Evaluation of Agronomic Performance and Genetic Diversity Analysis Using Simple Sequence Repeats Markers in Selected Wheat Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Experimental Design
2.3. Parameters under Consideration
2.4. DNA Isolation from Leaf Samples
2.5. Polymerase Chain Reaction (PCR)
2.6. SSR Makers
2.7. Data Analysis
2.7.1. Statistical Analysis
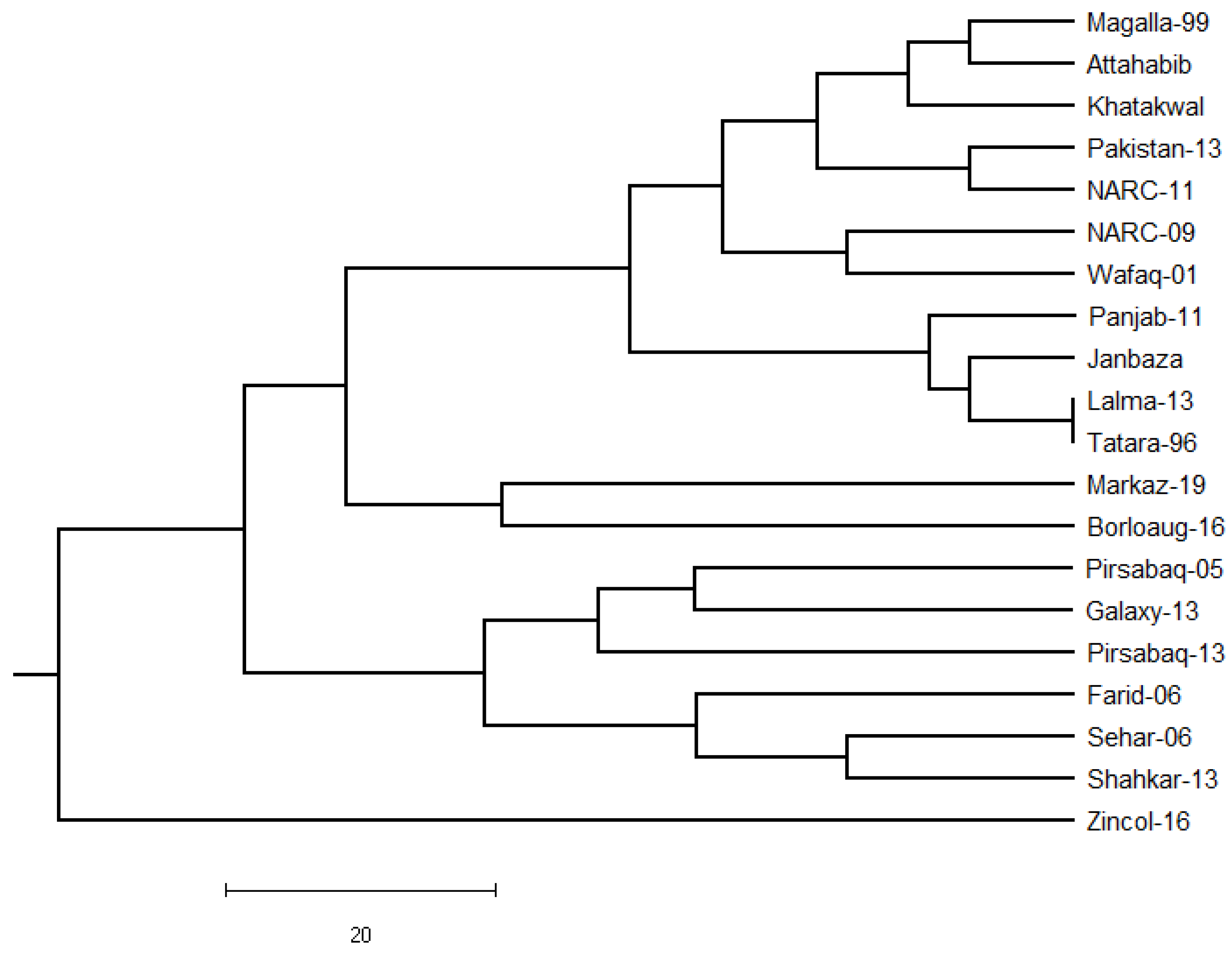
2.7.2. Phylogenetic Analysis
3. Results
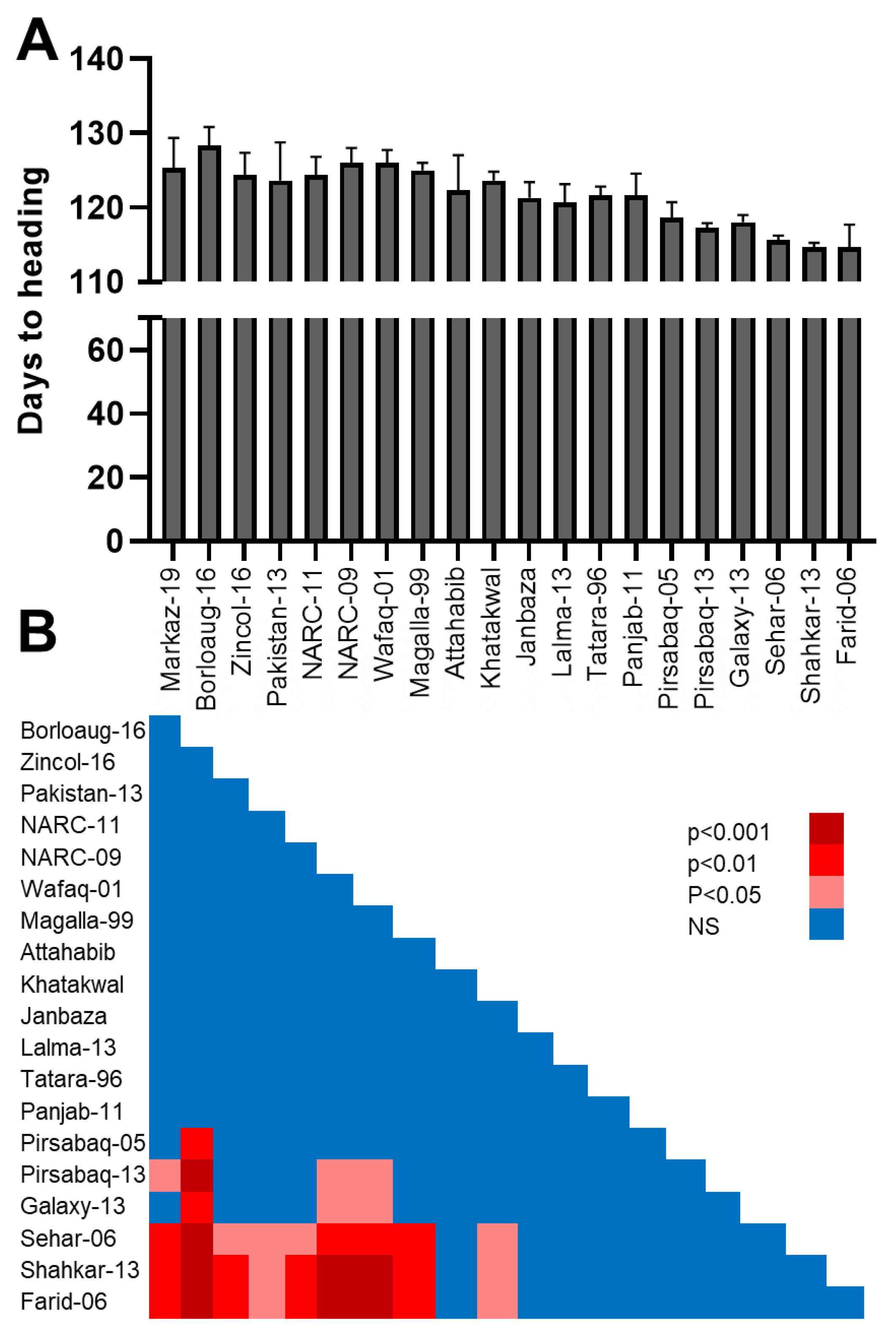
3.1. Variation in Days to 50% Heading
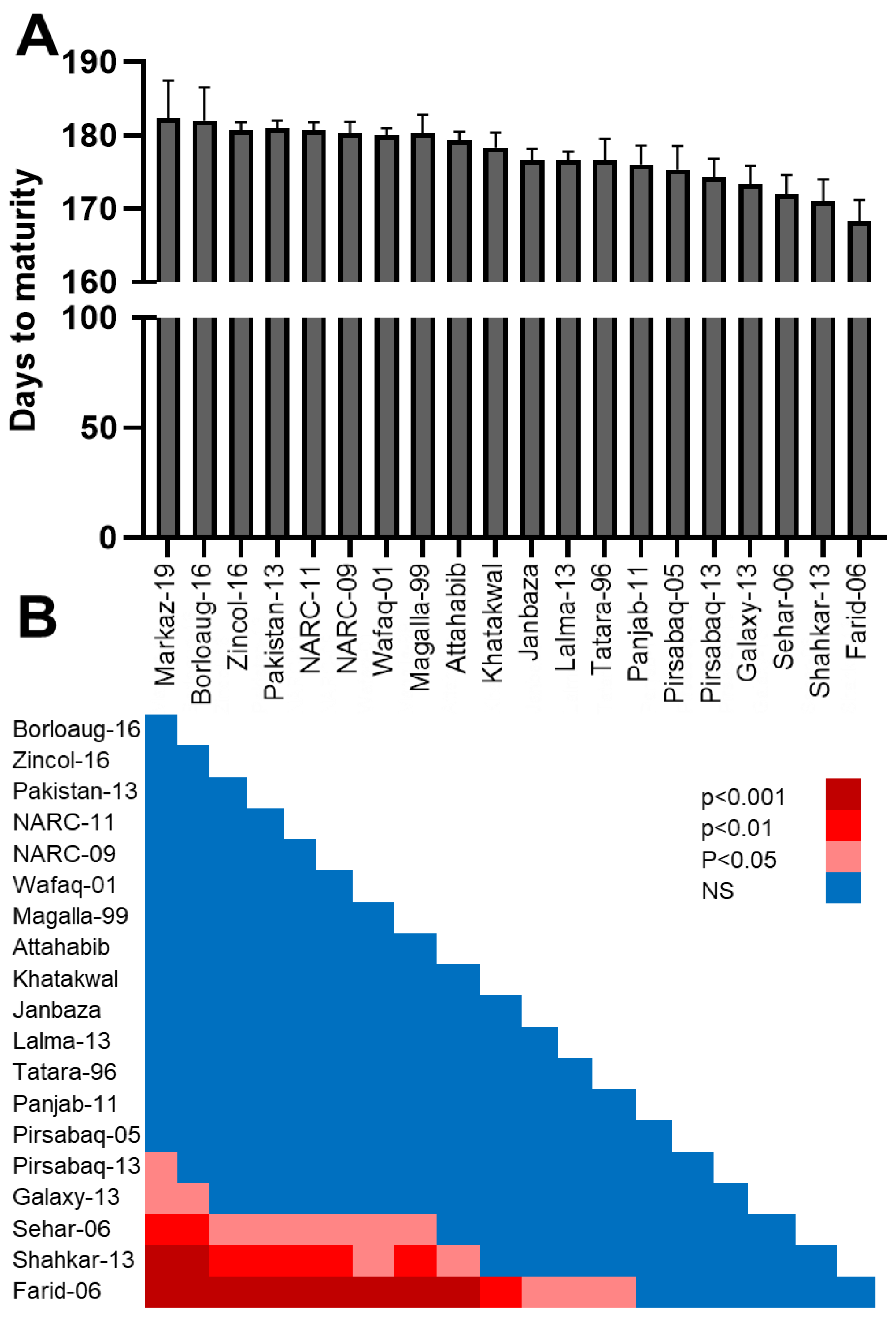
3.2. Days to Maturity in Different Wheat Lines
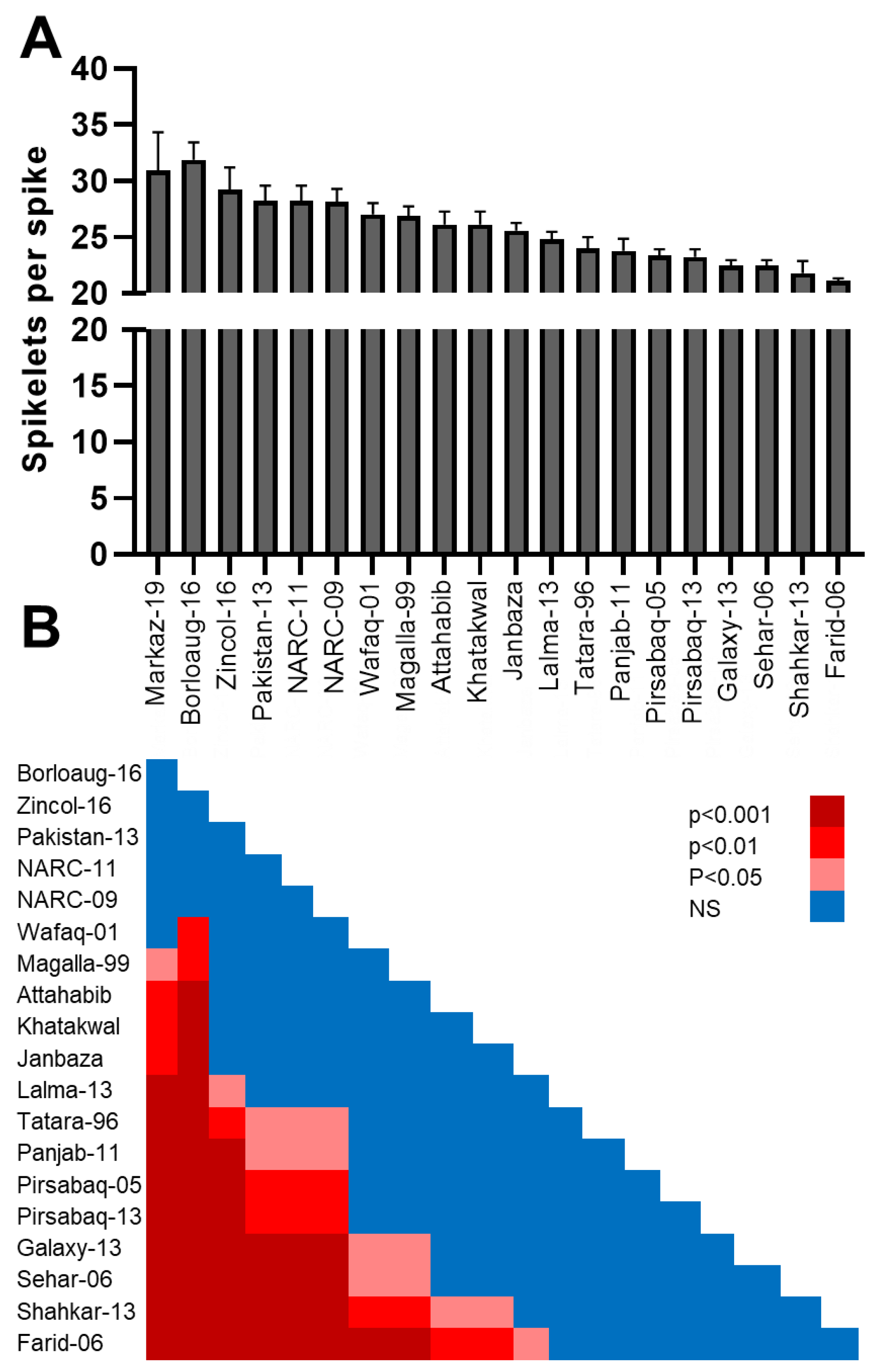
3.3. Number of Spikelets per Spike
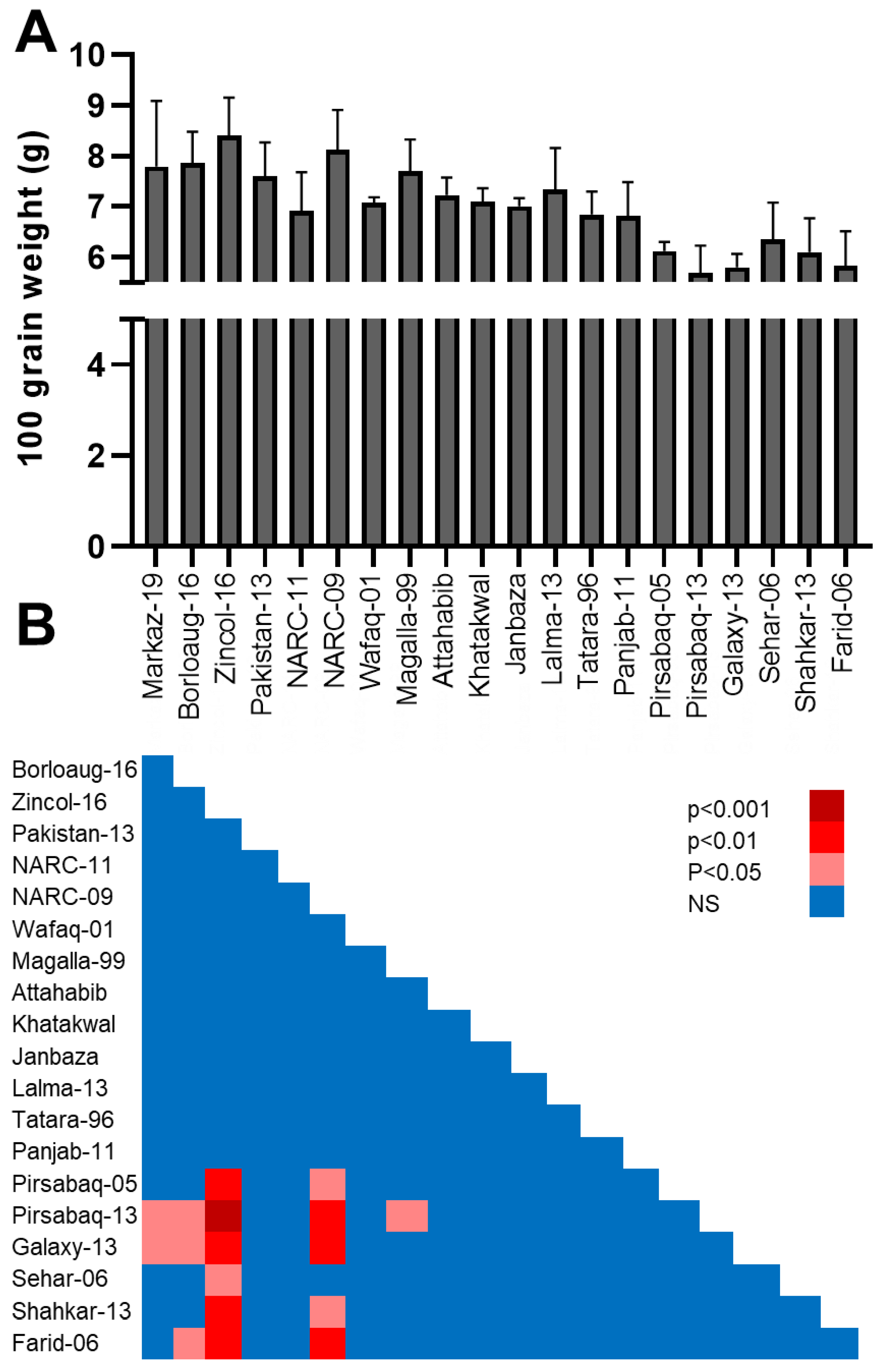
3.4. Hundred (100) Seeds Weight (g)
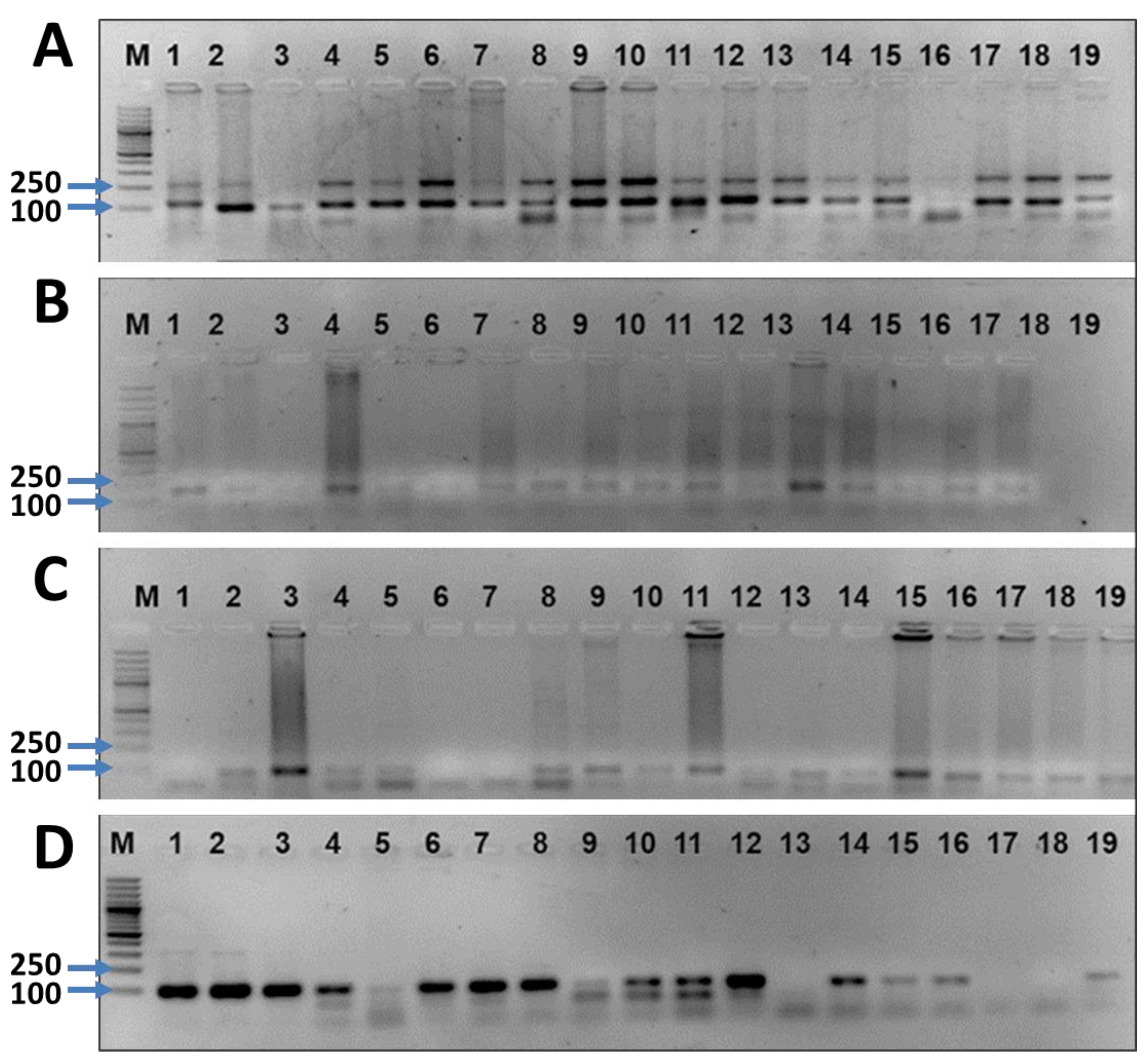
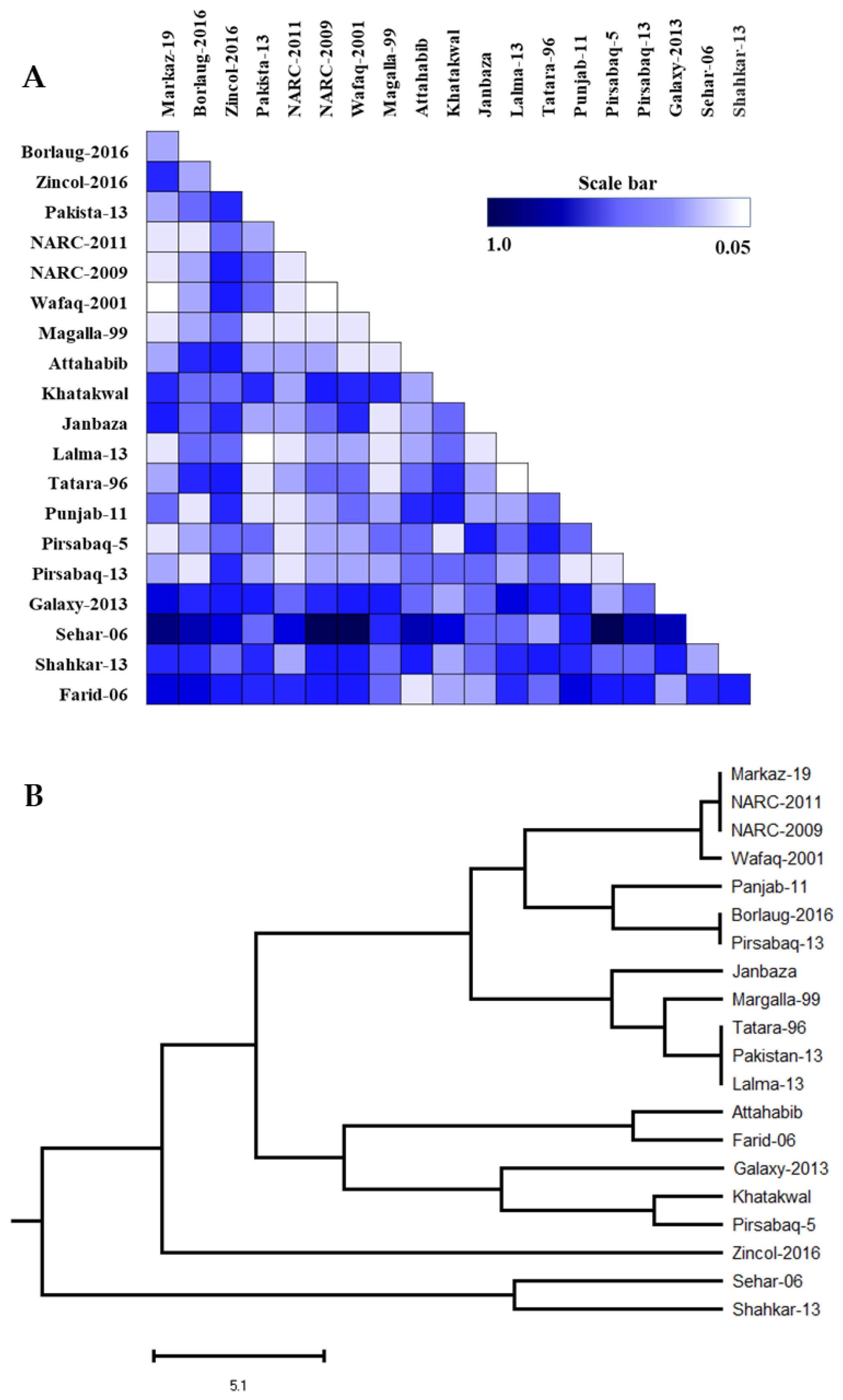
3.5. Diversity Assessment of 20 Wheat Lines Using SSR Markers
3.6. Genetic Distance
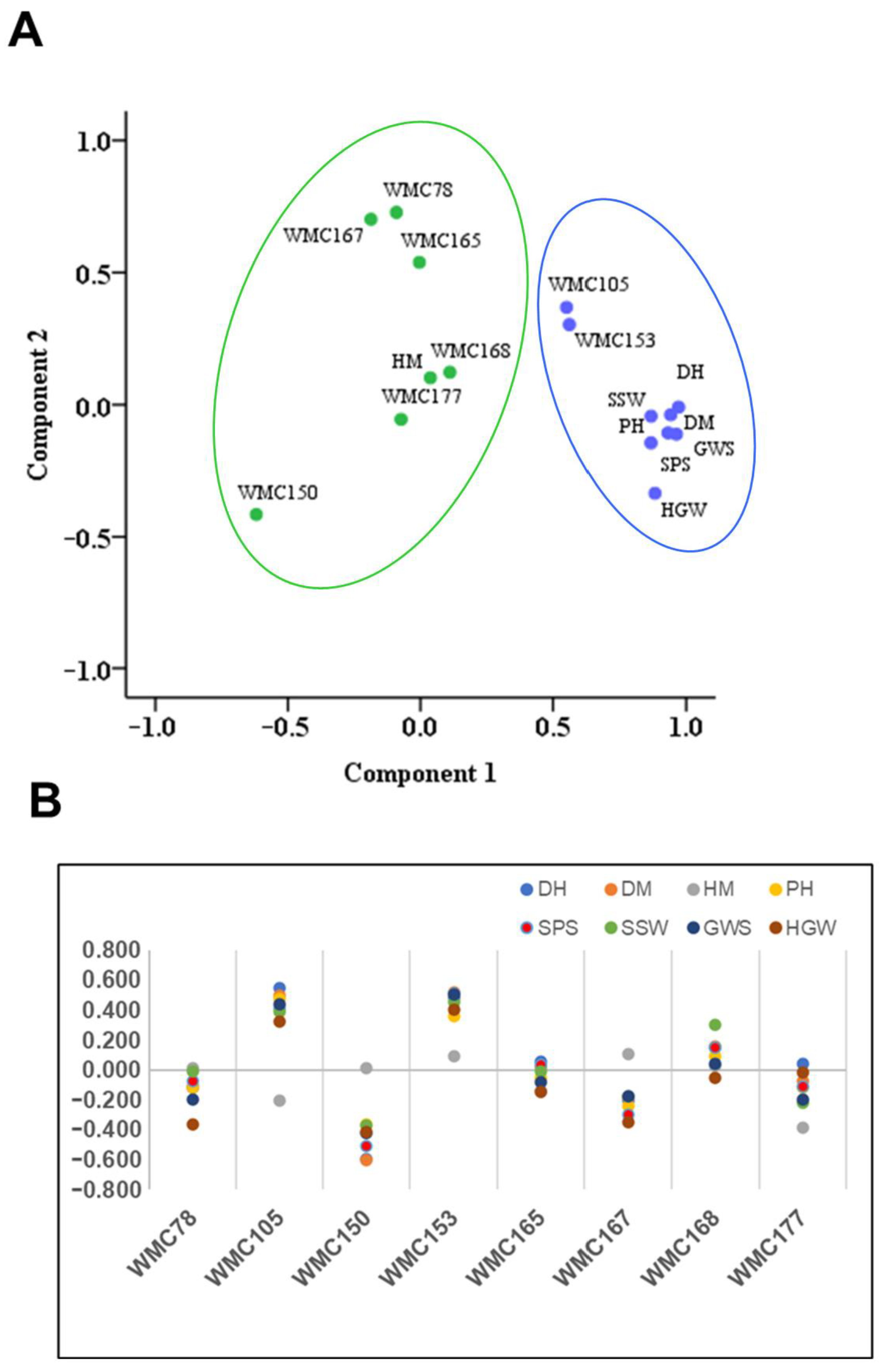
3.7. Correlation between SSR Markers and Morphological Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Saleem, M.H.; Ali, B.; Mussart, M.; Ullah, R.; Arif, M.; Ahmad, M.; Shah, W.A.; Romman, M. Comparative efficacy of phosphorous supplements with phosphate solubilizing bacteria for optimizing wheat yield in calcareous soils. Sci. Rep. 2022, 12, 11997. [Google Scholar] [CrossRef]
- Ahmad, M.; Ishaq, M.; Shah, W.A.; Adnan, M.; Fahad, S.; Saleem, M.H.; Khan, F.U.; Mussarat, M.; Khan, S.; Ali, B.; et al. Managing Phosphorus Availability from Organic and Inorganic Sources for Optimum Wheat Production in Calcareous Soils. Sustainability 2022, 14, 7669. [Google Scholar] [CrossRef]
- Khan, M.A.; Adnan, M.; Basir, A.; Fhad, S.; Hafeez, A.; Subhan, F.; Alamri, S.; Hashem, M.; Rehman, I.U. Impact of tillage, potassium levels and sources on growth, yield and yield attributes of wheat. Pak. J. Bot. 2022, 55. [Google Scholar] [CrossRef]
- Mahmood, N.; Arshad, M.; Kächele, H.; Ullah, A.; Müller, K. Economic efficiency of rainfed wheat farmers under changing climate: Evidence from Pakistan. Environ. Sci. Pollut. Res. 2020, 27, 34453–34467. [Google Scholar] [CrossRef]
- Nelson, A.R.L.E.; Ravichandran, K.; Antony, U. The impact of the Green Revolution on indigenous crops of India. J. Ethn. Foods 2019, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Al-Zaban, M.I.; Alhag, S.K.; Dablool, A.S.; Ahmed, A.E.; Alghamdi, S.; Ali, B.; Al-Saeed, F.A.; Saleem, M.H.; Poczai, P. Manufactured nano-objects confer viral protection against cucurbit chlorotic yellows virus (CCYV) infecting nicotiana benthamiana. Microorganisms 2022, 10, 1837. [Google Scholar] [CrossRef]
- Metayi, M.H.; Abd El-Naby, S.S.; El-Habal, N.A.; Fahmy, H.H.; Abdou, M.S.; Ali, B.; Abdel-Rheim, K.H.; Abdel-Megeed, A. Omani Frankincense nanoemulsion formulation efficacy and its latent effects on biological aspects of the spiny bollworm Earias insulana (Boisd.). Front. Physiol. 2022, 13, 2129. [Google Scholar] [CrossRef]
- Umar, U.d.; Ahmed, N.; Zafar, M.Z.; Rehman, A.; Naqvi, S.A.H.; Zulfiqar, M.A.; Malik, M.T.; Ali, B.; Saleem, M.H.; Marc, R.A. Micronutrients Foliar and Drench Application Mitigate Mango Sudden Decline Disorder and Impact Fruit Yield. Agronomy 2022, 12, 2449. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ Physio-Biochemical and Phyto-Hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Dola, D.B.; Mannan, M.A.; Sarker, U.; Al Mamun, M.A.; Islam, T.; Ercisli, S.; Saleem, M.H.; Ali, B.; Pop, O.L.; Marc, R.A. Nano-iron oxide accelerates growth, yield, and quality of Glycine max seed in water deficits. Front. Plant Sci. 2022, 13, 992535. [Google Scholar] [CrossRef] [PubMed]
