Slow Pyrolysis of Ulva lactuca (Chlorophyta) for Sustainable Production of Bio-Oil and Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Preparation
2.2. Feedstock Characterization
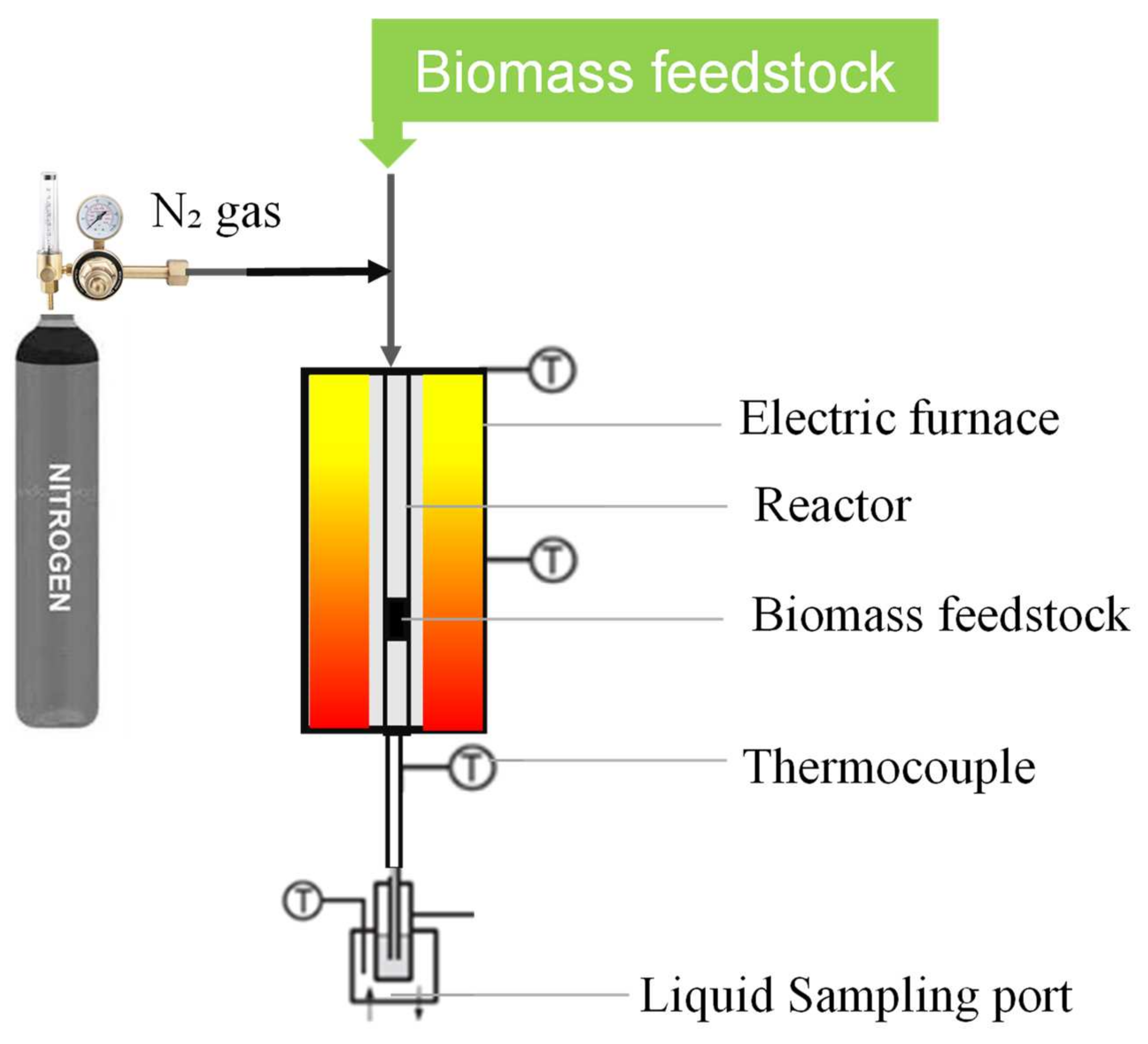
2.3. Pyrolysis of U. lactuca
2.4. Product Characterization
3. Results and Discussion
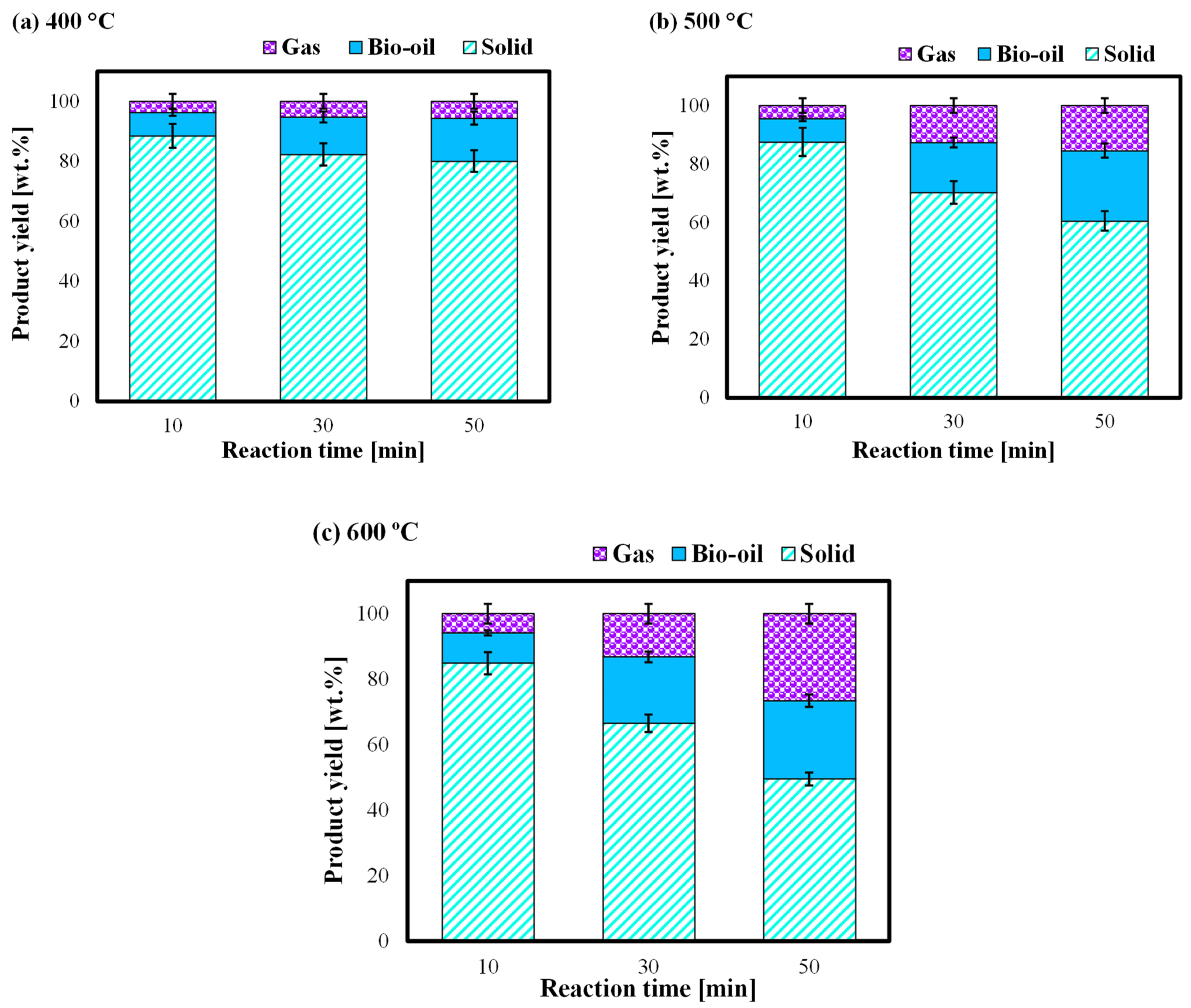
3.1. Product Yield Distribution at the Different Reaction Temperature
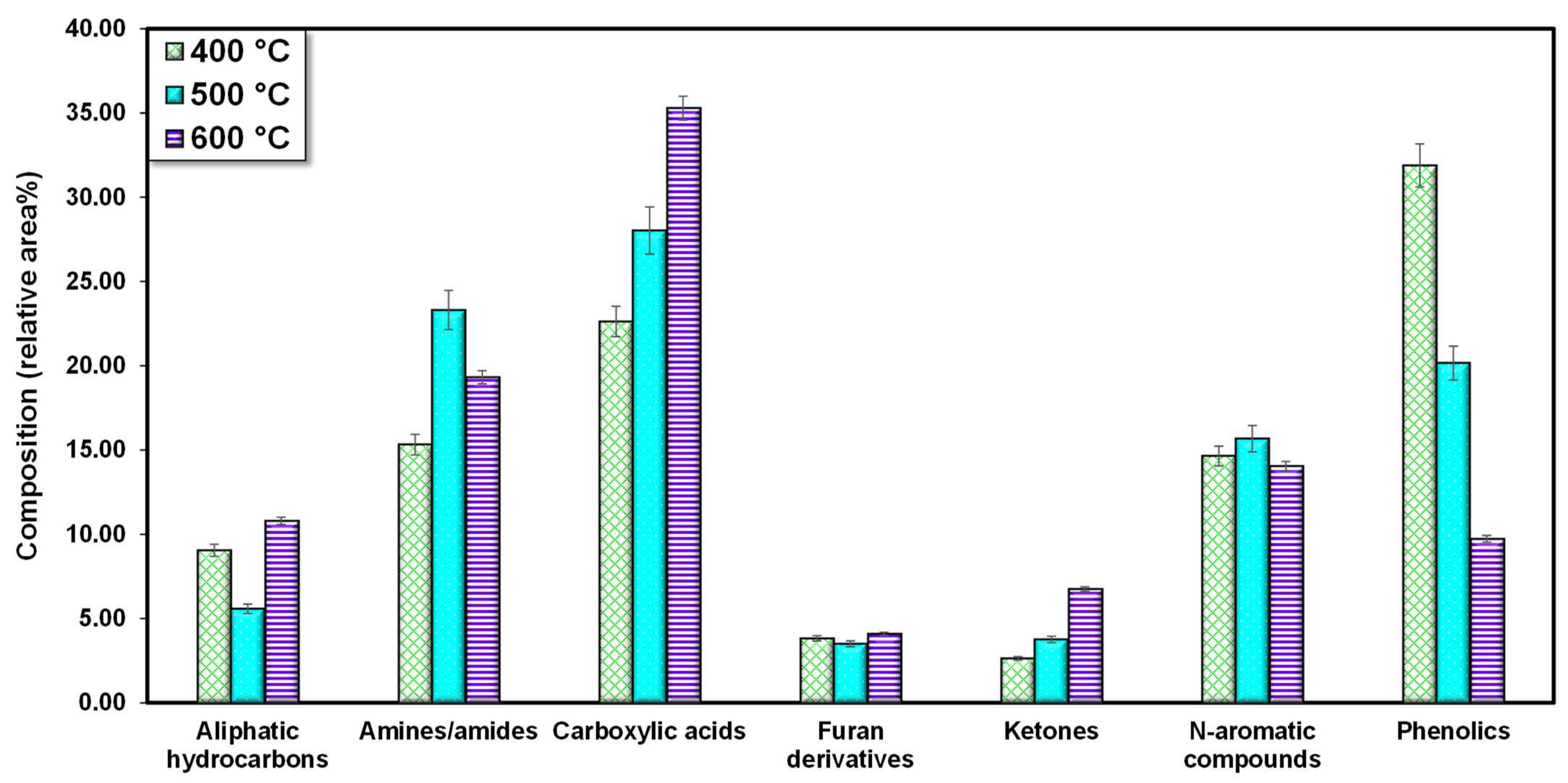
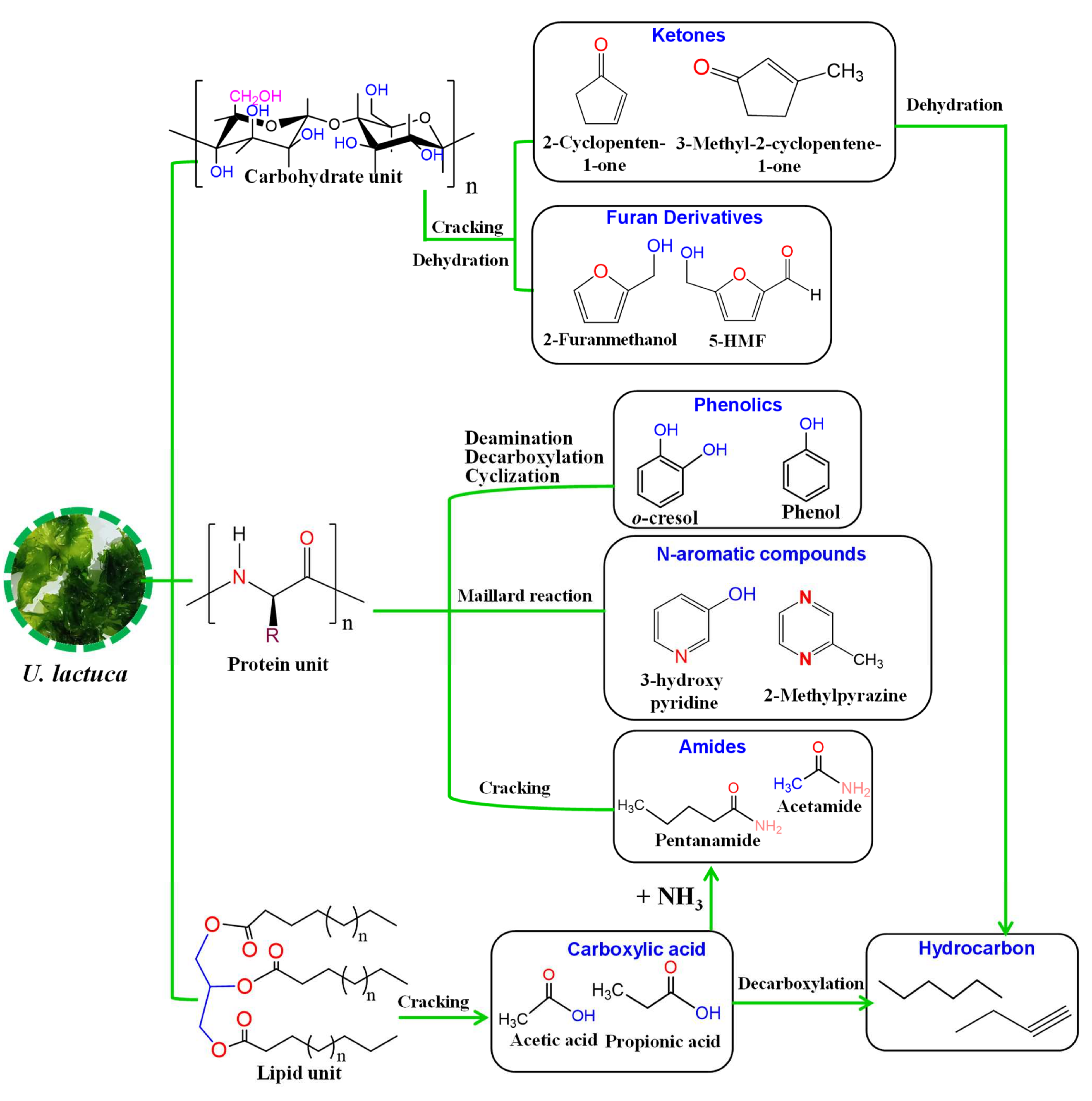
3.2. Bio-Oil Characteristics
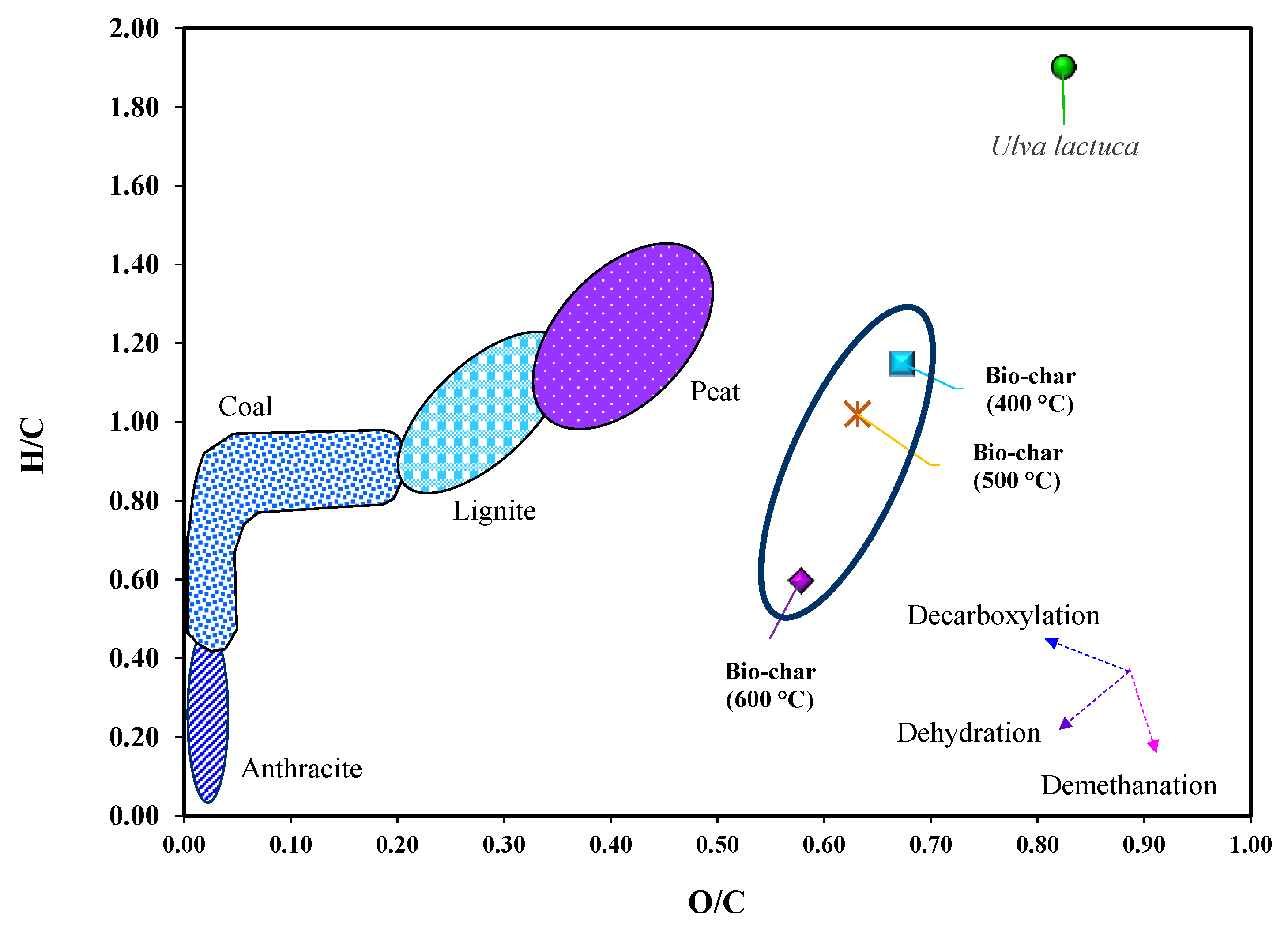
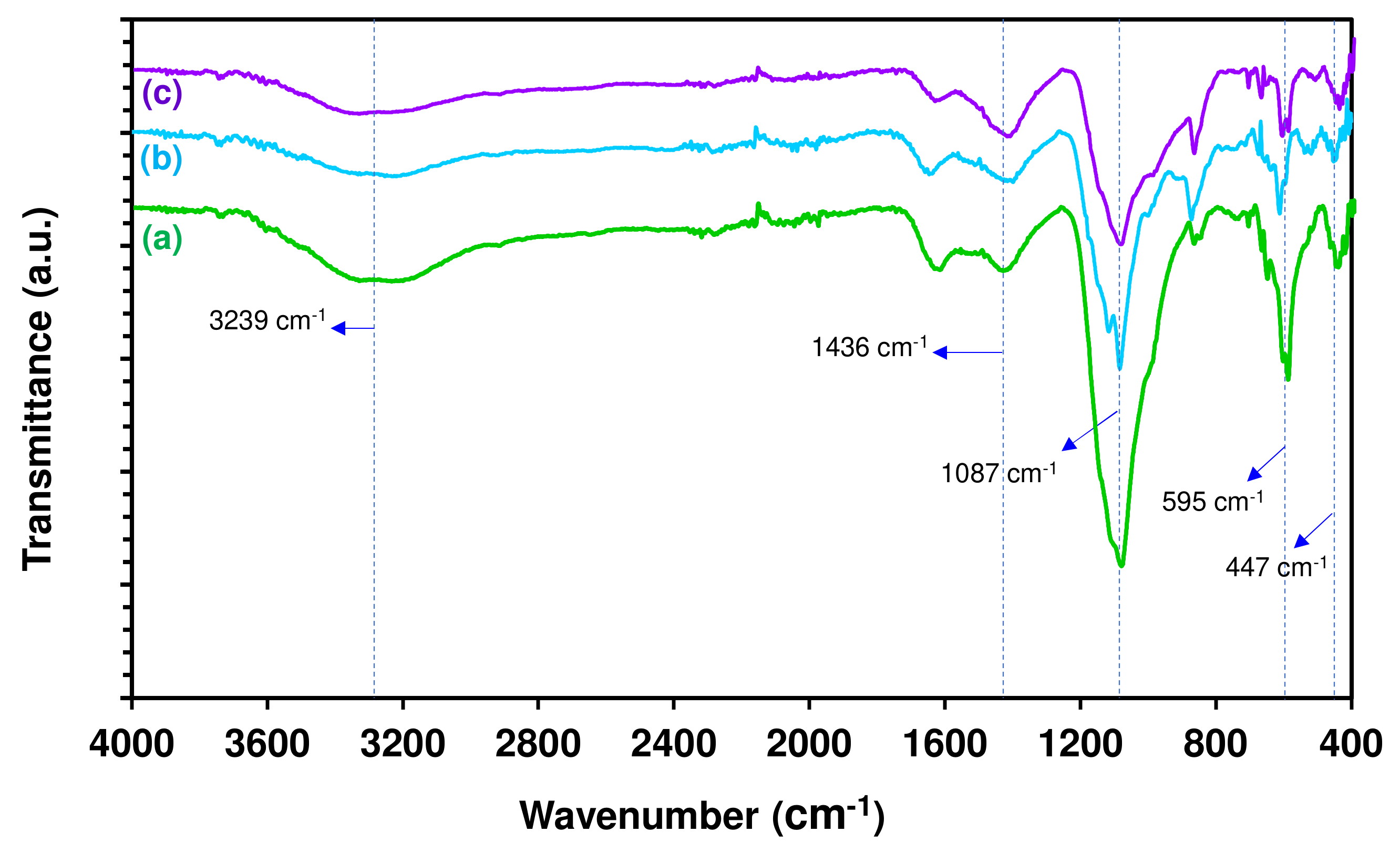
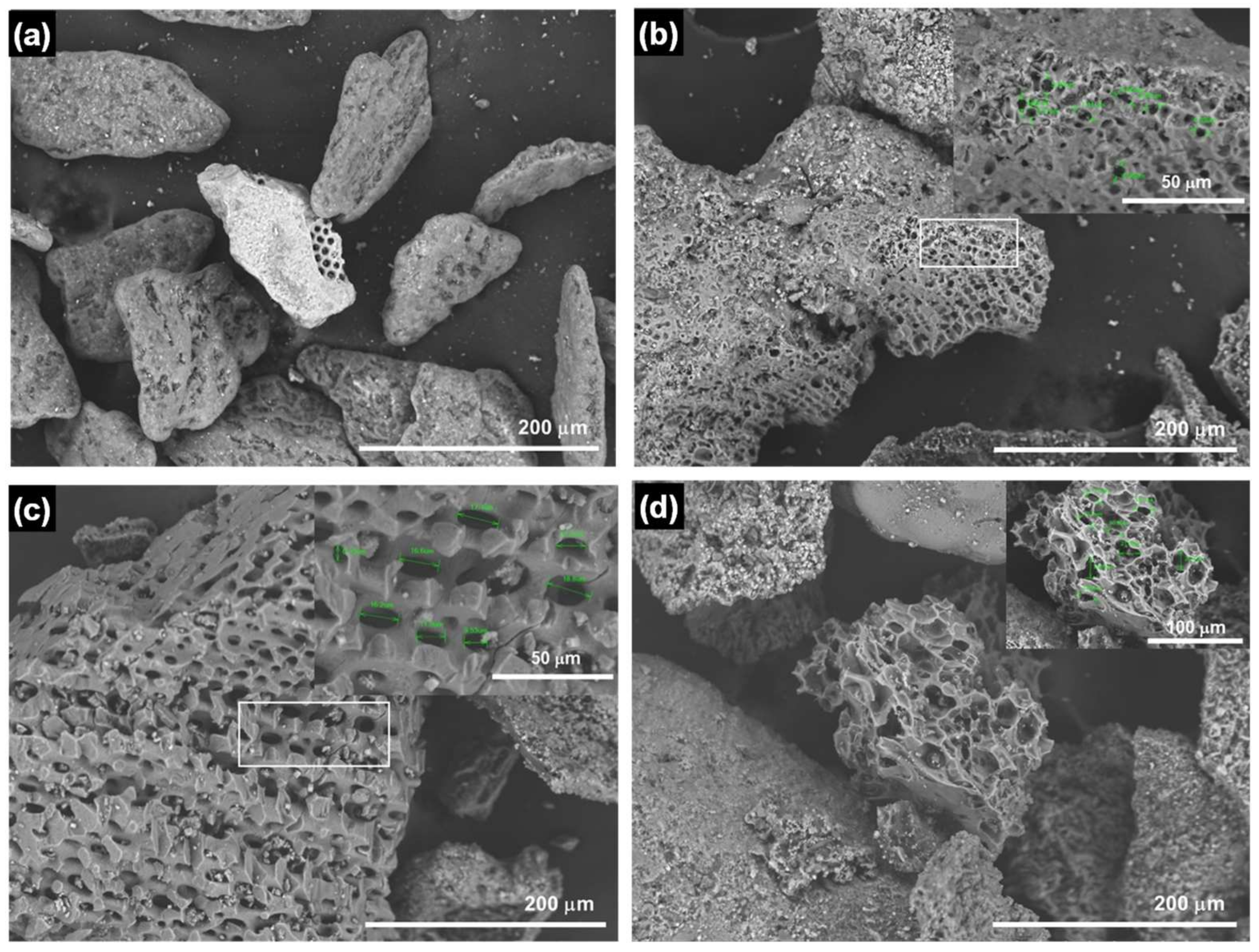
3.3. Biochar Product and Its Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Destek, M.A.; Sarkodie, S.A.; Asamoah, E.F. Does biomass energy drive environmental sustainability? An SDG perspective for top five biomass consuming countries. Biomass Bioenergy 2021, 149, 106076. [Google Scholar] [CrossRef]
- Biswas, B.; Arun Kumar, A.; Bisht, Y.; Krishna, B.B.; Kumar, J.; Bhaskar, T. Role of temperatures and solvents on hydrothermal liquefaction of Azolla filiculoides. Energy 2021, 217, 119330. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chen, W.-H.; Lee, D.-J.; Chang, J.-S. Supercritical water gasification (SCWG) as a potential tool for the valorization of phycoremediation-derived waste algal biomass for biofuel generation. J. Hazard. Mater. 2021, 418, 126278. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Zhang, C.; Tao, F.; Zhang, C.; Chen, W.H. Microalgal Torrefaction for Solid Biofuel Production. Trends Biotechnol. 2020, 38, 1023–1033. [Google Scholar] [CrossRef]
- Samanmulya, T.; Farobie, O.; Matsumura, Y. Gasification characteristics of aminobutyric acid and serine as model compounds of proteins under supercritical water conditions. J. Jpn. Pet. Inst. 2017, 60, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Reza, S.; Azad, A.K.; Bakar, M.S.A.; Karim, R.; Sharifpur, M.; Taweekun, J. Evaluation of Thermochemical Characteristics and Pyrolysis of Fish Processing Waste for Renewable Energy Feedstock. Sustainability 2022, 14, 1203. [Google Scholar] [CrossRef]
