The Codevelopment of Mangroves and Infaunal Community Diversity in Response to the Natural Dynamics of Mud Deposition in French Guiana
Abstract
1. Introduction
2. Materials and Methods
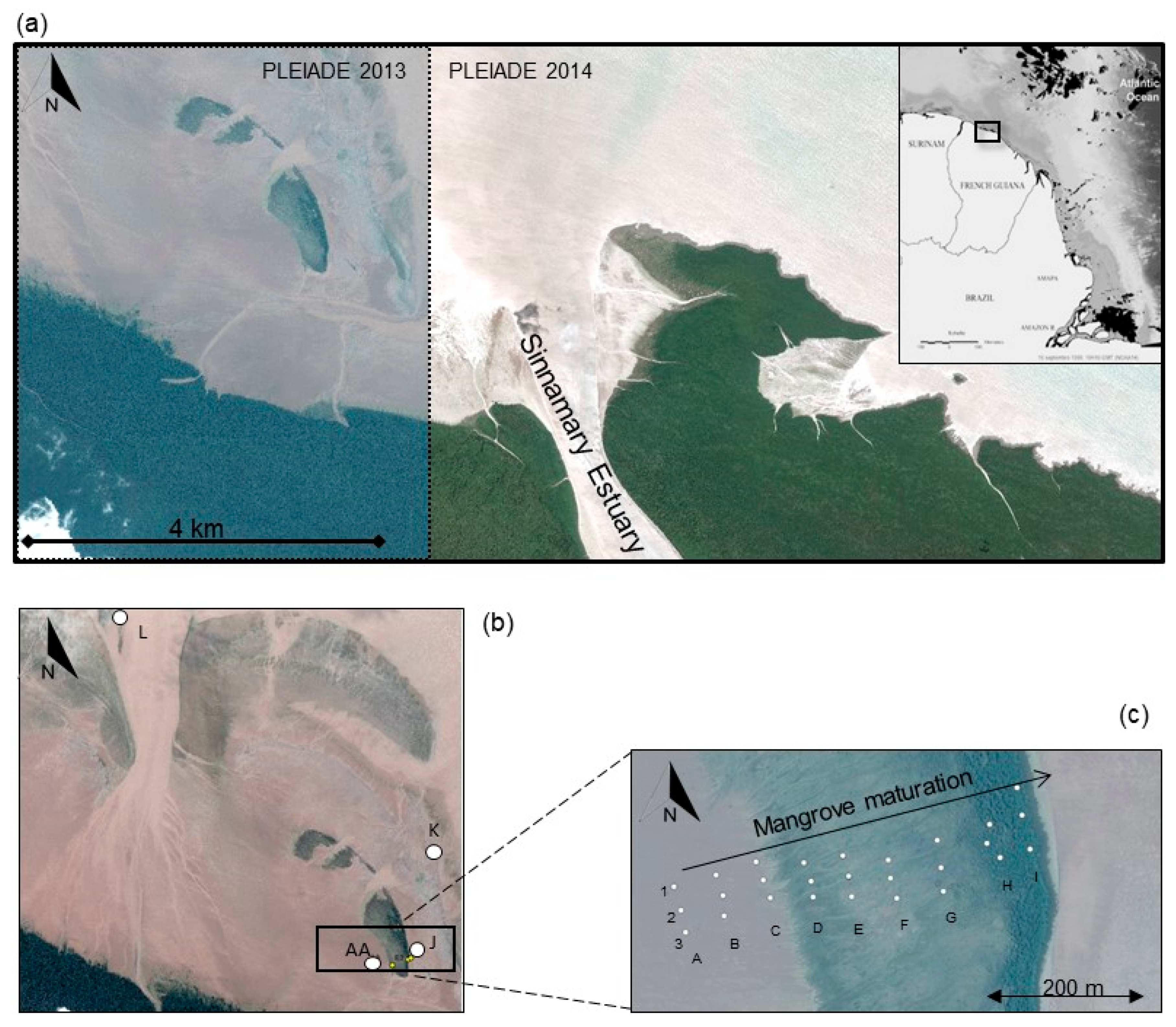
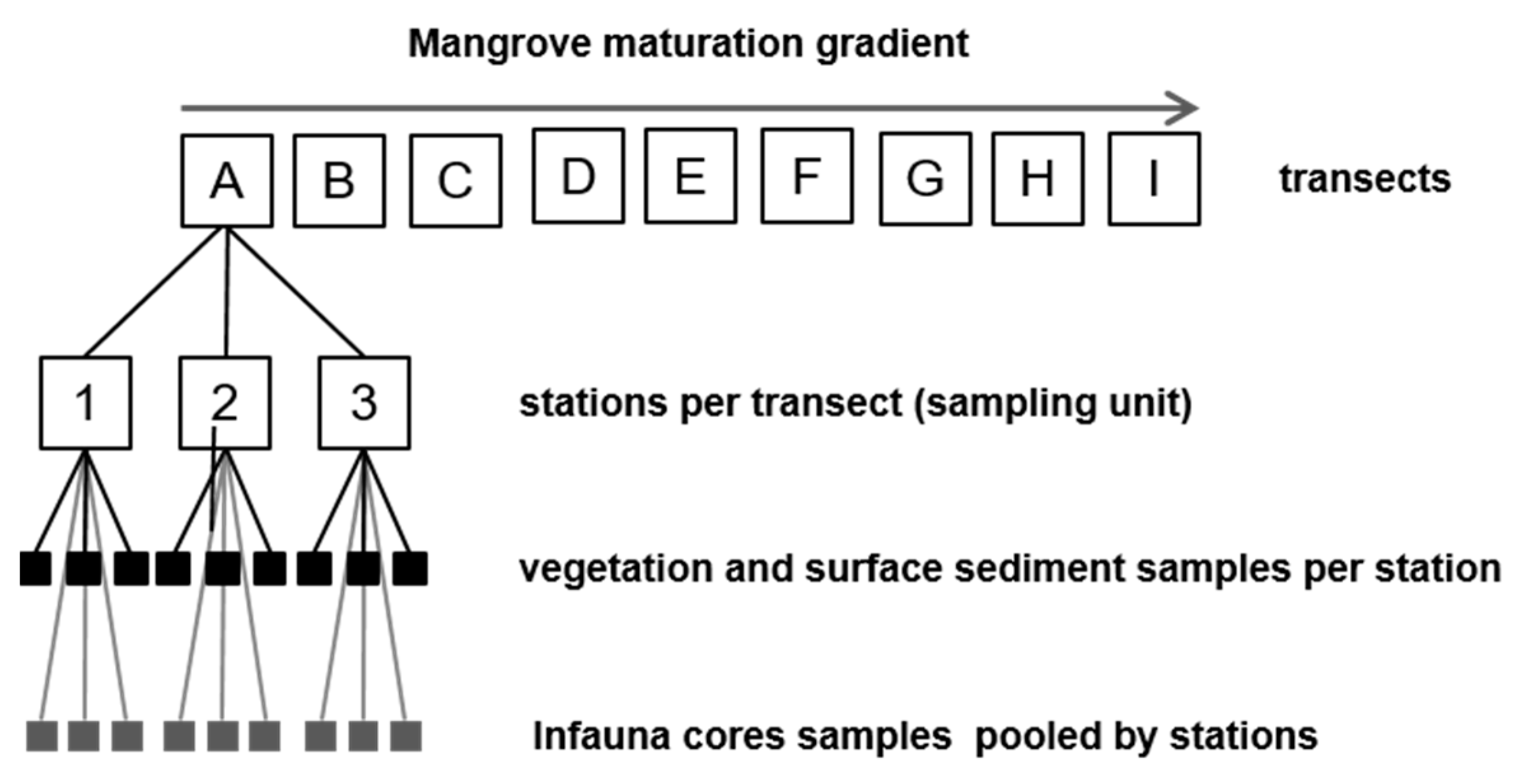
2.1. Study Area and Sampling Sites
2.2. Field Measurements and Analyses
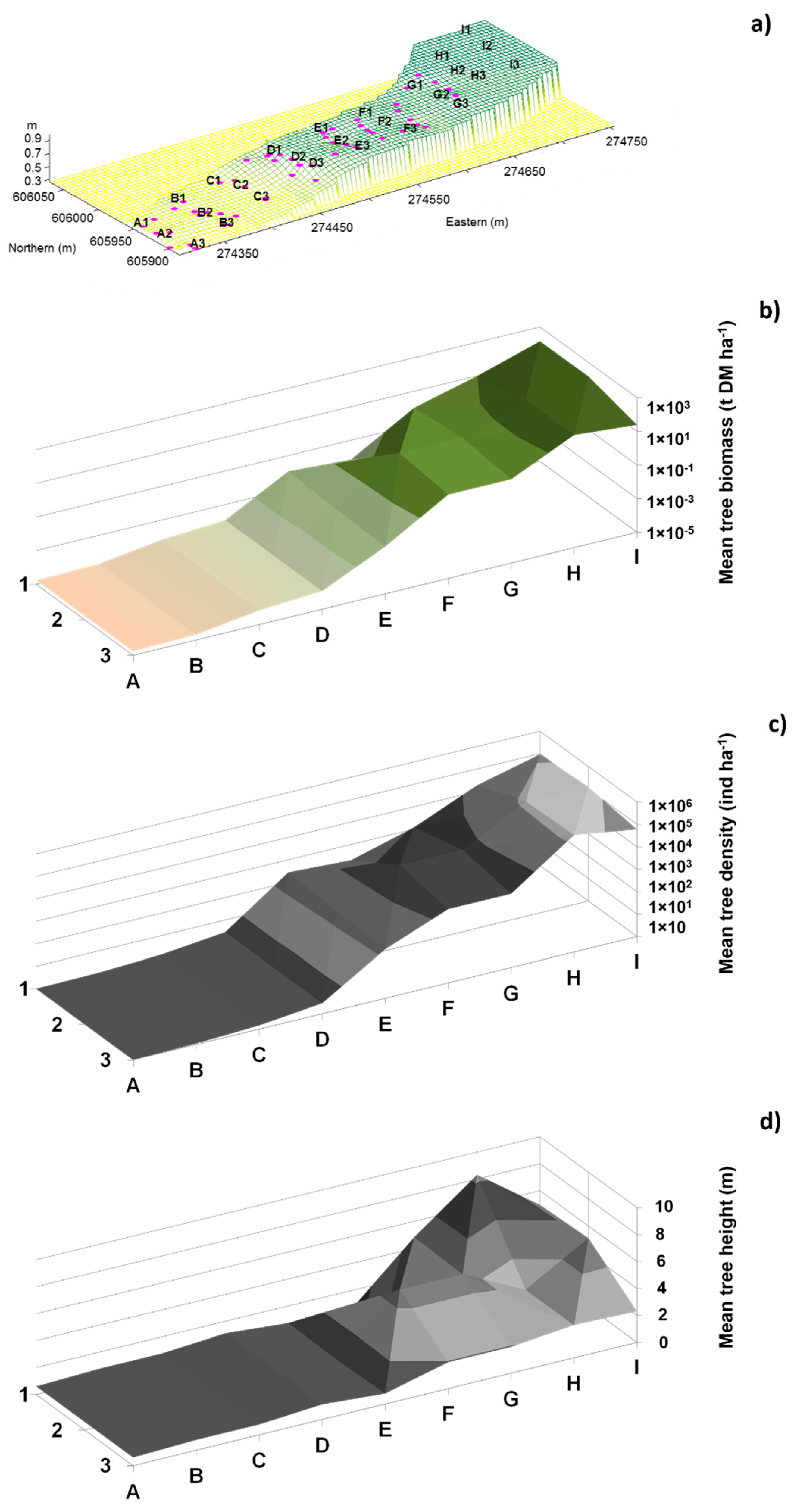
2.2.1. Topographic Measurements
2.2.2. Hydrodynamic Measurements
2.2.3. Vegetation Structure
2.2.4. Sediment Bulk Variables
2.2.5. Benthic Infauna
2.3. Statistical Analyses
3. Results
3.1. Environmental Variables
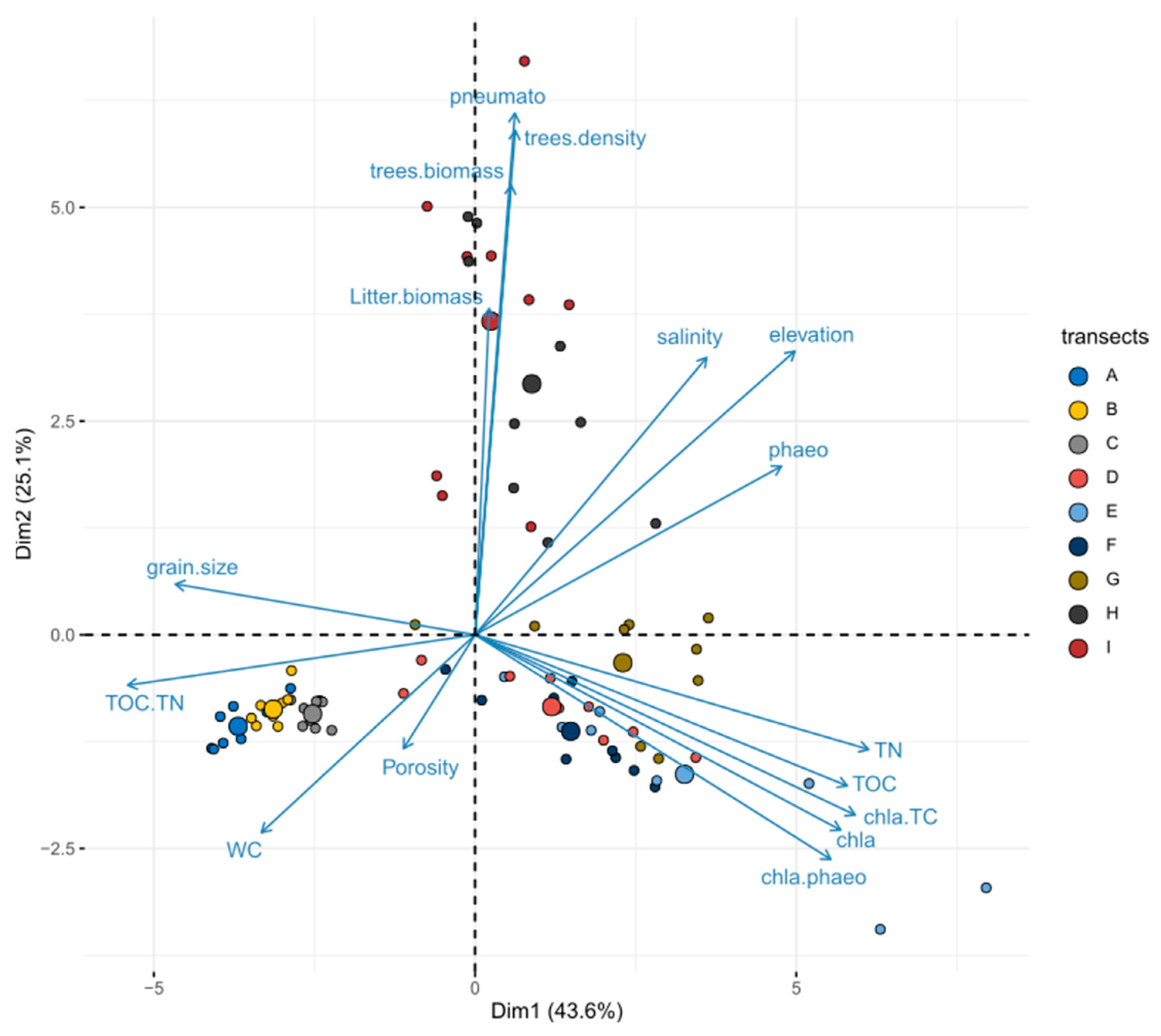
3.2. Validity of the Selected Surface Sediment Variables
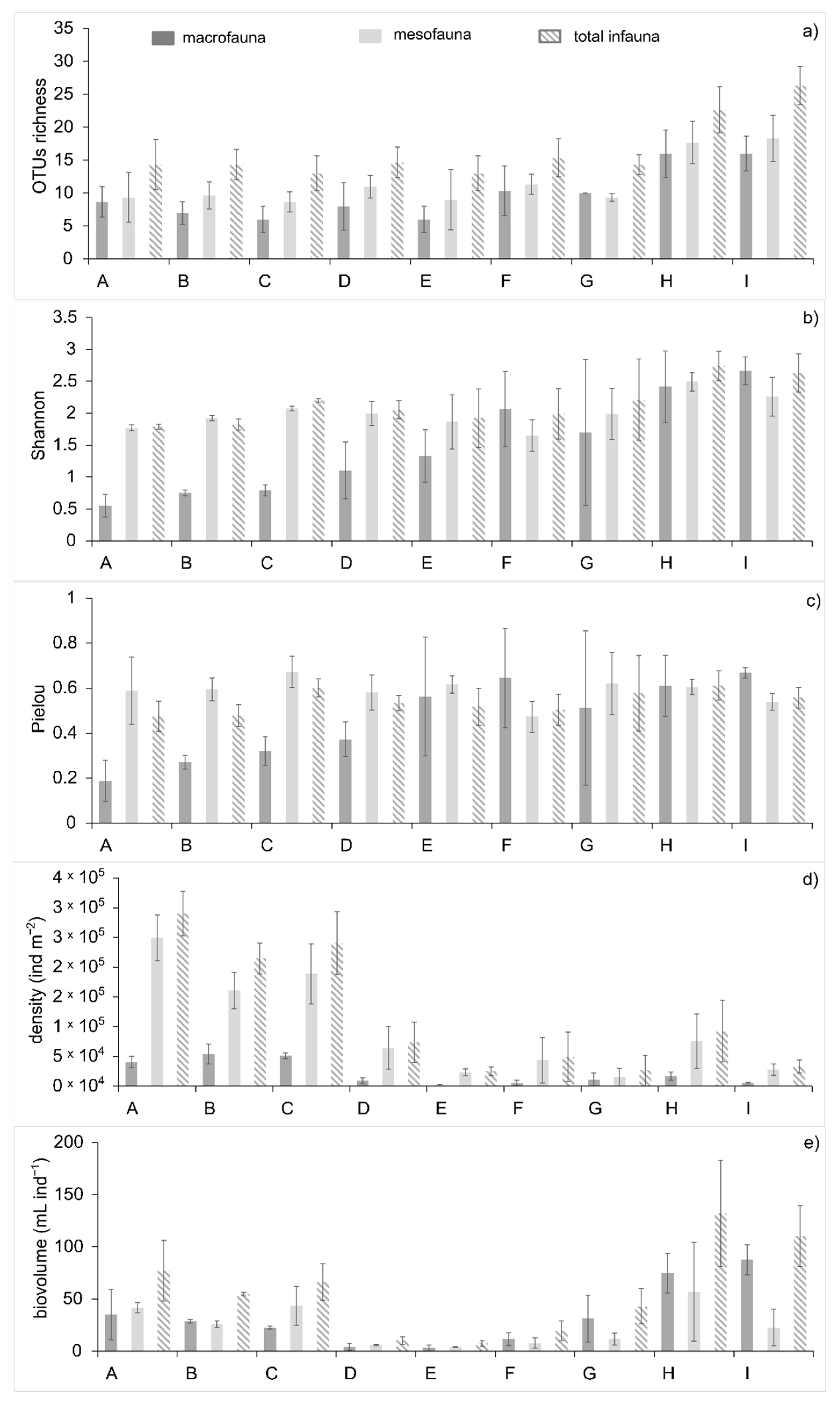
3.3. Infaunal Alpha Diversity
3.4. Infaunal Beta Diversity
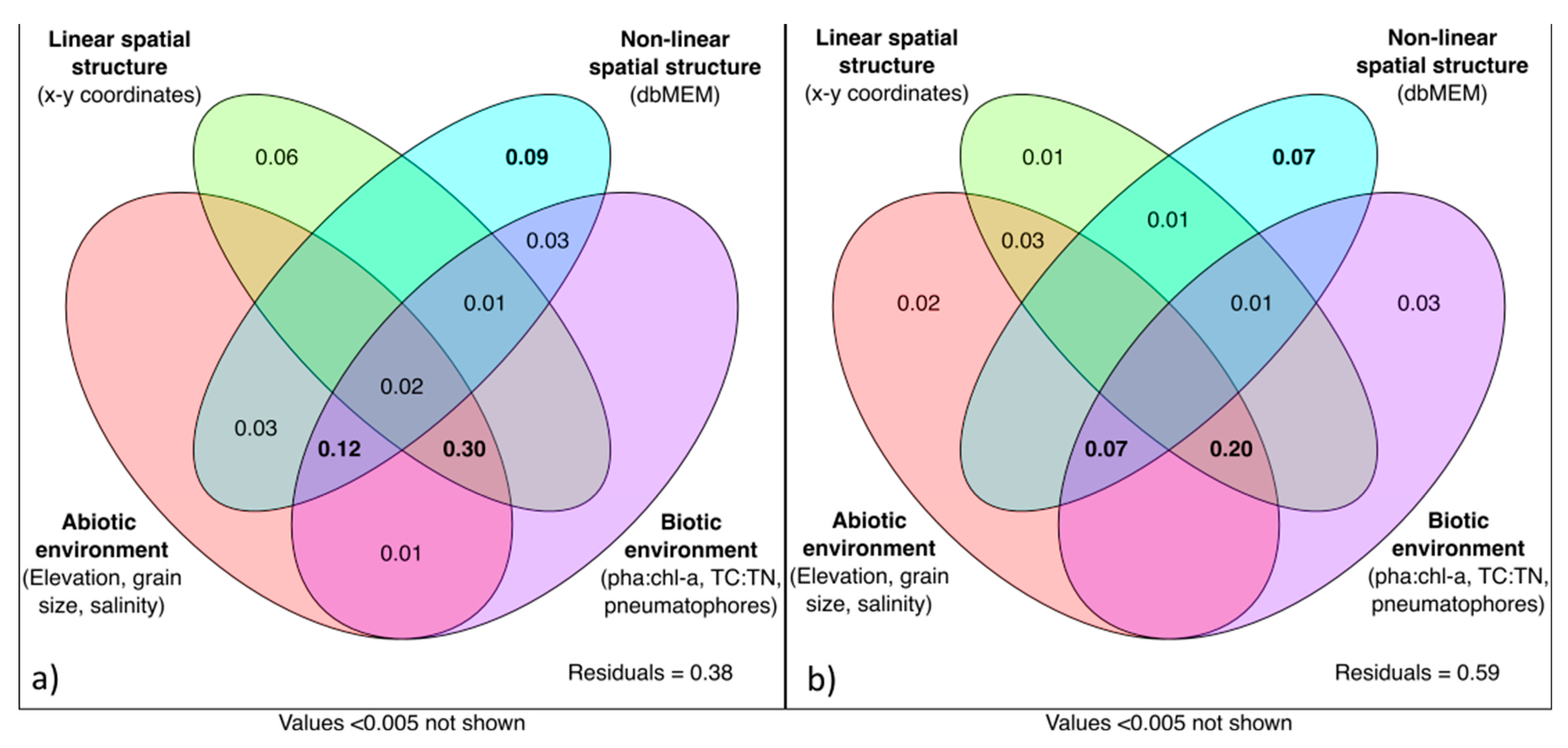
3.5. Disentangling the Effects of Environment Variables on Infaunal Multivariate Responses
4. Discussion
4.1. Environmental Changes versus Young Mangrove Development
4.2. Benthic Biodiversity Spatial Structure versus Young Mangrove Growth
4.3. Benthic Biodiversity Patterns in Highly Dynamic Amazon Influenced Mangroves
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Explanation Selection of Variables Prior to Variance Partitioning Analysis
Appendix A.1. Selection of Spatial Variables
Appendix A.2. Selection of Abiotic Variables
Appendix A.3. Selection of Biotic Variables
References
- Duke, N.C. Mangrove Floristics and Biogeography Revisited: Further Deductions from Biodiversity Hot Spots, Ancestral Discontinuities, and Common Evolutionary Processes. In Mangrove Ecosystems: A Global Biogeographic Perspective: Structure, Function, and Services; Rivera-Monroy, V.H., Lee, S.Y., Kristensen, E., Twilley, R.R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 17–53. ISBN 978-3-319-62206-4. [Google Scholar]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef] [PubMed]
- Cannicci, S.; Burrows, D.; Fratini, S.; Smith, T.J.; Offenberg, J.; Dahdouh-Guebas, F. Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquat. Bot. 2008, 89, 186–200. [Google Scholar] [CrossRef]
- Kristensen, E. Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. J. Sea Res. 2008, 59, 30–43. [Google Scholar] [CrossRef]
- Lee, S.Y. Mangrove macrobenthos: Assemblages, services, and linkages. J. Sea Res. 2008, 59, 16–29. [Google Scholar] [CrossRef]