- Farooq, T.H.; Rafy, M.; Basit, H.; Shakoor, A.; Shabbir, R.; Riaz, M.U.; Ali, B.; Kumar, U.; Qureshi, K.A.; Jaremko, M. Morpho-Physiological Growth Performance and Phytoremediation Capabilities of Selected Xerophyte Grass Species Towards Cr and Pb Stress. Front. Plant Sci. 2022, 13, 997120. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Ullah, S.; Hafeez, A.; Khan, M.N.; Javed, M.A.; Ali, B.; Din, I.U.; Bangash, S.A.K.; Wahab, S.; Wahid, N. Exogenous Ca/Mg quotient reduces the inhibitory effects of PEG induced osmotic stress on Avena sativa L. Braz. J. Biol. 2022, 84, e264642. [Google Scholar] [CrossRef]
- Naz, R.; Khan, M.S.; Hafeez, A.; Fazil, M.; Khan, M.N.; Ali, B.; Javed, M.A.; Imran, M.; Shati, A.A.; Alfaifi, M.Y. Assessment of phytoremediation potential of native plant species naturally growing in a heavy metal-polluted industrial soils. Braz. J. Biol. 2022, 84, e264473. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ali, S.; Saleem, M.H.; Mumtaz, S.; Yasin, G.; Ali, B.; Al-Ghamdi, A.A.; Elshikh, M.S.; Vodnar, D.C.; Marc, R.A.; et al. Short-term responses of Spinach (Spinacia oleracea L.) to the individual and combinatorial effects of Nitrogen, Phosphorus and Potassium and silicon in the soil contaminated by boron. Front. Plant Sci. 2022, 13, 983156. [Google Scholar] [CrossRef]
- Ma, J.; Saleem, M.H.; Ali, B.; Rasheed, R.; Ashraf, M.A.; Aziz, H.; Ercisli, S.; Riaz, S.; Elsharkawy, M.M.; Hussain, I.; et al. Impact of foliar application of syringic acid on tomato (Solanum lycopersicum L.) under heavy metal stress-insights into nutrient uptake, redox homeostasis, oxidative stress, and antioxidant defense. Front. Plant Sci. 2022, 13, 950120. [Google Scholar] [CrossRef]
- Ma, J.; Saleem, M.H.; Yasin, G.; Mumtaz, S.; Qureshi, F.F.; Ali, B.; Ercisli, S.; Alhag, S.K.; Ahmed, A.E.; Vodnar, D.C.; et al. Individual and combinatorial effects of SNP and NaHS on morpho-physio-biochemical attributes and phytoextraction of chromium through Cr-stressed spinach (Spinacia oleracea L.). Front. Plant Sci. 2022, 13, 973740. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Hafeez, A.; Ahmad, S.; Javed, M.A.; Cavalu, S.; Afridi, M.S. Bacillus thuringiensis PM25 Ameliorates Oxidative Damage of Salinity Stress in Maize via Regulating Growth, Leaf Pigments, Antioxidant Defense System, and Stress Responsive Gene Expression. Front. Plant Sci. 2022, 13, 921668. [Google Scholar] [CrossRef]
- Ali, B.; Hafeez, A.; Ammar Javed, M.; Ahmad, S.; Afridi, M.S.; Ullah, A.; Alwahibi, M.S.; Elsheikh, M.S.; Muresan, C.C.; Marc, R.A. Bacterial-Mediated Salt Tolerance in Maize: Insights into Plant Growth Promotion, Antioxidant Defenses System, Oxidative Stress, and Surfactant Production. Front. Plant Sci. 2022, 3170. [Google Scholar]
- Ali, B.; Hafeez, A.; Javed, M.A.; Afridi, M.S.; Abbasi, H.A.; Qayyum, A.; Batool, T.; Ullah, A.; Marc, R.A.; Al Jaouni, S.K.; et al. Role of endophytic bacteria in salinity stress amelioration by physiological and molecular mechanisms of defense: A comprehensive review. S. Afr. J. Bot. 2022, 151, 33–46. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Azeem, M.A.; Afridi, M.S.; Nadeem, M.; Ghazal, M.; Batool, T.; Qayyum, A.; Alatawi, A.; et al. Bacillus mycoides PM35 Reinforces Photosynthetic Efficiency, Antioxidant Defense, Expression of Stress-Responsive Genes, and Ameliorates the Effects of Salinity Stress in Maize. Life 2022, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Wang, X.; Saleem, M.H.; Hafeez, A.; Afridi, M.S.; Khan, S.; Ullah, I.; do Amaral Júnior, A.T.; Alatawi, A.; Ali, S. PGPR-Mediated Salt Tolerance in Maize by Modulating Plant Physiology, Antioxidant Defense, Compatible Solutes Accumulation and Bio-Surfactant Producing Genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Afridi, M.S.; Javed, M.A.; Ali, S.; De Medeiros, F.H.V.; Ali, B.; Salam, A.; Sumaira; Marc, R.A.; Alkhalifah, D.H.M.; Selim, S.; et al. New opportunities in plant microbiome engineering for increasing agricultural sustainability under stressful conditions. Front. Plant Sci. 2022, 13, 899464. [Google Scholar] [CrossRef]
