Abstract
The toxic effects of two commonly used glucocorticoids, the dexamethasone and prednisolone, on meiobenthic nematodes were assessed in a laboratory experiment for 30 days. Nine treatments were employed, comprised of two single and mixed concentrations of dexamethasone and prednisolone (i.e., 0.14 and 1.4 µg·L−1). The exposure to both glucocorticoids showed significant effects on the abundance and taxonomic diversity of nematodes. Such changes were mainly induced by the decrease in the abundance of the species Microlaimus honestus, considered to be sensitive to prednisolone and by the increase in Enoplolaimus longicaudatus abundance, which can be considered tolerant. The exposure to these glucocorticoids also led to a decrease in 2A feeding groups, 2–4 mm body-size interval, and c-p3 life history type in most treatments, with type of life history and shape of amphids as the most relevant functional traits impacted by these two glucocorticoids. The results could also be explained by the potential antagonism between these two pharmaceuticals.
1. Introduction
The pharmaceuticals released in aquatic ecosystems are consumed by wildlife, bioaccumulated and further transferred through food webs [1,2,3,4]. This environmental issue associated with potential toxic effects on aquatic biota has a long history of research [5,6]. However, currently, little is known about the fate and toxicological effects of synthetic glucocorticoids on aquatic habitats and biota. These drugs are used in the treatment of various autoimmune and inflammatory diseases [7] but are frequently excreted in the environment without undergoing physiological transformations into inactive metabolites [8]. Moreover, most glucocorticoids reach the aquatic habitats through wastewater effluents [9,10,11].
The prednisolone is one the most frequently consumed glucocorticoids [12], reaching mean concentrations in wastewaters within the range of ng·L−1. However, concentrations of prednisolone in the range of µg·L−1 in wastewater effluents from the hospital and industrial areas were also recorded [11]. Previous studies showed that prednisolone had deleterious effects on the physiological processes during the ontogenetic development of zebra fish larvae (Danio rerio, see [13]) or on the number of leukocytes from the adults of Pimephales promelas even at low concentrations as 1 µg·L−1 [14].
The dexamethasone is another glucocorticoid used primarily as an anti-inflammatory drug [15]. Ref. [16] proved that the dexamethasone induced developmental abnormalities in zebra fish, but the toxic effects of exposure in the range of ng·L−1 on aquatic habitats and biota are practically unknown, requiring additional investigations [11].
The current study investigated the toxicological effects of glucocorticoids on meiobenthic taxa, a crucial component of the ‘small food web’ (i.e., protists, bacteria and meiofauna). Thus, a closed microcosm study focused on the response of free-living marine nematodes following contamination with prednisolone and dexamethasone was carried in laboratory. The free-living marine nematodes are routinely used as reliable bioindicators in ecotoxicology [17,18,19,20]. Their small size and short life cycle, associated with their ease of laboratory maintenance, makes them ideal organisms in toxicology experiments [21,22]. Outcomes from our bioassay will allow us to identify tolerant nematodes towards glucocorticoids. To reach this goal, many factors should be considered, namely (1) the buccal armature affecting directly the manner of catching and the type of preys consumed, but also (2) other factors such as the body size and shapes of the organs involved in the feeding process like chemodetection efficacy and locomotion easiness for searching food items. The identification of tolerant morpho-functional groups is necessary for further applications where these nematodes will be reused not for bioindication purposes but as bioremediators in sustainable development projects. This aims to neutralize the harmful effects of pharmaceutical pollution in marine areas and to avoid its transmission to future generations.
2. Material and Methods
2.1. Collecting Site and Sediment Manipulation
Sediments were collected on the 14 August 2019 (7 a.m.) from a subtidal pristine beach in Bizerte Bay, Tunisia (37°15′07.34″ N, 9°56′26.75″ E). The sediment was collected at 50 cm below water surface with several hand cores (surface of 10 cm2, inner diameter 3.6 cm); just the first 5 cm of sediment was sampled. The sediment collection was restricted to the upper 5 cm layer because more than 90% of the meiobenthos live in this microhabitat [21]. The collected sediments were stored in dark at constant temperature (29 °C) for three days for acclimatization. The ambient temperature was inferred from meteorological data (http://www.infoclimat.fr; last accessed on 14 August 2019) of the previous month (14 July to 14 August 2019).
2.2. Sediment Contamination and Experimental Set-Up
Stock solutions of prednisolone and dexamethasone purchased from Sigma-Aldrich (Saint-Louis, MI, USA) were prepared by dissolution in filtered seawater (0.7 µm pore-size Glas Microfibre GF/F (Dutscher, Issy-les-Moulineaux, France). The glucocorticoids thus dissolved were gently mixed into the sediment with a food mixer during the first day of the experimental period with a pace of 10 min at the beginning of each hour [21]. The concentrations used in the current experiment (i.e., 0.14 and 1.4 µg·L−1) were derived from the minimum LC50s/24 h of the rotifer Brachiorus calyciflorus (i.e., 20.82 and 41.37 mg·L−1 for prednisolone and dexamethasone, respectively, see [23]). These concentrations were first divided by 30, which is the number of days for the current experiment, leading to 0.694 and 1.379 (~1.4) mg·L−1, respectively. Given the higher toxicity of prednisolone compared to dexamethasone for B. calyciflorus [23], a preliminary experiment was set by using four concentrations: 0.14, 1.4, 14, 140, and 1400 µg·L−1, respectively. Given that the sediment contaminated with 0.14, 1.4 and 14 µg·L−1 with prednisolone comprised meiofauna, the preliminary investigations were repeated, this time by spiking the sediment populated by a similar meiobenthic community with prednisolone and dexamethasone. This way, two final concentrations of 1.4 and 0.14 μg·L−1 for both glucocorticoids were obtained, representing realistic values that are to be found in nature. [24] detected prednisolone and dexamethasone at concentrations of 1918 and 90 ng·L−1, respectively, supporting the realistic nature of the chosen concentrations for the current experiment.
Overall, 27 microcosms were used (n = 3 per each type of treatment), as follows: one control set, two sets contaminated with prednisolone (hereafter P1 and P2) and two others with dexamethasone (hereafter D1 and D2). Finally, four sets were contaminated with mixtures of both employed concentrations of prednisolone and dexamethasone (hereafter P1D1, P1D2, P2D1, and P2D2).
The experimental microcosms were comprised of glass bottles (2 L) filled with 300 g of homogenized natural sediment and one liter of 40 μm pre-filtered (29 PSU) water, contaminated or not. Throughout the experiment, each microcosm was constantly aerated with an aquarium pump.
2.3. Structural and Functional Traits of Nematode Communities
Two stacked sieves of 1 mm and 40 µm mesh size, respectively [25], were used for collecting nematodes from sediment, followed by their fixation in 4% formalin solution [26] and staining with Rose-Bengal (0.2 g·L−1) [27]. Then, 100 individuals were randomly picked from each treatment under a dissecting microscope, transferred in 21% glycerol and mounted on microscope slides for taxonomic identification based on morphological features [28]. For taxonomic identification to genus and species levels, the keys of Platt and Warwick [29,30] and Warwick et al. [31] and the Nemys database developed and updated by nematologists at Ghent University [32], respectively, were used.
The appurtenance to trophic groups, tail and amphidial fovea shapes, as well as to type of life strategy, was established for each genus. The classification of amphids was based on the shape of fovea: circular (cr), spiral (sp), pocket (pk) or indistinct (id) (see [33]). The shape of tails was classified as conical (co), clavate/conico-cylindrical (cla), short/round (s/r) or elongated/filiform (e/f) [34]. The types of feeding group considered were epigrowth (2A), selective deposit (1A), nonselective deposit feeders (1B) and omnivores/predators (2B), based on the characteristics of the mouth opening [35]. The type of life strategy was ranked on a c-p scale as follows: c-p = 1 (i.e., short life cycle, high reproductive rates, tolerant to stress) to c-p = 5 (i.e., long life-cycles, low reproductive output, and sensitive to stress), analogous to the K/r-strategists following [36,37].
2.4. Data Processing
The abundance of nematodes (N), number of species (S), Margalef’s species richness (d), Shannon–Wiener index (H′), and evenness (Pielou) (J′) were calculated in PRIMER 5. [38,39]. Kolmogorov–Smirnov and Bartlett tests were applied to log10 (x + 1) transformed data [38,40]. One-way ANOVA, followed by Tukey’s HSD tests, were used to check for the overall and subsequent multiple comparisons among control and treatments with STATISTICA (v5.1). Square root transformed abundance data and the relative abundance of their functional traits, based on Bray–Curtis similarity measures, were used for nonmetric multidimensional scaling (nMDS, see [41]). SIMPER (i.e., similarity percentage analysis, see [38]) was used afterwards to assess the contribution of species and of their functional traits to the overall average dissimilarity among treatments.
