Typology of Smallholder and Commercial Shrimp (Penaeus vannamei) Farms, including Threats and Challenges in Davao Region, Philippines
Abstract
:1. Introduction
2. Materials and Methods
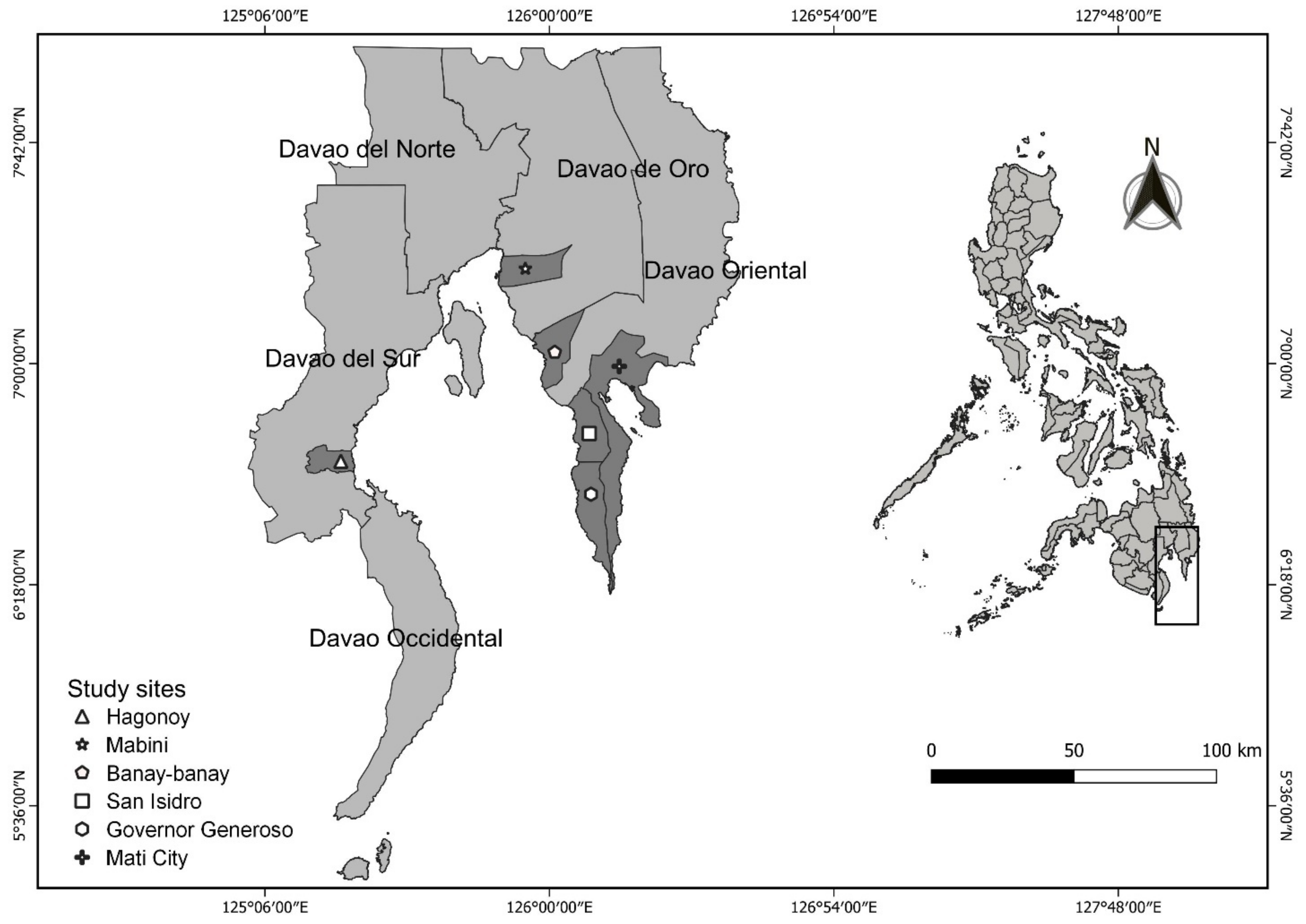
2.1. Study Site
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Sociodemographic Profile
3.2. Characteristics
3.2.1. Shrimp Farms
3.2.2. Variable Cost of Inputs
3.3. Prevalence of Disease and Probable Causes
3.4. Risks, Challenges, and Possible Solutions
4. Discussion
4.1. Farming Characteristics
4.2. Cultural Practices
4.3. Prevalence of Marine Pollution and Disease
4.4. Challenges in the Time of the COVID-19 Pandemic and Coping Strategies
5. Conclusions
6. Recommendation
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vergel, J.C.V. Current Trends in the Philippines’ Shrimp Aquaculture lndustry:A Booming Blue Economy in the Pacific. Oceanogr. Fish. Open Access J. 2017, 5, 555668. [Google Scholar] [CrossRef] [Green Version]
- BFAR. Philippine Fisheries Profile 2019; Bureau Of Fisheries And Aquatic Resources: Quezon City, Philippines, 2020; p. 76. [Google Scholar]
- PSA. Fisheries Statistics of the Philippines 2017–2019; Philippine Statistics Authority: Quezon City, Philippines, 2020; p. 321. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020; Volume 32, p. 244. [Google Scholar] [CrossRef]
- Loc, V.T.T. Quality management in shrimp supply chain in the Mekong Delta, Vietnam: Problems and measures. Qual. Assur. 2003, 43, 27. [Google Scholar]
- Martinez-Cordova, L.R.; Martinez-Porchas, M. Polyculture of Pacific white shrimp, Litopenaeus vannamei, giant oyster, Crassostrea gigas and black clam, Chione fluctifraga in ponds in Sonora, Mexico. Aquaculture 2006, 258, 321–326. [Google Scholar] [CrossRef]
- Guerrero, R.D. Farmed tilapia production in the Philippines is declining: What has happened and what can be done. Philipp. J. Sci. 2019, 148, xi–xv. [Google Scholar]
