Phenotypic and Molecular Characterization of Rice Genotypes’ Tolerance to Cold Stress at the Seedling Stage
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Materials Used
2.2. Evaluation of Near Isogenic Lines (NILs) for Cold Tolerance at Seedling Stage
2.3. Evaluation of Near Isogenic Lines (NILs) for Agronomic Performance
2.4. Genetic Analysis of Near-Isogenic Lines (NILs) of BRRI dhan29 Using SSR Markers
3. Results
3.1. Evaluation of NILs for Cold Tolerance at Seedling Stage
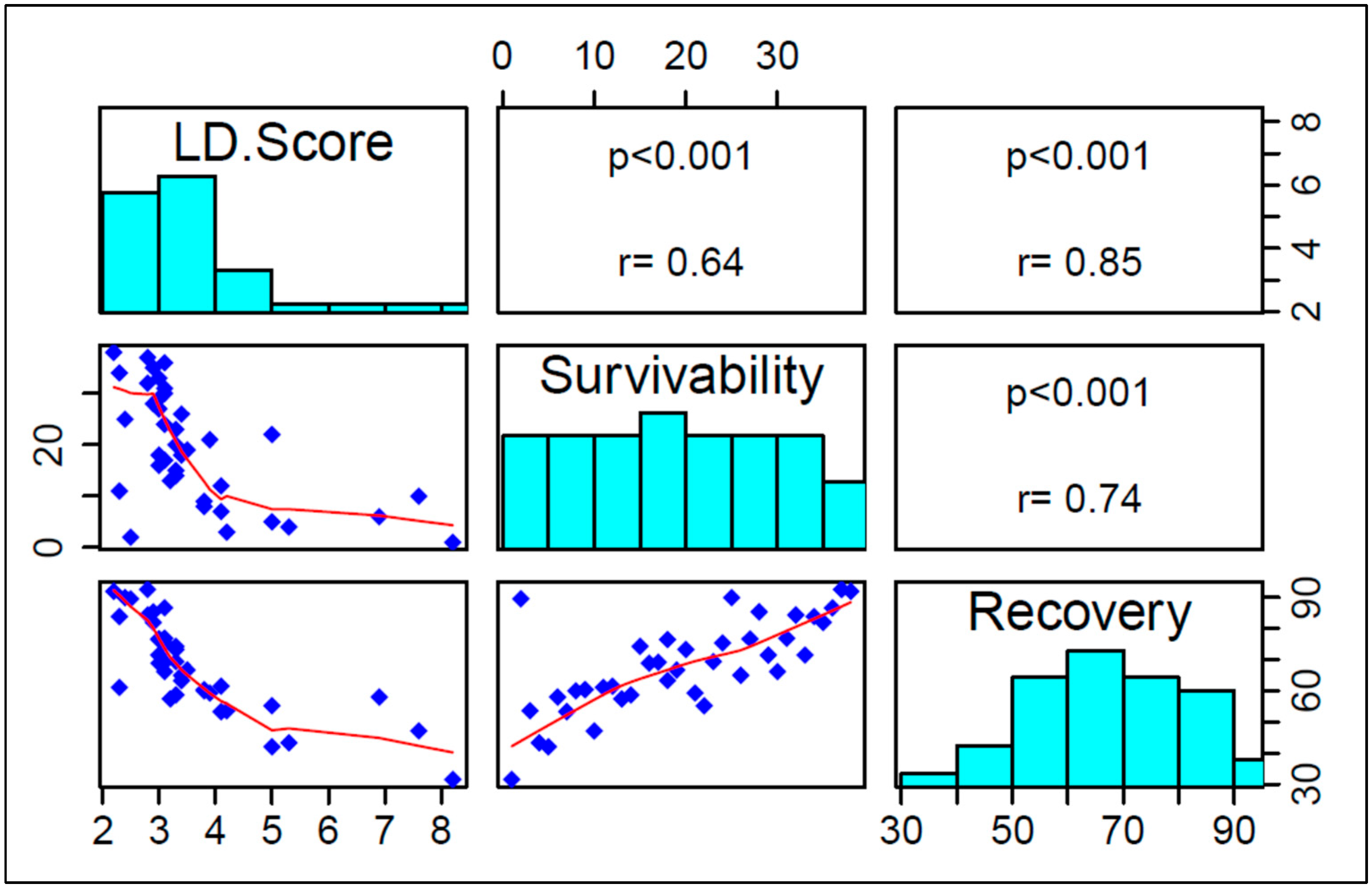
3.1.1. LD Score
3.1.2. Survivability Rate
3.1.3. Recovery Rate
3.1.4. Correlation among LD Score, Survivability, and Recovery Rate
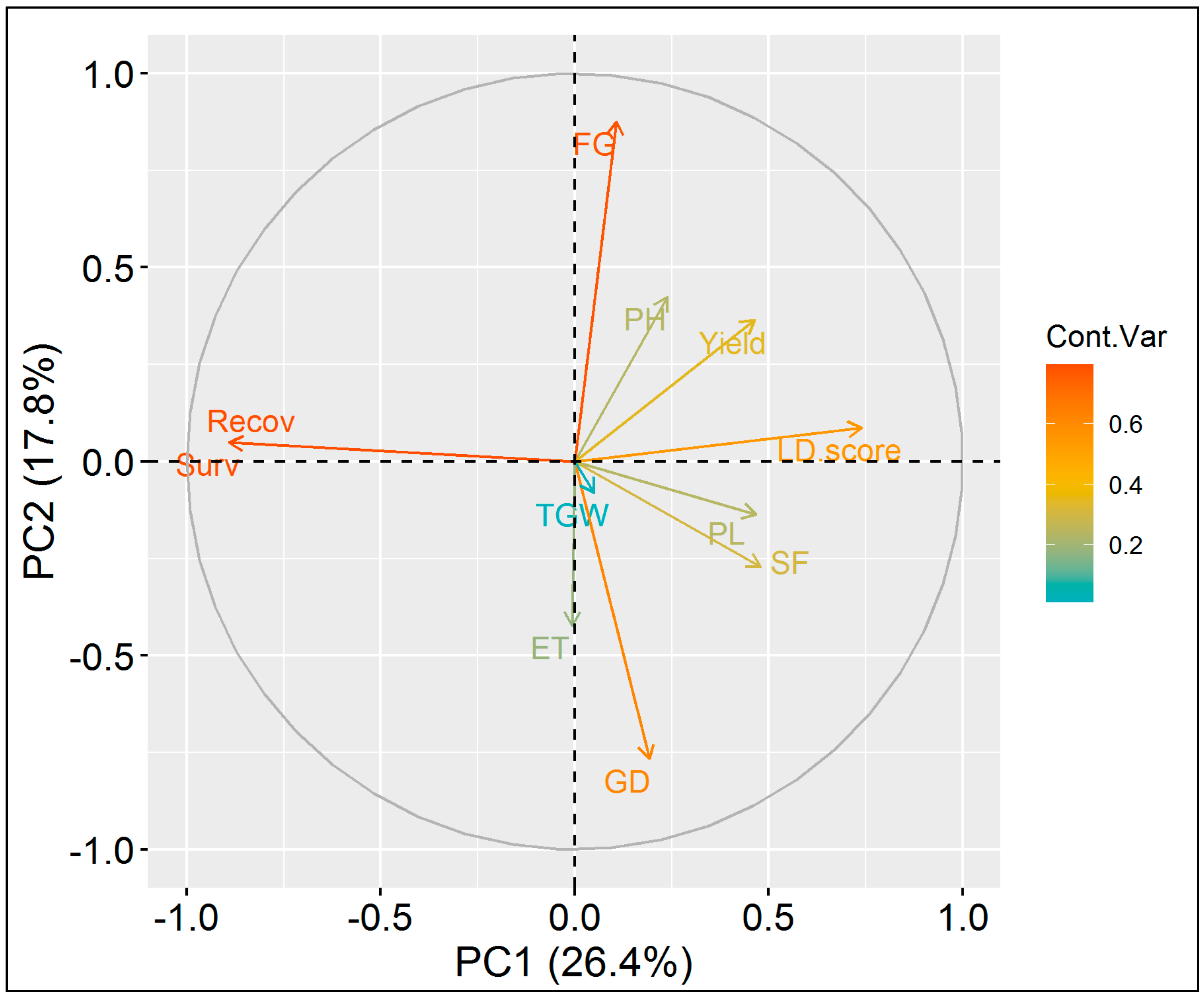
3.2. Evaluation of NILs for Agronomic Performance
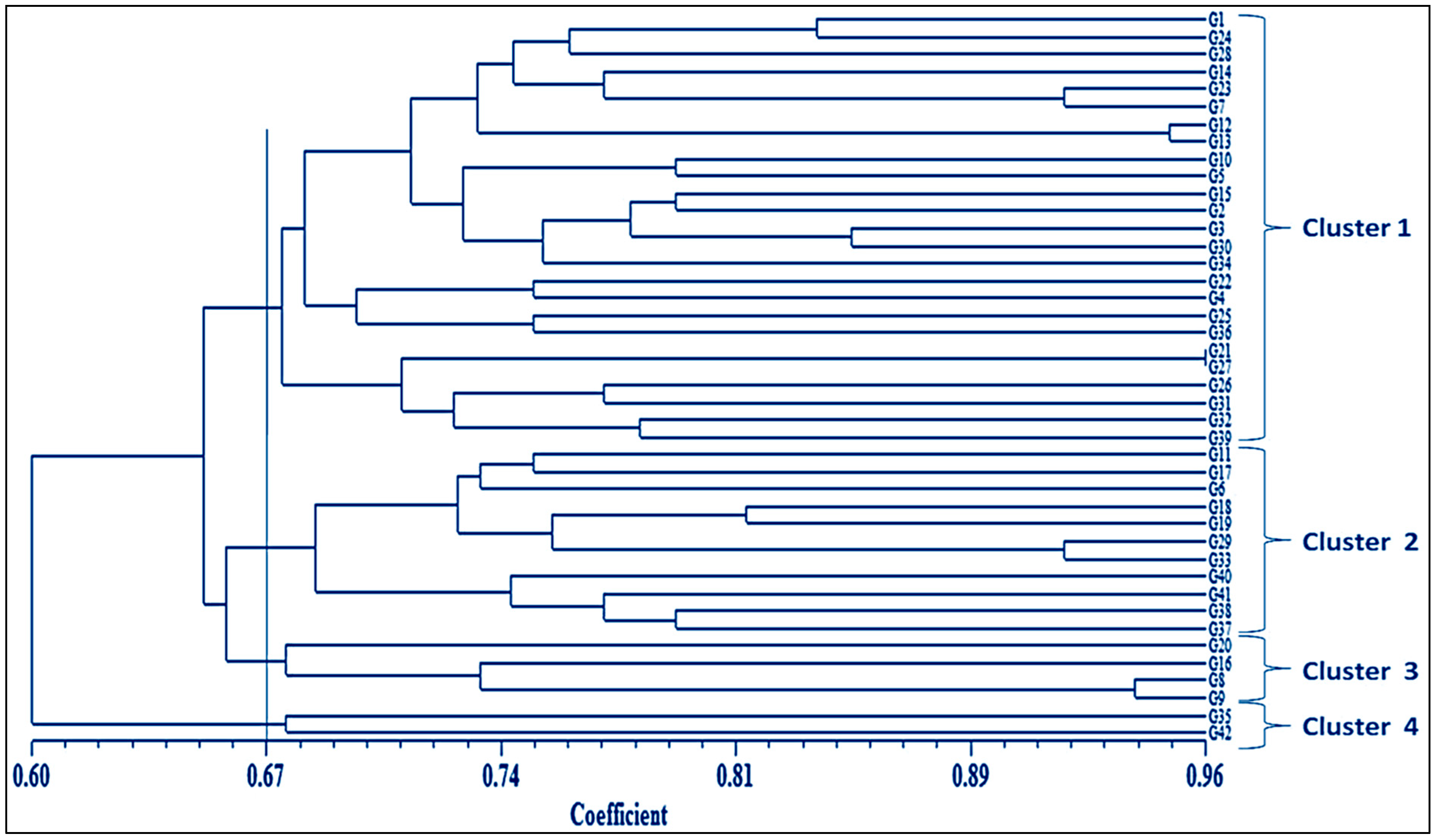
3.3. Genetic Analysis of NILs of BRRI dhan29 Using Microsatellite Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saito, K.; Miura, K.; Nagano, K.; Saito, Y.H.; Araki, H.; Kato, A. Identification of two closely linked quantitative trait loci for cold tolerance on chromosome 4 of rice and their association with anther length. Theor. Appl. Genet. 2001, 103, 862–868. [Google Scholar] [CrossRef]
- Nahar, K.; Biswas, J.K.; Shamsuzzaman, A.M.M.; Hasanuzzaman, M.; Barman, H.N. Screening of Indica Rice (Oryza sativa L.) genotypes against low temperature stress. Bot. Res. Int. 2009, 2, 295–303. [Google Scholar]
- Zeng, Y.; Zhang, Y.; Xiang, J.; Uphoff, N.T.; Pan, X.; Zhu, D. Effects of low temperature stress on spikelet-related parameters during anthesis in indica–japonica hybrid rice. Front. Plant Sci. 2017, 8, 1350. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.S.; Rashid, M.A.; Khatun, H.; Yasmeen, R.; Biswas, J.K. Scope and progress of rice research harnessing cold tolerance. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Elsevier Inc. & Woodland Publishing: Cambridge, UK, 2019; pp. 225–280. [Google Scholar]
- Ranawake, A.L.; Manangkil, O.E.; Yoshida, S.; Ishii, T.; Mori, N.; Nakamura, C. Mapping QTLs for cold tolerance at germination and the early seedling stage in rice (Oryza sativa L.). Biotechnol. Biotechnol. Equip. 2014, 28, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Eixarch, M.; Ellis, R.H. Temporal sensitivities of rice seed development from spikelet fertility to viable mature seed to extreme-temperature. Crop Sci. 2015, 55, 354–364. [Google Scholar] [CrossRef]
- Schläppi, M.R.; Jackson, A.K.; Eizenga, G.C.; Wang, A.; Chu, C.; Shi, Y.; Shimoyama, N.; Boykin, D.L. Assessment of five chilling tolerance traits and GWAS mapping in rice using the USDA mini-core collection. Front. Plant Sci. 2017, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, E.; Edwards, J.D.; Jodari, F.; Duke, S.E.; Baldo, A.M.; Korniliev, P.; McCouch, S.; Eizenga, G.C. Genetic architecture of cold tolerance in rice (Oryza sativa) determined through high resolution genome-wide analysis. PLoS ONE 2017, 12, e0172133. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Meng, L.; Lin, X.; Cui, Y.; Pang, Y.; Xu, J.; Li, Z. QTL and QTL networks for cold tolerance at the reproductive stage detected using selective introgression in rice. PLoS ONE 2018, 13, e0200846. [Google Scholar] [CrossRef]
- Xiao, N.; Gao, Y.; Qian, H.; Gao, Q.; Wu, Y.; Zhang, D.; Li, A. Identification of genes related to cold tolerance and a functional allele that confers cold tolerance. Plant Physiol. 2018, 177, 1108–1123. [Google Scholar] [CrossRef]