3. Results
3.1. Taxonomic Composition
The initial nematofauna comprised eight orders, 19 families, 24 genera and 26 species. The most diverse families were Xyalidae and Oncholaimidae (Table A1).
At the beginning of the experiment, the nematode community was dominated by Oncholaimus campylocercoïdes (19.3 ± 2.5%) and Microlaimus honestus (15 ± 2%), the other species comprising each less than 10%. By the end of the experiment, the control community was still dominated by O. campylocercoïdes (18.6 ± 4.7%) and M. honestus (14.6 ± 3.5%). The nematode O. campylocercoïdes dominated the communities from all microcosms by the end of the experiment. However, the nematodes Enoplolaimus longicaudatus represented 15.08 ± 3.8% and 14.01 ± 3.4% in P1 and P2, respectively. Cyartonema germanicum and Microlaimus cyatholaimoïdes represented 11.3 ± 1.5% and 11.3 ± 4.9%, respectively, in D1 and Trichotheristus mirabilis 11.3 ± 1.5% in D2. E. longicaudatus (12.6 ± 1.9% in P1D1; 13.2 ± 0.8% in P2D2; 16.7 ± 0.5% in P1D2) and T. mirabilis (12.5 ± 4.7% in P1D1, 14.7 ± 5.8% in P2D2 and 11.6 ± 4.1% in P1D2) were the co-dominant species in these microcosms. Finally, M. honestus represented 13.91 ± 4.15% in P2D1 (Table A1).
3.2. Abundance and Diversity
The mean abundance of nematodes ranged from 3114 ± 88 (I) and 2906 ± 72 (Utc) individuals in I to 78 ± 7 individuals in P2D2 (Figure 1). Overall, the abundance of nematodes in all types of treatments were significantly lower compared to control (p < 0.001, pairwise Tukey-HSD post hoc tests, see Figure 1).
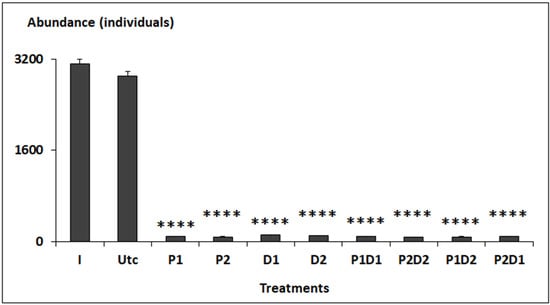
Figure 1.
Abundances of nematodes from the initial assemblage (I), and those from uncontaminated (Utc) and contaminated (P1, P2, D1, D2, P1D1, P1D2, P2D1, and P2D2) microcosms. P = prednisolone, D = dexamethasone. Stars above bars indicate significant differences in comparison to the corresponding controls (log-transformed data) using Tukey’s test: p < 0.0001 (****).
The initial diversity significantly decreased in most treatments during the experiment, excepting the control and P1 (Figure 2). At the end of the experiment, the diversity of control community was significantly higher compared to other treatments, excepting P1 and P2 (i.e., P1 vs. PD1, P2D2 and P1D2, as well as between P2 vs. P1D1 and P1D2). The Margalef’s species richness (d) showed significant differences among treatments, excepting the final control, P1 and P2 (i.e., P1 vs. P1D1 and P1D2, as well as P2 vs. P1D1 and P1D2, see Figure 2). However, the Shannon–Wiener (H′), and Pielou’s evenness (J′) indices were similar among all experimental microcosms.
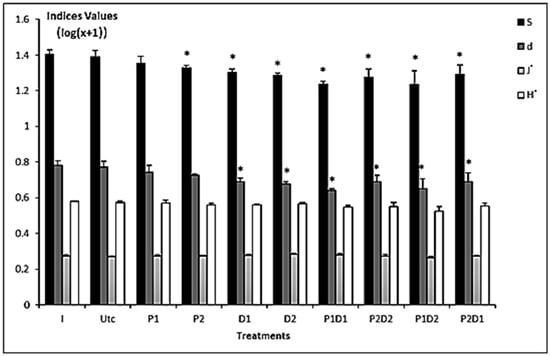
Figure 2.
Graphical summary of univariate indices for nematofauna from the initial assemblage (I), and those from uncontaminated (Utc) and contaminated (P1; P2; D1; D2; P1D1; P1D2; P2D1; P2D2) microcosms. P = prednisolone, D = dexamethasone, H′ = Shannon–Wiener index, d = species richness, J′ = evenness, S = number of species. Stars above bars indicate significant differences in comparison to the corresponding controls (log-transformed data) using Tukey’s test: p < 0.05 (*).
3.3. Multivariate Analyses
The nMDS ordination showed a clear differentiation among treatments (stress = 0.17, Figure 3). The initial and final control communities were situated very close in the ordination space. The P and D treatments were fully separated from control microcosms, along with their mixtures, except for P2D1 (Figure 3).
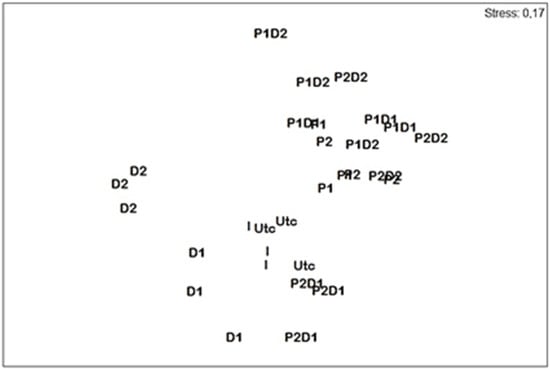
Figure 3.
Nonmetric multidimensional scaling (nMDS) 2D plot based on nematode species abundances from the initial assemblage (I), and those uncontaminated (Utc) and contaminated (P1; P2; D1; D2; P1D1; P1D2; P2D1; P2D2) microcosms. P = prednisolone, D = dexamethasone.
The average dissimilarity was high between the initial and final control nematode communities and other treatments (Table A2). The lowest dissimilarity was between initial and final control (25.1%) and the highest between initial control and P2D2 (48.3%). Despite the overall increase in dissimilarity, the mixture P2D1 showed very low dissimilarity with the initial (30.7%) and final control (27.2%) communities, respectively. The SIMPER results indicated that the dissimilarity between the initial and the final control community was mostly due to the decrease in the M. honestus abundance. Moreover, the increase in the abundance of T. mirabilis contributed to the dissimilarity observed with the initial community. The fluctuation among treatments of O. campylocercoïdes abundance also contributed to the average dissimilarity.
3.4. Functional Traits
The functional categories within and among treatments communities followed the following patterns (Figure 4):
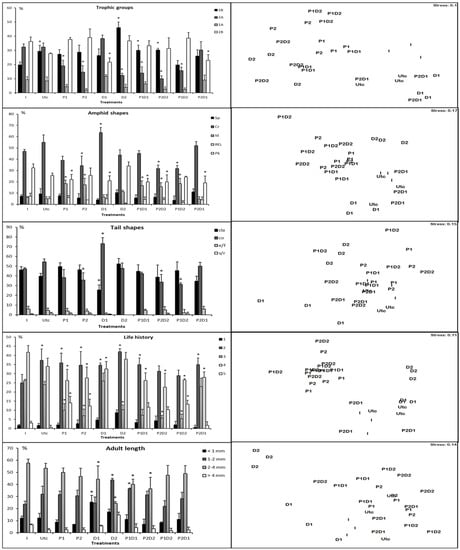
Figure 4.
Nonmetric multidimensional scaling (nMDS) 2D plots (left) and graphical summary (right) based on abundances of functional groups of nematode assemblages from each microcosm. P = prednisolone, D = dexamethasone. Selective deposit feeders (1A); nonselective deposit feeders (1B); epigrowth feeders (2A); omnivores–carnivores (2B); short/round (s/r); elongated/filiform (e/f); conical (co); clavate/conical-cylindrical (cla); spiral (sp); pocket-like (pk); indistinct (id); circular (cr). Stars indicate significant differences with the initial nematofauna (*).
