Abstract
The health of drinking water is an important criterion for developed countries and around half of the world’s population is deprived of sanitary and safe drinking water. By identifying the time of pollution occurrence and the places that are most sensitive to pollution the management of the quality of drinking water can be planned. Since the landfill for Yasouj, a city in Iran, was located in a higher place than the drinking water wells, which were drilled in a karst aquifer, the safety of the drinking water resources (including eight wells) of Yasouj were investigated in the present study. For this purpose, different parameters, comprising the concentration of eight heavy metals and eight ions, alkalinity, total harness, pH, biological oxygen demand (BOD5) and total coliform, were measured over 12 months and the obtained data were compared with the WHO’s and Iran’s drinking water standards. To assess the measured data statistically, SPSS software was applied. From the reported results, the water characterizations of the wells complied with the mentioned standards; however, four of the wells were more prone to supply higher quality water. It is noted that Hg, Cd, and the total coliform of wells were close to the permissible values reported by both the aforementioned standards. Therefore, the water obtained from wells should be disinfected before using and Hg and Cd concentrations need to be monitored regularly to prevent poisoning. Due to the rapid movement of pollutants in karst areas, it is very important to detect their presence in the water resources over time. Consequently, continuous monitoring and sampling is one of the most important protection dealings for karst aquifers.
1. Introduction
Water pollution can be defined as an undesirable change in the physical, chemical and biological properties of water that endangers the health and survival of humans or other living organisms. Various human issues threaten the quality of water resources and cause its pollution, these include increasing population growth, followed by increased production of waste, municipal and industrial waste and sewage, and uncontrolled fertilizers in agriculture.
With increasing industrialization and the release of hazardous industrial wastewater to the environment, ground and surface water sources are affected and it is necessary to assess water resources in terms of chemical and biological pollution [1,2]. Metals with atomic weights greater than 40.04, called heavy metals, exist all over the earth and they could be harmful for human health [3]. The presence of these heavy metals in water supply sources creates adverse effects on human bodies. Heavy metals include antimony, tellurium, bismuth, tin, thallium, gold, arsenic, cerium, gallium, cadmium, chromium, cobalt, copper, iron, lead, mercury, manganese, nickel, platinum, silver, uranium, vanadium, and zinc [4]. Some of these heavy metals are essential for different functions in human bodies. However, high dosages of them could be dangerous for human health. Drinking water has been observed as a primary source of the transport of heavy metals to the human body [5]. Therefore, some health organizations have released the safe ranges of different heavy metals in drinking water.
Heavy metals can aggregate in the cells of different organs in the human body and stop cellular functions by making changes in proteins and nucleic acids, resulting in several diseases in vital organs such as the lungs, liver, kidneys and even the central nervous system [6,7]. Lead, cadmium, mercury and arsenic are the major heavy metals that, if found in drinking water, should prompt those water sources to be investigated to prevent transporting these threats into the human body [8]. Anemia, brain and kidney damage are the complications of lead poisoning. Especially in children, it can cause an inhibition in physical and mental growth. Long-term arsenic poisoning via contaminated drinking water causes skin diseases such as lesions, swollen skin and cancer [9]. Short-term and long-term cadmium exposure is hazardous. The complications of cadmium poisoning are kidney damage, stomach cramps, fragile bones, and dehydration. Mercury is also known as a perilous heavy metal which creates different symptoms in the human body such as hearing, speaking and vision damage, muscle weakness, nervousness and memory loss. Its long-term complications are kidney damage and a reduction in brainpower [10,11].
Apart from heavy metals, drinking water sources such as wells should be evaluated in terms of other chemical parameters like pH, total hardness and common anion and cations such as sodium, potassium, nitrate, phosphate, sulfate etc. A number of reports about the quality of drinking water sources all over the world have been published regarding these parameters [12,13,14,15,16,17,18,19,20,21]. In one study, Javahershenas et al. studied the leachate effects of a landfill in Lahijan city on the quality of surface water and groundwater resources [20]. In another study, Khater et al. reported on the quality of bottled drinking water in Saudi Arabia [22].
