Examining the Socio-Economic and Natural Resource Risks of Food Estate Development on Peatlands: A Strategy for Economic Recovery and Natural Resource Sustainability
Abstract
1. Introduction
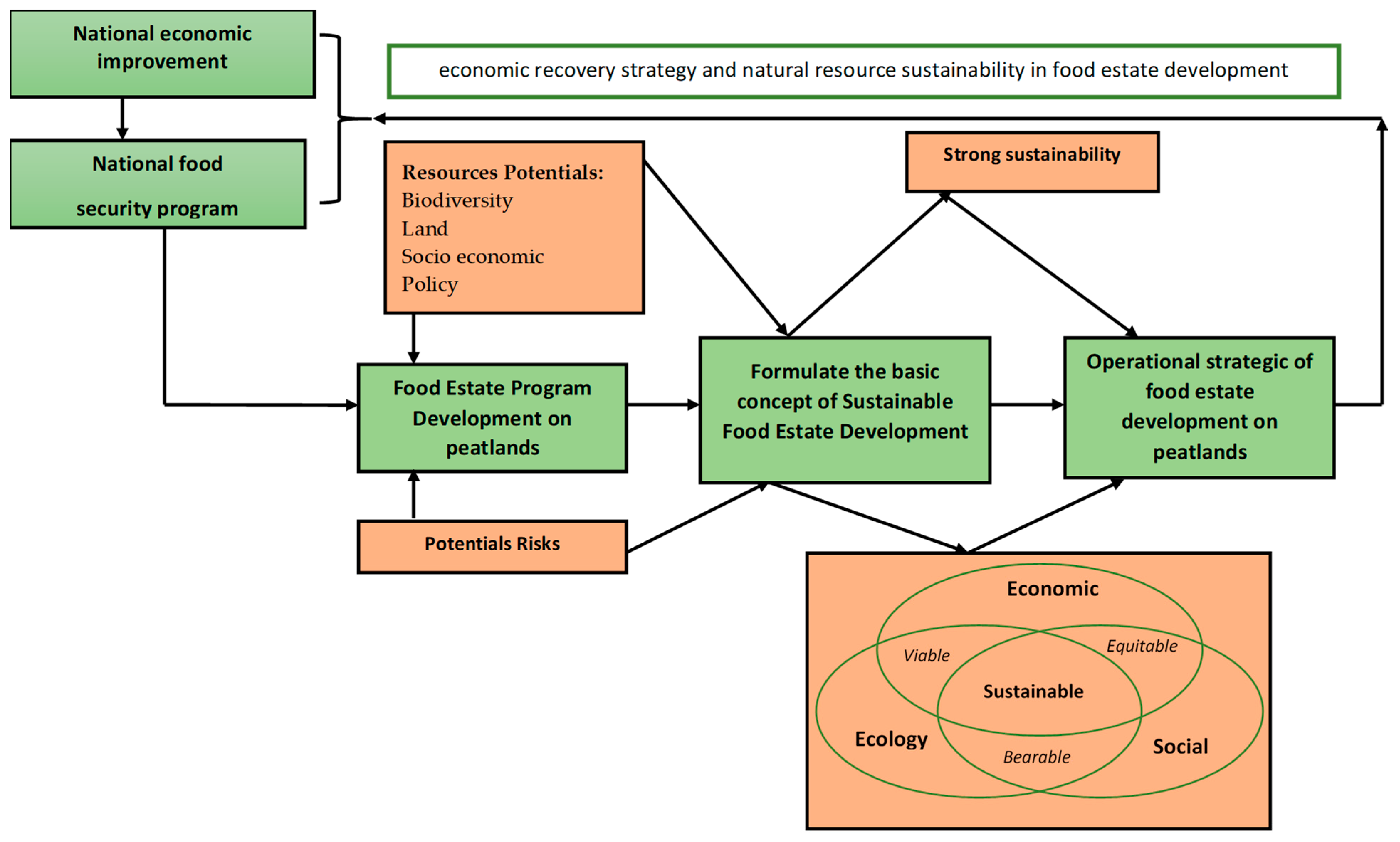
2. The Conceptual Framework
3. Methodology
3.1. Research Location
3.2. Data Collection Technique
3.3. Data Analysis
4. Results
4.1. National Policy
4.2. Socio-Economics of Local Communities: Potential and Threats
4.3. Biodiversity: Potential and Threats
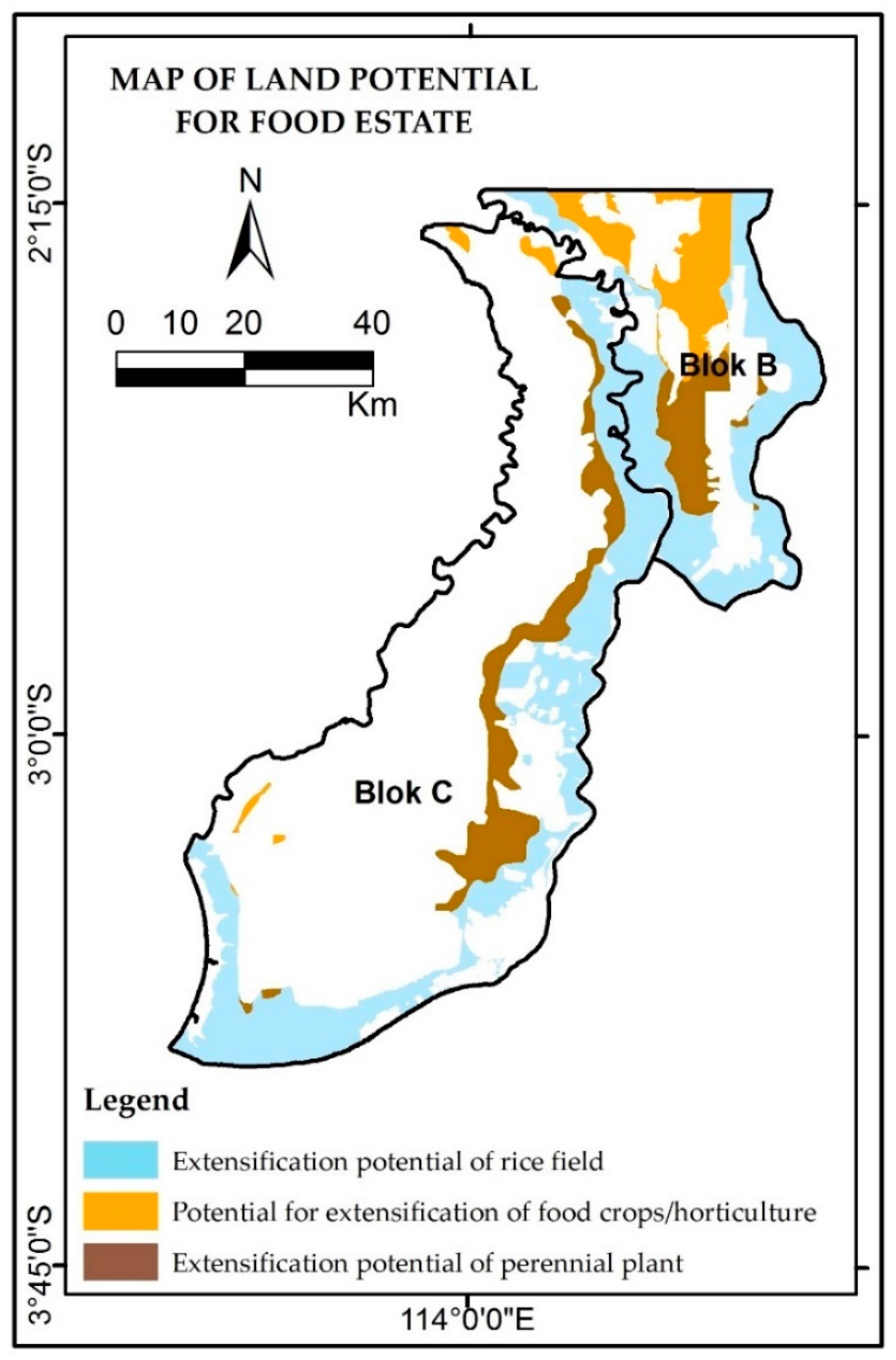
4.4. Land Resources: Potential and Threats
4.5. Risk Identification
5. Discussion
5.1. Risks Analysis and Mitigation
5.2. Operational Strategy
- (1)
- Landscape management;
- (2)
- Maintenance of conservation value and vulnerable areas as protected areas;
- (3)
- Prevention of fragmentation and maintenance of habitat connectivity via corridors;
- (4)
- Land management system with low impact (paludiculture);
- (5)
- Integrated multi-business development (agriculture, animal husbandry, captive breeding, fisheries, and forestry);
- (6)
- Community-based food estate.