- The feeding groups of the initial communities were dominated by omnivorous/carnivores (2B) and epistratum-feeders (2A), comprising 36.3 ± 3.05% and 32.3 ± 2.3% of the nematofauna. The 1B group significantly increased in final control, D2, P1D1 and P2D2, respectively. Conversely, the 2A group decreased in P, P2, D2, P1D1, P2D2 and P1D2, whereas the 2B group increased in D1 and P2D1. The nMDS results indicated that D2 and P2D2 groups were furthest from initial control. Conversely, the treatments D1 and P2D1 were situated closer to initial and final control, respectively.
- Amphid shapes of the initial community were dominated by circular (cr) and pocket-like (pk) amphids, comprising 47 ± 1.7% and 32.3 ± 3.5% of the nematofauna. The cr amphid shape types decreased in P2, D1, P2D2 and P1D2, whereas the id amphid shape significantly increased in P1, P2, P1D1, P2D2, and P1D2. The pk amphid shape significantly increased in P1, D1, P1D1, P2D2, and P2D1. The nMDS results indicated that P1D2 was furthest away from initial control, but P2D1 and final were the closest to initial control.
- Tail shapes were dominated by conical (co) and clavate (cla) types, comprising 47.3 ± 1.1% and 46 ± 3.4% of the initial nematofauna, respectively. The contamination induced a significant decrease of co tail shapes in P2, P2D2 and P1D2, as well as of cla tails shape in D1. The co tail shape increased significantly in D1. The nMDS results indicated that D1 was situated the furthest from initial control, but treatments P1 and P1D1 were the closest.
- The initial life history composition was c-p4, followed by c-p3 and c-p2, comprising 41.6 ± 3.7%, 26.3 ± 0.5% and 25 ± 4.58% of the nematofauna, respectively. The c-p2 types increased significantly in most treatments, excepting P1D2 and P2D2, as well as c-p5 in P1, P2 and P1D2. Conversely, the results showed a significant decrease of c-p3 in most treatments, except for final control, D1 and P2D1, and of c-p4 in most treatments except for final control and D1. The nMDS results indicated that P1D2 and P2D2 were situated the furthest from initial control, whereas the treatments P2D1 and final control were the closest.
- Body sizes were dominated by 2–4 mm and 1–2 mm species, comprising 57.6 ± 3.2% and 23.6 ± 2.5% of the initial nematofauna. The species smaller than 1 mm increased in D1, same for those between 1 and 2 mm interval in D2 and P1D1. Conversely, the species with body sizes between 2 and 4 mm intervals decreased in D1, D2, P1D1 and P2D2. The nMDS ordination indicated that D2 was situated the furthest from initial control, whereas D1 was the closest.
The dissimilarity values of all functional traits were lower than 30% compared to initial conditions, except for that of adults length in D2 (Table A2). SIMPER results highlighted significant decreases of 2A and 2B feeding groups, with 2–4 mm body-size interval, and of c-p3 life history type in most treatments compared to the initial conditions. Moreover, a significant modification of the co and cla tail shapes and cr and pk amphid shapes, respectively, were observed in treatments compared to initial conditions, but similar to final control.
The nMDS second-stage ordination showed that the responses of nematodes to different treatments depended mainly on life history (86.71%), amphid shape (84.17%), adult body size (81%) and trophic groups (80.12%) (Figure 5).
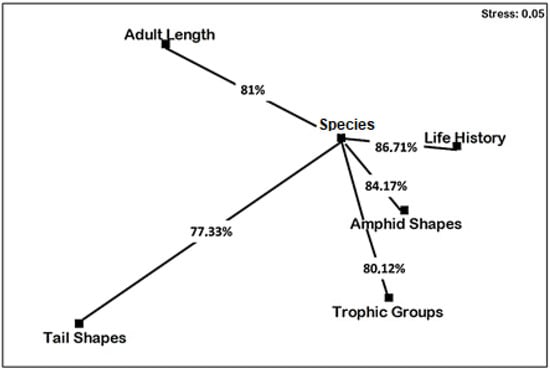
Figure 5.
Nonmetric multidimensional scaling (nMDS) second-stage ordination of intermatrix rank correlations. For matrices included. See Figure 3 (species) and Figure 4 (functional traits). Values indicate average similarity percentages between nMDS related to species and those related to functional traits.
4. Discussion
The knowledge of ecotoxicological effects of glucocorticoids in aquatic habitats, including the marine milieu, is scarce. The main objective of this experiment was to partially cover this knowledge gap by assessing the impact of prednisolone and dexamethasone on free-living marine nematodes. As such, several community-based indices were quantified following exposure of this phylogenetic group to various single and combined concentrations of these two glucocorticoids.
The decrease in the abundance of nematodes following their exposure to both dexamethasone and prednisolone, as well as to their mixtures, was in line with previous findings who assessed similar responses of nematofauna following exposure to hydrocarbons [42], heavy metals [43], and pesticides [44].
The initial community was dominated by O. campylocercoides (19.3 ± 2.5%) and M. honestus (15 ± 2%), respectively, by the end of the experiment and was similar after 30 days, indicating that the control community did not change during the experiment, assuring the representativeness of multiple comparisons with other treatments.
The contamination with prednisolone showed significant changes in diversity of nematofauna only for the highest concentration, P2. According to SIMPER results, the dissimilarity between the initial and final control and P2 treatments was mainly due to the disappearance of M. honestus and increased abundance of E. longicaudatus, suggesting sensitivity for the former and tolerance for the latter species to prednisolone. This pattern was also paralleled by changes in the composition of functional traits, showing a decrease in 2A feeding group frequency, cr amphid shapes and co tail shapes, but an increase in 2B feeding groups and id amphid shapes. A previous study suggested low acute toxicity of prednisolone, due to its photo-degradation byproducts [23]. Such byproducts may have occurred during the 30 days exposure and are potentially responsible for the decrease in abundance of intermediate size species (i.e., 1–4 mm) and the disappearance of M. honestus.
The contamination with both dosages of dexamethasone showed significant effects on the initial and control communities, suggesting higher toxicity compared to prednisolone. Additionally, according to SIMPER results, the dexamethasone impacted differently the initial nematofauna, by inducing a decrease in O. campylocercoïdes and an increase in Microlaimus cyatholaimoïdes abundances in D1 treatment, whereas in D2, the composition switch was due to the decrease in M. honestus and increase in Trichotheristus mirabilis abundances. Furthermore, the SIMPER results highlighted the contribution to the dissimilarity with final control community of Nudora gerlachi and Metoncholaimus pristiurus in D1 and D2, respectively. Despite the significant effect of dexamethasone on diversity indices, the nMDS ordination placed the D1 and D2 treatments close to the initial and final control microcosms, indicating a smaller change in the overall community composition compared to other treatments. Little is known about the fate and toxicity of dexamethasone on aquatic invertebrates, including meiofauna. To our knowledge, previous studies reported that the exposure to dexamethasone led to early hatching of zebra fish embryos [45]—impacting the overall ontogenetic development [46]—as well as to reducing C. dubia population growth by 50% at concentrations of 0.05 mg·L−1 [23].
All types of mixtures had a significant impact on nematofauna compared to initial and final control microcosms. However, the mixtures with the highest concentrations of prednisolone showed less effect on Margalef’s index, especially P2D1. Furthermore, according to SIMPER results, P2D1 recorded the lowest dissimilarity with the initial and control communities. The other mixtures led to higher dissimilarities compared to single glucocorticoid contamination. The species responsible for the average dissimilarity in P2D1 were the decreased abundances of T. mirabilis and O. campylocercoïdes, but with different rates in different mixtures and also due to the elimination of M. honestus and increased abundance of E. longicaudatus. The same conclusion is applicable for the functional traits, as reflected in the nMDS ordination of the P2D1 cluster, which was close to the initial and final control communities. The nMDS ordination and the opposite trends of the relative abundances of M. honestus and E. longicaudatus following exposure to the mixtures P1D1, P2D2, P1D2, and P2D1 support a potential antagonist interaction between glucocorticoids, as well as potentially higher toxicity of dexamethasone compared to prednisolone for the nematodes. The latter speculation seems also to be supported by a less severe impact on the nematofauna in P2D1 compared to P1D2.