In the present study, aquifers are the most important drinking water resources [23] so the quality of drinking water sources (eight wells) of Yasouj, a city in Iran, were investigated in terms of different chemical and biological parameters. The presence of eight heavy metals (As, Cd, Cu, Cr, Fe, Zn, Pb and Hg) and eight ions (Ca2+, Mg2+, Na+, K+, SO42−, PO43−, NO3− and Cl−) was checked and different chemical tests, comprising pH, dissolved oxygen (DO) concentration, turbidity, total hardness, alkalinity and microbial tests including biological oxygen demand (BOD) and total coliform, were performed and compared with WHO and Iranian drinking water standards. To collect the required data, the chemical and biological tests were undertaken on the samples of the eight studied wells for a year (12 months).
2. Materials and Methods
2.1. Studied Area
The study area, called Doposhteh landfill, is located in the southeast of Yasouj city (southwest of Iran) (Figure 1). This area is one of the sub-watersheds of the Beshar River, which is discharged into the Persian Gulf upon joining the great Karun River. The area is generally composed of carbonate and karst formations and is one of the most important drinking water supply points in Yasouj. By drilling eight deep wells, most of the required municipal water is pumped. Investigations have revealed that there was no precise planning and design for drilling wells; in fact, the wells were drilled based only on the water needs of Yasouj city and the very good discharge of the karst aquifer. Yasouj landfill is located 2 km away from the wells, so that the height of the landfill is about 300 m above the wells. Also, downstream of the landfill, a reservoir dam has been constructed to supply the water needed for agriculture and recharge the aquifer (Figure 1 and Figure 2). This karst aquifer is one of the most important sources of drinking water supply for the people of Yasouj, and has always been considered by experts, government officials and news agencies as at risk of pollution due to waste leachate above it. Since the nature and pollution content of leachate varies significantly from landfill to landfill and depends on various factors such as waste composition, seasons, area hydrology, compaction grade, waste age, landfill technology and sampling process [24,25] it is necessary to monitor the possible impact of the Yasouj landfill on the aquifer.
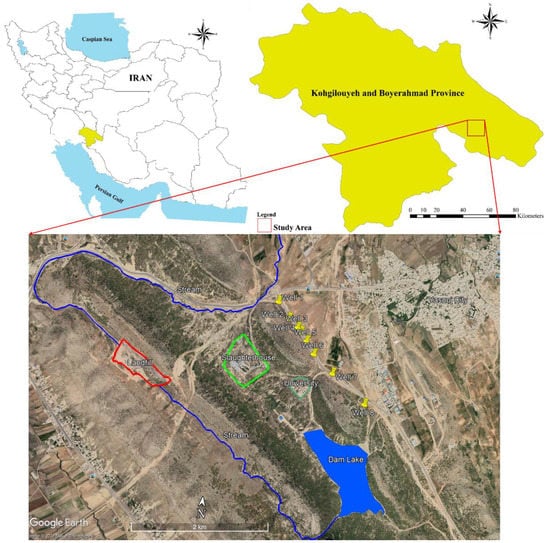
Figure 1.
The location of eight wells, landfill site and slaughter house.
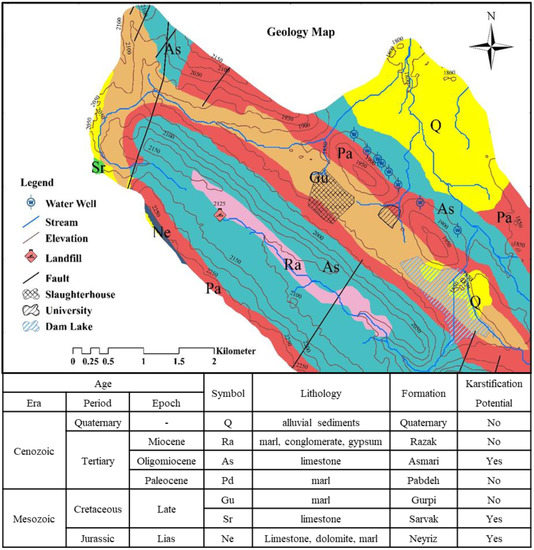
Figure 2.