- Nenciu, F.; Paraschiv, M.; Kuncser, R.; Stan, C.; Cocarta, D.; Vladut, V.N. High-Grade Chemicals and Biofuels Produced from Marginal Lands Using an Integrated Approach of Alcoholic Fermentation and Pyrolysis of Sweet Sorghum Biomass Residues. Sustainability 2022, 14, 402. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Anto, S.; Rene, E.R.; Sekar, M.; Mathimani, T.; Thuy Lan Chi, N.; Pugazhendhi, A. Effect of reaction temperature on the conversion of algal biomass to bio-oil and biochar through pyrolysis and hydrothermal liquefaction. Fuel 2021, 285, 119106. [Google Scholar] [CrossRef]
- Nazir, A.; Laila, U.-; Bareen, F.; Hameed, E.; Shafiq, M. Sustainable Management of Peanut Shell through Biochar and Its Application as Soil Ameliorant. Sustainability 2021, 13, 13796. [Google Scholar] [CrossRef]
- Iaccarino, A.; Gautam, R.; Sarathy, S.M. Bio-oil and biochar production from halophyte biomass: Effects of pre-treatment and temperature on Salicornia bigelovii pyrolysis. Sustain. Energy Fuels 2021, 5, 2234–2248. [Google Scholar] [CrossRef]
- Casoni, A.I.; Ramos, F.D.; Estrada, V.; Diaz, M.S. Sustainable and economic analysis of marine macroalgae based chemicals production—Process design and optimization. J. Clean. Prod. 2020, 276, 122792. [Google Scholar] [CrossRef]
- Adams, J.M.M.; Toop, T.A.; Donnison, I.S.; Gallagher, J.A. Seasonal variation in Laminaria digitata and its impact on biochemical conversion routes to biofuels. Bioresour. Technol. 2011, 102, 9976–9984. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Smith, B.; Dyer, P.W.; Harvey, P. Macroalgae-derived biofuel: A review of methods of energy extraction from seaweed biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef]