- Henriksson, P.J.G.; Banks, L.K.; Suri, S.K.; Pratiwi, T.Y.; Fatan, N.A.; Troell, M. Indonesian aquaculture futures—Identifying interventions for reducing environmental impacts. Environ. Res. Lett. 2019, 14, 124062. [Google Scholar] [CrossRef]
- Islam, M.; Yasmin, R. Impact of Aquaculture and Contemporary environmental issues in Bangladesh. Int. J. Fish. Aquat. Stud. 2017, 5, 100–107. [Google Scholar]
- Joffre, O.M.; Bosma, R.H. Typology of shrimp farming in Bac Lieu Province, Mekong Delta, using multivariate statistics. Agric. Ecosyst. Environ. 2009, 132, 153–159. [Google Scholar] [CrossRef]
- Weimin, M.; Mohan, C.V.; Ellis, W.; Davy, B. Adoption of Aquaculture Assessment Tools for Improving the Planning and Management of Aquaculture in Asia and the Pacific; FAO for Asia and the Pacific, Rap Publication: Bangkok, Thailand, 2013; p. 136. [Google Scholar]
- Walker, P.J.; Winton, J.R. Emerging viral diseases of fish and shrimp. Vet. Res. 2010, 41, 51. [Google Scholar] [CrossRef] [Green Version]
- Béné, C.; Barange, M.; Subasinghe, R.; Pinstrup-Andersen, P.; Merino, G.; Hemre, G.-I.; Williams, M. Feeding 9 billion by 2050—Putting fish back on the menu. Food Secur. 2015, 7, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Afroz, T.; Alam, S. Sustainable shrimp farming in Bangladesh: A quest for an Integrated Coastal Zone Management. Ocean Coast. Manag. 2013, 71, 275–283. [Google Scholar] [CrossRef]
- Tendencia, E.A.; Bosma, R.H.; Verdegem, M.C.; Verreth, J.A. The potential effect of greenwater technology on water quality in the pond culture of Penaeus monodon Fabricius. Aquac. Res. 2015, 46, 1–13. [Google Scholar] [CrossRef]
- Cruz, P.S.; Andalecio, M.N.; Bolivar, R.B.; Fitzsimmons, K. Tilapia-Shrimp Polyculture in Negros Island, Philippines: A Review. J. World Aquac. Soc. 2008, 39, 713–725. [Google Scholar] [CrossRef]
- Jickling, N. Shrimp Aquaculture in Aguadulce: Impacts on Mangrove Forest Health and Shrimp Larvae Populations in Two Sites on the Salado Coastline. Independent Study Project Collection 2729, 1–31. Available online: https://digitalcollections.sit.edu/isp_collection (accessed on 9 March 2022).
