Benthic Macroinvertebrates and Zooplankton Communities as Ecological Indicators in Urban Wetlands of Argentina
Abstract
:1. Introduction
2. Materials and Methods
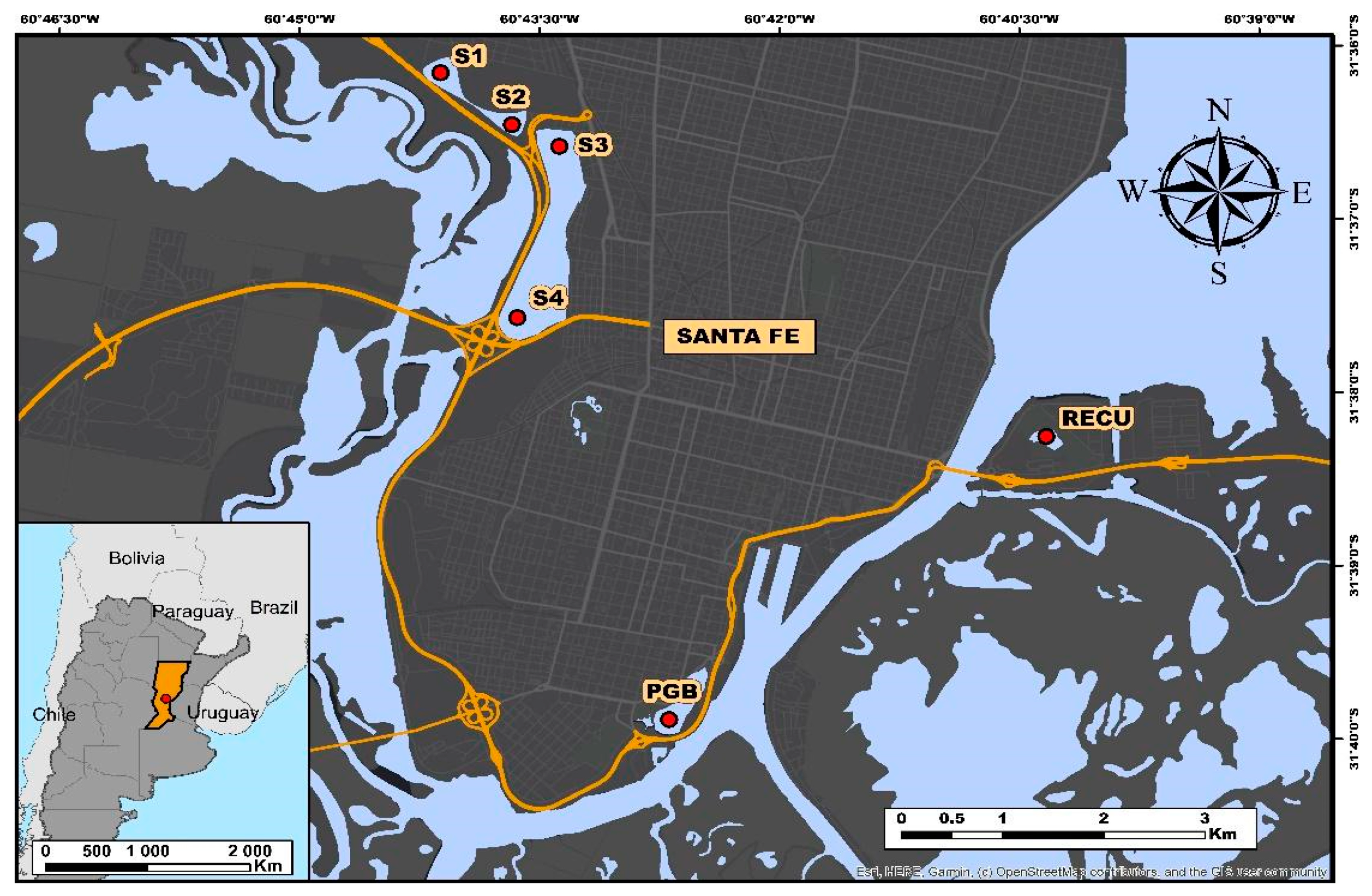
2.1. Study Area
2.2. Field Sampling
2.3. Environmental Variables
2.4. Data Analysis
3. Results
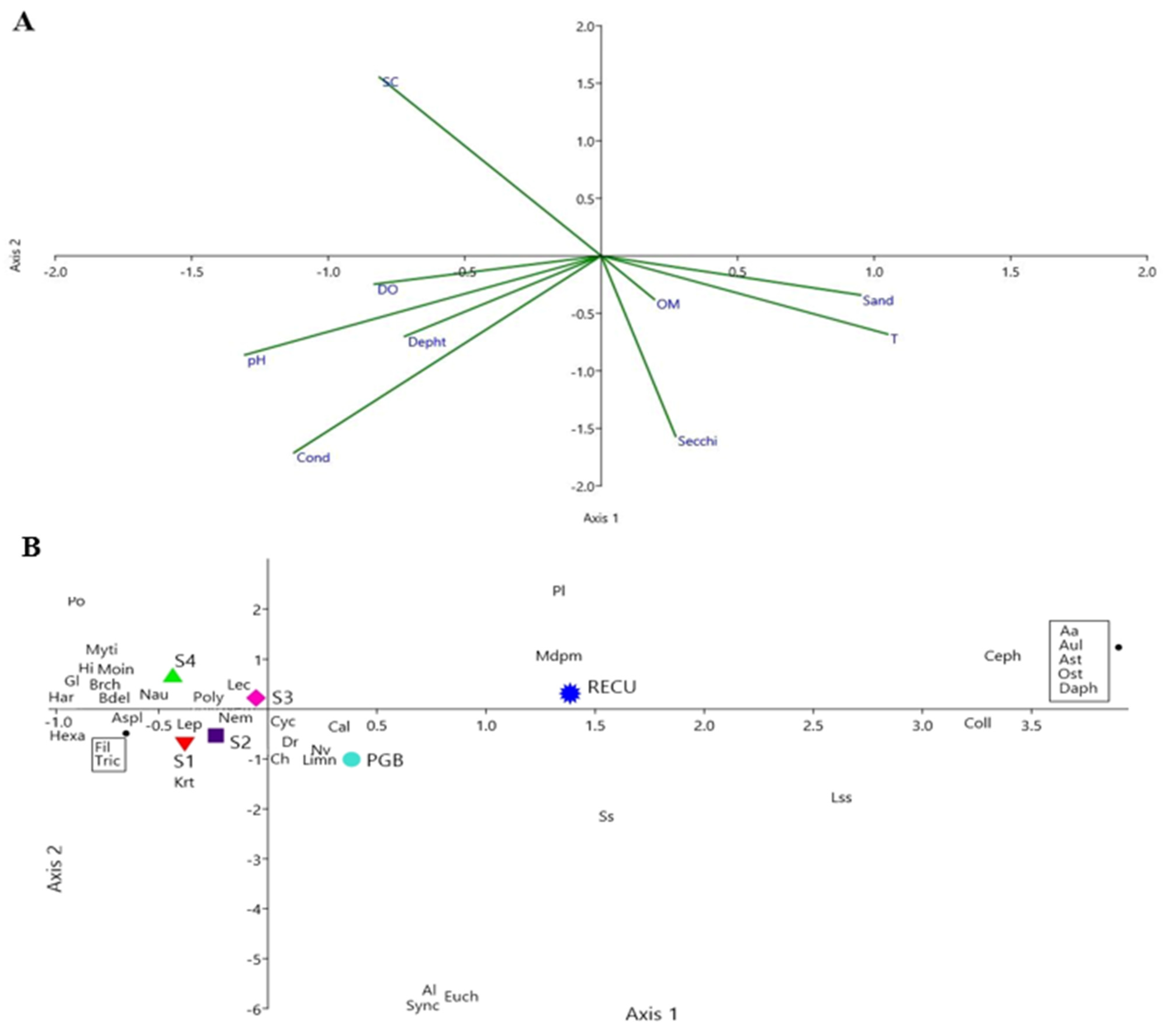
3.1. Environmental Variables
3.2. Benthic Macroinvertebrates and Zooplankton Communities
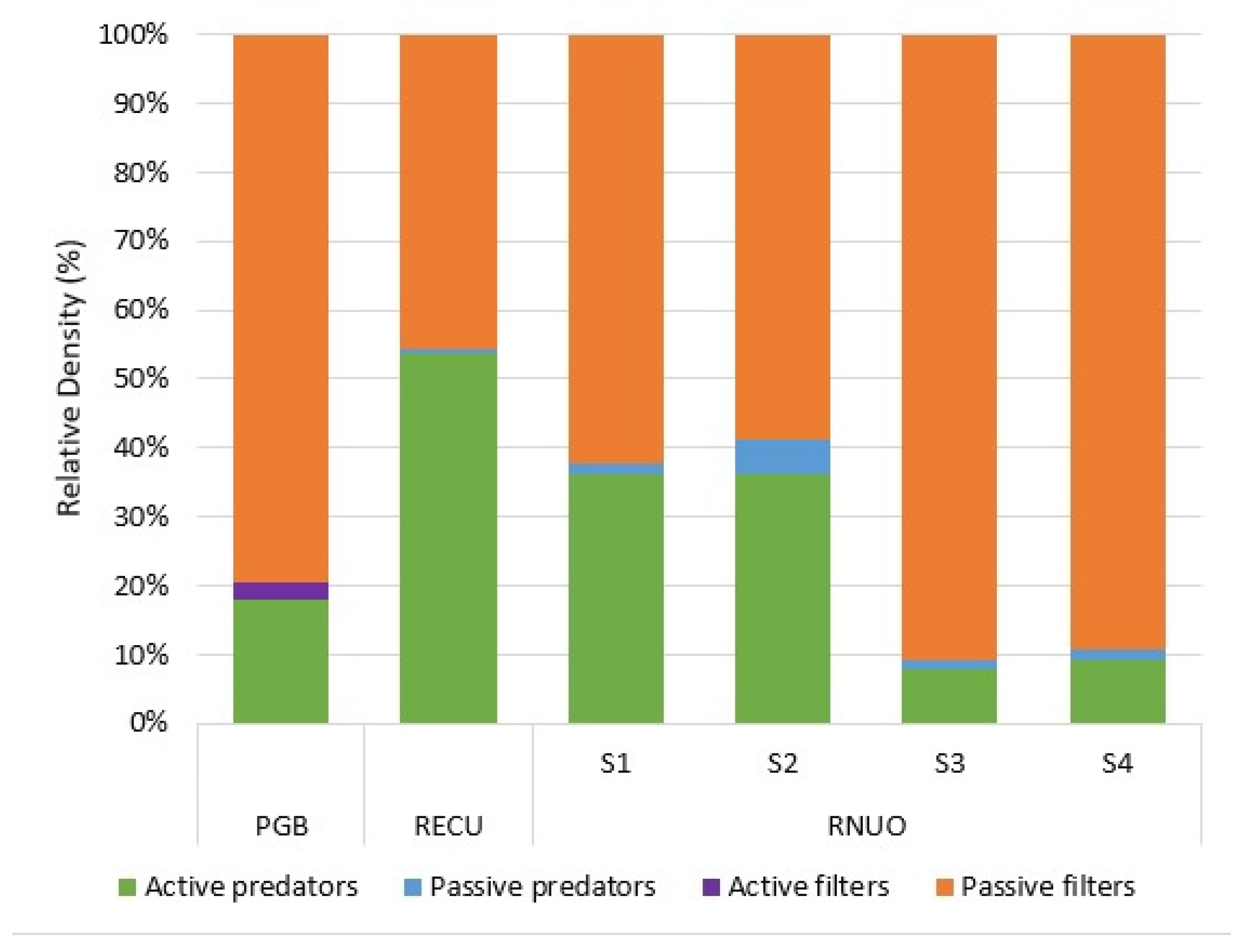
3.3. Functional Feeding Groups
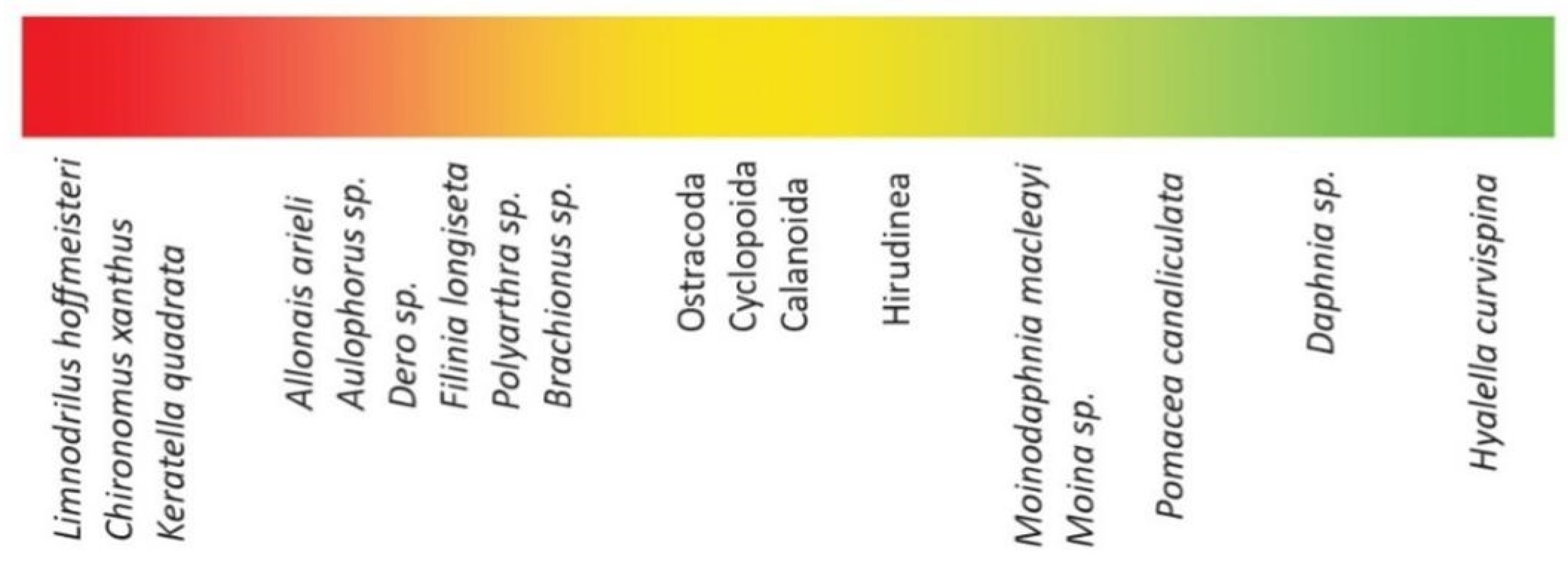
3.4. Biotic Indices
4. Discussion
4.1. Environmental Variables
4.2. Benthic Macroinvertebrates and Zooplankton Communities
4.3. Functional Feeding Groups
4.4. Biotic Indices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Physicochemical parameter | |
| T | Temperature (°C) |
| pH | pH |
| Cond | Conductivity (µS·cm−1) |
| DO | Dissolved Oxygen (mg·L−1) |
| Secchi | Transparency (cm) |
| Depth | Depth (cm) |
| Sand | % Sand |
| SC | % Silt and clay |
| CPOM | % Coarse Particulate Organic Matter |
| FPOM | % Fine Particulate Organic Matter |
| UFPOM | % Ultra Fine Particulate Organic Matter |
| Species | |
| Asc | Ascomorpha sp. |
| Aspl | Asplanchna sp. |
| Bdel | Bdelloidea sp. |
| Bang | Brachionus angularis |
| Bcau | Brachionus caudatus |
| Bhav | Brachionus havanensis |
| Bcal | Brachionus calyciforus |
| Bbud | Brachionus budapestinensis |
| Bqua | Brachionus quadridentatus |
| Brub | Brachionus rubens |
| Bsp | Brachionus sp. |
| Ceph | Cephalodella sp. |
| Coll | Collurela sp. |
| Euch | Euchlanis sp. |
| Flon | Filinia longiseta |
| Hexa | Hexarthra sp. |
| Kqua | Keratella quadrata |
| Ktro | Keratella tropica |
| Ksp | Keratella sp. |
| Lbul | Lecane bulla |
| Lecham | Lecane hamate |
| Lsp | Lecane sp. |
| Lphy | Lecane phyrra |
| Lpat | Lepadella patella |
| Lrho | Lepadella romboides |
| Meu | Manfredium eudactyla |
| Myti | Mytilina sp. |
| Ppat | Platyas patulus |
| Pqua | Platyas quadricornis |
| Ptyp | Platyonus patulus |
| Poly | Polyarthra sp. |
| Syn | Synchaeta sp. |
| Tric | Trichocerca sp. |
| Test | Testudinella sp. |
| Almon | Alona monocantha |
| Al | Alona sp. |
| Daphmdd | Daphnia middendorfiana |
| Jcla | Juvenile cladocera |
| Mmic | Moina micrura |
| Mmin | Moina minuta |
| Mret | Moina reticulata |
| Msp | Moina sp. |
| Mdpm | Moinodaphnia macleayi |
| Nau | Nauplio |
| Cal | Orden Calanoida |
| Cyc | Orden Cyclopoida |
| Har | Orden Harpactoidea |
| Ch | Chironomus sp. |
| Chs | Chironomus sancticaroli |
| Gl | Goeldichironomus luridus |
| Lss | Larsia sp. |
| Lh | Limnodrilus hoffmeisteri |
| Lc | Limnodrilus claparedeianus |
| Limn | Limnodrilus sp. |
| Do | Dero obtusa |
| Dd | Dero digitata |
| Ds | Dero sawayai |
| Aul | Aulophorus furcatus |
| Db | Dero botrytis |
| Dr | Dero sp. |
| Ss | Slavinia sawayai |
| Nv | Nais variabilis |
| Aa | Allonais arieli |
| Hi | Hirudinea |
| Me | Mermithidae |
| Ne | Nematoda |
| Ost | Ostracoda |
Appendix A
| Species | PGB | RECU | S1 | S2 | S3 | S4 |
|---|---|---|---|---|---|---|
| Chironomus sp. | x | x | ||||
| Chironomus xanthus | x | x | x | x | x | x |
| Goeldichironomus luridus | x | x | x | |||
| Polypedium sp. | x | |||||
| Ceratopogonidae | x | |||||
| Monopelopia sp. | x | |||||
| Larsia sp. | x | x | ||||
| Bothrioneurum sp. | x | |||||
| Limnodrilus hoffmeisteri | x | x | x | x | x | |
| Limnodrilus claparedeianus | x | x | x | x | x | |
| Dero obtuse | x | x | x | x | x | |
| Dero digitata | x | x | x | x | x | |
| Aulophorus furcatus | x | |||||
| Dero sawayai | x | x | x | x | x | x |
| Dero botrytis | x | x | ||||
| Slavina sawayai | x | x | x | |||
| Nais variabilis | x | x | x | x | x | x |
| Nais shubarti | x | |||||
| Allonais arieli | x | |||||
| Hirudinea | x | x | x | x | ||
| Mermithidae | x | x | ||||
| Nematoda | x | x | x | |||
| Cyclopoida | x | x | x | x | x | x |
| Calanoida | x | x | x | |||
| Daphnia sp. | x | |||||
| Moina reticulata | x | x | x | x | ||
