Removal of Reactive Black 5 Dye by Banana Peel Biochar and Evaluation of Its Phytotoxicity on Tomato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Banana Peel Biochar and Proximate Analysis
2.2. Preparation of Dye Stock Solution
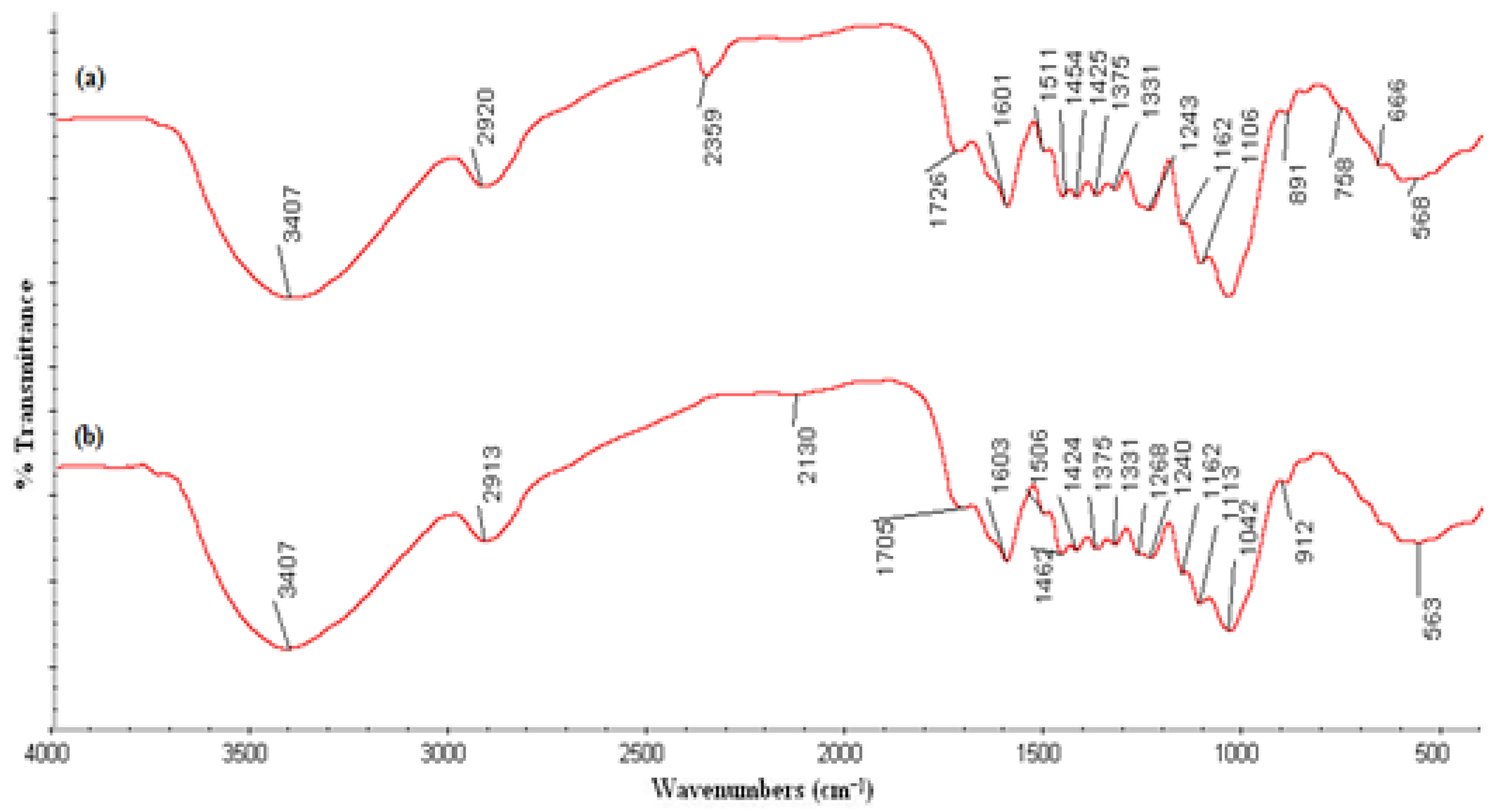
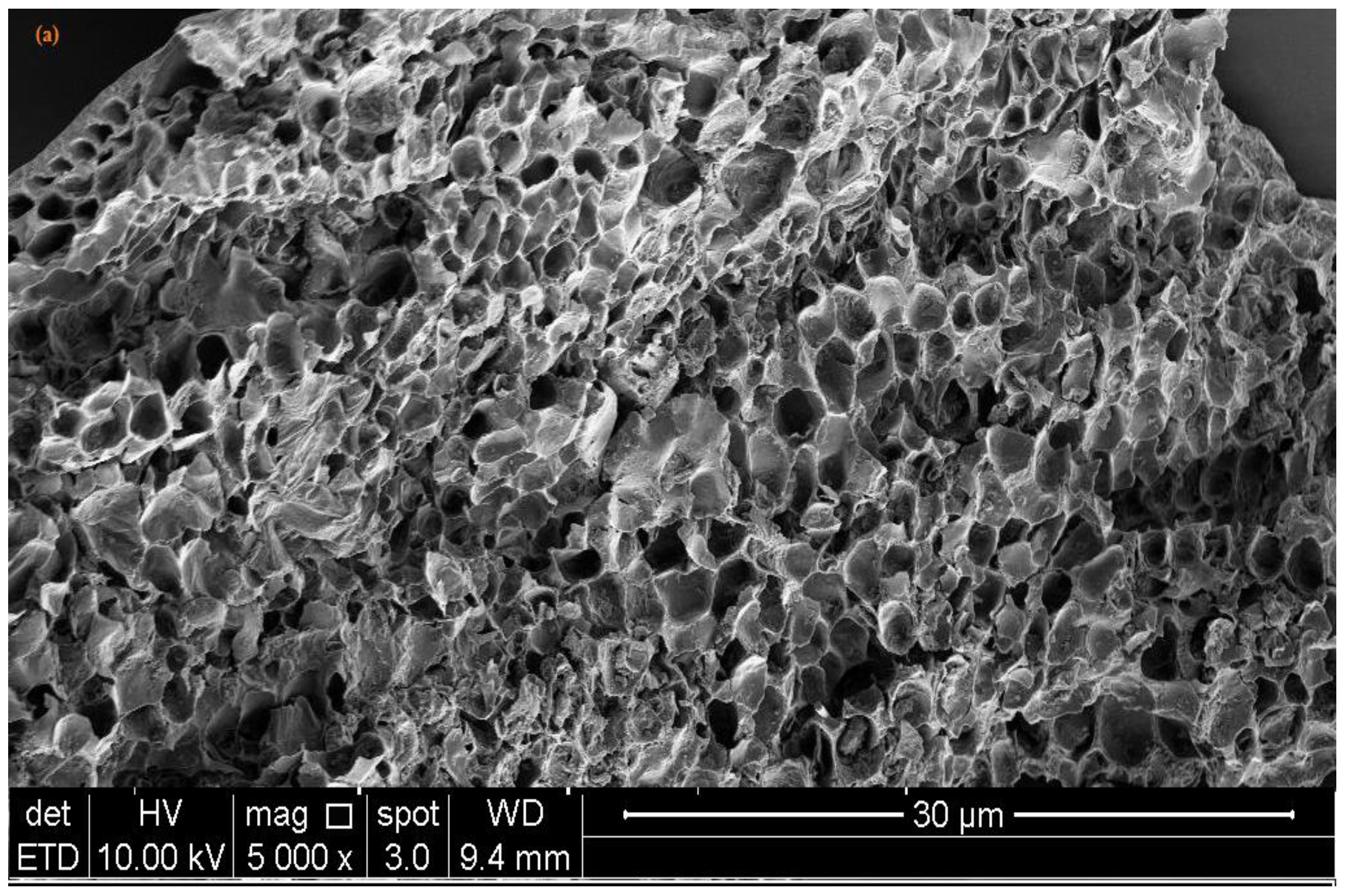
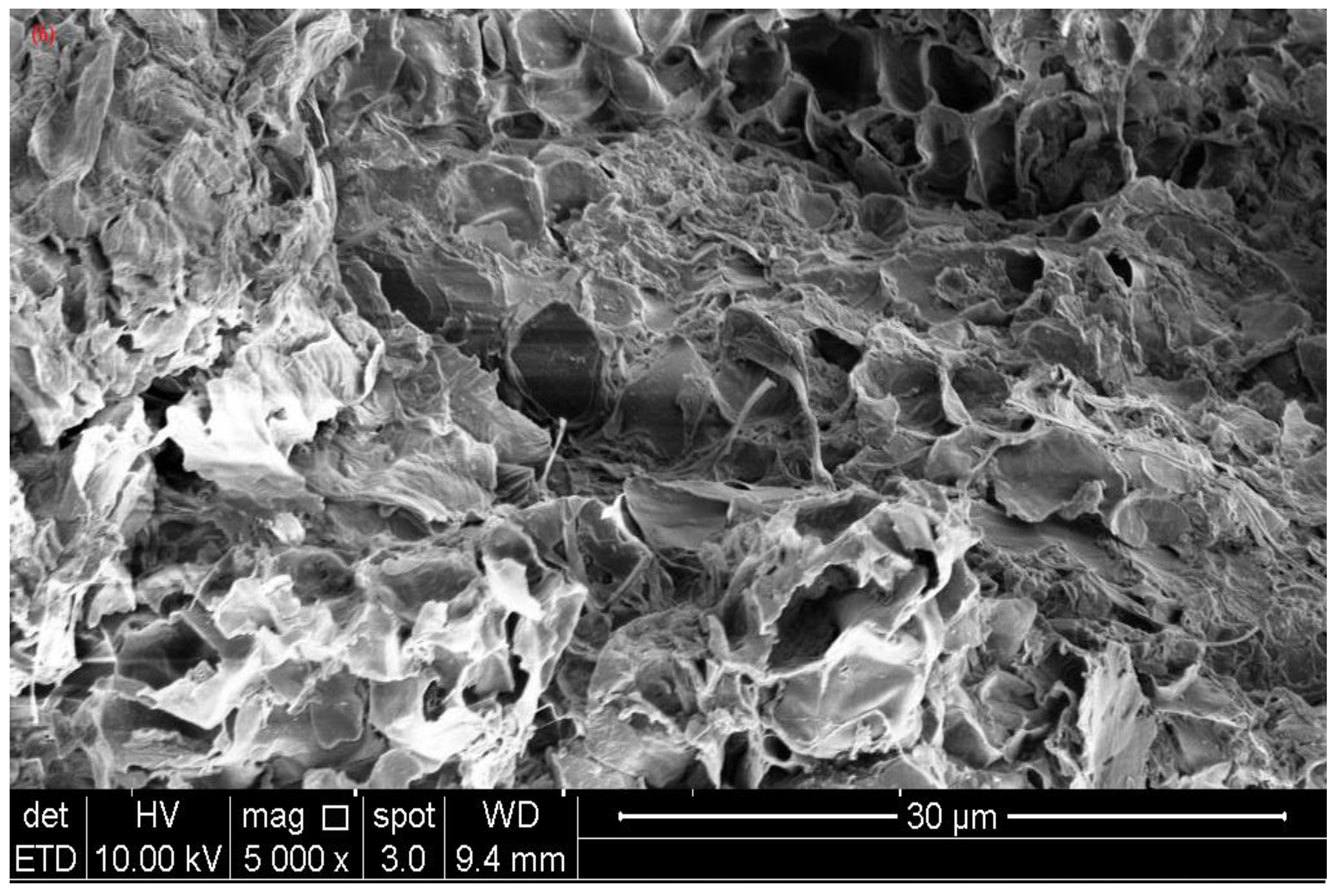
2.3. Characterization of Banana Peel Biochar
2.4. Batch Adsorption Experiments
2.5. Regeneration Analysis
2.6. Evaluation of Phytotoxicity
2.7. Estimation of Biochemical Components
2.8. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis of Banana Peel Biochars
3.2. Characterization of Banana Peel Biochars
3.3. Effect of Different Parameters on Reactive Black 5 Dye Adsorption by Banana Peel Biochar
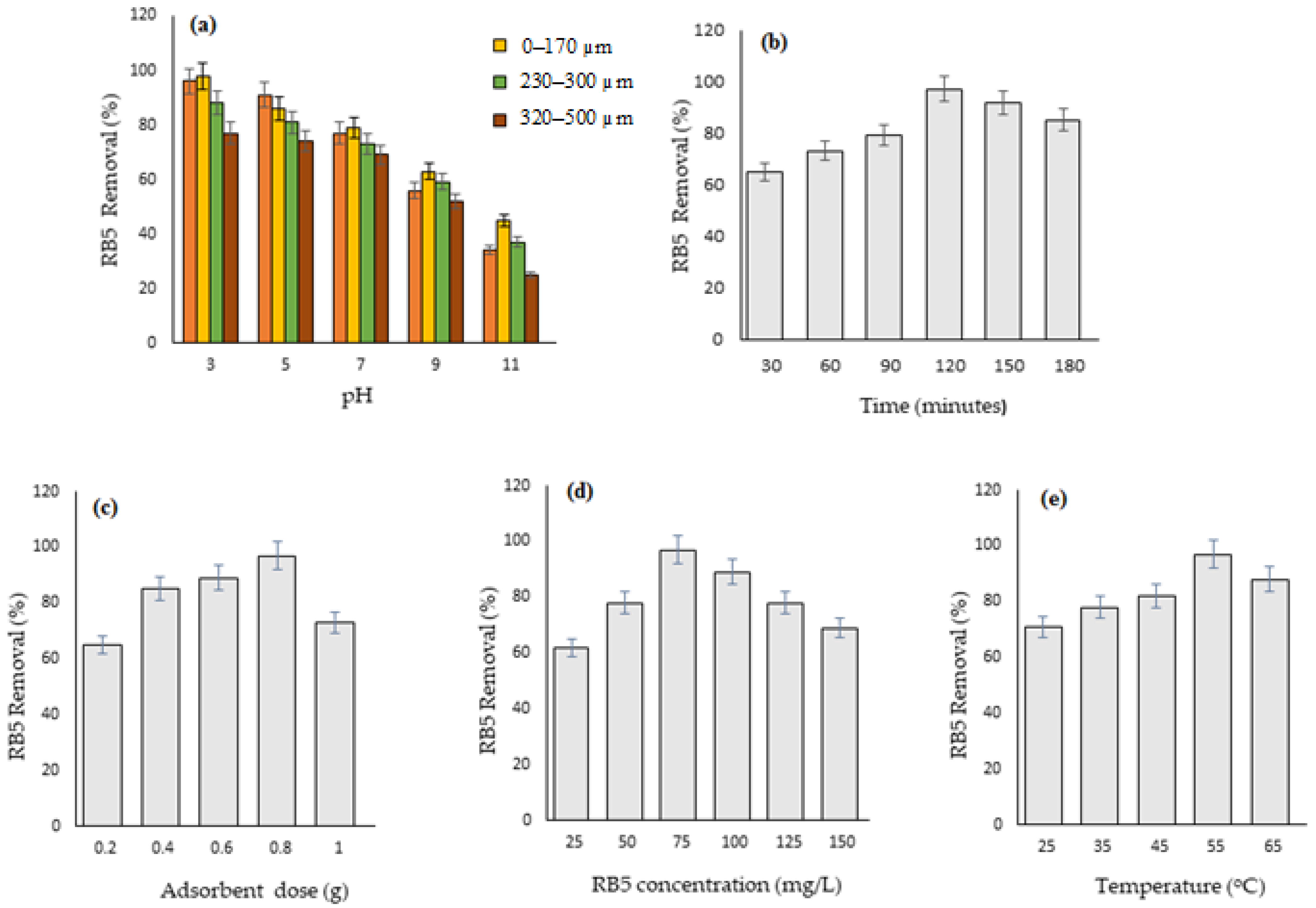
3.3.1. pH
3.3.2. Particle Size
3.3.3. Contact Time
3.3.4. Adsorbent Dose
3.3.5. Dye Concentration
3.3.6. Temperature
3.4. Adsorption Isotherm
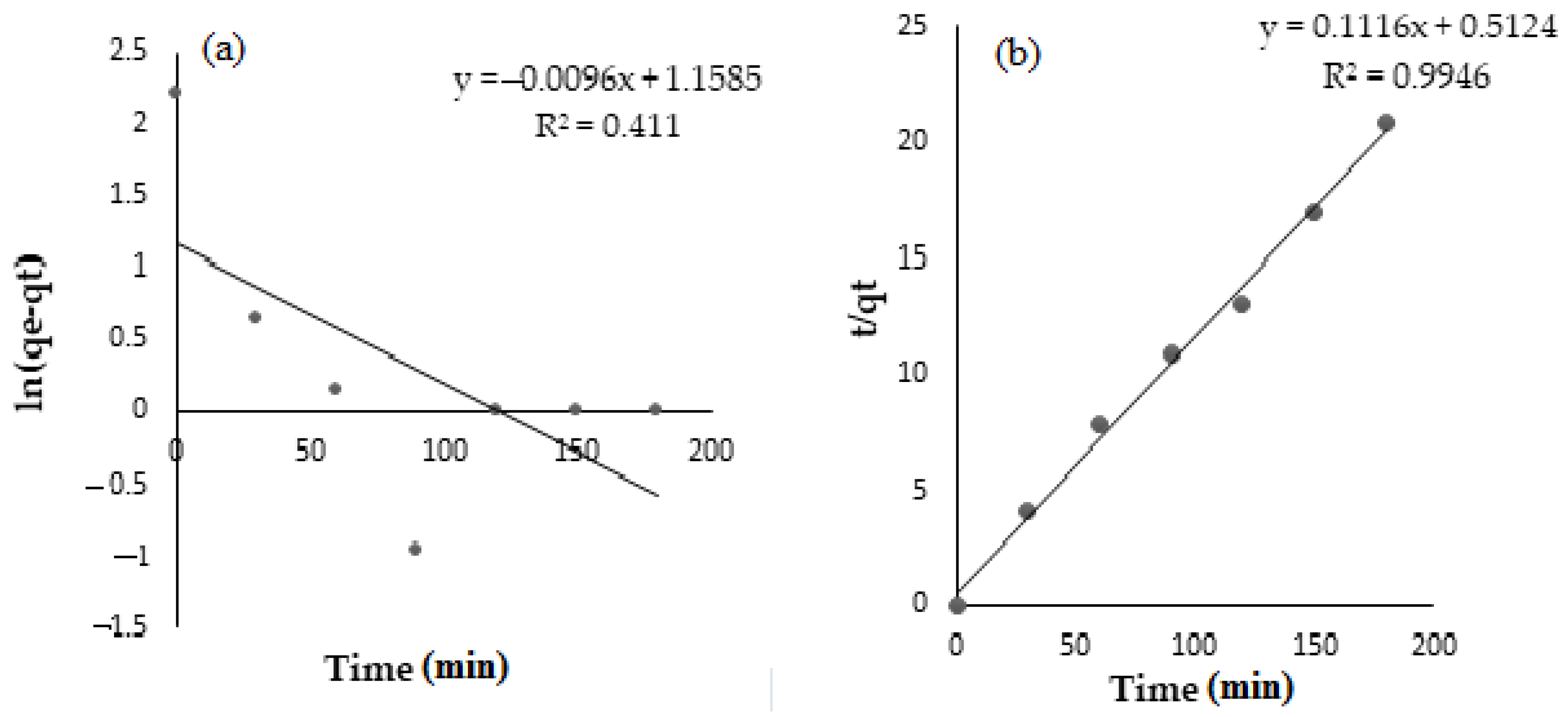
3.5. Adsorption Kinetic Models
3.6. Thermodynamic Analysis
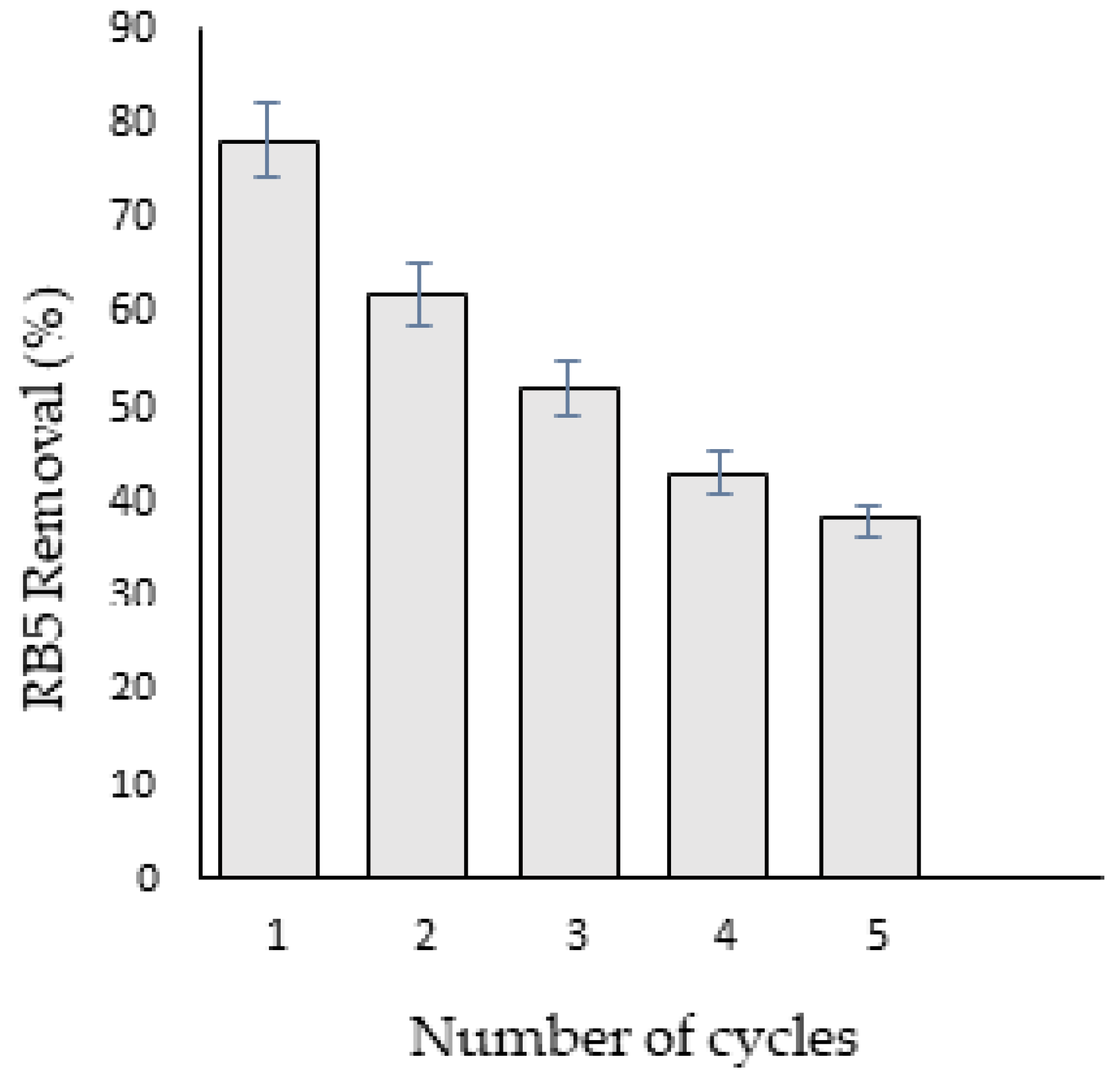
3.7. Regeneration Analysis
3.8. Evaluation of Phytotoxicity
3.9. Performance of Banana Peel Biochars
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BPB | Banana Peel Biochar |
| DMRT | Duncan’s Multiple Range Test |
| FTIR | Fourier Transform Infrared Spectroscopy |
| RB5 | Reactive Black 5 |
| SEM | Scanning Electron Micrograph |
References
- Chowdhary, P.; Bharagava, R.N.; Mishra, S.; Khan, N. Role of industries in water scarcity and its adverse effects on environment and human health. In Environmental Concerns and Sustainable Development: Air, Water and Energy Resources; Shukla, V., Kumar, N., Eds.; Springer: Singapore, 2020; Volume 1, pp. 235–256. [Google Scholar]
- Jawad, A.H.; Hum, N.N.M.F.; Farhan, A.M.; Mastuli, M.S. Biosorption of methylene blue dye by rice (Oryza sativa L.) straw: Adsorption and mechanism study. Desalin. Water Treat. 2020, 190, 322–330. [Google Scholar] [CrossRef]
- Varjani, S.; Rakholiya, P.; Yong, N.H.; You, S.; Teixeira, J.A. Microbial degradation of dyes: An overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef] [PubMed]
- Sackey, E.A.; Song, Y.; Yu, Y.; Zhuang, H. Biochars derived from bamboo and rice straw for sorption of basic red dyes. PLoS ONE 2021, 16, e0254637. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, R.S.; Acharya, S.; Abidi, N. Recent advances in biopolymer-based dye removal technologies. Molecules 2021, 26, 4697. [Google Scholar] [CrossRef]
- Ying, Z.; Chen, X.; Li, H.; Liu, X.; Zhang, C.; Zhang, J.; Yi, G. Efficient adsorption of methylene blue by porous biochar derived from soybean dreg using a one-pot synthesis method. Molecules 2021, 26, 661. [Google Scholar] [CrossRef] [PubMed]