Besides community-based indices, the functional traits also differed among treatments. The initial community was dominated by c-p4 species throughout the experiment in control microcosms. However, the emergence of c-p2 species was noticeable by the end of the experiment in control microcosms, slightly exceeding that of c-p4 species. The contamination induced a significant increase of c-p5 species, except for D1, D2 and P2D1 treatments, respectively. The only species belonging to c-p5 type in the current study was E. longicaudatus. This species has K type reproductive strategy and thrived in most treatments, excepting those contaminated only with D. These results suggest a potential higher tolerance of E. longicaudatus to prednisolone, but sensitivity to dexamethasone. Thus, this species of nematode could be considered as a positive bioindicator for prednisolone pollution and negative for dexamethasone.
The biomonitoring of marine areas is a controversial topic but necessary in establishing blue economy strategies [47,48]. In the case of meiobenthic nematodes, taxonomic diversity was commonly included, whereas functional tools such as Maturity index or Index of Trophic Diversity were less considered. In fact, it is not possible for the moment to develop a generally usable ecotoxicological model because experiments’ outcomes are different depending on the type of chemicals. Bioassays on nematodes, including the current one, belong to a primordial step with a main objective to classify taxa and/or functional groups in tolerant or sensitive categories. For several reasons, no absolute results are associated to taxa or functional traits [49,50,51]. First, the Maturity Index was developed by Bongers [52,53] and employed, like most functional descriptors, at a generic level. In our opinion, this could be the main reason of conflicting results regarding its usefulness (see [54,55,56]). Second, the response of any given species to stress will depend on its physiological, morphological and behavior characteristics. Several closely related (i.e., appurtenant to the same genus or family) nematode species from Tunisian waters have different responses to pollutants [57] due distinct tail shapes (e.g., O. campylocercoides vs. O. brevicaudatus), the presence/absence of sexual dimorphism (e.g., M. pristiurus vs. M. demani) and the presence/absence of long somatic setae (e.g., Spirinia gerlachi vs. S. parasitifera or T. mirabilis vs. Theristus modicus or Setosabatieria hilarula vs. Sabatieria granifer), etc. Third, during the last two decades, the use of molecular investigations in the systematics of nematodes became a very useful technique [58]. In particular, the results of [59,60,61] and [62], based on DNA barcoding of the mitochondrial marker Cytochrome c Oxidase I (COI), demonstrated for the marine nematode Pellioditis marina the existence of divergent lineages or haplotypes with different potentials for colonization/persistence and a wide spectrum of tolerance/sensitivity to pollutants. This body of evidence leads us to the conclusion that a second step is needed in the future in order to establish standard rearing methods for species with large spectra of tolerance or sensitivity and to launch bioassays on confirmed models and cell lines.
5. Conclusions
The current study explored for the first time the toxic effects of glucocorticoids on meiobenthic fauna, with a main focus on free-living marine nematodes. Therefore, single and mixtures of two commonly used glucocorticoids, prednisolone and dexamethasone, were used to study such toxicological interactions.
The results highlighted a significant toxic effect on nematode abundances. Diversity indices indicated higher toxicity of dexamethasone compared to prednisolone on nematofauna. SIMPER analysis showed significant modifications in the nematode’s communities, as well as on their functional traits. Thus, the results of the current experiment reveal the sensitivity of M. honestus to prednisolone. On the other hand, the results showed that E. longicaudatus was highly tolerant to prednisolone, but potentially sensitive to dexamethasone. Finally, the contradictory results obtained from the P2D1 treatment compared to P1D2 suggest potential antagonistic interactions between these pharmaceuticals, as well as potentially higher toxicity of dexamethasone compared to prednisolone for nematodes.
Author Contributions
M.A. and S.I.: Writing—original draft, Investigation, Review & editing, Methodology, Formal analysis, Visualisation. A.N.: Methodology, Data curation. A.H.H.: Funding acquisition, Writing—review & editing, Visualisation. S.A.: Conceptualisation, Resources, Methodology. H.B.: Writing—review & editing, Writing—review & editing. O.P.: Writing, Methodology, Formal analysis, Data curation. F.B.: Writing—original draft, Writing—review & editing, Supervision, Visualisation. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Researchers Supporting Project number (RSP-2021/17), King Saud University, Riyadh, Saudi Arabia. OP was funded by the National Core Program–Romanian Ministry of Research and Innovation Program, project 25 N/2019 BIODIVERS 19270103.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are not be shared due to restrictions, e.g., privacy and regulation.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP2021/17), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Species list and functional traits of nematode species identified in the initial assemblage (I), and those from uncontaminated (Utc) and contaminated (P1, P2, D1, D2, P1D1, P2D2, P2D1, and P1D2) microcosms. Prednisolone (P); dexamethasone (D); Colonizers-Persisters scores (c-p); tail shape (Tl): conical (co), elongated/filiform (e/f), clavate (cla); amphid shape (Am): pocket-like (pk), indistinct (id), spiral (sp), circular (cr), Rounded or Elongate Loop (REL); feeding groups (FG): selective deposit-feeders (1A), non-selective deposit-feeders (1B), epistratum-feeders (2A), omnivores carnivores (2B), adult length (AL).
Table A1.
