Heavy Metals Contaminants in Watercress (Nasturtium officinale R. BR.): Toxicity and Risk Assessment for Humans along the Swat River Basin, Khyber Pakhtunkhwa, Pakistan
Abstract
:1. Introduction
2. Materials and Methods
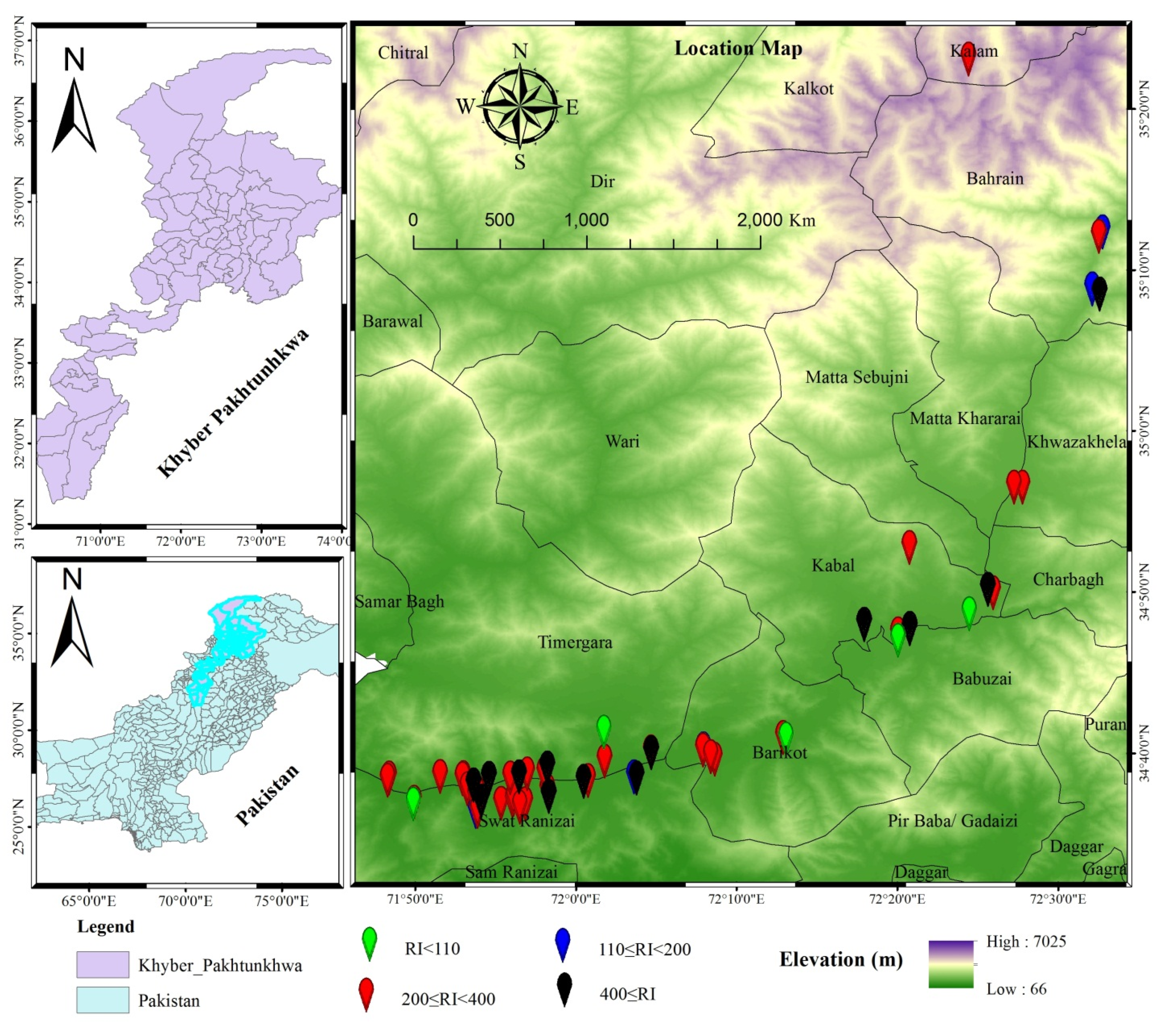
2.1. Study Area
2.2. Sample Collection
2.3. Acid Digestion and Metal Analysis
2.4. Data Analysis
2.4.1. Ecological Risk Index
2.4.2. Bioaccumulation Factor
2.4.3. Daily HM Intake
2.4.4. Health Risk Index
2.5. Statistical Analyses
3. Results and Discussion
3.1. Heavy Metals in Soil and Plant Samples
3.2. Bioaccumulation Factor
3.3. Daily Intake of Metal and Health Risk Index
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Food Policy Research. I MPACT Projections of Food Production, Consumption, and Hunger to 2050, with and without Climate Change: Extended Country-Level Results for 2019 GFPR Annex Table 5 (Trans: Cgiar Research Program on Policies I, Markets, Cgiar Research Program on Climate Change A, Food S, Bill, Melinda Gates F). V2, Deaccessioned Version ed. Harvard Datavers; International Food Policy Research: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Begum, H.A.; Hamayun, M.; Sadiq, A.; Ali, K.; Rauf, M.; Khan, W. Cholinesterase Enzyme Inhibition and Anti-oxidant Bioassays of Malus baccata (L.) Borkh.; An approach to cure Alzheimer’s disease. Biosci. Res. 2020, 17, 2799–2806. [Google Scholar]
- Manchali, S.; Chidambara, M.K.N.; Patil, B.S. Crucial facts about health benefits of popular cruciferous vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Łuczaj, L. Changes in the utilization of wild green vegetables in Poland since the 19th century: A comparison of four ethnobotanical surveys. J. Ethnopharmacol. 2010, 128, 395–404. [Google Scholar] [CrossRef]
- Misra, S.; Maikhuri, R.K.; Kala, C.P.; Rao, K.S.; Saxena, K.G. Wild leafy vegetables: A study of their subsistence dietetic support to the inhabitants of Nanda Devi Biosphere Reserve, India. J. Ethnobiol. Ethnomedicine 2008, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.-W.; Xu, Y.; Liu, S.-J.; He, J.-F.; Long, F.-Y. Concentration and potential health risk of heavy metals in market vegetables in Chongqing, China. Ecotoxicol. Environ. Saf. 2011, 74, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hesham, A.E.-L.; Qiao, M.; Rehman, S.; He, J.-Z. Effects of Cd and Pb on soil microbial community structure and activities. Environ. Sci. Pollut. Res. 2010, 17, 288–296. [Google Scholar] [CrossRef]
- Luo, C.; Liu, C.; Wang, Y.; Liu, X.; Li, F.; Zhang, G.; Li, X. Heavy metal contamination in soils and vegetables near an e-waste processing site, south China. J. Hazard. Mater. 2011, 186, 481–490. [Google Scholar] [CrossRef]
- Hawkes, J.S. Heavy metals. J. Chem. Educ. 1997, 74, 1369–1374. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M. Biological effects of heavy metals: An overview. J. Environ. Biol. 2005, 26, 301–313. [Google Scholar]
- Tongesayi, T.; Fedick, P.; Lechner, L.; Brock, C.; Beau, A.L.; Bray, C. Daily bio accessible levels of selected essential but toxic heavy metals from the consumption of non-dietary food sources. Food Chem. Toxicol. 2013, 62, 142–147. [Google Scholar] [CrossRef]
- Lin, A.-J.; Zhang, X.-H.; Wong, M.-H.; Ye, Z.-H.; Lou, L.-Q.; Wang, Y.-S.; Zhu, Y.-G. Increase of multi-metal tolerance of three leguminous plants by arbuscular mycorrhizal fungi colonization. Environ. Geochem. Health 2007, 29, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.U.; Goni, M.A. Heavy metal contamination in water, soil, and vegetables of the industrial areas in Dhaka, Bangladesh. Environ. Monit. Assess. 2010, 166, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Håkanson, L. An ecological risk index for aquatic pollution control: A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Pandey, R.; Shubhashish, K.; Pandey, J. Dietary intake of pollutant aerosols via vegetables influenced by atmospheric deposition and wastewater irrigation. Ecotoxicol. Environ. Saf. 2012, 76, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiao, M.; Liu, Y.; Zhu, Y. Health risk assessment of heavy metals in soils and vegetables from wastewater irrigated area, Beijing-Tianjin city cluster, China. J. Environ. Sci. 2012, 24, 690–698. [Google Scholar] [CrossRef]