- Farobie, O.; Matsumura, Y.; Syaftika, N.; Amrullah, A.; Hartulistiyoso, E.; Bayu, A.; Moheimani, N.R.; Karnjanakom, S.; Saefurahman, G. Recent advancement on hydrogen production from macroalgae via supercritical water gasification. Bioresour. Technol. Rep. 2021, 16, 100844. [Google Scholar] [CrossRef]
- Liu, J.; Tong, Y.; Xia, J.; Sun, Y.; Zhao, X.; Sun, J.; Zhao, S.; Zhuang, M.; Zhang, J.; He, P. Ulva macroalgae within local aquaculture ponds along the estuary of Dagu River, Jiaozhou Bay, Qingdao. Mar. Pollut. Bull. 2022, 174, 113243. [Google Scholar] [CrossRef]
- Bews, E.; Booher, L.; Polizzi, T.; Long, C.; Kim, J.H.; Edwards, M.S. Effects of salinity and nutrients on metabolism and growth of Ulva lactuca: Implications for bioremediation of coastal watersheds. Mar. Pollut. Bull. 2021, 166, 112199. [Google Scholar] [CrossRef]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Ulva rigida in the future ocean: Potential for carbon capture, bioremediation and biomethane production. GCB Bioenergy 2018, 10, 39–51. [Google Scholar] [CrossRef]
- Cheney, D.; Rajic, L.; Sly, E.; Meric, D.; Sheahan, T. Uptake of PCBs contained in marine sediments by the green macroalga Ulva rigida. Mar. Pollut. Bull. 2014, 88, 207–214. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Bruhn, A.; Rasmussen, M.B.; Olesen, B.; Larsen, M.M.; Møller, H.B. Cultivation of Ulva lactuca with manure for simultaneous bioremediation and biomass production. J. Appl. Phycol. 2012, 24, 449–458. [Google Scholar] [CrossRef]
- Rinehart, S.; Guidone, M.; Ziegler, A.; Schollmeier, T.; Thornber, C. Overwintering strategies of bloom-forming Ulva species in Narragansett Bay, Rhode Island, USA. Bot. Mar. 2014, 57, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Human, L.R.D.; Adams, J.B.; Allanson, B.R. Insights into the cause of an Ulva lactuca Linnaeus bloom in the Knysna Estuary. S. Afr. J. Bot. 2016, 107, 55–62. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Golden Tides: Problem or golden opportunity? The valorisation of Sargassum from beach inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef]
