Description of a Fe/Al Electrocoagulation Method Powered by a Photovoltaic System, for the (Pre-)Treatment of Municipal Wastewater of a Small Community in Northern Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Wastewater Sample
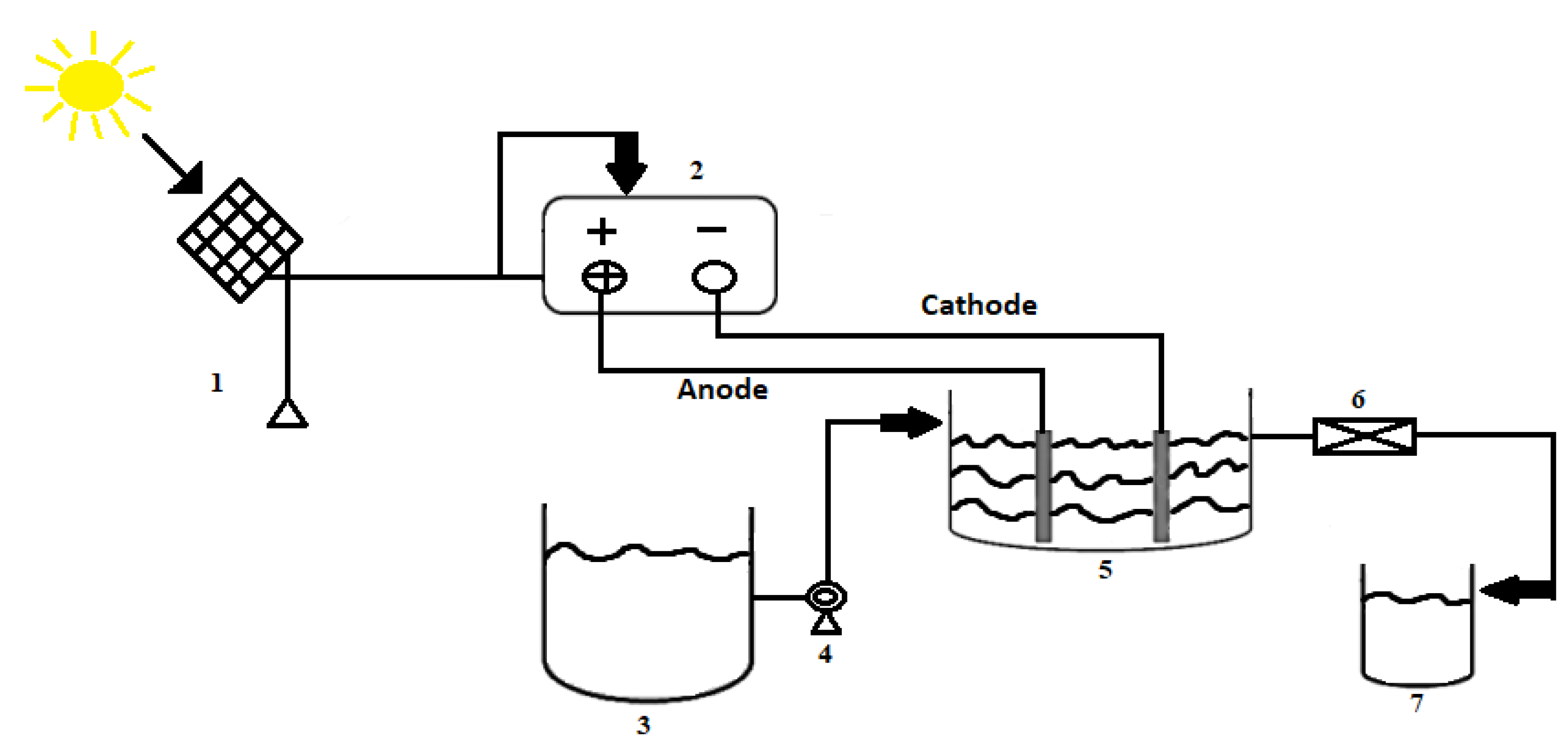
2.3. Experimental Design and Apparatus
2.4. Statistical Analysis
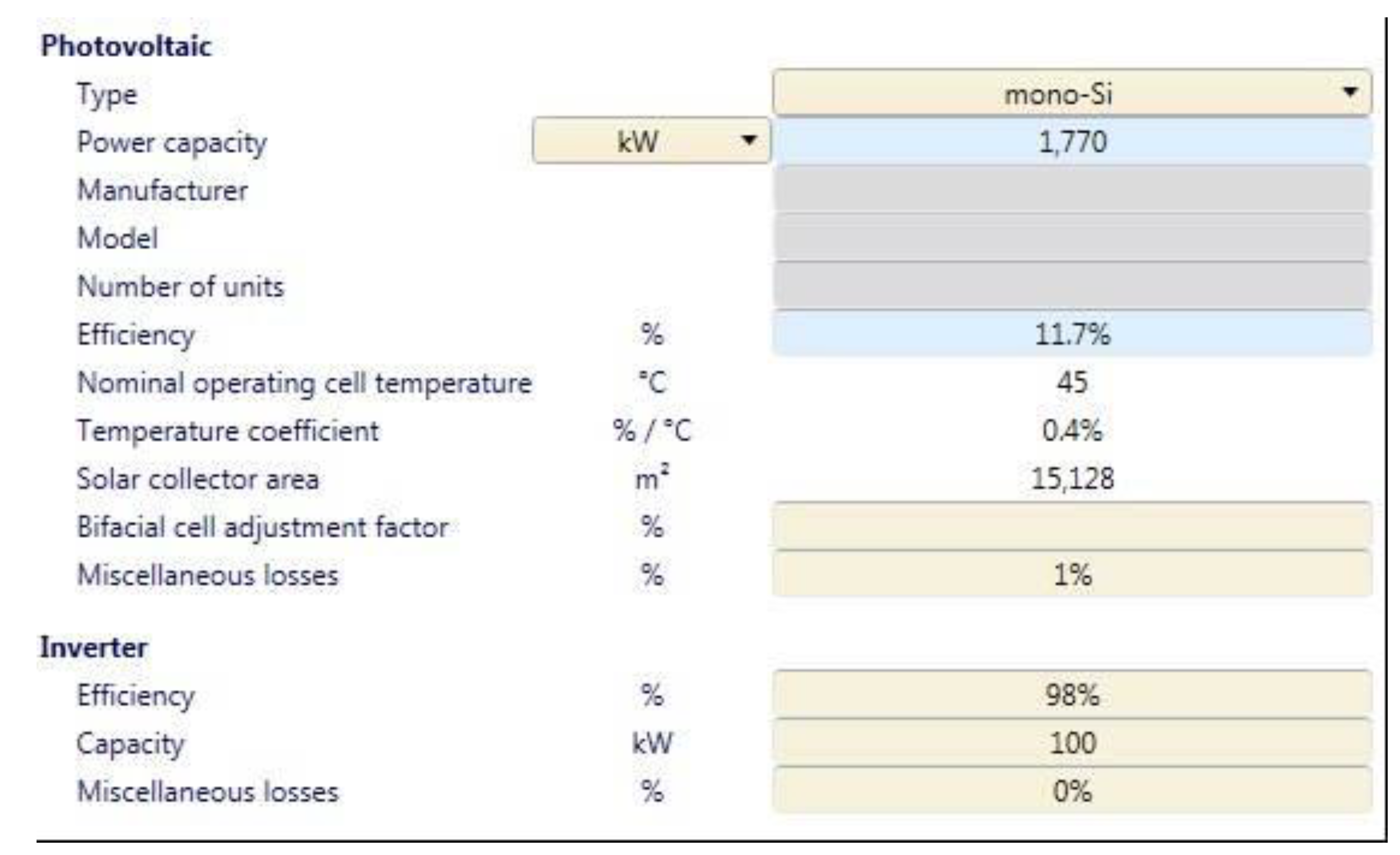
2.5. Electricity Needs Simulation of the Apparatus in the Small Community
3. Results
3.1. Sample Characteristics
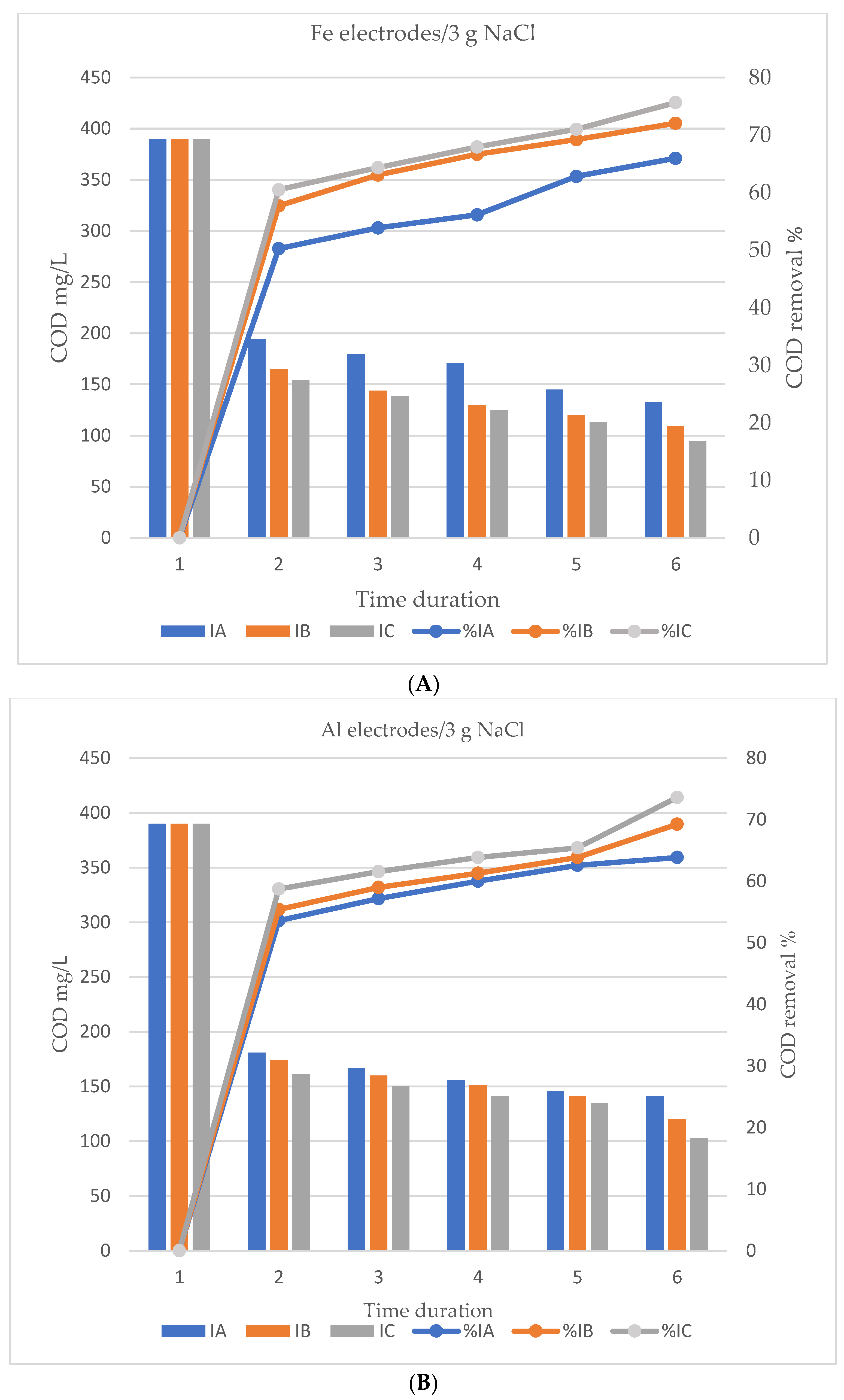
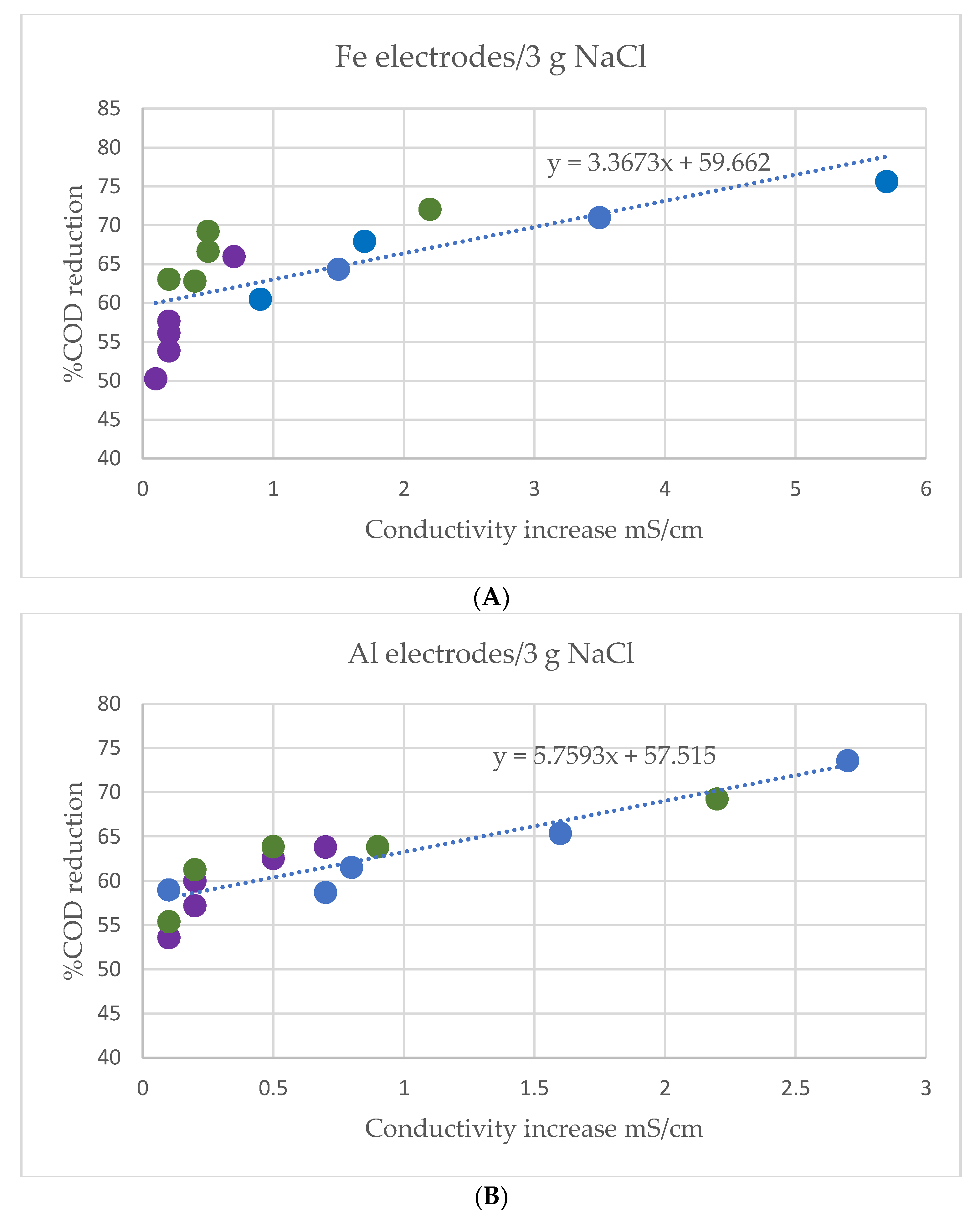
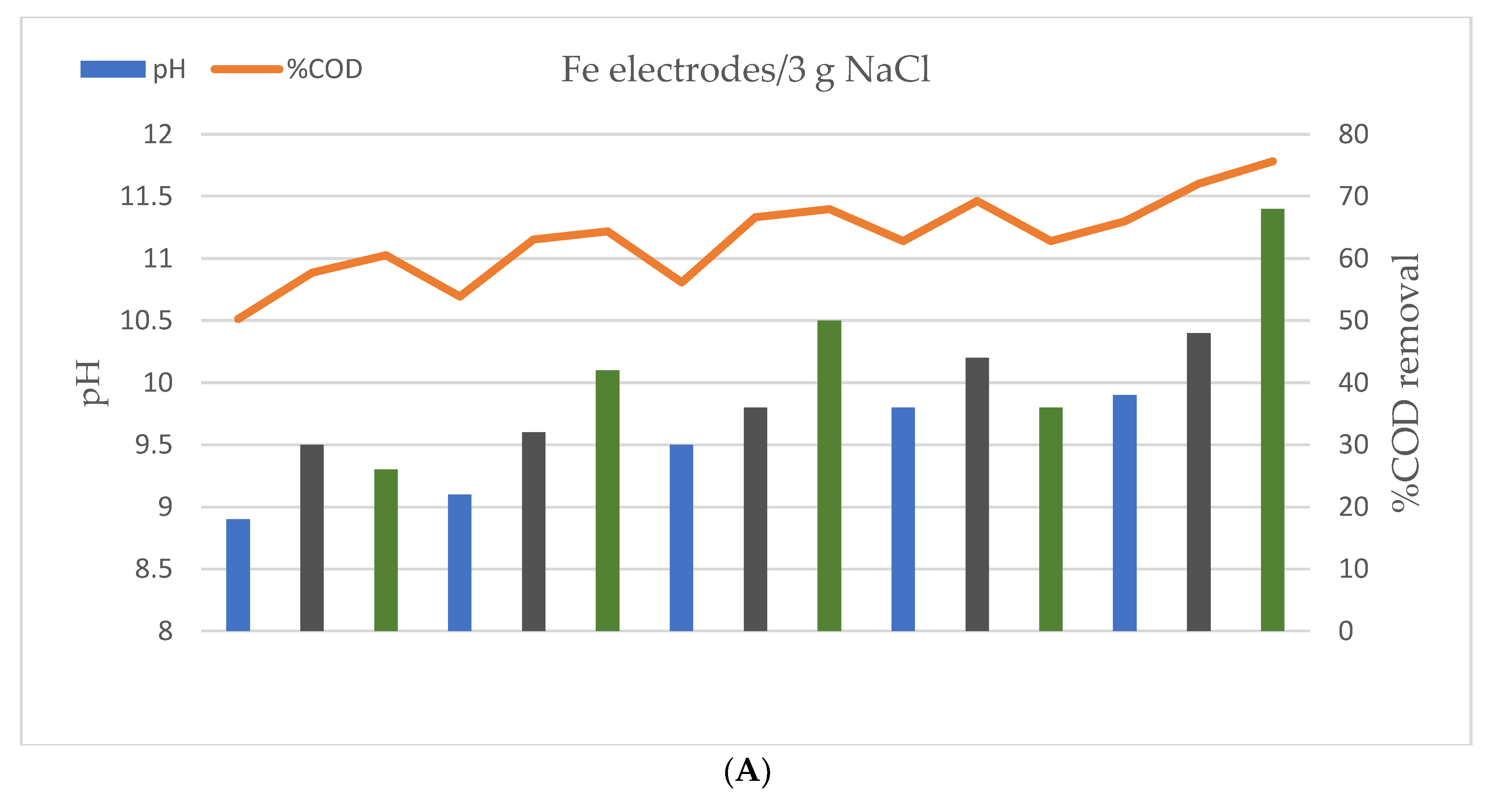