Species list and functional traits of nematode species identified in the initial assemblage (I), and those from uncontaminated (Utc) and contaminated (P1, P2, D1, D2, P1D1, P2D2, P2D1, and P1D2) microcosms. Prednisolone (P); dexamethasone (D); Colonizers-Persisters scores (c-p); tail shape (Tl): conical (co), elongated/filiform (e/f), clavate (cla); amphid shape (Am): pocket-like (pk), indistinct (id), spiral (sp), circular (cr), Rounded or Elongate Loop (REL); feeding groups (FG): selective deposit-feeders (1A), non-selective deposit-feeders (1B), epistratum-feeders (2A), omnivores carnivores (2B), adult length (AL).
| Tl | Am | FG | c-p | AL | I | Utc | P1 | P2 | D1 | D2 | P1D1 | P2D2 | P1D2 | P2D1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bathylaimus sp. | cla | sp | 1B | 2 | 1–2 mm | 1.33 ± 1.15 | 1.66 ± 1.52 | 0.74 ± 0.64 | 1.33 ± 1.52 | 3.66 ± 1.15 | 0.38 ± 0.67 | 1.82 ± 1.21 | |||
| Cyartonema germanicum | co | cr | 1A | 4 | <1 mm | 6.33 ± 1.52 | 7 ± 2.64 | 3.64 ± 1.41 | 0.71 ± 1.21 | 11.33 ± 1.52 | 4 ± 2 | 5.76 ± 1.29 | 1.73 ± 0.81 | 2.25 ± 1.81 | 8.92 ± 4.21 |
| Daptonema fallax | cla | cr | 1B | 2 | 1–2 mm | 0.66 ± 0.57 | 0.66 ± 0.57 | 0.66 ± 1.15 | 5.33 ± 2.08 | 0.45 ± 0.79 | 1.45 ± 1.63 | ||||
| Enoplolaimus longicaudatus | cla | id | 2B | 5 | 2–4 mm | 3 ± 1 | 0.66 ± 1.15 | 15.08 ± 3.85 | 14.01 ± 3.45 | 12.64 ± 1.93 | 13.21 ± 0.81 | 16.69 ± 0.53 | 1.81 ± 2.22 | ||
| Halalaimus gracilis | e/f | REL | 1A | 4 | 2–4 mm | 3 ± 1.73 | 1.33 ± 0.57 | 1.04 ± 1.06 | 1.53 ± 0.64 | 0.33 ± 0.57 | 1.09 ± 0.07 | 1.61 ± 1.76 | 0.76 ± 1.32 | 1.11 ± 1.08 | |
| Longicyatholaimus longicandatus | e/f | sp | 2A | 3 | 2–4 mm | 3 ± 1 | 3.33 ± 2.08 | 3.13 ± 1.94 | 2.93 ± 2.07 | 0.66 ± 0.57 | 3.99 ± 1.21 | 4.73 ± 4.12 | 1.53 ± 2.65 | 5.44 ± 2.77 | |
| Metoncholaimus pristiurus | cla | pk | 2B | 4 | >4 mm | 3.33 ± 057 | 0.66 ± 1.15 | 0.66 ± 0.57 | 8.33 ± 1.52 | ||||||
| Microlaimus cyatholaimoïdes | co | cr | 2A | 2 | 2–4 mm | 5.66 ± 3.05 | 7 ± 1.73 | 8.95 ± 1.36 | 7.31 ± 2.41 | 11.33 ± 4.93 | 2 ± 1 | 7.22 ± 3.01 | 5.66 ± 1.88 | 10.54 ± 1.13 | 8.15 ± 3.09 |
| Microlaimus honestus | co | cr | 2A | 3 | 2–4 mm | 15 ± 2 | 14.66 ± 3.51 | 15 ± 2 | 5.33 ± 0.57 | 13.91 ± 4.15 | |||||
| Nudora gerlachi | co | cr | 2A | 3 | <1 mm | 2.66 ± 2.08 | 1 ± 1.73 | 1.12 ± 1.16 | 1.71 ± 2.96 | 7.66 ± 2.51 | 2 ± 2 | 0.68 ± 1.19 | 0.45 ± 0.79 | 2.26 ± 2.2 | |
| Odontophora villoti | co | REL | 1B | 2 | 1–2 mm | 0.33 ± 0.57 | 1.33 ± 0.57 | 1.85 ± 1.41 | 1.96 ± 0.81 | 3 ± 2 | 0.33 ± 0.57 | 2.25 ± 2.35 | 3.86 ± 0.33 | 0.77 ± 0.67 | |
| Oncholaimellus calvadocicus | cla | pk | 2B | 4 | 1–2 mm | 3.33 ± 0.57 | 2 ± 1 | 1.73 ± 1.61 | 2.41 ± 2.1 | 1 ± 1 | 5.33 ± 1.52 | 1.71 ± 2.97 | 1.93 ± 3.35 | 1.16 ± 1.14 | 0.71 ± 1.24 |
| Oncholaimus campylocercoïdes | cla | pk | 2B | 4 | 2–4 mm | 19.33 ± 2.51 | 18.66 ± 4.72 | 16.96 ± 3.32 | 20.15 ± 5.83 | 13 ± 2.64 | 14 ± 2.64 | 15.31 ± 3.94 | 16.07 ± 4.43 | 24.52 ± 3.11 | 17.54 ± 4.26 |
| Paramonohystera proteus | cla | cr | 1B | 2 | 1–2 mm | 7.33 ± 0.57 | 7.33 ± 2.08 | 8.69 ± 2.67 | 7.46 ± 3.63 | 4 ± 1 | 8 ± 2 | 7.88 ± 2.76 | 7.28 ± 0.82 | 5.67 ± 2.69 | 7.363 ± 5.46 |
| Parasphaerolaimus paradoxus | cla | cr | 2B | 3 | 1–2 mm | 2.33 ± 0.57 | 2.33 ± 1.52 | 5.09 ± 1.63 | 3.01 ± 1.04 | 2 ± 2 | 1 ± 1 | 8.85 ± 2.79 | 4.66 ± 2.93 | 4.01 ± 2.29 | 2.58 ± 1.31 |
| Phanoderma sp. | s/r | pk | 2A | 4 | 2–4 mm | 0.66 ± 0.57 | 1.33 ± 1.15 | 1.12 ± 1.16 | 1.11 ± 1.13 | 0.33 ± 0.57 | 0.77 ± 1.34 | 1.19 ± 2.06 | |||
| Prochromadorella longicaudata | co | id | 2A | 2 | <1 mm | 1.33 ± 0.57 | 2.66 ± 1.52 | 2.46 ± 1.12 | 2.66 ± 0.45 | 1.66 ± 1.52 | 2.66 ±2.08 | 1.73 ± 2.13 | 1.36 ± 2.37 | 3.71 ± 0.93 | 2.64 ± 2.9 |
| Rhabditis sp. | co | id | 1B | 1 | <1 mm | 1.66 ± 0.57 | 1.66 ± 1.15 | 2.12 ± 1.01 | 3.023 ± 2.33 | 4.66 ± 1.15 | 8.66 ± 1.52 | 3.65 ± 1.66 | 5.63 ± 3.56 | 2.32 ± 2.29 | 0.71 ± 1.24 |
| Sabatiera splendens | cla | sp | 1B | 2 | 1–2 mm | 1.33 ± 1.52 | 1.66 ± 0.57 | 1.46 ± 0.074 | 1.08 ± 1.05 | 1 ± 1 | 4 ± 2 | 0.34 ± 0.59 | 0.77 ± 1.34 | 0.49 ± 084 | 2.18 ± 1.03 |
| Spirinia parasitifera | co | REL | 2A | 3 | 2–4 mm | 4 ± 1 | 2.33 ± 1.52 | 3.48 ± 2.05 | 1.12 ± 1.06 | 1.66 ± 1.52 | 0.33 ± 0.57 | 1.51 ± 1.3 | 0.39 ± 0.68 | 3.29 ± 1.85 | |
| Synonchiella edax | cla | sp | 2B | 3 | 2–4 mm | 1.66 ± 1.15 | 2.66 ± 1.15 | 2.84 ± 0.046 | 2.28 ± 1.03 | 1 ± 1.73 | 2.66 ± 0.57 | 1.82 ± 2.22 | 2.07 ± 1.83 | 2.32 ± 2.29 | 2.65 ± 3.71 |
| Thalassironus britannicus | co | pk | 2B | 4 | >4 mm | 3.33 ± 0.57 | 1.66 ± 1.15 | 2.81 ± 1.55 | 3.14 ± 1.99 | 5 ± 1 | 6.33 ± 1.52 | 4.81 ± 3.37 | 4.46 ± 3.55 | 2.34 ± 3.11 | 2.55 ± 2.49 |
| Theristus modicus | co | cr | 1B | 2 | 1–2 mm | 1.66 ± 0.57 | 3.66 ± 1.52 | 3.55 ± 1.46 | 4.14 ± 2.03 | 1.66 ± 1.52 | 4 ± 1.73 | 3.54 ± 2.09 | 2.06 ± 1.23 | 2.92 ± 0.53 | 2.95 ± 0.7 |
| Theristus pertenuis | co | cr | 1B | 2 | 1–2 mm | 2 ± 1.73 | 2 ± 1 | 3.18 ± 0.85 | 2.34 ± 1.19 | 0.66 ± 1.15 | 0.66 ± 1.15 | 2.57 ± 2.69 | 2.27 ± 2.85 | 0.383 ± 0.66 | 1.1 ± 1.07 |
| Thoonchus inermis | cla | pk | 2B | 4 | 2–4 mm | 2.33 ± 0.08 | 1.33 ± 1.15 | 1.12 ± 1.16 | 3.07 ± 1.29 | 1 ± 1.73 | 2.063 ± 1.23 | 1.65 ± 0.57 | |||
| Trichotheristus mirabilis | co | cr | 1B | 2 | 1–2 mm | 2 ± 1 | 7.33 ± 3.21 | 6.32 ± 2.8 | 9.81 ± 2.54 | 5.33 ± 2.88 | 11.33 ± 1.51 | 12.57 ± 4.74 | 14.71 ± 5.81 | 11.64 ± 4.14 | 7.37 ± 3.57 |
| Valvaelaimus maior | co | cr | 1B | 2 | 1–2 mm | 1.33 ± 1.15 | 2 ± 1 | 1.41 ± 1.63 | 2.96 ± 1.51 | 4 ± 1 | 1.72 ± 2.05 | 0.39 ± 0.68 | 3.66 ± 1.61 |

Table A2.
Dissimilarity percentages (bold values) between the initial assemblage (I) and treatments and results of Similarity Percentage analysis (SIMPER) based on square-root transformed data. Species and functional groups accounting for ∼70% of overall dissimilarity are ranked in order of importance of their contribution. Untreated control (Utc); Prednisolone (P); dexamethasone (D); Colonizers-Persisters scores (c-p); conical (co); elongated/filiform (e/f); clavate (cla); pocket-like (pk); indistinct (id); spiral (sp); circular (cr); Rounded or Elongate Loop (REL); selective deposit-feeders (1A); non-selective deposit-feeders (1B); epistratum-feeders (2A); omnivores carnivores (2B); more abundant (+); less abundant (−); eliminated (elim); no change (=).
Table A2.