- Kang, E.J.; Han, A.R.; Kim, J.H.; Kim, I.N.; Lee, S.; Min, J.O.; Nam, B.R.; Choi, Y.J.; Edwards, M.S.; Diaz-Pulido, G.; et al. Evaluating bloom potential of the green-tide forming alga Ulva ohnoi under ocean acidification and warming. Sci. Total Environ. 2021, 769, 144443. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Hu, C. To what extent can Ulva and Sargassum be detected and separated in satellite imagery? Harmful Algae 2021, 103, 102001. [Google Scholar] [CrossRef]
- Salimi, P.; Norouzi, O.; Pourhoseini, S.E.M.; Bartocci, P.; Tavasoli, A.; Di Maria, F.; Pirbazari, S.M.; Bidini, G.; Fantozzi, F. Magnetic biochar obtained through catalytic pyrolysis of macroalgae: A promising anode material for Li-ion batteries. Renew. Energy 2019, 140, 704–714. [Google Scholar] [CrossRef]
- Ly, H.V.; Choi, J.H.; Woo, H.C.; Kim, S.S.; Kim, J. Upgrading bio-oil by catalytic fast pyrolysis of acid-washed Saccharina japonica alga in a fluidized-bed reactor. Renew. Energy 2019, 133, 11–22. [Google Scholar] [CrossRef]
- Hao, J.; Qi, B.; Li, D.; Zeng, F. Catalytic co-pyrolysis of rice straw and ulva prolifera macroalgae: Effects of process parameter on bio-oil up-gradation. Renew. Energy 2021, 164, 460–471. [Google Scholar] [CrossRef]
- Brigljević, B.; Liu, J.J.; Lim, H. Comprehensive feasibility assessment of a poly-generation process integrating fast pyrolysis of S. japonica and the Rankine cycle. Appl. Energy 2019, 254, 113704. [Google Scholar] [CrossRef]
- Kostas, E.T.; Williams, O.S.A.; Duran-Jimenez, G.; Tapper, A.J.; Cooper, M.; Meehan, R.; Robinson, J.P. Microwave pyrolysis of Laminaria digitata to produce unique seaweed-derived bio-oils. Biomass Bioenergy 2019, 125, 41–49. [Google Scholar] [CrossRef]
