A Study on Temperature Distribution within HVDC Bushing Influenced by Accelerator Content during the Curing Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. DSC Measurement
2.3. Simulation Model
3. Results and Discussion
3.1. Analysis of Curing Kinetics
- (1)
- The accelerator attacks the anhydride to generate carboxylate anion, as shown in Figure 4a;
- (2)
- The carboxylate anion attacks the epoxy group and opens the epoxy group to generate an oxygen anion, as shown in Figure 4b;
- (3)
- Oxygen anions continue to attack other anhydride groups to form carboxylate anions, as shown in Figure 4c.
3.2. Determine the Curing Reaction Model
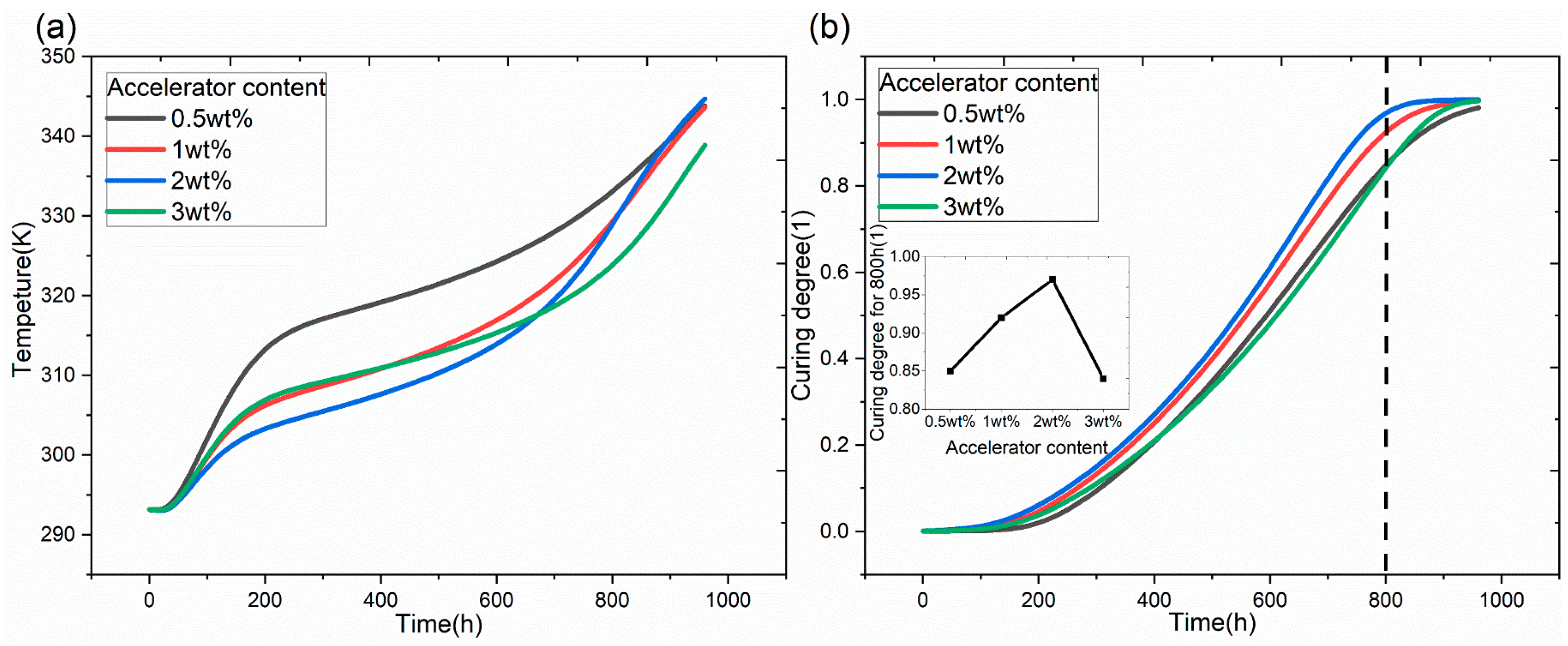
3.3. Simulation of a HVDC Bushing Capacitor Core
- (1)
- Positive and negative radicals collided to form molecules, which is the reverse reaction of the curing agent decomposition reaction. Due to the excessive use of an accelerator, the reverse reaction rate between the accelerator and the primary radical was greater than the rate of the primary radical initiating monomer. The conversion rate decreased, namely, the product performance was reduced [38].