5.2.1. Landscape Management
5.2.2. Maintenance of Conservation Value and Vulnerable Areas as Protected Areas
5.2.3. Prevention of Fragmentation and Maintenance of Habitat Connectivity via Corridors
5.2.4. Low-Impact Land Management System (Paludiculture)
5.2.5. Integrated Multi-Business Development (Agriculture, Animal Husbandry, Fisheries, and Forestry)
5.2.6. Community Based Food Estate
5.3. Constraints and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sources/Hazard Factors | Hazards and Risk Identification | Analyses | Evaluation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Likelihood (L) | Consequences | |||||||||||
| A | B | C | D | E | 1 | 2 | 3 | 4 | 5 | |||
| Human |
| A | 3 | A3 (Extreme) | ||||||||
| B | 2 | B2 (High) | |||||||||
| D | 3 | D3 (Moderate) | |||||||||
| D | 3 | D3 (Moderate) | |||||||||
| D | 5 | D5 (Extreme) | |||||||||
| C | 3 | C3 (High) | |||||||||
| D | 3 | D3 (Moderate) | |||||||||
| C | 3 | C3 (Hight) | |||||||||
| Paddy rice development on peatland |
| D | 3 | B3 (High) | ||||||||
| C | 3 | C3 (Moderate) | |||||||||
| Free-grazing livestock species |
| B | 3 | B3 (High) | ||||||||
| E | 3 | E3 (Moderate) | |||||||||
| Farmed fish species |
| B | 4 | B4 (Extreme) | ||||||||
| E | 2 | E2 (Low) | |||||||||
| Farming method |
| B | 5 | B5 (Extreme) | ||||||||
| C | 3 | C3 (High) | |||||||||
| A | 5 | A5 (extreme) | |||||||||
| D | 3 | D3 (Moderate) | |||||||||
| D | 3 | D3 (Moderate) | |||||||||
| C | 4 | C4 (Extreme) | |||||||||
| C | 3 | C3 (High) | |||||||||
References
- High Level Panel of Experts on Food Security and Nutrition (HLPE). Impacts of COVID-19 on Food Security and Nutrition: Developing Effective Policy Responses to Address the Hunger and Malnutrition Pandemic; FAO: Rome, Italy, 2020. [Google Scholar]
- Rowan, N.J.; Galanakis, C.M. Unlocking challenges and opportunities presented by COVID-19 pandemic for cross-cutting disruption in agri-food and green deal innovations: Quo Vadis? Sci. Total Environ. 2020, 748, 141362. [Google Scholar] [CrossRef] [PubMed]
- New Report Identifies 27 Countries Heading for COVID-19-Driven Food Crises. Available online: https://www.fao.org/news/story/en/item/1298468/icode/ (accessed on 30 October 2021).
- Badan Pusat Statistik (BPS). Statistik Indonesia; Badan Pusat Statistik: Jakarta, Indonesia, 2020. [Google Scholar]
- Masniadi, R.; Angkasa, M.A.Z.; Karmeli, E.; Esabella, S. Telaah Kritis Ketahanan Pangan Kabupaten Sumbawa dalam Menghadapi Pandemi COVID-19. Indones. J. Soc. Sci. Humanit. 2020, 1, 109–120. [Google Scholar]
- Fitriah, N.A. Dampak Pandemi COVID-19 Terhadap Ketahanan Pangan Indonesia: Sebuah Penelitian Eksploratif; IPB University: Bogor, Indonesia, 2021. [Google Scholar]
- Marwanto, S.; Pangestu, F. Food Estate program in Central Kalimantan Province as An Integrated and Sustainable Solution for Food Security in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 794, 012068. [Google Scholar] [CrossRef]
- Faizi, N.I.; Qurani, I.Z. Lesson learned from the development of sustainable rice farming in peatland. IOP Conf. Ser. Earth Environ. Sci. 2021, 752, 012037. [Google Scholar] [CrossRef]
- Rondhi, M.; Pratiwi, P.A.; Handini, V.T.; Sunartomo, A.F.; Budiman, S.A. Agricultural Land Conversion, Land Economic Value, and Sustainable Agriculture: A Case Study in East Java, Indonesia. Land 2018, 7, 148. [Google Scholar] [CrossRef]
- Harini, R.; Yunus, H.S.; Kasto; Hartono, S. Agricultural land conversion: Determinants and impact for food sufficiency in Sleman Regency. Indones. J. Geogr. 2012, 44, 120–133. [Google Scholar]
- Chofyan, I. The Dynamics of Rice Field Conversion Into Settlement In The District Of Bandung. J. Sos. Pembang. 2016, 32, 267–275. [Google Scholar] [CrossRef][Green Version]
- Arif, S.; Isdijoso, W.; Fatah, A.R.; Tamyis, A.R. Strategic Review of Food Security and Nutrition in Indonesia; The SMERU Research Institute: Jakarta, Indonesia, 2020. [Google Scholar]
- BAPPENAS (Badan Perencanaan dan Pembangunan Nasional). Rencana Induk 2020–2024, Pengembangan Kawasan Sentra Produksi Pangan (Food Estate) Kalimantan Tengah; Badan Perencanaan dan Pembangnan Nasional: Jakarta, Indonesia, 2020. [Google Scholar]
- Butarbutar, T. Contribution of forestry sector to support food security through agroforestry system. J. Anal. Kebijak. Kehutan. 2009, 6, 169–179. [Google Scholar]
- Dwiprabowo, H.; Effendi, R.; Hakim, I.; Bangsawan, I. Contribution of forest area in supporting food security: Case study of West Java Province. J. Anal. Kebijak. Kehutan. 2011, 8, 47–61. [Google Scholar] [CrossRef]
- Mayrowani, H. Agroforestry development to support food security and farmer’s empowerment nearby the forest. Forum Penelit. Agro Ekon. 2011, 29, 83–98. [Google Scholar] [CrossRef]
- Arisaputra, M.I. Reforma agraria untuk mewujudkan kedaulatan pangan. Rechtldee J. Huk. 2015, 10, 39–59. [Google Scholar]
- Nurlinda, I. Perolehan tanah obyek reformasi agraria (TORA) yang berasal dari kawasan hutan: Permasalahan dan pengaturannya. Veritas Justitia 2018, 4, 252–273. [Google Scholar] [CrossRef]
- Manik, S.S.; Martanto, R.; Salim, M.N. Potensi tanah untuk reforma grarian dalam kawasan hutan di Pakpak Bharat, Sumatera Utara. J. Tunas Agrar. 2021, 4, 32–39. [Google Scholar]
- Lasminingrat, L.; Efriza, E. The development of national food estate: The Indonesian food crisis anticipation strategy. J. Pertahanan Bela Negara 2020, 10, 229–249. [Google Scholar] [CrossRef]
- Nizami, A.F.; Sisgianto; Loilatu, M.J. The Urgency of Food Estate for National Food Security in The Middle of The COVID-19 Pandemic. J. Gov. Political Issues 2021, 1, 35–44. [Google Scholar] [CrossRef]
- Proyek Lahan Gambut Satu Juta Hektar. Available online: https://id.wikipedia.org/wiki/ (accessed on 17 March 2022).