- Alves, J.L.F.; Da Silva, J.C.G.; da Silva Filho, V.F.; Alves, R.F.; Ahmad, M.S.; Ahmad, M.S.; de Galdino, W.V.A.; De Sena, R.F. Bioenergy potential of red macroalgae Gelidium floridanum by pyrolysis: Evaluation of kinetic triplet and thermodynamics parameters. Bioresour. Technol. 2019, 291, 121892. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, X.; Wang, J.; Yang, J.; Xuanwei, P.; Feng, L.; Zu, B.; Xie, Y.; Li, M. Pyrolysis of aquatic fern and macroalgae biomass into bio-oil: Comparison and optimization of operational parameters using response surface methodology. J. Energy Inst. 2021, 97, 194–202. [Google Scholar] [CrossRef]
- Verma, T.N.; Shrivastava, P.; Rajak, U.; Dwivedi, G.; Jain, S.; Zare, A.; Shukla, A.K.; Verma, P. A comprehensive review of the influence of physicochemical properties of biodiesel on combustion characteristics, engine performance and emissions. J. Traffic Transp. Eng. 2021, 8, 510–533. [Google Scholar] [CrossRef]
- Ma, C.; Geng, J.; Zhang, D.; Ning, X. Non-catalytic and catalytic pyrolysis of Ulva prolifera macroalgae for production of quality bio-oil. J. Energy Inst. 2020, 93, 303–311. [Google Scholar] [CrossRef]
- Ceylan, S.; Goldfarb, J.L. Green tide to green fuels: TG-FTIR analysis and kinetic study of Ulva prolifera pyrolysis. Energy Convers. Manag. 2015, 101, 263–270. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Amrullah, A.; Farobie, O.; Pramono, G.P. Solid degradation and its kinetics on phenol-rich bio-oil production from pyrolysis of coconut shell and Lamtoro wood residue. Korean J. Chem. Eng. 2022, 39, 389–397. [Google Scholar] [CrossRef]
- Amrullah, A.; Farobie, O.; Widyanto, R. Pyrolysis of purun tikus (Eleocharis dulcis): Product distributions and reaction kinetics. Bioresour. Technol. Rep. 2021, 13, 100642. [Google Scholar] [CrossRef]