- Lee, S.Y.; Primavera, J.H.; Dahdouh-Guebas, F.; McKee, K.; Bosire, J.O.; Cannicci, S.; Diele, K.; Fromard, F.; Koedam, N.; Marchand, C.; et al. Ecological role and services of tropical mangrove ecosystems: A reassessment. Glob. Ecol. Biogeogr. 2014, 23, 726–743. [Google Scholar] [CrossRef]
- Hai, N.T.; Dell, B.; Phuong, V.T.; Harper, R.J. Towards a more robust approach for the restoration of mangroves in Vietnam. Ann. For. Sci. 2020, 77, 18. [Google Scholar] [CrossRef]
- Kodikara, K.A.S.; Mukherjee, N.; Jayatissa, L.P.; Dahdouh-Guebas, F.; Koedam, N. Have mangrove restoration projects worked? An in-depth study in Sri Lanka. Restor. Ecol. 2017, 25, 705–716. [Google Scholar] [CrossRef]
- Worthington, T.A.; zu Ermgassen, P.S.E.; Friess, D.A.; Krauss, K.W.; Lovelock, C.E.; Thorley, J.; Tingey, R.; Woodroffe, C.D.; Bunting, P.; Cormier, N.; et al. A global biophysical typology of mangroves and its relevance for ecosystem structure and deforestation. Sci. Rep. 2020, 10, 14652. [Google Scholar] [CrossRef]
- Ellison, A.M. Foundation Species, Non-trophic Interactions, and the Value of Being Common. iScience 2019, 13, 254–268. [Google Scholar] [CrossRef]
- Chapman, M.G.; Tolhurst, T.J. The relationship between invertebrate assemblages and bio-dependant properties of sediment in urbanized temperate mangrove forests. J. Exp. Mar. Biol. Ecol. 2004, 304, 51–73. [Google Scholar] [CrossRef]
- Procheş, Ş.; Marshall, D.J.; Ugrasen, K.; Ramcharan, A. Mangrove pneumatophore arthropod assemblages and temporal patterns. J. Mar. Biol. Assoc. UK 2001, 81, 545–552. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Verneirt, M.; Cannicci, S.; Kairo, J.G.; Tack, J.F.; Koedam, N. An exploratory study on grapsid crab zonation in Kenyan mangroves. Wetl. Ecol. Manag. 2002, 10, 179–187. [Google Scholar] [CrossRef]
- Kon, K.; Kurokura, H.; Tongnunui, P. Influence of a microhabitat on the structuring of the benthic macrofaunal community in a mangrove forest. Hydrobiologia 2011, 671, 205. [Google Scholar] [CrossRef]
