Carbon Soil Storage and Technologies to Increase Soil Carbon Stocks in the South American Savanna
Abstract
1. Introduction
- How much carbon can be sequestered by the soil in the Cerrado biome? What is its largest manageable carbon pool?
- What current agriculture technologies and land management models are capable of increasing soil carbon stocks in the Cerrado biome?
- What type of its native vegetation can store the most carbon in the soil?
2. Material and Methods
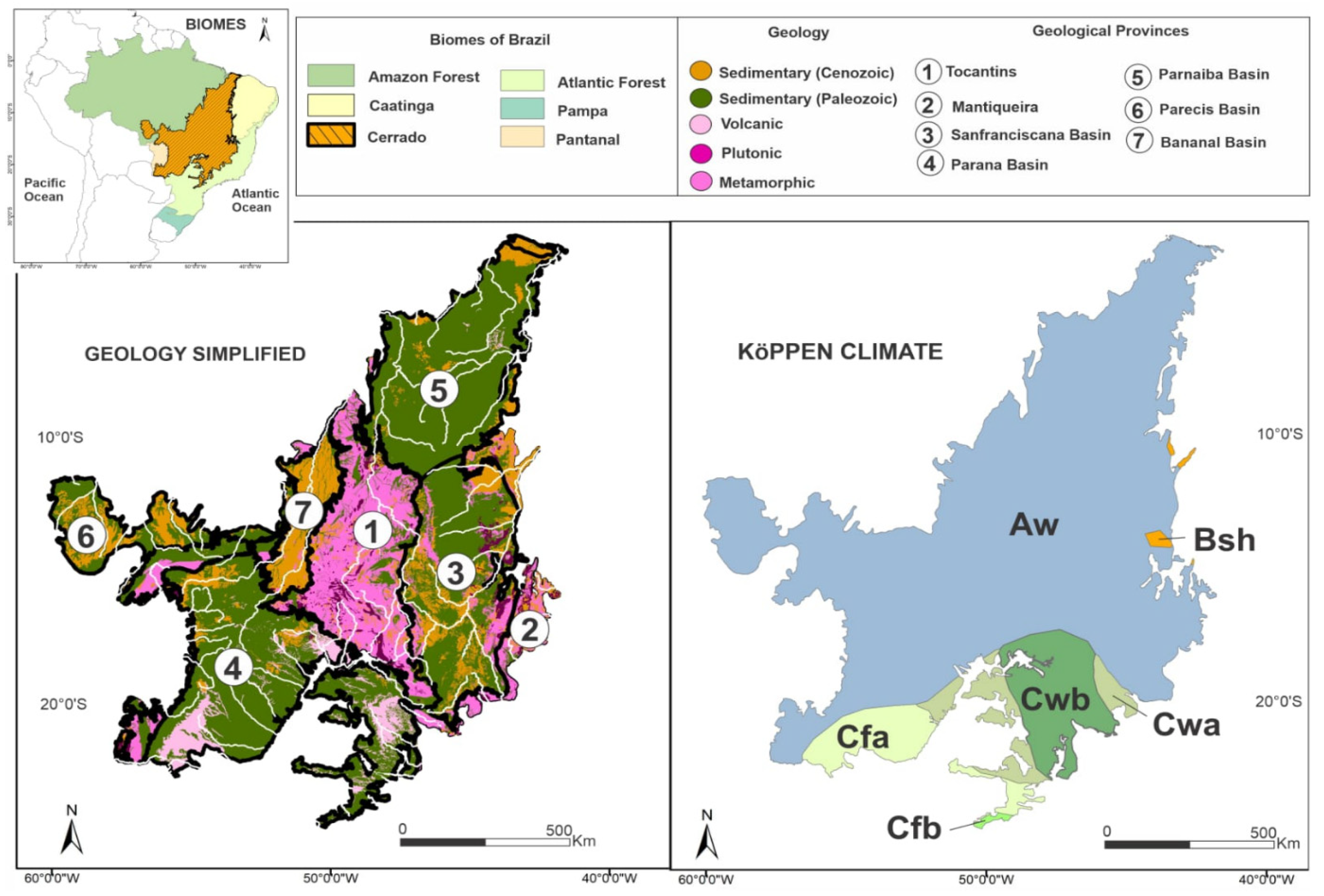
2.1. Study Area
2.2. Methods
3. Results and Discussion
3.1. Total Soil Carbon Stocks to Cerrado Area, Agricultural Land, and Native Vegetation
3.2. Vertical Distribution of Soil Carbon Stocks in the Cerrado Biome
3.3. Soil Carbon Stocks in Native Vegetation
3.4. Soil Carbon Stocks for the Planted Forest, Agroforest, Pasture, and Perennial Crops
3.5. Soil Carbon Stocks for Tillage, and No-Tillage Systems
3.6. Causes of the Decline of Soil Carbon Stocks in Pastures
3.7. Potential and Limitations of Soil Carbon Storage in No-Till
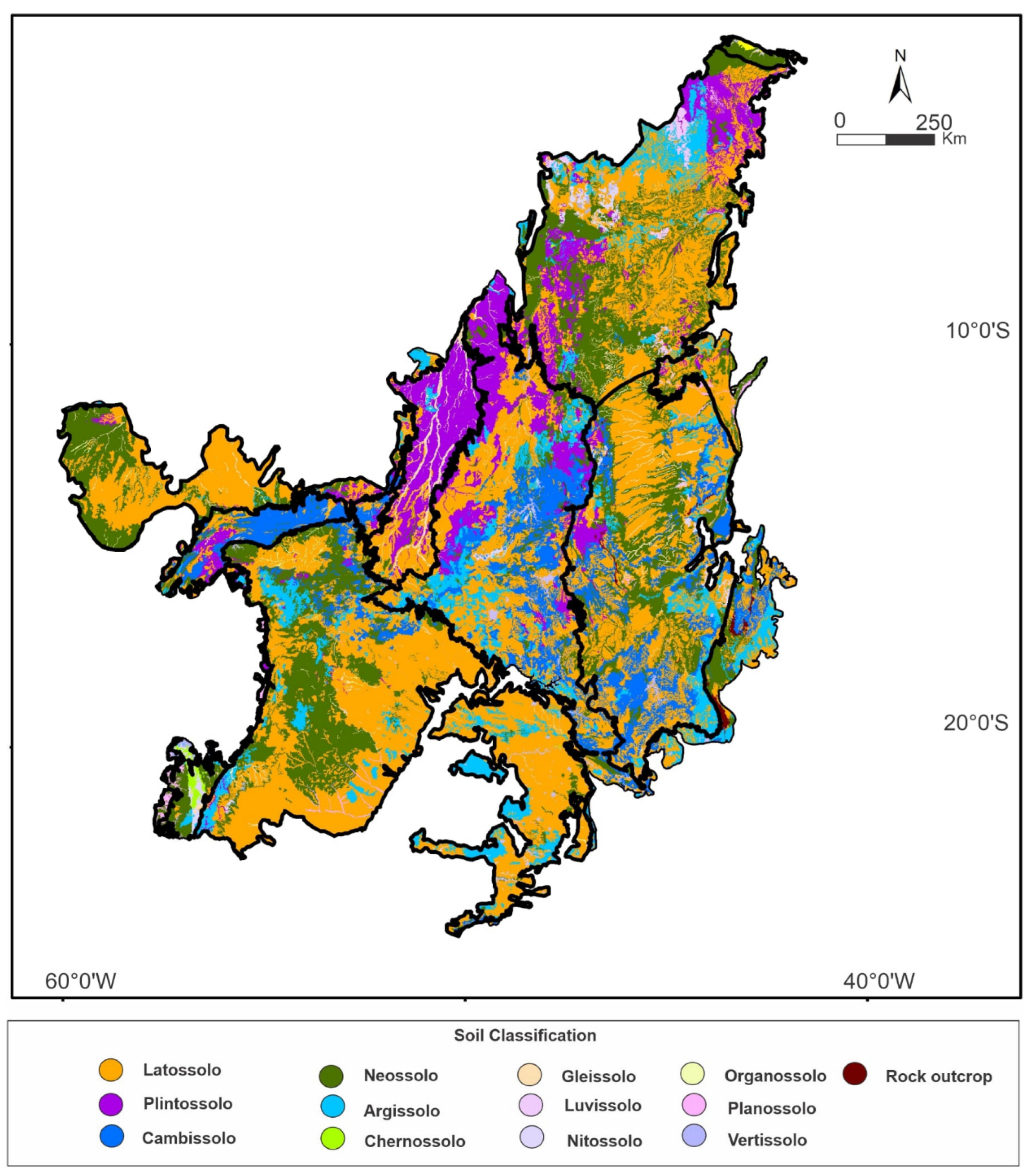
3.8. Relation of Soil Type and Roots Depth in the Soil Carbon Stocks
3.9. Role of Charcoal and the Variability among Soil Carbon Stocks Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Word Meteorological Organization. The State of Greenhouse Gases in the Atmosphere Based on Global Observations through 2018. WMO Greenh. Gas Bull. 2019, 15, 1–8. Available online: https://library.wmo.int/doc_num.php?explnum_id=10100 (accessed on 4 November 2021).
- Word Meteorological Organization. The State of Greenhouse Gases in the Atmosphere Based on Global Observations through 2020. WMO Greenh. Gas Bull. 2020, 17, 1–8. Available online: https://library.wmo.int/doc_num.php?explnum_id=10870 (accessed on 4 November 2021).
- Food and Agriculture Organization of the United Nations. 2016 The State of Food and Agriculture: Climate Change, Agriculture and Food Security; Food and Agriculture Organization: Rome, Italy, 2006. Available online: http://www.fao.org/3/a-i6030e.pdf (accessed on 4 November 2021).
- Beuchle, R.; Grecchi, R.C.; Shimabukuru, Y.E.; Seliger, R.; Eva, H.D.; Sano, E.; Achard, F. Land cover changes in the Brazilian Cerrado and Caatinga biomes from 1990 to 2010 based on a systematic remote sensing sampling approach. Appl. Geogr. 2015, 58, 116–127. [Google Scholar] [CrossRef]
- Souza, C.M., Jr.; Shimbo, J.Z.; Rosa, M.R.; Parente, L.L.; Alencar, A.A.; Rudor, B.F.T.; Hasenack, H.; Matsumoto, M.; Ferreira, L.G.; Souza-Filho, P.W.M.; et al. Reconstructing Three Decades of Land Use and Land Cover Changes in Brazilian Biomes with Landsat Archive and Earth Engine. Remote Sens. 2020, 12, 2735. [Google Scholar] [CrossRef]