- Aboulkas, A.; Hammani, H.; El Achaby, M.; Bilal, E.; Barakat, A.; El Harfi, K. Valorization of algal waste via pyrolysis in a fixed-bed reactor: Production and characterization of bio-oil and bio-char. Bioresour. Technol. 2017, 243, 400–408. [Google Scholar] [CrossRef]
- Yan, L.; Wang, Y.; Li, J.; Zhang, Y.; Ma, L.; Fu, F.; Chen, B.; Liu, H. Hydrothermal liquefaction of Ulva prolifera macroalgae and the influence of base catalysts on products. Bioresour. Technol. 2019, 292, 121286. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S.S.; Suh, D.J.; Jang, E.J.; Min, K., II; Woo, H.C. Characterization of the bio-oil and bio-char produced by fixed bed pyrolysis of the brown alga Saccharina japonica. Korean J. Chem. Eng. 2016, 33, 2691–2698. [Google Scholar] [CrossRef]
- Gautam, R.; Shyam, S.; Reddy, B.R.; Govindaraju, K.; Vinu, R. Microwave-assisted pyrolysis and analytical fast pyrolysis of macroalgae: Product analysis and effect of heating mechanism. Sustain. Energy Fuels 2019, 3, 3009–3020. [Google Scholar] [CrossRef]
- Wang, S.; Xia, Z.; Wang, Q.; He, Z.; Li, H. Mechanism research on the pyrolysis of seaweed polysaccharides by Py-GC/MS and subsequent density functional theory studies. J. Anal. Appl. Pyrolysis 2017, 126, 118–131. [Google Scholar] [CrossRef]
- Kim, J.Y.; Moon, J.; Lee, J.H.; Jin, X.; Choi, J.W. Conversion of phenol intermediates into aromatic hydrocarbons over various zeolites during lignin pyrolysis. Fuel 2020, 279, 118484. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Li, S.; Wang, X.; Lin, R. Comparative study on the two-step pyrolysis of different lignocellulosic biomass: Effects of components. J. Anal. Appl. Pyrolysis 2020, 152, 104966. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Mabon, R.; Broadbelt, L.J. A mechanistic model of fast pyrolysis of hemicellulose. Energy Environ. Sci. 2018, 11, 1240–1260. [Google Scholar] [CrossRef]