- Delfan, N.; Shojaei, M.G.; Naderloo, R. Patterns of structural and functional diversity of macrofaunal communities in a subtropical mangrove ecosystem. Estuar. Coast. Shelf Sci. 2021, 252, 107288. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Zhang, L.; Lu, H.; Ren, H.; Jian, S. Changes in the Macrobenthic Faunal Community during Succession of a Mangrove Forest at Zhanjiang, South China. Coas 2015, 31, 315–325. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Q.; Chen, P.; Lu, H.; Jian, S. Eco-exergy based self-organization of the macrobenthic faunal assemblage during mangrove succession in Zhanjiang, China. Ecol. Indic. 2018, 95, 887–894. [Google Scholar] [CrossRef]
- Metcalfe, K.N.; Glasby, C.J. Diversity of Polychaeta (Annelida) and other worm taxa in mangrove habitats of Darwin Harbour, northern Australia. J. Sea Res. 2008, 59, 70–82. [Google Scholar] [CrossRef]
- Alfaro, A.C. Benthic macro-invertebrate community composition within a mangrove/seagrass estuary in northern New Zealand. Estuar. Coast. Shelf Sci. 2006, 66, 97–110. [Google Scholar] [CrossRef]
- Alongi, D.M. Ecology of tropical soft-bottom benthos: A review with emphasis on emerging concepts. Rev. Biol. Trop. 1989, 37, 85–100. [Google Scholar]
- Dittmann, S. Benthos structure on tropical tidal flats of Australia. Helgo. Meeresunters 1995, 49, 539–551. [Google Scholar] [CrossRef]
- Dittmann, S.; Vargas, J.A. Tropical Tidal Flat Benthos Compared Between Australia and Central America. In Ecological Comparisons of Sedimentary Shores; Reise, K., Ed.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2001; pp. 275–293. ISBN 978-3-642-56557-1. [Google Scholar]
- Reise, K.; Herre, E.; Sturm, M. Biomass and abundance of macrofauna in intertidal sediments of Konigshafen in the northern Wadden Sea. Helgo. Meeresunters 1994, 48, 201–205. [Google Scholar] [CrossRef]
- Anthony, E.J.; Gardel, A.; Gratiot, N.; Proisy, C.; Allison, M.A.; Dolique, F.; Fromard, F. The Amazon-influenced muddy coast of South America: A review of mud-bank–shoreline interactions. Earth-Sci. Rev. 2010, 103, 99–121. [Google Scholar] [CrossRef]
- Walcker, R.; Anthony, E.J.; Cassou, C.; Aller, R.C.; Gardel, A.; Proisy, C.; Martinez, J.-M.; Fromard, F. Fluctuations in the extent of mangroves driven by multi-decadal changes in North Atlantic waves. J. Biogeogr. 2015, 42, 2209–2219. [Google Scholar] [CrossRef]
- Orseau, S.; Abascal Zorrilla, N.; Huybrechts, N.; Lesourd, S.; Gardel, A. Decadal-scale morphological evolution of a muddy open coast. Mar. Geol. 2020, 420, 106048. [Google Scholar] [CrossRef]