- Ministério da Ciência Tecnologia e Inovação. Quarto Inventario Nacional Emissões de GGE por Setor 2016. Available online: https://www.gov.br/mcti/pt-br/acompanhe-o-mcti/sirene/emissoes/emissoes-de-gee-por-setor-1 (accessed on 4 November 2021).
- Kutsch, W.; Banh, M.; Heinemeyer, A. Soil Carbon Dynamics: An Integrated Methodology; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Eiten, G. The Cerrado vegetation of Brazil. Bot. Rev. 1972, 38, 201–341. Available online: https://link.springer.com/content/pdf/10.1007/BF02859158.pdf (accessed on 4 November 2021). [CrossRef]
- Castro, E.A.; Kauffman, J.B. Ecosystem structure in the Brazilian Cerrado: A vegetation gradient of aboveground biomass. root mass and consumption by fire. J. Trop. Ecol. 1998, 14, 263–283. [Google Scholar] [CrossRef]
- Klink, C.A.; Machado, R.B. Conservation of the Brazilian Cerrado. Conserv. Biol. 2005, 19, 707–713. [Google Scholar] [CrossRef]
- Ribeiro, J.F.; Walter, B.M.T. As principais fitofisionomias do bioma Cerrado. In Cerrado: Ecologia e Flora; Sano, S.M., Almeida, S.P., Ribeiro, J.F., Eds.; EMBRAPA Cerrados: Brasília, Brazil, 2008; pp. 153–212. Available online: https://www.researchgate.net/publication/283072910_As_principais_fitofisionomias_do_bioma_Cerrado (accessed on 3 February 2022).
- Keller, M.; Palace, M.; Hurtt, G. Biomass in the Tapajos National Forest. Brazil: Examination of sampling and allometric uncertainties. For. Ecol. Manag. 2001, 154, 371–382. [Google Scholar] [CrossRef]
- Palace, M.; Keller, M.; Hurtt, G.; Frolking, S. A Review of Above Ground Necromass in Tropical Forests. In Tropical Forests; Sudarshana, P., Nageswara-Rao, M., Soneji, J.R., Eds.; InTech: Rijeka, Croatia, 2012; pp. 215–252. [Google Scholar] [CrossRef]
- Miranda, S.C.; Bustamante, M.; Palace, M.; Hagen, S.; Keller, M.; Ferreira, L.G. Regional Variations in Biomass Distribution in Brazilian Savanna Woodland. BIOTROPICA 2014, 46, 125–138. [Google Scholar] [CrossRef]
- Felfili, J.M.; Silva, M.C., Jr. Diversidade alfa e beta no cerrado sensu strictu, DF, GO, MG e BA. In Cerrado: Ecologia. Biodiversidade e Conservação; Scariot, A.A., Sousa-Silva, J.C., Felfili, J.M., Eds.; Ministério do Meio Ambiente: Brasília, Brazil, 2005; pp. 143–154. Available online: https://files.cercomp.ufg.br/weby/up/284/o/Cerrado_Parte1.pdf (accessed on 4 November 2021).
- IBGE. Biomas e Sistema Costeiro-Marinho do Brasil. Available online: https://www.ibge.gov.br/apps/biomas/ (accessed on 4 November 2021).
