Holistic Case Study on the Explosion of Ammonium Nitrate in Tianjin Port
Abstract
:1. Introduction
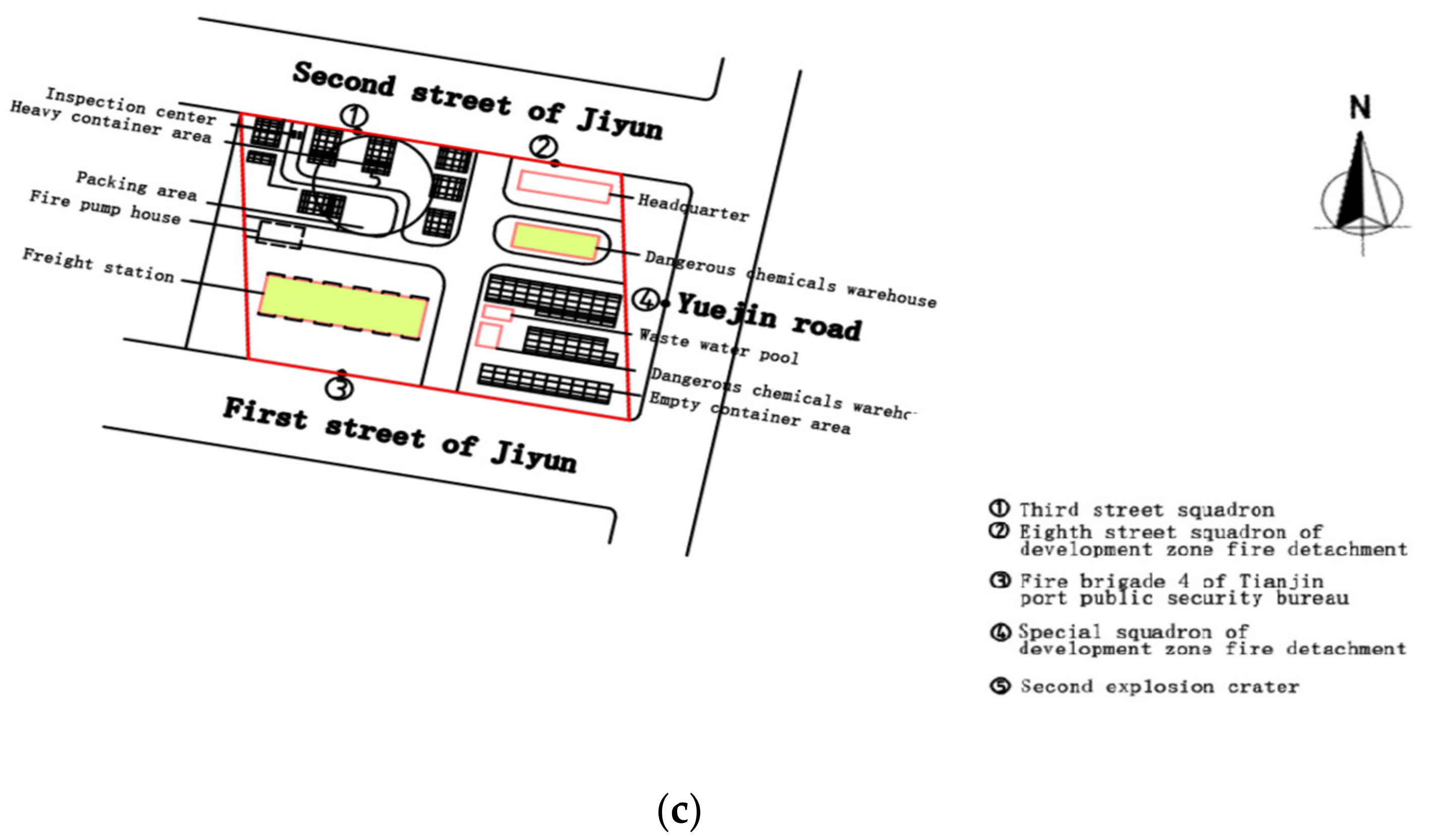
1.1. Occurrence of the Incident
1.2. Major Incidents Caused by Ammonium Nitrate
1.3. Thermal Hazards and Autocatalytic Decomposition of Nitrocellulose
1.4. Fire and Explosion Hazards of Ammonium Nitrate
1.5. Explosion Hazards and Susceptibility to Detonation of AN
2. Materials and Methods
2.1. Samples
2.2. Differential Scanning Calorimetry
2.3. Evaluation of Trinitrotoluene Equivalent
2.3.1. Trinitrotoluene Equivalent Model
2.3.2. Validation of Trinitrotoluene Equivalent
Damage–Distance Correlation