- Rashid, M.M.; Yasmeen, R. Cold Injury and Flash Flood Damage in Boro Rice Cultivation in Bangladesh, A Review. Bangladesh Rice J. 2017, 21, 13–25. [Google Scholar] [CrossRef]
- Biswas, J.K.; Mahbub, M.A.A.; Kabir, M.S. Critical temperatures and their probabilities on important growth stages of rice. In Annual Report of Bangladesh Rice Research Institute, 2008–2009; Bashar, M.K., Biswas, J.C., Kashem, M.A., Eds.; Bangladesh Rice Research Institute (BRRI): Gazipur, Bangladesh, 2011; pp. 127–129. [Google Scholar]
- Fuentes, J.L.; Escobar, F.; Alvarez, A.; Gallego, G.; Duque, M.C.; Ferrer, M.; Deus, J.E.; Tohme, J. Analyses of genetic diversity in Cuban rice varieties using isozyme, RAPD and AFLP markers. Euphytica 1999, 109, 107–115. [Google Scholar] [CrossRef]
- Rongwen, J.; Akakya, M.S.; Bhagwat, A.A.; Lavi, U.; Cregan, P.B. The use of microsatellite DNA markers for soybean genotype identification. Theor. Appl. Genet. 1995, 90, 43–48. [Google Scholar] [CrossRef]
- Sebastian, L.S.; Hipolito, L.R.; Tabanao, D.A.; Maramara, G.V.; Caldo, R.A. Molecular diversity of Philippines-based rice cultivars. SABRAO J. Breed. Genet. 1998, 30, 83–90. [Google Scholar]
- Chen, X.; Temnykh, S.; Xu, Y.; Choand, Y.G.; McCouch, S.R. Development of a microsatellite framework map providing genome wide coverage in rice (Oryza sativa L.). Theor. Appl. Genet. 1997, 95, 553–567. [Google Scholar] [CrossRef]
- Keurentjes, J.J.; Bentsink, L.; Alonso-Blanco, C.; Hanhart, C.J.; Blankestijn-De Vries, H.; Effgen, S.; Vreugdenhil, D.; Koornneef, M. Development of a Near-Isogenic Line Population of Arabidopsis Thaliana and Comparison of Mapping Power with a Recombinant Inbred Line Population. Genetics 2007, 175, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Andaya, V.C.; Mackill, D.J. Mapping of QTLs associated with cold tolerance during the vegetative stage in rice. J. Exp. Bot. 2003, 54, 2579–2585. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Chen, L.; Sun, Z.; Xing, Y.; Li, J.; Xu, X.; Mei, H.; Luo, L. A major QTL associated with cold tolerance at seedling stage in rice (Oryza sativa L.). Euphytica 2007, 158, 87–94. [Google Scholar] [CrossRef]
- Zhi, J.I.; Zeng, Y.X.; Zeng, D.I.; Ma, L.Y.; Li, X.M.; Liu, B.X.; Yang, C.D. Identification of QTLs for rice cold tolerance at plumule and 3-leaf-seedling stages by using QTL Network software. Rice Sci. 2010, 17, 282–287. [Google Scholar]
- Suh, J.; Lee, C.; Lee, J.; Kim, J.; Kim, S.; Cho, Y.; Park, S.; Shin, J.; Kim, Y.; Jena, K.K. Identification of quantitative trait loci for seedling cold tolerance using RILs derived from a cross between Japonica and tropical Japonica rice cultivars. Euphytica 2012, 184, 101–108. [Google Scholar] [CrossRef]
- Su, C.F.; Wang, Y.C.; Hsieh, T.H.; Lu, C.A.; Tseng, T.H.; Yu, S.M. A novel MYBS3-dependent pathway confers cold tolerance in rice. Plant Physiol. 2010, 153, 145–158. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, D.; Tang, W.; Zheng, Y.; Liang, K. Mapping of quantitative trait loci underlying cold tolerance in rice seedlings via high-throughput sequencing of pooled extremes. PLoS ONE 2013, 8, e68433. [Google Scholar] [CrossRef]