- (2)
- Owing to the accelerator content increase, the violent reaction resulted in a lack of adequate gel time for the system. Some of the monomers were not involved in the cross-linking reaction, which is one of the reasons for the low overall exothermic levels and the low rate of temperature rise [39].
- (3)
- The lack of a sufficient exothermic peak temperature triggered the continuous release of free radicals from the curing agent, reducing the cross-linking reaction and thus, resulted in permanent under-curing.
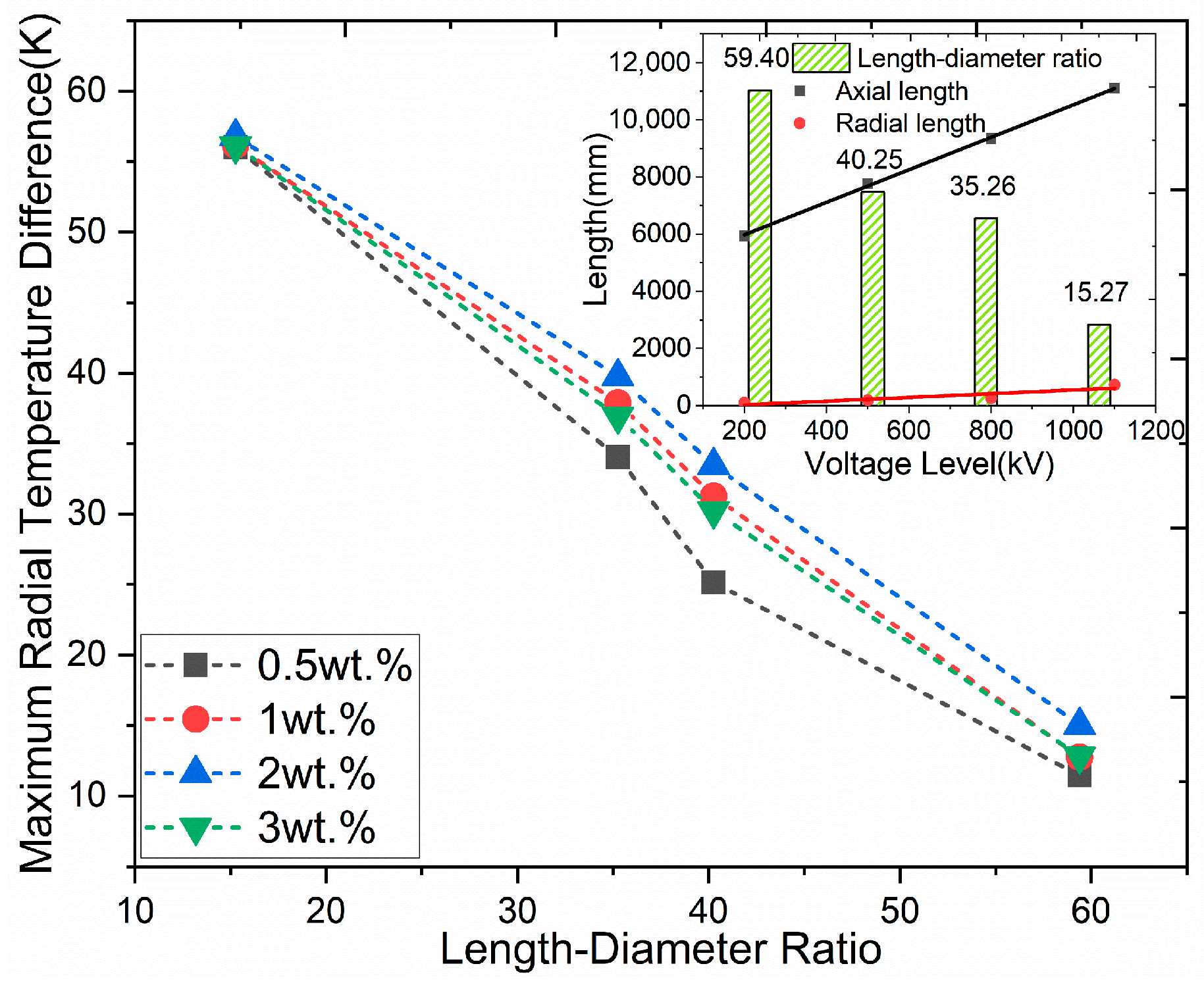
3.4. Effect of Bushing Size on the Curing Process
4. Conclusions
- (1)
- As the accelerator content increases, the enthalpy increases, the activation energy decreases, and the curing reaction occurs at a lower temperature.