Empirical Formula of Scaling Law
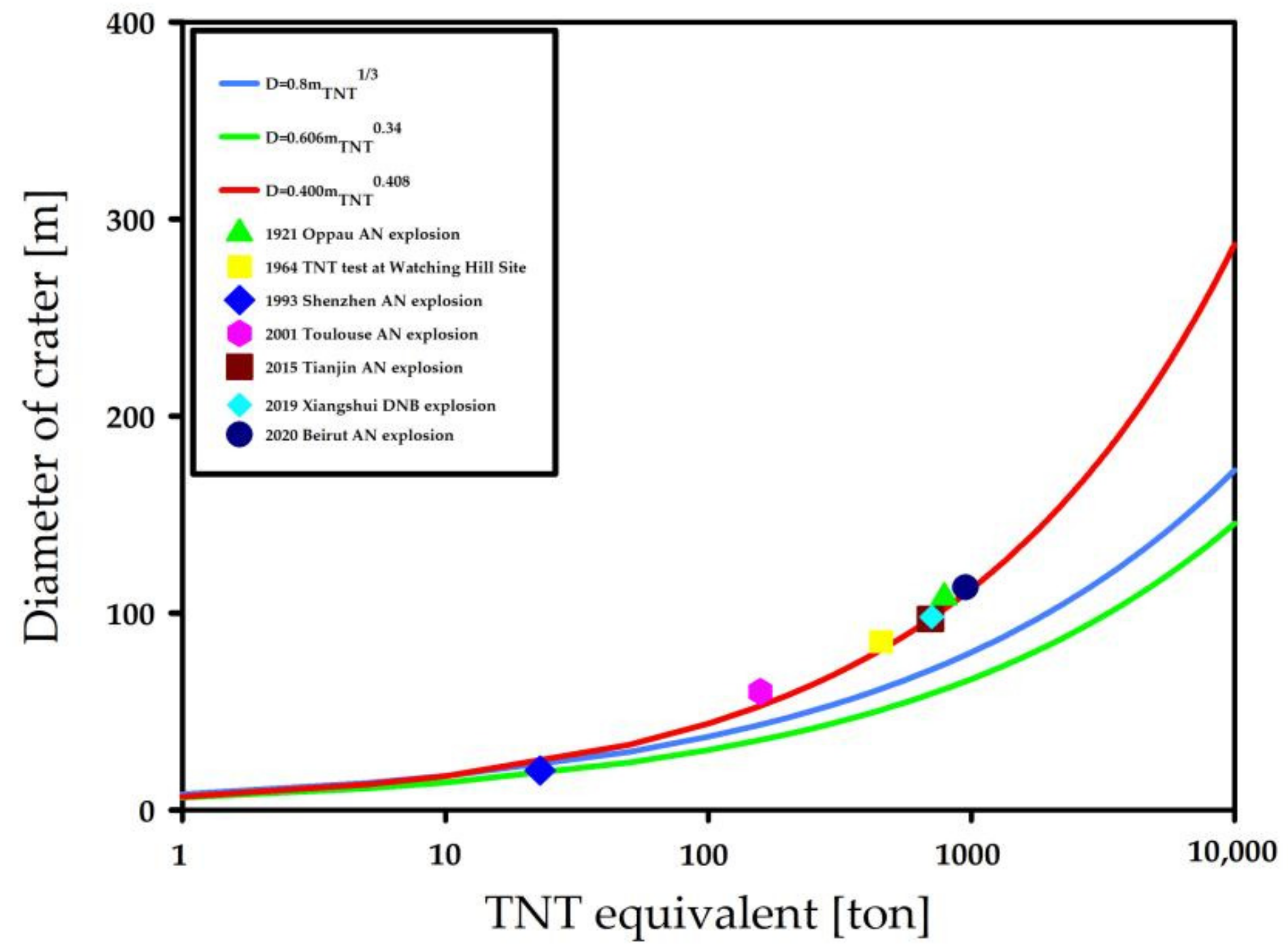
Inverse Analysis Based on Crater Size
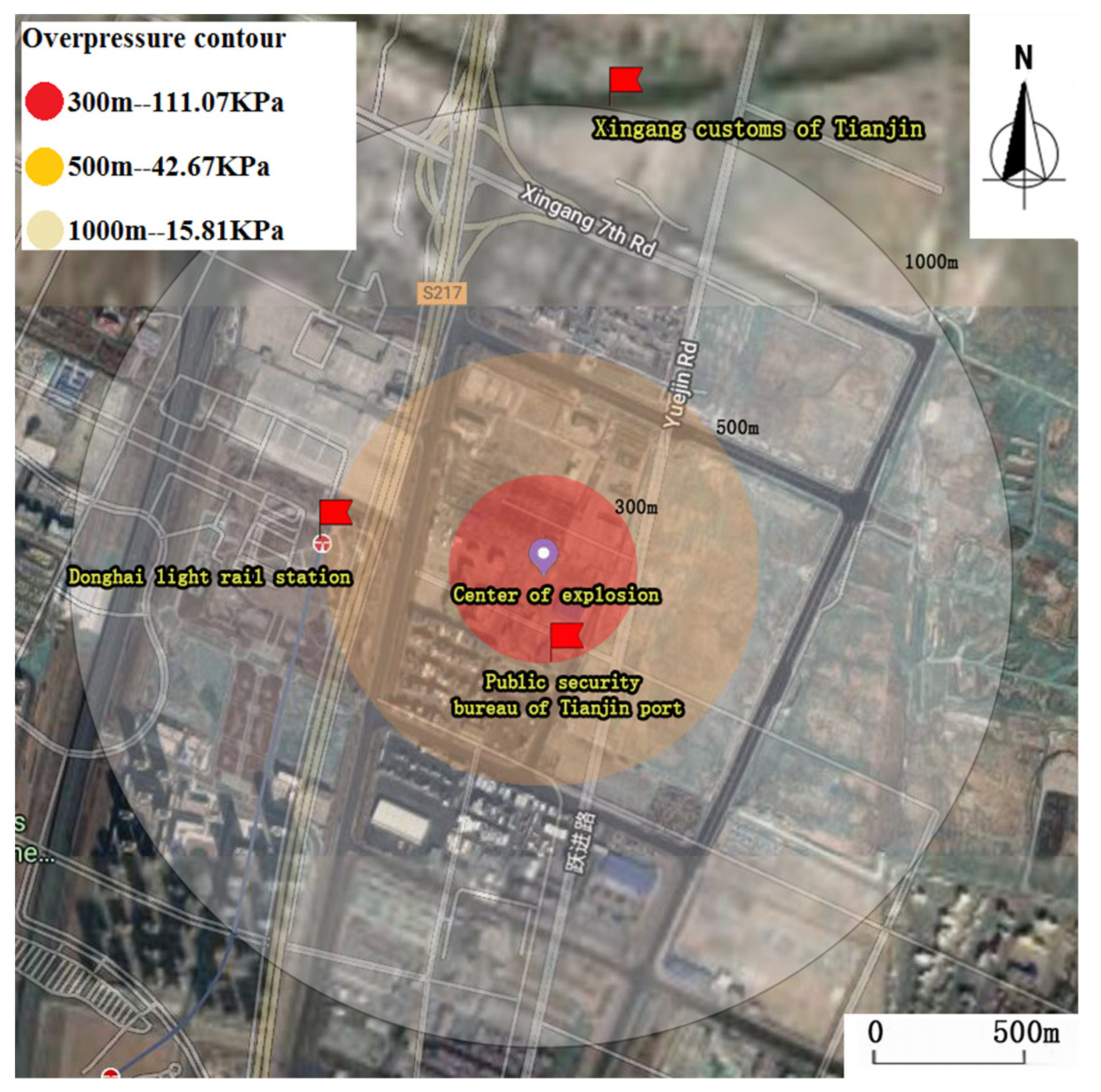
2.4. Influences of Blast Overpressure on Buildings and Personnel
2.4.1. Shock Wave Blast Effects
2.4.2. Baker’s Methodology
2.4.3. Sadovski’s Methodology
2.4.4. Alonso’s Methodology
3. Results and Discussion
3.1. Trinitrotoluene Equivalent
3.1.1. mTNT Evaluation by Overpressure Effects
3.1.2. mTNT Evaluation by Scaling Law
3.1.3. mTNT Evaluation by Inverse Analysis
3.2. Impact of Overpressure on Building and Human Body
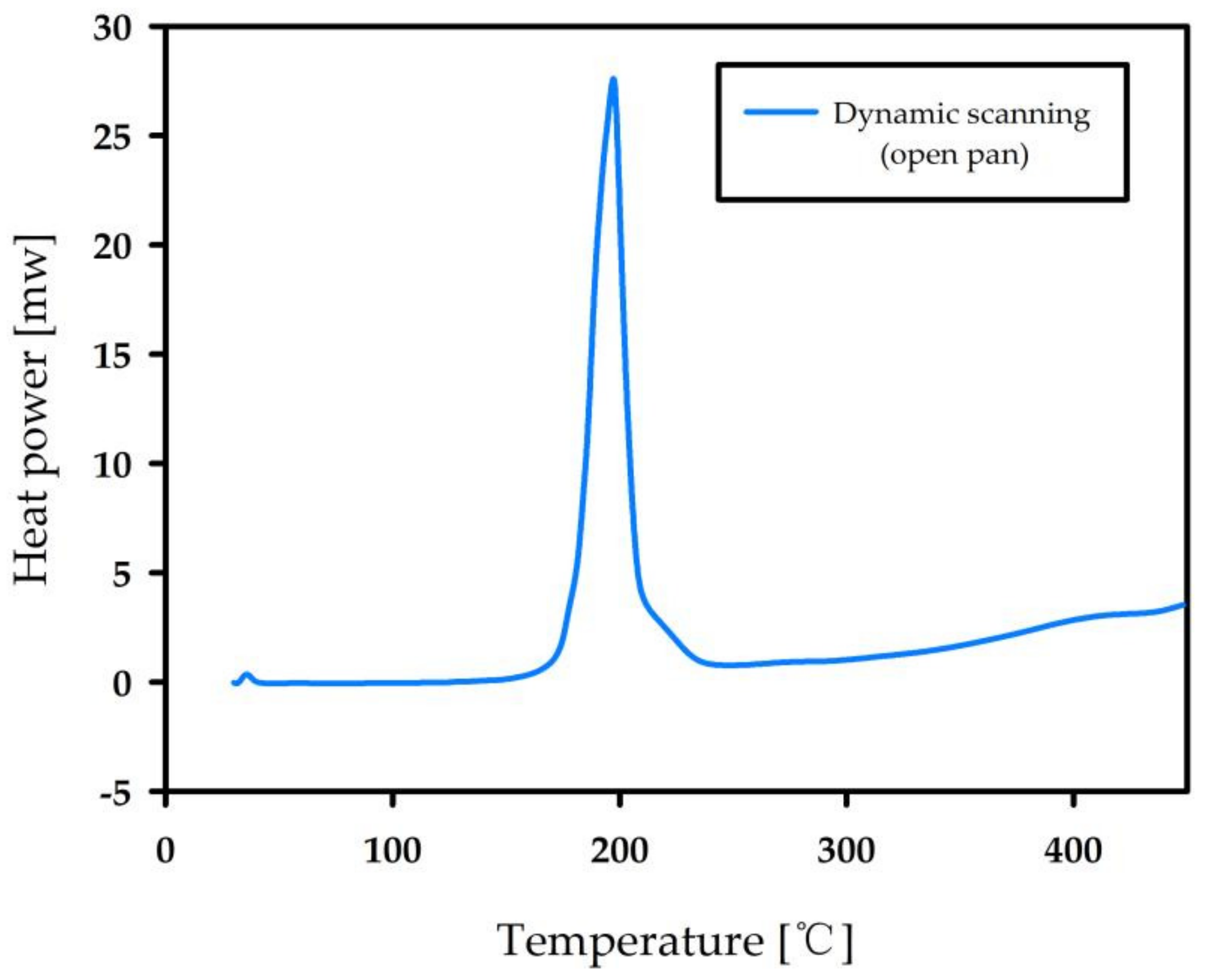