- Jamee, R.; Siddique, R. Biodegradation of synthetic dyes of textile effluent by microorganisms: An environmentally and economically sustainable approach. Eur. J. Microbiol. Immunol. 2019, 9, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golinska, P.; Belbahri, L. Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl, Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Desore, A.; Narula, S.A. An overview on corporate response towards sustainability issues in textile industry. Environ. Dev. Sustain. 2018, 20, 1439–1459. [Google Scholar] [CrossRef]
- Mohamed, R.R.; Abu Elella, M.H.; Sabaa, M.W.; Saad, G.R. Synthesis of an efficient adsorbent hydrogel based on biodegradable polymers for removing crystal violet dye from aqueous solution. Cellulose 2018, 25, 6513–6529. [Google Scholar] [CrossRef]
- Goswami, M.; Chaturvedi, P.; Sonwani, R.K.; Gupta, A.D.; Singhania, R.R.; Giri, B.S.; Rai, B.N.; Singh, H.; Yadav, S.; Singh, R.S. Application of Arjuna (Terminalia arjuna) seed biochar in hybrid treatment system for the bioremediation of Congo red dye. Bioresour. Technol. 2020, 307, 123203. [Google Scholar] [CrossRef]
- Miyah, Y.; Lahrichi, A.; Idrissi, M.; Boujraf, S.; Taouda, H.; Zerrouq, F. Assessment of adsorption kinetics for removal potential of crystal violet dye from aqueous solutions using Moroccan pyrophyllite. J. Assoc. Arab Univ. Basic Appl. Sci. 2017, 23, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Ardila-Leal, L.D.; Poutou-Pinales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. A brief history of colour, the environmental impact of synthetic dyes and removal by using laccases. Molecules. 2021, 26, 3813. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Emran, M.; El-Sadek, M.H.; El-Shanshory, A.A.; Soliman, H.M.A.; Akl, M.A.; Rashad, M. Enhanced removal of cationic dye by eco-friendly activated biochar derived from rice straw. Appl. Water Sci. 2020, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Barathi, S.; Karthik, C.; Nadanasabapathi, S.; Padikasan, I.A. Biodegradation of textile dye Reactive Blue 160 by Bacillus firmus (Bacillaceae: Bacillales) and non-target toxicity screening of their degraded Products. Toxicol. Rep. 2020, 7, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yu, J.; Pang, Y.; Zeng, G.; Deng, Y.; Wang, J.; Ren, X.; Ye, S.; Peng, B.; Feng, H. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Wanyonyi, W.C.; Mulaa, F.J. Alkaliphilic enzymes and their application in novel leather processing technology for next-generation tanneries. Adv. Biochem. Eng. Biotechnol. 2020, 172, 195–220. [Google Scholar] [PubMed]