- Fromard, F.; Puig, H.; Mougin, E.; Marty, G.; Betoulle, J.L.; Cadamuro, L. Structure, above-ground biomass and dynamics of mangrove ecosystems: New data from French Guiana. Oecologia 1998, 115, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Proisy, C.; Gratiot, N.; Anthony, E.J.; Gardel, A.; Fromard, F.; Heuret, P. Mud bank colonization by opportunistic mangroves: A case study from French Guiana using lidar data. Cont. Shelf Res. 2009, 29, 632–641. [Google Scholar] [CrossRef]
- Marchand, C.; Lallier-Vergès, E.; Baltzer, F. The composition of sedimentary organic matter in relation to the dynamic features of a mangrove-fringed coast in French Guiana. Estuar. Coast. Shelf Sci. 2003, 56, 119–130. [Google Scholar] [CrossRef]
- Swennen, C.; Duiven, P.; Spaans, A.L. Numerical density and biomass of macrobenthic animals living in the intertidal zone of Surinam, South America. Neth. J. Sea Res. 1982, 15, 406–418. [Google Scholar] [CrossRef]
- Aller, J.Y.; Stupakoff, I. The distribution and seasonal characteristics of benthic communities on the Amazon shelf as indicators of physical processes. Cont. Shelf Res. 1996, 16, 717–751. [Google Scholar] [CrossRef]
- Jourde, J.; Dupuy, C.; Nguyen, H.T.; Mizrahi, D.; de Pracontal, N.; Bocher, P. Low Benthic Macrofauna Diversity in Dynamic, Tropical Tidal Mudflats: Migrating Banks on Guiana’s Coast, South America. Estuaries Coasts 2017, 40, 1159–1170. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Dupuy, C.; Jourde, J.; Lefrançois, C.; Pascal, P.-Y.; Carpentier, A.; Chevalier, J.; Bocher, P. Persistent benthic communities in the extreme dynamic intertidal mudflats of the Amazonian coast: An overview of the Tanaidacea (Crustacea, Peracarida). Mar. Biodivers. 2018, 48, 1841–1853. [Google Scholar] [CrossRef]
- Aschenbroich, A.; Michaud, E.; Stieglitz, T.; Fromard, F.; Gardel, A.; Tavares, M.; Thouzeau, G. Brachyuran crab community structure and associated sediment reworking activities in pioneer and young mangroves of French Guiana, South America. Estuar. Coast. Shelf Sci. 2016, 182, 60–71. [Google Scholar] [CrossRef]
- Aschenbroich, A.; Michaud, E.; Gilbert, F.; Fromard, F.; Alt, A.; Le Garrec, V.; Bihannic, I.; De Coninck, A.; Thouzeau, G. Bioturbation functional roles associated with mangrove development in French Guiana, South America. Hydrobiologia 2017, 794, 179–202. [Google Scholar] [CrossRef]