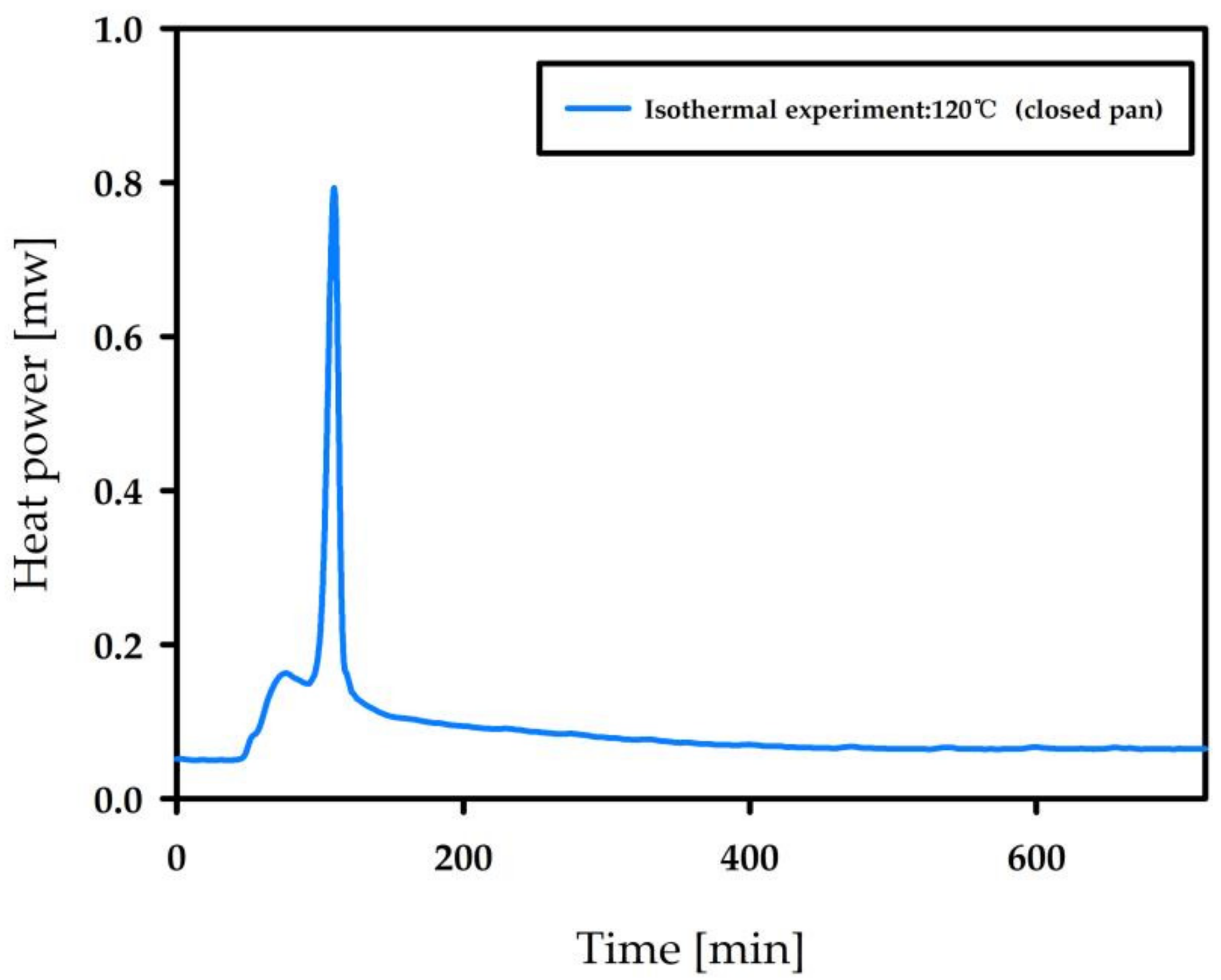
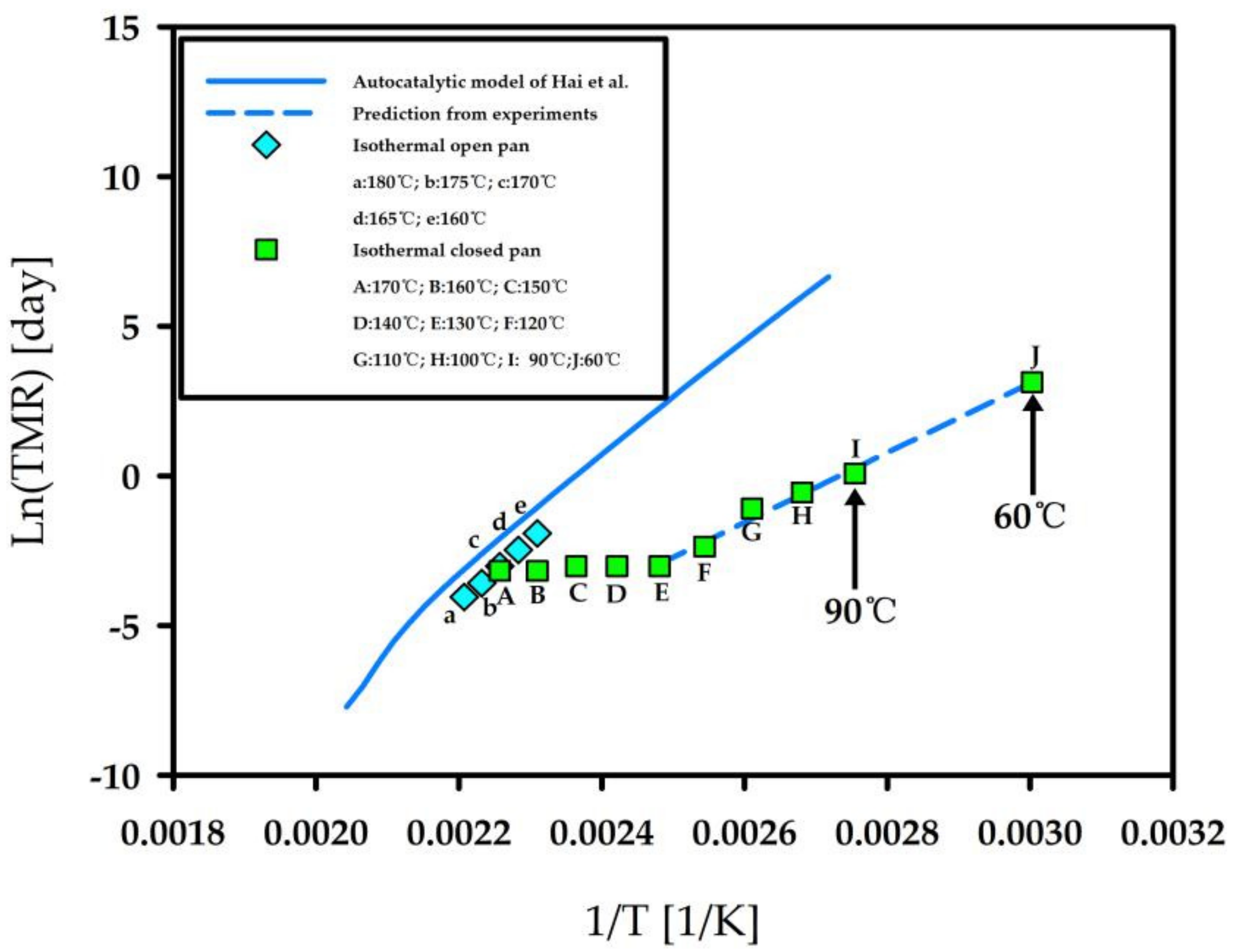
3.3. Autocatalytic Decomposition of Nitrocellulose
3.4. Incidents of Sympathetic Detonations
3.5. Scenario of Two-Successive-SD-Following-a-Fire in Tianjin Incident
4. Lessons Learned
- From the amount of fatality and injury, this is the most serious explosion of AN in the world since 1947 and before the incident at the Beirut Port occurred in 2020.
- Most of the victims and injured individuals were firefighters, therefore the guides for fire fighting in an industrial plant or warehouse storing AN must be carefully planned before their actions for emergency responses [62].
- The Ruihai company illegally stored too much AN and other dangerous chemicals.
- Reactive substances, NC and AN were stacked together to create the difficulties for fire-fighting and the risks of fire hazards.
- NC can be ignited by autocatalytic decomposition in containers during summertime due to poor heat removal.
- The hazards of autocatalytic decomposition of NC were proven to be more severe in closed than open status.
- NC is determined be ignited after about 9 days by autocatalytic decomposition in a container sustained at 60 °C.
- AN in a confined container can explode under external fire and propagate severe SD.
