Abstract
In the actual context of global change and biodiversity depletion, soil bioengineering represents an important tool for riparian ecosystem restoration and species conservation. Various techniques have already been implemented, but their adaptation still must be carried out in Caribbean Islands biodiversity hotspots, where suitable species remains unknown. Nitrogen-fixing legumes are particularly relevant for ecological restoration and the diversity of native Caribbean legume trees is promising in the search for suitable species for soil bioengineering. We hypothesized that Caribbean legume tree species present a growth performance and set of biotechnical traits compatible with their use in soil bioengineering. We selected five native legume trees, adapted to riparian environments, in different ecosystems (swamp forest, evergreen seasonal forest, rainforest) based on their ecology, resistance to disturbance and seed production characteristics. We measured root traits relevant for soil bioengineering on nursery grown 3-month-old seedlings. Despite their differences in sensitivity to herbivory and in growth strategies, the selected species have a high potential for use in soil bioengineering, with high seed production, high germination rates—from 88 to 100%—, and 100% survival rates, and are therefore compatible with large scale plant material production. We provided practical guidance tools for their integration into soil bioengineering techniques.
1. Introduction
In the context of global change and the erosion of biodiversity, ecosystem restoration and species conservation are priorities [1], particularly in places under high anthropogenic pressure such as riparian areas [2]. Riparian belts, at the interface between terrestrial and freshwater habitats, are of disproportionate importance relative to their spatial extent [3]. Although they only represent 1.4% of continental land surfaces, riparian zones and related floodplains contribute to more than 25% of all terrestrial ecosystem services [4]. Riparian areas worldwide are increasingly affected by urban development, industrial uses and agricultural expansion, which disrupt their structure and function [5,6]. In the “Caribbean Island hotspot”, i.e., one of the 35 world hotspots of biodiversity, wildlife is both rich and threatened, with a high level of endemism [7]; the Guadeloupe archipelago is remarkably biodiverse: native terrestrial vegetation comprises 1706 native vascular species [8], distributed over 34 ecosystem types [9,10]. The riparian ecosystems of Guadeloupe, particularly those close to human activities, are highly impacted by pollutions, deforestation, degradation and invasive alien species [11].
Soil bioengineering is a nature based solution that responds to societal challenges efficiently and adaptively and contributes to human wellbeing through its efficacy in controlling erosion and nurturing biodiversity [12]. It can be defined as the inclusion of vegetation into engineering designs to improve and protect slopes, embankments and structures from problems associated with erosion and other types of shallow slope failures [13]. Vegetation can positively impact soil degradation processes such as surface erosion and shallow landslides. Woody vegetation can exert a positive influence on the soil water regime and, thus, on slope stability [14]. Root systems of woody vegetation provides additional soil strength and cohesion, increasing the stability of shallow soils on steep slopes [15]. In shallow soils, tree roots may deeply penetrate the soil to anchor into a more stable substrate [16]. In the upper soil horizons, dense lateral root systems form a stabilizing membrane [17] and larger tree roots can provide reinforcement across planes of weakness along the flanks of potential slope failures [18]. Bioengineering techniques, such as brush layers, fascines, vegetated crib walls or brush mattresses, immediately protect stream banks and provide a combination of the benefits of immediate hazard control and long term stabilization due to plant reinforcement effects [19,20,21].
In soil bioengineering, the main construction materials are living plants or plant parts. The selection of adequate plant species with the biotechnical characteristics required by the projects conditions the success of works’ implementation [22,23,24]. Native and site specific plants well adapted to the local ecological conditions are recommended for the successful development of the works and for avoiding the introduction and development of invasive alien species, as well as biogenetical contamination [25]. Soil bioengineering is an important tool for the restoration of riparian ecosystems, as it promotes the recruitment and growth of native plant species along riverbanks [26]. The active introduction of early successional species can trigger successional trajectories of riparian communities [27,28,29,30]. It improves riparian habitat quality and allows the development of native plant communities during secondary succession [31,32]. Soil bioengineering facilitates the partial recovery of some of the main ecological functions previously provided by riverbanks and now degraded, e.g., ecological corridor, biodiversity support or depollution [28,33,34], and contributes to climate change mitigation through carbon sequestration [35]. Beyond its ability to restore ecosystems, soil bioengineering can also be used as a conservation tool for endangered plant populations [36].
Nitrogen-fixing species, such as legume species (family: Fabaceae) or Alnus incana (L.) Moench, are highly valuable in soil bioengineering contexts where disturbed soils tend to be poor. These species are capable of fixing N2 by bacterial symbiosis, increasing soil C and N contents as well as rates of N mineralization and other N transformations [14,37,38]. Thus, N-fixing species improve the local nutritional status of the soil and can increase the production of neighboring species [39,40,41]. Some legume species have been widely used as pioneer plants in the recovery and restoration of degraded areas in the tropics [42,43,44,45]. They also display a series of biotechnical traits that make them particularly useful for soil bioengineering, e.g., a fast growth rate and quick regeneration after disturbance. For soil bioengineering, species must also be easy to propagate, either by a sufficiently large number of seeds, or by cuttings [13,19,46].
Soil bioengineering is currently developing in the Neotropics [47,48,49], but there are remaining gaps in the necessary knowledge. Botanical knowledge strongly influences the development and transferability of soil bioengineering techniques and is one of the key aspects of successful project implementation [49]. Some Neotropical legume tree species have already been used in soil bioengineering, e.g., Gliricidia sepium (Jacq.) Walp. [13,50], or Erythrina sp. [19,49]. Others, such as Haematoxylon campechianum L., Leucaena leucocephala (Lam.) de Wit, Inga species or Albizia lebbeck (L.) Benth., have also been recommended [13,19]. However, in some areas, such as the Caribbean islands, recourse to native species is limited by the lack of knowledge available regarding their characteristics and their compatibility with soil bioengineering methods. The native flora of Guadeloupe includes 19 N-fixing legume tree species, which is promising in terms of suitable species for soil bioengineering. Recent experiments have shown the difficulty of propagating native Caribbean legume tree species by cuttings in conditions compatible with soil bioengineering [51], and further investigations on germination and seedling establishment are needed to provide adequate guidance for both researchers and practitioners.
Focusing on the seedling establishment phase, we hypothesized that the selected Caribbean legume tree species showed growth performance and biotechnical traits that make them suitable for use in soil bioengineering. Since available information is scarce, the objective of this study was to improve the scant knowledge of Caribbean legume trees in order to define the best way to use them, according to their ecosystem type and operational constraints. Germination, growth, survival, susceptibility to herbivory were assessed and biotechnical traits of interest for soil bioengineering were quantified.
