Effects of Silicic Acid on Leaching Behavior of Arsenic from Spent Magnesium-Based Adsorbents Containing Arsenite
Abstract
1. Introduction
2. Materials and Methods
2.1. Spent Mg-Based Adsorbents
2.1.1. Mg-Based Adsorbents (Unspent)
2.1.2. Synthetic As-Contaminated Water
2.1.3. Preparation of Spent Mg-Based Adsorbents
2.2. Silicic Acid Solution
2.3. Shaking Test with Spent Mg-Based Adsorbents and Silicic Acid Solution
3. Results
3.1. pH of Eluate
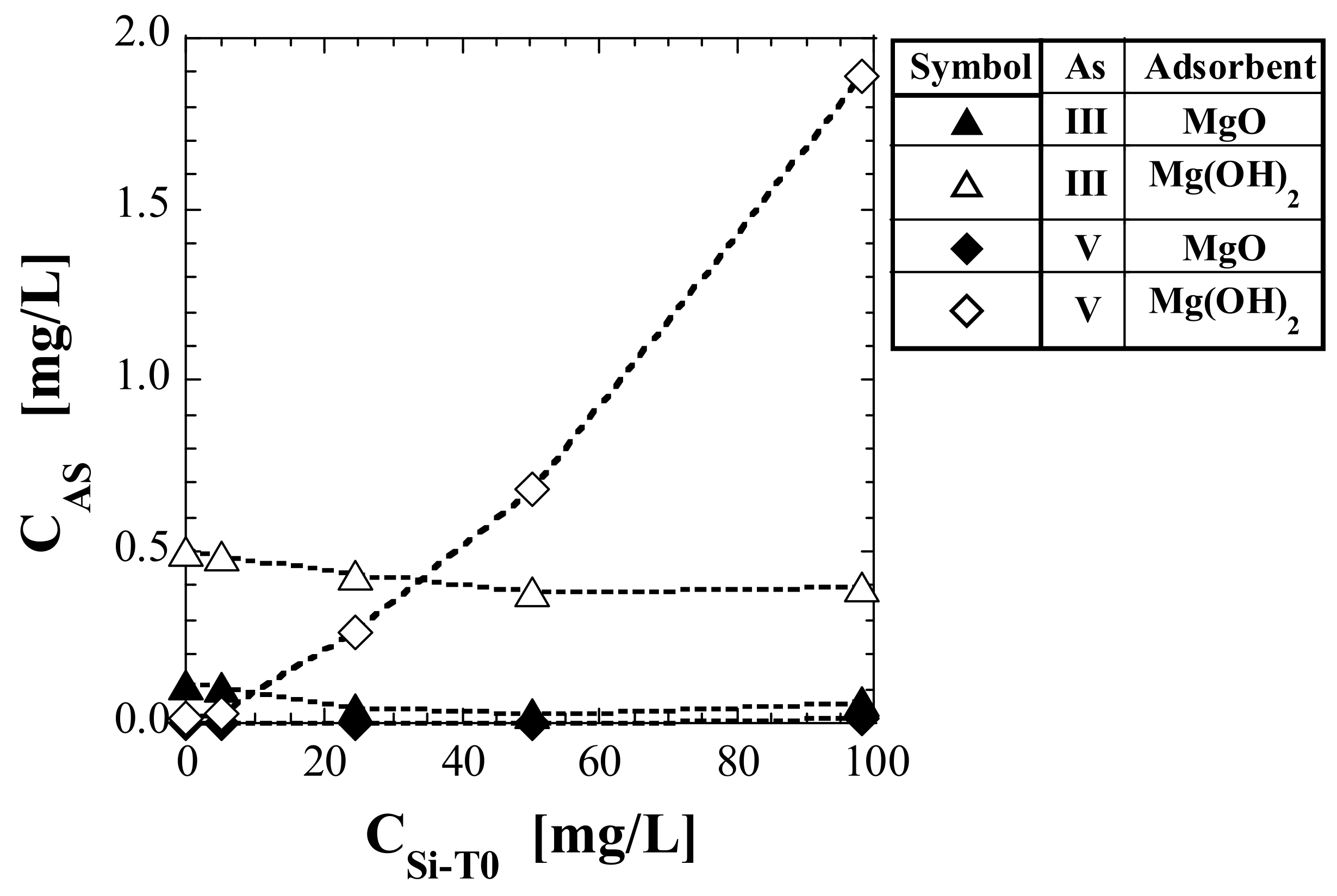
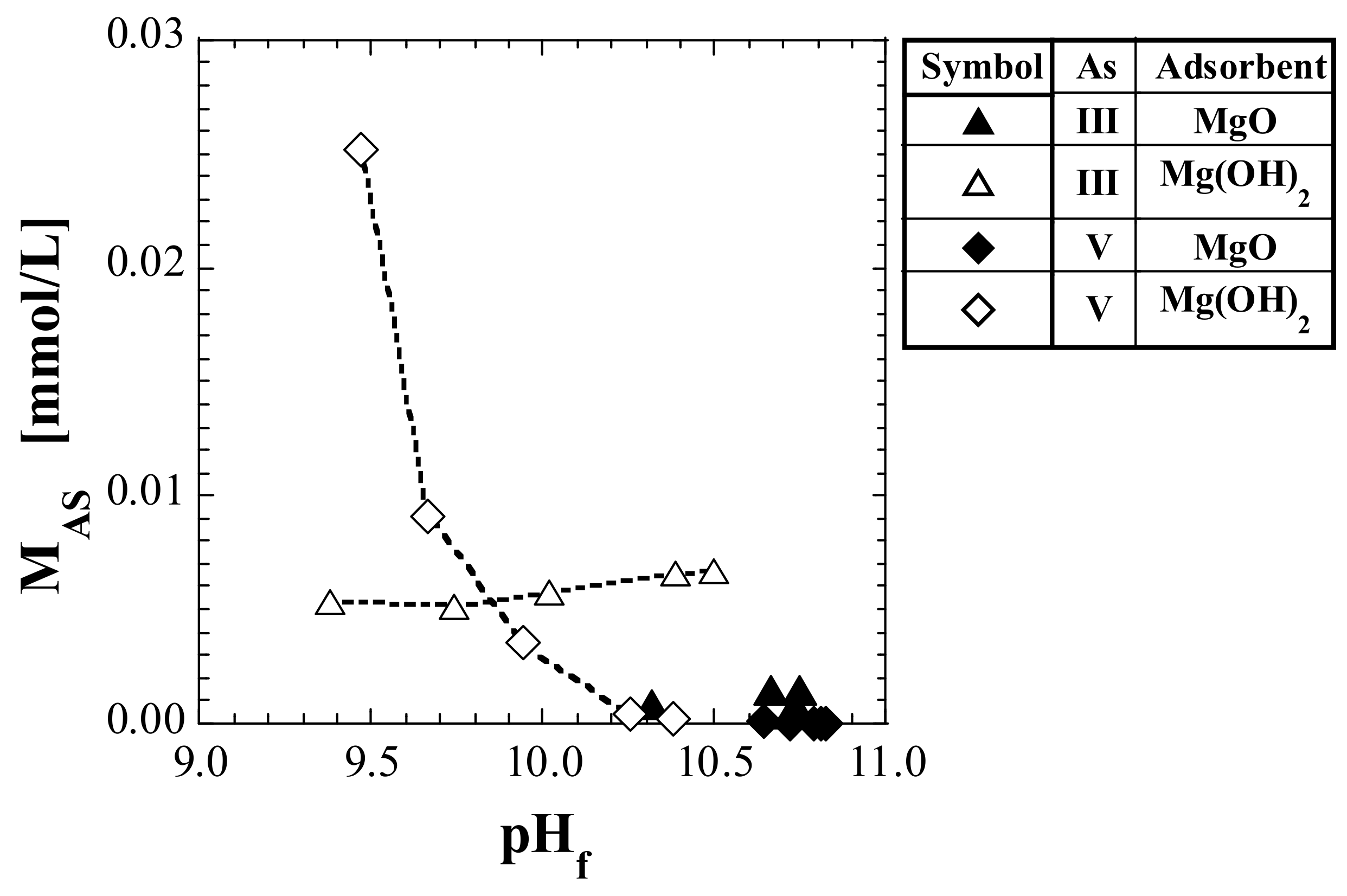
3.2. As Concentration in Eluate
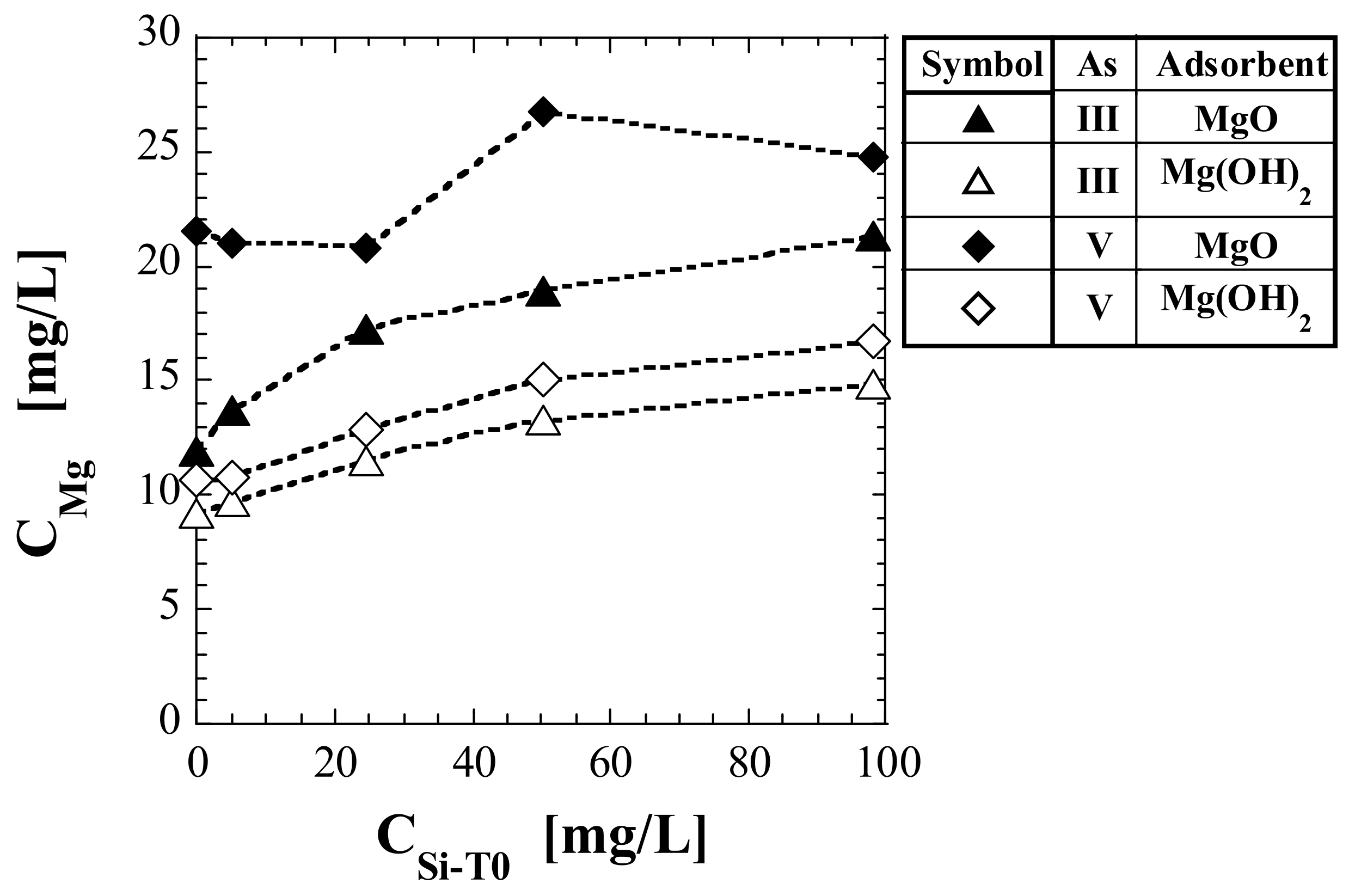
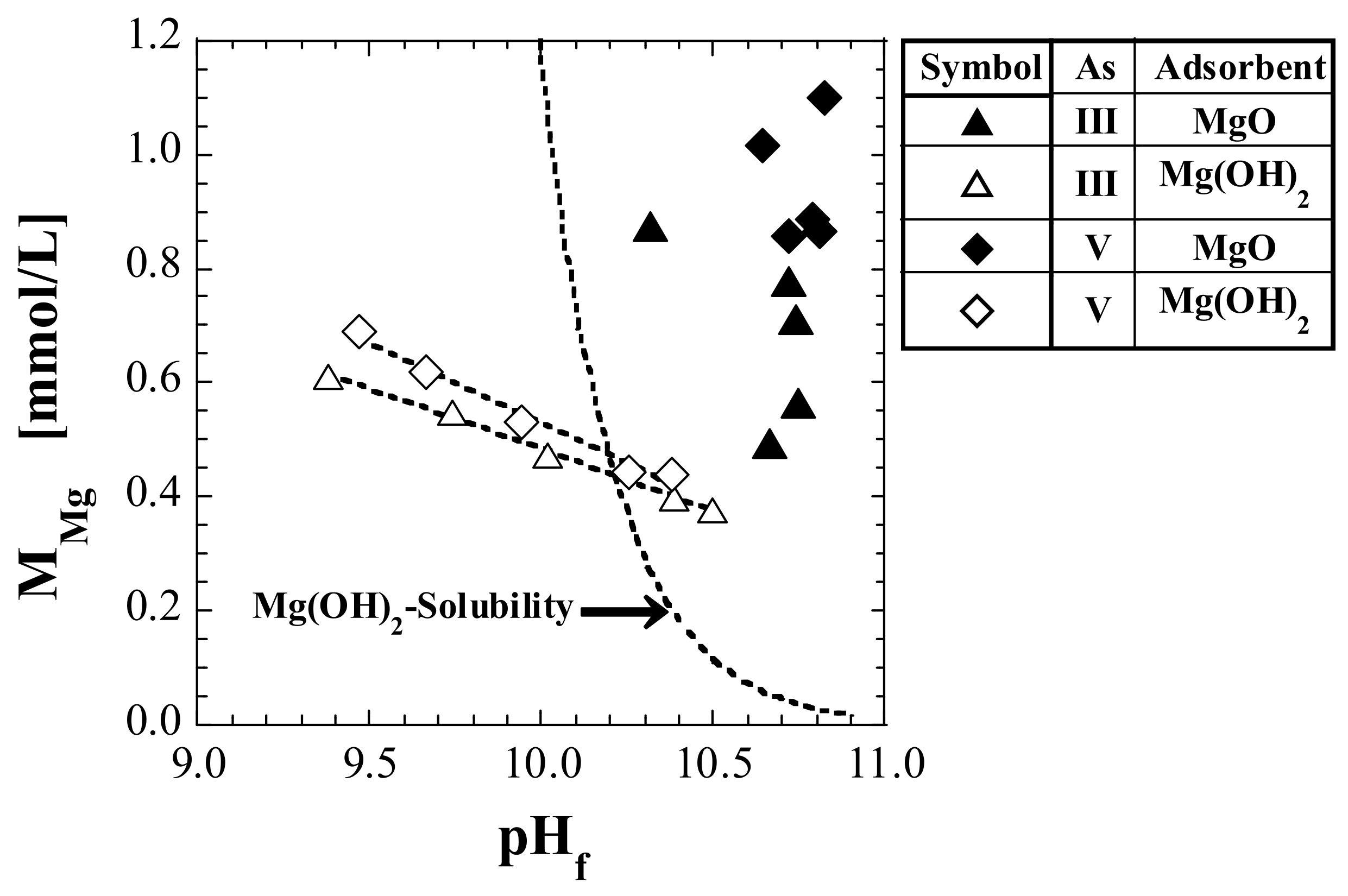
3.3. Mg Concentration in Eluate
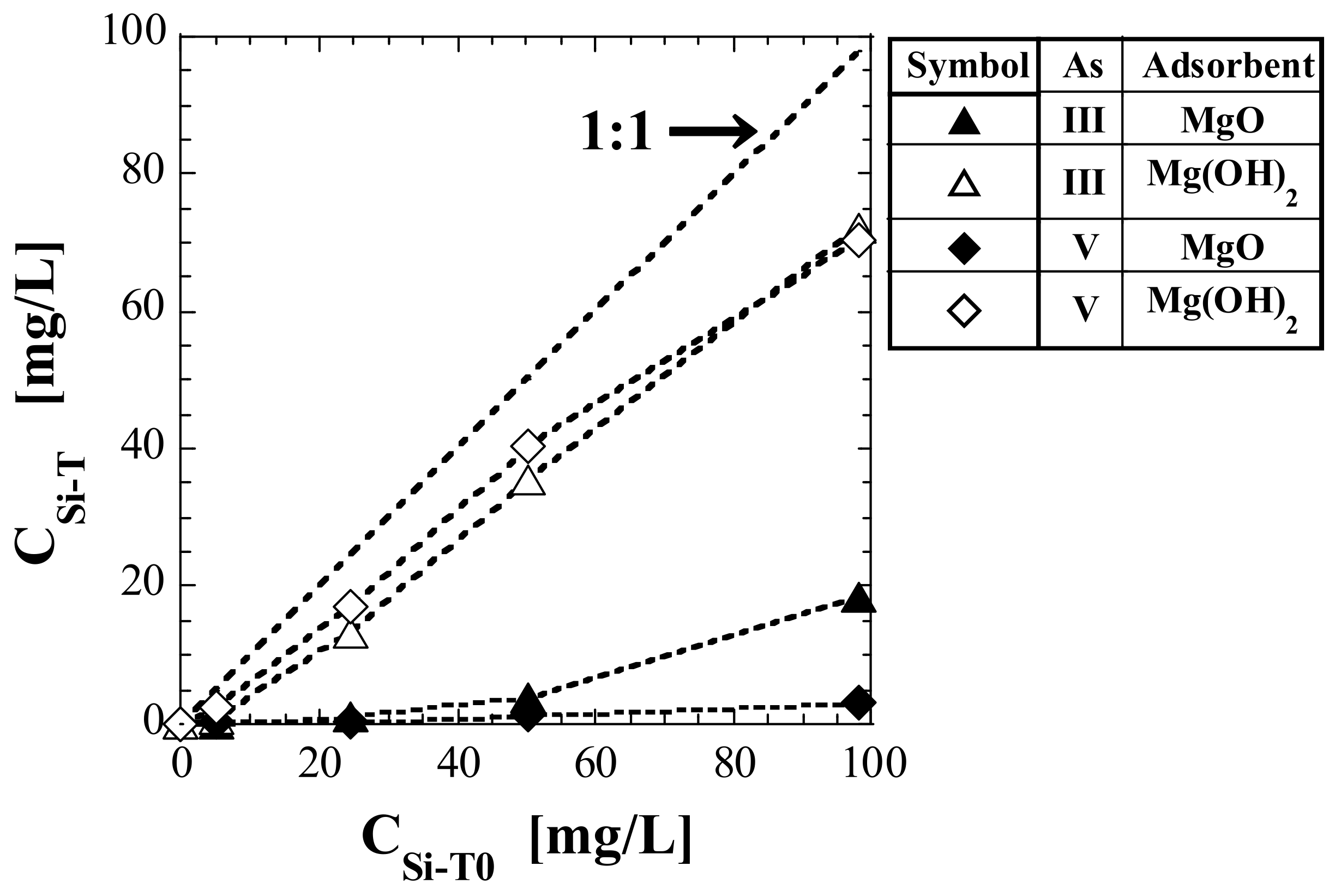
3.4. Total Si-Normalized Concentration in Eluate
3.5. Reproducibility of Experimental Data
4. Discussion
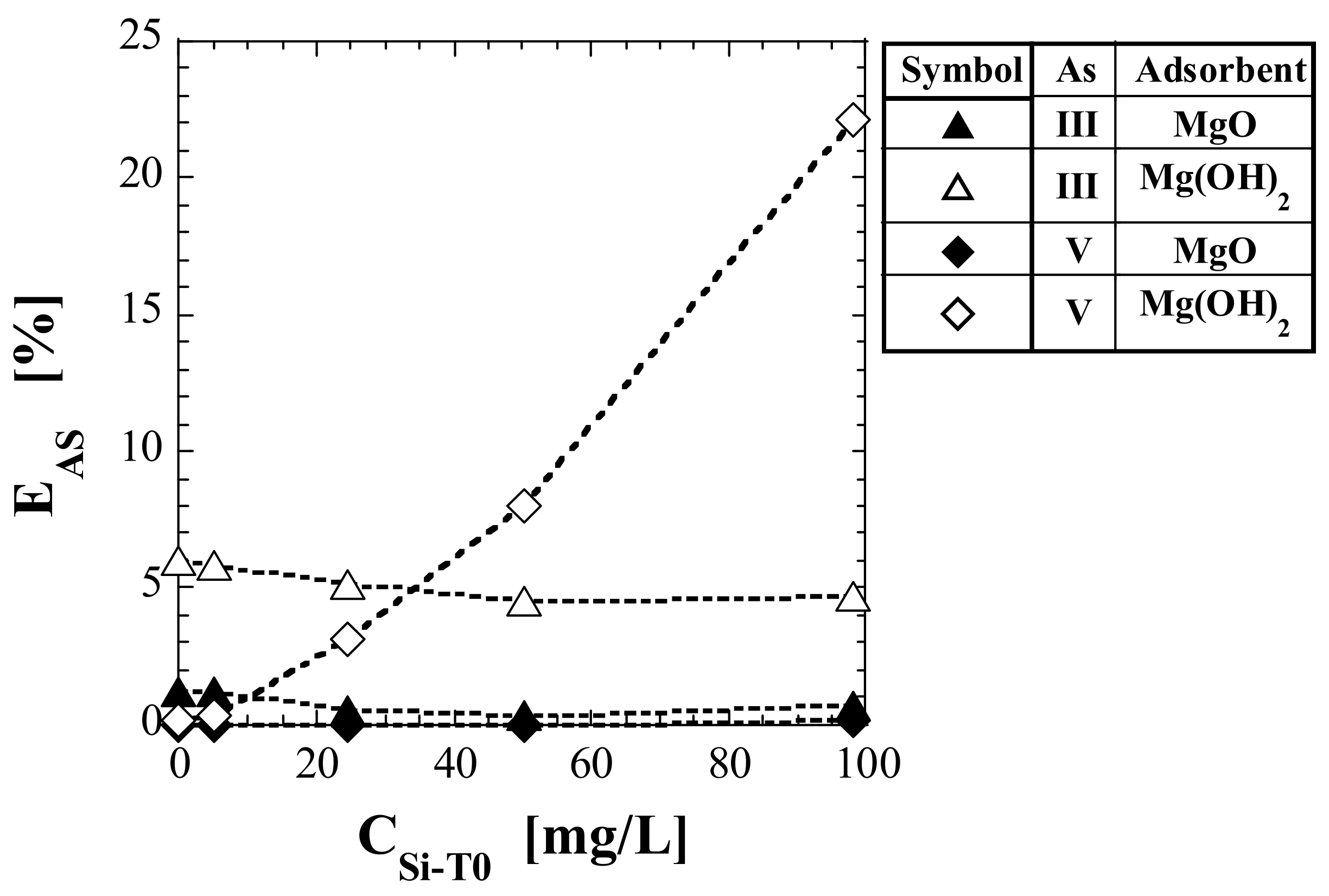
4.1. Relative Evaluation Using As Leaching Ratio
4.2. Dissolved Forms of Siliacic Acid in Liquid
4.3. Dissolved Forms of Arsenous Acid in Liquid
4.4. Stoichiometric Considerations on Spent Mg-Based Adsorbents
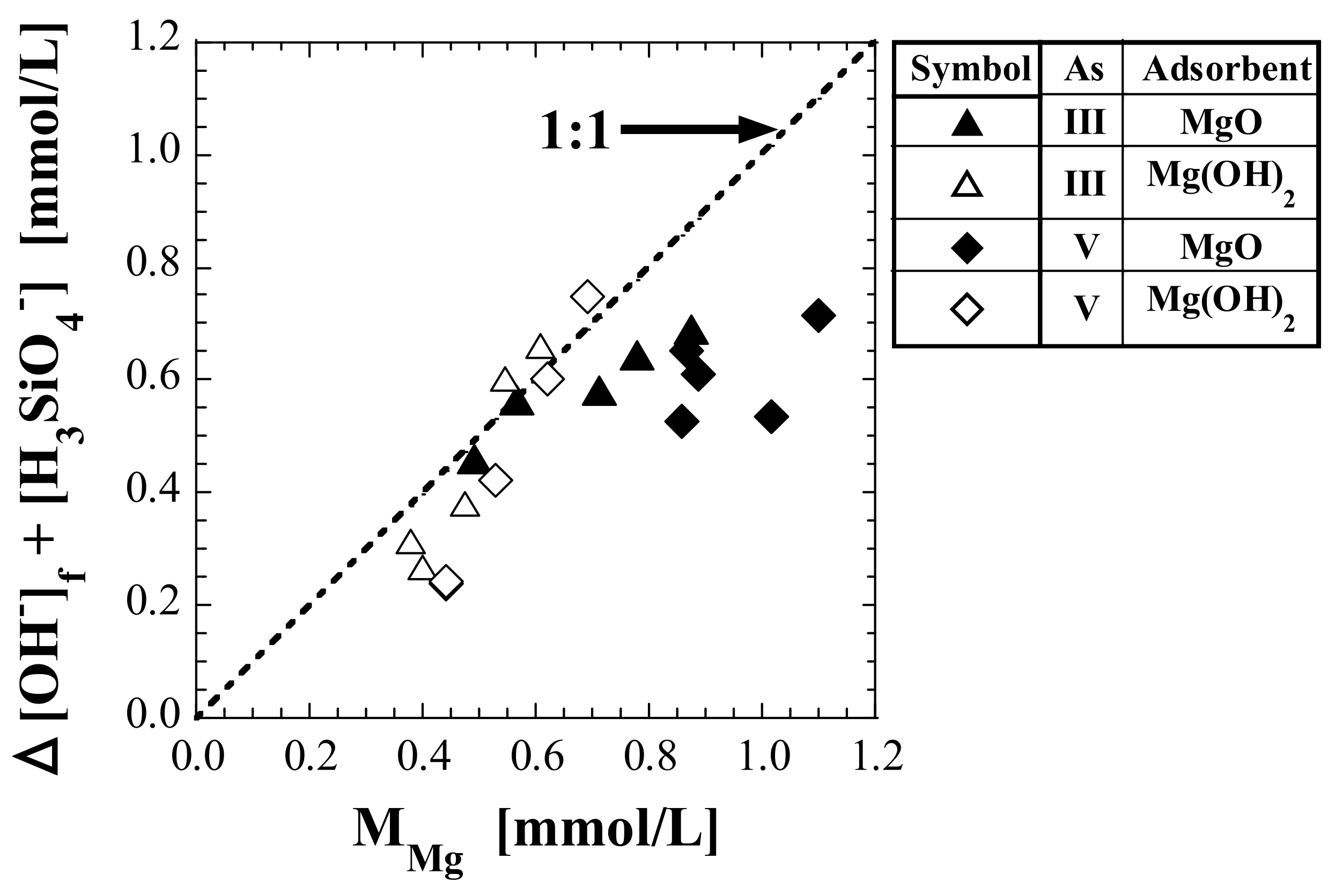
4.4.1. Relationship between Dissolved Components and pH of Eluate
4.4.2. Relationship between Mg Leaching and Silicic Acid Adsorption
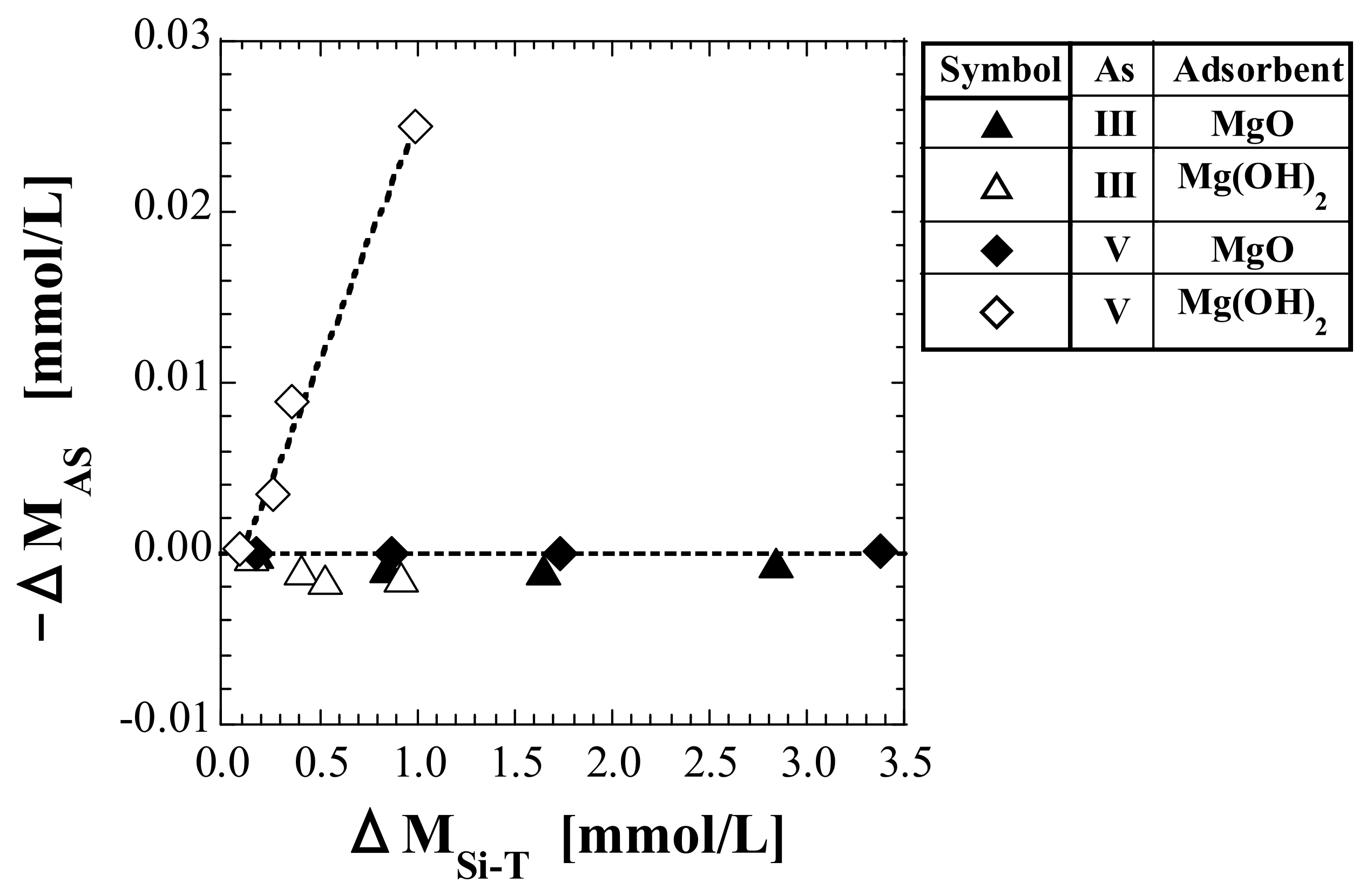
4.4.3. Relationship between As Leaching and Silicic Acid Adsorption
4.4.4. Comparison of Spent Mg-Based and Ca-Based Adsorbents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepch, V.; Divy, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 13, 101079. [Google Scholar] [CrossRef]