- Park, I.K.; Oh, C.S.; Kim, D.M.; Yeo, S.M.; Ahn, S.N. QTL mapping for cold tolerance at the seedling stage using introgression lines derived from an inter sub-specific cross in rice. Plant Breed. Biotech. 2013, 1, 18. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.; Zheng, J.; Liu, B.; Peng, S.; Leung, H.; Zhao, J.; Huang, Z. Identification of QTLs for cold tolerance at seedling stage in rice (Oryza sativa L.) using two distinct methods of cold treatment. Euphytica 2014, 195, 95–104. [Google Scholar] [CrossRef]
- Biswas, P.S.; Khatun, H.; Das, N.; Sarker, M.M.; Anisuzzaman, M. Mapping and validation of QTLs for cold tolerance at seedling stage in rice from an indica cultivar Habiganj Boro VI (Hbj. B. VI). 3 Biotech. 2017, 7, 359. [Google Scholar] [CrossRef]
- Andaya, V.; Tai, T. Fine mapping of the qCTS12 locus, a major QTL for seedling cold tolerance in rice. Theor. Appl. Genet. 2003, 113, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Glaszmann, J.C.; Kaw, R.N.; Khush, G.S. Genetic divergence among cold tolerant rices (Oryza sativa L.). Euphytica 1990, 5, 95–104. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Zhang, D.; Li, J.; Xiong, H.; Yu, J.; Li, J.; Rashid, M.A.R.; Li, G.; Ma, X.; et al. Genetic analysis of cold tolerance at the germination and booting stages in rice by association mapping. PLoS ONE 2015, 10, e0120590. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Guo, Z.; Li, X.; Ye, H.; Xiong, L. New insights into the genetic basis of natural chilling and cold shock tolerance in rice by genome-wide association analysis. Plant Cell Environ. 2016, 39, 556–570. [Google Scholar] [CrossRef]
- Kabir, M.S.; Salam, M.U.; Chowdhury, A.; Rahman, N.M.F.; Iftekharuddaula, K.M.; Rahman, M.S.; Rashid, M.H.; Dipti, S.S.; Islam, A.; Latif, M.A.; et al. Rice Vision for Bangladesh, 2050 and Beyond. Bangladesh Rice J. 2015, 19, 1–18. [Google Scholar] [CrossRef]
- Khatun, H.; Biswas, P.S.; Hwang, H.G.; Kim, K.M. A Quick and Simple In-house Screening Protocol forCold-Tolerance at Seedling Stage in Rice. Plant Breed. Biotech. 2016, 4, 373–378. [Google Scholar] [CrossRef]
- Federer, W.T. Augmented Design. Hawaiin Plant. Rec. 1956, 40, 191–207. [Google Scholar] [CrossRef]
- BRRI (Bangladesh Rice Research Institute). Annual Research Review; Bangladesh Rice Research Institute: Gazipur, Bangladesh, 2013. [Google Scholar]
- IRRI (International Rice Research Institute). Minimum List of Descriptors and Descriptor-States for Rice (Oryza sativa L.); IRRI: Los Baños, Philippines, 1980; p. 20. [Google Scholar]
- Federer, W.T.; Raghavarao, D. An augmented designs. Biometrics 1975, 31, 29–35. [Google Scholar] [CrossRef]
- Panse, V.G.; Sukhatme, P.V. Statistical Methods for Agricultural Workers, 2nd ed.; ICAR: New Delhi, India, 1964. [Google Scholar]
- Sharma, J.R. Augmented Design Model-II in Statistical and Biometrical Techniques in Plant Breeding; New Age International Publications: New Delhi, India, 1998. [Google Scholar]
- Syed, M.A.; Iftekharuddaula, K.M.; Mian, M.A.K.; Rasul, M.G.; Rahmam, G.K.M.M.; Panaullah, G.M.; Biswas, P.S. Main effect QTLs associated with arsenic phyto-toxicity tolerance at seedling stage in rice (Oryza sativa L.). Euphytica 2016, 209, 805–814. [Google Scholar] [CrossRef]
- Syed, M.A.; Iftekharuddaula, K.M.; Biswas, P.S.; Akter, N.; Hossain, M. Assessment of genetic diversity in arsenic contaminated rice using SSR markers. Trends Appl. Sci. Res. 2019, 14, 178–185. [Google Scholar]