2. Materials and Methods
2.1. Species Selection and Description
Species selection was based on a review of published and grey literature (technical reports, thesis, notes) [9,11,52,53] and expert evaluation of native nitrogen-fixing legume trees found in Caribbean riparian formations. Specific traits conducive to soil bioengineering, such as ecological status, strong response to disturbance, abundant seed production, and ease of seed harvest, were considered as selection criteria. The five species selected for their potential compatibility with soil bioengineering were Inga ingoides (Rich.) Willd., Inga laurina (Sw.) Willd., Lonchocarpus heptaphyllus (Poir.) DC., Lonchocarpus roseus (Mill.) DC., and Pterocarpus officinalis Jacq. All are widespread in the Neotropics, except L. roseus, whose range is limited to the Caribbean. They differ in ecology and ecological amplitude [53,54]. In Guadeloupe, Inga ingoides and Inga laurina are the species with the widest geographical and altitudinal range, between 0 and 700 m above sea level (asl), in seasonal evergreen forests and rainforests. L. heptaphyllus reaches 500 m asl. L. roseus, a strictly riparian species, and P. officinalis, the dominant species structuring swamp forests, have more restricted suitable environments and are only met at low elevations (0–150 m and 0–15 m asl, respectively). According to the literature, fruit production varies in season and duration from one species to the next (Table 1). I. ingoides can produce fruits all year long, whereas the other species produce seeds over shorter periods.

Table 1.
Fruiting phenology, ecology and geographical distribution of five legume tree species of the study area. Shaded areas indicate the fruiting period. Data extracted from [9,53,55].
2.2. Seed Collection
Seed collection was carried out from July to December 2020 in Guadeloupe between 0 and 300 m asl. Phenological data for these tree species were extracted from the literature to plan the harvest [9,53]. Fruits were collected from five or more healthy individuals from 3 to 6 distant populations, except for L. roseus, a critically endangered species, of which only two populations remain in Guadeloupe [8]. Mature fruits from I. ingoides and I. laurina were collected directly from the tree using a telescopic pole. Nondecayed fruits from L. heptaphyllus, L. roseus and P. officinalis were collected from the ground. Fruits were stocked in sealed plastic bags at ambient temperature, in the shade, and seeds were sown within the next 24 h. Seeds were separated from the fruit wall and, for I. ingoides and I. laurina, the sweet pulp was also removed. For L. roseus, it was empirically observed that removing a portion of the seed coat away from the root axis triggers germination. We therefore carried out this mechanical scarification of the seed integument for this species.
2.3. Experimental Conditions of Cultivation
Twenty to forty seeds, depending on the species, were planted 2 cm deep in 2 l containers filled with a mixture of pozzolana and top layer of agricultural ferralsols (v:v 3/4:1/4). Irrigation to field capacity maintained a favorable water balance throughout the experiment. After germination, the established seedlings were left in the containers for three months, protected from light stress under a shadehouse (60% light reduction).
2.4. Seed Mass, Germination, Herbivory and Survival
Prior to sowing, each fresh seed was weighed, and its dry mass was estimated from the following equation:
Mean fresh and dry mass were calculated on ten seeds, and dry mass was obtained after oven drying at 80 °C for 72 h.
Germination was recorded at cotyledon emission. At the end of the three-month period, survival and herbivory were recorded.
2.5. Biotechnical Traits of Seedlings
After three months, 15–24 healthy seedlings per species were uprooted. Roots were cleaned. Stem length and taproot length and diameter were measured. Aerial and root parts were oven dried at 80 °C for 72 h and then weighed (shoot and root biomass). The root to shoot ratio was calculated. These biotechnical traits were chosen for their importance for soil bioengineering purposes.
2.6. Statistical Analysis
Statistical analyses were performed with the Xlstat (Addinsoft) software. Nonparametric Kruskal Wallis tests, in combination with a posthoc Conover–Iman’s test, was used to reveal significant differences in traits between the five species. A principal component analysis (PCA) was conducted to position the species in relation to one another according to their traits.
3. Results
3.1. Germination, Herbivory and Survival
As indicated by their seed mass, the species studied had different seed resources, P. officinalis showing the highest value and L. heptaphyllus the lowest (Figure 1). The germination rate was high (>88%) in all species, and all the seedlings exhibited a remarkably high survival rate at 3 months, with 100% for all species. Germination was fairly synchronous within each species, the stem appearing within 4–5 days after sowing in all individuals. L. heptaphyllus showed a high rate of herbivory, as 75% of the seedlings displayed some trace of leaf-cutting ant or snail attacks; the other species appeared to sustain less damage (Figure 1).
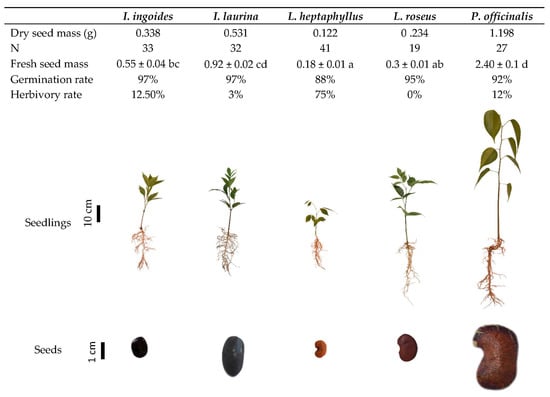
Figure 1.
Mean dry seed mass calculated from 10 oven dried seeds, fresh seed mass (± standard error, different alphabetic designations indicate significant differences between species according to Kruskal-Wallis’ test (p < 0.05) and Conover-Iman peer-to-peer comparison procedure), and germination and herbivory rates, reported for N individuals of the five species studied. Pictures represent typical 3-month-old seedlings, and seeds.
3.2. Biotechnical Traits of Seedlings
The first two axes produced by the PCA captured 79% of the total variance, i.e., 57% for the first axis and 22% for the second (Figure 2, Table 2). The main variables contributing to the first axis were shoot and root biomass, stem length and root diameter on the positive side. The main variables contributing to the second axis were root to shoot ratio and root length on the positive side. P. officinalis displayed the highest aboveground and belowground biomass, with the greatest mean stem length (reaching 37 cm at three months). Its root system was the most developed, with the highest values in both mean length (21 cm) and mean diameter (0.5 cm). At the other end of the range, L. heptaphyllus exhibited the lowest mean root diameter and mean biomass values. The other species had a mean stem height ranging between 14 and 18 cm and a mean root length ranging between 16 and 19 cm. I. laurina and L. roseus seedlings reached a significantly greater root diameter than I. ingoides. Interspecies differences in biomass allocation patterns were also noted. I. ingoides, I. laurina and L. heptaphyllus had the highest mean root to shoot ratio, ranging between 0.49 and 0.55, while, at the other end of the scale, L. roseus had the lowest (0.36). P. officinalis exhibited an intermediate mean value (0.45) (Figure 3). Seed mass was positively linked to aboveground (R2 = 0.65, p < 0.001) and belowground (R2 = 0.68, p < 0.001) biomass. The data presented in this study are available in Table S1 (supplementary material).