- (2)
- The obtained curing kinetic model shows that in the range from 0.5 wt.% to 3 wt.%, as the accelerator content increases, the preexponential factor A decreases, the order of reaction m increases, and n decreases. The value of n is always greater than m, and the curing process can be represented well by the Sestak–Berggren autocatalytic model.
- (3)
- The finite element simulation results show that as the accelerator content increases, the overall degree of curing of the capacitor core and the maximum radial temperature difference both increase and then decrease, and the strain peak decreases and then increases. As the voltage level increases, both the maximum radial temperature difference and the peak strain increase, which is mainly attributable to the larger radial length.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brent, A.C. Renewable energy for sustainable development. Sustainability 2021, 13, 6920. [Google Scholar] [CrossRef]
- Khan, N.; Sudhakar, K.; Mamat, R. Role of biofuels in energy transition, green economy and carbon neutrality. Sustainability 2021, 13, 12374. [Google Scholar] [CrossRef]
- Batrancea, L.; Pop, M.C.; Rathnaswamy, M.M.; Batrancea, I.; Rus, M.I. An empirical investigation on the transition process toward a green economy. Sustainability 2021, 13, 13151. [Google Scholar] [CrossRef]
- Wu, J.; Tan, Z.; Wang, K.; Liang, Y.; Zhou, J. Research on multi-objective optimization model for hybrid energy system considering combination of wind power and energy storage. Sustainability 2021, 13, 3098. [Google Scholar] [CrossRef]
- Wei, C.; Zhao, Y.B.; Zheng, Y.F.; Xie, L.Y.; Smedley, K. Analysis and design of a non-isolated high step-down converter with coupled inductor and ZVS operation. IEEE Trans. Ind. Electron. 2021, 1. [Google Scholar] [CrossRef]
- Du, B.X.; Wang, Q.; Tu, Y.P.; Tanaka, Y.; Li, J. Guest editorial: Advanced materials for HVDC insulation. High Volt. 2020, 5, 351–352. [Google Scholar]
- Liu, Y.X.; Hu, M.Z.; Dai, Q.M.; Le, H.F.; Liu, Y. Online recognition method of partial discharge pattern for transformer bushings based on small sample ultra-micro-CNN network. AIP Adv. 2021, 11, 045221. [Google Scholar] [CrossRef]