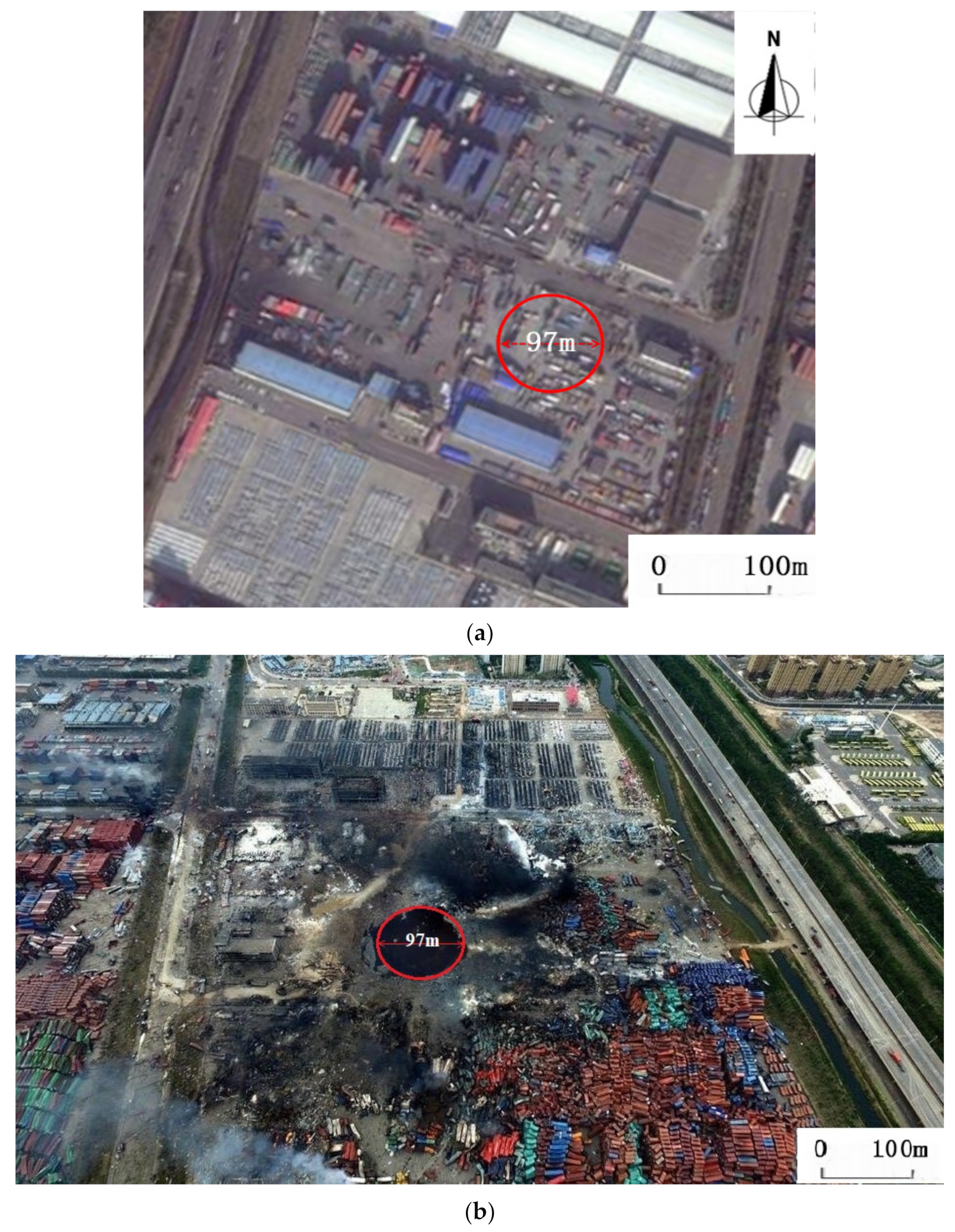
- A safety distance to prevent the SD caused by AN in a 20′GP container was determined to be 97 m in Dolah’s studies, indicating all the stacked AN containers will explode within a few seconds by SD if one of them ignites the first explosion.
- The mTNT in conjunction with the second explosion was averaged to be 577 tons using scaling law, damage-distance correlation and inverse analysis.
- NC and AN were reported to be the very materials to ignite the terrible first fire and disastrous explosion, respectively.
- The incident was a typical event of “two-successive-explosions-following-a-fire”.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Description |
| AIT | Auto-ignition temperature |
| AN-FO | Ammonium nitrate-fuel oil |
| ARC | Accelerating rate calorimetry |
| ASTM | American Society of Testing and Materials |
| DDT | deflagration-to-detonation |
| DSC | Differential scanning calorimetry |
| FGAN | Fertilizer grade ammonium nitrate |
| MPD | m-Phenylenediamine |
| NFPA | National Fire Protection Association |
| SD | Sympathetic detonation |
| TGA | Thermogravimetry analysis |
| TGAN | Technical grade ammonium nitrate |
| TNT | Trinitrotoluene |
| UCAT | Upper critical ambient temperature |
| UN | The United Nations |
| US-DOT | The United States Department of Transportation |
| Nomenclature | |
| D | Diameter of explosion pit (m) |
| mTNT | Mass of trinitrotoluene (kg) |
| R | Distance to the center of explosion (m) |
| P | Atmospheric pressure (Pa) |
| ΔP | Overpressure (Pa) |
| ΔHAN | Heat of decomposition of ammonium nitrate (J) |
| ΔHNC | Heat of decomposition of nitrocellulose (J) |
| ΔHTNT | Heat of decomposition of trinitrotoluene (J) |
| Tonset | Exothermic onset temperature (°C) |
| TMR | Time-to-maximum-rate (min) |
| Subscript | |
| AN | Ammonium nitrate |
| NC | Nitrocellulose |
| TNT | Trinitrotoluene |
References
- The State Council of China. Report on the Special Major Fire Explosion Accident of Ruihai Company in Tianjin Port. 5 February 2016. Available online: http://www.gov.cn/foot/2016-%2002/05/content%205039788.htm (accessed on 28 February 2021).
- Fire Protection Research Foundation. Variables Associated with the Classification of Ammonium Nitrate—A Literature Review. FRPF-2017-03. Available online: https://www.nfpa.org/-/media/Files/News-and-Research/Fire-statistics-and-reports/Hazardous-materials/RFANHazardClassification.pdf (accessed on 28 February 2021).
- Pittman, W.; Han, Z.; Harding, B.; Rosas, C.; Jiang, J.; Pineda, A.; Mannan, M.S. Lessons to be learned from an analysis of ammonium nitrate disasters in the last 100 years. J. Hazard. Mater. 2014, 280, 472–477. [Google Scholar] [CrossRef]
- Jiang, T. Explosion accident of warehouse storing Hazardous materials in Qingshuihe of Shenzhen City. Saf. Technol. Manag. 1998, 19, 18–21. [Google Scholar]
- Yu, G.D.; Wang, Y.; Zheng, L.; Huang, J.; Li, J.L.; Gong, L.Z.; Chen, R.G.; Li, W.; Huang, J.L.; Duh, Y.S. Comprehensive study on the catastrophic explosion of ammonium nitrate stored in the warehouse of Beirut port. Process Saf. Environ. 2021, 152, 201–219. [Google Scholar] [CrossRef]
- Guiochon, G. On the catastrophic explosion of the AZF plant in Toulouse. Process Saf. Prog. 2020, 39, e12197. [Google Scholar] [CrossRef]
- Laboureur, D.M.; Han, Z.; Harding, B.Z.; Pineda, A.; Pittman, W.C.; Rosas, C. Case study and lessons learned from the ammonium nitrate explosion at the west fertilizer facility. J. Hazard. Mater. 2016, 308, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Banks, J. Dangerously close: The CSB’s investigation into the fatal fire and explosion in West, Texas. Process Saf. Prog. 2016, 35, 312–316. [Google Scholar] [CrossRef]
- Pasman, H.J.; Fouchier, C.; Park, S.; Quddus, N.; Laboureur, D. Beirut ammonium nitrate explosion: Are not we really learning auything? Process Saf. Prog. 2020, 39, e12203. [Google Scholar] [CrossRef]
- Sivaraman, S.; Varadharajan, S. Investigative consequence analysis: A case study research of Beirut explosion accident. J. Loss Prev. Process 2021, 69, 104387. [Google Scholar] [CrossRef]
- Prugh, R.W. Historical record of Ammonium nitrate disasters. Process Saf. Prog. 2020, 39, e12210. [Google Scholar] [CrossRef]
- Boeck, L.R.; Mahan, P.W. Loss prevention learnings from Beirut and similar ammonium nitrate explosions. Process Saf. Prog. 2021, e12322. [Google Scholar] [CrossRef]
- Babrauskas, V. The ammonium nitrate explosion at West, Texas: A disaster that should have been avoided. Fire Mater. 2018, 42, 164–172. [Google Scholar] [CrossRef]
- The UN Numbers from UN2001 to UN2100 as Assigned by the United Nations Committee of Experts on the Transport of Dangerous Goods. List of UN numbers 2001 to 2100. Available online: https://en.wikipedia.org/wiki/ (accessed on 28 February 2021).