- Li, Q.; Cai, S.; Mo, C.; Chu, B.; Peng, L.; Yang, F. Toxic effects of heavy metals and their accumulation in vegetables grown in a saline soil. Ecotoxicol. Environ. Saf. 2010, 73, 84–88. [Google Scholar] [CrossRef]
- Adhikari, S.; Ghosh, L.; Giri, B.; Ayyappan, S. Distributions of metals in the food web of fishponds of Kolleru Lake, India. Ecotoxicol. Environ. Saf. 2009, 72, 1242–1248. [Google Scholar] [CrossRef]
- Chabukdhara, M.; Munjal, A.; Nema, A.K.; Gupta, S.K.; Kaushal, R.K. Heavy metal contamination in vegetables grown around peri-urban and urban-industrial clusters in Ghaziabad, India. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 736–752. [Google Scholar] [CrossRef]
- Khan, S.; Rehman, S.; Khan, A.Z.; Khan, M.A.; Shah, M.T. Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotoxicol. Environ. Saf. 2010, 73, 1820–1827. [Google Scholar] [CrossRef]
- Ali, M.; Al-Qahtani, K.M. Assessment of some heavy metals in vegetables, cereals and fruits in Saudi Arabian markets. Egypt. J. Aquat. Res. 2012, 38, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Balkhair, K.; Ashraf, M.A. Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, S32–S44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebrekidan, A.; Weldegebriel, Y.; Hadera, A.; Van der Bruggen, B. Toxicological assessment of heavy metals accumulated in vegetables and fruits grown in Ginfel river near Sheba Tannery, Tigray, Northern Ethiopia. Ecotoxicol. Environ. Saf. 2013, 95, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, A.; Magdas, D.A. Analysis of mineral and heavy metal content of some commercial fruit juices by inductively coupled plasma mass spectrometry. Sci. World J. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.M.; Iqbal, J.; Khan, M.A.; Shah, M.H. Health risk assessment and multivariate apportionment of trace metals in wild leafy vegetables from Lesser Himalayas, Pakistan. Ecotoxicol. Environ. Saf. 2013, 92, 237–244. [Google Scholar] [CrossRef]
- Can, H.; Ozyigit, I.I.; Can, M.; Hocaoglu-Ozyigit, A.; Yalcin, I.E. Environment-Based Impairment in Mineral Nutrient Status and Heavy Metal Contents of Commonly Consumed Leafy Vegetables Marketed in Kyrgyzstan: A Case Study for Health Risk Assessment. Biol. Trace Elem. Res. 2021, 199, 1123–1144. [Google Scholar] [CrossRef]
- Hussain, F.; Ilahi, I. Ecology and Vegetation of Lesser Himalayas Pakistan; Jadoon Printing Press: Peshawar, Pakistan, 1991. [Google Scholar]
- Abbasi, A.M.; Khan, M.A.; Ahmad, M.; Zafar, M. Medicinal Plant Biodiversity of Lesser Himalayas-Pakistan; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Reddy, M.; Moodley, R.; Kindness, A.; Jonnalagadda, S.B. Impact of soil quality on elemental uptake by, and distribution in, Colocasia esculenta (Amadumbe), an edible root. J. Environ. Sci. Health Part B 2011, 46, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Begum, H.A.; Hamayun, M.; Shad, N.; Khan, W.; Ahmad, J.; Khan, M.E.H.; Jones, D.A.; Ali, K. Effects of UV radiation on germination, growth, chlorophyll content, and fresh and dry weights of Brassica rapa L. and Eruca sativa L. Sarhad J. Agric. 2021, 37, 1016–1024. [Google Scholar] [CrossRef]
- Washington Department of Ecology. Aquatic Plant Identification Manual for Washington’s Freshwater Plants, Nasturtium Officinale, Watercress; Washington Department of Ecology: Lacey, WA, USA, 2009. [Google Scholar]