- Xie, L.Y.; Guo, X.; Wei, C.; Zhang, Y.B.; Chen, Y.; Liang, C.B.; Xue, Y.T.; Zhao, E. adaptive droop control of the MTDC system with high-capacity energy storage based on dynamic and static power decoupling method. Front. Energy Res. 2021, 10, 9. [Google Scholar] [CrossRef]
- Xie, Q.; Liang, S.D.; Liu, B.W.; Fu, K.X.; Zhan, Z.Y.; Lu, L.; Yang, X.M.; Lu, F.C.; Huang, Z.Y. Structure, microparameters and properties of crosslinked DGEBA/MTHPA: A molecular dynamics simulation. AIP Adv. 2018, 8, 075332. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.L.; Kuang, X.; Kong, L.; Dong, X.; Feng, Z.H.; Wang, D.J. A simplified chemorheological model of viscosity evolution for solvent containing resol resin in RTM process. J. Appl. Polym. Sci. 2017, 134, 45282. [Google Scholar] [CrossRef]
- Duan, Y.J.; Gao, Y.W. Delamination and current-carrying degradation behavior of epoxy-impregnated superconducting coil winding with 2G HTS tape caused by thermal stress. AIP Adv. 2020, 10, 025320. [Google Scholar] [CrossRef]
- Lin, P.Y.; Lee, S. Modeling and characterization of cure-dependent viscoelasticity of molded underfill in ultrathin packages. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 10, 1491. [Google Scholar] [CrossRef]
- Kiattipornpithak, K.; Thajai, N.; Kanthiya, T.; Rachtanapun, P.; Noppol Leksawasdi, P.; Phimolsiripol, Y.; Rohindra, D.; Ruksiriwanich, W.; Sommano, S.R.; Jantanasakulwong, K. Reaction mechanism and mechanical property improvement of poly(lactic acid) reactive blending with epoxy resin. Polymers 2021, 13, 2429. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.Y.; Zhou, Y.X.; Zhang, L.; Zhang, Y.X.; Huang, X.; Chen, J.N. Improved electrical resistivity-temperature characteristics of insulating epoxy composites filled with polydopamine-coated ceramic particles with positive temperature coefficient. Compos. Sci. Technol. 2022, 221, 109365. [Google Scholar] [CrossRef]
- Wei, W.; Sun, X.; Ye, W.T.; Zhang, B.W.; Fei, X.M.; Li, X.J.; Liu, X.Y. Thermal latent curing agent for epoxy resins from neutralization of 2-methylimidazole with a phosphazene–containing polyfunctional carboxylic acid. Polym. Adv. Technol. 2021, 31, 4884. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhang, R.; Wang, G.P.; Shi, Y.M. The mechanical properties of epoxy resin composites modified by compound modification. AIP Adv. 2018, 8, 105325. [Google Scholar] [CrossRef] [Green Version]