- U.S. Code of Federal Regulations Title 49, Section 172, Shipping Regulations and Proper Shipping Names of Class 4 “Flammable Solids”. Available online: https://www.ecfr.gov/current/title-49 (accessed on 1 February 2022).
- Phillips, R.W.; Orlick, C.A.; Steinberger, R. The kinetics of the thermal decomposition of nitrocellulose. J. Am. Chem. Soc. 1955, 59, 1034–1039. [Google Scholar] [CrossRef]
- De la Ossa, M.A.F.; López-López, M.; Torre, M.; García-Ruiz, C. Analytical techniques in the study of highly-nitrated nitrocellulose. Trends Anal. Chem. 2011, 30, 1740–1755. [Google Scholar] [CrossRef]
- Wang, K.; Liu, D.; Xu, S.; Cai, G. Research on the thermal history’s influence on the thermal stability of EHN and NC. Thermochim. Acta 2015, 610, 23–28. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Hosseini, S.G.; Rahimi-Nasrabadi, M.; Hajimirsadeghi, S.S.; Momenian, H. Effect of nitrate content on thermal decomposition of nitrocellulose. J. Hazard. Mater. 2009, 162, 1141–1144. [Google Scholar] [CrossRef]
- Sovizi, M.R.; Hajimirsadeghi, S.S.; Naderizadeh, B. Effect of particle size on thermal decomposition of nitrocellulose. J. Hazard. Mater. 2009, 168, 1134–1139. [Google Scholar] [CrossRef]
- Makashir, P.S.; Mahajan, R.R.; Agrawal, J.P. Studies on kinetics and mechanism of initial, thermal decomposition of nitrocellulose. J. Therm. Anal. Calorim. 1995, 45, 501–509. [Google Scholar] [CrossRef]
- Brill, T.B.; Gongwer, P.E. Thermal Decomposition of Energetic Materials 69. Analysis of the Kinetics of Nitrocellulose at 50–500 °C. Propellants Explos. Pyrotech. 1997, 22, 38–44. [Google Scholar] [CrossRef]
- Kimura, J. Kinetic Mechanism on Thermal Degradation of a Nitrate Ester Propellant. Propellants Explos. Pyrotech. 1988, 13, 8–12. [Google Scholar] [CrossRef]
- Hai, Z.; Zhiming, X.; Pengjiang, G.; Rongzu, H.; Shengli, G.; Binke, N.; Yan, F.; Qizhen, S.; Rong, L. Estimation of the critical rate of temperature rise for thermal explosion of first-order autocatalytic decomposition reaction systems using non-isothermal DSC. J. Hazard. Mater. 2002, A94, 205–210. [Google Scholar] [CrossRef]
- Ning, B.; Hu, R.; Zhang, H.; Xia, Z.; Guo, P.; Liu, R.; Lu, G.; Jiang, J. Estimation of the critical rate of temperature rise for thermal explosion of autocatalytic decomposing reaction of nitrocellulose using non-isothermal DSC. Thermochim. Acta 2004, 416, 47–50. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Hu, R.; Yao, E.; Guo, P. Estimation of the critical rate of temperature rise for the thermal explosion of nitrocellulose using non-isothermal DSC. J. Therm. Anal. Calorim. 2014, 115, 1099–1110. [Google Scholar] [CrossRef]
- Luo, Q.; Ren, T.; Shen, H.; Zhang, J.; Liang, D. The Thermal Properties of Nitrocellulose: From Thermal Decomposition to Thermal Explosion. Combust. Sci. Technol. 2018, 190, 579–590. [Google Scholar] [CrossRef]
- Oxley, J. Snapshot of ammonium nitrate: History and use. Process Saf. Prog. 2020, 39, e12204. [Google Scholar] [CrossRef]
- Babrauskas, V. The West, Texas, ammonium nitrate explosion: A failure of regulation. J. Fire Sci. 2017, 35, 396–414. [Google Scholar] [CrossRef]
- Babrauskas, V. Explosions of ammonium nitrate fertilizer in storage or transportation are preventable accidents. J. Hazard. Mater. 2016, 304, 134–149. [Google Scholar] [CrossRef] [PubMed]
- NFPA 400, Hazardous Materials Code. National Fire Protection Association: Quincy, MA, USA, 2016, 3rd ed. Available online: https://www.nfpa.org/Assets/files/AboutTheCodes/400/ProposedTIA1174_NFPA400.pdf (accessed on 28 February 2021).
- United Nations. European Agreement Concerning the International Storage of Dangerous Goods by Road (ADR). 2014. Available online: https://unece.org/DAM/trans/danger/publi/adr/ADRagree_e.pdf (accessed on 30 August 2016).
- Babrauskas, V.; Leggett, D. Thermal decomposition of ammonium nitrate. Fire Mater. 2020, 44, 250–268. [Google Scholar] [CrossRef]
- Willey, R.J. The nature of ammonium nitrate decomposition and explosions. Process Saf. Prog. 2020, 39, e12214. [Google Scholar] [CrossRef]
- Han, Z.; Sachdeva, S.; Papadaki, M.; Sam Mannan, M. Calorimetry studies of ammonium-Effect of inhibitors, confinement, and heating rate. J. Loss Prevent. Process 2015, 19, 724–728. [Google Scholar] [CrossRef]
- Han, Z.; Pasman, H.J.; Sam Mannan, M. Extinguishing fires involving ammonium nitrate stock with water: Possible complications. J. Fire Sci. 2017, 35, 457–483. [Google Scholar] [CrossRef]
- Babrauskas, V. Fire safety is the key to ammonium nitrate explosion safety. Process Saf. Prog. 2020, 39, e12200. [Google Scholar] [CrossRef]
- Van Dolah, R.W.; Gibson, F.C.; Murphy, J.N. Sympathetic Detonation of Ammonium Nitrate and Ammonium Nitrate-Fuel Oil, Report of Investigations 6746. Bureau of Mines, U.S. Department of the Interior. 1966. Available online: https://www.researchgate.net/publication/235114474_Sympathetic_detonation_of_ammonium_nitrate_and_ammonium_nitrate-fuel_oil (accessed on 28 February 2021).