- Mehmood, S.; Khatoon, Z.; Amna; Ahmad, I.; Muneer, M.A.; Kamran, M.A.; Ali, J.; Ali, B.; Chaudhary, H.J.; Munis, M.F.H. Bacillus sp. PM31 harboring various plant growth-promoting activities regulates Fusarium dry rot and wilt tolerance in potato. Arch. Agron. Soil Sci. 2021, 1–15. [Google Scholar] [CrossRef]
- Solanki, M.K.; Solanki, A.C.; Rai, S.; Srivastava, S.; Kashyap, B.K.; Divvela, P.K.; Kumar, S.; Yandigeri, M.S.; Kashyap, P.L.; Shrivastava, A.K.; et al. Functional interplay between antagonistic bacteria and Rhizoctonia solani in the tomato plant rhizosphere. Front. Microbiol. 2022, 13, 990850. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.W.; Nafees, M.; Ahmad, I.; Ali, B.; Maryam, B.; Iqbal, R.; Vodnar, D.C.; Marc, R.A.; Kamran, M.; Saleem, M.H.; et al. Postharvest dormancy-related changes of endogenous hormones in relation to different dormancy-breaking methods of potato (Solanum tuberosum L.) tubers. Front. Plant Sci. 2022, 13, 945256. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.; Halford, N. Food security: The challenge of increasing wheat yield and the importance of not compromising food safety. Ann. Appl. Biol. 2014, 164, 354–372. [Google Scholar] [CrossRef] [Green Version]
- Michel, S.; Löschenberger, F.; Ametz, C.; Pachler, B.; Sparry, E.; Bürstmayr, H. Simultaneous selection for grain yield and protein content in genomics-assisted wheat breeding. Theor. Appl. Genet. 2019, 132, 1745–1760. [Google Scholar] [CrossRef]
- Novoselovic, D.; Baric, M.; Drezner, G.; Gunjaca, J.; Lalic, A. Quantitative inheritance of some wheat plant traits. Genet. Mol. Biol. 2004, 27, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Dhillon, T.S.; Javed, T.; Singh, R.; Dobaria, J.; Dhankhar, S.K.; Kianersi, F.; Ali, B.; Poczai, P.; Kumar, U. Exploring the Genetic Diversity of Carrot Genotypes through Phenotypically and Genetically Detailed Germplasm Collection. Agronomy 2022, 12, 1921. [Google Scholar] [CrossRef]
- Amallah, L.; Taghouti, M.; Rhrib, K.; Gaboun, F.; Hassikou, R. Genetic variability in agro-morphological and quality traits of Mediterranean durum wheat landraces. Cereal Res. Commun. 2015, 43, 123–132. [Google Scholar] [CrossRef]
- Bashir, K.; Matsui, A.; Rasheed, S.; Seki, M. Recent advances in the characterization of plant transcriptomes in response to drought, salinity, heat, and cold stress. F1000Resarch 2019, 8, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodt, S.; Six, J.; Feenstra, G.; Ingels, C.; Campbell, D. Sustainable agriculture. Nat. Educ. Knowl. 2011, 3, 1. [Google Scholar]
- Saini, A.; Manuja, S.; Kumar, S.; Hafeez, A.; Ali, B.; Poczai, P. Impact of Cultivation Practices and Varieties on Productivity, Profitability, and Nutrient Uptake of Rice (Oryza sativa L.) and Wheat (Triticum aestivum L.) Cropping System in India. Agriculture 2022, 12, 1678. [Google Scholar] [CrossRef]
- Saeed, S.; Ullah, A.; Ullah, S.; Noor, J.; Ali, B.; Khan, M.N.; Hashem, M.; Mostafa, Y.S.; Alamri, S. Validating the Impact of Water Potential and Temperature on Seed Germination of Wheat (Triticum aestivum L.) via Hydrothermal Time Model. Life 2022, 12, 983. [Google Scholar] [CrossRef]
- Tao, Y.; Zhao, X.; Mace, E.; Henry, R.; Jordan, D. Exploring and exploiting pan-genomics for crop improvement. Mol. Plant 2019, 12, 156–169. [Google Scholar] [CrossRef] [Green Version]
- Akram, N.A.; Saleem, M.H.; Shafiq, S.; Naz, H.; Farid-ul-Haq, M.; Ali, B.; Shafiq, F.; Iqbal, M.; Jaremko, M.; Qureshi, K.A. Phytoextracts as Crop Biostimulants and Natural Protective Agents—A Critical Review. Sustainability 2022, 14, 14498. [Google Scholar] [CrossRef]