- The World Health Organization (WHO). Guidelines for Drinking-Water Quality, Fourth Edition; WHO: Tarxien, Malta, 2011; Arsenic, 315-318; Available online: https://apps.who.int/iris/bitstream/handle/10665/44584/9789241548151_eng.pdf (accessed on 27 December 2021).
- Wirth, G.S.; Gieskes, J.M. The Initial kinetics of the dissolution of vitreous silica in aqueous media. J. Colloid Interface Sci. 1979, 68, 492–500. [Google Scholar] [CrossRef]
- Rothbaum, H.P.; Rohde, A.G. Kinetics of silica polymerization and deposition from dilute solutions between 5 and 180 °C. J. Colloid Interface Sci. 1979, 71, 533–559. [Google Scholar] [CrossRef]
- Marshall, W.L. Amorphous silica solubilities I. Behavior in aqueous salt solutions at 25 °C. Geochim. Cosmochim. Acta 1980, 44, 907–913. [Google Scholar] [CrossRef]
- Bohlmann, E.G.; Mesmer, R.E.; Berlinski, P. Kinetics of silica deposition from simulated geothermal brines. Soc. Pet. Eng. J. 1980, 50, 239–248. [Google Scholar] [CrossRef][Green Version]
- Weres, O.; Yee, A.; Tsao, L. Kinetics of silica polymerization. J. Colloid Interface Sci. 1981, 84, 379–402. [Google Scholar] [CrossRef]
- Fleming, B.A.; Crerar, D.A. Silicic acid ionization and calculation of silica solubility at elevated temperature and pH application to geothermal fluid processing and reinjection. Geothermics 1982, 11, 15–29. [Google Scholar] [CrossRef]
- Fournier, R.O.; Marshall, W.L. Calculation of amorphous silica solubilities at 25° to 300 °C and apparent cation hydration numbers in aqueous salt solutions using the concept of effective density of water. Geochim. Cosmochim. Acta 1983, 47, 587–596. [Google Scholar] [CrossRef]
- Fleming, B.A. Kinetics of reaction between silicic acid and amorphous silica surfaces in NaCl solutions. J. Colloid Interface Sci. 1986, 110, 40–64. [Google Scholar] [CrossRef]