Dissimilarity percentages (bold values) between the initial assemblage (I) and treatments and results of Similarity Percentage analysis (SIMPER) based on square-root transformed data. Species and functional groups accounting for ∼70% of overall dissimilarity are ranked in order of importance of their contribution. Untreated control (Utc); Prednisolone (P); dexamethasone (D); Colonizers-Persisters scores (c-p); conical (co); elongated/filiform (e/f); clavate (cla); pocket-like (pk); indistinct (id); spiral (sp); circular (cr); Rounded or Elongate Loop (REL); selective deposit-feeders (1A); non-selective deposit-feeders (1B); epistratum-feeders (2A); omnivores carnivores (2B); more abundant (+); less abundant (−); eliminated (elim); no change (=).
| I vs. P1D1 | I vs. P2D2 | I vs. P1D2 | I vs. P2D1 | |
|---|---|---|---|---|
| Species | 44.87% | 48.32% | 44.44% | 30.72% |
| Microlaimus honestus (17.45%) elim | Microlaimus honestus (17.46%) elim | Microlaimus honestus (18.83%) elim | Trichotheristus mirabilis (7.98%) − | |
| Trichotheristus mirabilis (10.95%) + | Trichotheristus mirabilis (10.91%) + | Enoplolaimus longicaudatus (12.87%) + | Oncholaimus campylocercoïdes (6.9%) − | |
| Enoplolaimus longicaudatus (10.02%) + | Enoplolaimus longicaudatus (8.49%) + | Trichotheristus mirabilis (8.88%) + | Paramonohystera proteus (6.41%) − | |
| Parasphaerolaimus paradoxus (6.64%) + | Oncholaimus campylocercoïdes (7.86%) − | Cyartonema germanicum (5.8%) − | Microlaimus honestus (6.3%) − | |
| Oncholaimus campylocercoïdes (6.21%) − | Cyartonema germanicum (5.81%) − | Spirinia parasitifera (4.61%) − | Metoncholaimus pristiurus (5.69%) + | |
| Metoncholaimus pristiurus (3.88%) elim | Spirinia parasitifera (4.66%) elim | Metoncholaimus pristiurus (4.18%) elim | Microlaimus cyatholaimoïdes (5.15%) − | |
| Microlaimus cyatholaimoïdes (3.47%) + | Metoncholaimus pristiurus (3.88%) elim | Microlaimus cyatholaimoïdes (3.9%) + | Oncholaimellus calvadosicus (4.57%) + | |
| Oncholaimellus calvadosicus (3.25%) − | Oncholaimellus calvadosicus (3.26%) − | Paramonohystera proteus (3.45%) − | Nudora gerlachi (4.55%) + | |
| Spirinia parasitifera (3.08%) − | Rhabditis sp. (3.12%) + | Oncholaimellus calvadosicus (2.97%) − | Longicyatholaimus longigicandatus (4.53%) − | |
| Thalassironus britanicus (2.96%) + | Odontophora villoti (3.1%) + | Halalaimus gracilis (2.96%) − | Cyartonema germanicum (4.42%) − | |
| Thoonchus inermis (3.99%) + | ||||
| Synonchiella edax (3.83%) − | ||||
| Thalassironus britannicus (3.61%) − | ||||
| Feeding groups | 19.87% | 27.48% | 17.41% | 14.95% |
| 2A − | 2A − | 2A − | 2B − | |
| 1B + | ||||
| Tail shape | 6.91% | 16.71% | 16% | 9.6% |
| cla − | co − | co − | cla − | |
| Amphid shape | 16.62% | 24.2% | 25.36% | 14.5% |
| pk − | cr − | cr − | pk − | |
| pk − | id + | cr + | ||
| Adult length | 18.89% | 21.26% | 12.77% | 11.92% |
| 2–4 mm − | 2–4 mm − | 2–4 mm − | 2–4 mm − | |
| > 4 mm − | 1–2 mm + | |||
| c-p score | 30.13% | 32.86% | 30.16% | 16.92% |
| c-p3 − | c-p3 − | c-p3 − | c-p4 − | |
| c-p4 − | c-p4 − | c-p4 − | c-p2 + | |
| Utc vs. P1 | Utc vs. P2 | Utc vs. D1 | Utc vs. D2 | |
| Species | 34.62% | 36.76% | 32% | 41.67% |
| Microlaimus honestus (21.97%) elim | Microlaimus honestus (21.37%) elim | Nudora gerlachi (10.42%) + | Microlaimus honestus (11.2%) − | |
| Enoplolaimus longicaudatus (19.95%) + | Enoplolaimus longicaudatus (16.85%) + | Oncholaimus campylocercoïdes (9.2%) − | Metoncholaimus pristiurus(9.2%) + | |
| Oncholaimus campylocercoïdes (6.12%) − | Cyartonema germanicum (9.27%) − | Cyartonema germanicum (6.77%) + | Rhabditis sp. (8.4%) + | |
| Cyartonema germanicum (5.46%) − | Oncholaimus campylocercoïdes (6.42%) − | Microlaimus cyatholaimoïdes (6.77%) + | Oncholaimus campylocercoïdes (6.13%) − | |
| Trichotheristus mirabilis (4.69%) − | Trichotheristus mirabilis (4.48%) − | Thalassironus britannicus (5.21%) + | Microlaimus cyatholaimoïdes (6%) − | |
| Parasphaerolaimus paradoxus (3.53%) + | Paramonohystera proteus (3.39%) − | Trichotheristus mirabilis (5.21%) − | Thalassironus britannicus (5.6%) + | |
| Paramonohystera proteus (3.02%) + | Longicyatholaimus longicaudatus (2.93%) − | Paramonohystera proteus (5.21%) + | Daptonema fallax (5.6%) + | |
| Longicyatholaimus longicaudatus (2.84%) − | Microlaimus cyatholaimoïdes (2.59%) − | Rhabditis sp. (4.69%) + | Trichotheristus mirabilis (5.33%) − | |
| Theristus modicus (2.57%) = | Microlaimus honestus (4.34%) + | Cyartonema germanicum (4.13%) elim | ||
| Longicyatholaimus longicaudatus (4.17%) − | Longicyatholaimus longicaudatus (4%) + | |||
| Theristus modicus (3.47%) − | Oncholaimellus calvadosicus (4%) + | |||
| Synonchiella edax (3.3%) + | ||||
| Feeding groups | 16.9% | 21.78% | 10.3% | 25.52% |
| 2A − | 2A − | 2B − | 2A − | |
| 2A + | ||||
| Tail Shape | 15.3% | 15.25% | 18.78% | 12.89% |
| co − | co − | co + | cla + | |
| Amphid Shape | 20.32% | 23.47% | 12.44% | 17% |
| cr − | cr − | cr + | cr − | |
| sp − | pk + | |||
| Adult Length | 7.9% | 10.39% | 17.89% | 29% |
| 1–2 mm + | 2–4 mm − | < 1 mm + | 2–4 mm − | |
| 2–4 mm − | 1–2 mm − | 2–4 mm − | ||
| c-p score | 22.75% | 24.35% | 8% | 16.39% |
| c-p3 − | c-p3 − | c-p2 − | c-p3 − | |
| c-p5 + | c-p5 + | c-p4 − | c-p1 + | |
| Utc vs. P1D1 | Utc vs. P2D2 | Utc vs. P1D2 | Utc vs. P2D1 | |
| Species | 40.94% | 44.68% | 40.54% | 27.28% |
| Microlaimus honestus (18.7%) elim | Microlaimus honestus (18.46%) elim | Microlaimus honestus (20.18%) elim | Oncholaimus campylocercoïdes (9.45%) − | |
| Enoplolaimus longicaudatus (13.96%) + | Enoplolaimus longicaudatus (12.12%) + | Enoplolaimus longicaudatus (17.32%) + | Microlaimus honestus (7.72%) − | |
| Parasphaerolaimus paradoxus (7.28%) + | Oncholaimus campylocercoïdes (7.93%) − | Cyartonema germanicum (7.28%) − | Paramonohystera proteus (7.65%) − | |
| Oncholaimus campylocercoïdes (6.51%) − | Cyartonema germanicum (7.13%) − | Oncholaimus campylocercoïdes (5.19%) + | Trichotheristus mirabilis (6.4%) − | |
| Trichotheristus mirabilis (6.02%) + | Trichotheristus mirabilis (6.19%) + | Paramonohystera proteus (4.38%) − | Cyartonema germanicum (6.23%) − | |
| Thalassironus britannicus (3.99%) + | Rhabditis sp. (3.66%) + | Trichotheristus mirabilis (4.16%) + | Longicyatholaimus longicaudatus (5.32%) − | |
| Cyartonema germanicum (3.27%) − | Microlaimus cyatholaimoïdes (3.32%) − | Longicyatholaimus longicaudatus (3.71%) − | Synonchiella edax (4.93%) − | |
| Microlaimus cyatholaimoïdes (3.24%) − | Longicyatholaimus longicaudatus (3.24%) + | Spirinia parasitifera (2.76%) − | Microlaimus cyatholaimoïdes (4.07%) − | |
| Oncholaimellus calvadosicus (2.96%) − | Spirinia parasitifera (2.94%) elim | Parasphaerolaimus paradoxus (2.58%) + | Prochromadorella longicaudata (4.06%) − | |
| Paramonohystera proteus (2.86%) = | Oncholaimellus calvadosicus (2.92%) − | Theristus pertenuis (2.31%) − | Enoplolaimus longicaudatus (3.19%) − | |
| Oncholaimellus calvadosicus (3%) + | ||||
| Spirinia parasitifera (2.98%) − | ||||
| Thalassironus britannicus (2.98%) + | ||||
| Feeding groups | 16.81% | 21% | 24.96% | 9.84% |
| 2A − | 2A − | 2A − | 1B − | |
| 2B + | 2A − | |||
| Tail Shape | 10.91% | 19.24% | 20.43% | 7.81% |
