content and curing age, which was a different result than for pre-treatment with ferrous sulphate washing or mixing with organics. This result implies that the sugarcane ash effectively improved the strength of the cement soil. Furthermore, because of the high SiO2 content in the sugarcane ash, a large number of internal active substances were released, stimulating the activity of the FA and promoting secondary hydration of the cement.
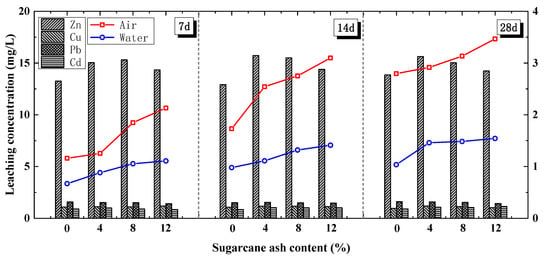
Figure 9.
UCS and leaching characteristics of FACS with sugarcane ash.
Figure 9 (bar chart) shows that the leaching ion concentrations of Cu, Zn, Pb, and Cd increased slightly with the sugarcane ash content. This result implies that additional sugarcane ash has negative effects on ion leaching.
4.2.4. Pre-Treatment Summary
The effects of pre-treatment of the FA on the resulting cement soil are briefly summarised in this section. Based on our investigations, a number of recommendations are provided, which may be useful for future analytical and statistical studies.
FACS peak strength and leaching concentrations after pre-treatment have been previously investigated. Thus, this section further summarises their similarities and differences in Table 5. In this table, the peak strength is divided into two parts (air and water), and the leaching concentration is divided into four parts (Zn, Cu, Pb, and Cd). The evaluation criteria are PE, NE, LC, NS, DWC, IWC, LCA, and LCA. Among these, compared with the untreated case, PE (positive effects) indicates that pre-treatment promoted strength or inhibited heavy metal leaching, and NE (negative effects) represents the opposite situation. LC (little change) indicates that pre-treatment had little influence on strength or leaching. NS (not significant) indicates that there was no relationship between strength or leaching and pre-treatment. DWC (decrease with content) and IWC (increase with content) indicate that the strength or leaching concentration decreased or increased, respectively, with the admixture contents (ferrous sulphate, organic chelating agent, and sugarcane ash). LCA (little change with age) and IWA (increase with age) indicate that the strength or leaching concentration changed little or increased with age, respectively.

Table 5.
Summary of pretreatment effect of fly ash cement soil, 28d.
In general, FACS strength increased with age and admixture content. Sugarcane ash and ferrous sulphate strengthened FA cement soil, while organics did not harm the strength. The positive effect of the sugarcane ash was better, indicating that the SiO2 in sugarcane ash was the most important factor promoting the strength of cement soil. On the other hand, the leaching concentration changed little with age. In most cases, the ferrous sulphate and organic chelating agent helped to stabilise the heavy metals; however, the sugarcane ash increased the leaching concentrations of Zn, Cu, and Cd to an extent.
5. Conclusions
This study experimentally investigated the UCS and leaching characteristics of FA cement soil under different mix proportions, ages, and curing methods. The FACS strength and environmental characteristics were researched with ferrous sulphate, organic chelation agent, and sugarcane ash. The major conclusions are summarised as follows:
- (1)
- The basic physical, chemical, and environmental characteristics of FA from Guangxi were systematically tested and compared with samples from different countries and other Chinese cities. The FA had similar characteristics overall. Moreover, MSWIFA from Hezhou, China was slightly different from other sources of MSWIFA. The chloride ion content of Hezhou’s FA (31.5%) was significantly higher than that from most Chinese cities, as well as that from the United States, Japan, and Italy.
- (2)
- The peak strength of the FA cement soil typically increased along with the curing age and decreased as the FA content was increased. This is because the MSWIFA produced hydrating C–S–H and had a certain pozzolanic activity, which is lower than that of cement. Chloride ions and heavy metals hinder C–S–H development and production in the soil skeleton, meaning that the cement soil strength only grew slowly.