- Katsaboxakis, K.Z. The influence of the degree of blanching on the quality of frozen vegetables. In Thermal Processing and Quality of Foods; Zeuthen, P., Cheftel, I.C., Erikson, C., Jul, M., Leniger, H., Linko, P., Varela, G., Vos, G., Eds.; Elsevier Applied Science: London, UK, 1984; pp. 559–565. [Google Scholar]
- Ahmad, H.; Ahmed, R. Agro ecology and biodiversity of the catchment area of Swat River. Nucleus 2004, 40, 67–75. [Google Scholar]
- Barinova, S.; Ali, N.; Barkatullah, S.F. Ecological adaptation to altitude of algal communities in the Swat Valley (Hindu Cush Mountains, Pakistan). Expert Opin. Environ. Biol. 2013, 2, 1–15. [Google Scholar] [CrossRef]
- Nafees, M.; Jan, M.R.; Khan, H.; Ali, A. Status of soil texture and required associated soil conservation measure of river swat catchments area, NWFP, Pakistan. Sarhad J. Agric. 2008, 24, 251–259. [Google Scholar]
- Singh, K.P.; Mohan, D.; Sinha, S.; Dalwani, R. Impact assessment of treated/untreated wastewater toxicants discharged by sewage treatment plants on health, agricultural, and environmental quality in the wastewater disposal area. Chemosphere 2004, 55, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Weldegebriel, Y.; Chandravanshi, B.S.; Wondimu, T. Concentration levels of metals in vegetables grown in soils irrigated with river water in Addis Ababa, Ethiopia. Ecotoxicol. Environ. Saf. 2012, 77, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Kiran, B.; Rani, S.; Rani, A.; Kaur, B.; Mittal, N. Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem. 2008, 111, 811–815. [Google Scholar] [CrossRef]
- Tiwari, K.K.; Singh, N.K.; Patel, M.P.; Tiwari, M.R.; Rai, U.N. Metal contamination of soil and translocation in vegetables growing under industrial wastewater irrigated agricultural field of Vadodara, Gujarat, India. Ecotoxicol. Environ. Saf. 2011, 74, 1670–1677. [Google Scholar] [CrossRef]
- Radojevic, M.; Bashkin, V.N. Practical Environmental Analysis; The Royal Society of Chemistry: Cambridge, UK, 1999. [Google Scholar]
- Singh, A.; Sharma, R.K.; Agrawal, M.; Marshall, F. Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem. Toxicol. 2010, 48, 611–619. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals in Soils; Blackie and Academic Professionals: London, UK, 1990. [Google Scholar]
- MacNicol, R.D.; Beckett, P.H.T. Critical tissue concentrations of potentially toxic elements. Plant Soil 1985, 85, 107–129. [Google Scholar] [CrossRef]
- Ul Islam, E.; Yang, X.-E.; He, Z.-L.; Mahmood, Q. Assessing potential dietary toxicity of heavy metals in selected vegetables and food crops. J. Zhejiang Univ. Sci. B 2007, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kabata-Pendias, A. Behavioural properties of trace metals in soils. Appl. Geochem. 1993, 8, 3–9. [Google Scholar] [CrossRef]
- Cui, Y.-J.; Zhu, Y.-G.; Zhai, R.-H.; Chen, D.-Y.; Huang, Y.-Z.; Qiu, Y.; Liang, J.-Z. Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China. Environ. Int. 2004, 30, 785–791. [Google Scholar] [CrossRef]
- Desiere, S.; Hung, Y.; Verbeke, W.; D’Haese, M. Assessing current and future meat and fish consumption in Sub-Sahara Africa: Learnings from FAO Food Balance Sheets and LSMS household survey data. Glob. Food Secur. 2018, 16, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Sato, T.; Xing, B.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yue, T.; Li, X.; Yuan, Y. Heavy metal levels in kiwifruit orchard soils and trees and its potential health risk assessment in Shaanxi, China. Environ. Sci. Pollut. Res. 2016, 23, 14560–14566. [Google Scholar] [CrossRef] [PubMed]
