Evaluation of the Current State of Preservation of Vaccinio uliginosi-Pinetum Kleist 1929 in Eastern Poland
Abstract
:1. Introduction
- -
- Whether herbaceous plants and the values of ecological indicators were typical of this habitat and indicative of spontaneous regeneration processes.
- -
- Whether pine regeneration had a sufficient density, height, and variability to ensure the sustainability of this habitat.
- -
- Whether the presence of herbaceous plants and undergrowth layers affected the density of pine regeneration and which of the analyzed factors determined the possibility of pine regeneration in pine marshy forest?
2. Materials and Methods
2.1. Study Area
2.2. Determination of the Abundance and Quality of Scots Pine Tree Natural Regeneration
2.3. Determination of Species Composition in Marshy Forests and Conditions of Pine Seedling Formation
2.4. Genetic Analysis
3. Results
3.1. Characteristics of Pine Regeneration
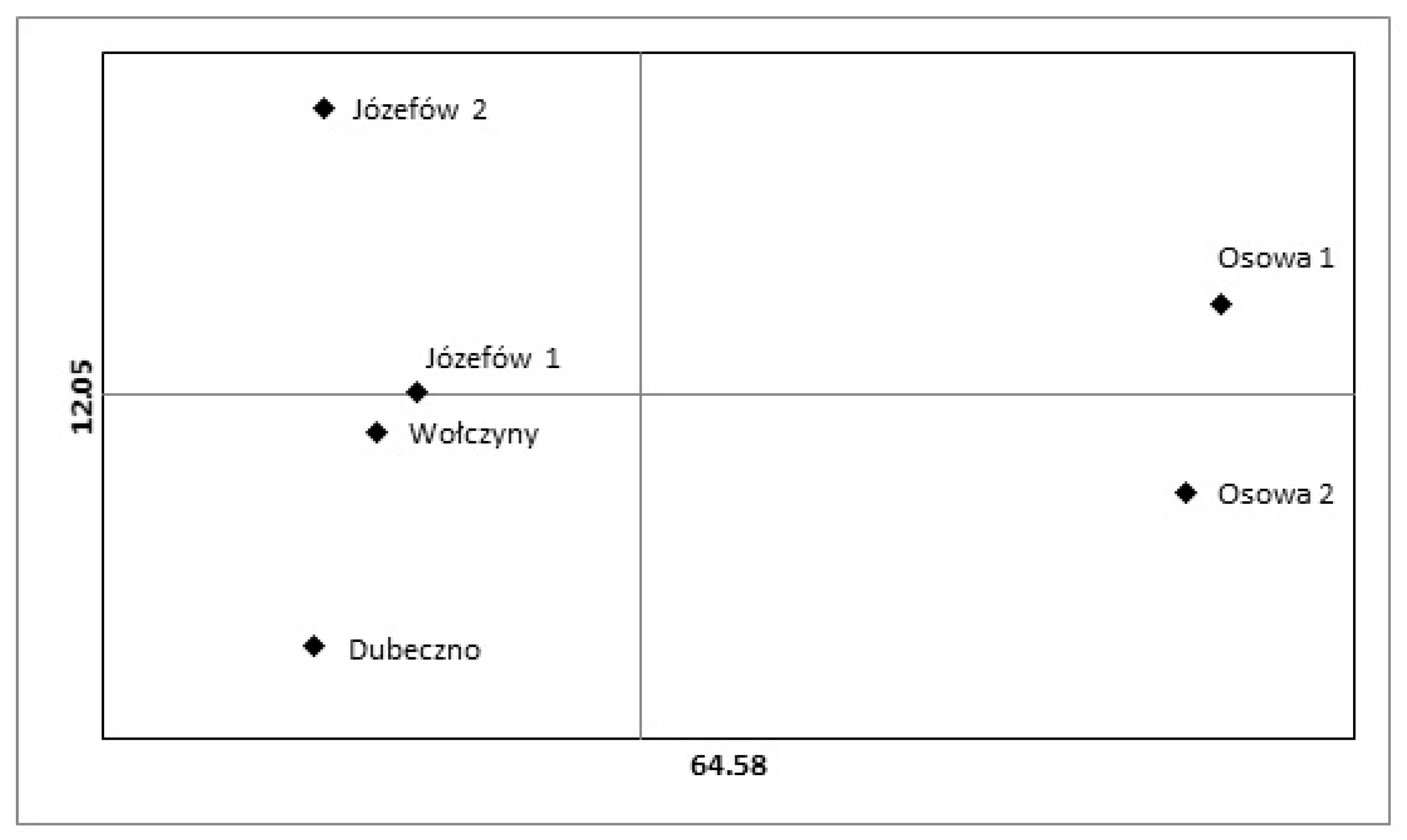