- Brunier, G.; Anthony, E.J.; Gratiot, N.; Gardel, A. Exceptional rates and mechanisms of muddy shoreline retreat following mangrove removal. Earth Surf. Processes Landf. 2019, 44, 1559–1571. [Google Scholar] [CrossRef]
- Anthony, E.J.; Gratiot, N. Coastal engineering and large-scale mangrove destruction in Guyana, South America: Averting an environmental catastrophe in the making. Ecol. Eng. 2012, 47, 268–273. [Google Scholar] [CrossRef]
- Toorman, E.A.; Anthony, E.; Augustinus, P.G.E.F.; Gardel, A.; Gratiot, N.; Homenauth, O.; Huybrechts, N.; Monbaliu, J.; Moseley, K.; Naipal, S. Interaction of Mangroves, Coastal Hydrodynamics, and Morphodynamics along the Coastal Fringes of the Guianas. In Threats to Mangrove Forests: Hazards, Vulnerability, and Management; Makowski, C., Finkl, C.W., Eds.; Coastal Research Library; Springer International Publishing: Cham, Switzerland, 2018; pp. 429–473. ISBN 978-3-319-73016-5. [Google Scholar]
- Filho, J.S.R.; Aviz, D. Macrobenthic communities of an Amazonian estuary (Guajará Bay, Brazil): Temporal and spatial changes. Coas 2013, 65, 123–128. [Google Scholar] [CrossRef]
- Bernardino, A.F.; Netto, S.A.; Pagliosa, P.R.; Barros, F.; Christofoletti, R.A.; Rosa Filho, J.S.; Colling, A.; Lana, P.C. Predicting ecological changes on benthic estuarine assemblages through decadal climate trends along Brazilian Marine Ecoregions. Estuar. Coast. Shelf Sci. 2015, 166, 74–82. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Fromard, F.; Vega, C.; Proisy, C. Half a century of dynamic coastal change affecting mangrove shorelines of French Guiana. A case study based on remote sensing data analyses and field surveys. Mar. Geol. 2004, 208, 265–280. [Google Scholar] [CrossRef]
- MacIntyre, H.L.; Geider, R.J.; Miller, D.C. Microphytobenthos: The ecological role of the “secret garden” of unvegetated, shallow-water marine habitats. I. Distribution, abundance and primary production. Estuaries 1996, 19, 186–201. [Google Scholar] [CrossRef]
- Marchand, C.; Baltzer, F.; Lallier-Vergès, E.; Albéric, P. Pore-water chemistry in mangrove sediments: Relationship with species composition and developmental stages (French Guiana). Mar. Geol. 2004, 208, 361–381. [Google Scholar] [CrossRef]