- Ashton, E.C. The impact of shrimp farming on mangrove ecosystems. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2008, 3, 1–12. [Google Scholar] [CrossRef]
- Barbier, E.B. Mangrove dependency and the livelihoods of coastal communities in Thailand. In Environment and Livelihoods in Tropical Coastal Zones: Managing Agriculture-Fishery-Aquaculture Conflicts; CAB International: Wallingford, UK, 2006; pp. 126–139. [Google Scholar] [CrossRef] [Green Version]
- Gopal, B.; Chauhan, M. Biodiversity and its conservation in the Sundarban Mangrove Ecosystem. Aquat. Sci. 2006, 68, 338–354. [Google Scholar] [CrossRef]
- Cienfuegos, H.H.A.; MacInnis, R. An Analysis of Mangrove Conservation and Land Use Management in the Pablo Arturo Barrios Wildlife Refuge; Smithsonian Tropical Research Institute: Calz. de Amador, Panama, 2015; pp. 1–47. [Google Scholar]
- TEEB—The Economics of Ecosystems and Biodiversity. 2009. Available online: http://teebweb.org/ (accessed on 9 March 2022).
- Samson, M.; Rollon, R. Growth Performance of Planted Mangroves in the Philippines: Revisiting Forest Management Strategies. AMBIO J. Hum. Environ. 2008, 37, 234–240. [Google Scholar] [CrossRef]
- Camacho, L.D.; Gevaña, D.T.; Carandang, A.P.; Camacho, S.C.; Combalicer, E.A.; Rebugio, L.L.; Youn, Y.-C. Tree biomass and carbon stock of a community-managed mangrove forest in Bohol, Philippines. For. Sci. Technol. 2011, 7, 161–167. [Google Scholar] [CrossRef]
- Garcia, K.B.; Malabrigo, P.L.; Gevaña, D.T. Philippines’ mangrove ecosystem: Status, threats and conservation. In Mangrove Ecosystems of Asia: Status, Challenges and Management Strategies; Springer: New York, NY, USA, 2014; pp. 81–94. [Google Scholar] [CrossRef]
- Orchard, S.E.; Stringer, L.C.; Quinn, C.H. Impacts of aquaculture on social networks in the mangrove systems of northern Vietnam. Ocean Coast. Manag. 2015, 114, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Blaber, S.J.M. Mangroves and fishes: Issues of diversity, dependence, and dogma. Bull. Mar. Sci. 2007, 80, 457–472. [Google Scholar]
- Nagelkerken, I.; Blaber, S.J.M.; Bouillon, S.; Green, P.; Haywood, M.; Kirton, L.G.; Meynecke, J.-O.; Pawlik, J.; Penrose, H.M.; Sasekumar, A.; et al. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquat. Bot. 2008, 89, 155–185. [Google Scholar] [CrossRef] [Green Version]
- Anh, P.T.; Kroeze, C.; Bush, S.; Mol, A. Water pollution by intensive brackish shrimp farming in south-east Vietnam: Causes and options for control. Agric. Water Manag. 2010, 97, 872–882. [Google Scholar] [CrossRef]
- Sheridan, P.; Hays, C. Are mangroves nursery habitat for transient fishes and decapods? Wetlands 2003, 23, 449–458. [Google Scholar] [CrossRef]
- Islam, S.; Wahab, A. A review on the present status and management of mangrove wetland habitat resources in Bangladesh with emphasis on mangrove fisheries and aquaculture. Hydrobiologia 2005, 542, 165–190. [Google Scholar] [CrossRef]