- Ito, T.; Rachman, F.N.; Savitri, L.A. Power to make land dispossession acceptable: A policy discourse analysis of the Merauke Integrated Food and Energy Estate (MIFEE), Papua, Indonesia. J. Peasant. Stud. 2014, 41, 29–50. [Google Scholar] [CrossRef]
- Mukti, A. Pemberdayaan Pertanian Lokal dalam menopang Keberhasilan program Food Estate di Kalimantan Tengah. J. Socio Econ. Agric. 2020, 15, 97–107. [Google Scholar] [CrossRef]
- Surahman, A.; Soni, P.; Shivakoti, G.P. Are peatland farming systems sustainable? Case study on assessing existing farming systems in the peatland of Central Kalimantan, Indonesia. J. Integr. Environ. Sci. 2018, 15, 1–19. [Google Scholar] [CrossRef]
- Kwasek, M. Threats to food Security and common agricultural policy. Econ. Agric. 2012, 59, 701–713. [Google Scholar]
- Goldstein, J. Carbon Bomb: Indonesia’s Failed Mega Rice Project. Arcadia 2016, 6. [Google Scholar] [CrossRef]
- Pertumbuhan Ekonomi Pulang Pisau Positif 2.69%. Available online: https://www.borneonews.co.id (accessed on 20 December 2021).
- Basundono, A.F.; Sulaeman, F.H. Meninjau pengembangan Food Estate sebagai strategi ketahanan nasional pada era pandemic COVID-19. J. Kaji. Lemhanas 2020, 8, 28–42. [Google Scholar]
- Setyo, P.; Elly, J. Problems Analysis on Increasing Rice Production Through Food Estate Program in Bulungan Regency, North Kalimantan. IOP Conf. Ser. Earth Environ. Sci. 2018, 147, 012043. [Google Scholar] [CrossRef]
- Eryan, A.; Shafira, D.; Wongkar, E.E.L.T. Analisis Hukum Pengembangan Food Estate di Kawasan Hutan Lindung. Seri Analisis Kebijakan Kehutanan dan Lahan; Indonesian Centre for Environment Law: Jakarta, Indonesia, 2020. [Google Scholar]
- Food Estate Kalimantan Tengah, Kebijakan Instan, Sarat Kontroversi. Available online: https://pantaugambut.id (accessed on 20 December 2021).
- Posa, M.R.C.; Wijedasa, L.S.; Corlett, R.T. Biodiversity and conservation of tropical peat swamp forests. BioScience 2011, 61, 49–57. [Google Scholar] [CrossRef]
- Kaye, Y.L. Toolkit and Guidance for Preventing and Managing Land and Natural Resources Conflicts; The United Nations Environment Programme: Nairobi, Kenya, 2012. [Google Scholar]
- Kildow, J.; Guo, J. The gap between science and policy: Assessing the use of nonmarket valuation in Estuarine management based on a case study of US federally managed estuaries. Ocean Coast. Manag. 2015, 108, 20–26. [Google Scholar] [CrossRef]
- Suharti, S.; Darusman, D.; Nugroho, B.; Sundawati, L. Economic Valuation As a Basis for Sustainable Mangrove Resource Management A Case in East Sinjai, South Sulawesi. J. Manag. Hutan Trop. 2016, 2, 13–23. [Google Scholar] [CrossRef]
- Samadhi, T.N.; Seymour, F. To Save Indonesia’s Carbon-Rich Peatlands, Start by Mapping Them. Available online: https://wri-indonesia.org (accessed on 20 December 2021).
- Daly, H.E. On Wilfred Beckerman’s Critique of Sustainable Development. Environ. Values 1995, 4, 49–55. [Google Scholar] [CrossRef]
- Jenkins, W. Encyclopedia of Sustainability, 1st ed.; Berkshire Publishing: Great Barrington, MA, USA, 2009. [Google Scholar]
- Neumayer, E. Weak versus Strong Sustainability Exploring the Limits of Two Opposing Paradigms, 4th ed.; Edward Elgar: Cheltenham, UK, 2013. [Google Scholar]
- Faucheux, S.; O’Connor, M.; Straaten, J.V.D. Sustainable Development: Concepts, Rationalities and Strategies; Springer: Dordrecht, The Netherlands, 1998. [Google Scholar] [CrossRef]
- Davies, G.R. Appraising Weak and Strong Sustainability: Searching for a Middle Ground. Cons. J. Sustain. Dev. 2013, 10, 111–124. [Google Scholar] [CrossRef]
- Norouzi, N.; Fani, M. Comparison of Weak and Strong Theories of Environmental Sustainability in the Conceptual Context of Sustainable Development. Res. J. Ecol. Environ. Sci. 2021, 1, 108–122. [Google Scholar] [CrossRef]
- Pelenc, J.; Ballet, J.; Dedeurwaerdere, T. Weak Sustainability versus Strong Sustainability; UN Division for Sustainable Development Goals: Incheon, Korea, 2015. [Google Scholar]
- Barua, A.; Khataniar, B. Strong or weak sustainability: A case study of emerging Asia. Asia Pac. Dev. J. 2015, 22, 1–31. [Google Scholar] [CrossRef]
- Harrison, M.E.; Ottay, J.B.; D’Arcy, L.J.; Cheyne, S.M.; Anggodo; Belcher, C.; Veen, F.J.F. Tropical forest and peatland conservation in Indonesia: Challenges and Directions. People Nat. 2020, 2, 4–28. [Google Scholar] [CrossRef]
- Swindles, G.; Blundell, A.; Roe, H.; Hall, V.A. A 4500-year proxy climate record from peatlands in the North of Ireland: The identification of widespread summer ‘drought phases’? Quat. Sci. Rev. 2010, 29, 1577–1589. [Google Scholar] [CrossRef]
- Onimisi, T. The Use of Qualitative Research Method in the Study of Policy Implementation in Nigerian: Sharing an Experience. Glob. J. Politics Law Res. 2020, 8, 1–10. [Google Scholar]
- Pertiwi, N. Implemtasi Sustainable Development di Indonesia; Pustaka Ramdhan: Bandung, Indonesia, 2017. [Google Scholar]
- Hediger, W. Weak and Strong Sustainability, Environmental Conservation and Economic Growth. Nat. Resour. Model. 2006, 19, 359–394. [Google Scholar] [CrossRef]
- BRG (Badan Restorasi Gambut). Peatland Restoration Information and Management System. Available online: https://en.prims.brg.go.id (accessed on 1 November 2020).
- Creswell, J.W.; Creswell, J.D. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches; Sage: Los Angeles, CA, USA, 2017. [Google Scholar]
- Morse, J.M. Determining Sample Size. Qual. Health Res. 2000, 10, 3–5. [Google Scholar] [CrossRef]
- Ministry of Environment and Forestry. Indonesian Forestry Thematic Mapping; Directorate of Forest Resources Inventory and Monitoring: Jakarta, Indonesia, 2020.
- Anda, M.; Ritung, S.; Suryani, E.; Hikmat, M.; Yatno, E.; Mulyani, A.; Subandiono, R.E. Revisiting tropical peatlands in Indonesia: Semi-detailed mapping, extent and depth distribution assessment. Geoderma 2021, 402, 115235. [Google Scholar] [CrossRef]
- Radha, W.; Soma, P.; Wendy, W. Feminist Institutionalism and Gendered Bureaucracies: Forestry Governance in Nepal. In Feminist Institutionalism and Gendered Bureaucracies: Forestry Governance in Nepal; Springer Nature: London, UK, 2020. [Google Scholar] [CrossRef]
- Lindgren, B.M.; Lundman, B.; Graneheim, U.H. Abstraction and interpretation during the qualitative content analysis process. Int. J. Nurs. Stud. 2020, 108, 103632. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Collins, A. Getting to Know ArcGIS Desktop; Esri Press: Redlands, CA, USA, 2018. [Google Scholar]
- AS/NZS 4360; Standards Australia:1999 Risk Management. Standards Association of Australia: Strathfield, Australia, 1999.