- Amna; Ali, B.; Azeem, M.A.; Qayyum, A.; Mustafa, G.; Ahmad, M.A.; Javed, M.T.; Chaudhary, H.J. Bio-Fabricated Silver Nanoparticles: A Sustainable Approach for Augmentation of Plant Growth and Pathogen Control. In Sustainable Agriculture Reviews 53; Springer: Berlin/Heidelberg, Germany, 2021; pp. 345–371. [Google Scholar]
- Faryal, S.; Ullah, R.; Khan, M.N.; Ali, B.; Hafeez, A.; Jaremko, M.; Qureshi, K.A. Thiourea-Capped Nanoapatites Amplify Osmotic Stress Tolerance in Zea mays L. by Conserving Photosynthetic Pigments, Osmolytes Biosynthesis and Antioxidant Biosystems. Molecules 2022, 27, 5744. [Google Scholar] [CrossRef]
- Fahad, S.; Chavan, S.B.; Chichaghare, A.R.; Uthappa, A.R.; Kumar, M.; Kakade, V.; Pradhan, A.; Jinger, D.; Rawale, G.; Yadav, D.K.; et al. Agroforestry Systems for Soil Health Improvement and Maintenance. Sustainability 2022, 14, 14877. [Google Scholar] [CrossRef]
- Hussain, S.S.; Rasheed, M.; Hamzah Saleem, M.; Ahmed, Z.I.; Hafeez, A.; Jilani, G.; Alamri, S.; Hashem, M.; Ali, S. Salt tolerance in maize with melatonin priming to achieve sustainability in yield on salt affected soils. Pak. J. Bot. 2023, 55. [Google Scholar] [CrossRef]
- Salam, A.; Afridi, M.S.; Javed, M.A.; Saleem, A.; Hafeez, A.; Khan, A.R.; Zeeshan, M.; Ali, B.; Azhar, W.; Sumaira; et al. Nano-Priming against Abiotic Stress: A Way Forward towards Sustainable Agriculture. Sustainability 2022, 14, 14880. [Google Scholar] [CrossRef]
- Ali, S.; Ullah, S.; Khan, M.N.; Khan, W.M.; Razak, S.A.; Wahab, S.; Hafeez, A.; Khan Bangash, S.A.; Poczai, P. The Effects of Osmosis and Thermo-Priming on Salinity Stress Tolerance in Vigna radiata L. Sustainability 2022, 14, 12924. [Google Scholar] [CrossRef]
- Nawaz, H.; Ali, A.; Saleem, M.H.; Ameer, A.; Hafeez, A.; Alharbi, K.; Ezzat, A.; Khan, A.; Jamil, M.; Farid, G. Comparative effectiveness of EDTA and citric acid assisted phytoremediation of Ni contaminated soil by using canola (Brassica napus). Braz. J. Biol. 2022, 82, e261785. [Google Scholar] [CrossRef]
- Zainab, N.; Khan, A.A.; Azeem, M.A.; Ali, B.; Wang, T.; Shi, F.; Alghanem, S.M.; Hussain Munis, M.F.; Hashem, M.; Alamri, S. PGPR-Mediated Plant Growth Attributes and Metal Extraction Ability of Sesbania sesban L. in Industrially Contaminated Soils. Agronomy 2021, 11, 1820. [Google Scholar] [CrossRef]
- Saleem, K.; Asghar, M.A.; Saleem, M.H.; Raza, A.; Kocsy, G.; Iqbal, N.; Ali, B.; Albeshr, M.F.; Bhat, E.A. Chrysotile-Asbestos-Induced Damage in Panicum virgatum and Phleum pretense Species and Its Alleviation by Organic-Soil Amendment. Sustainability 2022, 14, 10824. [Google Scholar] [CrossRef]
- Rehman, H.; Munir, M.; Ashraf, K.; Fatima, K.; Shahab, S.; Ali, B. Analysis of Drinking Water Quality in the Newly Developed. Water 2022, 14, 1–16. [Google Scholar]
- Brenchley, R.; Spannagl, M.; Pfeifer, M.; Barker, G.L.; D’Amore, R.; Allen, A.M.; McKenzie, N.; Kramer, M.; Kerhornou, A.; Bolser, D. Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature 2012, 491, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Chaudhry, S.; Sidhu, G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar] [CrossRef]
- Atlin, G.N.; Cairns, J.E.; Das, B. Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Glob. Food Sec. 2017, 12, 31–37. [Google Scholar] [CrossRef]
- Kim, H.; Ward, R. Patterns of RFLP-based genetic diversity in germplasm pools of common wheat with different geographical or breeding program origins. Euphytica 2000, 115, 197–208. [Google Scholar] [CrossRef]
- Burkhamer, R.L.; Lanning, S.P.; Martens, R.J.; Martin, J.M.; Talbert, L.E. Predicting progeny variance from parental divergence in hard red spring wheat. Crop Sci. 1998, 38, 243–248. [Google Scholar] [CrossRef]
- Joshi, C.P.; Nguyen, H.T. RAPD (random amplified polymorphic DNA) analysis based intervarietal genetic relationships among hexaploid wheats. Plant Sci. 1993, 93, 95–103. [Google Scholar] [CrossRef]