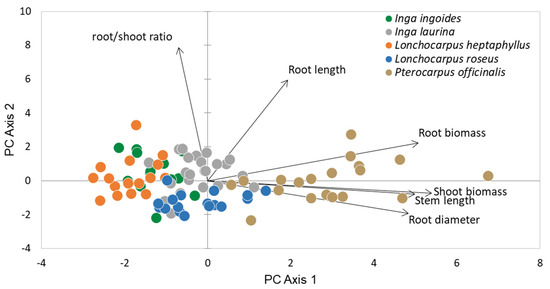
Figure 2.
Principal components analysis (PCA) on six traits of soil bioengineering interest, for 93 seedlings from five riparian legume tree species.

Table 2.
Eigenvector scores of plant traits on the three main PCA axes, obtained from a matrix of 6 traits × 90 individuals from 5 species. Values are ranked in order of absolute magnitude along PCA 1. The inertia accounted for by each axis is indicated between brackets.
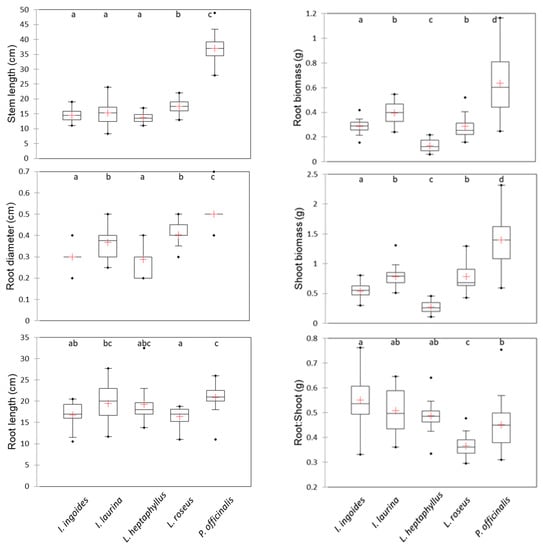
Figure 3.
Boxplot per trait for the five legume tree species studied (I. ingoides n = 15; I. laurina n = 24; L. heptaphyllus n = 16; L. roseus n = 17; P. officinalis n = 20). Boxplot mid lines represent medians, red crosses represent means, boxes represent the 25th and 75th quartile values, whiskers represent 1.5x the interquartile range, and points represent outliers. For each trait, different alphabetic designations indicate significant differences between types according to Kruskal–Wallis test (p < 0.05) and Conover–Iman peer to peer comparison procedure.
4. Discussion
The Caribbean riparian legume species studied exhibited high germination and survival rates in our experimental conditions. However, we identified differences in sensitivity to herbivory, growth and a set of morphological traits, reflecting differences in performance and allocation strategies between species during the establishment phase.
4.1. Species Traits and Strategies
The germination rates of the species studied were higher than 88% and are among the highest reported for neotropical tree species. In a previous large scale study, conducted in comparable experimental conditions, a large variability in the germination of tropical tree was reported. Of over 100 species from the seasonal evergreen forest, only 11% presented germination rates higher than 80%. Lower germination rates were recorded for congeneric Inga species (between 83 and 91%), Lonchocarpus species (54%) and Pterocarpus species (77%) [56]. However, our results are consistent with another study on tropical pioneer or early successional trees, in which 75% of the species (without dormancy) showed a germination rate higher than 70% [57]. The high germination rate of Caribbean riparian legume tree species can be linked to their early successional ecological status. Pioneer species are known to exhibit the frequent production of abundant small seeds that usually germinate after dispersal, a fast establishment strategy that can be useful in frequently disturbed ecosystems [57,58]. In addition, our results provide evidence that the high germination rates recorded in continental neotropics for I. ingoides (80–90%) [59,60] and I. laurina (90–100%) [61,62] are also a characteristic of Caribbean populations and reveals the high germination potential of these two Lonchocarpus species.
After three months of growth, all studied species presented a survival of 100%. In literature, seedling survival in tropical species is mostly addressed on in situ experiments, regarding plant response to environmental drivers such as resources availability [63,64,65] or community effects [66]. This high survival is consistent with previous results on neotropical species conducted in natural conditions with comparable climatic conditions [67]. Germination potential and seedling survival do not seem to be a constraint for natural regeneration or for large scale nursery production.
Our results highlight interspecific differences in sensitivity to herbivory, with L. heptaphyllus particularly sensitive (75% of seedlings damaged) compared with all the other species (less than 13% of seedlings damaged). These differences could be explained by the existence of defense mechanisms. It has been hypothesized that species interactions, including between plants and herbivores, are stronger in the tropics. This would promote the intensification and diversification of plant defenses [68,69] as well as plant plasticity in N-acquisition strategy [70,71], particularly in legume species, which are more attractive because of their higher N content [72]. I. laurina is known to produce chemical compounds involved in pest defense [45,73] and the seeds of P. officinalis contain hypaphorine, which is disliked by a wide range of seed-eating rodents [74]. Given their low herbivory rate, L. roseus seedlings can also be suspected to have developed defense mechanisms.
The selected species showed root to shoot ratios ranging from 0.3 to 0.75 g·g−1, this range is consistent with values reported in a greenhouse experiment for 44–60 days studying neotropical legume species from a lowland forest [75]. The root to shoot ratio of the selected seedlings appears also comparable with those reported in an in situ study on the growth and root traits of one year seedlings, encompassing 37 species of Bolivian moist forest [76]. However, the three month seedlings of the present study presented a much lower total dry biomass, between 0.8 to 2 g, than the 1 to 35 g recorded on the one-year seedlings of the Bolivian study. The average root length reported for the Bolivian seedling species where 7 ± 3 cm, a value two fold lower than those of the Caribbean seedlings species of the present study [76]. This difference could be explained by the competition occurring in natural conditions or by the differences in substrates characteristics. Indeed, the root length of the species from the present study displayed comparable values than those recorded in a greenhouse experiment on 6 month old African seedlings, ranking between 15 and 30 cm [77].
Data on seedling performance, biomass allocation and root traits are scarce for tropical forest species. The few studies available have used different, hardly comparable plant material and experimental designs, explaining the wide disparities reported in performance and traits [75,78,79]. As biomass allocation patterns and root traits are known to be influenced by environmental conditions and plant ontogeny [80,81], our results contribute to fill a gap in the knowledge of tropical legume tree seedlings establishment.
Species were distributed along the two main axes of the PCA, which reflected their variation in the studied traits. The first axis could be interpreted as the establishment strategy axis, along which species are ranked from slow to fast establishers. The second axis reflects resource allocation into the root system. P. officinalis was the best performing species, exhibiting the fastest seedling establishment (highest biomass) and rapid development of the root system (greatest root length), permitting quick anchorage that is well adapted to swamp forest conditions, where the water level can vary throughout the year [82]. This species, capable of withstanding brackish water and frequent floods [82], has promising potential for riverbank stabilization purposes. I. ingoides and L. heptaphyllus displayed opposite trait values to P. officinalis and seem less likely to achieve deep soil stabilization early on. However, both remain of interest for enriching species diversity on work sites and diversifying root system architectures, which would probably contribute to improve soil cohesion [15]. I. laurina and L. roseus exhibited intermediate growth characteristics. L. roseus invests in the development of its aerial system, whereas I. laurina gives precedence to the development of its root system.