- Rudianto, R.; Bengen, D.G.; Kurniawan, F. Causes and Effects of Mangrove Ecosystem Damage on Carbon Stocks and Absorption in East Java, Indonesia. Sustainability 2020, 12, 10319. [Google Scholar] [CrossRef]
- Alauddin, M.; Hamid, M.A. Shrimp culture in Bangladesh with emphasis on social and economic aspects. In Towards Sustainable Shrimp Culture in Thailand and the Region; Smith, P.T., Ed.; ACIAR Proceedings No. 90; Australian Centre for International Agricultural Research: Canberra, Australia, 2000; pp. 53–62. [Google Scholar]
- Bondad-Reantaso, M.G.; Subasinghe, R.P.; Josupeit, H.; Cai, J.; Zhou, X. The role of crustacean fisheries and aquaculture in global food security: Past, present and future. J. Invertebr. Pathol. 2012, 110, 158–165. [Google Scholar] [CrossRef]
- Gilbert, A.J.; Janssen, R. Use of environmental functions to communicate the values of a mangrove ecosystem under different management regimes. Ecol. Econ. 1998, 25, 323–346. [Google Scholar] [CrossRef]
- Macusi, E.D.; Katikiro, R.E.; Deepananda, K.H.M.A.; Jiminez, L.A.; Conte, A.R.; Fadli, N. Human Induced Degradation of Coastal Resources in Asia Pacific and Implications on Management and Food Security. J. Nat. Stud. 2011, 9, 10. [Google Scholar]
- Shinn, A.P.; Pratoomyot, J.; Griffiths, D.; Trong, T.Q.; Vu, N.T.; Jiravanichpaisal, P.; Briggs, M. Asian Shrimp Production and the Economic Costs of Disease. Asian Fish. Sci. 2018, 31S, 29–58. [Google Scholar] [CrossRef]
- Dabu, I.M.; Lim, J.J.; Arabit, P.M.T.; Orense, S.J.A.B.; Tabardillo, J.A.; Corre, V.L.; Maningas, M.B.B. The first record of acute hepatopancreatic necrosis disease in the Philippines. Aquac. Res. 2015, 48, 792–799. [Google Scholar] [CrossRef]
- De La Peña, L.D.; Lavilla-Pitogo, C.R.; Villar, C.B.R.; Paner, M.G.; Sombito, C.D.; Capulos, G.C. Prevalence of white spot syndrome virus (WSSV) in wild shrimp Penaeus monodon in the Philippines. Dis. Aquat. Org. 2007, 77, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Flegel, T.W.; Nielsen, L.; Thamavit, V.; Kongtim, S.; Pasharawipas, T. Presence of multiple viruses in non-diseased, cultivated shrimp at harvest. Aquaculture 2004, 240, 55–68. [Google Scholar] [CrossRef]
- Cao, L. Characterization of Chinese Shrimp Farming Systems towards Typology, Economic And Social Sustainability. Farming Shrimp For The Future: A Sustainability Analysis of Shrimp Farming In China. Doctoral Thesis, University of Michigan, Ann Arbor, MI, USA, 2012; pp. 103–131. [Google Scholar]
- White, P.; Phillips, M.; Beveridge, M. Review of Environmental Impact, Site Selection and Carrying Capacity Estimation for Small Scale Aquaculture in Asia FAO Expert Workshop on Site Selection and Carrying Capacity Estimates for Inland and Coastal Waterbodies. 2010. Available online: https://www.worldfishcenter.org/publication/review-environmental-impact-site-selection-and-carrying-capacity-estimation-small-scale (accessed on 9 March 2022).