- Sobhanardakani, S.; Tayebi, L.; Hosseini, S.V. Health risk assessment of arsenic and heavy metals (Cd, Cu, Co, Pb, and Sn) through consumption of caviar of Acipenser persicus from Southern Caspian Sea. Environ. Sci. Pollut. Res. 2018, 25, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Waqas, M.; Ding, F.; Shamshad, I.; Arpd, H.P.H.; Li, G. The influence of various biochars on the bioaccessibility and bioaccumulation of PAHs and potentially toxic elements to turnips (Brassica rapa L.). J. Hazard. Mater. 2015, 300, 243–253. [Google Scholar] [CrossRef]
- Bader, J.L.; González, G.; Goodell, P.C.; Pillai, S.D.; Ali, A.S. Chromium-Resistant Bacterial Populations from a Site Heavily Contaminated with Hexavalent Chromium. Water Air Soil Pollut. 1999, 109, 263–276. [Google Scholar] [CrossRef]
- Castro, E.; Mañas, P.; Heras, J.D.L. A comparison of the application of different waste products to a lettuce crop: Effects on plant and soil properties. Sci. Hortic. 2009, 123, 148–155. [Google Scholar] [CrossRef]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef]
- Rogival, D.; Scheirs, J.; Blust, R. Transfer and accumulation of metals in a soil–diet–wood mouse food chain along a metal pollution gradient. Environ. Pollut. 2007, 145, 516–528. [Google Scholar] [CrossRef]
- Itanna, F. Metal concentrations of some vegetables irrigated with industrial liquid waste at Akaki, Ethiopia. SINET Ethiop. J. Sci. 1998, 21, 133–144. [Google Scholar] [CrossRef]
- Itanna, F. Metals in leafy vegetables grown in Addis Ababa and toxicological implications. Ethiop. J. Health Dev. 2002, 16, 295–302. [Google Scholar] [CrossRef]
- Aschale, M.; Sileshi, Y.; Kelly-Quinn, M.; Hailu, D. Assessment of potentially toxic elements in vegetables grown along Akaki River in Addis Ababa and potential health implications. Assessment 2015, 40, 42–52. [Google Scholar]
- Ahmad, K.; Ashfaq, A.; Khan, Z.I.; Ashraf, M.; Akram, N.A.; Yasmin, S.; Batool, A.I.; Sher, M.; Shad, H.A.; Khan, A.; et al. Health risk assessment of heavy metals and metalloids via dietary intake of a potential vegetable (Coriandrum sativum L.) grown in contaminated water irrigated agricultural sites of Sargodha, Pakistan. 2016, 22, 597–610. Hum. Ecol. Risk Assess. 2016, 22, 597–610. [Google Scholar] [CrossRef]
- Xue, Z.-J.; Liu, S.-Q.; Liu, Y.-L.; Yan, Y.-L. Health risk assessment of heavy metals for edible parts of vegetables grown in sewage-irrigated soils in suburbs of Baoding City, China. Environ. Monit. Assess. 2012, 184, 3503–3513. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Hazra, G.C.; Saha, B.; Mandal, B. Assessment of heavy metals contamination in different crops grown in long-term sewage-irrigated areas of Kolkata, West Bengal, India. Environ. Monit. Assess. 2015, 187, 4087–4099. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Wu, W.; Liu, H.; Chen, H.; Yin, S. Impact of Long-Term Irrigation with Sewage on Heavy Metals in Soils, Crops, and Groundwater—A Case Study in Beijing. Pol. J. Environ. Stud. 2014, 23, 309–318. [Google Scholar]
- Muchuweti, M.; Birkett, J.; Chinyanga, E.; Zvauya, R.; Scrimshaw, M.; Lester, J. Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: Implications for human health. Agric. Ecosyst. Environ. 2006, 112, 41–48. [Google Scholar] [CrossRef] [Green Version]
- US Environmental Protection Agency (EPA). Guidance for Developing Ecological Soil Screening Levels. Office of Solid Waste and Emergency Response; US Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- Jensen, J.; Mesman, M.; Loibner, A.P.; Erlacher, E.; Rutgers, M.; Archibald, G.; Ehlers, C.; Dirven-van Breemen, L.; Bogolte, B.T.; Sorokin, N.; et al. Ecological Risk Assessment of Contaminated Land: Decision Support for Site Specific Investigations; RIVM: Bilthoven, The Netherlands, 2006. [Google Scholar]
