Effects of Enrofloxacin on Nutrient Removal by a Floating Treatment Wetland Planted with Iris pseudacorus: Response and Resilience of Rhizosphere Microbial Communities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Setup
2.2. Sampling and Analysis
2.2.1. Water Sampling and Analysis
2.2.2. Plant Tissue Sampling and Analysis
2.2.3. Microbial Sampling and Analysis
2.3. Statistical Analysis
3. Results
3.1. Effects of Enrofloxacin on Iris Pseudacorus
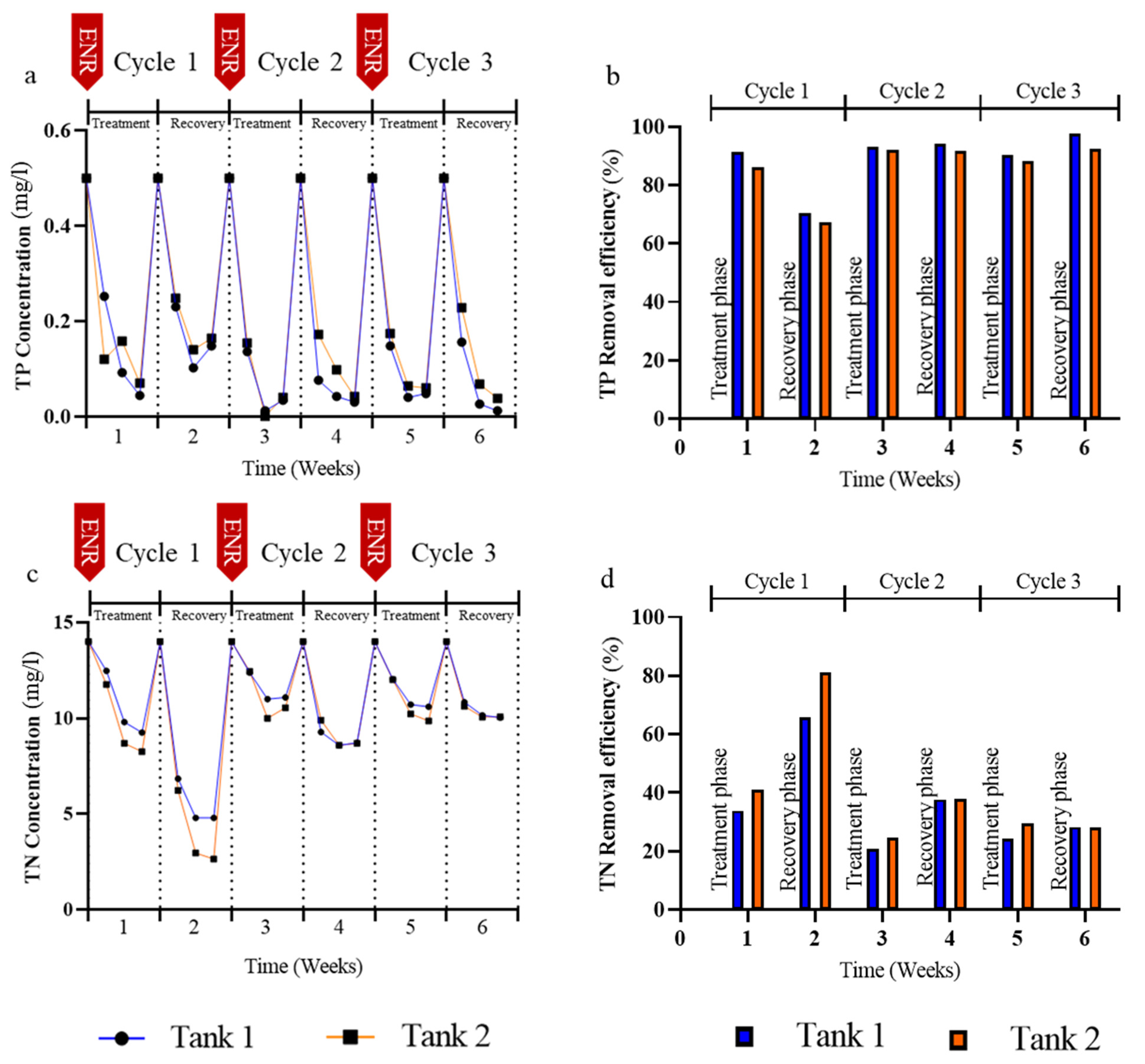
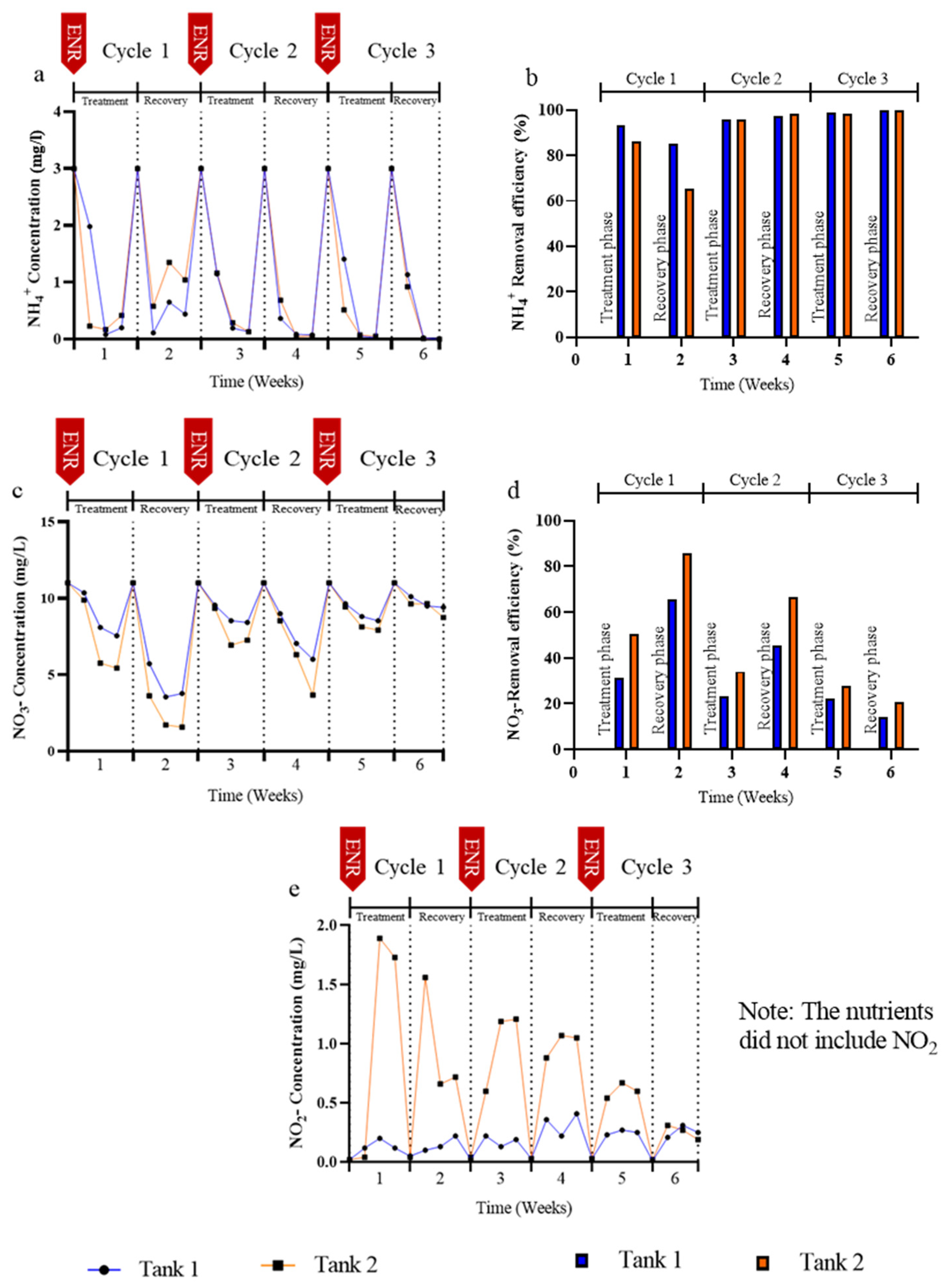
3.2. Effects of Enrofloxacin on Nutrient Removal
3.3. Removal of Enrofloxacin
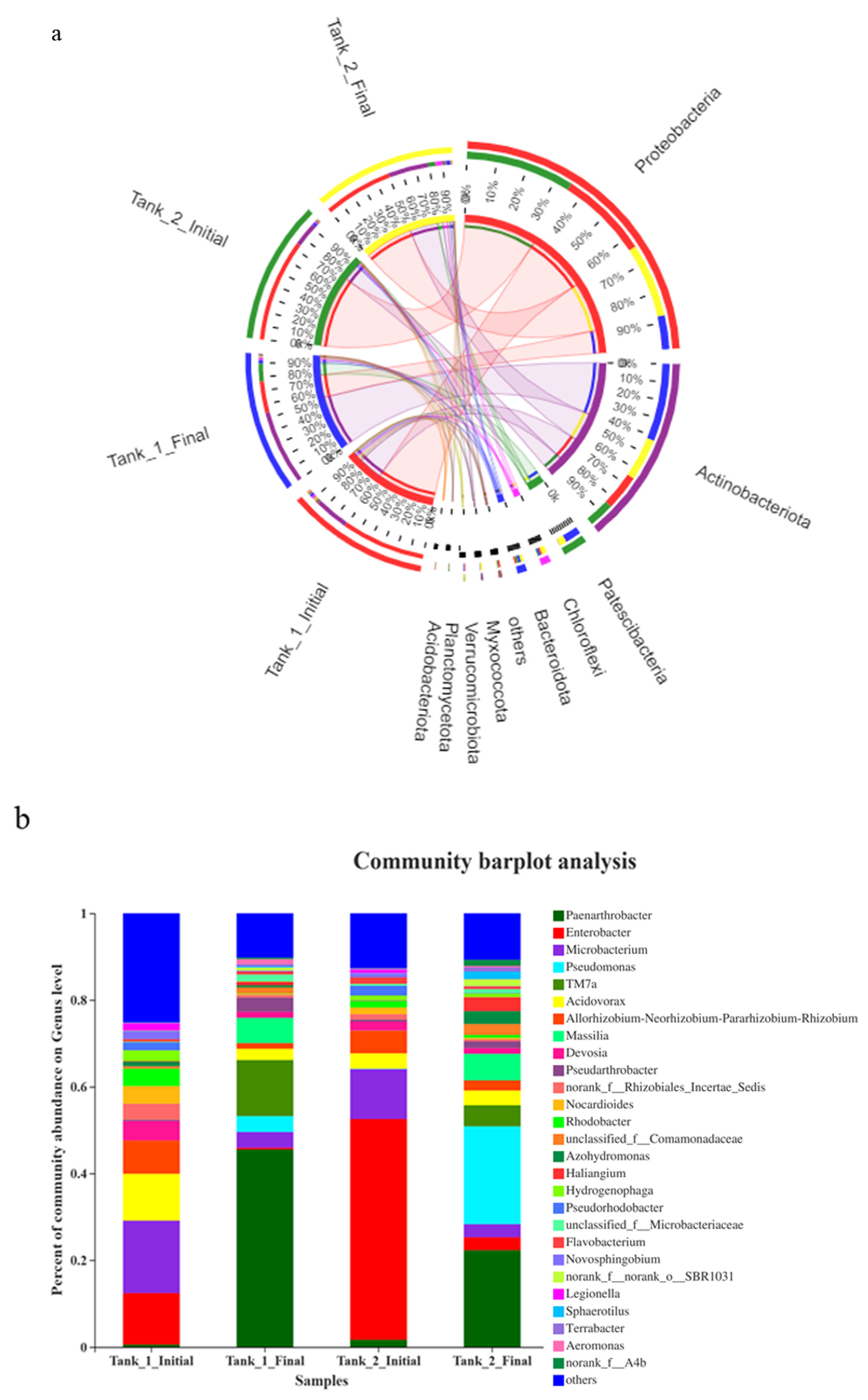
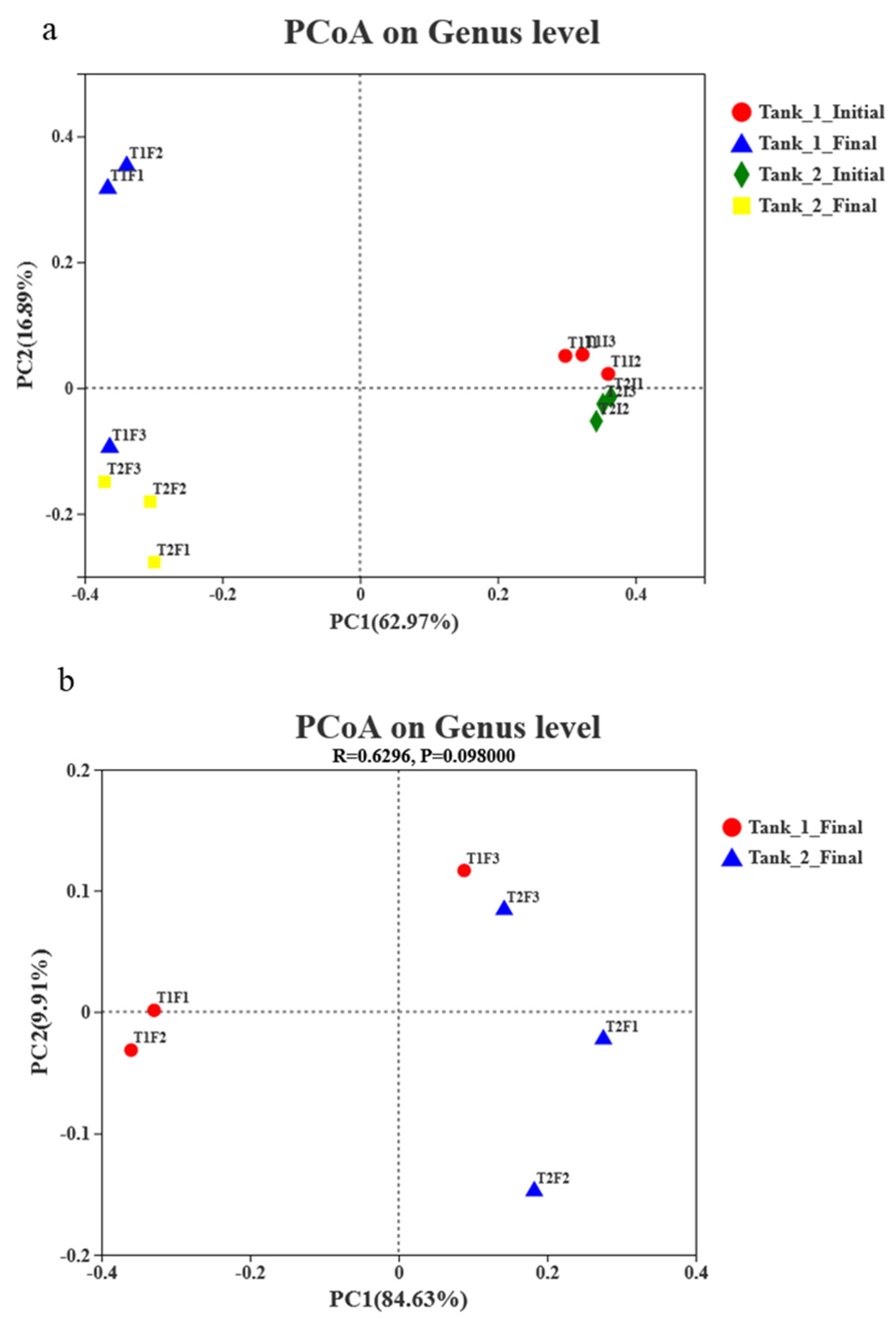
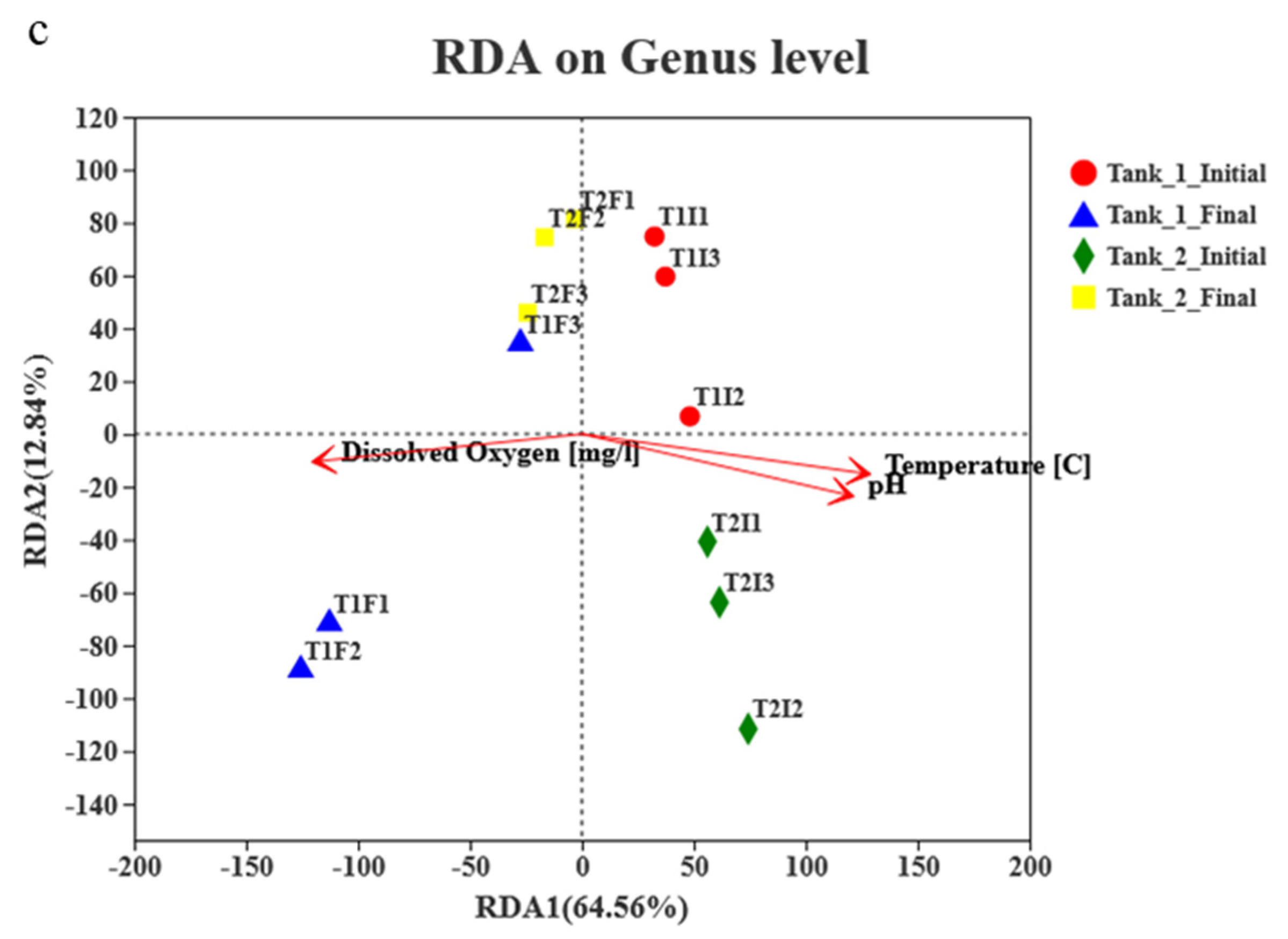
3.4. Microbial Community Richness, Diversity and Composition
3.5. Relative Abundance (%) of Significant Functional Groups Associated with N and P Removal
4. Discussion
4.1. Plant Growth and Photosynthetic Pigment
4.2. N and P Elimination in the Presence of Enrofloxacin
4.3. Enrofloxacin Degradation
4.4. Effect of Enrofloxacin on Microbial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Yang, X.; Wang, Z.; Shan, Y.; Zheng, Z. Comparison of four aquatic plant treatment systems for nutrient removal from eutrophied water. Bioresour. Technol. 2015, 179, 1–7. [Google Scholar] [CrossRef]
- González, J.E.; Roldán, G. Eutrophication and Phytoplankton: Some Generalities from Lakes and Reservoirs of the Americas. In Microalgae—From Physiology to Application; Vítová, M., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-83880-035-2. [Google Scholar]
- Lin, Z.; Wang, Y.; Huang, W.; Wang, J.; Chen, L.; Zhou, J.; He, Q. Single-stage denitrifying phosphorus removal biofilter utilizing intracellular carbon source for advanced nutrient removal and phosphorus recovery. Bioresour. Technol. 2019, 277, 27–36. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Lv, D.; Li, Y.; Wu, J. Nitrogen and phosphorus removal performance and bacterial communities in a multi-stage surface flow constructed wetland treating rural domestic sewage. Sci. Total Environ. 2020, 709, 136235. [Google Scholar] [CrossRef]
- Tong, X.; Wang, X.; He, X.; Xu, K.; Mao, F. Effects of ofloxacin on nitrogen removal and microbial community structure in constructed wetland. Sci. Total Environ. 2019, 656, 503–511. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, H.; Bañuelos, G.; Yan, B.; Shutes, B.; Cheng, X.; Chen, X. Removal of nutrients in saline wastewater using constructed wetlands: Plant species, influent loads and salinity levels as influencing factors. Chemosphere 2017, 187, 52–61. [Google Scholar] [CrossRef]