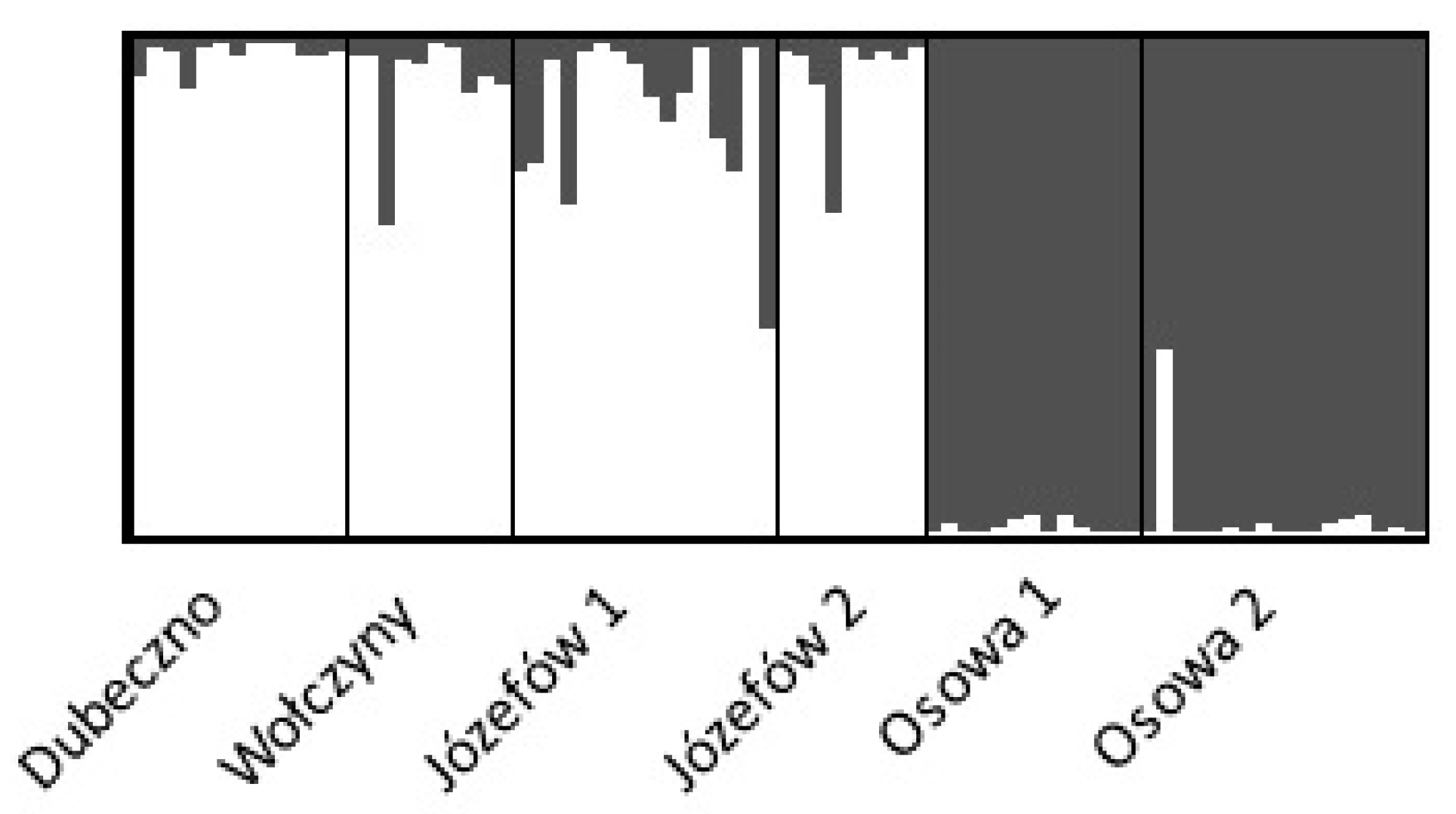
3.2. Genetic Variability of Regenerating Pines
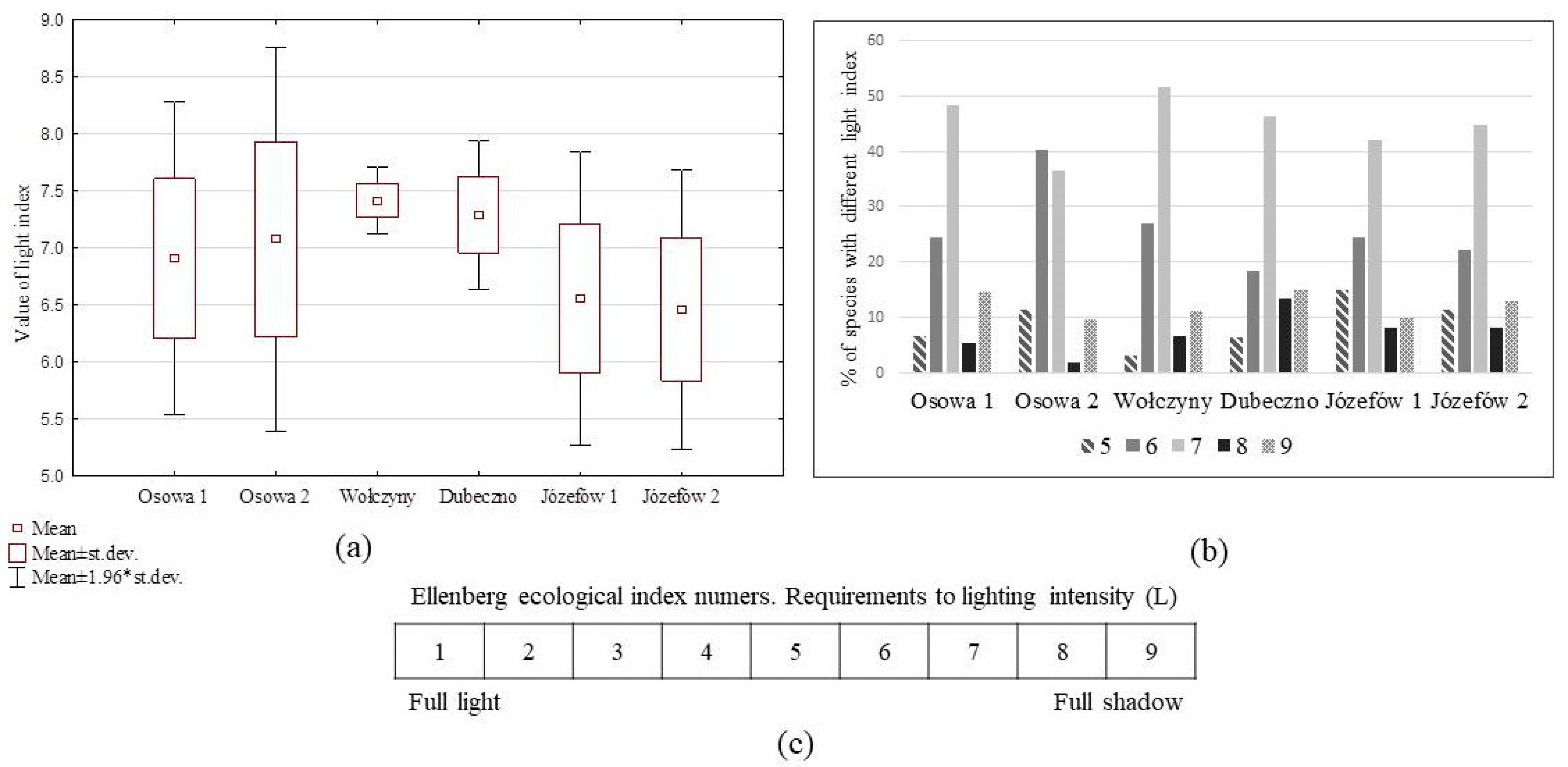
3.3. Forest Floor Vegetation and Conditions of Pine Seedling Emergence
4. Discussion
4.1. Abundance and Genetic Variability of Natural Pine Regenerations
4.2. Forest Floor Vegetation
4.3. Conditions of Pine Seedling Formation in Marshy Forests
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Bern Convention—The Convention of the Conservation of European Wildlife and Natural Habitats; Council of Europe: Strasbourg, France, 1979. [Google Scholar]
- European Commission. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats of Wild Fauna and Flora; As amended by the Accession Act of Austria, Finland and Sweden 1992; EEC Official Journal L 1, 1.1; European Commission: Brussels, Belgium, 1995; p. 135. [Google Scholar]
- Interpretation Manual of European Union Habitats; Version EUR 28; European Commission: Brussels, Belgium, 2013.