- Riviere, A. Méthodes Granulométriques-Techniques et Interprétations; Masson: Paris, France, 1977. [Google Scholar]
- Riaux-Gobin, C.; Klein, B. Microphytobenthic Biomass Measurement Using HPLC and Conventional Pigment Analysis. In Handbook of Methods in Aquatic Microbial Ecology; CRC Press: Boca Raton, FL, USA, 1993; ISBN 978-0-203-75274-6. [Google Scholar]
- Lund, J.W.G.; Kipling, C.; Le Cren, E.D. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Hasle, G.R. Using the Inverted Microscope. In Phytoplankton Manual; Sournia, A., Ed.; UNESCO: Paris, France, 1978; pp. 191–196. [Google Scholar]
- Warwick, R.M.; Gee, J.M. Community structure of estuarine meiobenthos. Mar. Ecol. Prog. Ser. 1984, 18, 97–111. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). Wiley StatsRef Stat. Ref. Online 2017, 1–15. [Google Scholar] [CrossRef]
- Young, S.G.; Bowman, A.W. Non-Parametric Analysis of Covariance. Biometrics 1995, 51, 920–931. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically Meaningful Transformations for Ordination of Species Data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Dray, S.; Legendre, P.; Peres-Neto, P.R. Spatial modelling: A comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol. Model. 2006, 196, 483–493. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P. Environmental control and spatial structure in ecological communities: An example using oribatid mites (Acari, Oribatei). Environ. Ecol Stat. 1994, 1, 37–61. [Google Scholar] [CrossRef]
- Gauch, H.G. Prediction, Parsimony and Noise. Am. Sci. 1993, 81, 468–478. [Google Scholar]
- Blanchet, F.G.; Legendre, P.; Borcard, D. Forward Selection of Explanatory Variables. Ecology 2008, 89, 2623–2632. [Google Scholar] [CrossRef]
- R: A language and Environment for Statistical Computing. Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 29 November 2021).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package R; R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 February 2022).
- Dray, S.; Bauman, D.; Blanchet, G.; Borcard, D.; Clappe, S.; Guenard, G.; Jombart, T.; Larocque, G.; Legendre, P.; Madi, N.; et al. Adespatial: Multivariate Multiscale Spatial Analysis; R Package Version 0.3-14. 2021. Available online: https://CRAN.R-project.org/package=adespatial (accessed on 15 February 2022).