- Tier, J.C. Förbättrade miljöriskbedömningar Naturvårdsverket 2006, Stockholm I: Screening phase. J. Soils Sediments 2006, 10, 1557–1571. [Google Scholar]
- Wang, Q.R.; Dong, Y.; Cui, Y.; Liu, X. Instances of soil and crop heavy metal contamination in China. Soil Sediment Contam. 2001, 10, 497–510. [Google Scholar]
- Chapman, P.M.; Wang, F.; Janssen, C.; Goulet, R.R.; Kamunde, C.N. Conducting Ecological Risk Assessments of Inorganic Metals and Metalloids: Current Status. Hum. Ecol. Risk Assess. Int. J. 2003, 9, 641–697. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry, 3rd ed.; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Lund, W. Speciation analysis—Why and how. Fresenius J. Anal. Chem. 1990, 337, 557–564. [Google Scholar] [CrossRef]
- Afolayan, A.J.; Jimoh, F.O. Nutritional quality of some wild leafy vegetables in South Africa. Int. J. Food Sci. Nutr. 2009, 60, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, J.; Joshi, M.; Giri, P. Zinc: The trace element of major importance in human nutrition and health. Int. J. Med. Sci. Public Health 2013, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Osendarp, S.J.M.; West, C.E.; Black, R. Maternal Zinc Supplementation Study Group the Need for Maternal Zinc Supplementation in Developing Countries: An Unresolved Issue. J. Nutr. 2003, 133, 817S. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.I.; Sharif, N. Mineral composition of some leafy vegetables consumed in Kano, Nigeria. Niger J. Basic Appl. Sci. 2011, 19, 208–212. [Google Scholar]
- Saikia, P.; Deka, D.C. Mineral content of some wild green leafy vegetables of North-East India. J. Chem. Pharm. Res. 2013, 5, 117–121. [Google Scholar]
- Akubugwo, I.E.; Obasi, N.A.; Chinyere, G.C.; Ugbogu, A.E. Nutritional and chemical value of Amaranthus hybridus L. leaves from Afikpo, Nigeria. Afr. J. Biotechnol. 2007, 6, 2833–2839. [Google Scholar] [CrossRef] [Green Version]
- Fismes, J.; Perrin-Ganier, C.; Empereur-Bissonnet, P.; Morel, J.L. Soil-to-Root Transfer and Translocation of Polycyclic Aromatic Hydrocarbons by Vegetables Grown on Industrial Contaminated Soils. J. Environ. Qual. 2002, 31, 1649–1656. [Google Scholar] [CrossRef]
- Huq, I.; Smith, E.; Correll, R.; Smith, L.; Smith, J.; Ahmed, M.; Roy, S.; Barnes, M.; Naidu, R. Arsenic transfer in water soil crop environments in Bangladesh. I: Assessing potential arsenic exposure pathways in Bangladesh. In Proceedings of the Arsenic in the Asia-Pacific Region Workshop 2001, “Managing arsenic for our future”, Adelaide, SA, Australia, 20–23 November 2001; pp. 50–51. [Google Scholar]
- Rattan, R.K.; Datta, S.P.; Chhonkar, P.K.; Suribabu, K.; Singh, A.K. Longterm impact of irrigation with waste water effluents on heavy metal content in soils, crops and groundwater—A case study. Agric. Ecosyst Environ. 2005, 109, 310–322. [Google Scholar] [CrossRef]
- Mapanda, F.; Mangwayana, E.; Nyamangara, J.; Giller, K. The effect of long-term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agric. Ecosyst. Environ. 2005, 107, 151–165. [Google Scholar] [CrossRef]
- Khan, S.; Aijun, L.; Zhang, S.; Hu, Q.; Zhu, Y.-G. Accumulation of polycyclic aromatic hydrocarbons and heavy metals in lettuce grown in the soils contaminated with long-term wastewater irrigation. J. Hazard. Mater. 2008, 152, 506–515. [Google Scholar] [CrossRef]
- Wang, G.; Su, M.-Y.; Chen, Y.-H.; Lin, F.-F.; Luo, D.; Gao, S.-F. Transfer characteristics of cadmium and lead from soil to the edible parts of six vegetable species in southeastern China. Environ. Pollut. 2006, 144, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Zhao, J.Z.; Ouyang, Z.Y.; Soderlund, L.; Liu, G.H. Impacts ofsewage irrigation on heavy metals distribution and contamination in Beijing, China. Environ. Int. 2005, 31, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Hooda, P.S.; McNulty, D.; Alloway, B.J. Plant availability of heavy metals in soils previously amended with heavy applications of sewage sludge. J. Sci. Food Agric. 1997, 73, 446–454. [Google Scholar] [CrossRef]