- Rapsomanikis, G. The Economic Lives of Smallholder Farmers. Food and Agriculture Organization. 2015. Available online: www.fao.org/publications (accessed on 9 March 2022).
- Eid, A.; Ali, B.; Al Sayed, K.; Marzok, S.; Khames, M.; Khames, D. Stocking density, Survival rate and Growth performance feed utilization and economic evaluation of Litopenaeus vannamei (Boon, 1931) in different cultured shrimp farms in Suez Canal Region. Egypt. J. Aquac. 2020, 10, 96–114. [Google Scholar] [CrossRef]
- Ruiz-Velazco, J.M.; Hernández-Llamas, A.; Gomez-Muñoz, V.M.; Magallon, F.J. Dynamics of intensive production of shrimp Litopenaeus vannamei affected by white spot disease. Aquaculture 2010, 300, 113–119. [Google Scholar] [CrossRef]
- Smith, M.D.; Roheim, C.A.; Crowder, L.B.; Halpern, B.S.; Turnipseed, M.; Anderson, J.L.; Asche, F.; Bourillón, L.; Guttormsen, A.G.; Khan, A.; et al. Sustainability and Global Seafood. Science 2010, 327, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.G.; Vogl, C.R. Impacts of shrimp farming in Bangladesh: Challenges and alternatives. Ocean Coast. Manag. 2011, 54, 201–211. [Google Scholar] [CrossRef]
- Graslund, S.; Bengtsson, B.E. Chemicals and biological products used in south-east Asian shrimp farming, and their potential impact on the environment—A review. Sci. Total Environ. 2001, 280, 93–131. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.; Mattii, G. Application of Zeolites in Agriculture and Other Potential Uses: A Review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Gräslund, S.; Holmström, K.; Wahlström, A. A field survey of chemicals and biological products used in shrimp farming. Mar. Pollut. Bull. 2003, 46, 81–90. [Google Scholar] [CrossRef]
- Maralit, B.A.; Caipang, C.M.A.; Santos, M.D.; Maningas, M.B.B. PCR detection of white spot syndrome virus (WSSV) from farmed Pacific white shrimp (Litopenaeus vannamei) in selected sites of the Philippines. Aquac. Aquar. Conserv. Legis. Int. J. Bioflux Soc. 2011, 4, 474–480. Available online: http://www.bioflux.com.ro/aacl (accessed on 9 March 2022).
- Lavilla Pitogo, C.R.; Lio Po, G.D.; Cruz Lacierda, E.R.; Alapide Tendencia, E.V.; de la Peña, L.D. Diseases of Penaeid Shrimps in the Philippines; SEAFDEC: Tigbauan, Iloilo, Philippines, 2000. [Google Scholar]
- Natividad, K.D.T.; Migo, M.V.P.; Albaladejo, J.D.; Magbanua, J.P.V.; Nomura, N.; Matsumura, M. Simultaneous PCR detection of two shrimp viruses (WSSV and MBV) in postlarvae of Penaeus monodon in the Philippines. Aquaculture 2006, 257, 142–149. [Google Scholar] [CrossRef]
- Corre, V. The Shrimp Farming Industry in The Philippines. In Proceedings of the Aquaculture Workshop for SEAFDEC/AQD Training Alumni, Iloilo, Philippines, 8–11 September 1992; SEAFDEC Aquaculture Department: Iloilo, Philippines; pp. 88–103. [Google Scholar]
- Hall, G.; Laddu, D.R.; Phillips, S.A.; Lavie, C.J.; Arena, R. A tale of two pandemics: How will COVID-19 and global trends in physical inactivity and sedentary behavior affect one another? Prog. Cardiovasc. Dis. 2021, 64, 108–110. [Google Scholar] [CrossRef]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, A.J.G.; Pomeroy, R.; Akester, M.J.; Muawanah, U.; Chumchuen, W.; Lee, W.C.; Hai, P.G.; Viswanathan, K.K. COVID-19 and Small-Scale Fisheries in Southeast Asia: Impacts and Responses. Asian Fish. Sci. 2021, 34, 99–113. [Google Scholar] [CrossRef]