- Liu, K.; Muse, S.V. Power Marker, an integrated analysis environment for genetic marker analysis. Bioin 2005, 21, 2128–2129. [Google Scholar] [CrossRef]
- Anderson, J.A.; Churchill, G.A.; Autique, J.E.; Tanksley, S.D.; Sorreils, M.E. Optimizing parental selection for genetic-linkage maps. Genome 1993, 36, 181–186. [Google Scholar] [CrossRef]
- Nei, M. The theory and estimation of genetic distance. In Genetic Structure of Populations; Morton, N.E., Ed.; University of Hawaii Press: Honolulu, HI, USA, 1973; pp. 45–54. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Rohlf, F.J. NTSYS-pc. In Numerical Taxonomy and Multivariate Analysis System; Exeter Software: Setauket, NY, USA, 2002. [Google Scholar]
- Botstein, D.; White, R.L.; Skalnick, M.H.; Davies, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Ye, C.; Fukai, S.; Godwin, D.I.; Reinke, R.; Snell, P.; Schiller, J.; Basnayake, J. Cold tolerance in rice varieties at different growth stages. Crop Pasture Sci. 2009, 60, 328–338. [Google Scholar] [CrossRef]
- International Rice Research Institute (IRRI). Standard Evaluation System (SES) for Rice, 5th ed.; IRRI: Manila, Philippines, 2013; p. 35. [Google Scholar]
- Suh, J.-P.; Cho, Y.-C.; Lee, J.-H.; Lee, S.-B.; Jung, J.-Y.; Choi, I.-S.; Kim, M.-K.; Kim, C.-K.; Jena, K.-K. SSR Analysis of Genetic Diversity and Cold Tolerance in Temperate Rice Germplasm. Plant Breed. Biotech. 2013, 106, 103–110. [Google Scholar] [CrossRef]
- Kundu, A. Haplotype Diversity Analysis in Cold Tolerant Rice (Oryza sativa L.). Master’s Thesis, Department of Biotechnology, Bangabandhu Sheikh MujiburRahman Agricultural University, Gazipur, Bangladesh, 2015. [Google Scholar]
- Shoroardi, M.; Mortuza, M.G.; Islam, M.M.; Samih, T. Phenotypic Screening and Molecular Characterization of 10 Rice (Oryza sativa) Landraces for Cold Tolerance. J. Environ. Sci. Nat. Resour. 2017, 10, 85–91. [Google Scholar] [CrossRef]
- Kim, S.M.; Suh, J.P.; Lee, C.K.; Lee, J.H.; Kim, Y.G.; Jena, K.K. QTL mapping and development of candidate gene-derived DNA markers associated with seedling cold tolerance in rice (Oryza sativa L.). Mol. Genet. Genom. 2014, 289, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Setter, T.L.; Laureles, E.V.; Mazaredo, A.M. Lodging reduces yield of rice by self-shading and reductions in canopy photosynthesis. Field Crops Res. 1997, 49, 95–106. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, C.; Lin, J.; Liu, J.; Liu, B.; Wang, J.; Zhao, T. OsMPH1 regulates plant height and improves grain yield in rice. PLoS ONE 2017, 12, e0180825. [Google Scholar] [CrossRef]
- Upadhyay, P.; Neeraja, C.N.; Kole, C.; Singh, V.K. Population structure and genetic diversity in popular rice varieties of India as evidenced from SSR analysis. Biochem. Genet. 2012, 50, 770–783. [Google Scholar] [CrossRef]
- Kostova, A.; Todorovska, E.; Christov, N.; Hristov, K.; Atanassov, A. Assessment of genetic variability induced by chemical mutagenesis in elite maize germplasm via SSR markers. J. Crop Improv. 2006, 16, 37–48. [Google Scholar] [CrossRef]
- Pachauri, V.; Taneja, N.; Vikram, P.; Singh, N.K.; Singh, S. Molecular and morphological characterization of Indian farmers rice varieties (Oryza sativa L.). Aust. J. Crop Sci. 2013, 7, 923–932. [Google Scholar]
