Sustainable Development: Use of Agricultural Waste Materials for Vanillic Acid Recovery from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Adsorbent Materials
2.3. Procedure for Adsorption Isotherms Assays
2.4. Determination of Vanillic Acid by Fluorescence Spectroscopy
2.5. Theoretical Background for Adsorption Isotherms Models
3. Results and Discussion
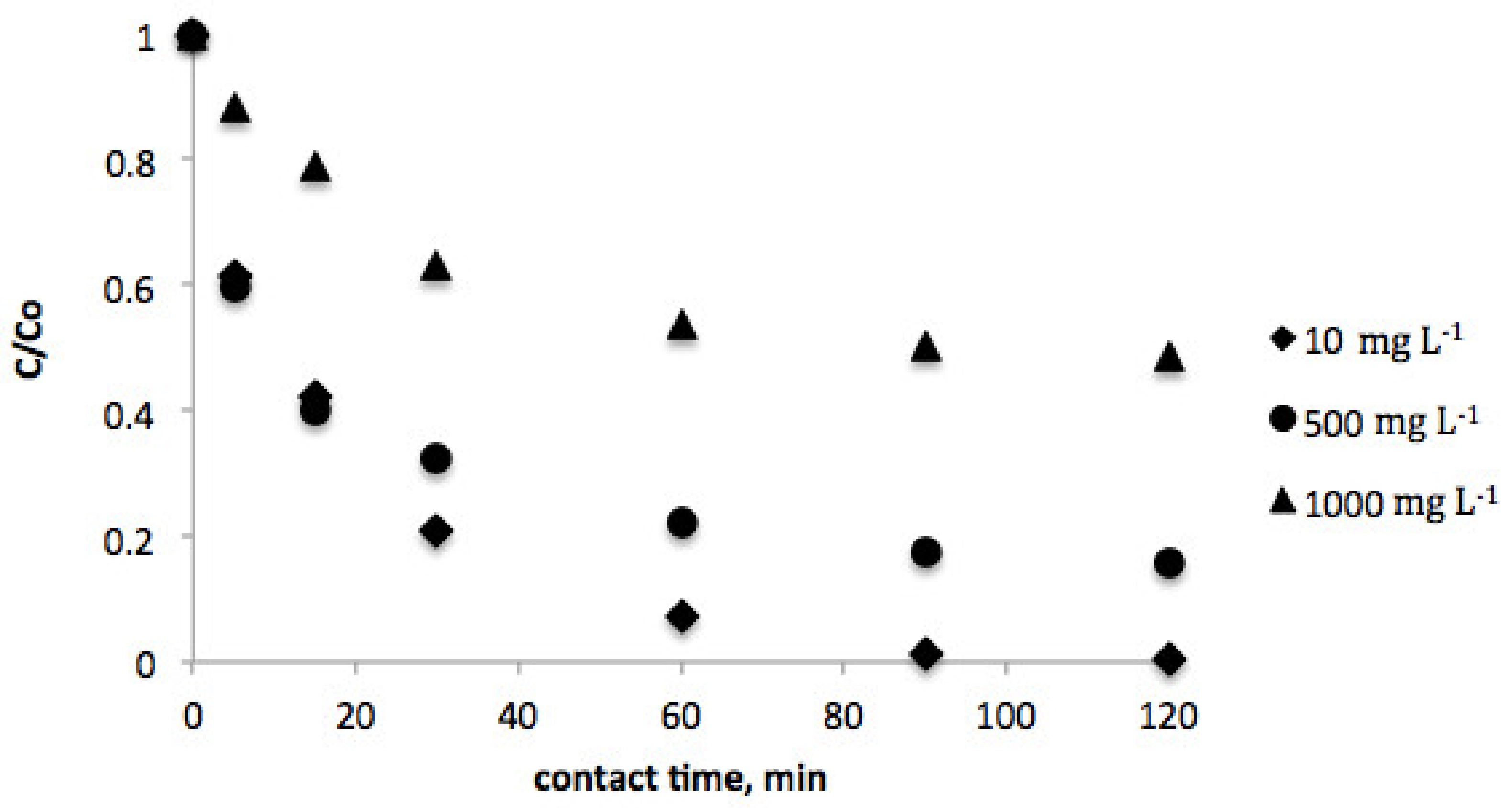
3.1. Effect of Contact Time
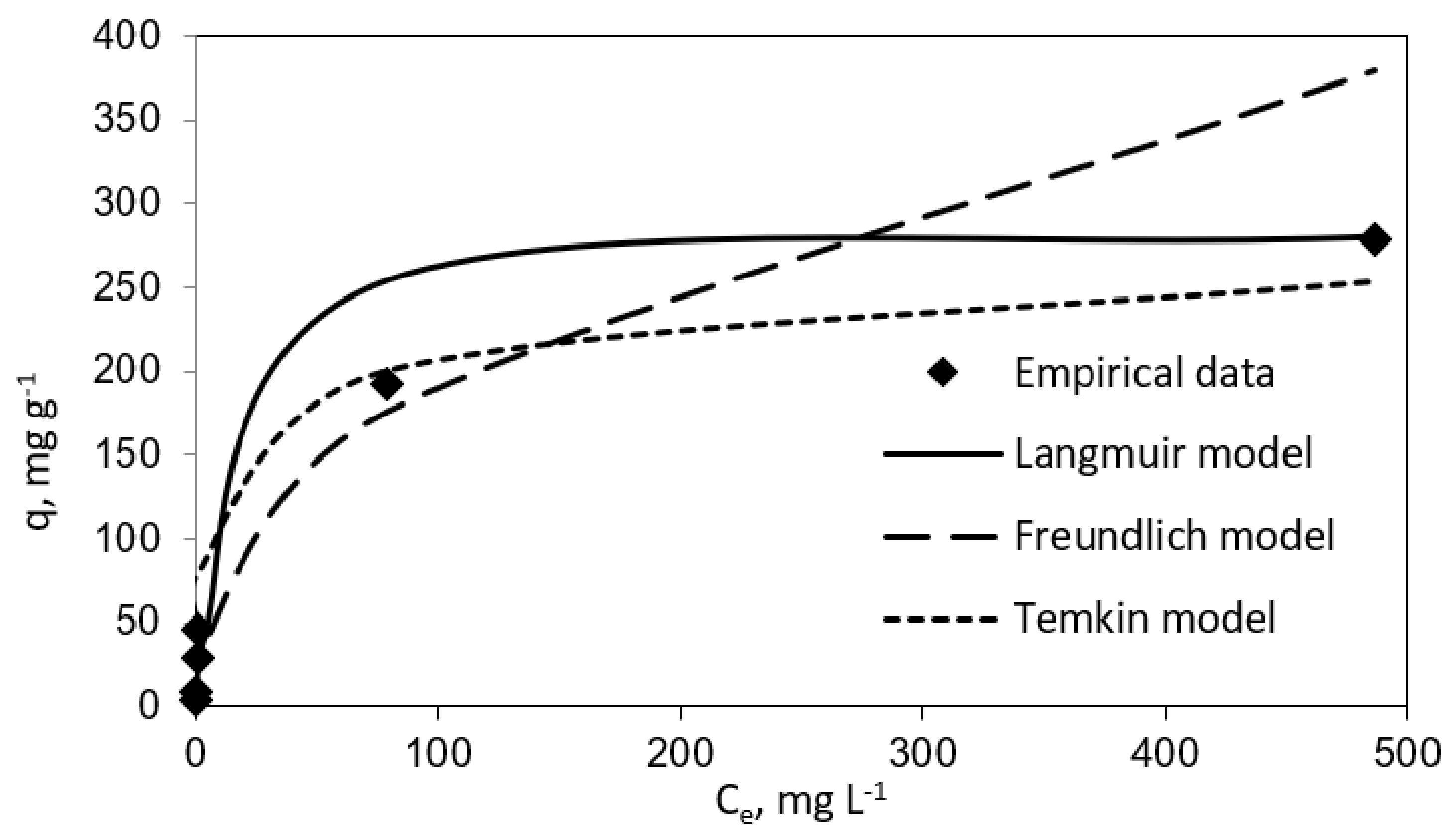
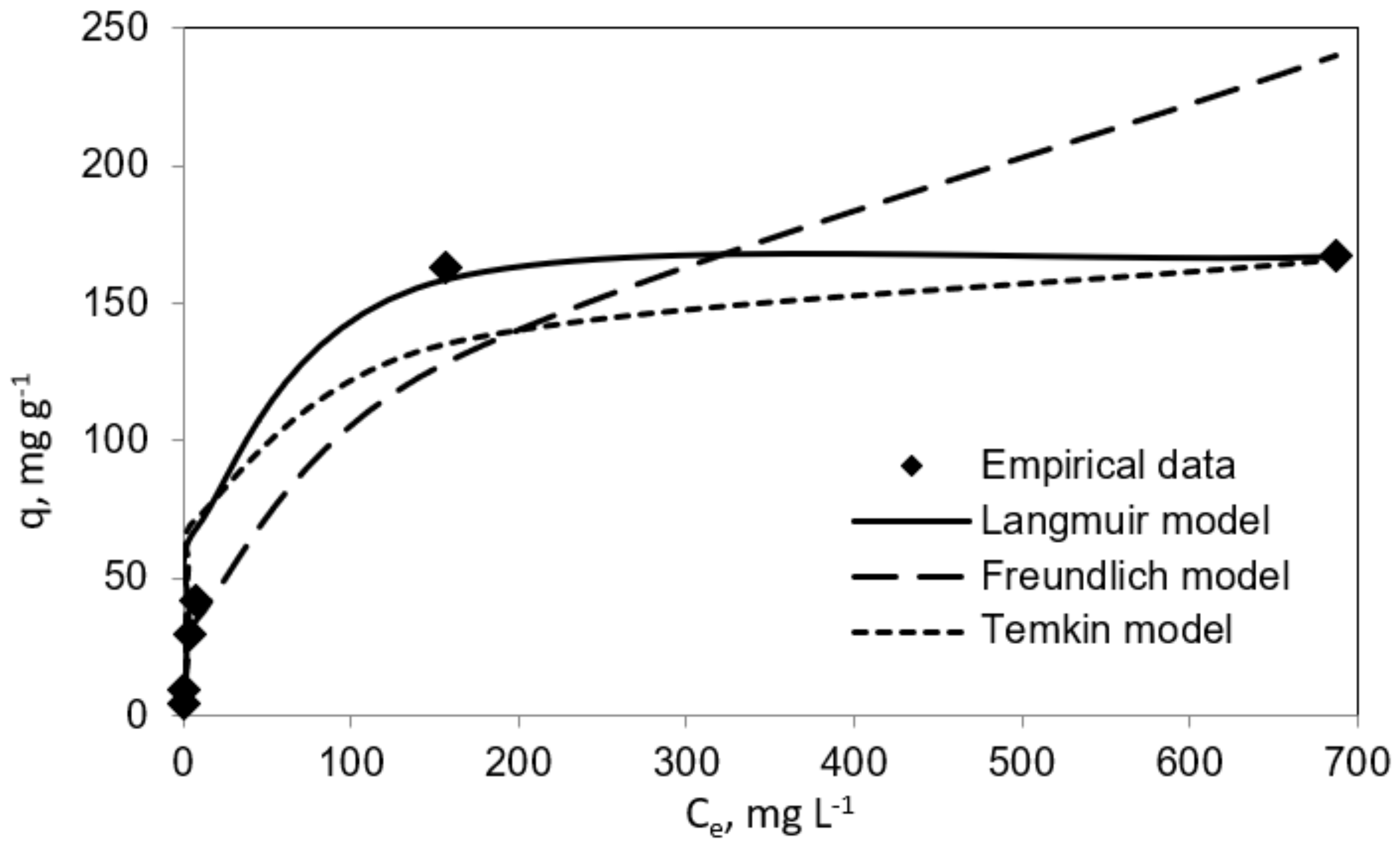
3.2. Adsorption Isotherms Studies
3.3. Gibbs Free Energy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jain, A.K.; Gupta, V.K.; Jain, V.K.; Suhas, S. Removal of chlorophenols using industrial wastes. Environ. Sci. Technol. 2004, 38, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Bazrafshan, E.; Mostafapour, F.K.; Mahvi, A.H. Phenol removal from aqueous solutions using pistachio-nut shell ash as a low cost adsorbent. Fresenius Environ. Bull. 2012, 21, 2962–2968. [Google Scholar]
- Medeiros, M.C.; dos Santos, E.V.; Martínez-Huitle, C.A.; Fajardo, A.S.; Castro, S.S.L. Obtaining high-added value products from the technical cashew-nut shell liquid using electrochemical oxidation with BDD anodes. Sep. Purif. Technol. 2020, 250, 117099. [Google Scholar] [CrossRef]
- Kujawski, W.; Warszawski, A.; Ratajczak, W.; Porebski, T.; Capała, W.; Ostrowska, I. Removal of phenol from wastewater by different separation techniques. Desalination 2004, 163, 287–296. [Google Scholar] [CrossRef]