- Nagaoka, T.; Ogihara, Y. Applicability of inter-simple sequence repeat polymorphisms in wheat for use as DNA markers in comparison to RFLP and RAPD markers. Theor. Appl. Genet. 1997, 94, 597–602. [Google Scholar] [CrossRef]
- Chen, H.; Martin, J.; Lavin, M.; Talbert, L. Genetic diversity in hard red spring wheat based on sequence-tagged-site PCR markers. Crop Sci. 1994, 34, 1628–1632. [Google Scholar] [CrossRef]
- Devos, K.; Gale, M.D. The use of random amplified polymorphic DNA markers in wheat. Theor. Appl. Genet. 1992, 84, 567–572. [Google Scholar] [CrossRef]
- The International Wheat Genome Sequencing Consortium (IWGSC); Appels, R.; Eversole, K.; Stein, N.; Feuillet, C.; Keller, B.; Rogers, J.; Pozniak, C.J.; Choulet, F.; Distelfeld, A.; et al. Shifting the Limits in Wheat Research and Breeding Using a Fully Annotated Reference Genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef] [Green Version]
- Akbari, M.; Wenzl, P.; Caig, V.; Carling, J.; Xia, L.; Yang, S.; Uszynski, G.; Mohler, V.; Lehmensiek, A.; Kuchel, H. Diversity arrays technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theor. Appl. Genet. 2006, 113, 1409–1420. [Google Scholar] [CrossRef]
- Khattak, S.H.; Begum, S.; Aqeel, M.; Fayyaz, M.; Bangash, S.A.K.; Riaz, M.N.; Saeed, S.; Ahmed, A.; Ali, G.M. Investigating the allelic variation of loci controlling rust resistance genes in wheat (Triticum aestivum L.) land races by SSR marker. Appl. Ecol. Environ. Res. 2020, 18, 8091–8118. [Google Scholar] [CrossRef]
- Chapman, J.A.; Mascher, M.; Buluç, A.; Barry, K.; Georganas, E.; Session, A.; Strnadova, V.; Jenkins, J.; Sehgal, S.; Oliker, L. A whole-genome shotgun approach for assembling and anchoring the hexaploid bread wheat genome. Genome Biol. 2015, 16, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Saintenac, C.; Jiang, D.; Wang, S.; Akhunov, E. Sequence-based mapping of the polyploid wheat genome. G3 Genes|Genomes|Genet. 2013, 3, 1105–1114. [Google Scholar] [CrossRef] [Green Version]
- Röder, M.S.; Korzun, V.; Wendehake, K.; Plaschke, J.; Tixier, M.-H.; Leroy, P.; Ganal, M.W. A microsatellite map of wheat. Genetics 1998, 149, 2007–2023. [Google Scholar] [CrossRef] [PubMed]
- Lelley, T.; Stachel, M.; Grausgruber, H.; Vollmann, J. Analysis of relationships between Aegilops tauschii and the D genome of wheat utilizing microsatellites. Genome 2000, 43, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Khanjari, S.A.; Filatenko, A.A.; Hammer, K.; Buerkert, A. Morphological spike diversity of Omani wheat. Genet. Resour. Crop Evol. 2008, 55, 1185–1195. [Google Scholar] [CrossRef]
- Thomson, M.J. High-throughput SNP genotyping to accelerate crop improvement. Plant Breed. Biotechnol. 2014, 2, 195–212. [Google Scholar] [CrossRef]
- Manifesto, M.M.; Schlatter, A.; Hopp, H.E.; Suárez, E.Y.; Dubcovsky, J. Quantitative evaluation of genetic diversity in wheat germplasm using molecular markers. Crop Sci. 2001, 41, 682–690. [Google Scholar] [CrossRef] [Green Version]
- Ott, A.; Trautschold, B.; Sandhu, D. Using microsatellites to understand the physical distribution of recombination on soybean chromosomes. PLoS ONE 2011, 6, e22306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.M. Mapping Salinity Tolerance Genes in Rice (Oryza sativa L.) at Reproduction Stage. Ph.D. Thesis, University of the Philippines Los Baños College, Laguna, Philippines, 2004. [Google Scholar]
- Miah, G.; Rafii, M.Y.; Ismail, M.R.; Puteh, A.B.; Rahim, H.A.; Islam, K.N.; Latif, M.A. A review of microsatellite markers and their applications in rice breeding programs to improve blast disease resistance. Int. J. Mol. Sci. 2013, 14, 22499–22528. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, A.; Ahmad, M.; Iqbal, M.; Ahmed, I.; Ali, G. Evaluation of wheat landrace genotypes for salinity tolerance at vegetative stage by using morphological and molecular markers. Genet. Mol. Res. 2012, 11, 679–692. [Google Scholar] [CrossRef]