- Schobbenhaus, C.; Bley, B.; Neves, B. A geologia do Brasil no contexto da plataforma Sul-americana. In Geologia, Tectônica e Recursos Minerais do Brasil; Bizzi, L.A., Schobbenhaus, C., Vidotti., R.M., Gonçalves., J.H., Eds.; CPRM: Brasília, Brazil, 2003; pp. 5–25. Available online: https://rigeo.cprm.gov.br/handle/doc/5006 (accessed on 3 February 2022).
- Köppen, W.; Geiger, R. Klimate der Erde (Wall-Map 150 cm × 200 cm); Verlag Justus Perthes: Gotha, Germany, 1928. [Google Scholar]
- Valente, C.R.; Lattubese, E.M. Fluvial archive of peculiar avulsive fluvial patterns in the largest Quaternary intracratonic basin of tropical South America: The Bananal Basin. Central-Brazil. Paleogeogr. Paleoclimatol. Paleoecol. 2012, 356–357, 62–74. [Google Scholar] [CrossRef]
- EMBRAPA. Cerrado: Correção do Solo e Adubação; Embrapa Informação Tecnológica: Brasília, Brazil, 2004; Available online: https://www.embrapa.br/en/busca-de-publicacoes/-/publicacao/555355/cerrado-correcao-do-solo-e-adubacao (accessed on 3 February 2022).
- EMBRAPA. Brazilian Soil Classification System, 5th ed.; Embrapa: Brasília, Brazil, 2018; Available online: https://www.redeilpf.org.br/arquivos/SiBCS-2018-ISBN-9788570358219-english.pdf (accessed on 3 February 2022).
- FAO. World Reference Base for Soil Resources 2014; International Soil Classification System for Naming Soils and Creating Legends for Soil Maps (Update 2015). Word Soil Resources Reports 106. 2014. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 3 February 2022).
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 1999. Available online: https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_051232.pdf (accessed on 3 February 2022).
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/survey/class/?cid=nrcs142p2_053580 (accessed on 3 February 2022).
- Santos, H.G.; Carvalho, W., Jr.; Áglio, M.L.D.; Silva, J.S.; Dart, R.O.; Pares, J.G.; Fontana, A.; Martins, A.L.S.; Oliveira, A.P. O novo Mapa de Solos do Brasil: Legenda Atualizada; Embrapa Solos: Rio de Janeiro, Brazil, 2011; Available online: https://www.embrapa.br/en/busca-de-publicacoes/-/publicacao/920267/o-novo-mapa-de-solos-do-brasil-legenda-atualizada (accessed on 3 February 2022).
- Sato, J.H.; de Figueiredo, C.C.; Marchão, R.L.; Madari, B.E.; Benedito, L.E.C.; Busato, J.G.; de Souza, D.M. Methods of soil organic carbon determination in Brazilian savannah soils Soils and Plant Nutrition. Sci. Agric. 2014, 71, 302–308. [Google Scholar] [CrossRef]
- Gatto, A.; de Barros, F.N.; Novais, R.F.; Silva, I.R.; Mendonça, E.S.; Villani, E.M.A. Comparison of methods for determination of organic carbon in soils under eucalypt plantations. Rev. Bras. Ciênc. Solo 2009, 33, 735–740. [Google Scholar] [CrossRef]
- Bernoux, M.; Cerri, C.C.; Cerri, C.E.P.; Neto, M.S.; Metay, A.; Perrin, A.-S.; Scopel, E.; Razafimbelo, T.; Blavet, D.; Piccolo, M.D.C.; et al. Cropping systems, carbon sequestration and erosion in Brazil, a review. Agron. Sustain. Dev. 2006, 26, 1–8. [Google Scholar] [CrossRef]
- Scaramuzza, C.A.M.; Sano, E.E.; Adami, M.; Bolfe, E.L.; Coutinho, A.C.; Esquerdo, J.C.D.M.; Maurano, L.E.P.; Narvaes, I.S.; Oliveira Filho, F.J.B.; Rosa, R.; et al. Land-use and land-cover mapping of the brazilian cerrado based mainly on landsat-8 satellite images. Rev. Bras. De Cartogr. 2017, 69, 1041–1051. Available online: https://seer.ufu.br/index.php/revistabrasileiracartografia/article/view/44309 (accessed on 3 February 2022).
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Dionizio, E.A.; Pimenta, F.M.; Lima, L.B.; Costa, M.H. Carbon stocks and dynamics of different land uses on the Cerrado agricultural frontier. PLoS ONE 2020, 15, e0241637. [Google Scholar] [CrossRef]
- Braz, S.P.; Urquiagua, S.; Alves, B.J.B.; Jantalia, C.P.; Guimarães, A.P.; Santos, C.A.; Santos, S.C.; Pinheiro, É.F.M.; Boddey, R.M. Soil Carbon Stocks under Productive and Degraded Pastures in the Brazilian Cerrado. Soil Sci. Soc. Am. J. 2013, 77, 914–928. [Google Scholar] [CrossRef]
- Bayer, C.; Martin-Neto, L.; Mielcizuk, J.; Pavinato, A.; Dieckow, J. Carbon sequestration in two Brazilian Cerrado soils under no-till. Soil Tillage Res. 2006, 86, 237–245. [Google Scholar] [CrossRef]
- Bogiani, J.C.; Ferreira, A.C.B.; Borin, A.L.D.C.; Sofiatti, V. Sequestro de Carbono em Sistemas de Produção de Soja. Milho e Algodão em Solo Arenoso do Cerrado da Bahia; EMBRAPA Territorial Campinas: Campinas, Brazil, 2020; Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/223222/1/5317.pdf (accessed on 3 February 2022).
- Zinn, Y.L.; Resck, D.V.S.; Silva, J.E. Soil Organic Carbon as Affected by Afforestation with Eucalyptus and Pinus in the Cerrado Region of Brazil. For. Ecol. Manag. 2002, 166, 285–294. [Google Scholar] [CrossRef]
- Lardy, L.C.; Brossard, M.; Assad, M.L.L.; Laurent, J.Y. Carbon and Phosphorus Stocks of Clayey Ferralsols in Cerrado Native and Agroecosystems. Brazil. Agric. Ecosyst. Environ. 2002, 92, 147–158. [Google Scholar] [CrossRef]
- Meirelles, M.L.; Ferreira, E.A.B.; Franco, A.C. Dinâmica Sazonal do Carbono em Campo Úmido do Cerrado; EMBRAPA Cerrados: Planaltina, DF, USA, 2016; Available online: https://www.embrapa.br/en/busca-de-publicacoes/-/publicacao/570281/dinamica-sazonal-do-carbono-em-campo-umido-do-cerrado (accessed on 3 February 2022).