| Moinodaphnia macleayi | x | x | x | x | ||
| Chydorus sp. | x | |||||
| Ostracoda | x | |||||
| Hyalella curvispina | x | x | ||||
| Pomacea sp. | x | x | ||||
| Planorbidae | x | x |
| Species | PGB | RECU | S1 | S2 | S3 | S4 |
|---|---|---|---|---|---|---|
| Ascomorpha sp. | x | x | ||||
| Asplanchna sp. | x | x | x | x | x | x |
| Bdelloidea sp. | x | x | x | x | x | x |
| Brachionus angularis | x | x | x | x | x | x |
| Brachionus caudatus | x | x | x | x | x | x |
| Brachionus caudatus alstromi | x | |||||
| Brachionus havanensis | x | x | x | x | x | |
| Brachionus calyciflorus | x | x | x | x | x | |
| Brachionus budapestinensis | x | x | x | x | x | |
| Brachionus quadridentatus | x | x | x | x | x | |
| Brachionus Rubens | x | x | ||||
| Cephalodella sp. | x | x | ||||
| Colurella sp. | x | x | ||||
| Euchlanis sp. | x | |||||
| Filinia sp. | x | |||||
| Filinia longiseta | x | x | x | x | x | x |
| Hexarthra sp. | x | x | x | x | x | |
| Keratella quadrata | x | x | ||||
| Keratella tropica | x | x | x | x | x | |
| Keratella valga | x | |||||
| Lecane acronycha | x | x | ||||
| Lecane bulla | x | x | x | |||
| Lecane closterocerca | x | x | ||||
| Lecane hamata | x | x | x | x | x | |
| Lecane hastata | x | |||||
| Lecane papuana | x | |||||
| Lecane phyrra | x | |||||
| Lecane sp. | ||||||
| Lepadella patella | ||||||
| Lepadella romboides | x | x | ||||
| Manfredium eudactyla | x | |||||
| Mytilina sp. | x | x | x | |||
| Platyas patulus | x | |||||
| Platyas quadricornis | x | |||||
| Platyonus patulus | x | x | ||||
| Polyarthra sp. | x | x | x | x | x | x |
| Synchaeta sp. | x | |||||
| Trichocerca sp. | x | x | x | x | x | |
| Testudinella sp. | x | x | x | |||
| Alona monocantha | x | x | x | |||
| Alona sp. | x | |||||
| Daphnia middendorfiana | x | |||||
| Daphnia sp. 1 | x | |||||
| Daphnia sp. 2 | x | |||||
| Guernella raphaelis | x | |||||
| Moina reticulata | x | x | x | x | ||
| Moina micrura | x | x | x | |||
| Moina minuta | x | x | x | x | ||
| Moina reticulata | x | |||||
| Moinodaphnia macleayi | x | x | x | x | ||
| Pleuroxus similis | x | |||||
| Pleuroxus sp. | x | |||||
| Juvenile Cladocera | x | |||||
| Nauplii | x | x | x | x | x | x |
| Juvenile Copepoda | x | x | ||||
| Cyclopoida | x | x | x | x | x | x |
| Calanoida | x | x | x | |||
| Harpacticoida | x | x | x |
References
- United Nations. World Urbanization Prospects: 2014 Revision, Department of Economic and Social Affairs, Population Division; United Nations: New York, NY, USA, 2014. [Google Scholar]
- OECD. OECD Environmental Outlook to 2050: The Consequences of Inaction; OECD: Paris, France, 2012. [Google Scholar]
- Ciacci, A.; Ivaldi, E.; González-Relaño, R. A Partially Non-Compensatory Method to Measure the Smart and Sustainable Level of Italian Municipalities. Sustainability 2021, 13, 435. [Google Scholar] [CrossRef]
- Cardinale, B.; Duffy, J.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2021, 486, 59–67. [Google Scholar] [CrossRef]
- Harrison, P.A.; Berry, P.M.; Simpson, G.; Haslett, J.R.; Blicharska, M.; Bucur, M.; Dunford, R.; Egoh, B.; Garcia-Llorente, M.; Geamănă, N.; et al. Linkages between biodiversity attributes and ecosystem services: A systematic review. Ecosyst. Serv. 2014, 9, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Kowarik, I.; Fischer, L.K.; Kendal, D. Biodiversity Conservation and Sustainable Urban Development. Sustainability 2020, 12, 4964. [Google Scholar] [CrossRef]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Ikin, C.; Shanahan, D.F.; Garrard, D.E.; Bekessy, S.A.; Fuller, R.A.; Mumaw, L.; Rayner, L.; et al. Cities are hotspots for threatened species. Glob. Ecol. Biogeogr. 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Nilon, C.H.; Berkowitz, A.R.; Hollweg, K.S. Understanding urban ecosystems: A new frontier for science and education. Urban Ecosyst. 1999, 3, 3–4. [Google Scholar] [CrossRef]