| co − | co − | co − | cla − | |
| Amphid Shape | 17.69% | 26.28% | 26.71% | 10.63% |
| id + | cr − | cr − | pk − | |
| cr − | cr − | |||
| Adult Length | 13.69% | 17.1% | 13.3% | 9.82% |
| 2–4 mm − | 2–4 mm − | 1–2 mm − | 1–2 mm − | |
| 2–4 mm − | ||||
| c-p score | 23.55% | 28.67% | 28.06% | 9.82% |
| c-p3 − | c-p3 − | c-p3 − | c-p4 − | |
| c-p5 + | c-p4 − | c-p5 + | c-p2 − |
References
- Han, G.H.; Hur, H.G.; Kim, S.D. Ecotoxicological risk of pharmaceuticals from wastewater treatment plants in Korea: Occurrence and toxicity to Daphnia magna. Environ. Toxicol. Chem. 2006, 25, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.M.L.M.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef] [PubMed]
- Godoy, A.A.; Oliveira, Á.C.D.; Silva, J.G.M.; Azevedo, C.C.D.J.; Domingues, I.; Nogueira, A.J.A.; Kummrow, F. Single and mixture toxicity of four pharmaceuticals of environmental concern to aquatic organisms, including a behavioral assessment. Chemosphere 2019, 235, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sorensen, B.; Nors Nielsen, S.; Lanzky, P.F.; Ingerslev, F.; Holten Loutzhoft, H.C.; Jorgensen, S.E. Occurrence, fate and effects of pharmaceutical substances in the environment––A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ternes, T.A. Special report: Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Kugathas, S.; Williams, R.J.; Sumpter, J.P. Prediction oenvironmental concentrations of glucocorticoids: The River Thames, UK, as an example. Environ. Int. 2012, 40, 15–23. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Chang, H.; Hu, J.; Shao, B. Occurrence of natural and synthetic glucocorticoids in sewage treatment plants and receiving river waters. Environ. Sci. Technol. 2007, 41, 3462–3468. [Google Scholar] [CrossRef]
- Chang, H.; Wan, Y.; Hu, J. Determination and source apportionment of five classes of steroid hormones in urban rivers. Environ. Sci. Technol. 2009, 43, 7691–7698. [Google Scholar] [CrossRef]
- Schriks, M.; van Leerdam, J.A.; van der Linden, S.C.; van der Burg, B.; van Wezel, A.P.; de Voogt, P. High-Resolution Mass Spectrometric Identification and Quantification of Glucocorticoid Compounds in Various Wastewaters in The Netherlands. Environ. Sci. Technol. 2010, 44, 4766–4774. [Google Scholar] [CrossRef] [PubMed]
- Runnalls, T.J.; Margiotta-Casaluci, L.; Kugathas, S.; Sumpter, J.P. Pharmaceuticals in the aquatic environment: Steroids and antisteroids as high priorities for research. Hum. Ecol. Risk Assess. 2010, 16, 1318–1338. [Google Scholar] [CrossRef]
- McNeil, P.L.; Nebot, C.; Cepeda, A.; Sloman, K.A. Environmental concentrations of prednisolone alter visually mediated responses during early life stages of zebrafish (Danio rerio). Environ. Pollut. 2016, 218, 981–987. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kugathas, S.; Sumpter, J.P. Synthetic glucocorticoids in the environment: First results on their potential impacts on fish. Environ. Sci. Technol. 2011, 45, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Maayan, R.; Segal, R.; Feuerman, E.J.; Sandbank, M.; Kaufman, H. Simple methods for estimation of prednisone intake and metabolism. Biomed. Pharmacother. 1988, 42, 409–414. [Google Scholar]
- Hillegass, J.M.; Villano, C.M.; Cooper, K.R.; White, L.A. Matrix metalloproteinase-13 is required for zebra fish (Danio rerio) development and is a target for glucocorticoids. Toxicol. Sci. 2007, 100, 168–179. [Google Scholar] [CrossRef]
- Guo, Y.; Somerfield, P.J.M.; Zhang, Z. Large-scale patterns in the community structure and biodiversity of free living nematodes in the Bohai Sea, China. J. Mar. Biol. Assoc. UK 2001, 81, 755–763. [Google Scholar] [CrossRef]
- Balsamo, M.; Albertelli, G.; Ceccherelli, V.U.; Coccionia, R.; Colangeloc, M.A.; Curini-Gallettid, M.; Danovaroe, R.; D’Addabbof, R.; De Leonardisf, C.; Fabianob, M.; et al. Meiofauna of the Adriatic Sea: Present knowledge and future perspectives. Chem. Ecol. 2010, 26, 45–63. [Google Scholar] [CrossRef]
- Moreno, M.; Semprucci, F.; Vezzulli, L.; Balsamo, M.; Fabiano, M.; Albertelli, G. The use of nematodes in assessing ecological quality status in the Mediterranean coastal ecosystems. Ecol. Indic. 2011, 11, 328–336. [Google Scholar] [CrossRef]
- Ben Ali, M.; Hedfi, A.; Almalki, M.; Karachle, P.K.; Boufahja, F. Toxicity of hydroxychloroquine, a potential treatment for COVID-19, on free-living marine nematodes. Mar. Pollut. Bull. 2021, 167, 112361. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Essid, E.; Beyrem, H.; Hedfi, A.; Boufahja, F.; Vitiello, P.; Aïssa, P. Individual and combined effects of lead and zinc of a free living marine nematode community: Results from microcosm experiments. J. Exp. Mar. Biol. Ecol. 2007, 343, 217–226. [Google Scholar] [CrossRef]
- Semprucci, F.; Balsamo, M. Key role of free-living nematodes in the marine ecosystem. In Nematodes: Morphology, Functions and Management Strategies; Boeri, F., Jordan, A.C., Eds.; NOVA Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 109–134. [Google Scholar]
- DellaGreca, M.; Fiorentino, A.; Isidori, M.; Lavorgna, M.; Previtera, L.; Rubino, M.; Temussi, F. Toxicity of prednisolone, dexamethasone and their photochemical derivatives on aquatic organisms. Chemosphere 2004, 54, 629–637. [Google Scholar] [CrossRef]
- Salgado, R.; Marques, R.; Noronha, J.P.; Mexia, J.T.; Carvalho, G.; Oehmen, A.; Reis, M.A.M. Assessing the diurnal variability of pharmaceutical and personal care products in a full-scale activated sludge plant. Environ. Pollut. 2011, 159, 2359–2367. [Google Scholar] [CrossRef] [PubMed]
- Wieser, W. Benthic studies in buzzards bay. II. The meiofauna. Limnol. Oceanogr. 1960, 5, 121–137. [Google Scholar] [CrossRef]
- Schratzberger, M.; Whomersley, P.; Warr, K.; Bolam, S.G.; Rees, H.L. Colonisation of various types of sediment by estuarine nematodes via lateral infaunal migration: A laboratory study. Mar. Biol. 2004, 145, 69–78. [Google Scholar] [CrossRef]
- Elarbaoui, S.; Richard, M.; Boufahja, F.; Mahmoudi, E.; Thomas-Guyonc, H. Effect of crude oil exposure and dispersant applicationon meiofauna: An intertidal mesocosm experiment. Environ. Sci. Process. Impacts 2015, 17, 997–1004. [Google Scholar] [CrossRef]
- Seinhorst, J.W. A rapid method for the transfer of nematodes from fixative to anhydrous glycerine. Nematologica 1959, 4, 67–69. [Google Scholar] [CrossRef]
- Platt, H.M.; Warwick, R.M. Free-Living Marine Nematodes. Part I. British Enoploids; Cambridge University: London, UK, 1983; 307p. [Google Scholar]
- Platt, H.M.; Warwick, R.M. Free-Living Marine Nematodes. Part II. British Chromadorids; Synopsis of the British Fauna (New Series); Cambridge University: London, UK, 1988; p. 38. [Google Scholar]
- Warwick, R.M.; Platt, H.M.; Somerfield, P.J. Free-Living Marine Nematodes. Part III. British Monohysterids; Synopsis of British Fauna (New Series No. 53); Field Studies Council: London, UK, 1998. [Google Scholar]
- Bezerra, T.N.; Decraemer, W.; Eisendle-Flockner, U.; Hodda, M.; Holovachov, O.; Leduc, D.; Miljutin, D.; Mokievsky, V.; Santiago, P.R.; Sharma, J.; et al. Nemys: World Database of Nematodes. 2020. Available online: http://nemys.ugent.be (accessed on 20 February 2021).