- Eveborn, D.; Kong, D.; Gustafsson, J.P. Wastewater treatment by soil infiltration: Long-term phosphorus removal. J. Contam. Hydrol. 2012, 140–141, 24–33. [Google Scholar] [CrossRef]
- Maucieri, C.; Salvato, M.; Borin, M. Vegetation contribution on phosphorus removal in constructed wetlands. Ecol. Eng. 2020, 152, 105853. [Google Scholar] [CrossRef]
- Su, C.; Zhu, X.; Shi, X.; Xie, Y.; Fang, Y.; Zhou, X.; Huang, Z.; Lin, X.; Chen, M. Removal efficiency and pathways of phosphorus from wastewater in a modified constructed rapid infiltration system. J. Clean. Prod. 2020, 267, 122063. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, W.; Huang, J.; He, S.; Yan, Y.; Zhu, W.; Wu, S.; Zhang, X. Nitrogen removal by the enhanced floating treatment wetlands from the secondary effluent. Bioresour. Technol. 2017, 234, 243–252. [Google Scholar] [CrossRef]
- Yu, B.; Huang, J.-C.; Zhou, C.; He, S.; Zhou, W. Selenium removal by clam shells and gravels amended with cattail and reed litter. Sci. Total Environ. 2020, 742, 140661. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, J.-C.; He, S.; Zhou, W. Enhancement of a constructed wetland water treatment system for selenium removal. Sci. Total Environ. 2020, 714, 136741. [Google Scholar] [CrossRef]
- Huang, X.; Xiong, W.; Liu, W.; Guo, X. Effect of reclaimed water effluent on bacterial community structure in the Typha angustifolia L. rhizosphere soil of urbanized riverside wetland, China. J. Environ. Sci. 2017, 55, 58–68. [Google Scholar] [CrossRef]
- Saumya, S. Construction and evaluation of prototype subsurface flow wetland planted with Heliconia angusta for the treatment of synthetic greywater. J. Clean. Prod. 2015, 91, 235–240. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.-S.; Su, H.-C.; Ying, G.-G.; Liu, F.; Liu, S.-S.; He, L.-Y.; Chen, Z.-F.; Yang, Y.-Q.; Chen, F.-R. Removal of antibiotics and antibiotic resistance genes in rural wastewater by an integrated constructed wetland. Environ. Sci. Pollut. Res. 2015, 22, 1794–1803. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Jiang, L.; Wagner, T.; Sutton, N.B.; Ji, R.; Langenhoff, A.A.M. Improving removal of antibiotics in constructed wetland treatment systems based on key design and operational parameters: A review. J. Hazard. Mater. 2021, 407, 124386. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, B.; Wang, H.; Lai, X.; Li, F.; Salam, M.M.A.; Pan, F.; Zhao, Y. The simultaneous antibiotics and nitrogen removal in vertical flow constructed wetlands: Effects of substrates and responses of microbial functions. Bioresour. Technol. 2020, 310, 123419. [Google Scholar] [CrossRef]