- What is a Hazard and What is Risk? Available online: https://www.dmp.wa.gov.au (accessed on 3 December 2020).
- About Risk Assessment. Available online: https://www.epa.gov/risk/about-risk-assessment#whatisrisk (accessed on 2 December 2020).
- Gurjar, B.R.; Manju, M. Environmental Risk Analysis: Problems and Perspectives in Different Countries. Risk 2002, 13, 3. [Google Scholar]
- Iacob, V.S. Risk management and evaluation and qualitative method within the projects. Ecoforum 2014, 3, 60–67. [Google Scholar]
- Grisham, T. The Delphi technique: A method for testing complex and multifaceted topics. Int. J. Manag. Proj. Bus. 2009, 2, 112–130. [Google Scholar] [CrossRef]
- Abie, H.; Borking, J.J. Risk Analysis Methods and Practices: Privacy Risk Analysis Methodology; Norsk Regnesentral: Oslo, Norway, 2012. [Google Scholar]
- Buckley, C.C. Delphi technique supplies the classic result? Aust. Libr. J. 1994, 43, 158–164. [Google Scholar] [CrossRef]
- Risk Assessment and Mapping Guidelines for Disaster Management. Available online: https://climate-adapt.eea.europa.eu/ (accessed on 2 December 2020).
- Husnain, H.; Mulyani, A. Dukungan data sumberdaya lahan dalam pengembangan kawasn sentra produksi pangan (Food Estate) di Provinsi Kalimantan Tengah. J. Sumberd. Lahan 2021, 15, 23–35. [Google Scholar] [CrossRef]
- Government of Indonesia. Law Number 41/1999 about Forestry; Government of Indonesia: Jakarta, Indonesia, 1999.
- Government of Indonesia. Law Number 26/2007 about Spatial Planning; Government of Indonesia: Jakarta, Indonesia, 2007.
- Government of Indonesia. Government Regulation Number 71/2014 about Protection and Management of Peatland Ecosystem; Government of Indonesia: Jakarta, Indonesia, 2014.
- Government of Indonesia. Presidential Decree Number 32/1990 about Environmental Management; Government of Indonesia: Jakarta, Indonesia, 1990.
- Government of Indonesia. Presidential Decree Number 80/1999 about General Guidelines for Planning and Management of Peatland Development Areas in Central Kalimantan; Government of Indonesia: Jakarta, Indonesia, 1990.
- Badan Pusat Statistik (BPS). Keadaan Tenagakerjaan Provinsi Kalimantan Tengah Februari 2021; Badan Pusat Statistik: Jakarta Pusat, Kalimantan Tengah, Indonesia, 2021.
- De Jong, W.; Van Noordwijk, M.; Sirait, M.; Liswanti, N. Farming Secondary Forests in Indonesia. J. Trop. For. Sci. 2001, 13, 705–726. [Google Scholar]
- Suriadikarta, D.A. Pemanfaatan dan strategi pengembangan lahan gambut eks PLG Kalimantan Tengah. J. Sumberd. Lahan 2008, 2, 31–44. [Google Scholar]
- Prayoga, K. Pengelolaan lahan gambut berbasis kearifan lokal Pulau Kalimantan. In Proceedings of the Proseding Seminar Nasional Lahan Basah, Banjarmasin, Indonesia, 5 November 2016. [Google Scholar]
- Sakuntaladewi, N.; Rachmanadi, D.; Mendham, D.; Yuwati, T.W.; Winarno, B.; Premono, B.T.; Lestari, S.; Ardhana, A.; Ramawati; Budiningsih, K.; et al. Can We Simultaneously Restore Peatlands and Improve Livelihoods? Exploring Community Home Yard Innovations in Utilizing Degraded Peatland. Land 2022, 11, 150. [Google Scholar] [CrossRef]
- Ekawati, S.; Sylviani; Surati; Ramawati; Handoyo; Prasetyo, B.D.; Lugina, M.; Hidayat, D.C.; Sumirat, B.K.; Hardiansyah, G.; et al. Strategi untuk mendorong masyarakat menerapkan Teknik budidaya gambut berkelanjutan di Pulang Pisau. Policy Brief 2020, 14, 1–70. [Google Scholar]
- Simangunsong, B.C.H.; Manurung, E.G.T.; Elias, E.; Hutagaol, M.P.; Tarigan, J.E.P.; Prabawa, S.P. Tangible economic value of non timber forest products from peat swamp forest in Kampar, Indonesia. Biodiversitas 2020, 21, 5954–5968. [Google Scholar] [CrossRef]
- Harrison, M.E. Using conceptual models to understand ecosystem function impacts of human activities in tropical peatswamp forest. Wetlands 2013, 33, 257–267. [Google Scholar] [CrossRef]
- Hoing, A.; Radjawali, I. Flexible livelihood strategies coming to an end? The case of forest-dependent communities in Central and West Kalimantan. In Continuity under Change in Dayak Societies; Arenz, C., Haug, M., Seitz, S., Venz, O., Eds.; Springer: Wiesbaden, Germany, 2017; pp. 73–95. [Google Scholar]
- O’Connor, A.; Sunderland, T.C.H. Non-timber forest products from tropical forests. In Achieving Sustainable Management of Tropical Forests; Blaser, J., Hardcastle, P.D., Eds.; Burleigh Dodds Science: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Nasrul, B. Program of community empowerment prevents forest fires in Indonesian peat land. Procedia Environ. Sci. 2013, 17, 129–134. [Google Scholar] [CrossRef]
- Minayeva, T.Y.; Sirin, A.A. Peatland biodiversity and climate change. Biol. Bull. Rev. 2012, 2, 164–175. [Google Scholar] [CrossRef]
- Anderson, B. Imagined Communities; The Thetford Press: Norfollk, UK, 1983. [Google Scholar]
- Phillips, D. Subjectivity and Objectivity: An Objective Inquiry. In Qualitative Inquiry in Education: The Continuing Debate; Teachers College Press: New York, NY, USA, 1990; pp. 19–37. [Google Scholar]
- Page, S.E.; Rieleys, J.O.; Doody, K.; Hodgson, S.; Husson, S.; Jenkins, P.; Morrogh-Bernard, H.; Otway, S.; Wilshaw. Biodiversity of tropical peat swamp forest a case study of animal diversity in Sungai Sebangau catcment of Central Kalimantan, Indonesia. In Biodiversity and Sustainable Management of Tropical Peatland; Rieley, J.O., Page, S.E., Eds.; Samara Publishing Ltd.: Samana, UK, 1997; pp. 231–242. [Google Scholar]
- Sweking; Mahyudin, I.; Mahareda, E.S.; Salawati, U. Produksi dan jumlah ikan yang tertangkap oleh nelayan di Dungai Kahayan, Kecamatan Palanduk, Kota Palangkaraya, Provinsi Kalimantan Tengah. Environ. Sci. 2011, 7, 39–49. [Google Scholar]
- Minggawati, I.; Madani; Marianti, R. Aspek biologi dan manfaat ekonomiikan yang tertangkap di Sungai Sebangau Kota Palangkaraya, Kalimantan Tengah. Ziraal’al 2020, 45, 335–340. [Google Scholar]
- Jannah, L. Kelimpahan Jenis Udang (Crustaceae) di Aliran Sungai Kahayan di Kota Palangkaraya; Institut Agama Islam Negeri Palangkaraya: Palangkaraya, Indonesia, 2015. [Google Scholar]
- Syafrudin, S. Identifikasi jenis udang (Crustaceae) di Daerah Aliran Sungai (DAS) Kahayan Kota Palangkaraya Provinsi Kalimantan Tengah; Institut Agama Islam Negeri Palangkaraya: Palangkaraya, Indonesia, 2016. [Google Scholar]
- Russon, A.E.; Erman, A.; Dennis, R. The population and distribution of orangutans (Pongo pygmaeus pygmaeus) in and around the Danau Sentarum Wildlife Reserve, West Kalimantan, Indonesia. Biol. Conserv. 2001, 97, 21–28. [Google Scholar] [CrossRef]
- Gaither, J.C., Jr. Understory avifauna of a Bornean peat swamp forest: Is it depauperate? Wilson Bull. 1994, 106, 381–390. [Google Scholar]