- 10 Messages for 2010 Climate Change and Biodiversity; European Environment Agency: Copenhagen, Denmark, 2010.
- Goddess, C.M.; Palutikof, J.P.; Davies, T.D. A First Approach to Assessing Future Climate States in the UK over Very Long Timescales: Input to Studies of the Integrity of Radioactive Waste Repositories. Clim. Chang. 1990, 16, 115–139. [Google Scholar] [CrossRef]
- Jaime, L.; Batllori, E.; Margalef-Marrase, J.; Pérez Navarro, M.Á.; Lloret, F. Scots Pine (Pinus sylvestris L.) Mortality Is Explained by the Climatic Suitability of Both Host Tree and Bark Beetle Populations. For. Ecol. Manag. 2019, 448, 119–129. [Google Scholar] [CrossRef]
- Van Halder, I.; Castagneyrol, B.; Ordóñez, C.; Bravo, F.; del Río, M.; Perrot, L.; Jactel, H. Tree Diversity Reduces Pine Infestation by Mistletoe. For. Ecol. Manag. 2019, 449, 117470. [Google Scholar] [CrossRef]
- Paluch, R. Gospodarka lesna na siedliskach silnie wilgotnych i bagiennych. Leśne Pr. Badaw. 2006, 2, 115–121. [Google Scholar]
- Savva, Y.; Oleksyn, J.; Reich, P.B.; Tjoelker, M.G.; Vaganov, E.A.; Modrzynski, J. Interannual Growth Response of Norway Spruce to Climate along an Altitudinal Gradient in the Tatra Mountains, Poland. Trees 2006, 20, 735–746. [Google Scholar] [CrossRef]
- Pfadenhauer, J.; Grootjans, A. Wetland Restoration in Central Europe: Aims and Methods. Appl. Veg. Sci. 1999, 2, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Diaci, J.; Györek, N.; Gliha, J.; Nagel, T.A. Response of Quercus robur L. Seedlings to North-South Asymmetry of Light within Gaps in Floodplain Forests of Slovenia. Ann. For. Sci. 2008, 65, 105. [Google Scholar] [CrossRef]
- Vizoso-Arribe, O.; Díaz-Maroto, I.; Vila-Lameiro, P.; Díaz-Maroto, M. Influence of the Canopy in the Natural Regeneration of Quercus robur in NW Spain. Biologia 2014, 69, 1678–1684. [Google Scholar] [CrossRef]
- Fussi, B.; Westergren, M.; Aravanopoulos, F.; Baier, R.; Kavaliauskas, D.; Finzgar, D.; Alizoti, P.; Bozic, G.; Avramidou, E.; Konnert, M.; et al. Forest Genetic Monitoring: An Overview of Concepts and Definitions. Environ. Monit. Assess. 2016, 188, 493. [Google Scholar] [CrossRef] [Green Version]
- Bragazza, L.; Gerdol, R. Are Nutrient Availability and Acidity-Alkalinity Gradients Related in Sphagnum-Dominated Peatlands? J. Veg. Sci. 2002, 13, 473–482. [Google Scholar] [CrossRef]
- Nygaard, P.; Ødegaard, T. Sixty Years of Vegetation Dynamics in South Boreal Coniferous Forest in Southern Norway. J. Veg. Sci. 2009, 10, 5–16. [Google Scholar] [CrossRef]
- Czerepko, J. Długookresowe zmiany roślinności w zespole sosnowego boru bagiennego Vaccinio uliginosi-Pinetum Kleist 1929. Leśne Pr. Badaw. 2011, 72, 21–29. [Google Scholar]
- Gmyz, R.; Skrzyszewski, J. Wpływ zróżnicowania mikrosiedliskowego boru świeżego na liczebność odnowienia naturalnego sosny zwyczajnej (Pinus sylvestris L.). Sylwan 2010, 154, 173–181. [Google Scholar]
- Sewerniak, P.; Gonet, S.S.; Quaium, M. Wpływ przygotowania gleby frezem leśnym na wzrost sadzonek sosny zwyczajnej w warunkach ubogich siedlisk Puszczy Bydgoskiej. Sylwan 2012, 156, 871–880. [Google Scholar]
- Dobrowolska, D. Warunki powstawania odnowien naturalnych sosny zwyczajnej (Pinus sylvestris L.) na terenie Nadlesnictwa Tuszyma. Leśne Pr. Badaw. 2010, 71, 217–224. [Google Scholar]
- Pigan, I. Odnowienie naturalne sosny (Pinus sylvestris L.) na siedliskach wilgotnych przy zastosowaniu różnych metod przygotowania gleby. Sylwan 2010, 154, 524–534. [Google Scholar]
- Jaworski, A. Hodowla Lasu. Tom 1. Sposoby Zagospodarowania, Odnawianie Lasu, Przebudowa i Przemiana Drzewostanów; PWRiL: Warsaw, Poland, 2011. [Google Scholar]
- Stat Soft, Inc. Statistica (Data Analysis Software System). Version 13.1. 2011. Available online: http://www.statsoft.com (accessed on 25 January 2022).