- Chen, J.; Wei, X.-D.; Liu, Y.-S.; Ying, G.-G.; Liu, S.-S.; He, L.-Y.; Su, H.-C.; Hu, L.-X.; Chen, F.-R.; Yang, Y.-Q. Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Optimization of wetland substrates and hydraulic loading. Sci. Total Environ. 2016, 565, 240–248. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.-H.; Liu, C.-X.; Wang, Z.; Dong, J.; Zhu, G.-F.; Huang, X. Potential effect and accumulation of veterinary antibiotics in Phragmites australis under hydroponic conditions. Ecol. Eng. 2013, 53, 138–143. [Google Scholar] [CrossRef]
- Tran, N.H.; Hoang, L.; Nghiem, L.D.; Nguyen, N.M.H.; Ngo, H.H.; Guo, W.; Trinh, Q.T.; Mai, N.H.; Chen, H.; Nguyen, D.D.; et al. Occurrence and risk assessment of multiple classes of antibiotics in urban canals and lakes in Hanoi, Vietnam. Sci. Total Environ. 2019, 692, 157–174. [Google Scholar] [CrossRef]
- Liu, X.-H.; Guo, X.; Liu, Y.; Lu, S.; Xi, B.; Zhang, J.; Wang, Z.; Bi, B. A review on removing antibiotics and antibiotic resistance genes from wastewater by constructed wetlands: Performance and microbial response. Environ. Pollut. 2019, 254, 112996. [Google Scholar] [CrossRef]
- Berglund, B.; Khan, G.A.; Weisner, S.E.; Ehde, P.M.; Fick, J.; Lindgren, P.-E. Efficient removal of antibiotics in surface-flow constructed wetlands, with no observed impact on antibiotic resistance genes. Sci. Total Environ. 2014, 476–477, 29–37. [Google Scholar] [CrossRef]
- Li, X.; Lu, S.; Liu, S.; Zheng, Q.; Shen, P.; Wang, X. Shifts of bacterial community and molecular ecological network at the presence of fluoroquinolones in a constructed wetland system. Sci. Total Environ. 2020, 708, 135156. [Google Scholar] [CrossRef]
- Santos, F.; de Almeida, C.M.R.; Ribeiro, I.; Ferreira, A.C.; Mucha, A.P. Removal of veterinary antibiotics in constructed wetland microcosms—Response of bacterial communities. Ecotoxicol. Environ. Saf. 2019, 169, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.P.; Mitzel, M.R.; Slawson, R.M.; Legge, R.L. Effect of ciprofloxacin on microbiological development in wetland mesocosms. Water Res. 2011, 45, 3185–3196. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.P.; Almeida, C.M.R.; Pereira, A.C.; Ribeiro, I.L.; Reis, I.; Carvalho, P.; Basto, M.C.P.; Mucha, A.P. Microbial community dynamics associated with veterinary antibiotics removal in constructed wetlands microcosms. Bioresour. Technol. 2015, 182, 26–33. [Google Scholar] [CrossRef]