- Sandifer, P.A.; Sutton-Grier, A.E.; Ward, B.P. Exploring connections among nature, biodiversity, ecosystem services, and human health and well-being: Opportunities to enhance health and biodiversity conservation. Ecosyst. Serv. 2015, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Aronson, M.F.; Lepczyk, C.A.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S.; Vargo, T. Biodiversity in the city: Key challenges for urban green space management. Front. Ecol. Environ. 2017, 15, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Häder, D.P.; Banaszak, A.T.; Villafañe, V.E.; Narvarte, M.A.; González, R.A.; Helbling, E.W. Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef]
- Assefa, W.W.; Eneyew, B.G.; Wondie, A. The impacts of land-use and land-cover change on wetland ecosystem service values in peri-urban and urban area of Bahir Dar City, Upper Blue Nile Basin, Northwestern Ethiopia. Ecol Process 2021, 10, 39. [Google Scholar] [CrossRef]
- Millenium Ecosystem Assessment. Ecosystems and Human Well-Being; Island Press: Washington, DC, USA, 2005; Volume 5. [Google Scholar]
- Dong, T.; Xu, W.; Zheng, H.; Xiao, Y.; Kong, L.; Ouyang, Z. A Framework for Regional Ecological Risk Warning Based on Ecosystem Service Approach: A Case Study in Ganzi, China. Sustainability 2018, 10, 2699. [Google Scholar] [CrossRef] [Green Version]
- Zhai, T.; Wang, J.; Fang, Y.; Liu, J.; Huang, L.; Chen, K.; Zhao, C. Identification and Prediction of Wetland Ecological Risk in Key Cities of the Yangtze River Economic Belt: From the Perspective of Land Development. Sustainability 2021, 13, 411. [Google Scholar] [CrossRef]
- Niesenbaum, R.A. The Integration of Conservation, Biodiversity, and Sustainability. Sustainability 2019, 11, 4676. [Google Scholar] [CrossRef] [Green Version]
- Campbell-Arvai, V. Engaging urban nature: Improving our understanding of public perceptions of the role of biodiversity in cities. Urban Ecosyst. 2019, 22, 409–423. [Google Scholar] [CrossRef]
- Chien, W.C.; Li, M.H.; Li, H.J. Appropriate management practices help enhance odonate species richness of small ponds in peri-urban landscapes. Urban Ecosyst. 2019, 22, 757–768. [Google Scholar] [CrossRef]
- Souza Costa, B.N.; Campelo Pinheiro, S.C.; Lund Amado, L.; de Oliveira Lima, M. Microzooplankton as a bioindicator of environmental degradation in the Amazon. Ecol. Indic. 2016, 61, 526–545. [Google Scholar] [CrossRef]
- Marchese, M.; Gagneten, A.M.; Montalto, L.; Gallardo, L.I.; Damborsky, M.P.; Poi, A.S.G. Aplicación de Indicadores Biológicos en el Nordeste Argentino. In Indicadores Biológicos, Calidad Biológica de Agua, Aplicaciones y Perspectivas de Uso; Dominguez, A., Giorgi, A., Eds.; Eudeba: Buenos Aires, Argentina, 2018. [Google Scholar]
- Forio, M.A.E.; Goethals, P.L.M. An Integrated Approach of Multi-Community Monitoring and Assessment of Aquatic Ecosystems to Support Sustainable Development. Sustainability 2020, 12, 5603. [Google Scholar] [CrossRef]
- Ruaro, R.; Gubiani, E.A. A scientometric assessment of 30 years of the Index of Biotic Integrity in aquatic ecosystems: Applications and main flaws. Ecol. Indic. 2013, 29, 105–110. [Google Scholar] [CrossRef]
- Serra, S.R.Q.; Calapez, A.R.; Simões, N.E.; Sá Marques, J.A.A.; Laranjo, M.; João Feio, M. Effects of variations in water quantity and quality in the structure and functions of invertebrates’ community of a Mediterranean urban stream. Urban Ecosyst. 2019, 22, 1173–1186. [Google Scholar] [CrossRef]
- Rosenberg, D.M.; Resh, V.H. Freshwater Biomonitoring and Benthic Macroinvertebrates; Chapman y Hall, Inc.: London, UK, 1993; 488p. [Google Scholar]
- Li, L.; Zheng, B.; Liu, L. Biomonitoring and bioindicators used for river ecosystems: Definitions, approaches and trends. Procedia Environ. Sci. 2010, 2, 1510–1524. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Chen, X.; Wang, Q. Local Assessment of Shanghai: Effects of Urbanization on the Diversity of Macrobenthic Invertebrates. In Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities; Elmqvist, T., Ed.; Springer Open: Berlin/Heidelberg, Germany, 2013; 771p. [Google Scholar] [CrossRef] [Green Version]