- França, A.M.S.; Paiva, R.J.O.; Sano, E.E.; Carvalho, A.M. Estimates for Carbon Stocks in Soil under Humid Grassland Areas in the Federal District of Brazil. Open J. Ecol. 2014, 4, 777–787. [Google Scholar] [CrossRef]
- Rossi, Q.C.; Pereira, M.G.; Giácomo, S.G.; Betta, M.; Polidoro, J.C. Frações orgânicas e índice de manejo de carbono do solo em Latossolo Vermelho sob plantio de soja no cerrado goiano. Rev. Bras. De Ciências Agrárias Recife 2021, 7, 2332–2341. [Google Scholar] [CrossRef]
- Tonussi, R.; Nair, P.K.R.; Nair, V.D.; Garcia, R.; Bernardino, F.S. Soil Carbon Storage in Silvopasture and Related Land-Use Systems in the Brazilian Cerrado. J. Environ. Qual. 2011, 40, 833–841. [Google Scholar] [CrossRef]
- Castro, E.A. Biomass, Nutrient Pools and Response to Fire in the Brazilian Cerrado. Master’s Dissertation, Oregon State University, Corvallis, OR, USA, 1996. [Google Scholar]
- Moraes, V.A.; Scolforo, J.R.S.; Silva, C.A.; de Mello, J.M.; Gomide, L.R.; Oliveira, A.D. Carbon and biomass stocks in a fragmente of cerradão in Minas Gerais. Brazil. Cerne 2013, 19, 237–245. [Google Scholar] [CrossRef]
- Brito, C.F.B.; Fonseca, V.A.; da Silva, N.P.; Braga, S.S.; Godinho, F.L.; Kondo, M.K.; Portugal, A.F.; Megda, M.X.V. Alterações físicas e biológicas de um Eutrustox (Latossolo) sob plantio direto e preparo convencional no semiárido brasileiro. Cienc. Del Suelo 2018, 36, 148–155. Available online: http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S1850-20672018000200014 (accessed on 3 February 2022).
- Neves, C.M.N.; Silva, M.L.N.; Curi, N.; Macedo, E.L.G.; Tokura, A.M. Estoque de carbono em sistemas agrossilvopastoril. pastagem e eucalipto sob cultivo convencional na região noroeste do estado de Minas Gerais. Ciência e Agrotecnologia 2004, 28, 1038–1046. [Google Scholar] [CrossRef]
- Leite, L.F.C.; de Arruda, F.P.; Costa, C.N.; Ferreira, J.S.; Netto, M.R.H. Qualidade química do solo e dinâmica de carbono sob monocultivo e consórcio de macaúba e pastagem. Rev. Bras. De Eng. Agrícola E Ambient. 2013, 17, 1257–1263. [Google Scholar] [CrossRef][Green Version]
- Fialho, R.C.; Teixeira, R.S.; Teixeira, A.P.M.; Reis, T.G.; Silva, I.R. Fertilization and Irrigation Affect Soil Carbon under Eucalyptus Plantation in the Cerrado. Floresta e Ambiente 2019, 26, e20170679. [Google Scholar] [CrossRef]
- Paiva, A.P.; Faria, G.E. Estoque de carbono do solo sob cerrado sensu stricto no Distrito Federal. Brasil. Rev. Trópica—Ciências Agrárias E Biológicas 2007, 1, 59–65. Available online: https://www.researchgate.net/publication/265583503_Estoque_de_carbono_do_solo_sob_cerrado_sensu_stricto_no_Distrito_Federal_Brasil (accessed on 3 February 2022).
- Corazza, E.J.; Silva, J.E.; Resck, D.V.S.; Gomes, A.C. Comportamento de diferentes sistemas de manejo como fonte ou depósito de carbono em relação à vegetação de Cerrado. Rev. Bras. De Ciências Do Solo 1999, 23, 425–432. [Google Scholar] [CrossRef]
- Silva, L.C.R.; Sternberg, L.; Haridasan, M.; Hoffmann, W.A.; Miralles-Wilhelm, F.; Franco, A.C. Expansion of Gallery Forests into Central Brazilian Savannas. Glob. Chang. Biol. 2008, 14, 2108–2118. [Google Scholar] [CrossRef]
- Ferreira, E.A.B.; Bustamante, M.M.C.; Resck, D.V.S.; Figueiredo, C.C.; Pinto, A.S.; Malaquias, J.V. Carbon Stocks in Compartments of Soil Organic Matter 31 Years after Substitution of Native Cerrado Vegetation by Agroecosystems. Rev. Bras. De Ciência Solo 2016, 40, e0150059. [Google Scholar] [CrossRef]
- Ozorio, J.M.B.O.; Rosset, J.S.; Schiavo, J.A.; Panachuki, E.; Souza, C.B.S.; Menezes, R.S.; Ximenes, T.S.; Castilho, S.C.P.; Marra, L.M. Estoque de carbono e agregação do solo sob fragmentos florestais nos biomas da Mata Atlântica e Cerrado. RBCIAM 2019, 53, 97–116. [Google Scholar] [CrossRef]
- Jantalia, C.P.; Resck, D.V.S.; Alves, B.J.R.; Zotarelli, L.; Urquiaga, S.; Boddey, R.M. Tillage effect on C stocks of a clayey Oxisol under a soybean-based crop rotation in the Brazilian Cerrado region. Soil Tillage Res. 2007, 95, 97–109. [Google Scholar] [CrossRef]
- Alves, R.P.; Junior, A.F.A.; Martins, E.S.; Nardoto, G.B. Role of soil carbon in the landscape functioning of the Alto São Bartolomeu watershed in the Cerrado region. Brazil. Pesqui. Agropecuária Bras. 2016, 51, 1241–1251. [Google Scholar] [CrossRef]
- Corbels, M.; Marchão, R.L.; Siqueira-Neto, M.; Ferreira, E.G.; Madari, B.E.; Scopel, E.; Brito, O.R. Evidence of limited carbon sequestration in soils under no-tillage systems in the cerrado of Brazil. Sci. Rep. 2016, 6, 21450. [Google Scholar] [CrossRef]
- Freixo, A.A.; Machado, P.L.O.A.; Guimarães, C.M.; Silva, C.A.; Fadigas, F.S. Estoques de Carbono e Nitrogênio e Distribuição de Frações Orgânicas de Latossolo do Cerrado sob Diferentes Sistemas de Cultivo. Rev. Bras. Ciênc. Solo 2002, 26, 425–434. [Google Scholar] [CrossRef]
- Bélizario, M.B.; Ferrão, G.E.; Cerri, C.C.; Siqueira-Neto, M. Soil carbon stocks cultivated with coffee in the Brazilian savanna: Effect of cultivation time and use of organic compost. Coffee Sci. 2018, 13, 53–62. [Google Scholar] [CrossRef]
- Freitas, R.C.A. Estoques de Carbono e Nitrogênio do solo e Fluxo de Gases do Efeito Estufa Em Solos Cultivados com Pinhão Mando (Jatropha spp.). Ph.D. Thesis, University of São Paulo, Piracicaba, Brazil, 2015. [Google Scholar]