- Van Dolah, R.W.; Gibson, F.C.; Murphy, J.N. Further Studies on Sympathetic Detonation, Report of Investigations 6903, Bureau of Mines, U.S. Department of the Interior. 1966. Available online: https://www.researchgate.net/publication/235184808_FURTHER_STUDIES_ON_SYMPATHETIC_DETONATION (accessed on 28 February 2021).
- Van Dolah, R.W. Large-Scale Investigation of Sympathetic Detonation. Ann. N. Y. Acad. Sci. 1968, 152, 792–801. [Google Scholar] [CrossRef]
- DSC 3 Operation Instructions; Mettler Company: Zurich, Switzerland, 2015.
- ASTM E537-20; Standard Test Method for the Thermal Stability of Chemicals by Differential Scanning Calorimetry. ASTM International: West Conshohocken, PA, USA, 2020. Available online: https://www.astm.org/e0537-20.html(accessed on 1 February 2022).
- ASTM E487-20; Standard Test Method for Constant-Temperature Stability of Chemical Materials. ASTM International: West Conshohocken, PA, USA, 2020. Available online: https://www.astm.org/e0487-20.html(accessed on 1 February 2022).
- ASTM E2070-13; Standard Test Method for Kinetic Parameters by Differential Scanning Calorimetry Using Isothermal Methods. ASTM International: West Conshohocken, PA, USA, 2018. Available online: https://www.astm.org/e2070-13r18.html(accessed on 1 February 2022).
- ASTM E2046-19; Standard Test Method for Reaction Induction Time by Thermal Analysis. ASTM International: West Conshohocken, PA, USA, 2019. Available online: https://www.astm.org/e2046-19.html(accessed on 1 February 2022).
- Török, Z.; Ozunu, A. Hazardous Properties of Ammonium nitrate and modeling of explosion using TNT equivalency. Environ. Eng. Manag. J. 2015, 14, 2671–2678. [Google Scholar] [CrossRef]
- Prugh, R.W. The effects of explosive blast on structures and personnel. Process Saf. Prog. 1999, 18, 5–16. [Google Scholar] [CrossRef]
- Vortman, L.J. Craters from surface explosions and scaling laws. J. Geophys. Res. 1968, 73, 4621–4636. [Google Scholar] [CrossRef]
- Lampson, C.W. Final Report on Effects of Underground Explosions; National Defense Research Committee of the Office of Scientific: Washington, DC, USA, 1946. [Google Scholar]
- Zhou, X.; Bai, C.; Zhang, Y.; Wang, Z. Inverse analysis of explosives based on crater size. Shock Waves 2017, 27, 27–35. [Google Scholar] [CrossRef]
- Baker, W.E.; Cox, P.A.; Westine, P.S.; Kulesz, J.J.; Strehlow, R.A. Explosion Hazards and Evaluation; Elsevier Scientific Publishing Company: New York, NY, USA, 1983; Available online: https://scholar.google.com/scholar_lookup?title=Explosion%20Hazards%20and%20Evaluation&author=W.E.%20Baker&publication_year=1983 (accessed on 28 February 2021).
- Orlenko, L.P. Fizika Vzriva; Fizmatlit: Moscow, Russia, 2002; Available online: http://refhub.elsevier.com/S0950-4230(20)30519-2/sref9 (accessed on 1 February 2022).
- Alonso, F.D.; Ferradas, E.G.; Perez, J.F.S.; Aznar, A.M.; Gimeno, J.R.; Alonso, J.M. Characteristic overpressure-impulse-distance curves for the detonation of explosives, pyrotechnics or unstable substances. J. Loss Prev. Process 2006, 19, 724–728. [Google Scholar] [CrossRef]
- Jeremić, R.; Bajić, Z. An approach to determining the TNT equivalent of high explosives. Sci. Tech. Rev. 2006, 6, 58–62. [Google Scholar]
- Török, Z.; Kovacs, L.A.; Ozunu, A. Ammonium nitrate explosion. Case study: The Mihăilesti accident (2004), Romania. J. Environ. Res. Prot. 2015, 12, 56–60. [Google Scholar]
- Jarrett, D.E. Derivation of the British explosives safety distances. Ann. N. Y. Acad. Sci. 1968, 152, 18–35. [Google Scholar] [CrossRef]
- Adushkin, V.V.; Khristoforov, B.D. Craters of large-scale surface explosions. Combust. Explo. Shock 2004, 40, 674–678. [Google Scholar] [CrossRef]
- Ambrosini, R.D.; Luccioni, B.M. Craters produced by explosions on the soil surface. J. Appl. Mech. 2006, 73, 890–900. [Google Scholar] [CrossRef]
- Kinney, G.F.; Graham, K.J. Expolsive Shocks in Air; Springer: Berlin, Germany; New York, NY, USA, 1985; Available online: http://refhub.elsevier.com/S0957-5820(21)00271-8/sbref0170 (accessed on 28 February 2021).