- Epele, L.B.; Manzo, L.M.; Grech, M.G.; Macchi, P.; Claverie, A.Ñ.; Lagomarsino, L.; Miserendino, M.L. Disentangling natural and anthropogenic influences on Patagonian pond water quality. Sci. Total Environ. 2018, 613, 866–876. [Google Scholar] [CrossRef]
- Lopes Costa, L.; Rosental Zalmon, I.; Fanini, L.; Defeo, O. Macroinvertebrates as indicators of human disturbances on sandy beaches: A global review. Ecol. Indic. 2020, 118, 106764. [Google Scholar] [CrossRef]
- Gagneten, A.M.; Plá, R.R.; Regaldo, L.; Paggi, J.C. Assesment of Bioconcentration Factor of Chromium by Instrumental Neutron Activation Analysis in Argyrodiaptomus falcifer Daday, a subtropical freshwater copepod. Water Air Soil Pollut. 2009, 204, 133–138. [Google Scholar] [CrossRef]
- Jeppesen, E.; Nõges, P.; Davidson, T.A.; Haberman, J.; Nõges, T.; Blank, K.; Johansson, L.S. Zooplankton as indicators in lakes: A scientific-based plea for including zooplankton in the ecological quality assessment of lakes according to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279. [Google Scholar] [CrossRef]
- Almeidal, R.; Formigo, N.E.; Sousa-Pinto, I.; Antunes, S.C. Contribution of zooplankton as a biological element in the assessment of reservoir water quality. Limnetica 2020, 39, 245–261. [Google Scholar] [CrossRef]
- Zannatul, F.; Muktadir, A.K.M. A Review: Potentiality of zooplankton as bioindicator. Am. J. Appl. Sci. 2009, 6, 1815–1819. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Q.; Wei, N.; Tang, C.; Sun, X.; Yang, Y. Biological indicators of ecological quality in typical urban river-lake ecosystems: The planktonic rotifer community and its response to environmental factors. Ecol. Indic. 2020, 112, 106127. [Google Scholar] [CrossRef]
- Resh, V.H. Which group is best? Attributes of different biological assemblages used in freshwater biomonitoring programs. Environ. Monit. Assess. 2008, 138, 131–138. [Google Scholar] [CrossRef]
- Sommer, U. (Ed.) Plankton Ecology: Succession in Plankton Commnunities; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar] [CrossRef]
- Trevisan, G.V.; Forsberg, B.R. Relationships among nitrogen and total phosphorus, algal biomass and zooplankton density in the central Amazonia lakes. Hydrobiologia 2007, 586, 357–365. [Google Scholar] [CrossRef]
- Rubright, J.S.; Harrell, J.L.; Holcomb, H.W.; Parker, J.C. Responses of planktonic and benthic communities to fertilizer and feed applications in shrimp mariculture ponds. J. World Maric. Soc. 1981, 12, 281–299. [Google Scholar] [CrossRef]
- Gagneten, A.M.; Marchese, M. Effect of Paraquat on freshwater zooplankton and zoobenthic assemblages in enclosure experiments. Int. J. Ecohydrol. Hydrobiol. 2003, 3, 389–398. [Google Scholar]
- Nowicki, C.J.; van Hees, E.H.; Kashian, D.R. Comparative effects of sediment versus aqueous polychlorinated biphenyl (PCB) exposure on benthic and planktonic invertebrates. Environ. Toxicol. Chem. 2014, 33, 641–647. [Google Scholar] [CrossRef]
- Marchese, M.R.; Rodriguez, A.R.; Pave, P.J.; Carignano, M.R. Benthic invertebrates structure in wetlands of a tributary of the middle Parana River (Argentina) affected by hydrologic and anthropogenic disturbances. J. Environ. Biol. 2008, 29, 343. [Google Scholar]
- Azevêdo, D.J.S.; Barbosa, J.E.L.; Gomes, W.I.A.; Porto, D.E.; Marques, J.C.; Molozzi, J. Diversity measures in macroinvertebrate and zooplankton communities related to the trophic status of subtropical reservoirs: Contradictory or complementary responses? Ecol. Indic. 2015, 50, 135–149. [Google Scholar] [CrossRef]
- Rodríguez Capítulo, A.R.; Tangorra, M.; Ocón, C. Use of benthic macroinvertebrates to assess the biological status of Pampean streams in Argentina. Aquat. Ecol. 2001, 35, 109–119. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Water Quality Indices; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Kuhlmann, M.L.; Johnscher-Fornasaro, G.; Ogura, L.L.; Imbimbo, H.R.V. Protocolo para o Biomonitoramento com as Comunidades Bentônicas de Rios e Reservatórios do Estado de São Paulo; CETESB: São Paulo, Brazil, 2012. [Google Scholar]