- Marchão, R.L.; Becquer, T.; Brunet, D.; Balbino, L.C.; Vilela, L.; Brossard, M. Carbon and nitrogen stocks in a Brazilian clayey Oxisol: 13-year effects of integrated crop-livestock management systems. Soil Tillage Res. 2009, 103, 442–450. [Google Scholar] [CrossRef]
- Corbel, M.; Scopel, E.; Cardoso, A.; Bernoux, M.; Douzet, J.; Siqueira-Neto, M. Soil carbon storage potential of direct seeding mulch-based cropping systems in the Cerrados of Brazil. Glob. Chang. Biol. 2006, 12, 1773–1787. [Google Scholar] [CrossRef]
- d’Andréa, A.F.; Silva, M.L.; Curi, N.; Guilherme, L.G. Estoque de carbono e nitrogênio e formas de nitrogênio mineral em um solo submetido a diferentes sistemas de manejo. Pesq. Agropec. Bras. 2004, 39, 179–186. [Google Scholar] [CrossRef]
- Dieckow, J.; Bayer, C.; Conceição, P.C.; Zanatta, J.A.; Martin-Neto, L.; Milori, D.B.M.; Salton, J.C.; Macedo, M.M.; Mielniczuk, J.; Hernani, L.C. Land use. tillage. texture and organic matter stock and composition in tropical and subtropical Brazilian soils. Eur. J. Soil Sci. 2009, 60, 240–249. [Google Scholar] [CrossRef]
- Salton, J.C. Matéria Orgânica E Agregação No Solo Na Rotação Lavoura-Pastagem Em Ambiente Tropical. Ph.D. Thesis, University of Rio Grande do Sul, Porto Alegre, Brazil, 2005. [Google Scholar]
- Roscoe, R.; Buurman, P. Tillage effects on soil organic matter in density fractions of a Cerrado Oxisol. Soil Tillage Res. 2003, 104, 107–119. [Google Scholar] [CrossRef]
- Silva, J.E.; Resck, D.V.S.; Corazza, E.J.; Vivaldi, L. Carbon Storage in Clayey Oxisol Cultivated Pastures in the Cerrado Region, Brazil. Agric. Ecosyst. Environ. 2004, 103, 357–363. [Google Scholar] [CrossRef]
- Freitas, P.L.; Blancaneux, P.; Gavinelli, E.; Larré-Larrouy, M.-C.; Feller, C. Nível e natureza do estoque orgânico de latossolos sob diferentes sistemas de uso e manejo. Pesq. Agropec. Bras. 2000, 35, 157–170. [Google Scholar] [CrossRef]
- Pausas, J.G.; Bond, W.J. Alternative Biome States in Terrestrial Ecosystems. Trends Plant Sci. 2020, 25, 3. [Google Scholar] [CrossRef]
- Stape, J.L.; Binkley, D.; Ryanc, M.G.; Fonseca, S.; Loos, R.A.; Takahashi, E.N. The Brazil Eucalyptus Potential Productivity Project: Influence of water, nutrients and stand uniformity on wood production. For. Ecol. Manag. 2010, 259, 1684–1694. [Google Scholar] [CrossRef]
- Gatto, A.; Barros, N.F.; Novais, R.F.; Silva, I.R.; Leite, H.G.; Leite, F.P. Estoques de carbono no solo e na biomassa em plantações de eucalipto. Rev. Bras. Ciênc. Solo 2010, 34, 1069–1079. [Google Scholar] [CrossRef]
- Sausen, T.S.; Schaefer, G.F.P.; Tomazi, M.; Santos, L.S.; Bayer, C.; Rosa, L.M.G. Clay content drives carbon stocks in soils under a plantation of Eucalyptus saligna Labill. in southern Brazil. Acta Bot. Bras. 2004, 28, 266–273. [Google Scholar] [CrossRef]
- Isernhagem, E.C.C.; Rodrigues, R.A.R.; Diel, D.; Matos, E.S.; Conceição, M.C.G. Estoques de carbono lábil e total em solo sob integração lavoura-pecuária-floresta na região de Transição Cerrado/Amazônia. Nativa 2017, 5, 515–521. [Google Scholar] [CrossRef][Green Version]
- Lorenz, K.; Lal, R. Soil organic carbon sequestration in agroforestry systems. A review. Agron. Sustain. Dev. 2014, 34, 443–454. [Google Scholar] [CrossRef]
- Ferreira, A.C.B.; Borin, A.L.D.C.; Lamas, F.M.; Bogiani, J.C.; Silva, M.A.S.; Filho, J.L.S.; Staut, L.A. Soil carbon accumulation in cotton production systems in the Brazilian Cerrado. Acta Scientiarum. Agron. 2020, 42, e43039. [Google Scholar] [CrossRef]
- Rosa, R.; Sano, E.S.; Rosendo, J.S. Estoque de carbono em solos sob pastagens cultivadas na bacia hidrográfica do Rio Paranaíba. Soc. Nat. 2014, 26, 333–351. [Google Scholar] [CrossRef]
- Bayer, C.; Martin-Neto, L.; Mielniczuk, J.; Pavinato, A. Armazenamento de carbono em frações lábeis da matéria orgânica de um Latossolo Vermelho sob plantio direto. Pesqui. Agropecuária Bras. 2004, 39, 677–683. [Google Scholar] [CrossRef]
- Bilibio, W.D.; Corrêa, G.F.; Borges, E.N. Atributos Físicos e Químicos de um Latossolo. sob diferentes sistemas de cultivo. Ciênc. Agrotec. 2010, 34, 817–822. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Marchão, R.L.; Souza, K.W.; Bustamante, M.M.C. Soil fertility status. carbon and nitrogen stocks under cover crops and tillage regimes. Revista Ciência Agronômica 2014, 45, 914–921. [Google Scholar] [CrossRef]
- Silva, B.M.; Oliveira, G.C.; Serafim, M.E.; Carducci, C.E.; da Silva, E.A.; Barbosa, S.M.; de Melo, L.B.B.; dos Santos, W.J.R.; Pereira Reis, T.H.P.; de Oliveira, C.H.C.; et al. Soil Management and Water-Use Efficiency in Brazilian Coffee Crops. In Coffee-Production and Research; Castanheira, D.T., Ed.; IntechOpen: London, UK, 2019; pp. 1–20. [Google Scholar] [CrossRef]
- Kluthcouski, J.; Cobucci, T.; Aidar, H.; Yokoyama, L.P.; de Oliveira, I.P.; Costa, J.L.S.; da Silva, J.G.; Vilela, L.; Barcellos, A.O.; Magnabosco, C.U. Integração Lavoura-Pecuária Pelo Consórcio de Culturas Anuais Com Forrageiras, em Áreas de Lavoura, nos Sistemas plantio Direto e Convencional; EMBRAPA Arroz e Feijão: Santo Antônio de Goiás, Brazil, 2000; Available online: https://www.embrapa.br/en/busca-de-publicacoes/-/publicacao/208449/sistema-santa-fe---tecnologia-embrapa-integracao-lavoura-pecuaria-pelo-consorcio-de-culturas-anuais-com-forrageiras-em-areas-de-lavoura-nos-sistemas-direto-e-convencional (accessed on 3 February 2022).