- Singleton, I.; van Schaik, C.P. Orangutan homerange size and its determinants in Sumatra Swamp. Forest. Int. J. Primatol 2001, 22, 877–911. [Google Scholar] [CrossRef]
- Struebig, M.J.; Harrison, M.E.; Cheyne, S.M.; Limin, S.H. Intensive hunting of large flung-foxes (Pteropus vampyrus natunae) in Central Kalimantan, Indonesia Borneo. Oryx 2007, 4, 390–393. [Google Scholar] [CrossRef]
- Langner, A.; Siegert, F. Spatiotemporal fire occurrence in Borneo over period of 10 years. Glob. Change Biol. 2009, 15, 48–62. [Google Scholar] [CrossRef]
- Page, S.; Hoscilo, A.; Langner, A.; Tansey, K.; Siegert, F.; Limin, S.; Rieley, J. Tropical peatland fires in Southeast Asia. In Tropical Fire Ecology; Cochrane, M.A., Ed.; Springer: Berlin, Heidelberg, 2009; pp. 263–287. [Google Scholar] [CrossRef]
- Minayeva, T.; Bragg, O.; Cherednichenko, O.; Couwenberg, J.; van Dunien, G.J.; Giesen, W.; Grootjans, A.; Grundling, P.I.; Nikoleye, V.; van der Schaaf, S. Peatland and Biodiversity. In Assessment on Peatlands, Biodiversity and Climate; Parish, F., Sirin, A., Chama, D., Jasten, H., Minayeva, T., Silvius, M., Stringer, Eds.; Global Environment Centre and Wetlands International: Kuala Lumpur, Malaysia; Wageningen, The Netherlands, 2008; pp. 60–78. [Google Scholar]
- Morrough-Bernard, H.C.; Morf, N.V.; Chivers, D.J.; Krutzu, M. Dispersal patterns of orangutan (Pongo spp.) in Borneon peat-swamp forest. Int. J. Primatol. 2011, 32, 362–376. [Google Scholar] [CrossRef]
- Cheyne, S.M.; Husson, P.J.; Dragiewicz, M.L.; Thormpson, L.J.; Adul, A.; Jeffers, K.A.; Limin, S.M.; Smith, D.A.E. Kalimantan tropical peat-swamp forests are important for storm’s stork (Ciconia storm) conservation. J. Indones. Nat. Hist. 2014, 2, 45–50. [Google Scholar]
- Kimmel, K.; Mander, U. Ecosystem services of peatlands: Implications for restoration. Prog. Phys. Geogr. Earth Environ. 2010, 34, 491–514. [Google Scholar] [CrossRef]
- Schaik, C.P.; Bastian, M.L.; Noordwijk, M.A. Innovative behaviour in wild borneo Orangutans revealed by targeted populations comparison. Behaviour 2012, 149, 275–297. [Google Scholar] [CrossRef]
- Hermansyah, H.; Salundih, S.; Priyanto, R. Phyciological response of reared Bali Cattle based on different peatland characteristics. Chalaza J. Anim. Husb. 2020, 5, 12–21. [Google Scholar] [CrossRef]
- Yule, C.M. Loss of biodiversity and ecosystem functioning in Indonesia-Malaya peat swamp forests. Biodivers. Conserv. 2010, 19, 393–409. [Google Scholar] [CrossRef]
- 10 Animals That Bad for Environment. Available online: https://www.treehugger.com (accessed on 10 December 2021).
- Lahtinen, L.; Mattila, T.; Myllyviita, T.; Seppala, J.; Vasander, H. Effects of paludiculture products on reducing greenhouse gas emissions from agricultural peatlands. Ecol. Eng. 2022, 175, 106502. [Google Scholar] [CrossRef]
- Yuwati, T.W.; Rachmanadi, D.; Qirom, M.A.; Santosa, P.B.; Kusin, K.; Tata, H.L. Peatland Restoration in Central Kalimantan by Rewetting and Rehabilitation with Shorea balangeran. In Tropical Peatland Eco-Management; Osaki, M., Tsuji, N., Foead, N., Rieley, J., Eds.; Springer: Singapore, 2021; pp. 595–611. [Google Scholar] [CrossRef]
- Firmansyah, M.A.; Mokhtar, M.S. Kearifan lokal pemanfaatan lahan gambut untuk usaha tani dalam mengantisipasi dampak perubahan iklim di Kalimantan Tengah. In Proceedings of the Workshop Nasional dan FGD (Focus Group Discussion) Adaptasi Perubahan Iklim di Sektor Pertanian, Bandung, Indonesia, 9–10 November 2011; pp. 1–11. [Google Scholar]
- Keenan, R.J. Climate change impacts and adaptation in forest management: A review. Ann. For. Sci. 2015, 72, 145–167. [Google Scholar] [CrossRef]
- Page, S.E.; Hooijer, A. In the line of fire: The peatlands of Southeast Asia. Phil. Trans. R. Soc. B 2016, 371, 20150176. [Google Scholar] [CrossRef] [PubMed]
- Khakim, M.Y.N.; Bama, A.A.; Yustian, I.; Poerwono, P.; Tsuji, T.; Matsuoka, T. Peatland subsidence and vegetation cover degradation as impacts of the 2015 El niño event revealed by Sentinel-1A SAR data. Int. J. Appl. Earth. Obs. Geoinf. 2020, 84, 101953. [Google Scholar] [CrossRef]
- Kelly, T.J.; Lawson, I.T.; Roucoux, K.H.; Baker, T.R.; Jones, T.D.; Sanderson, N.K. The vegetation history of an Amazonian domed peatland. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 468, 129–141. [Google Scholar] [CrossRef]
- Hartatik, W.; Subiksa, I.G.M.; Duirah, A.C. Sifat Fisik dan Kimia Tanah Gambut; Balai Penelitian Tanah: Bogor, Indonesia, 2011; pp. 45–56. [Google Scholar]
- Tata, H.L. Paludiculture: Can it be a trade-off between ecology and economic benefit on peatland restoration? IOP Conf. Ser. Earth Environ. Sci. 2019, 394, 012061. [Google Scholar] [CrossRef]
- Ribeiro, K.; Pacheco, F.S.; Ferreira, J.W.; de Sousa-Neto, E.R.; Hastie, A.; Filho, G.C.K.; Alvalá, P.C.; Forti, M.C.; Ometto, J.P. Tropical peatlands and their contribution to the global carbon cycle and climate change. Glob. Change Biol. 2021, 27, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Radu, D.D.; Duval, T.P. Precipitation frequency alters peatland ecosystem structure and CO2 exchange: Contrasting effects on moss, sedge, and shrub communities. Glob. Change Biol. 2018, 24, 2051–2065. [Google Scholar] [CrossRef]
- Molak, V. Fundamentals of Risk Analysis and Risk Management; Lewis Publishers: New York, NY, USA, 1997. [Google Scholar]
- Misra, K.B. Risk Analysis and Management: An Introduction. In Handbook of Performability Engineering; Misra, K.B., Ed.; Springer: London, UK, 2008. [Google Scholar] [CrossRef]
- Surahman, A.; Shivakoti, G.P.; Soni, P. Climate change mitigation through sustainable degraded peatland management in Central Kalimantan, Indonesia. Int. J. Commons 2019, 13, 859–866. [Google Scholar] [CrossRef]
- Dohong, A.; Aziz, A.A.; Dargusch, P. A review of the drivers of tropical peatland degradation in South-East Asia. Land Use Policy 2017, 69, 349–360. [Google Scholar] [CrossRef]
- Tan, Z.D.; Lupascu, M.; Wijedasa, L.S. Palidiculture as a sustinable land use alternative for tropical peatlands: Review. Sci. Environ. 2021, 753, 2–14. [Google Scholar] [CrossRef]
- Giesen, W.; Wijedasa, L.S.; Page, S. Unique Southeast Asian peat swamp forest habitats have relatively few distinctive peat species. Mires Peat 2018, 22, 1–13. [Google Scholar] [CrossRef]
- Salim, A.G.; Narendra, B.H.; Dharmawan, I.W.S.; Pratiwi, P. Chemical and hydro-physical peat characteristics under agricultural peat land management in Central Kalimantan, Indonesia. Pol. J. Environ. Stud. 2021, 30, 4647–4655. [Google Scholar] [CrossRef]
- Salim, A.G.; Narendra, B.H.; Lisnawati, Y.; Rachmat, H.H. Hydro-physical properties of agriculturally used peatlands in Liang Anggang Protected Forest, South Kalimantan, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 789, 012048. [Google Scholar] [CrossRef]