- Teng, C.Y.; Zhou, Y.X.; Li, S.H.; Zhang, L.; Zhang, Y.X.; Zhou, Z.L.; Zhao, L. Regulation of temperature resistivity characteristics of insulating epoxy composite by incorporating positive temperature coefficient material. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 512–520. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.M.; Tang, Y.Y.; Zhang, B.W.; Wei, W.; Li, X.J.; Fei, X.M.; Liu, X.Y. Synthesis ofisocyanurate-based imidazole carboxylate as thermal latent curing accelerator for thermosetting epoxy resins. J. Appl. Polym. Sci. 2020, 137, 49221. [Google Scholar] [CrossRef]
- Hubbard, R.L. Low-temperature processing of electronic materials using uniform microwave fields. IEEE Trans. Electron Devices 2021, 68, 3170. [Google Scholar] [CrossRef]
- Teng, C.Y.; Zhou, Y.X.; Wu, C.; Zhang, L.; Zhang, Y.X.; Zhou, W.J. Optimization of the temperature-dependent electrical resistivity in epoxy/positive temperature coefficient ceramic nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 468–475. [Google Scholar] [CrossRef]
- Nikzamir, M.; Mortezaei, M.; Jahani, M. Effect of surface area of nanosilica particles on the cure kinetics parameters of an epoxy resin system. J. Appl. Polym. Sci. 2019, 34, 136. [Google Scholar] [CrossRef]
- Dong, H.X.; Lia, Y.H.; Zhang, J.; Liu, L.J.; Cao, L.Y.; Ming, P.J.; Liu, W.J.; Zhang, C.H.; Liu, L.H.; Wei, H. Kinetics simulation and a novel curing procedure to avoid thermal shock during the curing process of epoxy composites. RSC Adv. 2016, 70, 65533. [Google Scholar] [CrossRef]
- Pi, B.S.; Yu, H.P.; Qin, F.; Chen, P.; Cai, A.W. Thermal conductivity of epoxy resin using molecular dynamics simulation. In Proceedings of the 2018 19th International Conference on Electronic Packaging Technology (ICEPT), Shanghai, China, 8–11 August 2018. [Google Scholar]
- Ma, B.; Wang, X.Q.; Zhou, X.Y.; Wei, K.; Huang, W. Measurement and analysis of thermophysical parameters of the epoxy resin composites shape-stabilized phase change material. Constr. Build. Mater. 2019, 223, 368. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lin, Y.; Wang, L.; Wang, H.N. Internal curing reaction and numerical analysis of temperature field of cycloaliphatic epoxy resin insulators. High Volt. Eng. 2020, 46, 1986. [Google Scholar]