- Reyes, L.; Abdelouahed, L.; Mohabeer, C.; Buvat, J.C.; Taouk, B. Energetic and exergetic study of the pyrolysis of lignocellulosic biomasses, cellulose, hemicellulose and lignin. Energy Convers. Manag. 2021, 244, 114459. [Google Scholar] [CrossRef]
| No. | Compounds | Molecular Formula | Relative Area (%) under Different | ||
|---|---|---|---|---|---|
| Pyrolysis Temperature | |||||
| 400 °C | 500 °C | 600 °C | |||
| 1 | Acetic acid | C2H4O2 | 18.011 | 22.582 | 32.908 |
| 2 | Propionic acid | C3H6O2 | 1.986 | 2.495 | 1.689 |
| 3 | Cyclopropane | C3H6 | 5.515 | 3.859 | 8.268 |
| 4 | Acetamide | C2H5NO | 5.030 | 8.535 | 5.061 |
| 5 | Butanoic acid | C4H8O2 | 1.168 | 1.248 | 0.283 |
| 6 | 2-Methylpyrazine | C5H6N2 | 1.370 | 1.918 | 2.243 |
| 7 | Isovaleric acid | C5H10O2 | 1.463 | 1.693 | 0.404 |
| 8 | 2-Furanmethanol | C5H6O2 | 0.462 | 0.263 | 0.283 |
| 9 | 3-Methylpyridine | C6H7N | 0.631 | 0.294 | 0.283 |
| 10 | 2-Hexen-1-ol | C6H12O | 2.144 | 0.314 | 0.566 |
| 11 | 2-Acetylfuran | C2H2O | 2.173 | 1.918 | 1.368 |
| 12 | 5-Methylfurfural | C6H6O2 | 1.195 | 1.329 | 2.447 |
| 13 | 3-Methyl-2-cyclopenten-1-one | C6H8O | 1.370 | 1.745 | 0.423 |
| 14 | Phenol | C6H6O | 27.671 | 18.130 | 8.106 |
| 15 | 2,3-Dimethyl-2-cyclopente-1-one | C7H10O | 0.713 | 0.593 | 1.324 |
| 16 | o-Cresol | C7H8O | 0.920 | 0.697 | 0.283 |
| 17 | p-Cresol | C7H8O | 3.295 | 1.337 | 1.346 |
| 18 | 3-Hydroxypyridine | C5H5NO | 9.864 | 8.483 | 9.015 |
| 19 | 2-(2-Butoxyethoxy) ethanol | C8H18O3 | 0.727 | 0.213 | 0.280 |
| 20 | Ethyl methacrylate | C6H10O2 | 0.660 | 1.195 | 1.681 |
| 21 | 2-Hyrodxy-5-methylacetophenone | C9H10O2 | 0.555 | 1.428 | 5.000 |
| 22 | 5-Methylhydantoin | C4H6N2O2 | 2.781 | 4.981 | 2.499 |
| 23 | 4-(2-Aminoethyl) morpholine | C6H14N2O | 1.458 | 1.445 | 1.763 |
| 24 | 4-Octadecylmorpholine | C22H45NO | 5.787 | 6.718 | 10.429 |
| 25 | Propanamide | C3H7NO | 0.622 | 1.767 | 0.799 |
| 26 | Hexanamide | C6H13NO | 0.917 | 1.871 | 0.722 |