- Rovira, J.; Domingo, J.L. Human health risks due to exposure to inorganic and organic chemicals from textiles: A review. Environ. Res. 2019, 168, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.Z.; Hussain, M.E.; Hakim, A.; Islam, K.; Uddin, M.N.; Azad, A.K. Biodegradation of reactive textile dye Novacron super black g by free cells of newly isolated Alcaligenes faecalis az26 and Bacillus spp. obtained from textile effluents. Heliyon 2019, 5, e02068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jozwiak, T.; Filipkowska, U.; Szymczyk, P.; Zyśk, M. Effect of the form and deacetylation degree of chitosan sorbents on sorption effectiveness of Reactive Black 5 from aqueous solutions. Int. J. Biol. Macromol. 2017, 95, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Viana, D.F.; Salazar-Banda, G.R.; Leite, M.S. Electrochemical degradation of Reactive Black 5 with surface response and artificial neural networks optimization models. Sep. Sci. Technol. 2018, 53, 2647–2661. [Google Scholar] [CrossRef]
- Parmar, A.; Shah, A. Cytogenotoxicity of azo dye Reactive Red 120 (RR120) on fish Catla catla. Environ. Exp. Biol. 2019, 17, 151–155. [Google Scholar]
- Munagapati, V.S.; Wen, J.C.; Pan, C.L.; Gutha, Y.; Wen, J.H.; Reddy, G.M. Adsorptive removal of anionic dye (Reactive Black 5) from aqueous solution using chemically modified banana peel powder: Kinetic, isotherm, thermodynamic and reusability studies. Int. J. Phytoremediation 2020, 22, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, D.; Sulthana, A.S.; Elangovan, N. Reactive black 5 induced developmental defects via potentiating apoptotic cell death in Zebrafish (Danio rerio) embryos. Pharm. Pharmacol. Int. J. 2018, 6, 449–452. [Google Scholar]
- Gurses, A.; Açıkyıldız, M.; Gunes, K.; Gurses, M.S. Colorants in health and environmental aspects. In Dyes and Pigments; Springer: New York, NY, USA, 2016; pp. 69–83. [Google Scholar]
- Mohanty, S.S.; Kumar, A. Biodegradation of Indanthrene Blue RS dye in immobilized continuous upflow packed bed bioreactor using corncob biochar. Sci. Rep. 2021, 11, 13390. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Meng, Y.; Wei, Z.; DeLaune, R.D.; Seo, D.C. Adsorption/desorption behavior of cationic and anionic dyes by biochars prepared at normal and high pyrolysis temperatures. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 274–282. [Google Scholar] [CrossRef]
- Maqbool, Z.; Shahid, M.; Azeem, F.; Shahzad, T.; Mahmood, F.; Rehman, M.; Ahmed, T.; Imran, M.; Hussain, S. Application of a dye-decolorizing Pseudomonas aeruginosa strain ZM130 for remediation of textile wastewaters in aerobic/anaerobic sequential batch bioreactor and soil columns. Water Air Soil Pollut. 2020, 231, 386. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Safa, Y.; Yakout, S.M.; Shair, O.H.; Iqbal, M.; Nazir, A. Efficient removal of dyes using carboxymethyl cellulose/alginate/polyvinyl alcohol/rice husk composite: Adsorption/desorption, kinetics and recycling studies. Int. J. Biol. Macromol. 2020, 150, 861–870. [Google Scholar] [CrossRef]