- Braun-Blanquet, J. Pflanzensoziologie; Springer: Wien, Vienna; New York, NY, USA, 1964. [Google Scholar]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski; PWN: Warsaw, Poland, 2008. [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering Plants and Pteridophytes of Poland: A Checklist; W. Szafer Institute of Botany; Polish Academy of Sciences: Cracow, Poland, 2002. [Google Scholar]
- Ochyra, R.; Żarnowiec, J.; Bednarek-Ochyra, H. Census Catalogue of Polish Mosses. Biodiversity of Poland; IB PAN: Cracow, Poland, 2003. [Google Scholar]
- Rogers, S.O.; Bendich, A.J. Extraction of DNA from plant tissue. In Plant Molecular Biology Mannual; Glevin, S.B., Schilperoort, R.A., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 1988; pp. 1–10. [Google Scholar]
- Bergmann, F.; Gregorius, H.R. Comparison of the genetic diversities of various populations of Norway spruce (Picea abies). In Proceedings of the Conference: Biochemical Genetics of Forest Trees, Umea, Sweden, 1978; Rudin, F., Ed.; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1979; pp. 99–107. [Google Scholar]
- Nei, M.; Roychoudhury, A.K. Sampling Variances of Heterozygosity and Genetic Distance. Genetics 1974, 76, 379–390. [Google Scholar] [CrossRef]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Yeh, F.C.; Yang, R.; Boyle, T. Popgene Version 1.31. Microsoft Windows–Based for Population Genetic Analysis; University of Alberta, Departament of Renewable Resources: Edmonton, AB, Canada, 1999. [Google Scholar]
- Peakall, R.; Smouse, P.E. Genalex 6: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the Number of Clusters of Individuals Using the Software STRUCTURE: A Simulation Study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earl, D.A.; von Holdt, B.M. STRUCTURE HARVESTER: A Website and Program for Visualizing STRUCTURE Output and Implementing the Evanno Method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Jakobsson, M.; Rosenberg, N.A. CLUMPP: A Cluster Matching and Permutation Program for Dealing with Label Switching and Multimodality in Analysis of Population Structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, N.A. Distruct: A Program for the Graphical Display of Population Structure. Mol. Ecol. Notes 2004, 4, 137–138. [Google Scholar] [CrossRef]
- CILP. Zasady Hodowli Lasu; CILP: Warszaw, Poland, 2012. [Google Scholar]
- Modrý, M.; Hubený, D.; Rejšek, K. Differential Response of Naturally Regenerated European Shade Tolerant Tree Species to Soil Type and Light Availability. For. Ecol. Manag. 2004, 188, 185–195. [Google Scholar] [CrossRef]
- Li, H.-Y.; Jing, J.; Liu, G.-F.; Ma, X.-J.; Dong, J.-X.; Jie, S.-J. Genetic Variation and Division of Pinus sylvestris Provenances by ISSR Markers. J. For. Res. 2008, 16, 216–218. [Google Scholar] [CrossRef]