- Medrilzam, M.; Smith, C.; Aziz, A.A.; Herbohn, J.; Dargusch, P. Smallholder farmers and the dynamics of degradation of peatland ecosystems in Central Kalimantan, Indonesia. Ecol. Econ. 2017, 136, 101–113. [Google Scholar] [CrossRef]
- Rachmanadi, D.; Faridah, E.; van DeerMeer, P.J. Keanekaragaman potensi regenerasi vegetasi pada hutan rawa gambut: Studi kasus di Kawasan Hutan dengan Tujuan Khusus (KHDTK) Tumbang Nusa, Kalimantan Tengah. J. Ilmu Kehutan. 2017, 11, 224–238. [Google Scholar] [CrossRef][Green Version]
- Agus, C.; Ilfana, Z.R.; Azmi, F.F.; Rachmanadi, D.; Wulandari, D.; Santosa, P.B.; Harun, M.K.; Yuwati, T.W.; Lestari, T. The effect of tropical peatland-use changes on plant diversity and soil properties. Int. J. Environ. Sci. Technol. 2020, 17, 1703–1712. [Google Scholar] [CrossRef]
- Hooijer, A.; Page, S.; Jauhiainen, J.; Lee, W.A.; Lu, X.X.; Idris, A.; Anshari, G. Subsidence and carbon loss in drained tropical peatlands. Biogeosciences 2012, 9, 1053–1071. [Google Scholar] [CrossRef]
- Tonks, A.J.; Aplin, P.; Beriro, D.J.; Cooper, H.; Evers, S.; Vane, C.H.; Sjögersten, S. Impacts of conversion of tropical peat swamp forest to oil palm plantation on peat organic chemistry, physical properties and carbon stocks. Geoderma 2017, 289, 36–45. [Google Scholar] [CrossRef]
- Nurulita, Y.; Adetutu, E.M.; Gunawan, H.; Zul, D.; Ball, A.S. Restoration of tropical peat soils: The application of soil microbiology for monitoring the success of the restoration process. Agric. Ecosyst. Environ. 2016, 216, 293–303. [Google Scholar] [CrossRef]
- Ziegler, R.; Wichtmann, W.; Abel, S.; Kemp, R.; Simard, M.; Joosten, H. Wet peatland utilization for climate protection—An international survey of paludiculture innovation. Clean. Eng. Technol. 2021, 5, 100305. [Google Scholar] [CrossRef]
- Kolinski, L.; Milich, K.M. Human-Wildlife Conflict Mitigation Impacts Community Perceptions around Kibale National Park, Uganda. Diversity 2021, 13, 145. [Google Scholar] [CrossRef]
- Sulistyawan, B.S.; Eichelberger, B.A.; Verweij, P.; Boot, R.A.A.; Hardian, O.; Adzan, G.; Suknartono, W. Connecting the fragmented habitat of endangered mammals in the landscape of Riau-Jambi-Sumatera Barat (RIMBA), Central Sumatra, Indonesia. Glob. Ecol. Conserv. 2017, 9, 116–130. [Google Scholar] [CrossRef]
- Ramiadantsoa, T.; Ovaskainen, O.; Rybicki, J.; Hanski, I. Large-Scale Habitat Corridors for Biodiversity Conservation: A Forest Corridor in Madagascar. PLoS ONE 2015, 10, e0132126. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M.; Bennett, A.F. Identifying wildlife corridors for the restoration of regional habitat connectivity: A multispecies approach and comparison of resistance surfaces. PLoS ONE 2018, 13, e0206071. [Google Scholar] [CrossRef] [PubMed]
- Curcic, N.; Djurdjic, S. The actual relevance of ecological corridors in nature conservation. J. Geogr. Inst. Jovan Cvijic SASA 2013, 63, 21–34. [Google Scholar] [CrossRef]
- Kelompok Kerja Pengelolaan KEE Bentang Alam Wehea-Kelay. Koridor Orangutan Bentang Alam Wehea-Kelay di Kabupaten Kutai Timur dan Kabupaten Berau Provinsi Kalimantan Timur; The Nature Conservancy: Samarinda, Indonesia, 2016. [Google Scholar]
- Astiani, D.; Ekamawanti, H.A.; Ekyastuti, W.; Widiastuti, T.; Tavita, G.E.; Suntoro, M.A. Tree species distribution in tropical peatland forest along peat depth gradients: Baseline notes for peatland restoration. Biodiversitas 2021, 22, 2571–2578. [Google Scholar] [CrossRef]
- Garsetiasih, R.; Heriyanto, N.M.; Adinugroho, W.C.; Gunawan, H.; Dharmawan, I.W.S.; Sawitri, R.; Yeny, I.; Mindawati, N. Connectivity of vegetation diversity, carbon stock, and peat depth in peatland ecosystems. Glob. J. Environ. Sci. Manage. 2022, 8, 369–388. [Google Scholar] [CrossRef]
- Tanneberger, F.; Appulo, L.; Ewert, S.; Lakner, S.; Ó Brolcháin, N.; Peters, J.; Wichtmann, W. The power of nature-based solutions: How peatlands can help us to achieve key EU sustainability objectives. Adv. Sustain. Syst. 2021, 5, 2000146. [Google Scholar] [CrossRef]
- Humpenoder, F.; Karstens, K.; Lotze-Campen, H.; Leifeld, J.; Menichetti, L.; Barthelmes, A.; Popp, A. Peatland protection and restoration are key for climate change mitigation. Environ. Res. Lett. 2020, 15, 104093. [Google Scholar] [CrossRef]
- Walton, C.R.; Zak, D.; Audet, J.; Petersen, R.J.; Lange, J.; Oehmke, C.; Hoffmann, C.C. Wetland buffer zones for nitrogen and phosphorus retention: Impacts of soil type, hydrology and vegetation. Sci. Total Environ. 2020, 727, 138709. [Google Scholar] [CrossRef] [PubMed]
- Wichtmann, W.; Schröder, C.; Joosten, H. Paludiculture—Productive Use of Wet Peatlands: Climate Protection—Biodiversity—Regional Economic Benefits; Schweizbart Science Publishers: Stuttgart, Germany, 2016. [Google Scholar]
- Giesen, W.; Sari, E.N. Tropical Peatland Restoration Report: The Indonesian Case; Millennium Challenge Account: Jakarta, Indonesia, 2018. [Google Scholar]
- Nong, Y.; Yin, C.; Yi, X.; Ren, J.; Chien, H. Farmers’ Adoption Preferences for Sustainable Agriculture Practices in Northwest China. Sustainability 2020, 12, 6269. [Google Scholar] [CrossRef]
- Awang, A.H.; Rela, I.Z.; Abas, A.; Johari, M.A.; Marzuki, M.E.; Mohd Faudzi, M.N.R.; Musa, A. Peat Land Oil Palm Farmers’ Direct and Indirect Benefits from Good Agriculture Practices. Sustainability 2021, 13, 7843. [Google Scholar] [CrossRef]
- Nuroniah, H.S.; Tata, H.L.; Mawazin; Martini, E.; Dewi, S. Assessment on the Suitability of Planting Non-Native Peatlands Species Falcataria moluccana (Miq.) Barneby & Grimes in Rewetted Peatlands. Sustainability 2021, 13, 7015. [Google Scholar] [CrossRef]
- Silvianingsih, Y.A.; Hairiah, K.; Suprayogo, D.; van Noordwijk, M. Kaleka Agroforest in Central Kalimantan (Indonesia): Soil Quality, Hydrological Protection of Adjacent Peatlands, and Sustainability. Land 2021, 10, 856. [Google Scholar] [CrossRef]
- Paleckiene, R.; Navikaite, R.; Slinksiene, R. Peat as a Raw Material for Plant Nutrients and Humic Substances. Sustainability 2021, 13, 6354. [Google Scholar] [CrossRef]
- Prastyaningsih, S.R.; Hardiwinoto, S.; Agus, C. Development Paludiculture on Tropical Peatland for Productive and Sustainable Ecosystem in Riau. IOP Conf. Ser. Earth Environ. Sci. 2019, 256, 012048. [Google Scholar] [CrossRef]
- Budiman, I.; Sari, E.N.; Hadi, E.E.; Siahaan, H.; Januar, R.; Hapsari, R.D. Progress of paludiculture projects in supporting peatland ecosystem restoration in Indonesia. Glob. Ecol. Conserv. 2020, 23, e01084. [Google Scholar] [CrossRef]
- Uda, S.K.; Hein, L.; Adventa, A. Towards better use of Indonesian peatlands with paludiculture and low-drainage food crops. Wetl. Ecol. Manage. 2020, 28, 509–526. [Google Scholar] [CrossRef]