- Sugita, H.; Oguma, T.; Hara, J.; Zhang, M.; Kawabe, Y. Effects of silicic acid on leaching behavior of arsenic from spent calcium-based adsorbents with arsenite. Sustainability 2021, 13, 12937. [Google Scholar] [CrossRef]
- Sugita, H.; Oguma, T.; Zhang, M.; Hara, J.; Kawabe, Y. Effects of silicic acid on environmental stability of spent calcium-based arsenic adsorbents. J. Jpn. Soc. Civ. Eng. Ser. G Environ. Res. 2018, 74, 493–502. [Google Scholar] [CrossRef]
- Ruhaimi, A.H.; Aziz, M.A.A.; Jalil, A.A. Magnesium oxide-based adsorbents for carbon dioxide capture: Current progress and future opportunities. J. CO2 Util. 2021, 43, 101357. [Google Scholar] [CrossRef]
- Tan, C.; Guo, Y.; Sun, J.; Li, W.; Zhang, J.; Zhao, C.; Lu, P. Structurally improved MgO adsorbents derived from magnesium oxalate precursor for enhanced CO2 capture. Fuel 2020, 278, 118379. [Google Scholar] [CrossRef]
- Cheng, W.; Fang, L.; Cheng, H.; Li, E.; Zhang, C.; Cheng, F. Formation of MgCO3 3H2O in the CO2 mineralization system using Mg(OH)2 as an intermediate at 20 °C. J. Ind. Eng. Chem. 2019, 76, 215–222. [Google Scholar] [CrossRef]
- Wu, S.; Tan, B.T.; Senevirathna, H.L.; Wu, P. Polarization of CO2 for improved CO2 adsorption by MgO and Mg(OH)2. Appl. Surf. Sci. 2021, 562, 150187. [Google Scholar] [CrossRef]
- Mohan, S.; Singh, D.K.; Kumar, V.; Hasan, S.H. Modelling of fixed bed column containing graphene oxide decorated by MgO nanocubes as adsorbent for Lead (II) removal from water. J. Water Process Eng. 2017, 17, 216–228. [Google Scholar] [CrossRef]
- Li, B.; Pu, S.; Mandal, S.; Li, M. Viscosity modification enhanced the migration and distribution of colloidal Mg(OH)2 in aquifers contaminated by heavy metals. Environ. Int. 2020, 138, 105658. [Google Scholar] [CrossRef]