- Sayen, S.; Rocha, C.; Silva, C.; Vulliet, E.; Guillon, E.; Almeida, C.M.R. Enrofloxacin and copper plant uptake by Phragmites australis from a liquid digestate: Single versus combined application. Sci. Total Environ. 2019, 664, 188–202. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Tam, N.F.-Y.; Yang, L.; Mei, X.-Q.; Yang, F.-J. Growth characteristics of six wetland plants and their influences on domestic wastewater treatment efficiency. Ecol. Eng. 2013, 60, 382–392. [Google Scholar] [CrossRef]
- Zhai, X.; Piwpuan, N.; Arias, C.A.; Headley, T.; Brix, H. Can root exudates from emergent wetland plants fuel denitrification in subsurface flow constructed wetland systems? Ecol. Eng. 2013, 61, 555–563. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.; Zuo, J.; Zhao, D.; Zou, X.; Zhu, Z.; Jeelani, N.; Leng, X.; An, S. A Hardy Plant Facilitates Nitrogen Removal via Microbial Communities in Subsurface Flow Constructed Wetlands in Winter. Sci. Rep. 2016, 6, 33600. [Google Scholar] [CrossRef] [Green Version]
- American Public Health Association (APHA). Standard Methods For the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Gogoi, M.; Basumatary, M.; Institutional Biotech Hub, Science College, Kokrajhar, BTC, Assam, India. Estimation of the Chlorophyll Concentration in Seven Citrus Species of Kokrajhar District, BTAD, Assam, India. Trop. Plant Res. 2018, 5, 83–87. [Google Scholar] [CrossRef]
- Sun, S.; Liu, J.; Zhang, M.; He, S. Simultaneous improving nitrogen removal and decreasing greenhouse gas emission with biofilm carriers addition in ecological floating bed. Bioresour. Technol. 2019, 292, 121944. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shao, Z.; Kong, Z.; Gu, L.; Fang, J.; Chai, H. Study of pyrite based autotrophic denitrification system for low-carbon source stormwater treatment. J. Water Process Eng. 2020, 37, 101414. [Google Scholar] [CrossRef]
- Lezcano, M.Á.; Velazquez, D.; Quesada, A.; El-Shehawy, R. Diversity and temporal shifts of the bacterial community associated with a toxic cyanobacterial bloom: An interplay between microcystin producers and degraders. Water Res. 2017, 125, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Gu, X.; Zhu, W.; He, S.; Wu, F.; Huang, J.; Zhou, W. Denitrification- and anammox-dominant simultaneous nitrification, anammox and denitrification (SNAD) process in subsurface flow constructed wetlands. Bioresour. Technol. 2019, 271, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, J.; Zhang, M.; He, S. Thiosulfate-driven autotrophic and mixotrophic denitrification processes for secondary effluent treatment: Reducing sulfate production and nitrous oxide emission. Bioresour. Technol. 