Geology map and lithological characteristics.
2.2. Geology Setting
Geology is one of the most important factors in the hydrology and hydrogeology of the watershed. Gentle folds and simple tectonics, in general, are prominent features of the study area; the faulting has caused the removal of some facies or their compaction. The presence of hard facies next to each other and their intense erosion has caused the outcrop of limestone formations, especially Asmari, and the depression of other formations such as Pabdeh-Gurpi. The streams of the region are mostly young and cause erosion along the flow. The region is generally composed of Asmari formation and limestone sediments with rough surfaces and dense to sparse vegetation. The formations of the region are: Gurpi, Pabdeh, Asmari, Sarvak, Neyriz, Razak and Quaternary alluvial (Figure 2).
2.3. Statistical Analysis of Samples
The attained results of the present study were analyzed by SPSS and compared with World Health Organization (WHO) guidelines. SPSS is a dominant software commonly used for statistical analysis, data management and data documentation with a graphical interface designed for ease of use. SPSS software is widely used in a range of disciplines for descriptive statistics such as frequency, cross-tabulation, and descriptive ratio statistics and also for bivariate statistics like analysis of variance (ANOVA), mean, correlation and nonparametric tests. In the present study, different statistical parameters such as mean, maximum, minimum and standard deviation for each measured case were calculated and T-tests were also applied for some parameters.
2.4. Analytical Methods
In order to determine possible contamination of the aquifer and reduce water quality over time, the samples were taken from eight wells and bottled in a polyethylene container, monthly and for one year. The samples were transferred to the laboratory in the shortest possible time to perform the required tests to measure heavy metals (As, Cd, Cu, Cr, Fe, Zn, Pb and Hg), ions (Ca2+, Mg2+, Na+, K+, SO42−, PO43−, NO3− and Cl−) temperature, pH, DO concentration, turbidity, total hardness, BOD5 and total coliform.
Heavy metals were measured by an inductively coupled plasma (ICP) device (OES model, Aligent, Waldbronn, Germany), after fixation of the samples by nitric acid. The pH and turbidity were analyzed with a pH-meter (PHL300, China). The DO concentration in wastewater was determined using a DO probe supplied by WTW DO Cell OX 330, electro DO probe, Germany. Turbidity was measured by a turbidity meter model 2100 P (Hach Co., ISO Loveland, CO 80539-0389, USA). Spectrophotometry technique was used for the detection of NO3−, PO43− and Cl−. Furthermore, the concentrations of Na+ and K+ were measured by a flame photometer. The concentrations of Ca2+ and Mg2+ were measured by titration. All chemical tests were performed according to standard methods [26].
3. Results and Discussion
The data of different parameters are shown in Table 1. Minimum, maximum, mean and standard deviation of different chemical and microbial parameters are reported in Table 1. Also, the WHO standard and Iran’s drinking water standard are presented in Table 1 [27]. The reported mean, maximum and minimum of pH data were approximately compared with the recommended range published by the WHO and Iran’s drinking water standards. The minimum pH value was 6.38, which is close to the minimum acceptable value (6.5).

Table 1.
Physical and chemical characteristics and the concentration of different measured parameters for the all eight wells.