3.2. Effect of Processing Time, Current Intensity and Sample Conductivity on COD Removal
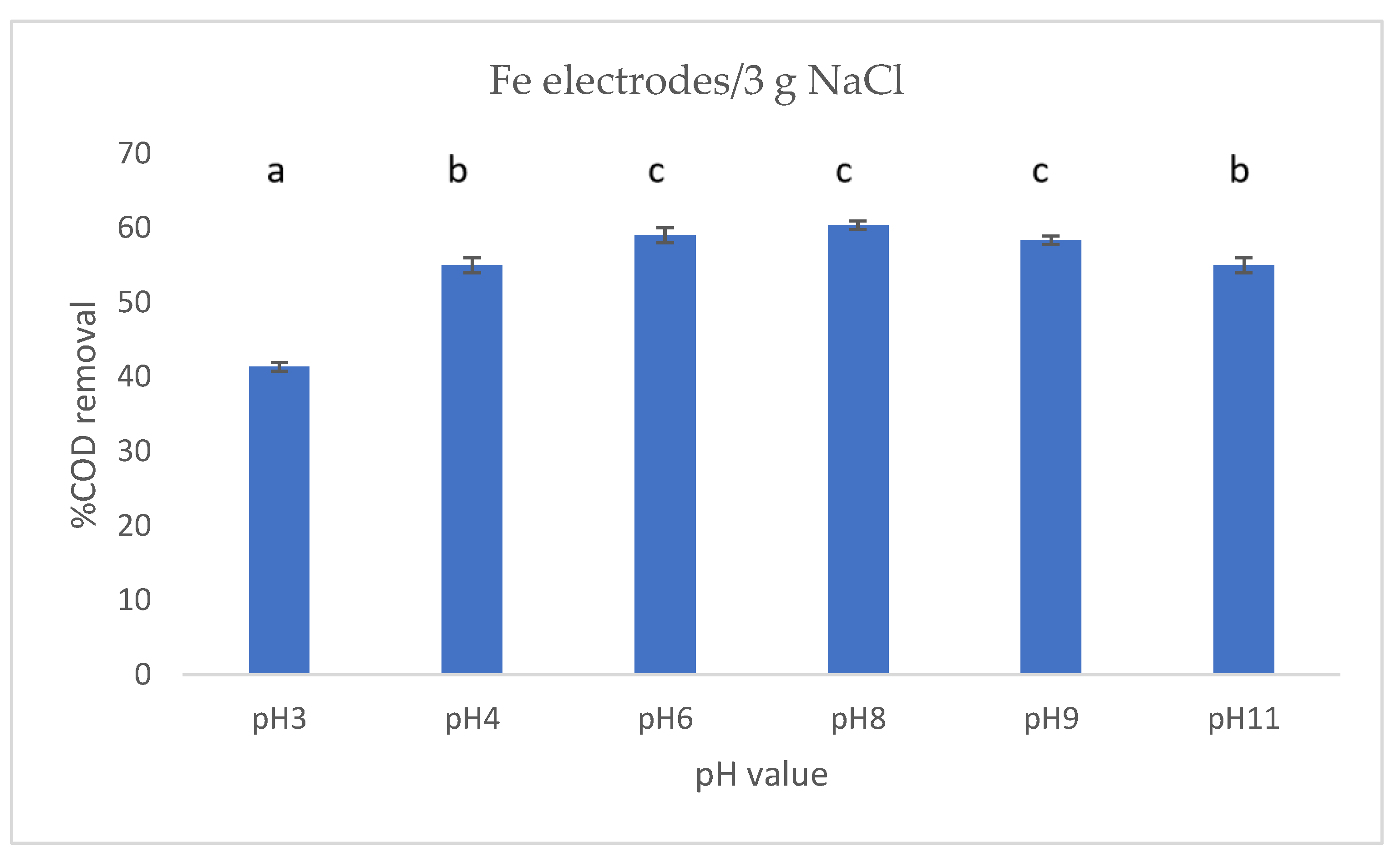
3.3. Effect of pH on COD Removal
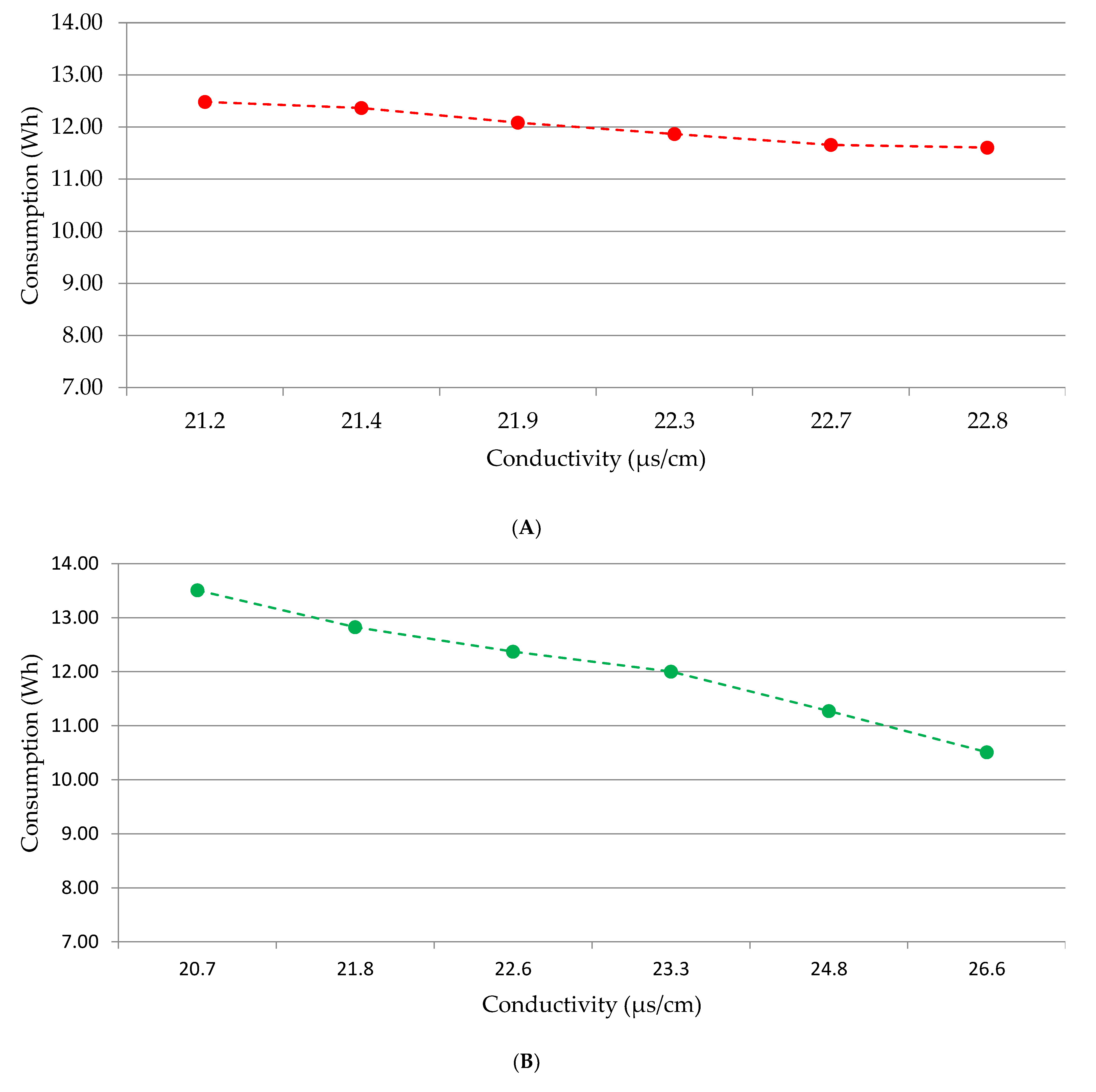
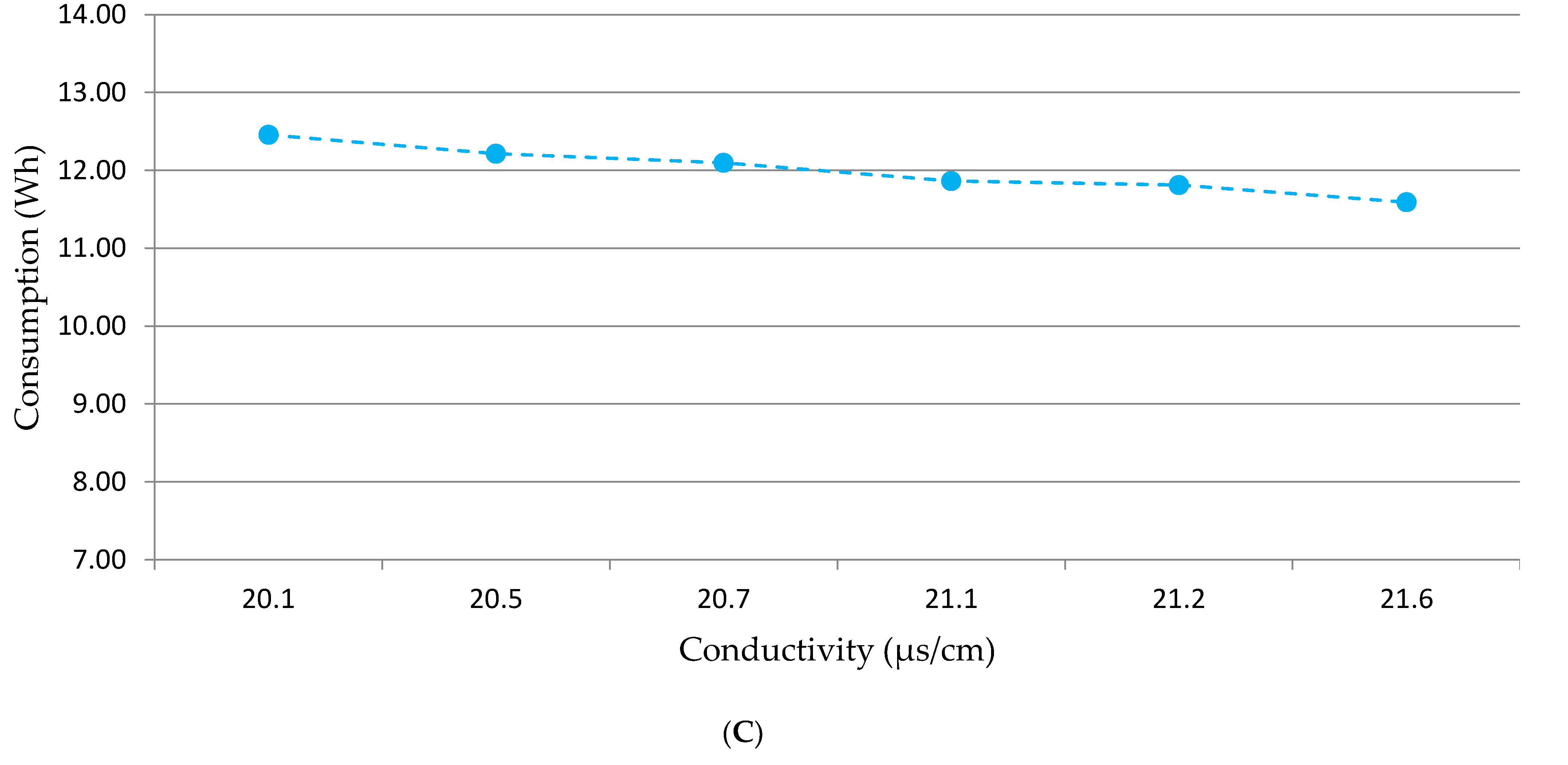
3.4. Material and Energy Consumption and Electricity Needs Simulation
4. Discussion
4.1. Sample Characteristics
4.2. Effect of Processing Time, Current Intensity and Sample Conductivity on COD Removal
4.3. Effect of pH on COD Removal
4.4. Electricity Needs Simulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nawarkar, C.J.; Salkar, V.D. Solar Power Electrocoagulation System for Municipal Wastewater Treatment. Fuel 2019, 237, 222–226. [Google Scholar] [CrossRef]