- Vidyakin, A.I.; Boronnikiva, S.V.; Nechayeva, Y.S.; Nechayeva, Y.S.; Prysimivskaya, Y.V.; Boboshina, I.V. Genetic Variation, Population Structure and Differentiation in Scots Pine (Pinus sylvestris L.) from the Northeast of the Russian Plain as Inferred from the Molecular Genetic Analysis Data. Genetika 2015, 51, 1401–1409. [Google Scholar] [CrossRef]
- Androsiuk, P.; Urbaniak, L. Genetic variability of Pinus sylvestris populations from IUFRO 1982 provenance trial. Dendrobiology 2014, 71, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Cipriano, J.; Carvalho, A.; Fernandes, C.; Gaspar, M.J.; Pires, J.; Bento, J.; Roxo, L.; Louzada, J.; Lima-Brito, J. Evaluation of genetic diversity of Portugese Pinus sylvestris L. populations based on molecular data and inferences about the future use of this germplasm. J. Genet. 2013, 92 (Suppl. S2), 41–48. [Google Scholar] [CrossRef]
- Li, C.; Chai, B.; Wang, M. Population Genetic Structure of Pinus tabulaeformis in Shanxi Plateau, China. Russ. J. Ecol. 2008, 39, 34–40. [Google Scholar] [CrossRef]
- Rubio-Moraga, A.; Candel-Perez, D.; Lucas-Borja, M.E.; Tiscar, P.A.; Viñegla, B.; Linares, J.C.; Gómez-Gómez, L.; Ahrazem, O. Genetic Diversity of Pinus nigra Arn. Populations in Southern Spain and Northern Morocco Revealed by Inter-Simple Sequence Repeat Profiles. Int. J. Mol. Sci. 2012, 13, 5645–5658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phong, D.T.; Lieu, T.T.; Hien, V.T.T.; Hiep, N.T. Genetic Diversity of the Endemic Flat-Needle Pine Pinus krempfii (Pinaceae) from Vietnam Revealed by SSR Markers. Genet. Mol. Res. 2015, 14, 7727–7739. [Google Scholar] [CrossRef]
- Masternak, K.; Banach, J.; Głębocka, K.; Wajdzik, M. Genetic Variability in Pitch Pine (Pinus rigida Mill.) Growing in the Niepołomice Forest as Determined by ISSR Markers. Acta Soc. Bot. Pol. 2018, 87, 3953. [Google Scholar] [CrossRef]
- Nowakowska, J. Genetic diversity of Scots pine (Pinus sylvestris L.) Polish provenances based on RAPD analysis. Sylwan 2003, 11, 26–37. [Google Scholar]
- Wachowiak, W. Genetic relationships between Polish and reference populations of Scots pine (Pinus sylvestris L.) in Europe based on nucleotide polymorphism study at nuclear loci. Sylwan 2015, 159, 26–37. [Google Scholar]
- Roo-Zielińska, E. Fitoindykacja Jako Narzędzie Oceny Środowiska Fizycznogeograficznego. Podstawy Teoretyczne i Analiza Porównawcza Stosowanych Metod; Prace Geograficzne: Warsaw, Poland, 2004. [Google Scholar]
- Van der Maarei, E. Relations between Sociological-Ecological Species Groups and Ellenberg Indicator Values. Phytocoenologia 1993, 23, 343–362. [Google Scholar] [CrossRef]
- Zarzycki, K.; Trzcińska-Tacik, H.; Różański, W.; Szeląg, Z.; Wołek, J.; Korzeniak, U. Ecological Indicator Values of Vascular Plants of Poland; W. Szafer Institute of Botany; Polish Academy of Sciences: Cracow, Poland, 2001. [Google Scholar]