Interspecies differences in performance can be linked to seed characteristics. The seedlings of larger seeded species, Pterocarpus officinalis and Inga laurina, tend to perform better than those of smaller seeded species, such as I. ingoides and L. heptaphyllus. They benefit from greater initial seed resources and are, therefore, better provisioned for their establishment [83]. The metabolism of L. roseus performed particularly well, since this small seeded species, by investing more in its shoots, acquired a comparatively large biomass.
4.2. Which Species, When and Where?
On Caribbean islands, highly diverse pedoclimatic conditions on small areas support complex floristic assemblages [9,10,84]. The species selected and studied here cover a broad ecological spectrum (Table 1), providing practitioners with a range of options adapted to different ecological conditions, from brackish estuarine to rainforest. In addition, most of these species have a geographical distribution that extends beyond the Caribbean territory [53,54] and are still relevant in a wider Neotropical context (Table 1).
In tropical regions, the time available for implementing soil bioengineering projects is mostly limited to the beginning of the rainy season. Seedling availability and seed stock management are therefore key aspects to consider when planning projects [19]. Whereas I. ingoides produces seeds all year round, for I. laurina and L. heptaphyllus, seed collection for seedling production is highly dependent on the phenology of in situ populations because both species have a short seed production period and, moreover, their recalcitrant seeds cannot endure the loss of only a small proportion of water and it is not possible to store them, for practical purposes [85,86]. Seed physiology in L. roseus is unknown but for the fact that germination is triggered by the scarification of its hard seed coat. This kind of dormancy has been reported in other legumes [87,88] and unpublished assays revealed that its germinative capacity can be retained for 6 months after seed collection, supporting the hypothesis that seeds of this species could be stored at least for a few months. As regards Pterocarpus officinalis seed conservation appears possible in certain conditions since seeds inside floating fruits have been found to retain their germinative capacity for more than 2 months in fresh water—but only for 2 weeks in sea water [82,89]. Further investigations on the longevity and storage conditions of L. roseus and P. officinalis seeds would help to design appropriate seed conservation protocols and guide seed storage management.
4.3. Using Legume Species in Soil Bioengineering
The active reintroduction of plants in degraded areas can be achieved by planting cuttings or seedlings, or by sowing seeds. The propagation of riparian Caribbean legume tree species by cuttings in soil bioengineering field conditions has been found to be very difficult [51], excluding this option for these N2-fixing species. However, since the five selected species produce sufficient quantities of seeds that are easy to collect, they can still be used in soil bioengineering and introduced as seedlings or saplings, with older established plantation sites usable as living stocks, providing donor trees for subsequent projects. Their synchronous cohort establishment, with seedling emergence within the first 3 months, is a bonus for nursery production and work site maintenance. In the field, rooted seedlings can be directly planted through a geotextile or incorporated into a range of soil bioengineering techniques, such as vegetated cribwalls, live gratings, brush layers, retaining walls, benches, hedge layerings or live spurs [13,19,90,91,92]. Even though a cultivated nursery stock is preferable, direct seeding is also possible in soil bioengineering [93] and we can imagine developing innovative methods such as the integration of legume tree seeds into dead fascines.
4.4. Caribbean Legume Tree Species for Restoring and Conserving Riparian Forests
In Guadeloupe, despite their globally recognized diversity and patrimonial value, natural ecosystems are being degraded at a critical rate and 150 ha of forest are lost every year [94]. Beyond the emergency need to conserve the remaining natural ecosystems and threatened species, the restoration of degraded forests is an important issue. Nitrogen-fixing legume tree species are recognized as pivotal in tropical forest restoration, in that they facilitate the establishment of more complex and resilient communities [45,95,96]. They are, consequently, of particular interest for riparian forest restoration using soil bioengineering techniques. All the species selected and studied here are highly compatible with soil bioengineering in low cost conditions, due to high seed availability, fast germination and seedling development, high seedling survival rates and effective N2-fixing root nodulation [52]. I. laurina and P. officinalis have already been used successfully in forest restoration programs in Brazil and the Caribbean [82,96,97]. Our results confirm the good performances of these species during their establishment phase. Beyond its ability to restore ecosystems, soil bioengineering can also be used as a conservation tool for endangered plant populations [36]. In Guadeloupe, the conservation of L. roseus is a major issue: endemic to the Caribbean, it is classified as critically endangered in the regional IUCN red list. This strictly riparian species is greatly threatened by the destruction of its natural habitat [8]. The few known stations concern limited areas, at low elevation and under strong anthropic pressure, persisting amid densely urbanized zones. Field observations in the remaining natural populations in Guadeloupe indicate a rapid spontaneous regeneration. This species is very common in riparian areas in Martinique [53]. Integrating L. roseus into soil bioengineering projects should, therefore, be encouraged for the additional purpose of its conservation.
In addition to being a biodiversity hot spot [7], the Caribbean is also a climate hot spot [98], i.e., a region for which potential climate change impacts on the environment or different activity sectors can be particularly pronounced. The species studied belong to different ecosystems along an altitudinal gradient from dry to moist forest. In the lesser Antilles, climate change scenarios predict an increase in temperature and a decrease in precipitation [99]. This will probably lead to the modification of plant community structure and composition, through the induced drought mortality of sensitive species [100,101] and the upward migration of species currently located at lower altitudes [102,103]. It then would be relevant to conduct further investigation on the drought response of selected species for soil bioengineering, in order to better identify the species adapted to the future drier conditions. Some riparian species of the present study (I. ingoides and I. laurina) display a wide ecological amplitude, a high dispersal ability, and can be distributed in different ecosystems, whereas others have a narrower distribution (P. officinalis and L. heptaphyllus) or are located in a few numbers of stations (L. roseus). Soil bioengineering, by creating the opportunity to plant whatever desired and adapted species, represents a promising tool to assist species migration, by expanding the range of species that are at risk of extinction by climate change to new locations [104,105]. That is the case of P. officinalis and L. roseus, which are threatened by the decrease and fragmentation in their habitats. Their current narrow habitats, located close from the coastline, are constrained by urbanization on one side, and by the rising ocean level at the other side [94]. Soil bioengineering and other planting opportunities are relevant, to contribute to the conservation of those species. Soil bioengineering may also have positive benefits for climate mitigation by the sequestration of carbon [35]. Vegetated land surfaces hold more carbon in their soil and biomass than do surfaces with civil engineering works that are sparsely vegetated or where vegetation is absent [106].