- Giesen, W. Utilisig non-timber forest products conserve Indonesia’s peat swamp forest and reduce carbon emission. J. Indones. Hist. 2015, 3, 17–26. [Google Scholar]
- Kamal, S.; Grodzińska-Jurczak, M.; Brown, G. Conservation on private land: A review of global strategies with a proposed classification system. J. Environ. Plann. Manag. 2014, 58, 576–597. [Google Scholar] [CrossRef]
- Wahyunto, W.; Supriatna, W.; Agus, F. Land use change and recommendation for sustainable development of peatland for agriculture: Case study at Kubu Raya and Pontianak Districts, West Kalimantan. Indones. J. Agric. Sci. 2010, 11, 32–40. [Google Scholar] [CrossRef]
- Syahza, A.; Bakce, D.; Irianti, M. Improved Peatlands Potential for Agricultural Purposes to Support Sustainable Development in Bengkalis District, Riau Province, Indonesia. J. Phys. Conf. Ser. 2019, 1351, 012114. [Google Scholar] [CrossRef]
- WALHI (Wahana Lingkungan Hidup Indonesia). Food Estate, Measuring Food Politics of Indonesia: A Study of Central Kalimantan Food Estate Project; Walhi-Kalimantan Tengah: Palangkaraya, Indonesia, 2021. [Google Scholar]
- Firmansyah, M.A.; Yuliani, N.; Nugroho, W.A.; Bhermana, A. Suitability of tidal swamp for rubber plantation in three villages of ex rice mega project, Pulang Pisau regency, Central Kalimantan Province. J. Suboptimum Land 2012, 1, 149–157. [Google Scholar]
- Joosten, H.; Tapio-Biström, M.; Tol, S. Peatlands—Guidance for Climate Change Mitigation through Conservation, Rehabilitation and Sustainable Use, 2nd ed.; FAO and the Wetlands International: Roma, Italy, 2012. [Google Scholar]
- Syahza, A.; Bauce, D.; Nasrul, B.; Irianti, M. Peatland policy and management strategy to support sustainable development in Indonesia. J. Phys. Conf. Ser. 2020, 1655, 012151. [Google Scholar] [CrossRef]
- Graham, L.L.B.; Giesen, W.; Page, S.E. A common-sense approach to tropical peat swamp forest restoration in Southeast Asia. Restor. Ecol. 2017, 25, 312–321. [Google Scholar] [CrossRef]


| No. | Scales | Description | |
|---|---|---|---|
| 1. | Severity level of risk | ||
| Insignificant | 1 | Minor impact that can be recovered quickly and naturally; there are no tenure and horizontal conflicts; alternative sources of livelihood outside the agricultural sector are available; FE does not affect the social capital of the community; shallow peat <100 cm is more suitable for agricultural crops in successful rewetting efforts | |
| Minor | 2 | Causes a slight seasonal decline in tree and/or animal populations; absorption of unskilled labor; limited involvement by local workers (only members of farmer groups); emergence of latent social conflicts; FE affects social capital among farmer groups | |
| Moderate | 3 | Causes a decrease in forest cover for animal habitat and/or causes many species of wildlife to move in some areas; causes damage to crops or/and depredation of livestock; limited involvement by local workers (only the heads of farmer groups); there is a social conflict that can be resolved by discussion; FE affects social capital among farmers in one village; peat with a depth of more than 100 cm faces tougher challenges in water level management, where a failure or an ineffective rewetting process will make the water level difficult to control such that the greenhouse gas (GHG) emissions will fluctuate. | |
| Major | 4 | Causes habitat loss and habitat fragmentation; many wildlife species populations decline or leave the site; high competition for space and feed between livestock and wild animals; limited involvement of local workers (only high achieving farmer groups); open conflict occurs and conflict resolution in the negotiation process is more difficult; FE affects social capital among farmers in one sub-district | |
| Extreme | 5 | Forest cover has changed completely into cultivation areas that can no longer be used as habitats; almost all terrestrial wildlife species disappear or are moved from these locations; no increase in income; local workers are not involved; open conflicts occur; FE causes the loss of local wisdom in managing peat; peat with a depth of >200 cm has low fertility; the excessive use of fertilizers accelerates peat decomposition and exacerbates the physiological damage to peat; on thick peat, rewetting is more difficult, and failure to do so causes the peat to remain exposed, thereby emitting greenhouse gases. | |
| 2. | Likelihood level of risk | ||
| Almost certain | A | Expected to occur in most circumstances; has occurred in this location; no specific protection identified and applied | |
| Likely | B | Will probably occur in most circumstances; has occurred in this location; specific protection identified and applied | |
| Possible | C | Might occur at some time; has occurred in this landscape but not in a food estate | |
| Unlikely | D | Could occur at some time; has occurred related to FE development | |
| Rare | E | May occur only in exceptional circumstances |
| Socio-Economic Aspect | Name of Village | |||
|---|---|---|---|---|
| Tumbang Nusa | Pilang | Garung | Gohong | |
| Main livelihood | Fishermen | Farmers and rubber tappers | Farmers and rubber tappers | Farmers and rubber tappers |
| Side livelihood | Traders | Traders, rattan crafts | Traders, rattan crafts | Traders, fishpond farmers, rattan craft |
| Arable area | No arable land | 2–5 ha | 2–5 ha | 2–5 ha |
| Mean income/month (USD) | 172.118 | 137.69 | 172.118 | 172.118 |
| No. | Institution Name | Number of Members in the Group (Person) | Purpose |
|---|---|---|---|
| 1. | Forest Farmer Group | ±15 | Conducting forestry business |
| 2. | Paddy and Vegetable Farmer Group | ±10 | Conducting agricultural business |
| 3. | Estate Crop Farmer Group | ±12 | Developing rubber or oil palms |
| 4. | Fishery Farmers Group | ±14 | Developing diversification and basic shares of marine and fishery production |
| 5. | Village-Owned Enterprises (Bumdes) | >3 | Improving the economies of village communities |
| 6. | Village Forest Management Institution (LPHD) | >16 | Managing forest area so that it can be of economic value for the welfare of its members |
| 7. | Fire Care Community groups | 20 | Controlling forest and land fires |
| 8. | Community groups that care about dams and water | 10 | Regulating the water level on peatlands |
| Land Status | Peat Depth (cm) | Potential Area (Ha) | Risk | |||
|---|---|---|---|---|---|---|
| Paddy Field | Horticulture | Perennial Crop | Total | |||
| APL | Mineral soil | 74,984 | 730 | 190 | 75,905 | Low |
| 50 < 100 | 5630 | 1 | 5631 | Low | ||
| 100–300 | 1457 | 926 | 5610 | 7993 | Moderate | |
| HL | Mineral soil | 19,052 | 45 | 17 | 19,114 | Moderate |