- Bicudo, B.; van Halem, D.; Trikannad, S.A.; Ferrero, G.; Medema, G. Low Voltage Iron Electrocoagulation as a Tertiary Treatment of Municipal Wastewater: Removal of Enteric Pathogen Indicators and Antibiotic–Resistant Bacteria. Water Res. 2021, 188, 116500. [Google Scholar] [CrossRef] [PubMed]
- Dal Magro Follmann, H.V.; Souza, E.; Battistelli, A.A.; Lapolli, F.R.; Lobo–Recio, M.A. Determination of the Optimal ElectroCoagulation Operational Conditions for Pollutant Removal and Filterability Improvement during the Treatment of Municipal Wastewater. J. Water Process. Eng. 2020, 36, 101295. [Google Scholar] [CrossRef]
- Mumbi, A.W.; Watanabe, T. Cost Estimations of Water Pollution for the Adoption of Suitable Water Treatment Technology. Sustain. 2022, 14, 649. [Google Scholar] [CrossRef]
- Fischer, K.; Majewsky, M. Cometabolic Degradation of Organic Wastewater Micropollutants by Activated Sludge and Sludge–Inherent Microorganisms. Appl. Microbiol. Biotechnol. 2014, 98, 6583–6597. [Google Scholar] [CrossRef]
- Official Journal of the European Communities. Council Directive of 21 May 1991 Concerning Urban Wastewater Treatment; No L135/40; Publications Office of the European Union: Luxembourg, 1991. [Google Scholar]