- Macusi, E.D.; Siblos, S.K.V.; Betancourt, M.E.; Macusi, E.S.; Calderon, M.N.; Bersaldo, M.J.I.; Digal, L.N. Impacts of COVID-19 on the Catch of Small-Scale Fishers and Their Families Due to Restriction Policies in Davao Gulf, Philippines. Front. Mar. Sci. 2022, 8, 770543. [Google Scholar] [CrossRef]
- Macusi, E.D.; Rafon, J.K.A.; Macusi, E.S. Impact of COVID-19 and closed fishing season on commercial fishers of Davao Gulf, Mindanao, Philippines. Ocean Coast. Manag. 2021, 217, 105997. [Google Scholar] [CrossRef]
- Manlosa, A.O.; Hornidge, A.-K.; Schlüter, A. Aquaculture-capture fisheries nexus under COVID-19: Impacts, diversity, and social-ecological resilience. Marit. Stud. 2021, 20, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.; Vishwakarma, D.K.; Singh, N. COVID-19 and its impact on education, social life and mental health of students: A survey. Child. Youth Serv. Rev. 2020, 121, 105866. [Google Scholar] [CrossRef]
- Kapasia, N.; Paul, P.; Roy, A.; Saha, J.; Zaveri, A.; Mallick, R.; Barman, B.; Das, P.; Chouhan, P. Impact of lockdown on learning status of undergraduate and postgraduate students during COVID-19 pandemic in West Bengal, India. Child. Youth Serv. Rev. 2020, 116, 105194. [Google Scholar] [CrossRef]
- Aucejo, E.M.; French, J.; Ugalde Araya, M.P.; Zafar, B. The impact of COVID-19 on student experiences and expectations: Evidence from a survey. J. Public Econ. 2020, 191, 104271. [Google Scholar] [CrossRef]
- Bestiantono, D.S.; Agustina, P.Z.R.; Cheng, T.-H. How Students’ Perspectives about Online Learning Amid the COVID-19 Pandemic? Stud. Learn. Teach. 2020, 1, 133–139. [Google Scholar] [CrossRef]
- Lee, J. Mental health effects of school closures during COVID-19. Lancet Child Adolesc. Health 2020, 4, 421. [Google Scholar] [CrossRef]
- Sunny, A.R.; Sazzad, S.A.; Prodhan, S.H.; Ashrafuzzaman, M.; Datta, G.C.; Sarker, A.K.; Rahman, M.; Mithun, M.H. Assessing impacts of COVID-19 on aquatic food system and small-scale fisheries in Bangladesh. Mar. Policy 2021, 126, 104422. [Google Scholar] [CrossRef] [PubMed]
- Roubík, H.; Lošťák, M.; Ketuama, C.T.; Procházka, P.; Soukupová, J.; Hakl, J.; Karlík, P.; Hejcman, M. Current coronavirus crisis and past pandemics—What can happen in post-COVID-19 agriculture? Sustain. Prod. Consum. 2022, 30, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Howarth, W.; Hernandez, R.; van Houtte, A. Legislation Governing Shrimp Aquaculture: Legal Issues, National Experiences and Options. FAO Legal Papers Online, 149. 2001. Available online: http://www.fao.org/fileadmin/user_upload/legal/docs/lpo18.pdf (accessed on 15 April 2022).
- Congress of the Philippines. The Philippine Fisheries Code of 1998 “An Act Providing for the Development, Management and Conservation of the Fisheries and Aquactic Resources, Integrating All Laws Pertinent Thereto and for Other Purposes” (Republic Act no. 8550); Congress of the Philippines: Quezon City, Philippines, 1998. Available online: https://www.officialgazette.gov.ph/1998/02/25/republic-act-no-8550/ (accessed on 15 April 2022).