- Saenger, P.; Snedaker, S.C. Pantropical Trends in Mangrove Above-Ground Biomass and Annual Litterfall. Oecologia 1993, 96, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.C.; Heilbrun, C.; Panzeca, C.; Zhu, Z.; Baltzer, F. Coupling between sedimentary dynamics, early diagenetic processes, and biogeochemical cycling in the Amazon–Guianas mobile mud belt: Coastal French Guiana. Mar. Geol. 2004, 208, 331–360. [Google Scholar] [CrossRef]
- Fabre, A.; Fromard, F.R.; Trichon, V. Fractionation of phosphate in sediments of four representative mangrove stages (French Guiana). Hydrobiologia 1999, 392, 13–19. [Google Scholar] [CrossRef]
- Brunier, G.; Michaud, E.; Fleury, J.; Anthony, E.J.; Morvan, S.; Gardel, A. Assessing the relationship between macro-faunal burrowing activity and mudflat geomorphology from UAV-based Structure-from-Motion photogrammetry. Remote Sens. Environ. 2020, 241, 111717. [Google Scholar] [CrossRef]
- Anthony, E.J. Sediment dynamics and morphological stability of estuarine mangrove swamps in Sherbro Bay, West Africa. Mar. Geol. 2004, 208, 207–224. [Google Scholar] [CrossRef]
- Reef, R.; Feller, I.C.; Lovelock, C.E. Nutrition of mangroves. Tree Physiol. 2010, 30, 1148–1160. [Google Scholar] [CrossRef]
- Sylvestre, F.; Guiral, D.; Debenay, J.P. Modern diatom distribution in mangrove swamps from the Kaw Estuary (French Guiana). Mar. Geol. 2004, 208, 281–293. [Google Scholar] [CrossRef]
- Alvarenga, D.O.; Rigonato, J.; Branco, L.H.Z.; Fiore, M.F. Cyanobacteria in mangrove ecosystems. Biodivers. Conserv. 2015, 24, 799–817. [Google Scholar] [CrossRef]
- Rigonato, J.; Kent, A.D.; Alvarenga, D.O.; Andreote, F.D.; Beirigo, R.M.; Vidal-Torrado, P.; Fiore, M.F. Drivers of cyanobacterial diversity and community composition in mangrove soils in south-east Brazil. Environ. Microbiol. 2013, 15, 1103–1114. [Google Scholar] [CrossRef]
- Bacescu, M.; Gutu, M. A new genus (Discapseudes N.G.) and three new species of Apseudidae (Crustacea, Tanaidacea) from the Northeastern coast of South America. Zool. Meded. 1975, 49, 95–113. [Google Scholar]
- Raffaelli, D.; Hawkins, S. Intertidal Ecology; Chapman and Hall: London, UK; Cambridge University Press: London, UK, 1996; Volume 77. [Google Scholar]
- de Lucas Pardo, M.A.; Bakker, M.; van Kessel, T.; Cozzoli, F.; Winterwerp, J.C. Erodibility of soft freshwater sediments in Markermeer: The role of bioturbation by meiobenthic fauna. Ocean. Dyn. 2013, 63, 1137–1150. [Google Scholar] [CrossRef]
- Thorp, J.H.; Covich, A.P. Ecology and Classification of North American Freshwater Invertebrates, 3rd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2009; ISBN 9780080889818. [Google Scholar]
- Blazewicz-Paszkowycz, M.; Bamber, R.; Anderson, G. Diversity of Tanaidacea (Crustacea: Peracarida) in the World’s Oceans–How Far Have We Come? PLoS ONE 2012, 7, e33068. [Google Scholar]
- Drumm, D.T. Comparison of Feeding Mechanisms, Respiration, and Cleaning Behavior in Two Kalliapseudids, Kalliapseudes Macsweenyi and Psammokalliapseudes Granulosus (Peracarida: Tanaidacea). J. Crustacean Biol. 2005, 25, 203–211. [Google Scholar] [CrossRef][Green Version]
- Hussain, S.M.; Noohu Nazeer, M.; Radhakrishnan, K.; Rajkumar, A.; Sivapriya, V. Mangrove Ostracoda species fluctuations, habitual adaptation, and its environmental implications—A review. In Holocene Climate Change and Environment; Kumaran, N., Damodara, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 429–440. ISBN 978-0-323-90085-0. [Google Scholar]
- Helal, S.A.; El-Wahab, M.A. Distribution of podocopid ostracods in mangrove ecosystems along the Egyptian Red Sea Coast. Crustaceana 2012, 85, 1669–1696. [Google Scholar]
- Erséus, C. Mangroves and marine oligochaete diversity. Wetl. Ecol. Manag. 2002, 10, 197–202. [Google Scholar] [CrossRef]
- Giere, O. Ecology and Biology of Marine Oligochaeta–an Inventory Rather than another Review. Hydrobiologia 2006, 564, 103–116. [Google Scholar] [CrossRef]
- De Wit, R.; Jonkers, H.M.; Van Den Ende, F.P.; Van Gemerden, H. In situ fluctuations of oxygen and sulphide in marine microbial sediment ecosystems. Neth. J. Sea Res. 1989, 23, 271–281. [Google Scholar] [CrossRef]
- Stal, L.J.; van Gemerden, H.; Krumbein, W.E. Structure and development of a benthic marine microbial mat. FEMS Microbiol. Lett. 1985, 31, 111–125. [Google Scholar] [CrossRef]
- Brown, M.R. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1991, 145, 79–99. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Role of Cyanobacterial Exopolysaccharides in Phototrophic Biofilms and in Complex Microbial Mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef] [PubMed]
- Debenay, J.-P.; Jouanneau, J.-M.; Sylvestre, F.; Weber, O.; Guiral, D. Biological Origin of Rhythmites in Muddy Sediments of French Guiana. Coas 2007, 2007, 1431–1442. [Google Scholar] [CrossRef]
- Gensac, E.; Gardel, A.; Lesourd, S.; Brutier, L. Morphodynamic evolution of an intertidal mudflat under the influence of Amazon sediment supply–Kourou mud bank, French Guiana, South America. Estuar. Coast. Shelf Sci. 2015, 158, 53–62. [Google Scholar] [CrossRef]
- Norkko, A.; Bonsdorff, E. Population responses of coastal zoobenthos to stress induced by drifting algal mats. Mar. Ecol. Prog. Ser. 1996, 140, 141–151. [Google Scholar] [CrossRef]