- Wang, L.; Yuan, D.P.; Hao, L.C.; Kan, C.H.; Wang, B. Effect of oven structure on insulator temperature field distribution during curing process. High Volt. Eng. 2019, 45, 2748. [Google Scholar]
- Kolyshkin, V.A.; Kerber, M.L.; Gorbunova, I.Y. Study of the effect of the accelerator content on the properties of an adhesive composite based on epoxy resin ED-22 and dicyanodiamine. Int. Polym. Sci. Technol. 2006, 33, 37. [Google Scholar] [CrossRef]
- Hayaty, M.; Honarkar, H.; Beheshty, M.H. Curing behavior of dicyandiamide/epoxy resin system using different accelerators. Iran. Polym. J. 2013, 22, 591–598. [Google Scholar] [CrossRef]
- Zhao, Y.S.; He, Y.H.; Yang, K.R.; Wang, X.P.; Bai, J.H.; Du, B. Improving the surface insulating performance of epoxy resin-Al2O3 composite materials by extending chain of liquid epoxy resin with Me-THPA. High Volt. 2020, 5, 472–481. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, C.F.; Zhang, J.Y.; Cheng, J. The influence of tertiary amine accelerators on the curing behaviors of epoxy/anhydride systems. Thermochim. Acta 2014, 577, 11. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, X.P.; Yu, X.H. Reaction mechanism, cure behavior and properties of a multifunctional epoxy resin, TGDDM, with latent curing agent dicyandiamidet. RSC Adv. 2018, 8, 8248. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.F.; Liu, X.D.; Cheng, J.; Zhang, J.Y. Study on curing kinetics of diglycidyl 1,2-cyclohexane dicarboxylate epoxy/episulfide resin system with hexahydro-4-methylphthalic anhydride as a curing agent. J. Therm. Anal. Calorim. 2015, 120, 1893. [Google Scholar] [CrossRef]
- Gibson, R.L.; Simmons, J.H.; Stitt, E.H.; West, J.; Wilkinson, S.K.; Gallen, R.W. Kinetic modelling of thermal processes using a modified Sestak-Berggren equation. Chem. Eng. J. 2021, 408, 127318. [Google Scholar] [CrossRef]
- Sakib, A.R.N.; Jha, V.; Mavinkurve, A.; Chopin, S. Reaction Kinetics and Rheological Model Coefficient Extraction for Epoxy Mold Compounds. In Proceedings of the 2018 17th IEEE Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), San Diego, CA, USA, 29 May–1 June 2018. [Google Scholar]