5. Conclusions
This study adds to the current knowledge on legume trees for riparian restoration including soil bioengineering techniques. All the species studied here display performances and traits that allow their inclusion in soil bioengineering techniques and the large scale plant material production necessary to implement soil bioengineering designs. Our experimental results open a new avenue for the use of native N-fixing species and are therefore innovative and important for soil bioengineering in the Caribbean, both for practitioners and researchers. Evidence from this study shows that using legume trees can conserve and restore Caribbean riparian forests. Their high seed availability, fast germination and seedling development, high seedling survival rates and effective N2-fixing root nodulation demonstrate their potential to induce the fast installation of early succession stage of riparian forests. The inclusion of threatened and structuring species in restoration programs can contribute to the conservation of an entire type of threatened riparian ecosystem.
Supplementary Materials
The following Supplementary Materials are available online at https://www.mdpi.com/article/10.3390/su14073709/s1, Table S1: dataset.
Author Contributions
Conceptualization, E.M., A.R. and A.E.; data curation, E.M.; formal analysis, E.M., A.R. and A.E.; funding acquisition, M.R. and A.E., investigation, E.M.; project administration, L.L.; resources, R.T.; supervision, A.R., R.T. and A.E.; validation, A.R. and A.E.; visualization, E.M.; writing—original draft, E.M.; writing—review and editing, A.R., M.R. and A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This paper is a scientific contribution to the “Protéger” project, funded by the European Union (European Regional Development Fund—ERDF, 2017-FED-639), the French Office for Biodiversity (Office Français pour la Biodiversité) and by the three project stakeholders: the National Park of Guadeloupe, the French National Institute for Agriculture, Food and the Environment (INRAE) and the University of Antilles.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the results reported can be found in the supplementary material.
Acknowledgments
The authors thank Jean-François Bernard for directing us to the location of Lonchocarpus roseus populations, Frédéric Berchel for his help in data collection and Anya Cockle Betian for English editing.
Conflicts of Interest
The authors declare no conflict of interest. The funding bodies have had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript, or in the decision to publish the results.
References
- United Nations Environment Program. Becoming #GenerationRestoration: Ecosystem Restoration for People, Nature and Climate. Nairobi, Kenya. 2021, p. 56. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/36251/ERPNC.pdf (accessed on 15 January 2022).
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef] [Green Version]
- González, E.; Felipe-Lucia, M.R.; Bourgeois, B.; Boz, B.; Nilsson, C.; Palmer, G.; Sher, A.A. Integrative conservation of riparian zones. Biol. Conserv. 2017, 211, 20–29. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine flood plains: Present state and future trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, C.; Reidy, C.A.; Dynesius, M.; Revenga, C. Fragmentation and flow regulation of the world’s large river systems. Science 2005, 308, 405–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feld, C.K.; Birk, S.; Bradley, D.C.; Hering, D.; Kail, J.; Marzin, A.; Melcher, A.; Nemitz, D.; Pedersen, M.L.; Pletterbauer, F.; et al. From natural to degraded rivers and back again: A test ofnrestoration ecology theory and practice. Adv. Ecol. Res. 2011, 44, 119–209. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Okada, K.I.; Mori, A.S. Reconsidering biodiversity hotspots based on the rate of historical land-use change. Biol. Conserv. 2019, 233, 268–275. [Google Scholar] [CrossRef]
- UICN. Liste Rouge de la Flore Vasculaire de Guadeloupe; UICN: Paris, France, 2019. [Google Scholar]
- Rollet, B. Description des espèces. In Arbres des Petites Antilles Tome 2; O.N.F.: Paris, France, 2010. [Google Scholar]
- Rousteau, A.; Portecop, J.; Rolle, B. Carte Écologique de la Guadeloupe; ONF, UAG, PNG, CGG: Jarry, Guadeloupe, 1996. [Google Scholar]
- Gayot, M.; Procopio, L.; Conjard, S.; Boulange, E.; Bernus, J. Étude de la Typologie des Ripisylves de Guadeloupe et Proposition D’espèces Utilisables en Génie Végétal sur les Berges; ONF PNG: Jarry, Guadeloupe, 2018. [Google Scholar]
- Cohen-Shacham, E.; Waters, G.; Janzen, C.; Maginnis, S. (Eds.) Nature Based Solutions to Address Global Societal Challenges; IUCN: Gland, Switzerland, 2016. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.; Hellin, J. Bioengineering for Effective Road Maintenance in the Caribbean; Natural Resources Institute: Chatham, UK, 1996. [Google Scholar]
- Stangl, R.; Hochbichler, E.; Bellos, P.N.; Florineth, F. Allometric estimation of the above-ground biomass components of Alnus incana (L.) Moench used for landslide stabilisation at Bad Goisern (Austria). Plant Soil 2009, 324, 115–129. [Google Scholar] [CrossRef]
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Wu, T.H.; McKinnell, W.P.; Swanston, D.N. Strength of tree roots and landslides on Prince of Wales Island, Alaska. Can. Geotech. J. 1979, 16, 19–33. [Google Scholar] [CrossRef]
- Schmidt, K.M.; Roering, J.J.; Stock, J.D.; Dietrich, W.E.; Montgomery, D.R.; Schaub, T. The variability of root cohesion as an influence on shallow landslide susceptibility in the Oregon Coast Range. Can. Geotech. J. 2001, 38, 995–1024. [Google Scholar] [CrossRef]
- Sidle, R.C.; Pearce, A.; O’Loughlin, J.C.L. Hillslope Stability and Land Use; American Geophysical Union: Washington, DC, USA, 1985. [Google Scholar]
- Diaz, J. Control de la Erosión en Zonas Tropicales; Universidad Industrial de Santander, Libreria UIS: Colombia, Bucaramanga, 2001. [Google Scholar]
- Zeh, H. Soil Bioengineering Construction Type Manual; European Federation for Soil Bioengineering: Zürich, Switzerland, 2007. [Google Scholar]
- Lachat, B. Guide de Protection en Techniques Végétales; Ministere de L’amenagement du Territoire et de L’environnement: Paris, France, 1994. [Google Scholar]
- Stokes, A. Selecting tree species for use in rockfall-protection forests. For. Snow Landsc. Res. 2006, 80, 77–86. [Google Scholar]
- De Baets, S.; Poesen, J.; Reubens, B.; Muys, B.; De Baerdemaeker, J.; Meersmans, J. Methodological framework to select plant species for controlling rill and gully erosion: Application to a Mediterranean ecosystem. Earth Surf. Process. Landforms 2009, 34, 1374–1392. [Google Scholar] [CrossRef]
- Ghestem, M.; Cao, K.; Ma, W.; Rowe, N.; Leclerc, R.; Gadenne, C.; Stokes, A. A framework for identifying plant species to be used as ‘ecological engineers’ for fixing soil on unstable slopes. PLoS ONE 2014, 9, e95876. [Google Scholar] [CrossRef]