- Xiang, J.; Lin, Q.; Yao, X.; Yin, G. Removal of Cd from aqueous solution by chitosan coated MgO-biochar and its in-situ remediation of Cd-contaminated soil. Environ. Res. 2021, 195, 110650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.-D.; Wang, Y.; Zou, X.-H.; Yin, W.-M.; Wang, X.-Y.; Guo, Y.-R.; Pan, Q.-J. Fabrication of cellulose@Mg(OH)2 composite filter via interfacial bonding and its trapping effect for heavy metal ions. Chem. Eng. J. 2021, 426, 130812. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, G.-S.; Xu, M.-D.; Wang, D.-C.; Wang, H.; Zhan, Y.; Jin, Z. Preparation of MgO porous nanoplates modified pumice and its adsorption performance on fluoride removal. J. Alloys Compd. 2021, 884, 160953. [Google Scholar] [CrossRef]
- Falyouna, O.; Bensaida, K.; Maamoun, I.; Ashik, U.P.M.; Tahara, A.; Tanaka, K.; Aoyagi, N.; Sugihara, Y.; Eljamal, O. Synthesis of hybrid magnesium hydroxide/magnesium oxide nanorods [Mg(OH)2/MgO] for prompt and efficient adsorption of ciprofloxacin from aqueous solutions. J. Clean. Prod. 2022, 342, 130949. [Google Scholar] [CrossRef]
- Cao, N.; Zhao, X.; Gao, M.; Li, Z.; Ding, X.; Li, C.; Liu, K.; Du, X.; Li, W.; Feng, J.; et al. Superior selective adsorption of MgO with abundant oxygen vacancies to removal and recycle reactive dyes. Sep. Purif. Technol. 2021, 275, 119236. [Google Scholar] [CrossRef]
- Yunessni, L.A.; Akbari, A. Membrane capsules with hierarchical Mg(OH)2 nanostructures as novel adsorbents for dyeing wastewater treatment in carpet industries. J. Taiwan Inst. Chem. Eng. 2017, 70, 391–400. [Google Scholar] [CrossRef]