| 27 | 2-Pyrrolidinone | C4H7NO | 1.206 | 1.964 | 0.291 |
| 28 | 4-methylpentanamide | C6H13NO | 0.310 | 1.009 | 0.253 |
| Sample | Ultimate Analysis (wt%, Dry Ash-Free) | HHV (MJ kg−1) | ||||
|---|---|---|---|---|---|---|
| % C | % H | % N | % S | % O | ||
| Ulva lactuca | 39.10 ± 0.30 | 6.20 ± 0.03 | 4.46 ± 0.02 | 7.28 ± 0.10 | 42.96 ± 0.45 | 12.04 ± 0.06 |
| Biochar (400 °C) | 44.30 ± 0.13 | 4.25 ± 0.17 | 3.33 ± 0.22 | 8.41 ± 0.04 | 39.72 ± 0.47 | 19.94 ± 0.08 |
| Biochar (500 °C) | 45.55 ± 0.06 | 3.87 ± 0.15 | 2.91 ± 0.15 | 9.34 ± 0.08 | 38.34 ± 0.16 | 20.50 ± 0.06 |
| Biochar (600 °C) | 48.03 ± 0.20 | 2.39 ± 0.15 | 2.54 ± 0.18 | 10.03 ± 0.04 | 37.01 ± 0.48 | 21.61 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amrullah, A.; Farobie, O.; Bayu, A.; Syaftika, N.; Hartulistiyoso, E.; Moheimani, N.R.; Karnjanakom, S.; Matsumura, Y. Slow Pyrolysis of Ulva lactuca (Chlorophyta) for Sustainable Production of Bio-Oil and Biochar. Sustainability 2022, 14, 3233. https://doi.org/10.3390/su14063233
Amrullah A, Farobie O, Bayu A, Syaftika N, Hartulistiyoso E, Moheimani NR, Karnjanakom S, Matsumura Y. Slow Pyrolysis of Ulva lactuca (Chlorophyta) for Sustainable Production of Bio-Oil and Biochar. Sustainability. 2022; 14(6):3233. https://doi.org/10.3390/su14063233
Chicago/Turabian StyleAmrullah, Apip, Obie Farobie, Asep Bayu, Novi Syaftika, Edy Hartulistiyoso, Navid R. Moheimani, Surachai Karnjanakom, and Yukihiko Matsumura. 2022. "Slow Pyrolysis of Ulva lactuca (Chlorophyta) for Sustainable Production of Bio-Oil and Biochar" Sustainability 14, no. 6: 3233. https://doi.org/10.3390/su14063233
APA StyleAmrullah, A., Farobie, O., Bayu, A., Syaftika, N., Hartulistiyoso, E., Moheimani, N. R., Karnjanakom, S., & Matsumura, Y. (2022). Slow Pyrolysis of Ulva lactuca (Chlorophyta) for Sustainable Production of Bio-Oil and Biochar. Sustainability, 14(6), 3233. https://doi.org/10.3390/su14063233