- Lim, H.-S.; Lee, J.-S.; Chon, H.-T.; Sager, M. Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. J. Geochem. Explor. 2008, 96, 223–230. [Google Scholar] [CrossRef]
- Li, Y.; Gou, X.; Wang, G.; Zhang, Q.; Su, Q.; Xiao, G. Heavy metal contamination and source in arid agricultural soil in central Gansu Province, China. J. Environ. Sci. 2008, 20, 607–612. [Google Scholar] [CrossRef]
- Montagne, D.; Cornu, S.; Bourennane, H.; Baize, D.; Ratié, C.; King, D. Effect of Agricultural Practices on Trace Element Distribution in Soil. Commun. Soil Sci. Plant Anal. 2007, 38, 473–491. [Google Scholar] [CrossRef]
- Wei, B.; Yang, L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 2010, 94, 99–107. [Google Scholar] [CrossRef]
- Liu, W.X.; Shen, L.F.; Liu, J.W.; Wang, Y.W.; Li, S.R. Uptake of toxic heavy metals by rice (Oryza sativa L.) cultivated in the agricultural soils near Zhengzhou City, People’s Republic of China. Bull. Environ. Contam. Toxicol. 2007, 79, 209–213. [Google Scholar] [CrossRef]
- Ahmed, F.; Ishiga, H. Trace metal concentrations in street dusts of Dhaka city, Bangladesh. Atmos. Environ. 2006, 40, 3835–3844. [Google Scholar] [CrossRef]
- De Miguel, E.; Iribarren, I.; Chacón, E.; Ordóñez-Alonso, A.; Charlesworth, S. Risk-based evaluation of the exposure of children to trace elements in playgrounds in Madrid (Spain). Chemosphere 2007, 66, 505–513. [Google Scholar] [CrossRef]
- Ferreira-Baptista, L.; De Miguel, E. Geochemistry and risk assessment of street dust in Luanda, Angola: A tropical urban environment. Atmos. Environ. 2005, 39, 4501–4512. [Google Scholar] [CrossRef] [Green Version]
- Sindern, S.; Lima, R.F.S.; Schwarzbauer, J.; Petta, R.A. Anthropogenic heavy metal signatures for the fast growing urban area of Natal (NE-Brazil). Environ. Earth Sci. 2006, 52, 731–737. [Google Scholar] [CrossRef]
- Amato, F.; Pandolfi, M.; Viana, M.; Querol, X.; Alastuey, A.; Moreno, T. Spatial and chemical patterns of PM10 in road dust deposited in urban environment. Atmos. Environ. 2009, 43, 1650–1659. [Google Scholar] [CrossRef]
- Oliva, S.R.; Espinosa, A.F. Monitoring of heavy metals in topsoils, atmospheric particles and plant leaves to identify possible contamination sources. Microchem. J. 2007, 86, 131–139. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, D.; Pan, J.; Nie, W.; Wu, K. Application of multivariate statistical approach to identify heavy metal sources in sediment and waters: A case study in Yangzhong, China. Environ. Geol. 2008, 54, 373–380. [Google Scholar] [CrossRef]
- Rosselli, W.; Rossi, M.; Sasu, I. Cd, Cu and Zn contents in the leaves of Taraxacum officinale. Swiss Federal Institute for Forest. Snow Landsc. Res. 2006, 80, 361–366. [Google Scholar]
- Wenzel, W.W.; Sattler, H.; Jockwer, F. Metal hyperaccumulator plants: A survey on species to be potentially used for soil remediation. Agron. Abstr. 1993, 52, 42–52. [Google Scholar]
- Blaylock, M.J.; Elles, M.P.; Nuttal, C.Y.; Zdimal, K.L.; Lee, C.R. Treatment of as contaminated soil and water using Pteris vittata. In Proceedings of the International Conferences of Biogeochemistry of Trace Elements, 7th ICOBTE 2003, Uppsala, Sweden, 15–19 June 2003. [Google Scholar]
- Ernst, W. Bioavailability of heavy metals and decontamination of soils by plants. Appl. Geochem. 1996, 11, 163–167. [Google Scholar] [CrossRef]
- Baker, A.; Mc Grath, S.; Reeves, R.; Smith, J. Metal hyper accumulator plants: A review of the ecology and physiology of a biological resource for phytoremediation of metal-polluted soils. In Phytoremediation of Contaminated Soils; Terry, N., Banuelos, G., Eds.; Lewis Publisher: London, UK, 2000; pp. 85–107. [Google Scholar]