- Krautzer, E.; Hacker, B. Soil-Bioengineering: Ecological Restoration with Native Plant and Seed Material. In Proceedings of the Conference HBLFA, Raumberg-Gumpenstein, Gumpenstein, Austria, 9 September 2006. HBLFA Raumberg-Gumpenstein. [Google Scholar]
- Li, X.; Zhang, L.; Zhang, Z. Soil bioengineering and the ecological restoration of riverbanks at the Airport Town, Shanghai, China. Ecol. Eng. 2006, 26, 304–314. [Google Scholar] [CrossRef]
- Cavaillé, P.; Ducasse, L.; Breton, V.; Dommanget, F.; Tabacchi, E.; Evette, A. Functional and taxonomic plant diversity for riverbank protection works: Bioengineering techniques close to natural banks and beyond hard engineering. J. Environ. Manag. 2015, 151, 65–75. [Google Scholar] [CrossRef]
- Janssen, P.; Cavaillé, P.; Bray, F.; Evette, A. Soil bioengineering techniques enhance riparian habitat quality and multi-taxonomic diversity in the foothills of the Alps and Jura Mountains. Ecol. Eng. 2019, 133, 1–9. [Google Scholar] [CrossRef]
- Polster, D.F.; Bio, R.P. Soil bioengineering for riparian restoration. In Proceedings of the Canadian Land Reclamation Association 2006 Conference, Ottawa, ON, Canada, 27–31 August 2006. [Google Scholar]
- Tisserant, M.; Janssen, P.; Evette, A.; González, E.; Cavaillé, P.; Poulin, M. Diversity and succession of riparian plant communities along riverbanks bioengineered for erosion control: A case study in the foothills of the Alps and the Jura Mountains. Ecol. Eng. 2020, 152, 105880. [Google Scholar] [CrossRef]
- Lennox, M.S.; Lewis, D.J.; Jackson, R.D.; Harper, J.; Larson, S.; Tate, K.W. Development of vegetation and aquatic habitat in restored riparian sites of California’s north coast rangelands. Restor. Ecol. 2011, 19, 225–233. [Google Scholar] [CrossRef]
- McClain, C.D.; Holl, K.D.; Wood, D.M. Successional models as guides for restoration of riparian forest understory. Restor. Ecol. 2011, 19, 280–289. [Google Scholar] [CrossRef]
- Martin, F.M.; Janssen, P.; Bergès, L.; Dupont, B.; Evette, A. Higher structural connectivity and resistance against invasions of soil bioengineering over hard-engineering for riverbank stabilisation. Wetl. Ecol. Manag. 2021, 29, 27–39. [Google Scholar] [CrossRef]
- Sudduth, E.B.; Meyer, J.L. Effects of Bioengineered Streambank Stabilization on Bank Habitat and Macroinvertebrates in Urban Streams. Environ. Manag. 2006, 38, 218–226. [Google Scholar] [CrossRef]
- Symmank, L.; Natho, S.; Scholz, M.; Schröder, U.; Raupach, K.; Schulz-Zunkel, C. The impact of bioengineering techniques for riverbank protection on ecosystem services of riparian zones. Ecol. Eng. 2020, 158, 106040. [Google Scholar] [CrossRef]
- Popoff, N.; Jaunatre, R.; Le Bouteiller, C.; Paillet, Y.; Favier, G.; Buisson, M.; Meyer, C.; Dedonder, E.; Evette, A. Optimization of restoration techniques: In-situ transplantation experiment of an endangered clonal plant species (Typha minima Hoppe). Ecol. Eng. 2020, 160, 106130. [Google Scholar] [CrossRef]
- Knoepp, D.; Swank, W.T. Rates of nitrogen mineralization across an elevation and vegetation gradient in the southern Appalachians. Plant Soil 1998, 204, 235–241. [Google Scholar] [CrossRef]
- Knops, J.M.H.; Bradley, K.L.; Wedin, D.A. Mechanisms of plant species impacts on ecosystem nitrogen cycling. Ecol. Lett. 2002, 5, 454–466. [Google Scholar] [CrossRef] [Green Version]
- Debell, D.S.; Whitesell, C.D.; Schubert, T.H. Mixed Plantations of Eucalyptus and Leguminous Trees; U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: Berkeley, CA, USA, 1985. [Google Scholar]
- Khanna, P.K. Comparison of growth and nutrition of young monocultures and mixed stands of Eucalyptus globulus and Acacia mearnsii. For. Ecol. Manag. 1997, 94, 105–113. [Google Scholar] [CrossRef]
- Binkley, D.; Senock, R.; Bird, S.; Cole, T.G. Twenty years of stand development in pure and mixed stands of Eucalyptus saligna and nitrogen-fixing Facaltaria moluccana. For. Ecol. Manag. 2003, 182, 93–102. [Google Scholar] [CrossRef]
- Fisher, R. Amelioration of degraded rain forest soils by plantations of native trees. Soil Sci. Soc. Am. J. 1995, 59, 544–549. [Google Scholar] [CrossRef] [Green Version]
- Franco, A.A.; De Faria, S.M. The contribution of N2-fixing tree legumes to land reclamation and sustainability in the tropics. Soil Biol. Biochem. 1997, 29, 897–903. [Google Scholar] [CrossRef]
- Johnson, D.W.; Curtis, P.S. Effects of forest management on soil C and N storage: Meta analysis. For. Ecol. Manag. 2001, 140, 227–238. [Google Scholar] [CrossRef]
- Macedo, M.O.; Resende, A.S.; Garcia, P.C.; Boddey, R.M.; Jantalia, C.P.; Urquiag, S.E.; Campello, F.C.; Franco, A.A. Changes in soil C and N stocks and nutrient dynamics 13 years after recovery of degraded land using leguminous nitrogen-fixing trees. For. Ecol. Manag. 2008, 255, 1516–1524. [Google Scholar] [CrossRef]
- Norris, J.E.; Stokes, A.; Mickovski, S.B.; Cammeraat, E.; Van Beek, R.; Nicoll, B.C.; Achim, A. (Eds.) Slope Stability and Erosion Control: Ecotechnological Solutions; Springer Science & Business Media: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Petrone, F.; Preti, A. Soil Bioengineering Measures in Latin America: Authocthonal Cuttings Suitability. In Landslide Science and Practice; Volume 7: Social and Economic Impact and Policies; Claudio, M.P., Canuti, K.S., Eds.; Springer: Berlin, Germany, 2013. [Google Scholar]
- Hostettler, S.; Jöhr, A.; Montes, C.; D’Acunzi, A. Community-based landslide risk reduction: A review of a Red Cross soil bioengineering for resilience program in Honduras. Landslides 2019, 16, 1779–1791. [Google Scholar] [CrossRef] [Green Version]
- Maxwald, M.; Crocetti, C.; Ferrari, R.; Petrone, A.; Rauch, H.P.; Preti, F. Soil and Water Bioengineering Applications in Central and South America: A Transferability Analysis. Sustainability 2020, 12, 10505. [Google Scholar] [CrossRef]
- Petrone, A.; Preti, F. Suitability of soil bioengineering techniques in Central America: A case study in Nicaragua. Hydrol. Earth Syst. Sci. 2008, 12, 1241–1248. [Google Scholar] [CrossRef] [Green Version]
- Mira, E.; Evette, A.; Labbouz, L.; Robert, M.; Rousteau, A.; Tournebize, R. Investigation of the asexual reproductive characteristics of native species for soil bioengineering in the west indies. J. Trop. For. Sci. 2021, 33, 333–342. [Google Scholar] [CrossRef]
- Saur, E.; Carcelle, S.; Guezennec, S.; Rousteau, A. Nodulation of legume species in wetlands of Guadeloupe (Lesser antilles). Wetlands 2000, 20, 730–734. [Google Scholar] [CrossRef]
- Fournet, J. Flore Illustrée des Phanérogames de Guadeloupe et de Martinique, Nouvelle Édition Revue et Augmentée; INRA: Paris, France, 1978. [Google Scholar]
- Acevedo, P.; Strong, M. Catalogue of Seed Plants of the West Indies. Smithson. Contrib. Bot. 2012, 98, 1–1192. [Google Scholar] [CrossRef]
- Tropicos v3. Missouri Botanical Garden. Available online: https://tropicos.org (accessed on 13 January 2022).