An imperative aspect of water quality that should be assessed is biological characteristics. For this, three parameters, DO concentration, BOD concentration, and total coliforms, were measured over 12 months. In order to inhibit septic conditions in water resources, the WHO standard recommends DO concentration in water samples higher than 1 mg/L, while, according to the measured data, the minimum DO concentration for Yasouj’s water samples was 7.21 mg/L over 12 months. Therefore, fresh water could be obtained from the eight wells. It is worth mentioning that BOD is a key factor in discerning organic compounds in water which ameliorate the conditions for microbial growth. The more BOD concentration, the more possibility of microbial growth. The permissible range for BOD has been reported as 3 mg/L by Iran’s drinking water standard. According to Table 1, the BOD concentration in Yasouj’s water was not detectable over 12 months and so it could be concluded that the water resources were not contaminated and organic matter had not penetrated into groundwater sources. Finally, the total coliform of the water samples is reported in Table 1 to evaluate the health of water in terms of biological domain. From the data, the mean of the coliform is less than the permissible value. However, the maximum value of total coliform data was over the range and more statistical analysis of the total coliform data was performed as follows (Section 3.2).
Another trait of drinking water quality that should be measured is total hardness, as hard water could threaten peoples’ health. The maximum hardness of Yasouj’s water during the year was 288 mg CaCO3/L, which is lower than the recommended value by WHO standards. Furthermore, the concentration of Ca2+ and Mg2+, two main cations involved in total hardness, was monitored as a validation for total hardness data. Eighty-two and 22 mg/L were reported as the maximum concentration for Ca2+ and Mg2+, respectively. The permissible values of WHO standards for Ca2+ and Mg2+ are 100 and 50 mg/L, respectively, and these data amply proved the harmless range of hardness of Yasouj’s water.
As TDS concentration should be lower than 500 mg/L as per the WHO standard, the water samples of Yasouj University, with a maximum TDS of 233 mg/L, meet the WHO standards. Moreover, the concentrations of common ions in water resources such as Na+, K+, PO43−, NO3−, SO42−, Cl− were evaluated and all of them were in the recommended range published by WHO standards.
As a result, a comparison between mean data and the standards values shows that all parameters for the eight wells meet the permissible range of both the WHO and Iran’s drinking water standards. However, it should be noted that the maximum data of Cd, Pb, Hg and total coliform were over their respective ranges. So, statistical analysis was applied to the aforementioned responses to investigate the variation trends over different months.
3.1. Heavy Metals
Figure 3a–c show pie plots of Pb, Cd, and Hg concentrations over 12 months.
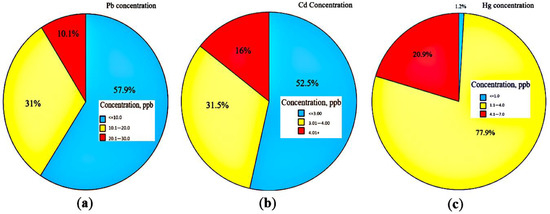
Figure 3.
Pie chart for the concentration of three heavy metals: (a) Pb, (b) Cd, (c) Hg.
The concentration data were categorized into three levels, represented with blue, yellow and red colors. Blue color represents the frequency of data on a safe concentration of heavy metals, which means the heavy metal concentration is less than the permissible range published by WHO and Iran’s drinking water standards. The yellow color indicates a range of heavy metal concentration close to the permissible range (by WHO and Iran’s drinking water standards). However, a high concentration range of heavy metals compared with the aforementioned standards is displayed in red. Moreover, Figure 4a–c presents more details about the concentrations of Pb, Cd, and Hg for each well, respectively.
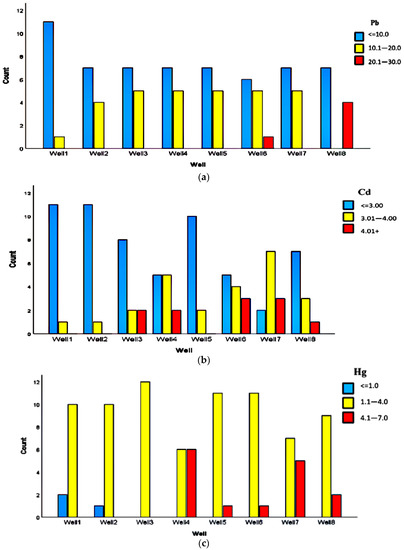
Figure 4.