- Li, C.; Liu, M.H.; Liu, Z.Y.; Qing, M.L.; Wang, G. DSC and curing kinetics of epoxy resin using cyclohexanediol diglycidyl ether as active diluents. J. Therm. Anal. Calorim. 2013, 116, 411. [Google Scholar] [CrossRef]
- Kelley, M.; Abdol, N.; Soroushian, P.; Keating, K.; Balachandra, A.M.; Meldrum, T. Monitoring real-time curing of epoxies in situ using single-sided NMR. J. Polym. Sci. 2020, 58, 616. [Google Scholar] [CrossRef]
- Lucio, B.; Fuente, J.L. Non-isothermal DSC and rheological curing of ferrocene-functionalized, hydroxyl-terminated polybutadiene polyurethane. React. Funct. Polym. 2016, 107, 60–68. [Google Scholar]
- Yang, Q.; Liang, G.Z. Sturdies on flexible unsaturated polyester resin from polyether gdycol and its flexible curing system. Thermosetting Resin 2007, 22, 9–12. [Google Scholar]
- Ma, J.X.; Hu, Z.Y.; Wang, Y.C. Rapid curing of epoxy resin /multithiol compounds at low temperature. Thermosetting Resin 2011, 26, 35–38. [Google Scholar]
- Li, X.Q.; Chen, W.B. Study of improving the adhesion of white unsaturated polyester primers. Shanghai Coat. 2015, 53, 6–10. [Google Scholar]
- Chen, M.; Liu, X.D.; Sun, Y.H.; Wu, Z.C.; Tang, H. Influence of material volume conductivity on electric field and surface charge of RIP valve-side bushing core under DC electro-thermal coupling stress. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 164–171. [Google Scholar] [CrossRef]
- Du, B.X.; Sun, H.L.; Jiang, J.P.; Kong, X.X.; Yang, W. Temperature-dependent electric field distribution in ±800 kV valve-side bushing insulation for a converter transformer. High Volt. 2021, 6, 106–115. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Xie, G.S.; Tian, H.D.; Peng, Z.R.; Yang, X. Impact of temperature on the transient DC field distribution of ±1100 kV UHVDC wall bushing. IEEE Trans. Power Deliv. 2021, 36, 1449–1457. [Google Scholar] [CrossRef]
- Yu, S.Z.; Li, X.D.; Zou, M.S.; Li, Z.R.; Wang, S.; Wang, D.H. Tetrafunctional epoxy resin-based buoyancy materials curing kinetics and properties. Polymers 2020, 12, 1732. [Google Scholar] [CrossRef]
- Geramil, G.; Bagheril, R.; Darvishi, R. Investigation of isothermal and dynamic cure kinetics of epoxy resin/nadic methyl anhydride/dicyandiamide by differential scanning calorimetry (DSC). J. Therm. Anal. Calorim. 2018, 7961. [Google Scholar]