- COM. Tenth Report on the Implementation Status and Programmes for Implementation (as Required by Article 17 of Council Directive 91/271/EEC, Concerning Urban Waste Water Treatment); 492 Final; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Raboni, M.; Torretta, V.; Urbini, G. Influence of Strong Diurnal Variations in Sewage Quality on the Performance of Biological Denitrification in Small Community Wastewater Treatment Plants (WWTPs). Sustainability 2013, 5, 3679–3689. [Google Scholar] [CrossRef] [Green Version]
- Stathatou, P.M.; Dedousis, P.; Arampatzis, G.; Grigoropoulou, H.; Assimacopoulos, D. Energy Savings and Reduced Emissions in Combined Natural and Engineered Systems for Wastewater Treatment and Reuse: The WWTP of Antiparos Island, Greece. Desalin. Water Treat. 2019, 159, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Ramos, N.d.F.S.; Borges, A.C.; Coimbra, E.C.L.; Gonçalves, G.C.; Colares, A.P.F.; de Matos, A.T. Swine Wastewater Treatment in Constructed Wetland Systems: Hydraulic and Kinetic Modeling. Water 2022, 14, 681. [Google Scholar] [CrossRef]
- Management Organisation Unit of Development Programmes (MOU). Available online: https://www.mou.gr/el/pages/OPWaste.aspx (accessed on 25 February 2022). (In Greek).