- Galdos, M.V.; Brown, E.; Rosolem, C.A.; Pires, L.F.; Hallett, P.D.; Mooney, S.J. Brachiaria species influence nitrate transport in soil by modifying soil structure with their root system. Sci. Rep. 2020, 10, 5072. [Google Scholar] [CrossRef]
- Timossi, P.C.; Durigan, J.C.; Leite, G.J. Formação de palhada por braquiárias para adoção do sistema plantio direto. Bragantia 2007, 66, 617–622. [Google Scholar] [CrossRef]
- Balbinot, A.A., Jr.; Moraes, A.; Backes, R.L. Efeito de coberturas de inverno e sua época de manejo sobre a infestação de plantas daninhas na cultura de milho. Planta Daninha 2007, 25, 473–480. [Google Scholar] [CrossRef]
- Dalmago, G.A.; Bergamaschi, H.; Krüger, C.A.M.B.; Bergonci, J.I.; Comiran, F.; Heckler, B.M.M. Evaporação da água na superfície do solo em sistemas de plantio direto e preparo convencional. Pesq. Agropec. Bras. 2010, 45, 780–790. [Google Scholar] [CrossRef]
- Merlin, A.; He, Z.L.; Rosolem, C.A. Ruzigrass affecting soil-phosphorus availability. Pesq. Agropec. Bras. 2013, 48, 1583–1588. [Google Scholar] [CrossRef]
- Zomer, R.J.; Bossio, D.A.; Sommer, R.; Verchot, L.V. Global Sequestration Potential of Increased Organic Carbon in Cropland Soils. Sci Rep. 2017, 7, 15554. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, D.S.; da Silva, L.C.M.; de Melo, L.B.B.; Azevedo, R.P.; Araújo, B.C.L.; de Carvalho, T.S.; Moreira, S.M.; Curi, N.; Silva, B.M. Occasional tillage in no-tillage systems: A global meta-analysis. Sci. Total Environ. 2020, 745, 140887. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.A.; de Oliveira, G.M.; de Carvalho, L.B.; da Silva, M.F.G.F. Herbicide Resistance in Brazil: Status, Impacts, and Future Challenges. In Pests, Weeds and Diseases in Agricultural Crop and Animal Husbandry Production; Kontogiannatos, D., Kourti, A., Mendes, K.F., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Moreira, S.G. Desafios para a sustentabilidade dos sistemas de produção com culturas anuais. Inf. Agron. 2019, 4, 1–12. Available online: https://www.npct.com.br/publication/IASite.nsf/pub/available/IA-2019-4?OpenDocument&toc=2019 (accessed on 3 February 2022).
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregate, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Pinheiro, R.C.; Bouillet, J.-P.; Pivello, V.R.; Aló, L.L.; Costa, V.E.; Meersche, K.V.; Guerrini, I.A.; Laclau, J.-P. Roots take up labeled nitrogen from a depth of 9 m in a wooded savanna in Brazil. Soil Biol. Biochem. 2021, 160, 108282. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, A.; Qui, X.; Liu, Z.; Sun, J.; Zhang, J.; Wang, H. Distribution of roots and root length density in a maize/soybean strip intercropping system. Agric. Water Manag. 2010, 98, 199–212. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G. The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils. Org. Geochem. 2000, 31, 711–725. [Google Scholar] [CrossRef]