- (3)
- The leaching ion concentrations of Cu, Zn, Pb, and Cd generally increased along with the FA content. The Cu and Zn were well below the limit value of the entry standards of domestic landfill waste under all the mix proportions. In an acetic acid environment, Pb and Cd may leach when sand is solidified with untreated cement and FA.
- (4)
- After water washing and ferrous sulphate washing, FACS strength development speed obviously increased in the later stage of curing. Appropriate FA pre-treatment with chlorine enhanced MSWIFA resource recovery. This was because water washing and ferrous sulphate washing greatly decreased the chlorine salt and free-CaO in the FA, improved its potential cementitious activity, and reduced obstructions due to impurities in cement hydration.
- (5)
- Ferrous sulphate had a better stabilisation effect on Pb and Cd. As the ferrous sulphate concentration increased from 2% to 6%, the decrease in Pb concentration increased from 11.80% to 30.95%, while the decrease in Cd concentration increased from 32.15% to 39.93%. Moreover, the organic chelating agent significantly reduced each of the heavy metals. This process ensures that MSWIFA can be reused.
- (6)
- Although the cement soil strength was slightly hindered by the addition of an organic chelating agent, the FACS UCS obviously increased with the sugarcane ash content. The 28-day UCS results of the FACS with 4%, 8%, and 12% sugarcane ash content were 41.08%, 43.51%, and 49.07% higher, respectively. Thus, a portion of cement can be replaced with MSWIFA that has undergone suitable pre-treatments.
In the future, the costs of harmless treatment processes are expected to decrease through continual in-depth research. This should further promote the practical application of MSWIFA in sandy and soft soil foundation reinforcement engineering.
Author Contributions
Conceptualization, Z.L., J.L. and X.Z.; Methodology, X.Z.; Software, L.H.; Validation, X.Z. and H.L.; Formal analysis, Z.L. and H.L.; Investigation, L.H. and X.Z.; Resources, Z.L. and S.D.; Data curation, H.L.; Writing—original draft, L.H.; Writing—review & editing, X.Z.; Visualization, H.L.; Supervision, Z.L.; Project administration, J.L. and S.D.; Funding acquisition, Z.L., J.L. and X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (42007250), Systematic Project of Guangxi Key Laboratory of Disaster Prevention and Engineering Safety (2019ZDK029), Key Scientific and Technological Project of Ministry of Transport of the People’s Republic of China (2020-MS4-110).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request from the authors.
Acknowledgments
This work was supported by the Key Scientific and Technological Project of the Ministry of Transportation of the People’s Republic of China (Grant No. 2020-MS4-110), the National Natural Science Foundation of China (Grant No. 42007250), and the Systematic Project of Guangxi Key Laboratory of Disaster Prevention and Engineering Safety (Grant No. 2019ZDK029).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dong, J.; Chi, Y.; Zou, D.; Fu, C.; Huang, Q.; Ni, M. Energy-environment-economy assessment of waste management systems from a life cycle perspective: Model development and case study. Appl. Energy 2014, 114, 400–408. [Google Scholar] [CrossRef]