- Matuszkiewicz, J.M. Zespoły Leśne Polski; PWN: Warsaw, Poland, 2001. [Google Scholar]
- Sokołowski, A.W. Zespoły Leśne Nadleśnictwa Balinka w Puszczy Augustowskiej. Monogr. Bot. 1969, 27, 3–79. [Google Scholar]
- Dobrowolska, D. Struktura drzewostanu glownego jako czynnik ksztaltujacy warunki swietlne w odnowieniu naturalnym jodly pospolitej [Abies alba Mill.]. Pr. Inst. Badaw. Leśnictwa Ser. A 1998, 843–851, 173–188. [Google Scholar]
- Obmiński, Z. Zarys ekologii. In Sosna Zwyczajna. Nasze Drzewa Leśne; Białobok, S., Ed.; PWN: Warsaw, Poland, 1970; pp. 152–231. [Google Scholar]
- Hale, S.E.; Edwards, C.; Mason, W.L.; Price, M.; Peace, A. Relationships between Canopy Transmittance and Stand Parameters in Sitka Spruce and Scots Pine Stands in Britain. For. Int. J. For. Res. 2009, 82, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Jaworski, A.; Zarzycki, K. Ekologia. In Jodła Pospolita Abies alba Mill. Nasze Drzewa Leśne; Białobok, S., Ed.; PWN: Warsaw, Poland; Poznan, Poland, 1983; pp. 317–430. [Google Scholar]
- Messier, C.; Doucet, R.; Ruel, J.-C.; Claveau, Y.; Kelly, C.; Lechowicz, M.J. Functional ecology of advance regeneration in relations to light in boreal forest. Can. J. For. Res. 1999, 29, 812–823. [Google Scholar] [CrossRef]
- Gil, W.; Kopryk, W.; Zachara, T. Growth dynamice of Scots pine natural regeneration under the shelter of the stand in Polish lowland—A case study Ostrów Mazowiecka. Folia For. Pol. Ser. A For. 2004, 26, 21–28. [Google Scholar]
- Zerbe, S.; Schmidt, I.; Betzin, J. Indicators for plant species richness in pine (Pinus sylvestris L.) forests of Germany. Biodivers. Conserv. 2007, 16, 3301–3316. [Google Scholar] [CrossRef]
- Tomczyk, S. Inicjowanie oraz wprowadzanie samosiewów sosnowych. Las Pol. 1989, 3, 16–17. [Google Scholar]
- Lust, N.; Geuden, G.; Olsthoorn, A.F.M. Scots pine in Belgium and the Netherlands. For. Syst. 2000, 9 (Suppl. S1), 213–231. [Google Scholar]
- Karlsson, M.; Nilsson, U. The Effects of Scarification and Shelterwood Treatments on Naturally Regenerated Seedlings in Southern Sweden. For. Ecol. Manag. 2005, 205, 183–197. [Google Scholar] [CrossRef]
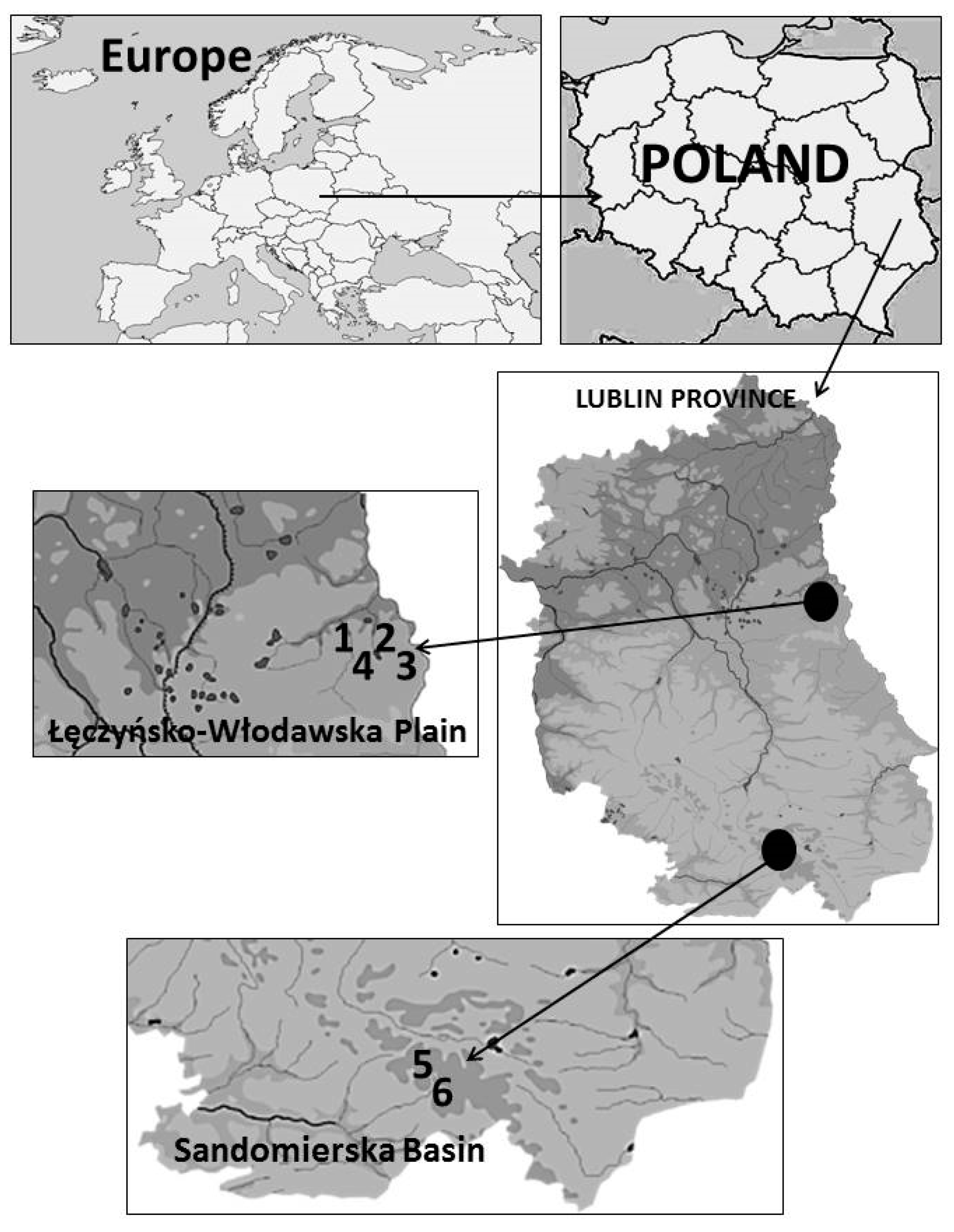
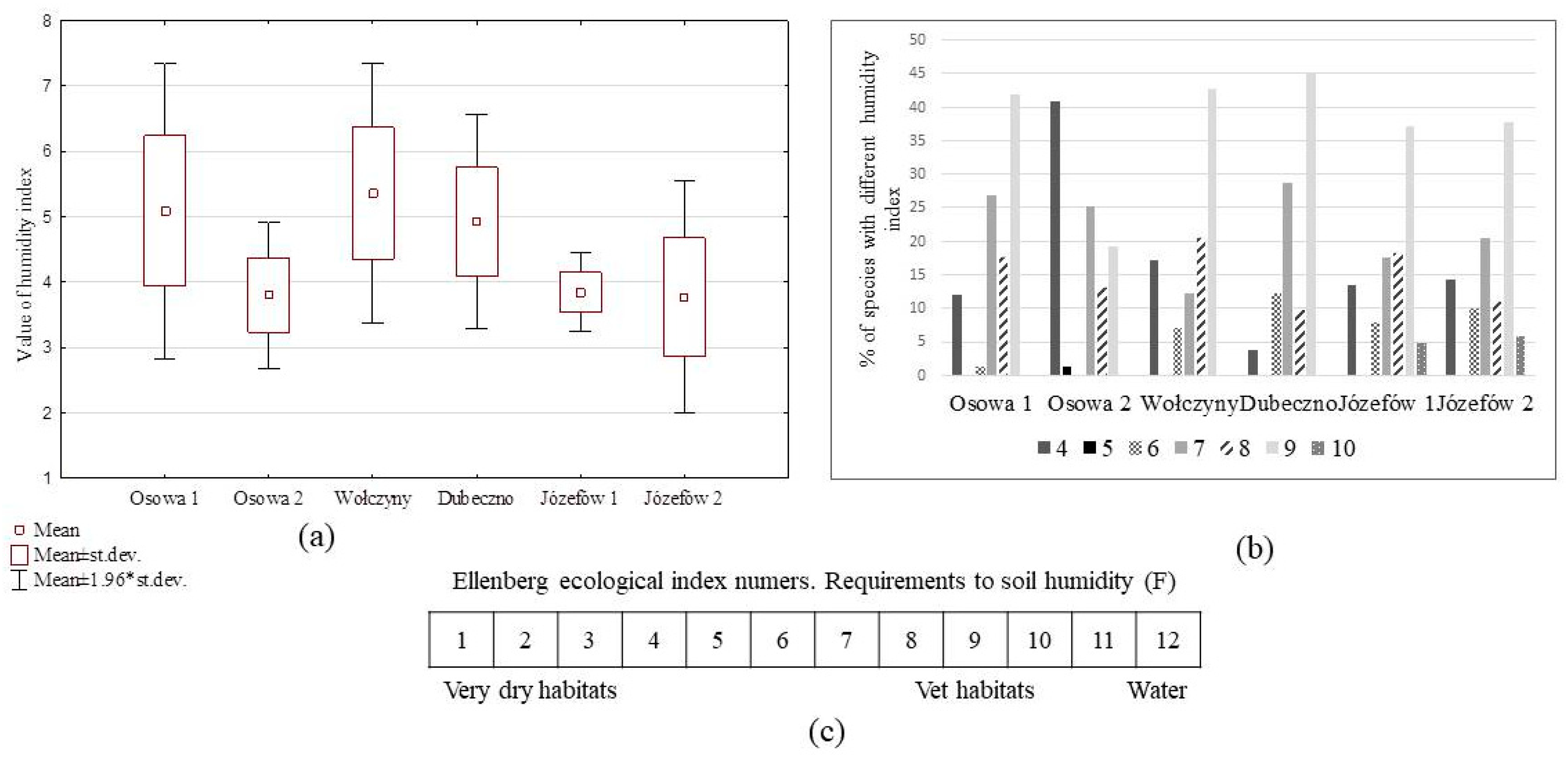
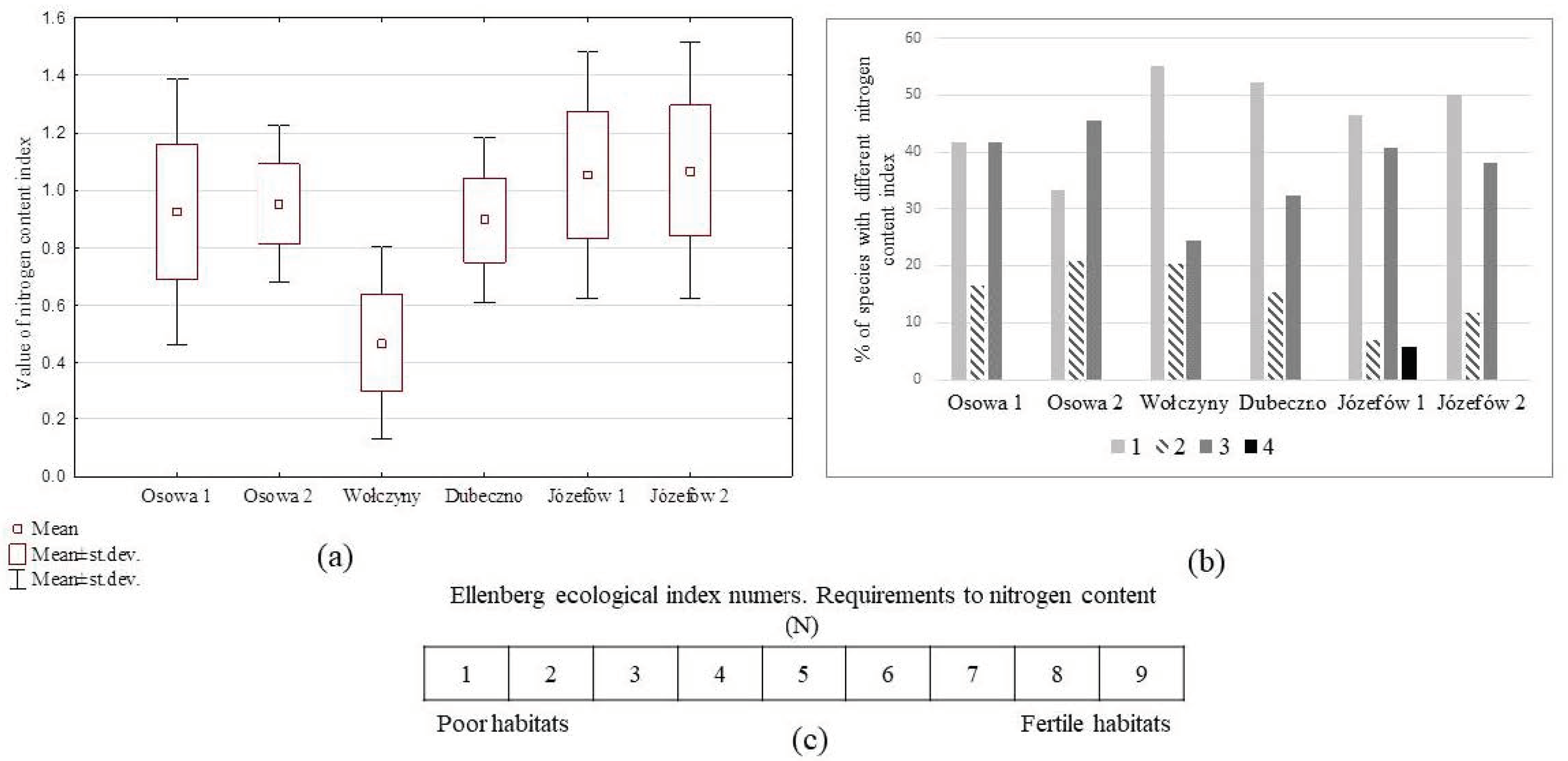
| Stand | Mean Height (cm) | Total Number | Number of Regeneration | ||
|---|---|---|---|---|---|
| Young Seedlings | Older Seedlings | Underwood | |||
| Osowa 1 | 56.83± e | 46 b | 4 | 19 | 23 |
| Osowa 2 | 28.97 c | 37 b | 18 | 11 | 8 |
| Dubeczno | 45.16 d | 49 b | 28 | 11 | 10 |
| Wołczyny | 3.80 a | 277 a | 277 | - | - |
| Józefów 1 | 16.43 b | 163 a | 114 | 45 | 4 |
| Józefów 2 | 14.17 b | 34 b | 26 | 7 | 1 |
| Test F (significance level) | 72.1796 (p < 0.001) | 20.8499 (p < 0.001) | - | - | - |