- DeMott, W.R.; Moxter, F. Foraging Cyanobacteria by Copepods: Responses to Chemical Defense and Resource Abundance. Ecology 1991, 72, 1820–1834. [Google Scholar] [CrossRef]
- Hogfors, H.; Motwani, N.H.; Hajdu, S.; El-Shehawy, R.; Holmborn, T.; Vehmaa, A.; Engström-Öst, J.; Brutemark, A.; Gorokhova, E. Bloom-Forming Cyanobacteria Support Copepod Reproduction and Development in the Baltic Sea. PLoS ONE 2014, 9, e112692. [Google Scholar] [CrossRef]
- To-orn, N. Distribution and Density of Polychaetes in Tha Chin Mangrove Estuary, Samut Sakhon Province. Burapha Sci. J. 2020, 25, 1120–1135. [Google Scholar]
- Odum, W.E.; Heald, E.J. Trophic analyses of an estuarine mangrove community. Bull. Mar. Sci. 1972, 22, 671–738. [Google Scholar]
- Torreias, S.R.S.; Ferreira-Keppler, R.L.; Ronderos, M.M. Biting midges (Ceratopogonidae: Diptera) present in aquatic macrophytes from wetlands of Marchantaria Island, Iranduba, Central Amazonia, Brazil. J. Nat. Hist. 2014, 48, 109–122. [Google Scholar] [CrossRef]
- Freitas-Júnior, F.; Christoffersen, M.L.; de Araújo, J.P.; Branco, J.O. Spatiotemporal Distribution and Population Structure of Monokalliapseudes schubarti (Tanaidacea: Kalliapseudidae) in an Estuary in Southern Brazil. Sci. World J. 2013, 2013, e363187. [Google Scholar] [CrossRef]
- Primavera, J.H. Fish predation on mangrove-associated penaeids: The role of structures and substrate. J. Exp. Mar. Biol. Ecol. 1997, 215, 205–216. [Google Scholar] [CrossRef]
- Reinsel, K.A. Impact of fiddler crab foraging and tidal inundation on an intertidal sandflat: Season-dependent effects in one tidal cycle. J. Exp. Mar. Biol. Ecol. 2004, 313, 1–17. [Google Scholar] [CrossRef]
- Graal, J.; Coic, N. Synthèse Des Méthodes D’évaluation De La Qualité Du Benthos En Milieu Côtier Rebent; Ifremer: Brest, France, 2006; p. 90. [Google Scholar]
- Michelet, C.; Zeppilli, D.; Hubas, C.; Baldrighi, E.; Cuny, P.; Dirberg, G.; Militon, C.; Walcker, R.; Lamy, D.; Jézéquel, R.; et al. First Assessment of the Benthic Meiofauna Sensitivity to Low Human-Impacted Mangroves in French Guiana. Forests 2021, 12, 338. [Google Scholar] [CrossRef]
- Chen, G.-C.; Ye, Y.; Lu, C.-Y. Changes of macro-benthic faunal community with stand age of rehabilitated Kandelia candel mangrove in Jiulongjiang Estuary, China. Ecol. Eng. 2007, 31, 215–224. [Google Scholar] [CrossRef]
- Ward, J.V.; Tockner, K.; Schiemer, F. Biodiversity of floodplain river ecosystems: Ecotones and connectivity1. Regul. Rivers Res. Manag. 1999, 15, 125–139. [Google Scholar] [CrossRef]
- Martin, B.; Ferrer, M. Temporally variable environments maintain more beta-diversity in Mediterranean landscapes. Acta Oecologica 2015, 68, 1–10. [Google Scholar] [CrossRef]
- Sasekumar, A.; Chong, V.C. Faunal diversity in Malaysian mangroves. Glob. Ecol. Biogeogr. Lett. 1998, 7, 57–60. [Google Scholar] [CrossRef]
- Bosire, J.O.; Dahdouh-Guebas, F.; Walton, M.; Crona, B.I.; Lewis, R.R.; Field, C.; Kairo, J.G.; Koedam, N. Functionality of restored mangroves: A review. Aquat. Bot. 2008, 89, 251–259. [Google Scholar] [CrossRef]
- Chen, G.C.; Ye, Y. Restoration of Aegiceras corniculatum mangroves in Jiulongjiang Estuary changed macro-benthic faunal community. Ecol. Eng. 2011, 37, 224–228. [Google Scholar] [CrossRef]
- Morrisey, D.J.; Skilleter, G.A.; Ellis, J.I.; Burns, B.R.; Kemp, C.E.; Burt, K. Differences in benthic fauna and sediment among mangrove (Avicennia marina var. australasica) stands of different ages in New Zealand. Estuar. Coast. Shelf Sci. 2003, 56, 581–592. [Google Scholar] [CrossRef]
- Holling, C.S. Resilience and Stability of Ecological Systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michaud, E.; Aschenbroich, A.; Gauthier, O.; Fromard, F.; Aller, J.Y.; Aller, R.C.; Brunier, G.; Anthony, E.J.; Gardel, A.; Le Garrec, V.; et al. The Codevelopment of Mangroves and Infaunal Community Diversity in Response to the Natural Dynamics of Mud Deposition in French Guiana. Sustainability 2022, 14, 2829. https://doi.org/10.3390/su14052829
Michaud E, Aschenbroich A, Gauthier O, Fromard F, Aller JY, Aller RC, Brunier G, Anthony EJ, Gardel A, Le Garrec V, et al. The Codevelopment of Mangroves and Infaunal Community Diversity in Response to the Natural Dynamics of Mud Deposition in French Guiana. Sustainability. 2022; 14(5):2829. https://doi.org/10.3390/su14052829
Chicago/Turabian StyleMichaud, Emma, Adélaïde Aschenbroich, Olivier Gauthier, François Fromard, Josephine Y. Aller, Robert C. Aller, Guillaume Brunier, Edward J. Anthony, Antoine Gardel, Vincent Le Garrec, and et al. 2022. "The Codevelopment of Mangroves and Infaunal Community Diversity in Response to the Natural Dynamics of Mud Deposition in French Guiana" Sustainability 14, no. 5: 2829. https://doi.org/10.3390/su14052829
APA StyleMichaud, E., Aschenbroich, A., Gauthier, O., Fromard, F., Aller, J. Y., Aller, R. C., Brunier, G., Anthony, E. J., Gardel, A., Le Garrec, V., Leynaert, A., & Thouzeau, G. (2022). The Codevelopment of Mangroves and Infaunal Community Diversity in Response to the Natural Dynamics of Mud Deposition in French Guiana. Sustainability, 14(5), 2829. https://doi.org/10.3390/su14052829