- Válio, I.F.; Scarpa, F.M. Germination of seeds of tropical pioneer species under controlled and natural conditions. Braz. J. Bot. 2001, 24, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Sautu, A.; Baskin, J.M.; Baskin, C.C.; Condit, R. Studies on the seed biology of 100 native species of trees in a seasonal moist tropical forest, Panama, Central America. For. Ecol. Manag. 2006, 234, 245–263. [Google Scholar] [CrossRef]
- Swaine, M.D.; Whitmore, T.C. On the definition of ecological species groups in tropical rain forests. Vegetatio 1988, 75, 81–86. [Google Scholar] [CrossRef]
- Laime, E.M.O.; Alves, E.U.; Guedes, R.S.; Silva, K.B.; de Souza Oliveira, D.C.; da Silva Santos, S. Emergency and initial growth of seedlings of Inga ingoides (Rich.) Willd. in function of the position and depth of sowing. Semina: Ciências Agrárias 2010, 31, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Palomino, J.; Barra, M. Especies forestales nativas con potencial para reforestación en la provincia de oxapampa y fichas técnicas de las especies de mayor prioridad. PRO Nat. 2003, 109. Available online: http://www.infobosques.com/descargas/biblioteca/70.pdf (accessed on 13 January 2022).
- Leão, J.R.; Lima, J.P.D.C.; Pinto, S.D.N.; de Paiva, A.V. Germinação de sementes e crescimento inicial de plântulas de ingá-mirim. Revsista Bras. Arborização Urbana 2012, 7, 11–19. [Google Scholar] [CrossRef]
- Mendes-Rodrigues, C.; Ferreira, W.R.; De Lima, J.A.; Dornelles, M.C.; Ranal, M.a.; De Santana, D.G. Germinação de embriões de duas espécies de Inga (Mimosaceae). Rev. Bras. Biociências 2007, 5, 561–563. [Google Scholar]
- Augspurger, C.K. Light Requirements of Neotropical Tree Seedlings: A Comparative Study of Growth and Survival. J. Ecol. 1984, 72, 777. [Google Scholar] [CrossRef]
- Poorter, L.; Markesteijn, L. Seedling Traits Determine Drought Tolerance of Tropical Tree Species. Biotropica 2008, 40, 321–331. [Google Scholar] [CrossRef]
- McLaren, K.P.; McDonald, M.A. The effects of moisture and shade on seed germination and seedling survival in a tropical dry forest in Jamaica. For. Ecol. Manag. 2003, 183, 61–75. [Google Scholar] [CrossRef]
- Queenborough, S.A.; Burslem, D.F.R.P.; Garwood, N.C.; Valencia, R. Neighborhood and community interactions determine the spatial pattern of tropical tree seedling survival. Ecology 2007, 88, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, B.M.; Kursar, T.A.; Tyree, M.T. Drought effects on seedling survival in a tropical moist forest. Trees 2005, 19, 312–321. [Google Scholar] [CrossRef]
- Schemske, D.W.; Mittelbach, G.G.; Cornell, H.V.; Sobel, J.M.; Roy, K. Is There a Latitudinal Gradient in the Importance of Biotic Interactions? Annu. Rev. Ecol. Evol. Syst. 2009, 40, 245–269. [Google Scholar] [CrossRef] [Green Version]
- Coley, P.D.; Barone, J.A. Herbivory and plant defenses in tropical forests. Annu. Rev. Ecol. Syst. 1996, 27, 305–335. [Google Scholar] [CrossRef]
- Coste, S.; Roggy, J.C.; Imbert, P.; Born, C.; Bonal, D.; Dreyer, E. Leaf photosynthetic traits of 14 tropical rain forest species in relation to leaf nitrogen concentration and shade tolerance. Tree Physiol. 2005, 25, 1127–1137. [Google Scholar] [CrossRef] [Green Version]
- Michalet, S.; Rohr, J.; Warshan, D.; Bardon, C.; Roggy, J.-C.; Domenach, A.M.; Czarnes, S.; Pommier, T.; Combourieu, B.; Guillaumaud, N.; et al. Phytochemical analysis of mature tree root exudates in situ and their role in shaping soil microbial communities in relation to tree N-acquisition strategy. Plant Physiol. Biochem. 2013, 72, 169–177. [Google Scholar] [CrossRef]
- Janzen, D.H. The defenses of legumes against herbivores. In Advances in Legume Systematics; Polhill, R.M., Raven, P.R., Eds.; Academic Press: London, UK, 1981; Available online: http://hdl.handle.net/11606/1296. (accessed on 13 January 2022).
- Macedo, M.L.R.; Garcia, V.A.; Maria das Graças, M.F.; Richardson, M. Characterization of a Kunitz trypsin inhibitor with a single disulfide bridge from seeds of Inga laurina (SW.) Willd. Phytochemistry 2007, 68, 1104–1111. [Google Scholar] [CrossRef]
- Janzen, D.H.; Lynn, D.G.; Fellowst, L.E.; Hallwachs, W. The indole alkaloid, hypaphorine and Pterocarpus seed protection. Phytochemistry 1982, 21, 1035–1037. [Google Scholar] [CrossRef]
- Nasto, M.K.; Winter, K.; Turner, B.L.; Cleveland, C.C. Nutrient acquisition strategies augment growth in tropical N2-fixing trees in nutrient-poor soil and under elevated CO2. Ecology 2019, 100, e02646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markesteijn, L.; Poorter, L. Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought-and shade-tolerance. J. Ecol. 2009, 97, 311–325. [Google Scholar] [CrossRef]
- Boonman, C.C.; van Langevelde, F.; Oliveras, I.; Couédon, J.; Luijken, N.; Martini, D.; Veenendaal, E.M. On the importance of root traits in seedlings of tropical tree species. New Phytol. 2020, 227, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Veneklaas, E.L.; Poorter, L. Growth and carbon partitioning of tropical tree seedlings in contrasting light environments. In Inherent Variation in Plant Growth. Physiological Mechanisms and Ecological Consequences; Van Vuuren, M.M.I., Ed.; Leiden, The Netherlands, 1998; pp. 337–361. Available online: https://agris.fao.org/agris-search/search.do?recordID=NL2012018952 (accessed on 13 January 2022).