| Stand | P% | Na | Ne | He | I |
|---|---|---|---|---|---|
| Osowa 1 | 64.71 | 1.490 | 1.338 | 0.200 | 0.304 |
| Osowa 2 | 63.40 | 1.444 | 1.351 | 0.205 | 0.310 |
| Dubeczno | 56.86 | 1.301 | 1.351 | 0.202 | 0.301 |
| Wołczyny | 58.82 | 1.379 | 1.343 | 0.199 | 0.299 |
| Józefów 1 | 66.67 | 1.516 | 1.359 | 0.207 | 0.312 |
| Józefów 2 | 52.29 | 1.275 | 1.329 | 0.188 | 0.279 |
| Mean | 60.46 | 1.401 | 1.345 | 0.200 | 0.301 |
| Source | df | SS | MS | Est. Var. | % |
|---|---|---|---|---|---|
| Among populations | 5 | 607.77 | 121.554 | 7.686 | 25% |
| Within populations | 72 | 1629.102 | 22.626 | 22.626 | 75% |
| Total | 77 | 2236.872 | 30.312 | 100% |
| Stand | Osowa 1 | Osowa 2 | Dubeczno | Wołczyny | Józefów 1 | Józefów 2 |
|---|---|---|---|---|---|---|
| a and b layer: | ||||||
| Pinus sylvestris a | 52.47 | 61.5 | 57.51 | 75.0 | 60.0 | 62.5 |
| Pinus sylvestris b | 7.27 | 6.55 | 0.14 | 0.01 | 0.03 | 0.52 |
| Pinus sylvestris c | 0.1 | 0.07 | 0.1 | 0.1 | 0.1 | 0.09 |
| Betula pubescens a | 1.53 | 5.51 | - | 0.02 | 1.76 | - |
| Betula pubescens b | 1.03 | 2.03 | 1.98 | 0.08 | 1.55 | 0.46 |
| Betula pubescens c | 0.04 | 0.04 | 0.04 | 0.07 | 0.03 | - |
| Frangula alnus b | 0.02 | 0.02 | - | - | 1.06 | 0.03 |
| Frangula alnus c | 0.02 | 0.01 | 0.01 | 0.03 | 0.04 | 0.02 |
| Quercus robur b | - | 0.01 | 0.01 | 0.03 | 0.01 | - |
| Quercus robur c | - | 0.01 | 0.07 | 0.02 | 0.01 | 0.01 |
| Quercus rubra b | - | 0.04 | - | - | - | - |
| Quercus rubra c | - | 0.01 | - | - | - | - |
| Padus serotina b | - | 0.01 | - | - | - | - |
| Sorbus aucuparia b | - | - | - | - | 0.01 | - |
| Sorbus aucuparia c | - | - | 0.01 | - | - | - |
| Salix cinerea b | - | - | - | - | 0.54 | - |
| Picea excelsa b | - | - | 0.01 | - | 1.57 | - |
| Picea excelsa c | - | - | - | - | 1.42 | 0.02 |
| Abies alba c | - | - | - | - | - | 0.02 |
| ChAss. Vaccinio uliginosi-Pinetum: | ||||||
| Ledum palustre | 65.5 | 67.5 | 39.75 | 34.26 | 5.0 | 26.25 |
| Vaccinium uliginosum | 28.76 | 22.01 | 35.25 | - | 48.0 | 40.5 |
| Vaccinio-Piceetea: | ||||||
| Vaccinium myrtillus | 7.53 | 24.25 | 6.51 | 2.76 | 30.75 | 39.0 |
| Pleurozium schreberi | 7.5 | 48.0 | 1.75 | 13.76 | 12.75 | 21.25 |
| Vaccinium vitis-idaea | 5.5 | 14.5 | 2.25 | - | 0.05 | 3.27 |
| Dicranum polysetum | 7.51 | 16.0 | - | 1.53 | - | - |
| Leucobryum glaucum | 0.5 | 2.26 | - | - | 0.01 | - |
| Hylocomium splendes | 1.75 | 10.0 | - | - | - | - |
| Melampyrum pratense | - | 0.02 | - | - | - | - |
| Oxycocco-Sphagnetea: | ||||||
| Eriophorum vaginatum | 24.76 | 2.77 | 16.25 | 22.75 | 24.25 | 25.5 |
| Andromeda polifolia | 2.26 | 0.01 | 4.54 | 8.51 | 0.16 | 1.54 |
| Oxycoccus palustris | 15.0 | - | 24.75 | 24.01 | 27.75 | 21.0 |
| Sphagnum fuscum | 19.75 | 7.26 | - | 14.75 | - | - |
| Sphagnum magellanicum | 27.0 | 5.5 | 55.5 | 53.5 | 8.0 | 10.25 |