- Otero, M.; Zabkova, M.; Rodriguez, A.L. Adsorptive purification of phenol wastewaters: Experimental basis and operation of a parametric pumping unit. Chem. Eng. J. 2005, 110, 101–111. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mohan, D.; Singh, K.P. Removal of 2-Aminophenol Using Novel Adsorbents. Ind. Eng. Chem. Res. 2006, 45, 1113–1122. [Google Scholar] [CrossRef]
- Cagnon, B.; Chedeville, O.; Cherrier, J.F.; Caqueret, V.; Porte, C. Evolution of adsorption kinetics and isotherms of gallic acid on an activated carbon oxidized by ozone: Comparison to the raw material. J. Taiwan Inst. Chem. Eng. 2011, 42, 996–1003. [Google Scholar] [CrossRef]
- Rosazza, J.P.N.; Huang, Z.; Dostal, L.; Volm, T.; Rosseau, B. Review: Biocatalytic transformation of ferulic acid: An abundant aromatic natural product. J. Ind. Microbiol. 1995, 15, 457–471. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Stratford, M.; Narbada, A. Analysis of the inhibition of food spoilage yeasts by vanillin. Int. J. Food Microbiol. 2003, 86, 113–122. [Google Scholar] [CrossRef]
- Delaquis, P.; Stanich, K.; Toivonen, P. Effect of pH on the inhibition of Listeria spp. by vanillin and vanillic acid. J. Food Prot. 2005, 68, 1472–1476. [Google Scholar] [CrossRef]
- Vetrano, A.M.; Heck, D.E.; Mariano, T.M.; Mishin, V.; Laskin, D.L.; Laskin, J.D. Characterization of the oxidase activity in mammalian catalas. J. Biol. Chem. 2005, 280, 35372–35381. [Google Scholar] [CrossRef] [Green Version]