The different ranges of three heavy metals’ concentration (ppb) based on each well: (a) Pb, (b) Cd, (c) Hg.
From Figure 3a–c, the portion of safe data is relatively small for Hg rather than both other metals. Furthermore, based on Figure 4c, a minor ratio of Hg data was in the range of Iran’s drinking water standard (≤1.0 ppb) which is related to wells 1 and 2 and the majority of the data were higher than 1 ppb. Hg data in the range of 4.1–7 ppb were reported for wells 4 to 8.
From Figure 3a, the obtained data for Pb concentration showed that the main portion of Pb data was in safe concentration. Also, it can be observed from Figure 3a that well 1 presented the lowest Pb concentrations and the highest Pb concentrations were reported for well 8 over a year.
Likewise, the measured Cd data over a year for eight wells were categorized into three levels according to Figure 3b. Based on the figure, similar to Pb data, the main portion of Cd data was in the harmless range. Also, according to Figure 4b, wells 1, 2 and 5 showed a safe range of Cd concentration, while wells 4 and 6–8 showed Cd concentration in a dangerous range.
Figure 3a–c, illustrate that the portion of safe data is relatively small for Hg rather than both other metals. According to Figure 4c, the trivial ratio of Hg data for wells 1 and 2 was shown in blue color with safe concentration (≤1.0 ppb), whereas the majority of the data were higher than 1 ppb. Hg concentrations were reported in the range of 4.1–7 ppb for wells 4 to 8, indicating a risky range for Hg concentrations related to the aforesaid wells.
From the reported concentration of Pb, Cd and Hg, we can conclude that wells 1–4 could be categorized as harmless wells, while wells 4 to 8 represent somewhat critical concentrations of heavy metals. In fact, by moving from well 1 to well 8 the hazardous range of heavy metals was bolded.
In order to present a decent evaluation of the obtained concentrations of different heavy metals, SPSS software was applied and the differences between wells and months over a year in terms of heavy metals concentrations were assessed. Table 2 shows statistical data for evaluating the differences between wells. It can be seen that there are significant differences between wells. Conversely, the reported data for Pb showed no significant difference between wells, verifying a normal distribution for Pb concentration in comparison with Figure 4a.

Table 2.
ANOVA results for three heavy metals’ concentrations reported from eight wells over a year (eight wells considered as eight groups).
Furthermore, to investigate the effect of weather on the heavy metals data, ANOVA results for three heavy metals concentrations over 12 months are reported in Table 3. Based on statistically significant probability calculated by SPSS, Hg data did not depend on months and Hg concentrations of the eight wells were approximately constant over 12 months. Nevertheless, Cd and Pb concentrations showed significant differences over 12 months.

Table 3.
ANOVA results for three heavy metals’ concentrations reported over 12 months (12 months considered as 12 groups).
To present a deeper assessment, the Cd and Pb data over different seasons are reported in Table 4. Overall, the concentrations of Pb are more substantial in winter and fall seasons (rainy months). As a result of more rainfall, the pollution could be dissolved in rainwater and penetrate to ground water. This result is a sign of the presence of pollution sources in nearby regions of the wells.

Table 4.
The mean concentration of Pb and Cd for each season.
Furthermore, one sample t-test was performed for the mentioned heavy metals (Table 5). A t-test is used to determine if there is a significant difference between the means of the heavy metals concentration and the aforementioned standards. The obtained significant probabilities were 0.05, 0.075, and 0 for Cd, Pb, and Hg, respectively. Therefore, there are significant differences between Cd and Hg data and the standard values. It should be noted that the recommended values for Iran’s drinking water standard were considered for one-sample t-test. Nonetheless, there is no significant difference between Pb concentration and the permissible value. Thus, it can be concluded that the concentration of Hg and Cd in the water resources of Yasouj city is critical and both these heavy metals should be considered as a dangerous potential which needs to be monitored frequently.