| Polytrichum strictum | 0.5 | - | 12.0 | 6.75 | 17.01 | 7.25 |
| Sphagnum fallax | 32.24 | 9.75 | 25.3 | 18.5 | 58.0 | 57.5 |
| Sphagnum rubellum | 1.75 | 5.75 | - | - | - | - |
| Aulacomium palustre | - | 0.02 | 0.52 | 5.03 | - | - |
| Other: | ||||||
| Calluna vulgaris | 0.55 | 0.02 | 2.02 | 3.5 | 1.0 | 6.77 |
| Molinia caerulea | 0.52 | 0.52 | - | - | 4.03 | 2.77 |
| Carex rostrata | - | - | - | - | 0.54 | 0.55 |
| Eriophorum angustifolium | - | - | - | - | 0.01 | - |
| Juncusef fusus | - | - | - | 0.51 | - | |
| Species and Layer | Osowa 1 | Osowa 2 | Dubeczno | Wołczyny | Józefów 1 | Józefów 2 | Średnio Dla Borów Bagiennych |
|---|---|---|---|---|---|---|---|
| A layer | −0.5372 | 0.1928 | −0.1424 | −0.2444 | −0.046 | −0.2659 | −0.3318 ** |
| Pinus sylvestris | −0.8777 *** | −0.5303 | −0.1542 | −0.2444 | −0.1762 | - | −0.4169 |
| B layer | 0.4712 | −0.1397 | −0.1335 | −0.1841 | −0.1441 | −0.3491 | −0.2162 |
| Pinus sylvestris | 0.5422 | 0.2296 | −0.1092 | −0.4656 | 0.7306 * | −0.2747 | −0.2748 |
| C layer | - | 0.1804 | 0.3172 | −0.4202 | −0.1762 | −0.4610 | −0.3732 ** |
| Eriophorum vaginatum | 0.7893 ** | 0.2082 | −0.2547 | 0.2481 | 0.3662 | 0.2895 | 0.3729 ** |
| Andromeda polifolia | 0.7193 * | −0.4124 | 0.2251 | −0.2773 | −0.5055 | 0.4807 | 0.2164 |
| Picea abies | - | - | −0.0591 | - | −0.1904 | - | 0.3016 * |
| Ledum palustre | −0.3873 | 0.3310 | −0.1936 | 0.1795 | 0.4595 | −0.1262 | −0.3016 * |
| Melampyrum pratense | - | −0.6187 | - | - | - | - | −0.3014 * |
| Oxycoccus palustris | 0.1645 | - | −0.1221 | 0.3025 | −0.0912 | −0.1460 | 0.3082 * |
| D layer | 0.7193 * | 0.1317 | −0.1215 | −0.1993 | - | −0.2289 | −0.1804 |
| Pleurozium schreberi | −0.1767 | −0.8879 *** | 0.5319 | −0.3137 | 0.5763 | −0.4744 | −0.1832 |
| Polytrichum strictum | −0.1767 | - | 0.6605 * | −0.028 | −0.3533 | 0.1602 | 0.2392 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masternak, K.; Urban, D.; Kowalczyk, K. Evaluation of the Current State of Preservation of Vaccinio uliginosi-Pinetum Kleist 1929 in Eastern Poland. Sustainability 2022, 14, 5387. https://doi.org/10.3390/su14095387
Masternak K, Urban D, Kowalczyk K. Evaluation of the Current State of Preservation of Vaccinio uliginosi-Pinetum Kleist 1929 in Eastern Poland. Sustainability. 2022; 14(9):5387. https://doi.org/10.3390/su14095387
Chicago/Turabian StyleMasternak, Katarzyna, Danuta Urban, and Krzysztof Kowalczyk. 2022. "Evaluation of the Current State of Preservation of Vaccinio uliginosi-Pinetum Kleist 1929 in Eastern Poland" Sustainability 14, no. 9: 5387. https://doi.org/10.3390/su14095387
APA StyleMasternak, K., Urban, D., & Kowalczyk, K. (2022). Evaluation of the Current State of Preservation of Vaccinio uliginosi-Pinetum Kleist 1929 in Eastern Poland. Sustainability, 14(9), 5387. https://doi.org/10.3390/su14095387