- Francois, L.; Maas, E.; Donovan, T.; Youngs, V. Effect of Salinity on Grain Yield and Quality, Vegetative Growth, and Germination of Semi-Dwarf and Durum Wheat1. Agron. J. 1986, 78, 1053–1058. [Google Scholar] [CrossRef]
- Yeh, F.C.; Yang, R.C.; Boyle, T.; Mao, J.X. PopGene: Microsoft Window-Based Freeware for Population Genetic Analysis; Version 1.31; University of Alberta: Edmonton, AB, Canada, 1999. [Google Scholar]
- Thakur, A.; Singh, S.; Singh, N.; Ali, B.; Hafeez, A.; Vodnar, D.C.; Marc, R.A. Nutritional evaluation, phytochemical makeup, antibacterial and antioxidant properties of wild plants utilized as food by the Gaddis-a tribal tribe in the Western Himalayas. Front. Agron. 2022, 4, 1010309. [Google Scholar] [CrossRef]
- Bhandari, H.; Bhanu, A.N.; Srivastava, K.; Singh, M.; Shreya, H.A. Assessment of genetic diversity in crop plants-an overview. Adv. Plants Agric. Res. 2017, 7, 279–286. [Google Scholar]
- Mohammed, A.; Amsalu, A.; Geremew, B. Genetic variability, heritability and trait associations in durum wheat (Triticum turgidum L. var. durum) genotypes. Afr. J. Agric. Res. 2011, 6, 3972–3979. [Google Scholar]
- Langer, S.M.; Longin, C.F.H.; Würschum, T. Flowering time control in European winter wheat. Front. Plant Sci. 2014, 5, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gürcan, E.C. Political geography of Turkey’s intervention in Syria: Underlying causes and consequences (2011–2016). J. Aggress. Confl. Peace Res. 2017, 11. [Google Scholar] [CrossRef]
- Acevedo, E.; Silva, P.; Silva, H. Wheat Growth and Physiology. Bread Wheat: Improvement and Production; 2002; Available online: https://www.fao.org/3/y4011e/y4011e06.htm (accessed on 18 October 2022).
- Tewolde, H.; Fernandez, C.J.; Erickson, C.A. Wheat cultivars adapted to post-heading high temperature stress. J. Agron. Crop Sci. 2006, 192, 111–120. [Google Scholar] [CrossRef]
- Hossain, A.; da Silva, J.A.T. Phenology, growth and yield of three wheat (Triticum aestivum L.) varieties as affected by high temperature stress. Not. Sci. Biol. 2012, 4, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Ormoli, L.; Costa, C.; Negri, S.; Perenzin, M.; Vaccino, P. Diversity trends in bread wheat in Italy during the 20th century assessed by traditional and multivariate approaches. Sci. Rep. 2015, 5, 8574. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, R.; Sayre, K.D.; Govaerts, B.; Gupta, R.; Subbarao, G.; Ban, T.; Hodson, D.; Dixon, J.M.; Ortiz-Monasterio, J.I.; Reynolds, M. Climate change: Can wheat beat the heat? Agric. Ecosyst. Environ. 2008, 126, 46–58. [Google Scholar] [CrossRef]
- Egamberdiyeva, D.; Höflich, G. Influence of growth-promoting bacteria on the growth of wheat in different soils and temperatures. Soil Biol. Biochem. 2003, 35, 973–978. [Google Scholar] [CrossRef]
- Royo, C.; Ammar, K.; Villegas, D.; Soriano, J.M. Agronomic, physiological and genetic changes associated with evolution, migration and modern breeding in durum wheat. Front. Plant Sci. 2021, 12, 674470. [Google Scholar] [CrossRef]
- Mollasadeghi, V.; Elyasi, S.; Mirzamasoumzadeh, B. Genetic variation of 12 bread wheat genotypes based on number of phonological and morphological traits. Ann. Biol. Res. 2012, 3, 4734–4740. [Google Scholar]
- Kuang, C.-H.; Zhao, X.-F.; Yang, K.; Zhang, Z.-P.; Ding, L.; Pu, Z.-E.; Ma, J.; Jiang, Q.-T.; Chen, G.-Y.; Wang, J.-R. Mapping and characterization of major QTL for spike traits in common wheat. Physiol. Mol. Biol. Plants 2020, 26, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Knežević, D.; Radosavac, A.; Zelenika, M. Variability of grain weight per spike in wheat grown in different ecological conditions. Acta Agric. Serbica 2015, 20, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Ujala, M.; Muhammad, A. In vitro and greenhouse response of different wheat lines against salinity stress. Int. J. Biol. Biotechnol. 2016, 13, 377–383. [Google Scholar]