- Duxburry, J.M.; Smith, M.S.; Doran, J.W. Soil organic matter as a source and a sink of plant nutrients. In Dynamics of Soil Organic Matter in Tropical Ecosystems; Coleman, D.C., Oades, J.M., Uehara, G., Eds.; University of Hawaii Press: Honolulu, HI, USA, 1989; p. 3367. [Google Scholar]
- Cambardella, C.A.; Elliot, E.T. Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Batlle-Bayer, L.; Batjes, N.H.; Bindraban, P.S. Changes in organic carbon stocks upon land use conversion in the Brazilian Cerrado: A review. Agric. Ecosyst. Environ. 2010, 137, 47–58. [Google Scholar] [CrossRef]
- Costa Junior, C.; Corbeels, M.; Bernoux, M.; Píccolo, M.C.; Siqueira-Neto, M.; Feigl, B.J.; Cerri, C.E.P.; Cerri, C.C.; Scopel, E.; Lal, R. Assessing soil carbon storage rates under no-tillage: Comparing the synchronic and diachronic approaches. Soil Tillage Res. 2013, 134, 207–212. [Google Scholar] [CrossRef]
- Sisti, C.P.J.; Santos, H.P.; Kohhan, R.; Albes, B.J.R.; Urquiaga, S.; Bodey, R.M. Change in carbon and nitrogen stocks in soil under 13 years of conventional or zero tillage in Southern Brazil. Soil Tillage Res. 2004, 76, 39–58. [Google Scholar] [CrossRef]
- Carvalho, J.L.N.; Cerri, C.E.P.; Feigel, B.J.; Piccolo, M.C.; Godinho, V.P.; Cerri, C.C. Carbon sequestration in agricultural soils in the Cerrado region of the Brazil Amazon. Soil Tillage Res. 2009, 103, 342–349. [Google Scholar] [CrossRef]
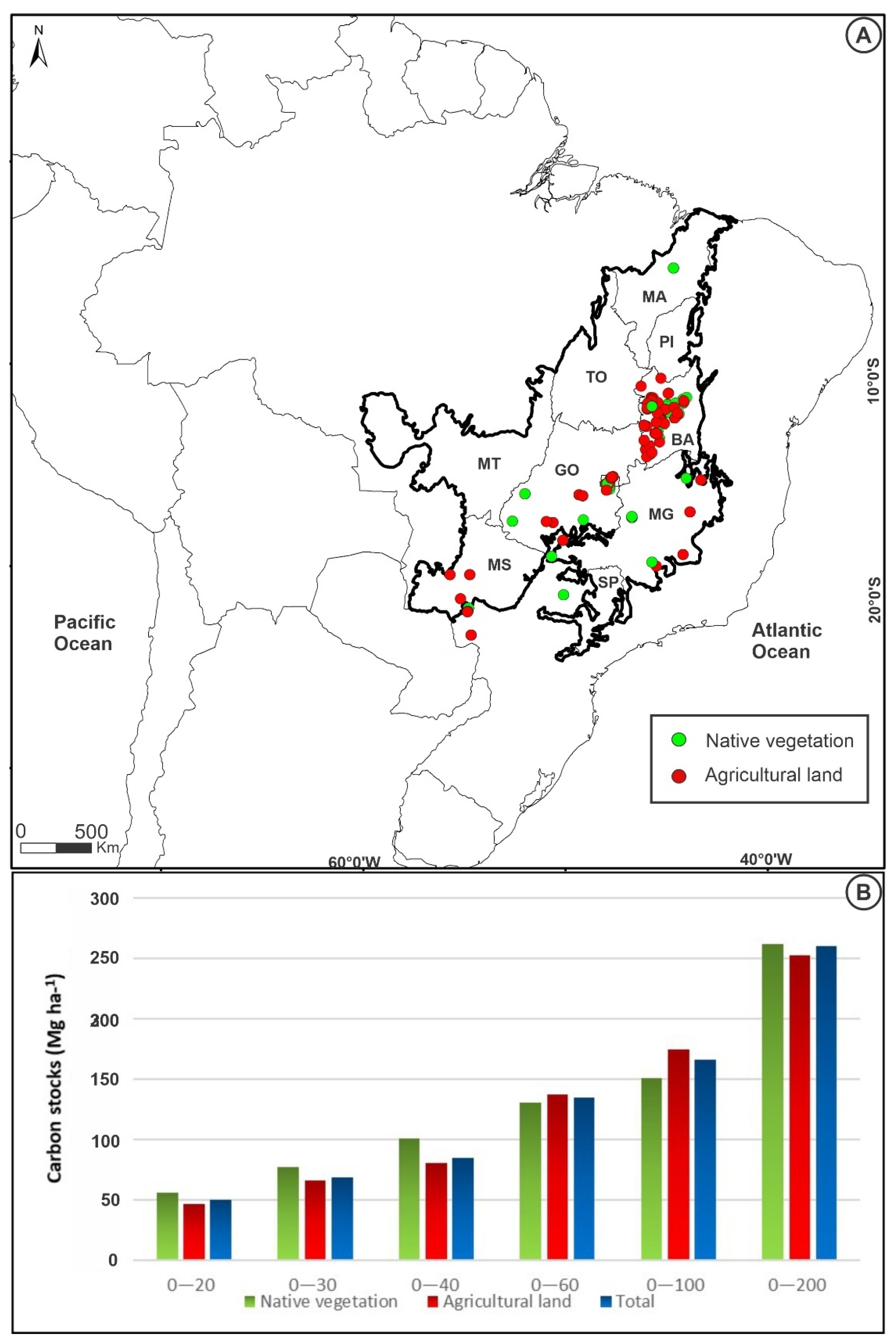
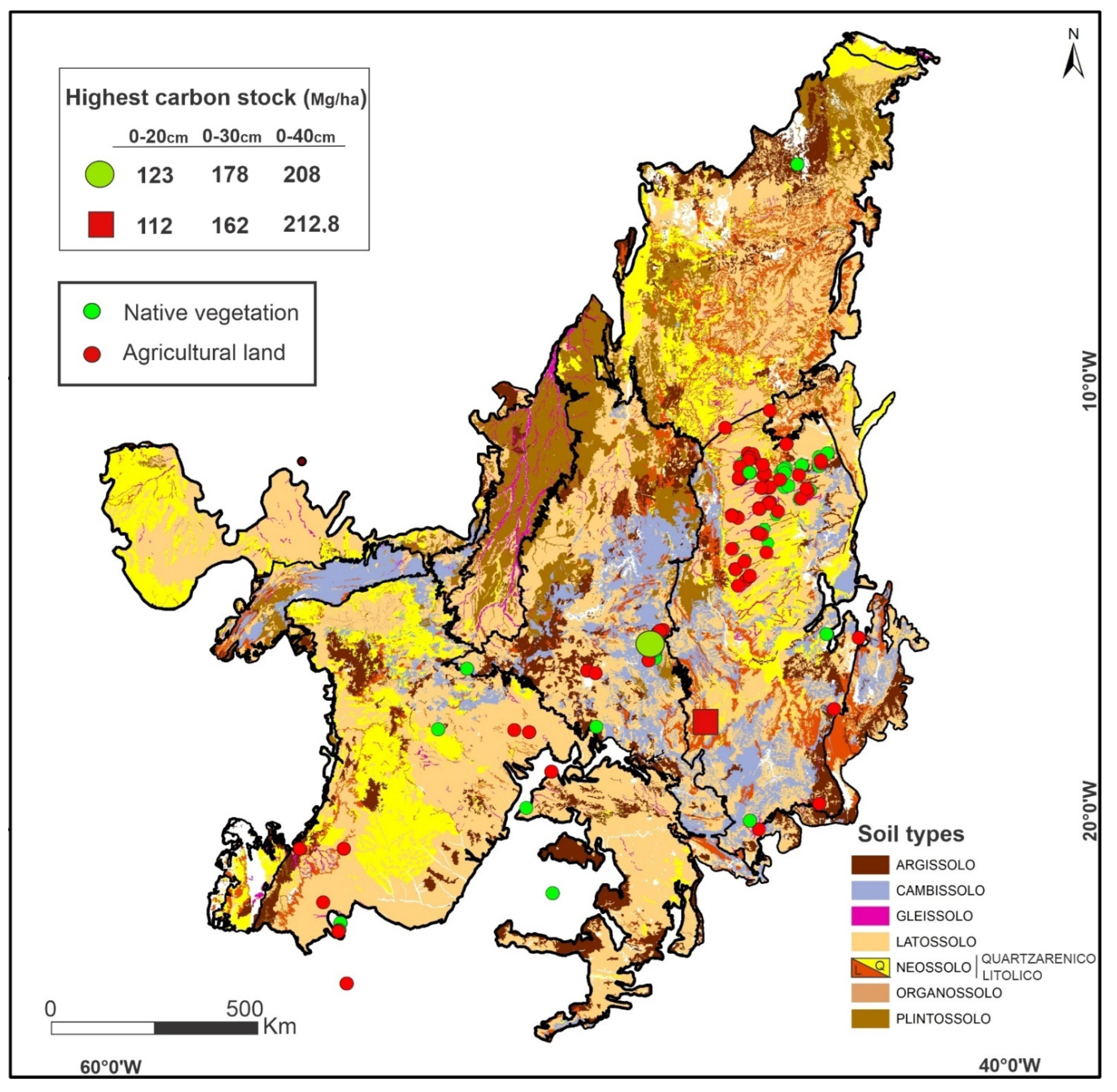
| Land-Use | Soil Layer | Number of Sites | Min | Mean | Max | CV (%) |
|---|---|---|---|---|---|---|
| Native vegetation | Mg ha−1 | |||||
| 0–20 | 44 | 16.2 | 55.7 | 123.8 | 49.4 | |
| 0–30 | 17 | 21.7 | 77.0 a | 178.2 | 60.3 | |
| 0–40 | 18 | 28.9 | 100.7 a | 208.9 | 54.4 | |
| 0–60 | 16 | 50.4 | 130.3 a | 278.1 | 60.6 | |
| 0–100 | 22 | 54.6 | 150.4 a | 414.4 | 124 | |
| 0–200 | 8 | 230.0 | 261.2 a | 297.0 | 9.3 | |
| Agricultural land | ||||||
| 0–20 | 94 | 16.1 | 46.7 | 112.2 | 43.7 | |
| 0–30 | 54 | 23.5 | 65.5 a | 162.5 | 45.5 | |
| 0–40 | 62 | 29.6 | 79.9 a | 212.8 | 115.7 | |
| 0–60 | 24 | 36.8 | 137.3 a | 303.7 | 82.4 | |
| 0–100 | 42 | 51.7 | 174.0 a | 466.1 | 118.7 | |
| 0–200 | 1 | 252.0 | 252.0 a | 252.0 | ||
| Land Cover | Soil Layer | Number of Sites | Min | Mean | Max | CV (%) |
|---|---|---|---|---|---|---|
| Forestland | Mg ha−1 | |||||
| 0–20 | 7 | 33.1 | 65.2 bc | 112.8 | 51.0 | |
| 0–30 | 2 | 51.0 | 100.4 bc | 149.7 | 69.5 | |
| 0–40 | 6 | 45.6 | 104.6 a | 189.7 | 54.0 | |
| 0–60 | 5 | 61.5 | 124.0 bc | 266.6 | 67.6 | |
| 0–100 | 5 | 82.5 | 182.4 a | 414.4 | 74.1 | |
| 0–200 | 2 | 248.0 | 251.2 a | 254.3 | 2 | |
| Shrubland | ||||||
| 0–20 | 27 | 16.2 | 48.9 b * | 79.0 | 29.1 | |
| 0–30 | 11 | 21.7 | 54.3 b | 96.6 | 40.4 | |
| 0–40 | 7 | 28.9 | 71.4 a | 119.9 | 38.2 | |
| 0–60 | 7 | 50.4 | 86.3 b | 154.4 | 45.6 | |
| 0–100 | 12 | 54.6 | 130.5 a | 203.1 | 42.0 | |
| 0–200 | 3 | 230.0 | 169.0 a | 271.2 | 13.9 | |