- Baker, A.M.J.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements—A review of their distribution, ecology and phytochemistry. Bio. Recovery 1989, 1, 81–126. [Google Scholar]
- Zhuang, P.; Zou, B.; Li, N.Y.; Li, Z.A. Heavy metal contamination in soils and food crops around Dabaoshan mine in Guangdong, China: Implication for human health. Environ. Geochem. Health 2009, 31, 707–715. [Google Scholar] [CrossRef]
- National Research Council. Recommended Dietary Allowances; National Research Council: Ottawa, ON, Canada, 1989. [Google Scholar]
- Mahmood, A.; Malik, R.N. Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arab. J. Chem. 2014, 7, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Chopra, A.K.; Pathak, C. Accumulation of heavy metals in the vegetables grown in wastewater irrigated areas of Dehradun, India with reference to human health risk. Environ. Monit. Assess. 2015, 187, 445. [Google Scholar] [CrossRef] [PubMed]
- Bahemuka, T.E.; Mubofu, E.B. Heavy metals in edible green vegetables grown along the sites of the Sinza and Msimbazi Rivers in Darselam, Tanzania. Food Chem. 1999, 66, 63–66. [Google Scholar] [CrossRef]
- Maleki, A.; Zarasvand, M.A. Heavy metals in selected edible vegetables and estimation of their daily intake in Sanandaj, Iran. Southeast Asian J. Trop. Med. Public Health 2008, 39, 335. [Google Scholar] [PubMed]
- Gupta, S.; Satpati, S.; Nayek, S.; Garai, D. Effect of wastewater irrigation on vegetables in relation to bioaccumulation of heavy metals and biochemical changes. Environ. Monit. Assess. 2010, 165, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Wang, Q.; Zhang, X.; Zheng, D.; Zhang, Z.; Zhang, S. Population health risk due to dietary intake of heavy metals in the industrial area of Huludao city, China. Sci. Total Environ. 2007, 387, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.S.; Hussain, M.I.; Ismail, S.; Khan, Q.M. Evaluating heavy metal accumulation and potential health risks in vegetables irrigated with treated wastewater. Chemosphere 2016, 163, 54–61. [Google Scholar] [CrossRef]

| Index | Category | Risk Grade | HM | SRV (mg/kg) | PRV (mg/kg) | DL (mg/kg) | QL (mg/kg) |
|---|---|---|---|---|---|---|---|
| Ecological Risk Index (RI) | RI < 110 | Low risk | Cd | 0.31 (a) | 0.10 (c) | 0.0082 | 0.027 |
| 110 ≤ RI < 200 | Moderate | Pb | 8.15 (b) | 5 (d) | 0.0027 | 0.0091 | |
| 200 ≤ RI < 400 | Considerable risk | Cu | 8.39 (b) | 10 (d) | 0.0006 | 0.002 | |
| 400 ≤ RI | Very high risk | Zn | 44.19 (b) | 60 (e) | 0.0049 | 0.016 |
| Variable | RI < 110 | 110 ≤ RI < 200 | 200 ≤ RI< 400 | 400 ≤ RI | F-Value | p-Value |
|---|---|---|---|---|---|---|
| CLY | 10.4 ± 2.03 | 12.33 ± 1.5 | 10.38 ± 0.64 | 11.38 ± 1.09 | 0.55 | 0.65 |
| SLT | 49.2 ± 4.45 | 47 ± 3.13 | 46.88 ± 1.43 | 50.69 ± 2.89 | 0.61 | 0.61 |
| SND | 40 ± 5.29 | 40.33 ± 4.17 | 42.88 ± 1.59 | 38.53 ± 2.99 | 0.66 | 0.58 |
| pH | 7.96 ± 0.10 | 7.9 ± 0.11 | 7.94 ± 0.02 | 7.94 ± 0.04 | 0.026 | 0.99 |
| EC | 0.21 ± 0.03 | 0.32 ± 0.032 | 0.31 ± 0.01 | 0.24 ± 0.01 | 4.50 | 0.007 |
| OM% | 0.92 ± 0.06 | 0.91 ± 0.05 | 0.953 ± 0.01 | 0.88 ± 0.04 | 1.16 | 0.33 |
| Cd (mg/kg) | 0 ± 0 | 1.5 ± 0.08 | 2.6 ± 0.1 | 4.7 ± 0.2 | 76.2 | 9.20 × 10−20 |
| Pb (mg/kg) | 61.6 ± 18.1 | 51.1 ± 7.8 | 62 ± 4.3 | 53 ± 8.1 | 0.5 | 0.64 |
| Cu (mg/kg) | 3.09 ± 0.5 | 2.6 ± 0.3 | 2.8 ± 0.1 | 4.2 ± 1.2 | 1.3 | 0.27 |
| Zn (mg/kg) | 8.38 ± 0.74 | 7.3 ± 2.2 | 6.1 ± 0.4 | 7.7 ± 1.03 | 1.4 | 0.24 |
| Metal | Groups-RI < 110 | 110 ≤ RI < 200 | 200 ≤ RI < 400 | 400 ≤ RI | F-Value | p-Value |
|---|---|---|---|---|---|---|
| M ± SE | M ± SE | M ± SE | M ± SE | |||
| Cd (mg/kg) | 0 ± 0 | 0.6 ± 0.09 | 0.9 ± 0.06 | 1.1 ± 0.1 | 69.59 | 6.90 × 10−19 |