- Mota, M.I.F.; Pinto, P.C.R.; Loureiro, J.M.; Rodrigues, A.E. Recovery of Vanillin and Syringaldehyde from Lignin Oxidation: A Review of Separation and Purification Processes. Sep. Purif. Rev. 2016, 45, 227–259. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Madureira, J.; Melo, R.; Cabo Verde, S.; Matos, I.; Bernardo, M.; Noronha, J.P.; Margaça, F.M.A.; Fonseca, I.M. Recovery of phenolic compounds from multi-component solution by a synthesized activated carbon using resorcinol and formaldehyde. Water Sci. Technol. 2018, 77, 456–466. [Google Scholar] [CrossRef]
- García-Pérez, P.; Losada-Barreiro, S.; Gallego, P.P.; Bravo-Díaz, C. Adsorption of gallic acid, propyl gallate and polyphenols from Bryophyllum extracts on activated carbon. Sci. Rep. 2019, 9, 14830. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Chen, R.; Du, H.; Zhang, J.; Shi, L.; Qin, Y.; Yue, L.; Wang, J. Synthesis of activated carbon from peanut shell as dye adsorbents for wastewater treatment. Adsorpt. Sci. Technol. 2019, 37, 34–48. [Google Scholar] [CrossRef] [Green Version]
- Sumboja, A.; Prakoso, B.; Ma, Y.; Irwan, F.R.; Hutani, J.J.; Mulyadewi, A.; Mahbub, M.A.A.; Zong, Y.; Liu, Z. FeCo Nanoparticle-Loaded Nutshell-Derived Porous Carbon as Sustainable Catalyst in Al-Air Batteries. Energy Mater. Adv. 2021, 2021, 7386210. [Google Scholar] [CrossRef]