- Navarro-Ortega, A.; Acuña, V.; Bellin, A.; Burek, P.; Cassiani, G.; Choukr-Allah, R.; Grathwohl, P.; Jones, C.; Rault, P.K.; Kok, K.; et al. Managing the effects of multiple stressors on aquatic ecosystems under water scarcity. The GLOBAQUA project. Sci. Total Environ. 2015, 503–504, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.; Cao, Y.; Sass, G.G.; Epifanio, J. Large river fish functional diversity responses to improved water quality over a 28 year period. Ecol. Indic. 2018, 88, 322–331. [Google Scholar] [CrossRef]
- Kalarus, K.; Halecki, W.; Skalski, T. Both semi-natural and ruderal habitats matter for supporting insect functional diversity in an abandoned quarry in the city of Kraków (S Poland). Urban Ecosyst. 2019, 22, 943–953. [Google Scholar] [CrossRef] [Green Version]
- Vieira, N.K.M.; Poff, N.L.; Carlisle, D.M.; Moulton, S.R.; Koski, M.L.; Kondratieff, B.C. A Database of Lotic Invertebrate Traits for North America; U.S. Geological Survey Data Series 187; US Department of the Interior: Reston, VA, USA, 2006. [Google Scholar] [CrossRef]
- Wentworth, C. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. Trophic Relationships of Macroinvertebrates. In Methods in Stream Ecology; Academic Press: Cambridge, MA, USA, 2017; Volume 1, pp. 413–433. [Google Scholar]
- Gallardo, B.; Gascón, S.; Quintana, X.; Comín, F.A. How to choose a biodiversity indicator-Redundancy and complementarity of biodiversity metrics in a freshwater ecosystem. Ecol. Indic. 2011, 11, 1177–1184. [Google Scholar] [CrossRef]
- Gagneten, A.M.; Paggi, J.C. Effects of heavy metal contamination (Cr, Cu, Pb, Cd) and eutrophication on zooplankton in the lower basin of the Salado River (Argentina). Water Air Soil Pollut. 2009, 198, 317–334. [Google Scholar] [CrossRef]
- Barnett, A.J.; Finlay, K.; Beisner, B.E. Functional diversity of crustacean zooplankton communities: Towards a trait-based classification. Freshw. Biol. 2007, 52, 796–813. [Google Scholar] [CrossRef]
- DeMott, W.R.; Kerfoot, W.C. Competition among cladocerans: Nature of the interaction between Bosmina and Daphnia. Ecology 1982, 63, 1949–1966. [Google Scholar] [CrossRef]
- Bogdan, K.G.; Gilbert, J.J. Body size and food size in freshwater zooplankton. Proc. Natl. Acad. Sci. USA 1984, 81, 6427–6431. [Google Scholar] [CrossRef] [Green Version]
- Stemberger, R.S.; Gilbert, J.J. Body size, food concentration, and population growth in planktonic rotifers. Ecology 1985, 66, 1151–1159. [Google Scholar] [CrossRef]
- Obertegger, U.; Smith, H.A.; Flaim, G.; Wallace, R.L. Using the guild ratio to characterize pelagic rotifer communities. Hydrobiologia 2011, 662, 157–162. [Google Scholar] [CrossRef]
- Regaldo, L.; Gutierrez, M.; Reno, U.; Fernández, V.; Gervasio, S.; Repetti, M.; Gagneten, A.M. Water and sediment quality assessment in the Colastiné-Corralito stream system (Santa Fe, Argentina): Impact of industry and agriculture on aquatic ecosystems. Environ. Sci. Pollut. Res. 2018, 25, 6951–6968. [Google Scholar] [CrossRef]
- Vaschetto, P.; Regaldo, L.; Polla, W.; Andrade, V.; Gervasio, S.; Gagneten, A.M. Plankton community responses to anthropogenic pollution in an Argentinian urban reserve. Water Air Soil Pollut. 2021, 232, 427. [Google Scholar] [CrossRef]
- Andrade, V.S.; Gutierrez, M.F.; Regaldo, L.; Paira, A.R.; Repetti, M.R.; Gagneten, A.M. Influence of rainfall and seasonal crop practices on nutrient and pesticide runoff from soybean dominated agricultural areas in Pampean streams, Argentina. Sci. Total Environ. 2021, 788, 147676. [Google Scholar] [CrossRef]
- Caino, A. Alternativas de Gestión De una Laguna Urbana Eutrofizada de Santa Fe (Argentina) Utilizando el Fitoplancton Como Indicador de Calidad Trófica. In Tesina de Licenciatura en Biodiversidad; Facultad de Humanidades y Ciencias, Universidad Nacional del Litoral: Santa Fe, Argentina, 2016. [Google Scholar]