- Milbrath, M.O.; Wenger, Y.; Chang, C.-W.; Emond, C.; Garabrant, D.; Gillespie, B.W.; Jolliet, O. Apparent Half-Lives of Dioxins, Furans, and Polychlorinated Biphenyls as a Function of Age, Body Fat, Smoking Status, and Breast-Feeding. Environ. Health Perspect. 2009, 117, 417–425. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, L.; Zhou, J.; Liu, J.; Qian, G.; Ohtsuka, N.; Motegi, M.; Oh, K.; Hosono, S. Characteristics of dioxins content in fly ash from municipal solid waste incinerators in China. Chemosphere 2013, 92, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Min, X.-B.; Wang, Z.-X.; Pang, Z.-H.; Liang, Y.-J.; Ke, Y. Health and ecological risk assessment of heavy metals pollution in an antimony mining region: A case stu-dy from South China. Environ. Sci. Pollut. Res. 2017, 24, 27573–27586. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, J.; Zhu, G.; Zhang, D.; Zhu, Y.; Chen, Z.; Li, J.; Zhang, H.; Tang, J.; Nie, J.; et al. PCDD/Fs distribution characteristics and health risk assessment in fly ash discharged from MSWIs in China. Ecotoxicol. Environ. Saf. 2017, 139, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Huet, B.; L’Hostis, V.; Miserque, F.; Idrissi, H. Electrochemical behavior of mild steel in concrete: Influence of pH and carbonate content of concrete pore solution. Electrochim. Acta 2006, 51, 172–180. [Google Scholar] [CrossRef]
- Birninyauri, U.A.; Garba, S. Effect of Mechanism of Chloride Ion Attack on Portland Cement Concrete and the Structural Steel Reinforcement. Res. J. Appl. Sci. 2007, 8, 131–135. [Google Scholar]
- Inseok, Y. Binding Behavior of Chloride Ion to React with Cement Hydration Products. J. Am. Chem. Soc. 2010, 101, 1634–1635. [Google Scholar] [CrossRef]
- Jin, S.H.; Yang, H.J.; Hwang, J.P.; Ann, K.Y. Corrosion behaviour of steel in CAC-mixed concrete containing different concentrations of chloride. Constr. Build. Mater. 2016, 110, 227–234. [Google Scholar] [CrossRef]
- Ghazy, A.; Bassuoni, M.T. Resistance of concrete to different exposures with chloride-based salts. Cem. Concr. Res. 2017, 101, 144–158. [Google Scholar] [CrossRef]
- Gao, X.; Wang, W.; Ye, T.; Wang, F.; Lan, Y. Utilization of washed MSWI fly ash as partial cement substitute with the addition of dithiocarbamic chelate. J. Environ. Manag. 2008, 88, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Inoue, K.; Harada, H.; Kawakita, H.; Ohto, K. Leaching behavior of heavy metals with hydrochloric acid from fly ash generated in municipal waste incineration plants. Trans. Nonferr. Met. Soc. China 2011, 21, 1422–1427. [Google Scholar] [CrossRef]
- Bai, J.J.; Zhang, Z.Q.; Yan, D.H.; Li, L. Study on the removal of chlorine and heavy metals in incineration fly ash during water-washing process. Environ. Eng. 2012, 30, 104–108. (In Chinese) [Google Scholar] [CrossRef]
- Chen, W.-S.; Chang, F.-C.; Shen, Y.-H.; Tsai, M.-S.; Ko, C.-H. Removal of chloride from MSWI fly ash. J. Hazard. Mater. 2012, 237–238, 116–120. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.D.; Li, Y.L.; Wei, L.H. Release of Soluble Salts and Heavy Metals during the Short-Time Washing Process of MSWI Fly Ash. Adv. Mater. Res. 2012, 518–523, 3247–3251. [Google Scholar] [CrossRef]
- Wang, X.; Li, A.; Zhang, Z. The Effects of Water Washing on Cement-based Stabilization of MWSI Fly Ash. Procedia Environ. Sci. 2016, 31, 440–446. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, S.; Liu, L.; Wang, X.; Zhang, Z. Application of washed MSWI fly ash in cement composites: Long-term environmental impacts. Environ. Sci. Pollut. Res. 2018, 25, 12127–12138. [Google Scholar] [CrossRef]