Table 5.
The results of one sample t-test for all three heavy metals.
3.2. Microbial Analysis
In order to evaluate the quality of water resources, a total coliform test was accomplished for all eight wells over 12 months. The diagnostic test was based on the identification of three or more bacteria; In other words, the minimum detection value was three and less than that was considered zero. From Table 1, the maximum value of total coliform data was higher than the standard value of WHO. Therefore, the statistical analysis of total coliform data was performed. A one-sample t-test was undertaken to perceive the threat of microbial pollution as shown in Table 6. From the statistically significant probability data reported for total data obtained from all the wells (0.00), it can be concluded that there is a significant difference between total coliform data and WHO standards. Furthermore, one-sample t-test was carried out for each well displayed in Table 6. According to the statistically significant probability for each well, significant differences between total coliform data and WHO standards for wells 1, 2, 5 and 8 were observed. For other wells, statistically significant probability was not computed by SPSS as the standard deviation was zero for their data. Furthermore, to portray more profound evaluation from total coliform data, the obtained data of all eight wells over 12 months are depicted in Figure 5. Based on Figure 5, Wells 1, 2, 5, and 8 showed over range values of total coliform over May, September, June, July and December in good agreement with statistically significant probability data shown in Table 6.

Table 6.
The results of one-sample t-test for total coliform data.
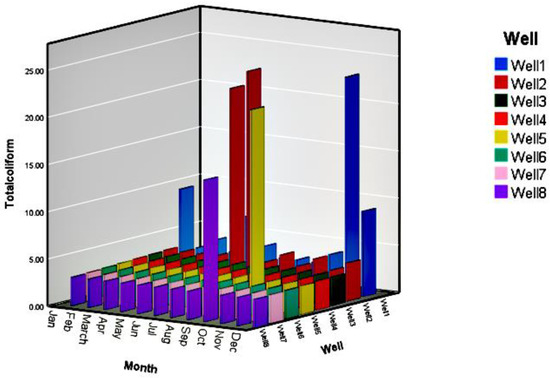
Figure 5.
Total coliform data for all eight wells over 12 months (detection limit is three and less).
It is needless to say that, as microbial pollution is indeed a vital matter to serve citizens’ health, so a proper disinfection method is essential to be applied. Overall, the water sources (eight wells) were not meaningfully influenced by the dumping zone and slaughterhouse wastewater and the quality of water will be acceptable after a disinfection method like chlorination.
4. Conclusions
It is necessary to assess the risk of waste disposal in groundwater resources by determining the chemical properties and monitoring water quality over time and at the place. In other words, the study of spatial and temporal changes in groundwater quality parameters is important in understanding the aquifer quality status, pollutant sources and determining the most appropriate management strategy. In fact, by identifying the time (here, month) of contamination occurrence and the most sensitive place (here, wells) to pollution can be planned to manage the quality of drinking water. Since the landfill of Yasouj city is located at a higher elevation than the drinking water wells and the karst aquifer is also very vulnerable to pollution, it is possible that the landfill affects the quality of drinking water in the wells. Hence, this study investigated the quality of the drinking water resources, including the eight wells. In this manner, the concentration of eight heavy metals and eight ions and also various chemical and biological parameters were monitored for all eight wells over 12 months and compared with both the WHO standard and Iran’s drinking water standard. From the obtained data, drinking water resources were not significantly influenced by landfill site at a distance of 2 km. However, the maximum values of three heavy metals (Pb, Cd and Hg) and total coliform were over the permissible values reported from the aforementioned standards. Therefore, a t-test was performed, and it was concluded that there were significant differences between Hg and Cd concentrations along with total coliform at the recommended levels. This is a significant difference and so increasing groundwater pollution very probably is in limestone areas with karst aquifers. Consequently, the drinking water resources should be disinfected before being released in a water distribution network. Further, Hg and Cd concentration should be considered as potential threats and monitored frequently. Due to the rapid movement of pollutants in karst formations, it is very important to detect their presence in the water resources over time. Therefore, continuous monitoring and sampling is one of the most important protections for karst aquifers. Finally, protecting groundwater resources is not only environmentally friendly but also cost-effective because the cost of preventing pollution is lower than its treatment.