- Sulehria, A.Q.K.; Qamar, M.F.; Haider, S.; Ejaz, M.; Hussain, A. Water quality and Rotifer diversity in the fish pond at District Mianwali Pakistan. Biologia 2009, 55, 79–85. [Google Scholar]
- Sarkar, R.; Ghosh, A.R.; Mondal, N.K. Comparative study on physicochemical status and diversity of macrophytes and zooplanktons of two urban ponds of Chandannagar, WB, India. Appl. Water Sci. 2020, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Cummins, K.W.; Merritt, R.W.; Andrade, P.C. The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Stud. Neotrop. Fauna Environ. 2005, 40, 69–89. [Google Scholar] [CrossRef]
- Rodríguez, P.; Arrate, J.; Martinez-Madrid, M.; Reynoldson, T.B.; Schumacher, V.; Viguri, J. Toxicity of Santander Bay Sediments to the Euryhaline Freshwater Oligochaete Limnodrilus hoffmeisteri. In Aquatic Oligochaete Biology IX; Springer: Dordrecht, The Netherlands, 2006; pp. 157–169. [Google Scholar]
- Martins, R.T.; Stephan, N.N.C.; Alves, R.G. Tubificidae (Annelida: Oligochaeta) as an indicator of water quality in an urban stream in southeast Brazil. Acta Limnol. Bras. 2008, 20, 221–226. [Google Scholar]
- Mugni, H.; Ronco, A.; Bonetto, C. Insecticide toxicity to Hyalella curvispina in runoff and stream water within a soybean farm (Buenos Aires, Argentina). Ecotoxicol. Environ. Saf. 2011, 74, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Anguiano, O.L.; Vacca, M.; Araujo, M.E.R.; Montagna, M.; Venturino, A.; Ferrari, A. Acute toxicity and esterase response to carbaryl exposure in two different populations of amphipods Hyalella curvispina. Aquat. Toxicol. 2017, 188, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Gagneten, A.M. Effects of Contamination by Heavy Metals and Eutrophication on Zooplankton, and Their Possible Effects on the Throphic Webs of Freshwater Aquatic Ecosystems. In Eutrophication: Causes, Consequences and Control, 1st ed.; Ansari, A.A., Singh Gill, S., Lanza, G.R., Rast, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; 394p, ISBN 97890-481-9624-1. [Google Scholar]
- Hanazato, T. Response of a zooplankton community to insecticide application in experimental ponds: A review and the implications of the effects of chemicals on the structure and functioning of freshwater communities. Environ. Pollut. 1998, 101, 361–373. [Google Scholar] [CrossRef]
- Sterner, R.W. Role of zooplankton in aquatic ecosystems. In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Academic Press: Oxford, UK, 2009; pp. 678–688. [Google Scholar]
- Mukhopadhyay, S.K.; Chattopadhyay, B.; Goswami, A.R. Spatial variations in zooplankton diversity in waters contaminated with composite effluents. J. Limnol. 2007, 66, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.K.; Saini, M. Impact of industrial effluents on the diversity and population structure of rotifer fauna in river basantar, jammu (j&k, India). Int. J. Adv. Res. 2016, 4, 1458–1472. [Google Scholar] [CrossRef] [Green Version]
- Pejler, B. Zooplanktic indicators of trophy and their food. Hydrobiologia 1983, 101, 111–114. [Google Scholar] [CrossRef]
- Von der Ohe, P.C.; Liess, M. Relative sensitivity distribution of aquatic invertebrates to organic and metal compounds. Environ. Toxicol. Chem. Int. J. 2004, 23, 150–156. [Google Scholar] [CrossRef]
- Dalla Bona, M.; Di Leva, V.; De Liguoro, M. The sensitivity of Daphnia magna and Daphnia curvirostris to 10 veterinary antibacterials and to some of their binary mixtures. Chemosphere 2014, 115, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Petchey, O.L.; Gaston, K.J. Functional diversity (FD), species richness and community composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Vogt, R.J.; Beisner, B.E.; Prairie, Y.T. Functional diversity is positively associated with biomass for lake diatoms. Freshw. Biol. 2010, 55, 1636–1646. [Google Scholar] [CrossRef]