- Abdelkhalik, S.M.; Salem, A.K.M.; Abdelaziz, A.R.; Ammar, M.H. Morphological and sequence-related amplified polymorphism-based molecular diversity of local and exotic wheat genotypes. Genet. Mol. Res. 2016, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vaiman, D.; Mercier, D.; Moazami-Goudarzi, K.; Eggen, A.; Ciampolini, R.; Lépingle, A.; Velmala, R.; Kaukinen, J.; Varvio, S.-L.; Martin, P. A set of 99 cattle microsatellites: Characterization, synteny mapping, and polymorphism. Mamm. Genome 1994, 5, 288–297. [Google Scholar] [CrossRef]
- Fu, X.S.; Choi, E.; Bubley, G.J.; Balk, S.P. Identification of hypoxia-inducible factor-1α (HIF-1α) polymorphism as a mutation in prostate cancer that prevents normoxia-induced degradation. Prostate 2005, 63, 215–221. [Google Scholar] [CrossRef]
- Hammer, K.; Filatenko, A.; Korzun, V. Microsatellite markers—A new tool for distinguishing diploid wheat species. Genet. Resour. Crop Evol. 2000, 47, 497–505. [Google Scholar] [CrossRef]
- Dreisigacker, S.; Zhang, P.; Warburton, M.; Van Ginkel, M.; Hoisington, D.; Bohn, M.; Melchinger, A. SSR and pedigree analyses of genetic diversity among CIMMYT wheat lines targeted to different megaenvironments. Crop Sci. 2004, 44, 381–388. [Google Scholar] [CrossRef]
- Christov, N.K.; Tsonev, S.; Dragov, R.; Taneva, K.; Bozhanova, V.; Todorovska, E.G. Genetic diversity and population structure of modern Bulgarian and foreign durum wheat based on microsatellite and agronomic data. Biotechnol. Biotechnol. Equip. 2022, 36, 637–652. [Google Scholar] [CrossRef]
- Kumar, D.; Chhokar, V.; Sheoran, S.; Singh, R.; Sharma, P.; Jaiswal, S.; Iquebal, M.A.; Jaiswar, A.; Jaisri, J.; Angadi, U.B. Characterization of genetic diversity and population structure in wheat using array based SNP markers. Mol. Biol. Rep. 2020, 47, 293–306. [Google Scholar] [CrossRef] [PubMed]
| Wheat Variety | Pedigree |
|---|---|
| Markaz-2019 | SOKOLL//FRTL/2*PIFED |
| Borlaug-2016 | SOKOLL/3/PASTOR//HXL7573/2*BAU |
| Zincol-2016 | OASIS/SKAUZ//4*BCN/3/2*PASTOR/4/T.SPELTA PI348449/5/BACEU #1/6/WBLL1*2/CHAPIO |
| Pakistan-13 | MSA04M00552S-040ZTP0Y-040ZTM-040SY-19ZTM-03Y-0B-01D |
| NARC-2011 | OASIS/SKAUZ//4*BCN/3/2*PASTOR |
| NARC-2009 | INQALAB 91*2/TUKURU |
| Wafaq-2001 | OPATA/RAYON//KAUZ |
| Magalla-99 | OPATA/BOW’S’ |
| Attahabib | INQALAB 91*2/TUKURU |
| Khatakwal | Land race of Southern KPK, Pakistan |
| Janbaza | GEN*2//BUC/FILK/3/BUCHIN |
| Lalma-13 | “PASTOR/3/ALTAR84/AE.SQUARROSA(TAUS)//OPATA(SOKOLL) |
| Tatara-96 | JUP/ALDS??KLTS/3VEES |
| Punjab-11 | SA 42 *2/4CC/INIA//BB/3/INIA/HD832 |
| Pirsabaq-5 | CMSS93B00729S-23Y-010M-010Y-010M-7Y-1M-0Y |
| Pirsabaq-13 | CS/TH.SC//3*PVN/3/MIRLO/BUC/4/MILAN/5/TILHI |
| Galaxy-2013 | CMH84.3379/CMH78.578//MILAN |
| Sehar-06 | CHILL/2* STAR/4/BOW//BUC/PVN/3/2*VEE#10 |
| Shahkar-13 | CMH84.3379/CMH78.578//MILAN |
| Farid-06 | PT’S’/3/TOB/LFN//BB/4/BB/HD-832-5//ON/5/G-V/ALD’S’//HPO |
| Marker | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Length (bp) | Annealing (°C) |
|---|---|---|---|---|
| WMC177 | AGGGCTCTCTTTAATTCTTGCT | GGTCTATCGTAATCCACCTGTA | 22 | 61.7 |
| WMC168 | AACACAAAAGATCCAACGACAC | CAGTATAGAAGGATTTTGAGAG | 22 | 56.8 |
| WMC167 | AGTGGTAATGAGGTGAAAGAAG | TCGGTCGTATATGCATGTAAAG | 22 | 61.0 |
| WMC165 | CACACTCGCACGATTTTCCTAT | TCGGTTACACTGGAAGTGGTCT | 22 | 63.9 |
| WMC153 | ATGAGGACTCGAAGCTTGGC | CTGAGCTTTTGCGCGTTGAC | 20 | 65.2 |
| WMC150 | CATTGATTGAACAGTTGAAGAA | CTCAAAGCAACAGAAAAGTAAA | 22 | 58.4 |
| WMC105 | AATGTCATGCGTGTAGTAGCCA | AAGCGCACTTAACAGAAGAGGG | 22 | 65.1 |