- Mniszewski, K.R. The Pepcon Plant Fire/Explosion: A Rare Opportunity in Fire/Explosion Investigation. J. Fire Prot. Eng. 1994, 6, 63–78. [Google Scholar] [CrossRef]
- Marlair, G.; Michot, C.; Turcotte, R.; Singh, S. Comments about the paper entitled “Lessons to be learned from an analysis of ammonium nitrate disasters in the last 100 years” by Pittman et al. (J. Hazard. Mater. 2014, 280, 472–477). J. Hazard. Mater. 2016, 303, 177–180. [Google Scholar] [CrossRef]
- Babrauskas, V. Will Firefighters be Any Safer under the New Hazardous Materials code? Fire Eng. 2015, 168, 66–70. [Google Scholar]



| Incident Number | Location | Date | Quantity (Tons) | Fatalities/Injuries | Reference |
|---|---|---|---|---|---|
| 1 | Morgan, NJ, USA | 4 October 1918 | 1000 | 64/100 | [2] |
| 2 | Oppau, Germany | 21 September 1921 | 450 | 561/1952 | [3] |
| 3 | Tessenderloo, Belgium | 29 April 1942 | 150 | >100/NA | [2] |
| 4 | Texas City (TX, USA) | 16 April 1947 | 2300 | >500/3500 | [3] |
| 5 | Brest, France | 28 July 1947 | 3000 | 30/>1000 | [2] |
| 6 | Shenzen, China | 5 August 1993 | 65 | 18/873 | [4] |
| 7 | Toulouse, France | 21 September 2001 | 300 | 30/3000 | [3] |
| 8 | Ryongchon, North Korea | 22 April 2004 | NA | 161/1300 | [3] |
| 9 | Monclova, Coahuila, Mexico | 4 September2007 | 22–25 | 28/130 | [3] |
| 10 | Tianjin Port, China | 12 August 2015 | 800 | 173/798 | [1] |
| 11 | Beirut Port, Lebaron | 4 August 2020 | 2750 | 204/>7000 | [5] |
| Author | Scaling Law | mTNT (kg) | Description |
|---|---|---|---|
| Vortman | D = 0.56 mTNT0.375 | 3.6, 29, 116, 454, 2720, 18,144 | 16 explosion tests on the surface of dry-lake at Nevada test site |
| Vortman | D = 0.40 mTNT0.408 | 232, 4564, 18,144, 90,718, 453,592 | 5 explosion tests at the Watching Hill site |
| Vortman | D = 0.32 mTNT0.426 | 3.63, 3.86, 13.83, 236, 250, 272, 4565, 4574, 4579, 18,144 | 30 explosion tests at the Downing Ford site |
| Adushkin and Khristoforov | D = 0.606 mTNT0.34 | 1000–5,000,000 | 54 explosion tests on surface |
| Ambrosini and Luccioni | D = 0.606 mTNT0.34 | 1, 2, 4, 7, 10, 250 | 6 explosion tests on surface |
| Ambrosini et al. | D = 0.80 mTNT1/3 | 1–10 | 30 explosion tests; Scaling law revised from tests by Kinney and Graham |
| mTNT (kg) | D = 0.606 mTNT0.34 (m) | D = 0.80 mTNT1/3 (m) | D = 0.40 mTNT0.408 (m) |
|---|---|---|---|
| 1 | 0.6 | 0.8 | 0.4 |
| 10 | 1.3 | 1.7 | 1.0 |
| 50 | 2.3 | 2.9 | 2.0 |
| 100 | 2.9 | 3.7 | 2.6 |
| 500 | 5.0 | 6.3 | 5.0 |
| 1000 | 6.3 | 8.0 | 6.7 |
| 5000 | 11.0 | 13.4 | 12.9 |
| 10,000 | 13.9 | 17.2 | 17.1 |
| 50,000 | 24.0 | 29.5 | 33.0 |
| 100,000 | 30.3 | 37.0 | 43.9 |
| 500,000 | 52.5 | 63.5 | 84.6 |
| 1,000,000 | 66.4 | 80.0 | 112.2 |
| 2,000,000 | 84.1 | 100.8 | 148.9 |
| 5,000,000 | 114.8 | 136.8 | 216.4 |
| 8,000,000 | 134.7 | 160.0 | 262.1 |
| 10,000,000 | 145.4 | 172.4 | 287.1 |
| Approach | ΔP (MPa) (300 m) | ΔP (MPa) (400 m) | ΔP (MPa) (500 m) | ΔP (MPa) (1000 m) | ΔP (MPa) (3000 m) |
|---|---|---|---|---|---|
| Baker’s | 0.1111 | 0.0692 | 0.0425 | 0.0158 | 0.0047 |
| Sadovski’s | 0.0638 | 0.0379 | 0.0262 | 0.0096 | 0.0026 |
| Alonso’s | 0.0859 | 0.0482 | 0.0308 | 0.0076 | 0.0008 |
| Temperature (°C) | Mass (mg) | TMR (min) |
|---|---|---|
| 180 | 1.2 | 25.4 |
| 175 | 2.2 | 36.4 |
| 170 | 2.2 | 65.5 |
| 165 | 4.0 | 120.3 |
| 160 | 4.0 | 195.3 |
| Temperature (°C) | Mass (mg) | TMR (min) |
|---|---|---|
| 170 | 2.2 | 46.1 |
| 160 | 1.5 | 47.1 |
| 150 | 1.5 | 65.0 |
| 140 | 1.5 | 78.9 |
| 130 | 1.5 | 106.5 |
| 120 | 1.5 | 132.8 |
| 110 | 1.5 | 357.3 |
| 100 | 2.2 | 859.0 |
| 90 | 1.5 | 1985.8 |
| Incident/Year | Chemical | Quantity (ton) | Ignition (Time) | First Explosion (Time) | Major Explosion (Time) |
|---|---|---|---|---|---|
| Oppau/1921 | (NH4NO3)(NH4)2SO4 | 5000 | Blasting at 07:00 | 07:31 | 07:32 |
| Nevada/1988 | NH4ClO4 | Several hundred tons | Fire at 11:15 | 11:53 | 11:57 |
| Shenzhen/1993 | AN | 65 | Fire at 13:10 | 13:26 | 14:27 |
| Queensland/2014 | AN | 53 | Fire by truck rollover at 09:00 | 10:10 | 10:12 |
| Tianjin/2015 | AN | 430 | Fire at 22:51:46 | 23:34:06 | 23:34:37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Duh, Y.-S.; Yang, X.; Li, Y.; Chen, Y.; Li, Y.; Li, J.; Chen, R.; Gong, L.; Yang, B.; et al. Holistic Case Study on the Explosion of Ammonium Nitrate in Tianjin Port. Sustainability 2022, 14, 3429. https://doi.org/10.3390/su14063429
Yu G, Duh Y-S, Yang X, Li Y, Chen Y, Li Y, Li J, Chen R, Gong L, Yang B, et al. Holistic Case Study on the Explosion of Ammonium Nitrate in Tianjin Port. Sustainability. 2022; 14(6):3429. https://doi.org/10.3390/su14063429
Chicago/Turabian StyleYu, Gending, Yih-Shing Duh, Xiaodong Yang, Yongzhao Li, Yangqing Chen, Yuqi Li, Jingling Li, Rongguo Chen, Lingzhu Gong, Bin Yang, and et al. 2022. "Holistic Case Study on the Explosion of Ammonium Nitrate in Tianjin Port" Sustainability 14, no. 6: 3429. https://doi.org/10.3390/su14063429
APA StyleYu, G., Duh, Y.-S., Yang, X., Li, Y., Chen, Y., Li, Y., Li, J., Chen, R., Gong, L., Yang, B., & Huang, J. (2022). Holistic Case Study on the Explosion of Ammonium Nitrate in Tianjin Port. Sustainability, 14(6), 3429. https://doi.org/10.3390/su14063429