| 50–<100 | 11,787 | 125 | 406 | 12,318 | Moderate | |
| 100–300 | 1584 | 26,219 | 29,426 | 57,229 | High | |
| HP | Mineral soil | 30,177 | 270 | 239 | 30,686 | Low |
| 200–300 | 2026 | 814 | 15,106 | 17,946 | High | |
| Total | 226,823 | |||||
| No. | Activity | Risk Identification |
|---|---|---|
| 1 | Hunting or harvesting wildlife | Populations declining and threatened species to become extinct |
| 2 | Human activities in wildlife habitats | Edge effects and reduced space for animal activities |
| 3 | Conventional farming | Low productivity, low income |
| 4 | Free grazing husbandry | Competition with wild animals, transfer of disease |
| 5 | Farmer institutions have not been effective | Difficult to develop on a large scale or industrial scale (plantation) |
| 6 | Limited agricultural infrastructure, roads, and bridges | Distribution of food production is not optimal |
| No. | Activity | Risk Identification |
|---|---|---|
| 1 | Drainage system | Habitat fragmentation, drought, fire risk |
| 2 | Extensive peat swamp forest conversion | Loss of habitat, reduction in peat swamp tree species, animal–human conflicts |
| 3 | Introduction of exotic animal and fish species |
|
| 4 | Monoculture rice crop development |
|
| 5 | Determination of FE area and infrastructure development does not accommodate the interests of all communities | Social conflicts that hinder the acceleration of local economic recovery |
| 6 | Mechanization of agriculture and modernization of the agricultural system |
|
| 7 | Development of FE requires a large investment | Possession of assets by several parties (corporations) |
| 8 | FE development location is not clear or clean | Potential for tenurial conflicts |
| 9 | Use of fire-prone peat areas | Degradation of habitat quality, destruction of habitat, destruction of various types of animals |
| 10 | Use of 100–300 cm depth peatlands in protected forest areas | High risk to surrounding peatlands |
| Risks to Biodiversity | Category of Severity | Mitigation Management |
|---|---|---|
| Extreme | Foster animal husbandry, the captive breeding of wildlife, and freshwater fisheries to meet animal protein needs |
| High | Minimize the activities of food estate workers on the edge of the conservation area and create a vegetation buffer on the conservation area |
| High | Cattle should not be freely grazed in the wild, but rather should be raised and reared in fenced areas, and a feed garden should be created to meet the needs of animal feed |
| Medium | Cattle should not be freely grazed, but rather should be raised and reared in fenced areas |
| Extreme | Conduct fish farming with a pond system that is not directly connected to open waters |
| Low | Conduct fish farming with a pond system that is not directly connected to open waters |
| Extreme | Connect the swamp landscape by creating canal corridors and green belts |
| High | Forbid slash and burn farming. Cultivate suitable swamp habitat plants so there is no need to dry the swamp |
| Extreme | Leave intact vegetated areas to conserve endemic tree species, especially in deep peat areas. Apply an agroforestry system that combines agricultural crops with local forest trees |
| Socio-Economic and Land Use Risks | Category of Severity | Mitigation Management |
|---|---|---|
| Moderate | Increase community capacity through training and education in peatland protection and management |
| Moderate | Form institutions among stakeholders related to food estate development programs, both at the local and the central levels |
| Extreme | Develop incentives and access to capital to manage and protect peatlands |
| High | Socialize the FE program in every village that has administrative boundaries with the prospective FE development area |
| Moderate | Socialize the FE program in every village that has administrative boundaries with the prospective FE development area |
| High | Identification of potential FE areas with related stakeholder involvement |
| Extreme | Increase skill activities in various forms of business that can be developed |
| High | Develop appropriate technology for the management and utilization of peatlands based on community knowledge |
| Moderate | Manage degraded peatlands in the development of FE in line with peat restoration efforts. |
| Moderate | Form specific formulations for peatland farming that can maintain the organic cycle |
| High | Construct road and bridge adopting peat-friendly technology |
| Moderate | The use of various species in the context of integrated multi-business (agriculture, livestock, fisheries, and forestry) to increase the variety of income sources |
| Sources/Hazard Factors | Risk Analysis | Total | ||||
|---|---|---|---|---|---|---|
| Low | Moderate | High | Extreme | |||
| 1. | Human | 3 | 3 | 2 | 8 | |
| 2. | Paddy development in peatland | 1 | 1 | 2 | ||
| 3. | Free-grazing livestock species | 1 | 1 | 2 | ||
| 4. | Farmed fish species | 1 | 1 | 2 | ||
| 5 | Farming method | 2 | 2 | 3 | 7 | |
| Total | 1 | 7 | 7 | 6 | 21 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeny, I.; Garsetiasih, R.; Suharti, S.; Gunawan, H.; Sawitri, R.; Karlina, E.; Narendra, B.H.; Surati; Ekawati, S.; Djaenudin, D.; et al. Examining the Socio-Economic and Natural Resource Risks of Food Estate Development on Peatlands: A Strategy for Economic Recovery and Natural Resource Sustainability. Sustainability 2022, 14, 3961. https://doi.org/10.3390/su14073961
Yeny I, Garsetiasih R, Suharti S, Gunawan H, Sawitri R, Karlina E, Narendra BH, Surati, Ekawati S, Djaenudin D, et al. Examining the Socio-Economic and Natural Resource Risks of Food Estate Development on Peatlands: A Strategy for Economic Recovery and Natural Resource Sustainability. Sustainability. 2022; 14(7):3961. https://doi.org/10.3390/su14073961
Chicago/Turabian StyleYeny, Irma, Raden Garsetiasih, Sri Suharti, Hendra Gunawan, Reny Sawitri, Endang Karlina, Budi Hadi Narendra, Surati, Sulistya Ekawati, Deden Djaenudin, and et al. 2022. "Examining the Socio-Economic and Natural Resource Risks of Food Estate Development on Peatlands: A Strategy for Economic Recovery and Natural Resource Sustainability" Sustainability 14, no. 7: 3961. https://doi.org/10.3390/su14073961
APA StyleYeny, I., Garsetiasih, R., Suharti, S., Gunawan, H., Sawitri, R., Karlina, E., Narendra, B. H., Surati, Ekawati, S., Djaenudin, D., Rachmanadi, D., Heriyanto, N. M., Sylviani, & Takandjandji, M. (2022). Examining the Socio-Economic and Natural Resource Risks of Food Estate Development on Peatlands: A Strategy for Economic Recovery and Natural Resource Sustainability. Sustainability, 14(7), 3961. https://doi.org/10.3390/su14073961