| Variables | Smallholder | Commercial |
|---|---|---|
| Farm area (ha) | 0.3–3.0 | 4.8–50 |
| Number of croppings per year | 1–3 | 2–3 |
| Yield per cropping (kg/ha) | 10,000 | 24,000 |
| Revenue per ha/cropping (US$) | 62,814 | 150,754 |
| Average income per ha/cropping (US$) | 20,938 | 60,302 |
| Number of employed individuals | 1–10 | 8–71 |
| Stocking density (* PL/m2) | 25–200 | 60–350 |
| Aeration | Paddle wheel | Paddle wheel, Blower |
| Number of ponds | 1–5 | 3–38 |
| Average pond size (m2) | 4000–5000 | 2000–3000 |
| Shape of pond | Rectangular, Irregular | Rectangular, Circular |
| Use of supplements | Probiotic, Molasses, Fermentation | Probiotic, Molasses |
| Commercial feed (kg/100,000PL/crop) | 1000–3000 | 850–2650 |
| Days of culture period | 70–110 | 80–100 |
| Average body weight (g) | 14–30 | 27–35 |
| Factors | Impacts | Suggested Solution |
|---|---|---|
| Disease | Shrimp death | Proper cultural management Strict implementation of biosecurity and government intervention |
| Overstocking | Lack of oxygen | Following the standard stocking density suitable to the capacity of the pond area and availability of aerator |
| Waste disposal | Increased contamination | Total removal of excess solid or liquid waste inside the pond Construction of sludge pond/reservoir for solid and liquid pond waste Treating the wastewater before the release into the waterways |
| Water quality | Causes diseases on shrimp Stunted/low growth rate | Water quality testing kit/lab to regularly monitor the optimum level of water parameters in the pond. |
| Pollution | Low survival rate of shrimp post-larvae | Good source of water |
| Source of fry | Insufficient supply Potential in carrying disease | Accessible hatchery Disease-free, high-quality, and cheaper source of fry |
| High cost of inputs | Limited and insufficient supply of inputs required for better production Production declines | Access to a cheaper supply input for production Other alternatives to lessen the cost of production |
| Lack of capital | Disable the farmers to increase their production Unable to support the needs of the farm | Access to credit (money/in kind) |
| Low market demand | Low profitability | Processing plant for shrimp produced. |
| Pandemic | Decreasing buyer due to mobility restriction It prolongs the culture period of shrimps Led to an increase in input cost Low market price | Government policy to loosen the mobility restriction to allow the people to work and earn for their necessities, as well as to enable the transportation of goods in and out of the town Government assistance to help the shrimp farmers that are affected by the pandemic overcome associated obstacles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clapano, M.B.; Diuyan, J.M.T.; Rapiz, F.G.B.; Macusi, E.D. Typology of Smallholder and Commercial Shrimp (Penaeus vannamei) Farms, including Threats and Challenges in Davao Region, Philippines. Sustainability 2022, 14, 5713. https://doi.org/10.3390/su14095713
Clapano MB, Diuyan JMT, Rapiz FGB, Macusi ED. Typology of Smallholder and Commercial Shrimp (Penaeus vannamei) Farms, including Threats and Challenges in Davao Region, Philippines. Sustainability. 2022; 14(9):5713. https://doi.org/10.3390/su14095713
Chicago/Turabian StyleClapano, Misael B., Jenie Mae T. Diuyan, France Guillian B. Rapiz, and Edison D. Macusi. 2022. "Typology of Smallholder and Commercial Shrimp (Penaeus vannamei) Farms, including Threats and Challenges in Davao Region, Philippines" Sustainability 14, no. 9: 5713. https://doi.org/10.3390/su14095713
APA StyleClapano, M. B., Diuyan, J. M. T., Rapiz, F. G. B., & Macusi, E. D. (2022). Typology of Smallholder and Commercial Shrimp (Penaeus vannamei) Farms, including Threats and Challenges in Davao Region, Philippines. Sustainability, 14(9), 5713. https://doi.org/10.3390/su14095713