- USEPA. Decentralized Wastewater Management Memorandum of Understanding among the U.S. Environmental Protection Agency and Partner Organizations. Available online: https://www.epa.gov/sites/default/files/2020–09/documents/2020_mou_agreement.pdf (accessed on 25 February 2022).
- Chen, E.G.; Chen, X.; Yue, P.L. Electrocoagulation and Electroflotation of Restaurant Wastewater. J. Environ. Eng. 2000, 126, 858–863. [Google Scholar] [CrossRef]
- Lacasa, E.; Cotillas, S.; Saez, C.; Lobato, J.; Cañizares, P.; Rodrigo, M.A. Environmental Applications of Electrochemical Technology. What is Needed to Enable Full–Scale Applications? Curr. Opin. Electrochem. 2019, 16, 149–156. [Google Scholar] [CrossRef]
- Smoczyński, L.; Kalinowski, S.; Ratnaweera, H.; Kosobucka, M.; Trifescu, M.; Pieczulis-Smoczyńska, K. Electrocoagulation of Municipal Wastewater—a Pilot-Scale Test. Desalination Water Treat. 2017, 72, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Dermentzis, K.; Davidis, A.; Chatzichristou, C.; Dermentzi, A. Ammonia Removal from Fertilizer Plant Effluents by a Coupled Electrostatic Shielding Based Electrodialysis/Electrodeionization Process. Glob. Nest J. 2012, 14, 468–476. [Google Scholar]
- Rakhmania; Kamyab, H.; Yuzir, M.A.; Abdullah, N.; Quan, L.M.; Riyadi, F.A.; Marzouki, R. Recent Applications of the Electrocoagulation Process on Agro–Based Industrial Wastewater: A Review. Sustainability 2022, 14, 1985. [Google Scholar] [CrossRef]
- Sher, F.; Hanif, K.; Zafar Iqbal, S.; Imran, M. Implications of Advanced Wastewater Treatment: Electrocoagulation and Electroflocculation of Effluent Discharged from a Wastewater Treatment Plant. J. Water Process. Eng. 2020, 33, 101101. [Google Scholar] [CrossRef]
- Koshla, N.K.; Venkachalam, S.; Sonrasundaram, P. Pulsed Electrogeneration of Bubbles for Electroflotation. J. Appl. Electrochem. 1991, 21, 986–990. [Google Scholar]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of Coagulation/Flocculation in Oily Wastewater Treatment: A Review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef] [PubMed]
- Dermentzis, K.; Christoforidis, A.; Valsamidou, E.; Lazaridou, A.; Kokkinos, N. Removal of Hexavalent Chromium from Electroplating Wastewater by Electrocoagulation with Iron Electrodes. Glob. Nest J. 2011, 13, 412–418. [Google Scholar]
- Khanitchaidecha, W.; Ratananikom, K.; Yangklang, B.; Intanoo, S.; Sing–Aed, K.; Nakaruk, A. Application of Electrocoagulation in Street Food Wastewater. Water 2022, 14, 655. [Google Scholar] [CrossRef]
- Sarala, C. Domestic Wastewater Treatment by Electrocoagulation with Fe–Fe Electrodes. Int. J. Eng. Trends Technol. 2012, 3, 530–533. [Google Scholar]
- DIN 17100; Steels for General Structural Purposes Quality Standards. Deutsches Institut fur Normung E.V.: Berlin, Germany, 1980.
- APHA (American Public Health Association). Standard Methods for Examination of Water and Wastewater; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC USA, 1999. [Google Scholar]
- Kumari, S.; Kumar, R.N. and Kumar. Electrocoagulation for COD, Turbidity, Ammonia and Phosphate Removal from Municipal Wastewater. J. Indian Chem. Soc. 2020, 97, 527–532. [Google Scholar]
- Common Ministerial Decree 5673/400/1997. Measures and Limits for Municipal Wastewater Treatment, Official Government Gazette 192/B/14-3-1997. Available online: http://www.et.gr/idocs-nph/search/pdfViewerForm.html?args=5C7QrtC22wEWFzYWFtEvQndtvSoClrL8V8YeS8scMDB5MXD0LzQTLf7MGgcO23N88knBzLCmTXKaO6fpVZ6Lx3UnKl3nP8NxdnJ5r9cmWyIq-BTkXB0ftEAEhATUkJb0x1LIdQ163nV9K--td6SIuSL1V86_WUbADgzI1tHV81NRFdX-aie6i9tD9Q3FX6Te (accessed on 4 April 2022). (In Greek).