- Noreen, S.; Bhatti, H.N.; Iqbal, M.; Hussain, F.; Sarim, F.M. Chitosan, starch, polyaniline and polypyrrole biocomposite with sugarcane bagasse for the efficient removal of Acid Black dye. Int. J. Biol. Macromol. 2020, 147, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Wekoye, J.N.; Wanyonyi, W.C.; Wangila, P.T.; Tonui, M.K. Kinetic and equilibrium studies of Congo red dye adsorption on cabbage waste powder. Environ. Chem. Ecotoxicol. 2020, 2, 24–31. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Abukhadra, M.R.; Shahien, M.G.; Ibrahim, S.S. Novel bentonite/zeolite-NaP composite efficiently removes methylene blue and Congo red dyes. Environ. Chem. Lett. 2018, 16, 275–280. [Google Scholar] [CrossRef]
- Degermenci, G.D.; Degermenci, N.; Ayvaoglu, V.; Durmaz, E.; Çakır, D.; Akan, E. Adsorption of reactive dyes on lignocellulosic waste; characterization, equilibrium, kinetic and thermodynamic studies. J. Cleaner Prod. 2019, 225, 1220–1229. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Comparative study for adsorption of methylene blue dye on biochar derived from orange peel and banana biomass in aqueous solutions. Environ. Monit Assess. 2019, 191, 735. [Google Scholar] [CrossRef] [PubMed]
- Astuti, W.; Sulistyaningsih, T.; Kusumastuti, E.; Thomas, G.; Kusnadi, R.Y. Thermal conversion of pineapple crown leaf waste to magnetized activated carbon for dye removal. Bioresour. Technol. 2019, 287, 121426. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Tumari, H.H.; Ngadi, N.; Mohamed, N.B.; Hassan, O.; Mat, R.; Saidina Amin, N.A. Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine (PEI-STL). J. Clean Prod. 2019, 206, 394–406. [Google Scholar] [CrossRef]
- Li, F.; Zimmerman, A.R.; Hu, X.; Yu, Z.; Huang, J.; Gao, B. One-pot synthesis and characterization of engineered hydrochar by hydrothermal carbonization of biomass with ZnCl2. Chemosphere. 2020, 254, 126866. [Google Scholar] [CrossRef]
- Masto, R.E.; Ansari, M.A.; George, J.; Selvi, V.A.; Ram, L.C. Co-application of biochar and lignite fly ash on soil nutrients and biological parameters at different crop growth stages of Zea mays. Ecol. Eng. 2013, 58, 314–322. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Onyango, M.S.; Wolkersdorfer, C. Application of banana peels nanosorbent for the removal of radioactive minerals from real mine water. J. Environ. Radioact. 2016, 164, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Vilardi, G.; Di Palma, L.; Verdone, N. Heavy metals adsorption by banana peels micro-powder: Equilibrium modeling by non-linear models. Chin. J. Chem. Eng. 2018, 26, 455–464. [Google Scholar] [CrossRef]