| Grassland | ||||||
| 0–20 | 7 | 31.1 | 54.2 b | 72.0 | 28.3 | |
| 0–40 | 2 | 54.7 | 68.3 a | 81.9 | 28.2 | |
| 0–100 | 3 | 99.7 | 170.2 a | 209.0 | 28.9 | |
| 0–200 | 4 | 277.0 | 285.0 a | 297.0 | 3.7 | |
| Wet grassland | ||||||
| 0–20 | 3 | 76.0 | 98.5 c * | 123.8 | 24.4 | |
| 0–30 | 3 | 113.3 | 145.0 c | 178.2 | 22.4 | |
| 0–40 | 3 | 148.8 | 182.6 a | 208.9 | 16.8 | |
| 0–60 | 3 | 211.4 | 243.5 c | 278.1 | 13.7 | |
| Land Cover | Soil Layer | Number of Sites | Min | Mean | Max | CV (%) |
|---|---|---|---|---|---|---|
| Planted Forest | Mg ha−1 | |||||
| 0–20 | 7 | 44.9 | 86.2 b * | 102.5 | 23 | |
| 0–30 | 2 | 135.4 | 138.8 bc | 142.1 | 3 | |
| 0–40 | 7 | 78.4 | 150.8 b * | 183.3 | 24 | |
| 0–60 | 9 | 41.6 | 169.7 a | 262.4 | 52 | |
| 0–100 | 7 | 148.2 | 303.7 b * | 414.0 | 29 | |
| Agroforest | ||||||
| 0–20 | 7 | 25.0 | 48.9 bc | 98.2 | 69.4 | |
| 0–30 | 3 | 71.2 | 118.7 b | 144.1 | 34.7 | |
| 0–40 | 7 | 41.1 | 89.0 bc | 208.0 | 82.6 | |
| 0–60 | 3 | 65.3 | 209.8 a | 301.0 | 60.3 | |
| 0–100 | 2 | 407.8 | 430.4 b | 453.0 | 7.4 | |
| Pasture | ||||||
| 0–20 | 24 | 24.4 | 42.8 c * | 112.2 | 40 | |
| 0–30 | 14 | 23.5 | 54.2 c | 162.5 | 65 | |
| 0–40 | 14 | 39.0 | 74.3 c * | 212.8 | 55 | |
| 0–60 | 3 | 36.8 | 129.8 a | 303.7 | 116 | |
| 0–100 | 18 | 51.7 | 122.3 * | 466.1 | 199 | |
| 0–200 | 1 | 252.0 | 252.0 | 252.0 | ||
| Land Cover | Soil Layer | Number of Sites | Min | Mean | Max | CV (%) |
|---|---|---|---|---|---|---|
| Tillage | Mg ha−1 | |||||
| 0–20 | 22 | 16.1 | 39.7 | 84.9 | 37 | |
| 0–30 | 19 | 45.4 | 63.3 a | 81.5 | 18 | |
| 0–40 | 14 | 29.6 | 61.2 a | 116.3 | 39 | |
| 0–60 | 2 | 85.7 | 91.5 a | 97.2 | 9 | |
| 0–100 | 8 | 125.3 | 147.8 | 176.4 | 14 | |
| No-tillage | ||||||
| 0–20 | 28 | 20.7 | 46.0 | 87.1 | 30 | |
| 0–30 | 10 | 40.2 | 63.5 a | 85.9 | 24 | |
| 0–40 | 19 | 37.5 | 70.3 a | 124.3 | 28 | |
| 0–60 | 4 | 92.9 | 102.4 a | 116.1 | 10 | |
| 0–100 | 3 | 135.8 | 160.0 a | 186.3 | 13 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellano, G.R.; Santos, L.A.; Menegário, A.A. Carbon Soil Storage and Technologies to Increase Soil Carbon Stocks in the South American Savanna. Sustainability 2022, 14, 5571. https://doi.org/10.3390/su14095571
Castellano GR, Santos LA, Menegário AA. Carbon Soil Storage and Technologies to Increase Soil Carbon Stocks in the South American Savanna. Sustainability. 2022; 14(9):5571. https://doi.org/10.3390/su14095571
Chicago/Turabian StyleCastellano, Gabriel Ribeiro, Landerlei Almeida Santos, and Amauri Antonio Menegário. 2022. "Carbon Soil Storage and Technologies to Increase Soil Carbon Stocks in the South American Savanna" Sustainability 14, no. 9: 5571. https://doi.org/10.3390/su14095571
APA StyleCastellano, G. R., Santos, L. A., & Menegário, A. A. (2022). Carbon Soil Storage and Technologies to Increase Soil Carbon Stocks in the South American Savanna. Sustainability, 14(9), 5571. https://doi.org/10.3390/su14095571