- Pan, J.R.; Huang, C.; Kuo, J.-J.; Lin, S.-H. Recycling MSWI bottom and fly ash as raw materials for Portland cement. Waste Manag. 2008, 28, 1113–1118. [Google Scholar] [CrossRef]
- Ning, S.; Haiyang, W.; Huanan, W.; Hac, K.J.; Qiyong, X. Study of compressive strength and leachability of cement mortars containing municipal solid waste incineration fly ash. Chin. J. Environ. Eng. 2016, 10, 3207–3214. (In Chinese) [Google Scholar] [CrossRef]
- Lee, T.-C.; Chang, C.-J.; Rao, M.-K.; Su, X.-W. Modified MSWI ash-mix slag for use in cement concrete. Constr. Build. Mater. 2011, 25, 1513–1520. [Google Scholar] [CrossRef]
- Tyrer, M. Municipal solid waste incinerator (MSWI) concrete. Eco-Effic. Concr. 2013, 59, 273–310. [Google Scholar] [CrossRef]
- Rafieizonooz, M.; Mirza, J.; Salim, M.R.; Hussin, M.W.; Khankhaje, E. Investigation of coal bottom ash and fly ash in concrete as replacement for sand and cement. Constr. Build. Mater. 2016, 116, 15–24. [Google Scholar] [CrossRef]
- Zheng, L.; Gao, X.; Wang, W.; Li, Z.; Zhang, L.; Cheng, S. Utilization of MSWI fly ash as partial cement or sand substitute with focus on cementing efficiency and health risk assessment. Front. Environ. Sci. Eng. 2019, 14, 5. [Google Scholar] [CrossRef]
- Hayashi, T.; Ono, S. Immobilization of Lead in Fly Ash by Phosphate Compounds. Bunseki Kagaku 2019, 68, 65–69. [Google Scholar] [CrossRef]
- Liang, S.H.; Chen, J.T.; Guo, M.X. Utilization of pretreated municipal solid waste incineration fly ash for cement-stabilized soil. Waste Manag. 2020, 105, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Marruzzo, G.; Medici, F.; Panei, L. Characteristics and properties of a mixture containing fly ash, hydrated lime, and an organic additive. Environ. Eng. Sci. 2001, 18, 159–166. [Google Scholar] [CrossRef]
- Huang, W.J.; Lo, J.S. Synthesis and efficiency of a new chemical fixation agent for stabilizing MSWI fly ash. J. Hazard. Mater. 2004, 112, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, H.B.; Jin, X. An Analysis Summary of Urban Waste Incineration Technology in Our and Foreign Countries. Ind. Boil. 2003, 5, 15–19. [Google Scholar]
- Qiu, Q.; Jiang, X.; Chen, Z.; Lu, S.; Ni, M. Microwave-Assisted Hydrothermal Treatment with Soluble Phosphate Added for Heavy Metals Solidification in MS-WI Fly Ash. Energy Fuels 2017, 31, 5222–5232. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Z.; Fang, Z.; Qian, Y.; Zhong, P.; Yan, J. Review of harmless treatment of municipal solid waste incineration fly ash. Waste Dispos. Sustain. Energy 2020, 2, 25. [Google Scholar] [CrossRef]
- An, J.; Golestani, B.; Nam, B.H.; Lee, J.L. Sustainable Utilization of MSWI Bottom Ash as Road Construction Materials, Part I: Physical and Mechanical Evaluation. Airfld. Highw. Pavements 2015, 225–235. [Google Scholar] [CrossRef]
- Zhang, Y.; Soleimanbeigi, A.; Likos, W.J.; Edil, T.B. Geotechnical and Leaching Properties of Municipal Solid Waste Incineration Fly Ash for Use as Embankment Fill Material. Transp. Res. Rec. J. Transp. Res. Board 2016, 2579, 70–78. [Google Scholar] [CrossRef]
- Zhipeng, T.; Bingru, Z.; Chengjun, H.; Rongzhi, T.; Huangpu, Z.; Fengting, L. The physiochemical properties and heavy metal pollution of fly ash from municipal solid waste incineration. Process Saf. Environ. Prot. 2015, 98, 333–341. [Google Scholar] [CrossRef]
- Tang, Q.; Pan, L.L.; Gao, Y.F.; Chen, S. Strength and environmental behaviors of solidified fly ash under carbonation effect. Chin. J. Geotech. Eng. 2018, 40, 645–654. (In Chinese) [Google Scholar] [CrossRef]