- Semprucci, F.; Balsamo, M.; Appolloni, L.; Sandulli, R. Assessment of ecological quality status along the Apulian coasts (eastern Mediterranean Sea) based on meiobenthic and nematode assemblages. Mar. Biodivers. 2018, 48, 105–115. [Google Scholar] [CrossRef]
- Thistle, D.; Lambshead, P.J.D.; Sherman, K.M. Nematode tail-shape groups respond to environmental differences in the deep-sea. Vie Milieu 1995, 45, 107–115. [Google Scholar]
- Wieser, W. Die Beziehung zwischen Mundhöhlengestalt, Ernäh rungsweiseund Vorkommen bei freilebenden marinen Nematoden. Arkiv. För. Zool. 1953, 2, 439–484. [Google Scholar]
- Bongers, T.; Alkemade, R.; Yeates, G.W. Interpretation of disturbance-induced maturity decrease in marine nematode assemblages by means of the maturity index. Mar. Ecol. Prog. Ser. 1991, 76, 135–142. [Google Scholar] [CrossRef]
- Bongers, T.; de Goede, R.G.M.; Korthals, G.W.; Yeates, G.W. An update to the cprating of nematode genera can be found in proposed changes of c-p classification for nematodes. Russ. J. Nematol. 1995, 3, 61–62. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E., Ltd., Plymouth Marine Laboratory: London, UK, 2001. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v5: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2001; p. 91. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Essid, N.; Beyrem, H.; Hedfi, A.; Boufahja, F.; Vitiello, P.; Aissa, P. Effects of hydrocarbon contamination on a free-living marine nematode community: Results from microcosm experiments. Mar. Pollut. Bull. 2005, 50, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Hedfi, A.; Mahmoudi, E.; Boufahja, F.; Beyrem, H.; Aïssa, P. Effects of increasing levels of nickel contamination on structure of offshore nematode communities in experimental microcosms. Bull. Environ. Contam. Toxicol. 2007, 79, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Boufahja, F.; Sellami, B.; Dellali, M.; Aïssa, P.; Mahmoudi, E.; Beyrem, H. A microcosm experiment on the effects of permethrin on a free-living nematode assemblage. Nematology 2011, 13, 901–909. [Google Scholar]
- Wilson, K.S.; Matrone, G.; Livingstone, D.E.W.; Al-Dujaili, E.A.S.; Mullins, J.J.; Tucker, C.S.; Hadoke, P.W.F.; Kenyon, C.J.; Denvir, M.A. Physiological roles of glucocorticoids during early embryonic development of the zebrafish (Danio rerio). J. Physiol. 2013, 15, 6209–6220. [Google Scholar] [CrossRef]
- Barrett, R.; Chappell, C.; Quick, M.; Fleming, A. A rapid, high content, in vivo model of glucocorticoid-induced osteoporosis. Biotechnol. J. 2006, 1, 651–655. [Google Scholar] [CrossRef]
- Mesut, S. Blue Economy and Blue Ocean Strategy. J. Ecol. Nat. Resour. 2021, 5, 000263. [Google Scholar]
- Mesut, S. The Effects of the Ports and Water Transportation on the Aquatic Ecosystem. J. Biogen. Sci. Res. 2021, 10, 1–8. [Google Scholar]
- Hedfi, A.; Ben Ali, M.; Hassan, M.M.; Albogami, B.; Al-Zahrani, S.S.; Mahmoudi, E.; Karachle, P.K.; Rohal-Lupher, M.; Boufahja, F. Nematode traits after separate and simultaneous exposure to Polycyclic Aromatic Hydrocarbons (anthracene, pyrene and benzo [a] pyrene) in closed and open microcosms. Environ. Pollut. 2021, 276, 116759. [Google Scholar] [CrossRef] [PubMed]
- Semprucci, F.; Moreno, M.; Sbrocca, S.; Rocchi, M.; Albertelli, G.; Balsamo, M. The nematode assemblage as a tool for the assessment of marine ecological quality status: A case-study in the Central Adriatic Sea. Mediterr. Mar. Sci. 2013, 14, 48–57. [Google Scholar] [CrossRef][Green Version]
- Ürkmez, D.; Sezgin, M.; Bat, L. Use of nematode maturity index for the determination of ecological quality status: A case study from the Black Sea. J. Black Sea/Mediterr. Environ. 2014, 20, 96–107. [Google Scholar]
- Bongers, T. The Maturity Index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Bongers, T. The Maturity Index, the evolution of nematode life history traits, adaptive radiation and cp-scaling. Plant Soil 1999, 212, 13–22. [Google Scholar] [CrossRef]
- Armenteros, M.; Pérez-García, J.A.; Ruiz-Abierno, A.; Díaz-Asencio, L.; Helguera, Y.; Vincx, M.; Decraemer, W. Effects of organic enrichment on nematode assemblages in a microcosm experiment. Mar. Environ. Res. 2010, 70, 374–382. [Google Scholar] [CrossRef]
- Losi, V.; Montefalcone, M.; Moreno, M.; Giovannetti, E.; Gaozza, L.; Grondona, M.; Albertelli, G. Nematodes as indicators of environmental quality in seagrass (Posidonia ceanic) meadows of the NW Mediterranean Sea. Adv. Oceanogr. Limnol. 2012, 3, 69–91. [Google Scholar] [CrossRef]
- Patrício, J.; Adão, H.; Neto, J.M.; Alves, A.S.; Traunspurger, W.; Marques, J.C. Do nematode and macrofauna assemblages provide similar ecological assessment information? Ecol. Indic. 2012, 14, 124–137. [Google Scholar] [CrossRef]
- Boufahja, F.; Vitiello, P.; Aïssa, P. More than 35 years of studies on marine nematodes from Tunisia: A checklist of species and their distribution. Zootaxa 2014, 3786, 269–300. [Google Scholar] [CrossRef]
- Rodrigues Da Silva, N.R.; Da Silva, M.C.; Fonseca Genevois, V.; De Esteves, A.M.; De Ley, P.; Decraemer, W.; Rieger, T.T.; Dos Santos Correia, M.T. Marine nematode taxonomy in the age of DNA: The present and future of molecular tools to assess their biodiversity. Nematology 2010, 12, 661–672. [Google Scholar] [CrossRef]
- Derycke, S.; Remerie, T.; Vierstraete, A.; Backeljau, T.; Vanfleteren, J.; Vincx, M.; Moens, T. Mitochondrial DNA variation and cryptic speciation within the free-living marine nematode Pellioditis marina. Mar. Ecol. Prog. Ser. 2005, 300, 91–103. [Google Scholar] [CrossRef]
- Derycke, S.; Hendrickx, F.; Backeljau, T.; D’Hondt, S.; Camphijn, L.; Vincx, M.; Moens, T. Effects of sublethal abiotic stressors on population growth and genetic diversity of Pellioditis marina (Nematoda) from the Westerschelde estuary. Aquat. Toxicol. 2007, 82, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Derycke, S.; Van Vynckt, R.; Vanaverbecke, J.; Vincx, M.; Moens, T. Colonization patterns of nematode on decomposing algae in the estuarine environment: Community assembly and genetic structure of the dominant species Pellioditis marina. Limnol. Oceanogr. 2007, 52, 992–1001. [Google Scholar] [CrossRef]
- De Meester, N.; Derycke, S.; Bonte, D.; Moens, T. Salinity effects on the coexistence of cryptic species: A case study on marine nematodes. Mar. Biol. 2011, 158, 2717–2726. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).