- El-Zawahry, M.M.; Abdelghaffar, F.; Abdelghaffar, R.A.; Hassabo, A.G. Equilibrium and kinetic models on the adsorption of Reactive Black 5 from aqueous solution using Eichhornia crassipes/chitosan composite. Carbohydr. Polym. 2016, 136, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Shahzad, T.; Sahar, A.; Hussain, S.; Mahmood, F.; Siddique, M.H.; Siddique, M.A.; Rashid, M.I. Effect of Reactive Black 5 azo dye on soil processes related to C and N cycling. PeerJ 2018, 6, e4802. [Google Scholar] [CrossRef] [PubMed]
- Celebi, H. The applicability of evaluable wastes for the adsorption of Reactive Black 5. Int. J. Environ. Sci. Technol. 2018, 16, 135–146. [Google Scholar] [CrossRef]
- Giri, B.S.; Gun, S.; Pandey, S.; Trivedi, A.; Kapoor, R.T.; Singh, R.P.; Abdeldayem, O.M.; Rene, E.R.; Yadav, S.; Chaturvedi, P.; et al. Reusability of brilliant green dye contaminated wastewater using corncob biochar and Brevibacillus parabrevis: Hybrid treatment and kinetic studies. Bioengineered 2020, 11, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Cebrian, G.; Condon, S.; Manas, P. Physiology of the inactivation of vegetative bacteria by thermal treatments: Mode of action, influence of environmental factors and inactivation kinetics. Foods 2017, 6, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharathi, K.S.; Ramesh, S.P.T. Fixed-bed column studies on biosorption of crystal violet from aqueous solution by Citrullus lanatus rind and Cyperus rotundus. Appl. Water Sci. 2013, 3, 673–687. [Google Scholar] [CrossRef] [Green Version]
- Ng, J.C.Y.; Cheung, W.H.; McKay, G. Equilibrium studies of the sorption of Cu(II) ions onto chitosan. J. Colloid Interface Sci. 2002, 255, 64–74. [Google Scholar]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. Pseudo-second-order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Selvaraju Sivamani, S. Exploring the potential of Eucalyptus citriodora biochar against direct red 31 dye and its phytotoxicity assessment. Biomass Convers. Biorefin. 2021, 24, 1–12. [Google Scholar] [CrossRef]
- Yaashikaaa, P.R.; Senthil Kumar, P.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef]
- Ipeaiyeda, A.R.; Okeoghene Tesi, G. Sorption and desorption studies on toxic metals from brewery effluent using eggshell as adsorbent. Adv. Nat. Sci. 2014, 7, 15–24. [Google Scholar]
- ISTA. International Rules for Seed Testing; International Seed Testing Association, ISTA Secretariat: Bassersdorf, Switzerland, 2008. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Viability and leaching of sugars from germinating seeds by textile, leather and distillery industries. Ind. J. Environ. Protec. 1973, 11, 592–594. [Google Scholar]