- Camacho, J.; Wee, H.-Y.; Kramer, T.A.; Autenrieth, R. Arsenic stabilization on water treatment residuals by calcium addition. J. Hazard. Mater. 2009, 165, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Montes-Hernandeza, G.; Concha-Lozan, N.; Renard, F.; Quirico, E. Removal of oxyanions from synthetic wastewater via carbonation process of calcium hydroxide: Applied and fundamental aspects. J. Hazard. Mater. 2009, 166, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Olyaie, E.; Banejad, H.; Afkhami, A.; Rahmani, A.; Khodaveisi, J. Development of a cost-effective technique to remove the arsenic contamination from aqueous solutions by calcium peroxide nanoparticles. Separ. Purif. Technol. 2012, 95, 10–15. [Google Scholar] [CrossRef]
- Hua, C.-Y.; Lo, S.-L.; Kuan, W.-H. High concentration of arsenate removal by electrocoagulation with calcium. Separ. Purif. Technol. 2014, 126, 7–14. [Google Scholar] [CrossRef]
- Park, Y.Y.; Tran, T.; Lee, Y.H.; Nam, Y., II; Senanayake, G.; Kim, M.J. Selective removal of arsenic (V) from a molybdate plant liquor by precipitation of magnesium arsenate. Hydrometallurgy 2010, 104, 290–297. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Katsikini, M.; Paloura, E.C.; Bantsis, G.; Mitrakas, M. A novel approach for arsenic adsorbents regeneration using MgO. J. Hazard. Mater. 2014, 265, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Luo, T.; Jia, Y.; Zhang, Y.X.; Liu, J.H.; Huang, X.J. Porous hierarchically micro/nanostructured MgO: Morphology control and their excellent performance in As (III) and As (V) removal. J. Phys. Chem. C 2011, 115, 22242–22250. [Google Scholar] [CrossRef]