- Syed, M.A.; Iftekharuddaula, K.M.; Akter, N.; Biswas, P.S. Molecular diversity analysis of some selected BBRI released rice varieties using SSR markers. Int. Res. J. Biol. Sci. 2019, 1, 51–58. [Google Scholar]

| SL | Designation | LD Score | % Survivability | % Recovery | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Diff. from BR1 | Diff. from BR18 | Mean | Diff. from BR1 | Diff. from BR18 | Mean | Diff. from BR1 | Diff. from BR18 | ||
| 1 | IR90688-5-1-1-1-1-1 | 2.3 | −5.9 * | −2.7 * | 67.60 | 28.9 | −9.3 | 61.10 | 29.6 | 5.9 |
| 2 | IR90688-13-1-1-1-1-p1 | 3.2 | −5 * | −1.8 * | 70.00 | 31.3 | −6.9 | 57.40 | 25.9 | 2.2 |
| 3 | IR90688-15-1-1-1-1-1 | 3.1 | −5.1 * | −1.9 * | 71.90 | 33.2 | −5 | 69.20 | 37.7 * | 14 |
| 4 | IR90688-19-1-1-1-1-1 | 3.1 | −5.1 * | −1.9 * | 79.20 | 40.5 * | 2.3 | 75.40 | 43.9 * | 20.2 |
| 5 | IR90688-20-1-1-1-1-1 | 2.8 | −5.4 * | −2.2 * | 86.30 | 47.6 * | 9.4 | 84.40 | 52.9 * | 29.2 |
| 6 | IR90688-27-1-1-1-1-1 | 3.8 | −4.4 * | −1.2 | 62.20 | 23.5 | −14.7 | 60.00 | 28.5 | 4.8 |
| 7 | IR90688-30-1-1-1-1-1 | 3.4 | −4.8 * | −1.6 * | 73.30 | 34.6 | −3.6 | 63.30 | 31.8 | 8.1 |
| 8 | IR90688-42-1-1-1-1-p1 | 3.3 | −4.9 * | −1.7 * | 78.30 | 39.6 * | 1.4 | 69.40 | 37.9 * | 14.2 |
| 9 | IR90688-42-1-1-1-1-p2 | 4.1 | −4.1 * | −0.9 | 68.10 | 29.4 | −8.8 | 61.50 | 30 | 6.3 |
| 10 | IR90688-43-1-1-1-1-P1 | 3.5 | −4.7 * | −1.5 * | 74.30 | 35.6 | −2.6 | 66.70 | 35.2 * | 11.5 |
| 11 | IR90688-52-1-1-1-1-1 | 2.9 | −5.3 * | −2.1 * | 82.40 | 43.7 * | 5.5 | 85.40 | 53.9 * | 30.2 |
| 12 | IR90688-54-1-1-1-1-1 | 3.9 | −4.3 * | −1.1 | 76.70 | 38 | −0.2 | 59.30 | 27.8 | 4.1 |
| 13 | IR90688-56-1-1-1-1-1 | 3.8 | −4.4 * | −1.2 | 63.10 | 24.4 | −13.8 | 60.40 | 28.9 | 5.2 |
| 14 | IR90688-62-1-1-1-1-1 | 4.2 | −4 * | −0.8 | 52.70 | 14 | −24.2 | 53.60 | 22.1 | -1.6 |
| 15 | IR90688-64-1-1-1-1-1 | 3.3 | −4.9 * | −1.7 * | 71.50 | 32.8 | −5.4 | 74.30 | 42.8 * | 19.1 |
| 16 | IR90688-73-1-1-1-1-P1 | 3.0 | −5.2 * | −2 | 71.70 | 33 | −5.2 | 68.90 | 37.4 * | 13.7 |
| 17 | IR90688-74-1-1-1-1-1 | 2.8 | −5.4 * | −2.2 | 95.00 | 56.3 * | 18.1 | 92.60 | 61.1 * | 37.4 * |
| 18 | IR90688-77-1-1-1-1-1 | 3.3 | −4.9 * | −1.7 * | 71.10 | 32.4 | −5.8 | 58.70 | 27.2 | 3.5 |
| 19 | IR90688-81-1-1-1-1-1 | 2.5 | −5.7 * | −2.5 * | 100.00 | 61.3 * | 23.1 | 89.60 | 58.1 * | 34.4 * |
| 20 | IR90688-82-1-1-1-1-1 | 3.0 | −5.2 * | −2 * | 86.80 | 48.1 * | 9.9 | 71.50 | 40 * | 16.3 |
| 21 | IR90688-82-1-1-1-1-1 | 3.1 | −5.1 * | −1.9 * | 84.30 | 45.6 * | 7.4 | 66.20 | 34.7 * | 11 |
| 22 | IR90688-92-1-1-1-1-1 | 3.4 | −4.8 * | −1.6 * | 80.60 | 41.9 * | 3.7 | 65.00 | 33.5 * | 9.8 |
| 23 | IR90688-94-1-1-1-1-1 | 3.1 | −5.1 * | −1.9 * | 90.90 | 52.2 * | 14 | 86.70 | 55.2 * | 31.5 |
| 24 | IR90688-95-1-1-1-1-1 | 3.0 | −5.2 * | −2 * | 81.90 | 43.2 * | 5 | 76.70 | 45.2 * | 21.5 |
| 25 | IR90688-96-1-1-1-1-1 | 4.1 | −4.1 * | −0.9 | 59.00 | 20.3 | −17.9 | 53.30 | 21.8 | −1.9 |
| 26 | IR90688-103-1-1-1-1-1 | 2.2 | −6 * | −2.8 * | 98.30 | 59.6 * | 21.4 | 92.00 | 60.5 * | 36.8 * |
| 27 | IR90688-105-1-1-1-1-1 | 2.3 | −5.9 * | −2.7 * | 90.00 | 51.3 * | 13.1 | 83.90 | 52.4 * | 28.7 |
| 28 | IR90688-106-1-1-1-1-1 | 3.0 | −5.2 * | −2 * | 83.30 | 44.6 * | 6.4 | 71.50 | 40 * | 16.3 |
| 29 | IR90688-108-1-1-1-1-1 | 3.3 | −4.9 * | −1.7 * | 75.00 | 36.3 * | −1.9 | 73.30 | 41.8 * | 18.1 |
| 30 | IR90688-109-1-1-1-1-1 | 3.1 | −5.1 * | −1.9 * | 85.00 | 46.3 * | 8.1 | 76.90 | 45.4 * | 21.7 |