- Lichtenthaler, H.K. Chlorophyll and carotenoids: Pigments of photosynthetic biomembranes. In Methods Enzymology; Packer, L., Douce, R., Eds.; Academic Press: San Diego, CA, USA, 1987; pp. 350–382. [Google Scholar]
- Hedge, J.E.; Hofreiter, B.T. Estimation of carbohydrate. In Methods in Carbohydrate Chemistry; Whistler, R.L., Be Miller, J.N., Eds.; Academic Press: New York, NY, USA, 1962; pp. 17–22. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Oyekanmi, A.A.; Ahmad, A.; Hossain, K.; Rafatullah, M. Adsorption of Rhodamine B dye from aqueous solution onto acid treated banana peel: Response surface methodology, kinetics and isotherm studies. PLoS ONE 2019, 14, e0216878. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Park, J.H.; Kim, S.H.; Kang, S.W.; Cho, J.S.; Jeon, J.R.; Lee, Y.B.; Seo, D.C. Sorption behavior of malachite green onto pristine lignin to evaluate the possibility as a dye adsorbent by lignin. Appl. Biol. Chem. 2019, 62, 37. [Google Scholar] [CrossRef]
- Regti, A.; Laamari, M.R.; Stiriba, S.E.; Haddad, M.E. Removal of Basic Blue 41 dyes using Persea americana-activated carbon prepared by phosphoric acid action. Int. J. Ind. Chem. 2017, 8, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Kowalkowska, A.; Jozwiak, T. Utilization of pumpkin (Cucurbita pepo) seed husks as a low-cost sorbent for removing anionic and cationic dyes from aqueous solutions. Desalin. Water Treat. 2019, 171, 397–407. [Google Scholar] [CrossRef]
- Wong, S.; Ghafar, N.A.; Ngadi, N.; Razmi, F.A.; Inuwa, I.M.; Mat, R.; Amin, N.A.S. Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci. Rep. 2020, 10, 2928. [Google Scholar] [CrossRef] [Green Version]
- Felista, M.M.; Wanyonyi, W.C.; Ongera, G. Adsorption of anionic dye (Reactive black 5) using macadamia seed husks: Kinetics and equilibrium studies. Sci. Afr. 2020, 7, e00283. [Google Scholar] [CrossRef]
- Karthick, K.; Namasivayam, C.; Pragasam, L.A. Removal of direct red 12B from aqueous medium by ZnCl2 activated Jatropha husk carbon: Adsorption dynamics and equilibrium studies. Ind. J. Chem. Technol. 2017, 24, 73–81. [Google Scholar]
- Akter, M.; Rahman, F.B.A.; Abedin, M.Z.; Kabir, S.M.F. Adsorption characteristics of banana peel in the removal of dyes from textile effluent. Textiles 2021, 1, 361–375. [Google Scholar] [CrossRef]
- Jozwiak, T.; Filipkowska, U.; Bugajska, P.; Kalkowski, T. The use of coconut shells for the removal of dyes from aqueous solutions. J. Ecol. Eng. 2018, 19, 129–135. [Google Scholar] [CrossRef]