- Liu, J.; Hu, L.; Tang, L. Utilisation of Municipal Solid Waste Incinerator (MSWI) Fly Ash with Metakaolin for Preparation of Alkali-Activated Cementitious Material. J. Hazard. Mater. 2020, 402, 123451. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hu, Y.; Cheng, H. Municipal solid waste (MSW) incineration fly ash as an important source of heavy metal pollution in China. Environ. Pollut. 2019, 252, 461–475. [Google Scholar] [CrossRef]
- Suthatta, D.; Suched, L.; Dao, J. Mechanisms of chloride and sulfate removalfrommunicipal-solid-waste-incinerationfly ash (MSWIFA): Effect of acid-base solutions. Waste Manag. 2020, 101, 44–53. [Google Scholar]
- Saikia, N.; Kato, S.; Kojima, T. Production of cement clinkers from municipal solid waste incineration (MSWI) fly ash. Waste Manag. 2007, 27, 1178–1189. [Google Scholar] [CrossRef]
- Cobo, M.; Gálvez, A.; Conesa, J.A.; de Correa, C.M. Characterization of fly ash from a hazardous waste incinerator in Medellin, Colombia. J. Hazard. Mater. 2009, 168, 1223–1232. [Google Scholar] [CrossRef]
- Shi, H.S.; Kan, L.L. Leaching behavior of heavy metals from municipal solid wastes incineration (MSWI) fly ash used in concrete. J. Hazard. Mater. 2009, 164, 750–754. [Google Scholar] [CrossRef]
- Guo, X.L.; Shi, H.S.; Huang, J.B. Effects of Cement Additives on Alinite Cement-Based Materials from Municipal Solid Waste Incineration (MSW-I) Fly Ash. Key Eng. Mater. 2017, 727, 1046–1053. [Google Scholar] [CrossRef]
- Ebert, B.A.R.; Steenari, B.-M.; Geiker, M.R.; Kirkelund, G.M. Screening of untreated municipal solid waste incineration fly ash for use in cement-based materials: Chemical and physical properties. SN Appl. Sci. 2020, 2, 802. [Google Scholar] [CrossRef]
- Wang, B.; Fan, C. Hydration behavior and immobilization mechanism of MgO-SiO2-H2O cementitious system blended with MSWI fly ash. Chemosphere 2020, 250, 126269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Chen, L.; Ma, B.; Zhang, Y.; Ni, W.; Tsang, D.C. Treatment of municipal solid waste incineration fly ash: State-of-the-art technologies and future perspectives—ScienceDirect. J. Hazard. Mater. 2021, 411, 125–132. [Google Scholar] [CrossRef]
- Shim, Y.S.; Rhee, S.W.; Lee, W.K. Comparison of leaching characteristics of heavy metals from bottom and fly ashes in Korea and Japan. Waste Manag. 2005, 25, 473–480. [Google Scholar] [CrossRef]
- Jin, M.T.; Huang, C.J.; Jin, Z.F. The Physical and Chemical Properties of Fly Ash fom Municipal Solid Wastes Incineration. Adv. Mater. Res. 2011, 194–196, 2065–2071. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Soluble salt removal from MSWI fly ash and its stabilization for safer disposal and recovery as road basement material. Waste Manag. 2012, 32, 1179–1185. [Google Scholar] [CrossRef]
- Tasneem, K.M.; Nam, B.H.; Eun, J. Sustainable Utilization of MSWI Bottom Ash as Road Construction Materials, Part II: Chemical and Environmental Characterization. Airfld. Highw. Pavements 2015, 2015, 593–604. [Google Scholar] [CrossRef]
- Xinghua, H.; Shujing, Z.; Hwang, J.Y. Physical and Chemical Properties of MSWI Fly Ash; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 451–459. [Google Scholar]
- Aziz, M.; Sheikh, F.N.; Qureshi, M.U.; Rasool, A.M.; Irfan, M. Experimental Study on Endurance Performance of Lime and Cement-Treated Cohesive Soil. Geotech. Eng. 2021, 25, 3306–3318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).