| 31 | IR90688-114-1-1-1-1-1 | 3.0 | −5.2 * | −2 * | 73.30 | 34.6 | −3.6 | 76.50 | 45 * | 21.3 |
| 32 | IR90688-118-1-1-1-1-1 | 2.4 | −5.8 * | −2.6 * | 80.00 | 41.3 * | 3.1 | 90.00 | 58.5 * | 34.8 * |
| 33 | IR90688-120-1-1-1-1-1 | 5.3 | −2.9 * | 0.3 | 53.30 | 14.6 | −23.6 | 43.30 | 11.8 | −11.9 |
| 34 | IR90688-124-1-1-1-1-P2 | 5.0 | −3.2 * | 0 | 54.50 | 15.8 | −22.4 | 42.00 | 10.5 | −13.2 |
| 35 | IR90688-125-1-1-1-1-1 | 2.9 | −5.3 * | −2.1 * | 90.30 | 51.6 * | 13.4 | 82.00 | 50.5 * | 26.8 |
| 36 | BR1 | 8.2 | 0 | 3.2 * | 38.70 | 0 | −38.2 | 31.50 | 0 | −23.7 |
| 37 | BR18 | 5.0 | −3.2 * | 0 | 76.90 | 38.2 * | 0 | 55.20 | 23.7 | 0 |
| 38 | BRRI dhan28 | 7.6 | −0.6 | 2.6 * | 64.70 | 26 | −12.2 | 47.10 | 15.6 | −8.1 |
| 39 | BRRI dhan29 | 6.9 | −1.3 | 1.9 * | 58.70 | 20 | −18.2 | 58.00 | 26.5 | 2.8 |
| Variance | 3.43 ** | 526.69 ** | 604.45 * | |||||||
| Mean | 3.49 | 76.18 | 69.07 | |||||||
| CV | 15.17 | 19.20 | 23.72 | |||||||
| Mean | 3.49 | 76.18 | 69.07 | |||||||
| LSD (0.05) | 1.4 | 37.70 | 32.20 | |||||||
| SN | Genotype | GD (Days) | PH (cm) | PL (cm) | ET | FG | % SF | TGW | Yield (g/plant) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | IR90688-5-1-1-1-1-1 | 143.7 | 94.1 | 22.7 | 17.7 | 61.6 | 38.3 | 22.7 | 11.5 |
| 2 | IR90688-13-1-1-1-1-P1 | 150.7 | 98.2 | 22.4 | 14.7 | 71.4 | 97.4 | 18.5 | 27.7 |
| 3 | IR90688-15-1-1-1-1-1 | 146.7 | 95.2 | 22.4 | 14.6 | 82.4 | 73.6 | 28.2 | 30.5 |
| 4 | IR90688-19-1-1-1-1-1 | 146.7 | 94.1 | 19.9 | 14.5 | 56.8 | 56.1 | 19.0 | 11.9 |
| 5 | IR90688-20-1-1-1-1-1 | 158.7 | 97.3 | 23.0 | 15.1 | 54.1 | 47.1 | 18.5 | 14.0 |
| 6 | IR90688-27-1-1-1-1-1 | 146.7 | 97.2 | 24.7 | 17.1 | 91.5 | 41.3 | 19.2 | 24.4 |
| 7 | IR90688-30-1-1-1-1-1 | 163.7 | 91.5 | 20.7 | 17.3 | 49.3 | 63.2 | 17.0 | 18.1 |
| 8 | IR90688-42-1-1-1-1-P1 | 154.7 | 88.2 | 19.6 | 15.8 | 49.9 | 49.7 | 20.1 | 19.3 |
| 9 | IR90688-42-1-1-1-1-P2 | 157.7 | 90.9 | 21.8 | 16.9 | 48.9 | 71.4 | 19.3 | 12.5 |
| 10 | IR90688-43-1-1-1-1-P1 | 147.2 | 74.6 | 22.1 | 23.2 | 45.1 | 39.0 | 17.4 | 16.9 |
| 11 | IR90688-52-1-1-1-1-1 | 161.2 | 84.8 | 22.2 | 13.5 | 65.2 | 88.3 | 18.7 | 23.2 |
| 12 | IR90688-54-1-1-1-1-1 | 151.2 | 102.4 | 24.6 | 13.2 | 54.0 | 33.4 | 15.4 | 17.6 |
| 13 | IR90688-56-1-1-1-1-1 | 154.2 | 99.9 | 24.0 | 29.8 | 46.9 | 37.5 | 19.6 | 15.7 |
| 14 | IR90688-62-1-1-1-1-1 | 147.2 | 92.5 | 23.2 | 17.1 | 71.7 | 65.1 | 20.6 | 27.6 |
| 15 | IR90688-64-1-1-1-1-1 | 149.2 | 81.7 | 23.2 | 15.5 | 118.7 | 37.8 | 16.5 | 22.2 |
| 16 | IR90688-73-1-1-1-1-P1 | 153.2 | 86.6 | 23.5 | 15.7 | 44.0 | 49.2 | 18.6 | 11.8 |
| 17 | IR90688-74-1-1-1-1-1 | 151.2 | 84.7 | 23.6 | 19.0 | 96.0 | 73.2 | 20.2 | 27.6 |
| 18 | IR90688-77-1-1-1-1-1 | 155.2 | 82.9 | 23.3 | 22.3 | 44.3 | 42.1 | 25.0 | 20.7 |
| 19 | IR90688-81-1-1-1-1-1 | 155.4 | 107.0 | 23.6 | 15.9 | 65.6 | 65.2 | 23.9 | 12.8 |
| 20 | IR90688-82-1-1-1-1-1 | 144.4 | 98.2 | 25.4 | 19.3 | 87.9 | 50.9 | 15.6 | 13.0 |
| 21 | IR90688-91-1-1-1-1-1 | 143.4 | 105.7 | 22.3 | 15.3 | 107.1 | 53.5 | 17.4 | 17.3 |
| 22 | IR90688-92-1-1-1-1-1 | 138.4 | 93.5 | 14.2 | 15.7 | 160.2 | 60.2 | 16.1 | 23.8 |
| 23 | IR90688-94-1-1-1-1-1 | 149.4 | 99.3 | 25.8 | 19.4 | 80.4 | 30.3 | 25.8 | 18.1 |
| 24 | IR90688-95-1-1-1-1-1 | 152.4 | 79.7 | 21.0 | 18.8 | 80.8 | 33.5 | 18.3 | 12.4 |
| 25 | IR90688-96-1-1-1-1-1 | 146.4 | 104.0 | 24.4 | 21.7 | 68.3 | 49.7 | 20.7 | 20.5 |