- Jozwiak, T.; Filipkowska, U.; Brym, S.; Kope’c, L. Use of aminated hulls of sunflower seeds for the removal of anionic dyes from aqueous solutions. Int. J. Environ. Sci. Technol. 2020, 17, 1211–1224. [Google Scholar] [CrossRef] [Green Version]
- Samarghandy, M.R.; Hoseinzade, E.; Taghavi, M.; Hoseinzadeh, S. Biosorption of reactive black 5 from aqueous solution using acid-treated biomass from potato peel waste. Bioresources 2011, 6, 4840–4855. [Google Scholar]
- Eren, Z.; Acar, F.N. Adsorption of Reactive Black 5 from an aqueous solution: Equilibrium and kinetic studies. Desalination 2006, 194, 1–10. [Google Scholar] [CrossRef]
- Do Nascimento, B.F.; de Araujo, C.M.B.; do Nascimento, A.C.; da Costa, G.R.B.; Gomes, B.F.M.L.; da Silva, M.P.; da Motta Sobrinho, M.A. Adsorption of Reactive Black 5 and Basic Blue 12 using biochar from gasification residues: Batch tests and fixed-bed breakthrough predictions for wastewater treatment. Bioresour. Technol. Rep. 2021, 15, 100767. [Google Scholar] [CrossRef]
- Shah, J.A.; Mirza, C.R.; Butt, T.A.; Khalifa, W.M.A.; Gasmi, H.H.; Haroon, H.; Khan, M.S.; Ali, M.A.; Zeb, I.; Shah, S.H.; et al. Tobacco stalk waste biomass holds multilayer and spontaneous adsorption capabilities for Reactive Black 5 dye: Equilibrium modelling and error function analysis. Pol. J. Environ. Stud. 2021, 30, 2301–2312. [Google Scholar] [CrossRef]

| Dye | RB5 |
|---|---|
| Chemical name | Remazol Black B |
| Solubility | High solubility in water, easily forms covalent bonds with cellulosic fibers, resistant to sunlight and aerobic decomposition |
| Melting point | >300 °C |
| CAS number | 17095-24-8 |
| Dye type | Anionic dye |
| Appearance | Black colored powder |
| IUPAC Name | 4-amino-5-hydroxy-3,6-bis[[4-(2-sulfonatooxyethylsulfonyl)phenyl]diazenyl]naphthalene-2,7-disulfonate |
| Empirical Formula | C26H21N5Na4O19S6 |
| Molecular Weight | 991.82 g/mol |
| Molecular Structure | 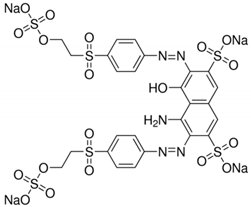 |
| λmax | 597 nm |
| Isotherm | Equation | Parameters | Value |
|---|---|---|---|
| Langmuir | Ce/qe = 1/qe KL + Ce/qm | qm (mg/g) | 7.58 |
| KL (l/mg) | 0.0053 | ||
| R2 | 0.9489 | ||
| Freundlich | In qe = In KF + (1/n) In Ce | 1/n | 7.813 |
| KF (mg/g) | 1.90294 | ||
| R2 | 0.4471 |
| Model | Equation | Parameters | Value |
|---|---|---|---|
| Pseudo-first order | In (qe−qt) = In qe − k1 t | k1 (min−1) | 0.0096 |
| qe (mg/g) | 3.1852 | ||
| R2 | 0.4111 | ||
| Pseudo-second order | t/qt = 1/k2 qe + t/qe | K2 (g/mg min) | 0.0243 |
| qe (mg/g) | 8.9606 | ||
| R2 | 0.9946 |
| S.No. | Temperature (°C) | ∆G° (kJ/mol) | ΔH° (kJ mol−1) | ΔS° (J/K) |
|---|---|---|---|---|
| 1. | 25 | −2363.79 | −11.223 | 30.457 |
| 2. | 35 | −1506.13 | ||
| 3. | 45 | −30.21 | ||
| 4. | 55 | −3754.22 | ||
| 5. | 65 | −596.03 |