2020, 300, 122651. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total Environ. 2017, 599–600, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ying, G.-G.; Tao, R.; Zhao, J.-L.; Yang, J.-F.; Zhao, L.-F. Effects of six selected antibiotics on plant growth and soil microbial and enzymatic activities. Environ. Pollut. 2009, 157, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Batchelder, A.R. Chlortetracycline and Oxytetracycline Effects on Plant Growth and Development in Soil Systems. J. Environ. Qual. 1982, 11, 675–678. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, F.; Langenkämper, G.; Grote, M. Studies on uptake and distribution of antibiotics in red cabbage. J. Verbr. Lebensm. 2016, 11, 61–69. [Google Scholar] [CrossRef]
- Verhagen, F.J.M.; Laanbroek, H.J.; Woldendorp, J.W. Competition for ammonium between plant roots and nitrifying and heterotrophic bacteria and the effects of protozoan grazing. Plant Soil 1995, 170, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, P.N.; Basto, M.C.P.; Almeida, C.M.R. Potential of Phragmites australis for the removal of veterinary pharmaceuticals from aquatic media. Bioresour. Technol. 2012, 116, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Dordio, A.V.; Duarte, C.; Barreiros, M.; Carvalho, A.J.P.; Pinto, A.P.; da Costa, C.T. Toxicity and removal efficiency of pharmaceutical metabolite clofibric acid by Typha spp.—Potential use for phytoremediation? Bioresour. Technol. 2009, 100, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, T.; Lu, S.; Liu, H.; Wang, H.; Li, C.; Liu, X.; Guo, X.; Zhao, X.; Liu, F. Performance and bacterial community dynamics of hydroponically grown Iris pseudacorus L. during the treatment of antibiotic-enriched wastewater at low/normal temperature. Ecotoxicol. Environ. Saf. 2021, 213, 111997. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ding, J.; Xie, H.; Hao, D.; Du, Y.; Zhao, C.; Xu, F.; Kong, Q.; Wang, B. Phosphorus removal performance of microbial-enhanced constructed wetlands that treat saline wastewater. J. Clean. Prod. 2021, 288, 125119. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, A.; Rodriguez-Sanchez, A.; Martinez-Toledo, M.V.; Garcia-Ruiz, M.-J.; Hontoria, E.; Osorio-Robles, F.; Gonzalez–Lopez, J. Effect of ciprofloxacin antibiotic on the partial-nitritation process and bacterial community structure of a submerged biofilter. Sci. Total Environ. 2014, 476–477, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Yin, G.; Liu, M.; Zhou, J.; Zheng, Y.; Gao, J.; Zong, H.; Yang, Y.; Gao, L.; Tong, C. Effects of Sulfamethazine on Denitrification and the Associated N2O Release in Estuarine and Coastal Sediments. Environ. Sci. Technol. 