| 26 | IR90688-103-1-1-1-1-1 | 149.4 | 90.2 | 20.8 | 21.9 | 73.1 | 16.0 | 22.1 | 14.1 |
| 27 | IR90688-104-1-1-1-1-1 | 145.4 | 100.1 | 22.3 | 18.1 | 82.0 | 68.9 | 19.1 | 23.0 |
| 28 | IR90688-105-1-1-1-1-1 | 151.7 | 83.3 | 19.0 | 18.2 | 51.0 | 29.5 | 16.4 | 9.8 |
| 29 | IR90688-106-1-1-1-1-1 | 148.7 | 84.0 | 20.9 | 19.6 | 57.2 | 22.0 | 17.3 | 19.6 |
| 30 | IR90688-108-1-1-1-1-1 | 144.7 | 84.7 | 19.7 | 17.4 | 65.0 | 33.5 | 21.4 | 17.4 |
| 31 | IR90688-109-1-1-1-1-1 | 139.7 | 82.3 | 16.9 | 19.6 | 64.0 | 14.8 | 17.9 | 7.7 |
| 32 | IR90688-114-1-1-1-1-1 | 148.7 | 88.9 | 21.9 | 19.7 | 35.7 | 23.1 | 16.4 | 8.1 |
| 33 | IR90688-118-1-1-1-1-1 | 146.7 | 90.3 | 18.1 | 17.3 | 68.9 | 28.5 | 23.4 | 13.4 |
| 34 | IR90688-120-1-1-1-1-1 | 153.7 | 72.3 | 18.9 | 18.7 | 50.8 | 53.3 | 18.1 | 12.0 |
| 35 | IR90688-124-1-1-1-1-P2 | 146.7 | 91.3 | 22.5 | 17.6 | 95.6 | 81.2 | 17.0 | 30.6 |
| 36 | IR90688-125-1-1-1-1-1 | 143.7 | 89.2 | 22.4 | 20.3 | 86.7 | 63.8 | 17.9 | 22.6 |
| 37 | BR1 (Ck) | 148.8 | 77.9 | 23.1 | 24.1 | 57.3 | 59.8 | 18.6 | 18.1 |
| 38 | BR18 (Ck) | 160.8 | 89.0 | 24.9 | 23.1 | 73.3 | 67.8 | 19.5 | 20.3 |
| 39 | BRRI dhan28 (Ck) | 139.8 | 99.2 | 24.8 | 12.2 | 143.7 | 77.0 | 18.4 | 18.2 |
| 40 | BRRI dhan29 (Ck) | 157.5 | 93.8 | 22.8 | 19.1 | 92.9 | 65.0 | 17.7 | 32.6 |
| LSD (Ck Mean) | 1.19 | 2.69 | 0.36 | 0.74 | 8.24 | 4.10 | 1.27 | 1.80 | |
| LSD (Ck Mean vs. NIL) | 2.11 | 4.75 | 0.64 | 1.30 | 14.57 | 7.25 | 2.24 | 3.19 | |
| Range of NILs | 138.4–163.7 | 72.3–107 | 14.2–25.8 | 13.2–29.8 | 45.1–160.2 | 14.8–97.4 | 15.4–28.2 | 7.7–30.6 | |
| CV (%) | 1.11 | 4.22 | 2.13 | 5.31 | 12.7 | 8.61 | 9.67 | 11.43 |
| Marker ID | Chr. No. | Position (Mb) | Allele No. | Allele Size (bp) | Highest Frequency Allele | PIC Value | ||
|---|---|---|---|---|---|---|---|---|
| Range | Difference | Size (bp) | Frequency % | |||||
| RM7278 | 1 | 1.79 | 3 | 173–240 | 67 | 201 | 57.14 | 0.45 |
| RM220 | 1 | 4.42 | 4 | 110–126 | 16 | 120 | 71.43 | 0.40 |
| RM259 | 1 | 7.45 | 3 | 158–174 | 16 | 158 | 52.38 | 0.44 |
| RM581 | 1 | 9.11 | 3 | 134–141 | 7 | 141 | 45.24 | 0.51 |
| RM572 | 1 | 9.87 | 3 | 165–187 | 22 | 165 | 61.90 | 0.44 |
| RM10649 | 1 | 10.28 | 3 | 449–498 | 49 | 477 | 61.90 | 0.39 |
| RM10800 | 1 | 12.73 | 2 | 130–144 | 14 | 144 | 57.14 | 0.37 |
| RM5638 | 1 | 20.93 | 4 | 190–235 | 45 | 207 | 52.38 | 0.44 |
| RM11570 | 1 | - | 4 | 351–424 | 73 | 351 | 61.90 | 0.52 |
| RM297 | 1 | 32.1 | 4 | 147–187 | 40 | 147 | 50.00 | 0.47 |
| RM11874 | 1 | - | 3 | 181–195 | 14 | 195 | 66.67 | 0.37 |
| RM3703 | 2 | 3.86 | 4 | 100–118 | 18 | 100 | 57.14 | 0.51 |
| RM12769 | 2 | 7.35 | 5 | 151–164 | 13 | 151 | 52.38 | 0.47 |
| RM424 | 2 | 11.39 | 3 | 235–278 | 43 | 235 | 50.00 | 0.46 |
| RM13155 | 2 | 15.26 | 3 | 450–551 | 101 | 450 | 57.14 | 0.40 |
| RM3421 | 2 | 29.91 | 3 | 142–147 | 5 | 142 | 54.76 | 0.41 |
| RM266 | 2 | 35.43 | 4 | 112–125 | 13 | 125 | 50.00 | 0.49 |
| RM3202 | 3 | 0.81 | 4 | 194–211 | 17 | 194 | 57.14 | 0.48 |
| RM546 | 3 | 6.16 | 3 | 156–167 | 11 | 156 | 61.90 | 0.39 |
| RM14795 | 3 | 10.36 | 4 | 126–139 | 13 | 139 | 45.24 | 0.51 |
| RM1164 | 3 | 14.86 | 3 | 192–200 | 8 | 192 | 83.33 | 0.27 |
| RM7134 | 3 | 22.01 | 4 | 177–289 | 112 | 201 | 66.67 | 0.40 |
| RM7097 | 3 | 26.87 | 3 | 168–176 | 8 | 176 | 69.05 | 0.38 |
| RM16686 | 4 | 14.72 | 2 | 87–90 | 3 | 87 | 76.19 | 0.30 |
| RM273 | 4 | 23.86 | 3 | 195–204 | 9 | 195 | 64.29 | 0.41 |