- Tsakalos, G.; Thysiadou, A.; Fantidis, J.G. Feasibility Study of a 400kW PV Power Plant via Virtual Net Metering Scheme for the Center of Social Welfare of Eastern Macedonia and Thrace. J. Electr. Electron. Eng. 2021, 14, 44–47. [Google Scholar]
- Mukherjee, S.; Razzak, M.A. Design, analysis and optimization of grid–connected solar photovoltaic system using RETScreen in eight divisions of Bangladesh. In Proceedings of the 3rd International Conference on Electrical Engineering and Information & Communication Technology, Dhaka, Bangladesh, 24 September 2016; pp. 1–6. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, L.; Zhu, T.; Zhang, T.; Zhang, J. Feasibility Analysis on Distributed Energy System of Chongming County based on RETScreen Software. Energy 2017, 130, 298–306. [Google Scholar] [CrossRef]
- Huang, C.-H.; Shen, S.-Y.; Chen, C.-W.; Dong, C.-D.; Kumar, M.; Dakshinamoorthy, B.; Chang, J.-H. Effect of Chloride Ions on Electro–Coagulation to Treat Industrial Wastewater Containing Cu and Ni. Sustainability 2020, 12, 7693. [Google Scholar] [CrossRef]
- Moreno, H.; Parga, J.R.; Gomes, A.J.; Rodríguez, M. Electrocoagulation Treatment of Municipal Wastewater in Torreon Mexico. Desalin. Water Treat. 2013, 51, 2710–2717. [Google Scholar] [CrossRef]
- Ozyonar, F.; Karagozoglu, B. Operating Cost Analysis and Treatment of Domestic Wastewater by Electrocoagulation Using Aluminum Electrodes. Polish J. Environ. Stud. 2011, 20, 173–179. [Google Scholar]
- Kabdaşlı, I.; Arslan–Alaton, I.; Ölmez–Hancı, T.; Tünay, O. Electrocoagulation Applications for Industrial Wastewaters: A Critical Review. Environ. Technol. Rev. 2012, 1, 2–45. [Google Scholar] [CrossRef]
- Bouhezila, F.; Hariti, M.; Lounici, H.; Mameri, N. Treatment of the OUED SMAR Town Landfill Leachate by an Electrochemical Reactor. Desalination 2011, 280, 347–353. [Google Scholar] [CrossRef]
- Bakopoulou, S.; Emmanouil, C.; Kungolos, A. Assessment of Wastewater Effluent Quality in Thessaly Region, Greece, for Determining its Irrigation Reuse Potential. Ecotoxicol. Environ. Saf. 2011, 74, 188–194. [Google Scholar] [CrossRef]
- Silva, P.; Matos, M. Assessment of the Impact of Aluminum on Germination, Early Growth and Free Proline Content in Lactuca sativa L. Ecotoxicol. Environ. Saf. 2016, 131, 151–156. [Google Scholar] [CrossRef]
- Dermentzis, K.; Marmanis, D.; Christoforidis, A.; Moumtzakis, A. Photovoltaic Electrocoagulation Process for Remediation of Chromium Plating Wastewaters. Desalin. Water Treat. 2014, 1–6. [Google Scholar] [CrossRef]
- Bukhari, A.A. Investigation of the Electro–Coagulation Treatment Process for the Removal of Total Suspended Solids and Turbidity from Municipal Wastewater. Bioresour. Technol. 2008, 99, 914–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Parameter | Value |
|---|---|
| COD (mg/L) | 390 |
| Conductivity (mS/cm) | 21 |
| Temperature (°C) | 24.5 |
| pH | 7.4 |
| N-NH3 (mg/L) | 43.50 |
| N total (mg/L) | 75.60 |
| p total (mg/L) | 7.41 |
| Suspended Solids (mg/L) | 128.00 |
| BOD (mg/L) | 195 |
| Electrode Consumption C | |
|---|---|
| Al | Fe |
| I = 300 mA | I = 300 mA |
| Mw = 26.98 g/mol | Mw = 55.85 g/mol |
| t = 3600 s | t = 3600 s |
| F = 96.487 c/mol | F = 96.487 c/mol |
| V = 300 mL | V = 300 mL |
| z = 3 e | Z = 2 e |
| Cal = 0.0932 kg/m3 | Cfe = 0.289 kg/m3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marmanis, D.; Emmanouil, C.; Fantidis, J.G.; Thysiadou, A.; Marmani, K. Description of a Fe/Al Electrocoagulation Method Powered by a Photovoltaic System, for the (Pre-)Treatment of Municipal Wastewater of a Small Community in Northern Greece. Sustainability 2022, 14, 4323. https://doi.org/10.3390/su14074323
Marmanis D, Emmanouil C, Fantidis JG, Thysiadou A, Marmani K. Description of a Fe/Al Electrocoagulation Method Powered by a Photovoltaic System, for the (Pre-)Treatment of Municipal Wastewater of a Small Community in Northern Greece. Sustainability. 2022; 14(7):4323. https://doi.org/10.3390/su14074323
Chicago/Turabian StyleMarmanis, D., C. Emmanouil, J. G. Fantidis, A. Thysiadou, and K. Marmani. 2022. "Description of a Fe/Al Electrocoagulation Method Powered by a Photovoltaic System, for the (Pre-)Treatment of Municipal Wastewater of a Small Community in Northern Greece" Sustainability 14, no. 7: 4323. https://doi.org/10.3390/su14074323
APA StyleMarmanis, D., Emmanouil, C., Fantidis, J. G., Thysiadou, A., & Marmani, K. (2022). Description of a Fe/Al Electrocoagulation Method Powered by a Photovoltaic System, for the (Pre-)Treatment of Municipal Wastewater of a Small Community in Northern Greece. Sustainability, 14(7), 4323. https://doi.org/10.3390/su14074323