- Opiso, E.M.; Sato, T.; Morimoto, K.; Asai, A.; Anraku, S.; Numako, C.; Yoneda, T. Incorporation of arsenic during the formation of Mg-bearing minerals at alkaline condition. Miner. Eng. 2010, 23, 230–237. [Google Scholar] [CrossRef]
- Sugita, H.; Oguma, T.; Zhang, M.; Hara, J.; Kawabe, Y. Effects of silicic acid on environmental stability of spent magnesium-based arsenic adsorbents. J. Jpn. Soc. Civ. Eng. Ser. G Environ. Res. 2017, 73, 407–418. [Google Scholar] [CrossRef]
- Sugita, H.; Oguma, T.; Hara, J.; Zhang, M.; Kawabe, Y. Effects of solution pH on spent Mg-based and Ca-based adsorbents-leaching behavior of arsenite and arsenate acids. Jpn. Geotech. J. 2020, 15, 441–453. [Google Scholar] [CrossRef]
- Sugita, H.; Oguma, T.; Zhang, M.; Hara, J.; Takahashi, S. Environmental stability of spent magnesium-based and calcium-based arsenic adsorbents-Effects of soils. J. Jpn. Soc. Civ. Eng. Ser. G Environ. Res. 2016, 72, 437–448. [Google Scholar] [CrossRef]
- The Chemical Society of Japan (CSJ). Kagaku Binran (Handbook of Chemistry), Pure Chemistry II, 4th ed.; Maruzen: Tokyo, Japan, 1993; p. 317. [Google Scholar]
- Fujinaga, T.; Sewkido, E. Ionic Equilibria; Kagakudojin: Kyoto, Japan, 1967; p. 252. [Google Scholar]




| No. | Adsorbent | As(Valence) | WAD/V (g/L) | pH0 | CAS0 (mg/L) | CAS (mg/L) | RAS (%) |
|---|---|---|---|---|---|---|---|
| (1) 1 | MgO | As(III) | 5.004 | 6.99 | 21.792 | 0.149 | 99.3 |
| (2) 1 | Mg(OH)2 | As(III) | 5.007 | 7.10 | 21.854 | 1.013 | 95.4 |
| (3) 2 | MgO | As(V) | 5.028 | 7.14 | 21.160 | 0.016 | 99.9 |
| (4) 2 | Mg(OH)2 | As(V) | 5.012 | 7.11 | 21.065 | 0.024 | 99.9 |
| No. | Adsorbent | As(Valence) | αMg (%) | WMg/V (mg/L) | CMg (mg/L) | βMg (%) |
|---|---|---|---|---|---|---|