2015, 49, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, Y.; Qu, M.; Hao, M.; Yang, D.; Yang, Q.; Wang, X.C.; Dzakpasu, M. Fate of an antibiotic and its effects on nitrogen transformation functional bacteria in integrated vertical flow constructed wetlands. Chem. Eng. J. 2021, 417, 129272. [Google Scholar] [CrossRef]
- Shan, A.; Wang, W.; Kang, K.J.; Hou, D.; Luo, J.; Wang, G.; Pan, M.; Feng, Y.; He, Z.; Yang, X. The Removal of Antibiotics in Relation to a Microbial Community in an Integrated Constructed Wetland for Tail Water Decontamination. Wetlands 2020, 40, 993–1004. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. EXBOTJ 2016, 68, erw449. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhou, W.; Wu, S.; He, S.; Huang, J.; Zhang, X. Nitrogen removal by thiosulfate-driven denitrification and plant uptake in enhanced floating treatment wetland. Sci. Total Environ. 2018, 621, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.N.; Araújo, J.L.D.S.; Mucha, A.P.; Basto, M.C.P.; Almeida, C.M.R. Potential of constructed wetlands microcosms for the removal of veterinary pharmaceuticals from livestock wastewater. Bioresour. Technol. 2013, 134, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Roose-Amsaleg, C.; Laverman, A.M. Do antibiotics have environmental side-effects? Impact of synthetic antibiotics on biogeochemical processes. Environ. Sci. Pollut. Res. 2016, 23, 4000–4012. [Google Scholar] [CrossRef] [PubMed]
- Picó, Y.; Andreu, V. Fluoroquinolones in soil—risks and challenges. Anal. Bioanal. Chem. 2007, 387, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Conkle, J.L.; Lattao, C.; White, J.R.; Cook, R.L. Competitive sorption and desorption behavior for three fluoroquinolone antibiotics in a wastewater treatment wetland soil. Chemosphere 2010, 80, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Mucha, A.P.; Alexandrino, D.A.M.; Almeida, C.M.R.; Carvalho, M.F. Biodegradation of enrofloxacin by microbial consortia obtained from rhizosediments of two estuarine plants. J. Environ. Manag. 2019, 231, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.J.R.; Loh, J.; Davidson, N.C.; Beltrame, C.; Freeman, R.; Walpole, M. Tracking global change in ecosystem area: The Wetland Extent Trends index. Biol. Conserv. 2016, 193, 27–35. [Google Scholar] [CrossRef]