| Pb (mg/kg) | 0.7 ± 0.2 | 0.8 ± 0.1 | 1.07 ± 0.09 | 2.1 ± 0.5 | 4.12 | 0.010 |
| Cu (mg/kg) | 0.5 ± 0.1 | 0.7 ± 0.08 | 1.02 ± 0.07 | 1.4 ± 0.1 | 6.37 | 9 × 10−4 |
| Zn (mg/kg) | 0.9 ± 0.07 | 2.1 ± 0.4 | 3.9 ± 0.4 | 5.7 ± 0.7 | 6.49 | 7 × 10−4 |
| Metals | Cd | Pb | Cu | Zn |
|---|---|---|---|---|
| M ± SE | M ± SE | M ± SE | M ± SE | |
| DIM for Children | ||||
| Group-RI < 110 | 0 ± 0 | 1.6 × 10−2 ± 68 × 10−4 | 7.7 × 10−4 ± 5 × 10−5 | 4 × 10−3 ± 1.7 × 10−4 |
| 110 ≤ RI < 200 | 7 × 10−4 ± 4 × 10−5 | 1.8 × 10−2 ± 5.5 × 10−4 | 1.4 × 10−3 ± 1.8 × 10−5 | 6.4 × 10−3 ± 1.4 × 10−4 |
| 200 ≤ RI < 400 | 1.32 × 10−3 ± 1.7 × 10−5 | 3.5 × 10−2 ± 4.6 × 10−4 | 1.5 × 10−3 ± 8 × 10−5 | 1.1 × 10−2 ± 1.1 × 10−4 |
| 400 ≤ RI | 3.3 × 10−3 ± 1.8 × 10−4 | 4.8 × 10−2 ± 1.6 × 10−3 | 2 × 10−3 ± 3 × 10−5 | 2.2 × 10−2 ± 3 × 10−4 |
| ANOVA | *** | *** | *** | *** |
| DIM for Adults | ||||
| Group-RI < 110 | 0 ± 0 | 1.5 × 10−2 ± 1.7 × 10−3 | 9.4 × 10−4 ± 8.1 × 10−4 | 5.5 × 10−3 ± 5.8 × 10−4 |
| 110 ≤ RI < 200 | 1 × 10−3 ± 1.6 × 10−4 | 2.1 × 10−2 ± 1.6 × 10−3 | 8.6 × 10−4 ± 5 × 10−5 | 7.6 × 10−3 ± 5.7 × 10−4 |
| 200 ≤ RI < 400 | 1.1 × 10−3 ± 1 × 10−5 | 2.6 × 10−2 ± 3.6 × 10−4 | 1.1 × 10−3 ± 1.7 × 10−5 | 9.5 × 10−3 ± 1.2 × 10−4 |
| 400 ≤ RI | 1 × 10−3 ± 13 × 10−4 | 1.9 × 10−2 ± 9.8 × 10−4 | 1.1 × 10−3 ± 4.2 × 10−5 | 7.4 × 10−3 ± 3.5 × 10−4 |
| ANOVA | * | NS | NS | NS |
| Metals | Cd | Pb | Cu | Zn |
|---|---|---|---|---|
| M ± SE | M ± SE | M ± SE | M ± SE | |
| Children HRI | ||||
| Group-RI < 110 | 0 ± 0 | 4.75 ± 1.8 × 10−1 | 1.9 × 10−1 ± 1 × 10−2 | 1.5 × 10−2 ± 5.9 × 10−4 |
| 110 ≤ RI < 200 | 7.1 × 10−1 ± 4 × 10−2 | 5.13 ± 1.5 × 10−1 | 2.5 × 10−2 ± 4.6 × 10−4 | 2.1 × 10−2 ± 3.3 × 10−4 |
| 200 ≤ RI < 400 | 1.39 ± 1 × 10−2 | 9.93 ± 1.2 × 10−1 | 3.9 × 10−2 ± 2.2 × 10−5 | 9.6 × 10−2 ± 3.9 × 10−4 |
| 400 ≤ RI | 3.36 ± 1.8 × 10−1 | 13.55 ± 4 × 10−1 | 6 × 10−2 ± 8.4 × 10−4 | 7.5 × 10−2 ± 15 × 10−3 |
| ANOVA | *** | *** | *** | *** |
| Adults HRI | ||||
| Group-RI < 110 | 0 ± 0 | 4.37 ± 4 × 10−1 | 2.3 × 10−2 ± 2.1 × 10−3 | 1.8 × 10−2 ± 1.9 × 10−3 |
| 110 ≤ RI < 200 | 1.23 ± 1.6 × 10−2 | 5.85 ± 4.4 × 10−1 | 2.1 × 10−2 ± 1.4 × 10−4 | 2.1 × 10−2 ± 6.9 × 10−3 |
| 200 ≤ RI < 400 | 1.14 ± 18 × 10−2 | 7.38 ± 1.3 × 10−1 | 2.4 × 10−2 ± 2.5 × 10−4 | 3.1 × 10−2 ± 4.1 × 10−4 |
| 400 ≤ RI | 1.71 ± 1.3 × 10−1 | 5.35 ± 2.7 × 10−1 | 2.8 × 10−2 ± 1.1 × 10−3 | 2 × 10−2 ± 1.1 × 10−3 |
| NS | NS | NS | NS | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.; Khan, J.; Ullah, R.; Ali, K.; Jones, D.A.; Khan, M.E.H. Heavy Metals Contaminants in Watercress (Nasturtium officinale R. BR.): Toxicity and Risk Assessment for Humans along the Swat River Basin, Khyber Pakhtunkhwa, Pakistan. Sustainability 2022, 14, 4690. https://doi.org/10.3390/su14084690
Khan N, Khan J, Ullah R, Ali K, Jones DA, Khan MEH. Heavy Metals Contaminants in Watercress (Nasturtium officinale R. BR.): Toxicity and Risk Assessment for Humans along the Swat River Basin, Khyber Pakhtunkhwa, Pakistan. Sustainability. 2022; 14(8):4690. https://doi.org/10.3390/su14084690
Chicago/Turabian StyleKhan, Nasrullah, Jawad Khan, Rafi Ullah, Kishwar Ali, David Aaron Jones, and Muhammad Ezaz Hasan Khan. 2022. "Heavy Metals Contaminants in Watercress (Nasturtium officinale R. BR.): Toxicity and Risk Assessment for Humans along the Swat River Basin, Khyber Pakhtunkhwa, Pakistan" Sustainability 14, no. 8: 4690. https://doi.org/10.3390/su14084690
APA StyleKhan, N., Khan, J., Ullah, R., Ali, K., Jones, D. A., & Khan, M. E. H. (2022). Heavy Metals Contaminants in Watercress (Nasturtium officinale R. BR.): Toxicity and Risk Assessment for Humans along the Swat River Basin, Khyber Pakhtunkhwa, Pakistan. Sustainability, 14(8), 4690. https://doi.org/10.3390/su14084690