| (1) 1 | MgO | As(III) | 59.08 | 2956 | 6.23 | 0.21 |
| (2) 1 | Mg(OH)2 | As(III) | 40.55 | 2030 | 7.80 | 0.38 |
| (3) 2 | MgO | As(V) | 59.08 | 2971 | 10.37 | 0.35 |
| (4) 2 | Mg(OH)2 | As(V) | 40.55 | 2032 | 9.51 | 0.47 |
| No. | Adsorbent | As(Valence) | QAS (mg-As/g) |
|---|---|---|---|
| (1) 1 | MgO | As(III) | 4.32 |
| (2) 1 | Mg(OH)2 | As(III) | 4.16 |
| (3) 2 | MgO | As(V) | 4.20 |
| (4) 2 | Mg(OH)2 | As(V) | 4.20 |
| CSi-T0 (mg/L) | 0 | 5 | 25 | 50 | 100 | ||
|---|---|---|---|---|---|---|---|
| No. | Adsorbent | As(Valence) | pHf | ||||
| (1) | MgO | As(III) | 10.66 | 10.75 | 10.74 | 10.72 | 10.32 |
| (2) | Mg(OH)2 | As(III) | 10.49 | 10.39 | 10.02 | 9.74 | 9.38 |
| (3) 1 | MgO | As(V) | 10.79 | 10.81 | 10.72 | 10.83 | 10.64 |
| (4) 1 | Mg(OH)2 | As(V) | 10.38 | 10.26 | 9.95 | 9.66 | 9.47 |
| CSi-T0 (mg/L) | 0 | 5 | 25 | 50 | 100 | ||
|---|---|---|---|---|---|---|---|
| No. | Adsorbent | As(Valence) | CAS (mg/L) | ||||
| (1) | MgO | As(III) | 0.108 | 0107 | 0.043 | 0.030 | 0.061 |
| (2) | MgOH)2 | As(III) | 0.503 | 0.490 | 0.432 | 0.383 | 0.395 |
| (3) 1 | MgO | As(V) | 0.000 | 0.000 | 0.000 | 0.001 | 0.010 |
| (4) 1 | Mg(OH)2 | As(V) | 0.012 | 0.028 | 0.265 | 0.677 | 1.89 |
| CSi-T0 (mg/L) | 0 | 5 | 25 | 50 | 100 | ||
|---|---|---|---|---|---|---|---|
| No. | Adsorbent | As(Valence) | CMg (mg/L) | ||||
| (1) | MgO | As(III) | 11.9 | 13.7 | 17.2 | 18.9 | 21.3 |
| (2) | Mg(OH)2 | As(III) | 9.1 | 9.7 | 11.5 | 13.3 | 14.8 |
| (3) 1 | MgO | As(V) | 26.9 | 21.0 | 20.8 | 26.7 | 24.7 |
| (4) 1 | Mg(OH)2 | As(V) | 10.7 | 10.7 | 12.9 | 15.1 | 16.7 |
| CSi-T0 (mg/L) | 0 | 5 | 25 | 50 | 100 | ||
|---|---|---|---|---|---|---|---|
| No. | Adsorbent | As(Valence) | CSi-T (mg/L) | ||||