| WMC78 | AGTAAATCCTCCCTTCGGCTTC | AGCTTCTTTGCTAGTCCGTTGC | 22 | 64.9 |
| Wheat Lines | WMC | |||||||
|---|---|---|---|---|---|---|---|---|
| 78 | 105 | 150 | 153 | 165 | 167 | 168 | 177 | |
| Markaz-19 | BC | AA | AA | BB | AB | AB | ||
| NARC-2011 | BC | AA | AB | BB | AB | AB | AB | |
| NARC-2009 | BC | AA | AA | BB | AB | AB | BB | |
| Wafaq-2001 | BC | AA | AA | BB | AB | BB | ||
| Punjab-11 | BC | AA | AB | BB | AA | BB | AA | AB |
| Borlaug-2016 | AC | AA | AB | BB | AA | AB | AB | |
| Pirsabaq-13 | CC | AA | AB | BB | AB | AB | AB | AB |
| Janbaza | BC | AA | BB | AB | AB | AA | BB | |
| Margalla-99 | BC | AA | AB | BB | AA | BB | ||
| Tatara-96 | BC | AA | AB | AA | AB | |||
| Pakistan-13 | BC | AA | AB | AB | AB | AA | AB | |
| Lalma-13 | BC | AA | AB | AB | AA | AB | ||
| Attahabib | BC | AA | AB | AB | BB | |||
| Farid-06 | BC | AA | BB | BB | ||||
| Galaxy-2013 | BC | AA | BB | AB | AB | BB | BB | |
| Khatakwal | BC | AA | BB | AB | BB | AB | ||
| Pirsabaq-5 | BC | AA | AB | BB | AB | BB | AB | |
| Zincol-2016 | AA | AA | BB | BB | AB | AB | ||
| Sehar-06 | BC | BB | AA | AB | ||||
| Shahkar-13 | BC | BB | BB | AB | AB | |||
| Locus | Sample Size | Na | Ne | I | Ho | He | PIC |
|---|---|---|---|---|---|---|---|
| WMC78 | 20 | 3 | 2.292 | 0.904 | 0.900 | 0.578 | 0.403 |
| WMC105 | 18 | 1 | 1.00 | 0 | 0 | 0 | - |
| WMC150 | 20 | 2 | 1.923 | 0.673 | 0.500 | 0.492 | 0.365 |
| WMC153 | 16 | 2 | 1.358 | 0.433 | 0.312 | 0.272 | 0.229 |
| WMC165 | 7 | 2 | 1.849 | 0.651 | 0.714 | 0.494 | 0.354 |
| WMC167 | 5 | 2 | 1.923 | 0.673 | 0.800 | 0.533 | 0.365 |
| WMC168 | 18 | 2 | 1.905 | 0.668 | 0.444 | 0.488 | 0.362 |
| WMC177 | 20 | 2 | 1.781 | 0.630 | 0.650 | 0.450 | 0.342 |
| Mean | 2.00 | 1.754 | 0.579 | 0.586 | 0.395 | 0.346 | |
| St. Dev | 0.534 | 0.397 | 0.266 | 0.189 | 0.180 | 0.055 |
| Locus | Alleles | Genotypes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | AA | AB | BB | BC | AC | CC | |
| WMC78 | 0.075 | 0.425 | 0.5 | 1 | - | - | 17 | 1 | 1 |
| WMC105 | 1 | - | - | 18 | - | - | - | - | - |
| WMC150 | 0.4 | 0.6 | - | 3 | 10 | 7 | - | - | - |
| WMC153 | 0.1562 | 0.8438 | - | - | 5 | 11 | - | - | - |
| WMC165 | 0.6429 | 0.3571 | - | 2 | 5 | - | - | - | - |
| WMC167 | 0.4 | 0.6 | - | - | 4 | 1 | - | - | - |
| WMC168 | 0.6111 | 0.3889 | - | 7 | 8 | 3 | - | - | - |
| WMC177 | 0.325 | 0.675 | - | - | 13 | 7 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, O.; Bangash, S.A.K.; Ibrahim, M.; Shahab, S.; Khattak, S.H.; Ud Din, I.; Khan, M.N.; Hafeez, A.; Wahab, S.; Ali, B.; et al. Evaluation of Agronomic Performance and Genetic Diversity Analysis Using Simple Sequence Repeats Markers in Selected Wheat Lines. Sustainability 2023, 15, 293. https://doi.org/10.3390/su15010293
Tahir O, Bangash SAK, Ibrahim M, Shahab S, Khattak SH, Ud Din I, Khan MN, Hafeez A, Wahab S, Ali B, et al. Evaluation of Agronomic Performance and Genetic Diversity Analysis Using Simple Sequence Repeats Markers in Selected Wheat Lines. Sustainability. 2023; 15(1):293. https://doi.org/10.3390/su15010293
Chicago/Turabian StyleTahir, Osama, Sajid Ali Khan Bangash, Muhammad Ibrahim, Sana Shahab, Sahir Hameed Khattak, Israr Ud Din, Muhammad Nauman Khan, Aqsa Hafeez, Sana Wahab, Baber Ali, and et al. 2023. "Evaluation of Agronomic Performance and Genetic Diversity Analysis Using Simple Sequence Repeats Markers in Selected Wheat Lines" Sustainability 15, no. 1: 293. https://doi.org/10.3390/su15010293
APA StyleTahir, O., Bangash, S. A. K., Ibrahim, M., Shahab, S., Khattak, S. H., Ud Din, I., Khan, M. N., Hafeez, A., Wahab, S., Ali, B., Makki, R. M., & Harakeh, S. (2023). Evaluation of Agronomic Performance and Genetic Diversity Analysis Using Simple Sequence Repeats Markers in Selected Wheat Lines. Sustainability, 15(1), 293. https://doi.org/10.3390/su15010293