Author Contributions
Conceptualization, M.F., M.Z. and A.A.; methodology, M.F. and A.A.; validation, M.F. and A.A.; formal analysis, M.F. and A.A.; investigation, M.F. and M.Z.; data curation, M.F.; writing—original draft preparation, M.F. and A.A.; writing—review and editing, M.F., A.A., M.Z. and K.P.; supervision, M.F. and K.P.; project administration, M.F.; funding acquisition, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Slovak Research and Development Agency under the Contract no. APVV-20-0281, and a project funded by the Ministry of Education of the Slovak Republic VEGA1/0308/20 “Mitigation of hydrological hazards, floods, and droughts by exploring extreme hydroclimatic phenomena in river basins”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are not publicly available due to institutional property rights.
Acknowledgments
We would like to thank the Kohgiluyeh va Boyerahmad Provincial Government and Department of Environment of Iran for their financial support of the project under Contract no. 1400/74/06/1139.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global water pollution and human health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Chowdhary, P.; Bharagava, R.N.; Mishra, S.; Khan, N. Role of industries in water scarcity and its adverse effects on environment and human health. In Environmental Concerns and Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2020; pp. 235–256. [Google Scholar]
- Giagnorio, M.; Steffenino, S.; Meucci, L.; Zanetti, M.C.; Tiraferri, A. Design and performance of a nanofiltration plant for the removal of chromium aimed at the production of safe potable water. J. Environ. Chem. Eng. 2018, 6, 4467–4475. [Google Scholar] [CrossRef]
- Hussain, J.; Husain, I.; Arif, M.; Gupta, N. Studies on heavy metal contamination in Godavari river basin. Appl. Water Sci. 2017, 7, 4539–4548. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Flora, S.J. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxidative Med. Cell. Longev. 2009, 2, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans, in Poisoning in the Modern World—New Tricks for an Old Dog? IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/64762 (accessed on 16 March 2022).
- Unsal, V.; Dalkıran, T.; Çiçek, M.; Kölükçü, E. The role of natural antioxidants against reactive oxygen species produced by cadmium toxicity: A review. Adv. Pharm. Bull. 2020, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Singh, W.S.; Manna, K. Sources and toxicological effects of lead on human health. Indian J. Med. Spec. 2019, 10, 66. [Google Scholar]
- Hyman, M. Mercury: Get This Heavy-Metal Poison out of Your Body. 2010. Available online: https://drhyman.com/blog/2010/05/20/mercury-get-this-poison-out-of-your-body/ (accessed on 15 August 2021).