| Treatment | Germination (%) | Length of Plumule (cms) | Length of Radicle (cms) | Vigor index | Total Chlorophyll (mg/g FW) | Sugar Content (mg/g DW) | Protein Content (mg/g FW) |
|---|---|---|---|---|---|---|---|
| Control | 96 ± 1.41 a | 13.67 ± 0.57 a | 2.97 ± 0.29 a | 15974.4 a | 3.28 ± 0.36 a | 3.74 ± 0.32 a | 19.03 ± 0.57 a |
| RB5 dye solution (75 mg/L) | 10 ± 0.71 c | 1.93 ± 0.18 d | 0.19 ± 0.01 c | 212 d | 0.99 ± 0.09 c | 1.07 ± 0.06 c | 4.53 ± 0.16 d |
| BPB-treated RB5 dye solution | 81 ± 1.1 b | 11.4 ± 0.74 a | 2.4 ± 0.49 a | 11178 b | 2.33 ± 0.35 b | 2.31 ± 0.32 b | 15.57 ± 0.47 b |
| Biochars | Optimum Experimental Conditions | qmax (mg/g) | References |
|---|---|---|---|
| Coconut shell | pH = 2; exposure time = 60 min | 0.82 | Jozwiak et al. [69] |
| Pumpkin seed husk | pH = 3; exposure time = 60 min | 1 | Kowalkowska and Jozwiak [64] |
| Macadamia seed husk | pH = 3; exposure time = 510 min | 1.21 | Felista et al. [66] |
| Cotton fibers | pH = 3; exposure time = 240 min | 2.74 | Jozwiak et al. [70] |
| Potato peel | pH = 3; exposure time = 120 min | 3.61 | Samarghandy et al. [71] |
| Fly ash | pH = 5.64; exposure time = 60 min | 7.94 | Eren and Acar [72] |
| Pumpkin seed hulls | pH = 2; exposure time = 30 min | 9.18 | Celebi [46] |
| Eggshells | pH = 6; exposure time = 15 min | 18.46 | Celebi [46] |
| Coffee waste | pH = 7; exposure time = 50 min | 77.52 | Wong et al. [65] |
| Wood waste | Temperature = 25 °C; exposure time = 90 min | 35.67 | Figueiredo Do Nascimento [73] |
| Tobacco stalk biomass | pH = 2; exposure time = 120 min | 92.84 | Shah et al. [74] |
| Banana peel biochars | pH = 3; exposure time = 120 min | 7.58 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapoor, R.T.; Rafatullah, M.; Siddiqui, M.R.; Khan, M.A.; Sillanpää, M. Removal of Reactive Black 5 Dye by Banana Peel Biochar and Evaluation of Its Phytotoxicity on Tomato. Sustainability 2022, 14, 4176. https://doi.org/10.3390/su14074176
Kapoor RT, Rafatullah M, Siddiqui MR, Khan MA, Sillanpää M. Removal of Reactive Black 5 Dye by Banana Peel Biochar and Evaluation of Its Phytotoxicity on Tomato. Sustainability. 2022; 14(7):4176. https://doi.org/10.3390/su14074176
Chicago/Turabian StyleKapoor, Riti Thapar, Mohd Rafatullah, Masoom Raza Siddiqui, Moonis Ali Khan, and Mika Sillanpää. 2022. "Removal of Reactive Black 5 Dye by Banana Peel Biochar and Evaluation of Its Phytotoxicity on Tomato" Sustainability 14, no. 7: 4176. https://doi.org/10.3390/su14074176
APA StyleKapoor, R. T., Rafatullah, M., Siddiqui, M. R., Khan, M. A., & Sillanpää, M. (2022). Removal of Reactive Black 5 Dye by Banana Peel Biochar and Evaluation of Its Phytotoxicity on Tomato. Sustainability, 14(7), 4176. https://doi.org/10.3390/su14074176