| (1) | MgO | As(III) | 0.00 | 0.08 | 0.95 | 3.90 | 18.3 |
| (2) | Mg(OH)2 | As(III) | 0.00 | 0.77 | 13.1 | 35.4 | 72.2 |
| (3) 1 | MgO | As(V) | 0.00 | 0.15 | 0.15 | 1.41 | 3.24 |
| (4) 1 | Mg(OH)2 | As(V) | 0.00 | 2.43 | 17.1 | 40.3 | 70.2 |
| CSi-T0 (mg/L) | 0 | 5 | 25 | 50 | 100 | ||
|---|---|---|---|---|---|---|---|
| No. | Adsorbent | As(Valence) | EAS (%) | ||||
| (1) | MgO | As(III) | 1.24 | 1.22 | 0.50 | 0.34 | 0.69 |
| (2) | Mg(OH)2 | As(III) | 5.97 | 5.85 | 5.12 | 4.55 | 4.71 |
| (3) 1 | MgO | As(V) | >0.01 | >0.01 | >0.01 | >0.01 | 0.12 |
| (4) 1 | Mg(OH)2 | As(V) | 0.14 | 0.34 | 3.13 | 7.98 | 22.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugita, H.; Oguma, T.; Hara, J.; Zhang, M.; Kawabe, Y. Effects of Silicic Acid on Leaching Behavior of Arsenic from Spent Magnesium-Based Adsorbents Containing Arsenite. Sustainability 2022, 14, 4236. https://doi.org/10.3390/su14074236
Sugita H, Oguma T, Hara J, Zhang M, Kawabe Y. Effects of Silicic Acid on Leaching Behavior of Arsenic from Spent Magnesium-Based Adsorbents Containing Arsenite. Sustainability. 2022; 14(7):4236. https://doi.org/10.3390/su14074236
Chicago/Turabian StyleSugita, Hajime, Terumi Oguma, Junko Hara, Ming Zhang, and Yoshishige Kawabe. 2022. "Effects of Silicic Acid on Leaching Behavior of Arsenic from Spent Magnesium-Based Adsorbents Containing Arsenite" Sustainability 14, no. 7: 4236. https://doi.org/10.3390/su14074236
APA StyleSugita, H., Oguma, T., Hara, J., Zhang, M., & Kawabe, Y. (2022). Effects of Silicic Acid on Leaching Behavior of Arsenic from Spent Magnesium-Based Adsorbents Containing Arsenite. Sustainability, 14(7), 4236. https://doi.org/10.3390/su14074236