- Litvinenko-Bhopal, A. Mercury poisoning. Singh, N.; Sharma, M. Assessment of the Quality of Drinking Water Sources and Human Health in a Rural Area of Solan. N. India Mapan 2020, 35, 301–308. [Google Scholar]
- Saleh, M.A.; Ewane, E.; Jones, J.; Wilson, B.L. Chemical evaluation of commercial bottled drinking water from Egypt. J. Food Compos. Anal. 2001, 14, 127–152. [Google Scholar] [CrossRef]
- Pant, N.D.; Poudyal, N.; Bhattacharya, S.K. Bacteriological quality of bottled drinking water versus municipal tap water in Dharan municipality, Nepal. J. Health Popul. Nutr. 2016, 35, 17. [Google Scholar] [CrossRef]
- Karavoltsos, S.; Sakellari, A.; Mihopoulos, N.; Dassenakis, M.; Scoullos, M.J. Evaluation of the quality of drinking water in regions of Greece. Desalination 2008, 224, 317–329. [Google Scholar] [CrossRef]
- Nogueira, G.; Nakamura, C.V.; Tognim, M.C.; Abreu Filho, B.A.; Dias Filho, B.P. Microbiological quality of drinking water of urban and rural communities, Brazil. Revista Saúde Pública 2003, 37, 232–236. [Google Scholar] [CrossRef]
- Ewaid, S.H.; Rabee, A.M.; Al-Naseri, S.K. Carcinogenic risk assessment of trihalomethanes in major drinking water sources of Baghdad City. Water Resour. 2018, 45, 803–812. [Google Scholar] [CrossRef]
- Barenboim, G.; Kozlova, M. Pollution of Sources of Drinking Water Supply of Large Cities with Pharmaceuticals (the Example of Moscow, Russia). Water Resour. 2018, 45, 941–952. [Google Scholar] [CrossRef]
- Demir, V.; Dere, T.; Ergin, S.; Cakır, Y.; Celik, F. Determination and health risk assessment of heavy metals in drinking water of Tunceli, Turkey. Water Resour. 2015, 42, 508–516. [Google Scholar] [CrossRef]
- Abu-Hashim, M.; Zeleňáková, M.; Vranayová, Z.; Khalil, M. Soil Water Erosion Vulnerability and Suitability under Different Irrigation Systems Using Parametric Approach and GIS, Ismailia, Egypt. Sustainability 2021, 13, 1057. [Google Scholar] [CrossRef]
- Zeleňáková, M.; Purcz, P.; Pintilii, R.D.; Blišťan, P.; Hluštík, P.; Oravcova, A.; Hashim, M.A. Spatio-temporal variations in water quality parameter trends in river waters. Rev. Chim. 2018, 69, 2940–2947. [Google Scholar] [CrossRef]
- Javahershenas, M.; Nabizadeh, R.; Alimohammadi, M.; Mahvi, A.H. The effects of Lahijan landfill leachate on the quality of surface and groundwater resources. Int. J. Environ. Anal. Chem. 2020, 102, 558–574. [Google Scholar] [CrossRef]
- Khater, A.E.; Al-Jaloud, A.; El-Taher, A. Quality level of bottled drinking water consumed in Saudi Arabia. J. Environ. Sci. Technol. 2014, 7, 90. [Google Scholar] [CrossRef][Green Version]
- Farzin, M.; Avand, M.; Ahmadzadeh, H.; Zelenakova, M.; Tiefenbacher, J.P. Assessment of Ensemble Models for Groundwater Potential Modeling and Prediction in a Karst Watershed. Water 2021, 13, 2540. [Google Scholar] [CrossRef]
- Bjerg, P.L.; Albrechtsen, H.J.; Kjeldsen, P.; Christensen, T.H.; Cozzarelli, I.M. The groundwater geochemistry of waste disposal facilities A2—Holland, Heinrich, D. In Treatise on Geochemistry; Turekian, K.K., Ed.; Pergamon: Oxford, UK, 2003; pp. 579–612. [Google Scholar]
- Christensen, T.H.; Kjeldsen, P.; Bjerg, P.L.; Jensen, D.L.; Christensen, J.B.; Baun, A.; Albrechtsen, H.J.; Heron, G. Biogeochemistry of landfill leachate plumes. Appl. Geochem. 2001, 16, 659–718. [Google Scholar] [CrossRef]
- Federation, W.E.; Association, A. Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association, Water Environment Federation Publisher: Washington, DC, USA, 2017. [Google Scholar]
- WHO. A Global Overview of National Regulations and Standards for Drinking-Water Quality; WHO: Geneva, Switzerland, 2018. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).