- Yan, Q.; Min, J.; Yu, Y.; Zhu, Z.; Feng, G. Microbial community response during the treatment of pharmaceutically active compounds (PhACs) in constructed wetland mesocosms. Chemosphere 2017, 186, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, J.-C.; Sun, S.; Ur Rehman, M.M.; He, S.; Zhou, W. Impact of functional microbes on nitrogen removal in artificial tidal wetlands in the Yangtze River estuary: Evidence from molecular and stable isotopic analyses. J. Clean. Prod. 2021, 287, 125077. [Google Scholar] [CrossRef]
- Cao, S.; Du, R.; Peng, Y.; Li, B.; Wang, S. Novel two stage partial denitrification (PD)-Anammox process for tertiary nitrogen removal from low carbon/nitrogen (C/N) municipal sewage. Chem. Eng. J. 2019, 362, 107–115. [Google Scholar] [CrossRef]
- Xiong, W.; Sun, Y.; Ding, X.; Zhang, Y.; Zhong, X.; Liang, W.; Zeng, Z. Responses of plasmid-mediated quinolone resistance genes and bacterial taxa to (fluoro)quinolones-containing manure in arable soil. Chemosphere 2015, 119, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, K.J.; Patel, S.; Gibson, M.K.; Lauber, C.L.; Knight, R.; Fierer, N.; Dantas, G. Bacterial phylogeny structures soil resistomes across habitats. Nature 2014, 509, 612–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Zhai, H.; Cheng, S. Fate of antibiotics and antibiotic resistance genes in home water purification systems. Water Res. 2021, 190, 116762. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yang, J.; Yu, H.; Lan, Z.; Ye, X.; Yang, G.; Yang, Q.; Zhou, J. Response of denitrifying community, denitrification genes and antibiotic resistance genes to oxytetracycline stress in polycaprolactone supported solid-phase denitrification reactor. Bioresour. Technol. 2020, 308, 123274. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Liu, H.; Lan, T.; Dong, L.; Hu, H.; Zhao, S.; Zhang, Y.; Zheng, N.; Wang, J. Antibiotic Resistance Patterns of Pseudomonas spp. Isolated From Raw Milk Revealed by Whole Genome Sequencing. Front. Microbiol. 2020, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Rezakhani, L.; Motesharezadeh, B.; Tehrani, M.M.; Etesami, H.; Mirseyed Hosseini, H. Phosphate–solubilizing bacteria and silicon synergistically augment phosphorus (P) uptake by wheat (Triticum aestivum L.) plant fertilized with soluble or insoluble P source. Ecotoxicol. Environ. Saf. 2019, 173, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, M.; Li, N.; Du, S.; Yang, J.; Li, Y. Effects of CeO2 nanoparticles on bacterial community and molecular ecological network in activated sludge system. Environ. Pollut. 2018, 238, 516–523. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Gall, D.L.; McMahon, K.D. “Candidatus Accumulibacter” Population Structure in Enhanced Biological Phosphorus Removal Sludges as Revealed by Polyphosphate Kinase Genes. Appl. Environ. Microbiol. 2007, 73, 5865–5874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mino, T.; van Loosdrecht, M.C.M.; Heijnen, J.J. Microbiology and biochemistry of the enhanced biological phosphate removal process. Water Res. 1998, 32, 3193–3207. [Google Scholar] [CrossRef]
- Peterson, S.B.; Warnecke, F.; Madejska, J.; McMahon, K.D.; Hugenholtz, P. Environmental distribution and population biology of Candidatus Accumulibacter, a primary agent of biological phosphorus removal: Environmental Distribution of Accumulibacter. Environ. Microbiol. 2008, 10, 2692–2703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, M.; Loy, A.; Nogueira, R.; Purkhold, U.; Lee, N. Microbial community composition and function in wastewater treatment plants. Antonie van Leeuwenhoek 2002, 81, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zeng, W.; Fan, Z.; Wang, C.; Meng, Q.; Peng, Y. Effects of polyaluminium chloride addition on community structures of polyphosphate and glycogen accumulating organisms in biological phosphorus removal (BPR) systems. Bioresour. Technol. 2020, 297, 122431. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramdat, N.; Wang, Z.-J.; Huang, J.-C.; Wang, Y.; Chachar, A.; Zhou, C.; Wang, Z. Effects of Enrofloxacin on Nutrient Removal by a Floating Treatment Wetland Planted with Iris pseudacorus: Response and Resilience of Rhizosphere Microbial Communities. Sustainability 2022, 14, 3358. https://doi.org/10.3390/su14063358
Ramdat N, Wang Z-J, Huang J-C, Wang Y, Chachar A, Zhou C, Wang Z. Effects of Enrofloxacin on Nutrient Removal by a Floating Treatment Wetland Planted with Iris pseudacorus: Response and Resilience of Rhizosphere Microbial Communities. Sustainability. 2022; 14(6):3358. https://doi.org/10.3390/su14063358
Chicago/Turabian StyleRamdat, Naven, Zi-Jing Wang, Jung-Chen Huang, Yikun Wang, Azharuddin Chachar, Chuanqi Zhou, and Zhiping Wang. 2022. "Effects of Enrofloxacin on Nutrient Removal by a Floating Treatment Wetland Planted with Iris pseudacorus: Response and Resilience of Rhizosphere Microbial Communities" Sustainability 14, no. 6: 3358. https://doi.org/10.3390/su14063358
APA StyleRamdat, N., Wang, Z.-J., Huang, J.-C., Wang, Y., Chachar, A., Zhou, C., & Wang, Z. (2022). Effects of Enrofloxacin on Nutrient Removal by a Floating Treatment Wetland Planted with Iris pseudacorus: Response and Resilience of Rhizosphere Microbial Communities. Sustainability, 14(6), 3358. https://doi.org/10.3390/su14063358