- Esteves, B.M.; Morales-Torres, S.; Madeira, L.M.; Maldonado-H’odar, F.J. Specific adsorbents for the treatment of OMW phenolic compounds by activation of bio-residues from the olive oil industry. J. Environ. Manag. 2022, 306, 114490. [Google Scholar] [CrossRef]
- Yang, T.; Lua, A.C. Characteristics of activated carbons prepared from pistachio-nut shells by physical activation. J. Colloid Interface Sci. 2003, 267, 408–417. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Cui, K.; Karpuzov, D.; Tan, X.; Kohandehghan, A.; Mitlin, D. Peanut shell hybrid sodium ion capacitor with extreme energy–power rivals lithium ion capacitors. Energy Environ.Sci. 2015, 8I, 941–955. [Google Scholar] [CrossRef]
- Xu, S.-D.; Zhao, Y.; Liu, S.; Ren, X.; Chen, L.; Shi, W.; Wang, X.; Zhang, D. Curly hard carbon derived from pistachio shells as high-performance anode materials for sodium-ion batteries. J. Mater. Sci. 2018, 53, 12334–12351. [Google Scholar] [CrossRef]
- Boumchita, S.; Lahrichi, A.; Benjelloun, Y.; Lairini, S.; Nenov, V.; Zerrouq, F. Application of Peanut shell as a low-cost adsorbent for the removal of anionic dye from aqueous solutions. J. Mater. Environ. Sci. 2017, 8, 2353–2364. [Google Scholar]
- Igwegbe, C.A.; Ighalo, J.O.; Ghosh, S.; Ahmadi, S.; Ugonabo, V.I. Pistachio (Pistacia vera) waste as adsorbent for wastewater treatment: A review. Biomass Convers. Biorefnery 2021, 1–19. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Temkin, M.J.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Physicochim. URSS 1940, 12, 217–222. [Google Scholar]