- Archaimbault, V.; Usseglio-Polatera, P.; Garric, J.; Wasson, J.G.; Babut, M. Assessing pollution of toxic sediment in streams using bio-ecological traits of benthic macroinvertebrates. Freshw. Biol. 2010, 55, 1430–1446. [Google Scholar] [CrossRef]
- Statzner, B.; Bêche, L.A. Can biological invertebrate traits resolve effects of multiple stressors on running water ecosystems? Freshw. Biol. 2010, 55, 80–119. [Google Scholar] [CrossRef]
- Peng, F.J.; Pan, C.G.; Zhang, N.S.; ter Braak, C.J.; Salvito, D.; Selck, H.; Ying, G.G.; Van den Brink, P.J. Benthic invertebrate and microbial biodiversity in sub-tropical urban rivers: Correlations with environmental variables and emerging chemicals. Sci. Total Environ. 2020, 709, 136281. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.; Sabater, S.; Barata, C. Evaluating ecological integrity in multistressed rivers: From the currently used biotic indices to newly developed approaches using biofilms and invertebrates. In Emerging and Priority Pollutants in Rivers; Springer: Berlin/Heidelberg, Germany, 2012; pp. 219–241. [Google Scholar]
- Lencioni, V.; Bellamoli, F.; Paoli, F. Multi-level effects of emerging contaminants on macroinvertebrates in Alpine streams: From DNA to the ecosystem. Ecol. Indic. 2020, 117, 106660. [Google Scholar] [CrossRef]
- Gabriels, W.; Lock, K.; De Pauw, N.; Goethals, P.L. Multimetric Macroinvertebrate Index Flanders (MMIF) for biological assessment of rivers and lakes in Flanders (Belgium). Limnol. Ecol. Manag. Inland Waters 2010, 40, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Rocchetta, I.; Pasquevich, M.Y.; Heras, H.; de Molina, M.D.C.R.; Luquet, C.M. Effects of sewage discharges on lipid and fatty acid composition of the Patagonian bivalve Diplodon chilensis. Mar. Pollut. Bull. 2014, 79, 211–219. [Google Scholar] [CrossRef]
- Muñoz-Colmenares, M.E.; Soria, J.M.; Vicene, E. Can zooplankton species be used as indicators of trophic status and ecological potential of reservoirs? Aquat. Ecol. 2021, 55, 1143–1156. [Google Scholar] [CrossRef]
- Whiting, E.R.; Clifford, H.F. Invertebrates and urban runoff in a small northern stream, Edmonton, Alberta, Canada. Hydrobiologia 1983, 102, 73–80. [Google Scholar] [CrossRef]
- Zimmer, K.D.; Hanson, M.A.; Butler, M.G.; Duffy, W.G. Size distribution of aquatic invertebrates in two prairie wetlands, with and without fish, with implications for community production. Freshw. Biol. 2001, 46, 1373–1386. [Google Scholar] [CrossRef]
- Gascón, S.; Brucet, S.; Sala, J.; Boix, D.; Quintana, X.D. Comparison of the effects of hydrological disturbance events on benthos and plankton salt marsh communities. Estuar. Coast. Shelf Sci. 2007, 74, 419–428. [Google Scholar] [CrossRef]
- Scharler, U.M.; Lechman, K.; Radebe, T.; Jerling, H.L. Effects of prolonged mouth closure in a temporarily open/closed estuary: A summary of the responses of invertebrate communities in the uMdloti Estuary, South Africa. Afr. J. Aquat. Sci. 2020, 45, 121–130. [Google Scholar] [CrossRef]
- Chapman, P.M.; Brinkhurst, R.O. Lethal and Sublethal Tolerances of Aquatic Oligochaetes with Reference to Their Use as a Biotic Index of Pollution. In Aquatic Oligochaeta; Springer: Dordrecht, The Netherlands, 1984; pp. 139–144. [Google Scholar]
- Andersen, T.; Hessen, D.O. Carbon, nitrogen and phosphorus content of freshwater zooplankton. Limnol. Oceanogr. 1991, 36, 807–814. [Google Scholar] [CrossRef]
- Vinebrooke, R.D.; Schindler, D.W.; Findlay, D.L. Trophic dependence of ecosystem resistance and species compensation in experimentally acidified Lake 302S (Canada). Ecosystems 2003, 6, 101–113. [Google Scholar] [CrossRef]
- Beghelli, I.F.G.; Cetra, M.; Marchese, M.; López-Dovál, J.C.; Rosa, A.H.; Pompêo, M.L.; Moschini-Carlos, V. Taxonomic and non-taxonomic responses of benthic macroinvertebrates to metal toxicity in tropical reservoirs. The case of Cantareira Complex, São Paulo, Brazil. An. Acad. Bras. Ciências 2020, 92, e20180962. [Google Scholar] [CrossRef] [Green Version]

| PGB | RECU | S1 | S2 | S3 | S4 | Kruskal-Wallis Test | |