- Yamaguchi, H.; Enomoto, T.; Sato, T. Stress Variation Analysis during Curing Process of Epoxy Underfill. In Proceedings of the 2014 International Conference on Electronics Packaging (ICEP), Toyama, Japan, 23–25 April 2014. [Google Scholar]

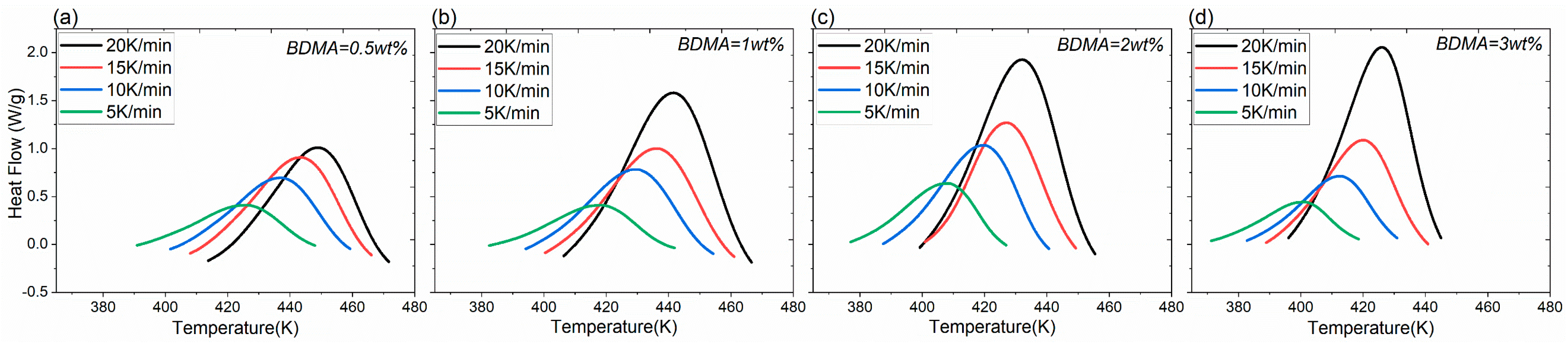

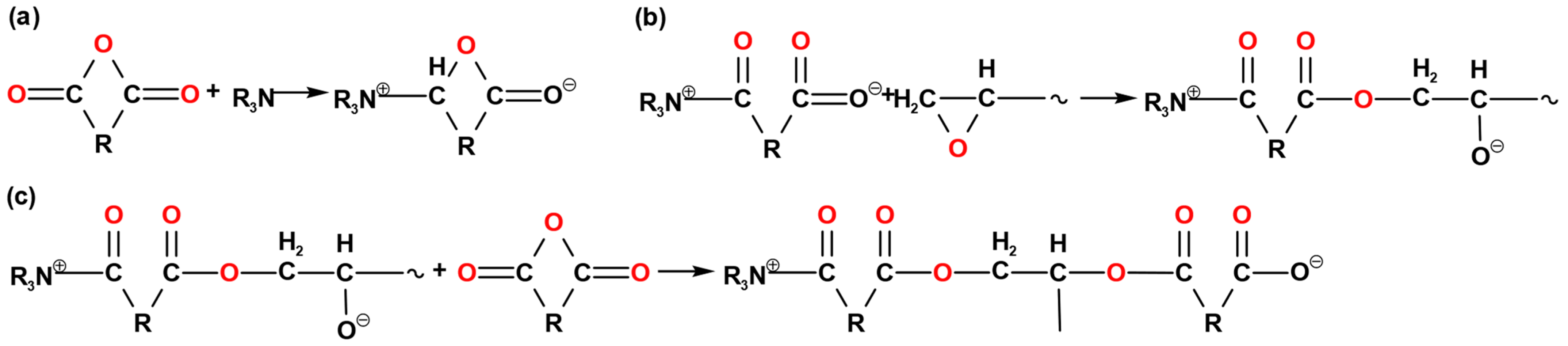
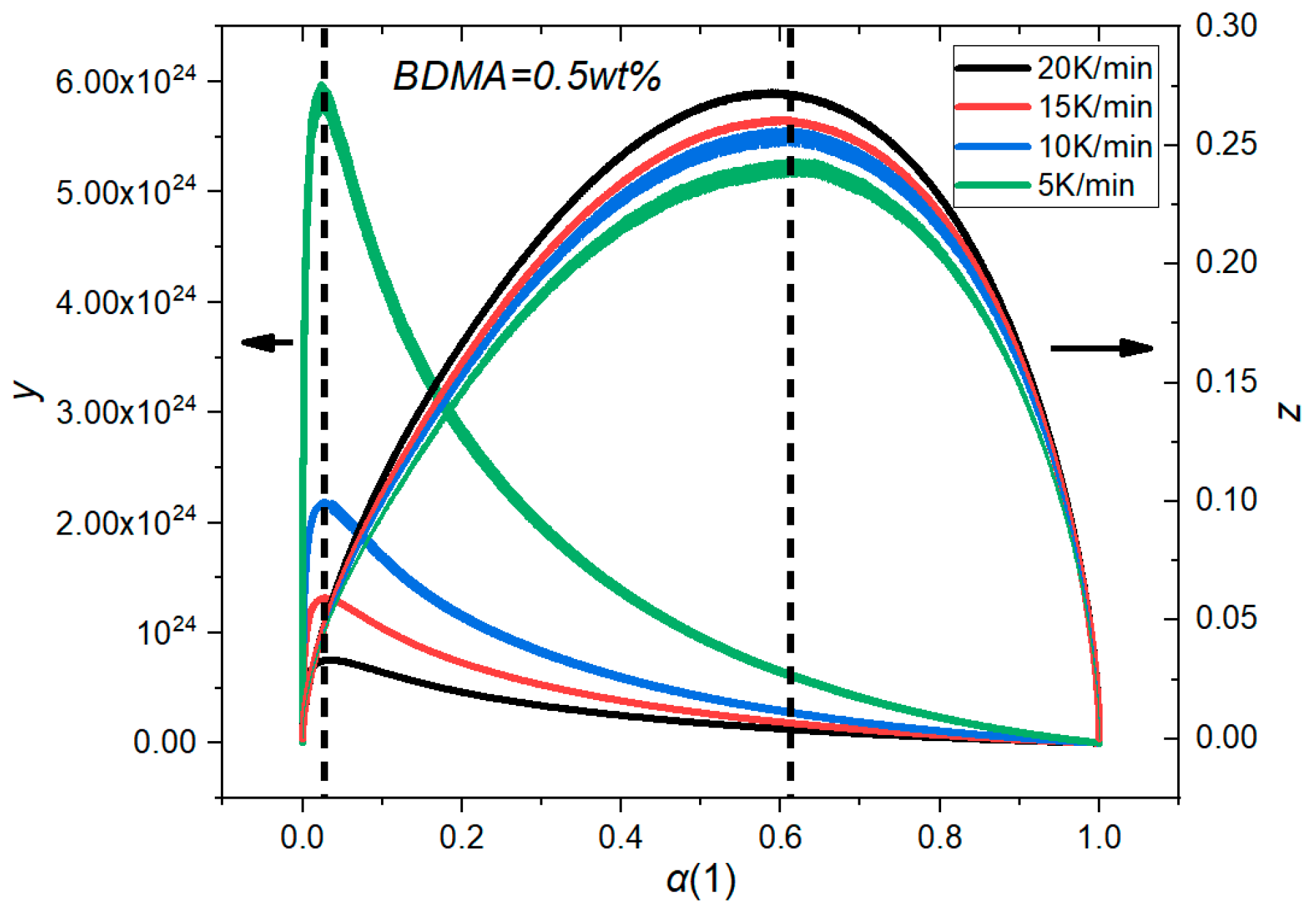
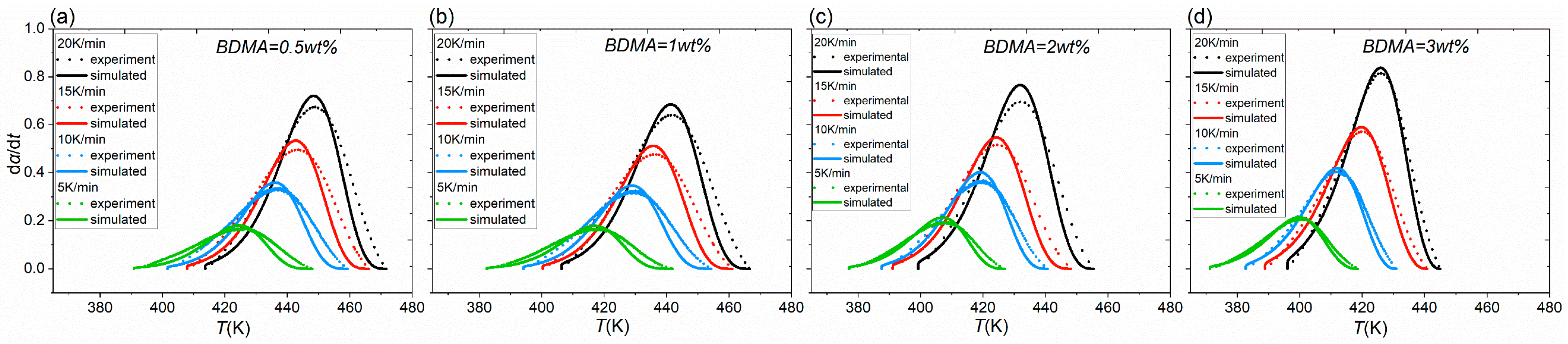




| Matrix Material | kx,y,z/[W (m K)−1] | C/[J (kg K)−1] | ρ/[kg m−3] |
|---|---|---|---|
| Epoxy Resin | 0.16 × (1 − α) + 0.22α | 1763 × (1 − α) + 1171α | 1109 × (1 − α) + 1169α |
| Accelerator Content | 0.5 wt.% | 1 wt.% | 2 wt.% | 3 wt.% |
|---|---|---|---|---|
| Enthalpy [J g−1] | 126.52 | 153.39 | 161.56 | 167.89 |
| Accelerator Content | 0.5 wt.% | 1 wt.% | 2 wt.% | 3 wt.% |
|---|---|---|---|---|
| Activation Energy [kJ mol−1] | 186.36 | 174.02 | 165.05 | 160.90 |
| Accelerator Content | A | m | n |
|---|---|---|---|
| 0.5 wt.% | 1.79 × 1024 | 4.07 × 10−2 | 1.75 |
| 1 wt.% | 1.15 × 1023 | 5.33 × 10−2 | 1.74 |
| 2 wt.% | 6.98 × 1021 | 6.10 × 10−2 | 1.51 |
| 3 wt.% | 5.04 × 1020 | 7.39 × 10−2 | 1.31 |
| 0.5 wt.% | 1 wt.% | ||
| 2 wt.% | 3 wt.% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wang, X.; Teng, C.; Zhang, Y.; Huang, X.; Chen, J. A Study on Temperature Distribution within HVDC Bushing Influenced by Accelerator Content during the Curing Process. Sustainability 2022, 14, 3393. https://doi.org/10.3390/su14063393
Zhou Y, Wang X, Teng C, Zhang Y, Huang X, Chen J. A Study on Temperature Distribution within HVDC Bushing Influenced by Accelerator Content during the Curing Process. Sustainability. 2022; 14(6):3393. https://doi.org/10.3390/su14063393
Chicago/Turabian StyleZhou, Yuanxiang, Xuewei Wang, Chenyuan Teng, Yunxiao Zhang, Xin Huang, and Jianning Chen. 2022. "A Study on Temperature Distribution within HVDC Bushing Influenced by Accelerator Content during the Curing Process" Sustainability 14, no. 6: 3393. https://doi.org/10.3390/su14063393
APA StyleZhou, Y., Wang, X., Teng, C., Zhang, Y., Huang, X., & Chen, J. (2022). A Study on Temperature Distribution within HVDC Bushing Influenced by Accelerator Content during the Curing Process. Sustainability, 14(6), 3393. https://doi.org/10.3390/su14063393