| RM317 | 4 | 29.06 | 3 | 154–160 | 6 | 154 | 50.00 | 0.49 |
| RM127 | 4 | 34.53 | 3 | 208–217 | 9 | 217 | 61.90 | 0.39 |
| RM413 | 5 | 2.21 | 4 | 72–100 | 28 | 75 | 52.38 | 0.44 |
| RM3916 | 5 | - | 3 | 233–289 | 56 | 289 | 47.62 | 0.47 |
| RM588 | 6 | 1.61 | 4 | 115–128 | 13 | 115 | 50.00 | 0.44 |
| RM6811 | 6 | 29.23 | 3 | 165–187 | 22 | 165 | 54.76 | 0.41 |
| RM295 | 7 | 0.41 | 3 | 186–193 | 7 | 186 | 47.62 | 0.47 |
| RM6427 | 7 | 17.45 | 4 | 156–170 | 14 | 160 | 50.00 | 0.47 |
| RM336 | 7 | 21.87 | 4 | 145–202 | 57 | 145 | 52.38 | 0.44 |
| RM234 | 7 | 25.47 | 3 | 131–153 | 22 | 153 | 66.67 | 0.37 |
| RM172 | 7 | 29.56 | 3 | 158–164 | 6 | 164 | 50.00 | 0.41 |
| RM547 | 8 | 5.59 | 3 | 197–253 | 56 | 197 | 54.76 | 0.46 |
| RM331 | 8 | 12.29 | 3 | 150–171 | 21 | 150 | 57.14 | 0.45 |
| RM264 | 8 | 27.92 | 3 | 165–179 | 14 | 165 | 50.00 | 0.51 |
| RM5515 | 9 | 7.15 | 3 | 115–126 | 11 | 115 | 59.52 | 0.42 |
| RM24087 | 9 | - | 2 | 246–259 | 13 | 246 | 64.29 | 0.35 |
| RM566 | 9 | 14.74 | 4 | 239–271 | 32 | 249 | 50.00 | 0.47 |
| RM553 | 9 | 19.32 | 4 | 160–173 | 13 | 160 | 42.86 | 0.54 |
| RM216 | 10 | 5.35 | 3 | 133–149 | 16 | 149 | 54.76 | 0.43 |
| RM6142 | 10 | 12.8 | 3 | 82–97 | 15 | 94 | 66.67 | 0.40 |
| RM3773 | 10 | 19.9 | 3 | 137–162 | 25 | 150 | 50.00 | 0.44 |
| RM26063 | 11 | 2.26 | 2 | 129–137 | 8 | 137 | 52.38 | 0.37 |
| RM26243 | 11 | 5.56 | 3 | 150–160 | 10 | 160 | 64.29 | 0.38 |
| RM7283 | 11 | 9.12 | 3 | 149–182 | 33 | 149 | 66.67 | 0.37 |
| RM26501 | 11 | 11.1 | 2 | 137–146 | 9 | 137 | 71.43 | 0.32 |
| RM3428 | 11 | 13.48 | 3 | 140–157 | 17 | 140 | 64.29 | 0.38 |
| RM229 | 11 | 18.41 | 3 | 114–129 | 15 | 114 | 59.52 | 0.40 |
| RM224 | 11 | 21.85 | 4 | 123–160 | 37 | 160 | 59.52 | 0.43 |
| RM206 | 11 | 22.01 | 4 | 132–171 | 39 | 165 | 54.76 | 0.44 |
| RM20 | 12 | 0.97 | 3 | 111–135 | 24 | 111 | 54.76 | 0.43 |
| RM512 | 12 | 5.1 | 2 | 221–226 | 5 | 226 | 52.38 | 0.37 |
| RM28502 | 12 | 23.41 | 3 | 124–131 | 7 | 124 | 50.00 | 0.46 |
| RM7558 | 12 | 27.02 | 2 | 134–139 | 5 | 134 | 57.14 | 0.37 |
| Mean | 3.21 | 57.31 | 0.42 | |||||
| Max | 5.00 | 83.33 | 0.54 | |||||
| Min | 2.00 | 42.86 | 0.27 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, N.; Biswas, P.S.; Syed, M.A.; Ivy, N.A.; Alsuhaibani, A.M.; Gaber, A.; Hossain, A. Phenotypic and Molecular Characterization of Rice Genotypes’ Tolerance to Cold Stress at the Seedling Stage. Sustainability 2022, 14, 4871. https://doi.org/10.3390/su14094871
Akter N, Biswas PS, Syed MA, Ivy NA, Alsuhaibani AM, Gaber A, Hossain A. Phenotypic and Molecular Characterization of Rice Genotypes’ Tolerance to Cold Stress at the Seedling Stage. Sustainability. 2022; 14(9):4871. https://doi.org/10.3390/su14094871
Chicago/Turabian StyleAkter, Nasira, Partha Sarathi Biswas, Md. Abu Syed, Nasrin Akter Ivy, Amnah Mohammed Alsuhaibani, Ahmed Gaber, and Akbar Hossain. 2022. "Phenotypic and Molecular Characterization of Rice Genotypes’ Tolerance to Cold Stress at the Seedling Stage" Sustainability 14, no. 9: 4871. https://doi.org/10.3390/su14094871
APA StyleAkter, N., Biswas, P. S., Syed, M. A., Ivy, N. A., Alsuhaibani, A. M., Gaber, A., & Hossain, A. (2022). Phenotypic and Molecular Characterization of Rice Genotypes’ Tolerance to Cold Stress at the Seedling Stage. Sustainability, 14(9), 4871. https://doi.org/10.3390/su14094871