|---|---|---|---|---|---|---|---|
| T (°C) | 23.87 ± 5.70 | 23.57 ± 5.06 | 19.43 ± 6.06 | 19.58 ± 6.61 | 20.58 ± 5.53 | 19.63 ± 6.47 | 0.821 |
| pH | 8.09 ± 0.29 | 7.20 ± 0.18 | 7.91 ± 0.46 | 8.60 ± 0.21 | 7.64 ± 0.25 | 8.51 ± 1.11 | 0.044 |
| Ω (µS cm−1) | 2158 ± 432.54 | 823 ± 26.63 | 1449 ± 323.99 | 2627 ± 715.60 | 1029 ± 21.49 | 1102 ± 309.88 | 0.003 |
| DO (mg L−1) | 8.98 ± 2.87 | 6.28 ± 0.25 | 6.85 ± 0.77 | 10.69 ± 1.49 | 4.78 ± 0.98 | 10.66 ± 3.17 | 0.008 |
| Secchi (cm) | 32.05 ± 28.65 | 28.33 ± 2.89 | 29.25 ± 12.20 | 23.00 ± 4.00 | 43.25 ± 10.11 | 28.50 ± 11.36 | 0.228 |
| Depth (cm) | 33.67 ± 27.21 | 32.33 ± 8.74 | 59.75 ± 29.67 | 33.25 ± 10.11 | 47.00 ± 8.72 | 52.38 ± 16.00 | 0.162 |
| PGB | RECU | S1 | S2 | S3 | S4 | |
|---|---|---|---|---|---|---|
| Sand (%) | 98 | 86 | 47 | 76 | 91 | 76 |
| Silt-Clay (%) | 2 | 14 | 53 | 24 | 9 | 24 |
| CPOM (%) | 64 | 42 | 31 | 34 | 35 | 37 |
| FPOM (%) | 17 | 34 | 35 | 52 | 45 | 39 |
| UFPOM (%) | 19 | 24 | 34 | 14 | 20 | 24 |
| Benthos | Zooplankton | |||||||
|---|---|---|---|---|---|---|---|---|
| Density | S | H | J | Density | S | H | J | |
| Sites | (Ind·m−2) | (Ind·L−1) | ||||||
| JPGB | 115–5298 | 4–7 | 0.61–1.08 | 0.31–0.72 | 12.29–22.62 | 18–21 | 1.93–2.37 | 0.58–0.69 |
| RECU | 796–7136 | 9–17 | 1.48–1.84 | 0.52–0.84 | 7.53–23.85 | 21–27 | 1.72–2.29 | 0.47–0.66 |
| S1 | 332–8430 | 3–8 | 0.91–1.60 | 0.44–0.86 | 37.84–2231 | 12–18 | 1.60–1.98 | 0.56–0.65 |
| S2 | 1098–10,746 | 7–12 | 1.29–1.74 | 0.52–0.84 | 535.56–3092 | 11–23 | 1.14–2.14 | 0.52–0.63 |
| S3 | 3197–13,765 | 7–11 | 1.09–1.69 | 0.53–0.70 | 9.43–1028.8 | 7–16 | 1.61–2.12 | 0.59–0.85 |
| S4 | 1365–18,397 | 5–10 | 0.55–1.01 | 0.26–0.57 | 342.23–1469 | 9–18 | 0.99–1.95 | 0.50–0.67 |
| Score | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sampling Site | Chironomidae | Naidinae | Tubificinae | Cyclpoida | Calanoida | Hirudinae | Ostracoda | Planorbidae | Ampullriidae | Nematoda | Cladocera | Hyallelidae | IMRP | BCI |
| PGB | 0.45 | 0.35 | 0.14 | 0.35 | 0.35 | 1.64 | 4 | |||||||
| RECU | 0.45 | 0.35 | 0.14 | 0.35 | 0.35 | 0.55 | 0.40 | 0.55 | 3.14 | 3.25 | ||||
| S1 | 0.45 | 0.35 | 0.14 | 0.35 | 0.35 | 0.55 | 0.10 | 2.29 | 3.75 | |||||
| S2 | 0.45 | 0.35 | 0.14 | 0.35 | 0.35 | 0.55 | 0.10 | 0.55 | 2.84 | 3.25 | ||||
| S3 | 0.45 | 0.35 | 0.14 | 0.35 | 0.55 | 0.55 | 0.55 | 0.90 | 3.84 | 3.25 | ||||
| S4 | 0.45 | 0.35 | 0.14 | 0.35 | 0.35 | 0.55 | 0.40 | 0.55 | 0.10 | 0.90 | 4.14 | 3.25 | ||
| Sampling Site | Rotifers/Total Zooplankton | Microcrustaceans/ Total Zooplankton | Cladocerans/ Total Zooplankton | Macrocrustaceans/ Microcrustaceans |
|---|---|---|---|---|
| PGB | 0.94 | 0.05 | 0.04 | 0.03 |
| RECU | 0.65 | 0.34 | 0.21 | 0.26 |
| S1 | 0.85 | 0.14 | 0.11 | 0.04 |
| S2 | 0.70 | 0.29 | 0.19 | 0.13 |
| S3 | 0.90 | 0.09 | 0.08 | 0.01 |
| S4 | 0.80 | 0.19 | 0.17 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias, M.J.; Vaschetto, P.A.; Marchese, M.; Regaldo, L.; Gagneten, A.M. Benthic Macroinvertebrates and Zooplankton Communities as Ecological Indicators in Urban Wetlands of Argentina. Sustainability 2022, 14, 4045. https://doi.org/10.3390/su14074045
Arias MJ, Vaschetto PA, Marchese M, Regaldo L, Gagneten AM. Benthic Macroinvertebrates and Zooplankton Communities as Ecological Indicators in Urban Wetlands of Argentina. Sustainability. 2022; 14(7):4045. https://doi.org/10.3390/su14074045
Chicago/Turabian StyleArias, María Julieta, Pablo Andrés Vaschetto, Mercedes Marchese, Luciana Regaldo, and Ana María Gagneten. 2022. "Benthic Macroinvertebrates and Zooplankton Communities as Ecological Indicators in Urban Wetlands of Argentina" Sustainability 14, no. 7: 4045. https://doi.org/10.3390/su14074045
APA StyleArias, M. J., Vaschetto, P. A., Marchese, M., Regaldo, L., & Gagneten, A. M. (2022). Benthic Macroinvertebrates and Zooplankton Communities as Ecological Indicators in Urban Wetlands of Argentina. Sustainability, 14(7), 4045. https://doi.org/10.3390/su14074045