- Smith-Martin, C.M.; Gei, M.G.; Bergstrom, E.; Becklund, K.K.; Becknell, J.M.; Waring, B.G.; Werden, L.K.; Powers, J.S. Effects of soil type and light on height growth, biomass partitioning, and nitrogen dynamics on 22 species of tropical dry forest tree seedlings: Comparisons between legumes and nonlegumes. Am. J. Bot. 2017, 104, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortunel, C.; Stahl, C.; Heuret, P.; Nicolini, E.; Baraloto, C. Disentangling the effects of environment and ontogeny on tree functional dimensions for congeneric species in tropical forests. New Phytol. 2020, 226, 385–395. [Google Scholar] [CrossRef]
- McConnaughay, K.D.M.; Coleman, J.S. Biomass allocation in plants: Ontogeny or optimality? A test along three resource gradients. Ecology 1999, 80, 2581–2593. [Google Scholar] [CrossRef]
- Imbert, D.; Dulormne, M. Restauration du Couvert Forestier dans les ESPACES inondables D’arrière-Mangrove: Comment Faciliter le Retour de la Forêt à Pterocarpus Officinalis; UAG: Pointe à Pitre, France, 2013. [Google Scholar]
- Green, P.T.; Juniper, P.A. Seed–seedling allometry in tropical rain forest trees: Seed mass-related patterns of resource allocation and the ‘reserve effect’. J. Ecol. 2004, 92, 397–408. [Google Scholar] [CrossRef]
- Acevedo-Rodríguez, P.; Strong, M.T. Floristic Richness and Affinities in the West Indies. Bot. Rev. 2008, 74, 5–36. [Google Scholar] [CrossRef]
- Silva, C.C. Conservação de Sementes de Inga laurina (Sw.) Willd. 2016. Available online: https://repositorio.ufpb.br/jspui/handle/123456789/12085 (accessed on 13 January 2022).
- Barrozo, L.M.; Ursulino, E.A.; de Araujo, L.R.; Sena, D.D.A.; de Medeiros, D.S.; dos Santos, J.C. Quality seeds Inga physiological function of drying. Biosci. J. 2014, 30, 645–654. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Elsevier: New York, NY, USA, 1998. [Google Scholar]
- Cruz, E.D.; Martins, F.D.O.; Carvalho, J.E.U.D. Biometria de frutos e sementes e germinação de jatobá-curuba (Hymenaea intermedia Ducke, Leguminosae—Caesalpinioideae). Rev. Bras. Botânica 2001, 24, 161–165. [Google Scholar] [CrossRef]
- Muller., F. Diversité Génétique, Adaptation de Pterocarpus Officinalis Jacq. et de ses Symbiotes dans des Forêts Marécageuses de la Région Caraïbe. Ph.D. Thesis, Université des Antilles la Guyane, Pointe à Pitre, France, 2006. [Google Scholar]
- Gao, J.; Wang, F.; Gao, Y.; Stangl, R. Root architecture characteristics of plant inlay in live slope grating. For. Stud. China 2007, 9, 177–181. [Google Scholar] [CrossRef]
- Shah, B.H. Field Guide on Soil Bioengineering for Slope Stabilization in Timor-Leste; Exch. South–South Exp. TF011068, Trust Fund; World Bank Office: Dili, Timor-Leste, 2012. [Google Scholar]
- Stangl, R. Hedge brush layers and live crib walls—stand development and benefits. In Eco-and Ground Bio-Engineering: The Use of Vegetation to Improve Slope Stability. Developments in Plant and Soil Sciences; Stokes, A., Spanos, I., Norris, J.E., Cammeraat, E., Eds.; Springer: Dordrecht, The Netherland, 2007; Volume 103. [Google Scholar] [CrossRef]
- Dhital, Y.P.; Kayastha, R.B.; Shi, J. Soil bioengineering application and practices in Nepal. Environ. Manag. 2013, 51, 354–364. [Google Scholar] [CrossRef]
- IGN Diagnostic des Forêts de la Guadeloupe. CD971-; IGN-ONF Deal Guadeloupe: Basse-Terre, Guadeloupe, 2015. [Google Scholar]
- Chaer, G.M.; Resende, A.S.; Campello, E.F.C.; de Faria, S.M.; Boddey, R.M. Nitrogen-fixing legume tree species for the reclamation of severely degraded lands in Brazil. Tree Physiol. 2011, 31, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Lima, E.M.; Curcio, G.R.; Bonnet, A.; Uhlmann, A.; Palma, V.H. Initial growth of native tree species in a degraded soil with presence of plinthite in Biome Cerrado, Brasília-DF. Nativa: Pesquisas Agrárias e Ambientais 2018, 6, 787–794. [Google Scholar] [CrossRef]
- Cruz-Neto, O.; Machado, I.C.; Duarte, J.A.; Lopes, A.V. Synchronous phenology of hawkmoths (Sphingidae) and Inga species (Fabaceae–Mimosoideae): Implications for the restoration of the Atlantic forest of northeastern Brazil. Biodiv. Conserv. 2011, 20, 751–765. [Google Scholar] [CrossRef]
- Giorgi, F. Climate change hot-spots. Geophys. Res. Lett. 2006, 33, 1866–1878. [Google Scholar] [CrossRef]
- Campbell, J.D.; Taylor, M.A.; Stephenson, T.S.; Watson, R.A.; Whyte, F.S. Future climate of the Caribbean from a regional climate model. Int. J. Climatol. 2011, 31, 1866–1878. [Google Scholar] [CrossRef]
- Lowry, J.B. Effect of drought on Mount Kinabalu. Malayan Nat. J. 1973, 26, 178–179. [Google Scholar]
- Werner, W.L. Canopy dieback in the upper montane rain forests of Sri Lanka. GeoJournal 1988, 17, 245–248. [Google Scholar] [CrossRef]
- Pounds, J.A.; Fogden, M.P.L.; Campbell, J.H. Biological response to climate change on a tropical mountain. Nature 1999, 398, 611–615. [Google Scholar] [CrossRef]
- Joseph, P. La végétation des Petites Antilles: Principaux traits floristiques et effets plausibles du changement climatique. VertigO-la Rev. Électronique en Sci. L’environnement 2011, 11, 1. [Google Scholar] [CrossRef]
- Ste-Marie, C.; Nelson, E.A.; Dabros, A.; Bonneau, M.E. Assisted migration: Introduction to a multifaceted concept. For. Chron. 2011, 87, 724–730. [Google Scholar] [CrossRef] [Green Version]
- McLachlan, J.S.; Hellmann, J.J.; Schwartz, M.W. A framework for debate of assisted migration in an era of climate change. Conserv. Biol. 2007, 21, 297–302. [Google Scholar] [CrossRef]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Chang. Biol. 2000, 6, 317–327. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).