- Kopinke, F.-D.; Georgi, A.; Goss, K.-U. Comment on “Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solution: A critical review”. Water Res. 2018, 129, 520–521. [Google Scholar] [CrossRef]
- Alzaydien, A.S. Adsorption of methylene blue from aqueous solution onto a low-cost natural Jordanian Tripoli. Am. J. Appl. Sci. 2009, 5, 197–208. [Google Scholar] [CrossRef] [Green Version]

| Langmuir Model | Freundlich Model | Temkin Model | ||||||
|---|---|---|---|---|---|---|---|---|
| KL | qm | R2 | Kf | n | R2 | A | B | R2 |
| 0.102 | 285.7 | 0.995 | 27.714 | 2.37 | 0.961 | 0.0849 | 29.26 | 0.944 |
| Langmuir Model | Freundlich Model | Temkin Model | ||||||
|---|---|---|---|---|---|---|---|---|
| KL | qm | R2 | Kf | n | R2 | A | B | R2 |
| 0.094 | 169.49 | 0.999 | 15.248 | 2.36 | 0.971 | 0.2258 | 20.72 | 0.901 |
| Shell | KL, L mmol−1 | ln KC | ΔG° (kJ mol−1) |
|---|---|---|---|
| Pistachio | 15.81 | 13.68 | 33.90 |
| Peanut | 17.15 | 13.77 | 34.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Víctor-Ortega, M.D.; Fajardo, A.S.; Airado-Rodríguez, D. Sustainable Development: Use of Agricultural Waste Materials for Vanillic Acid Recovery from Wastewater. Sustainability 2022, 14, 2818. https://doi.org/10.3390/su14052818
Víctor-Ortega MD, Fajardo AS, Airado-Rodríguez D. Sustainable Development: Use of Agricultural Waste Materials for Vanillic Acid Recovery from Wastewater. Sustainability. 2022; 14(5):2818. https://doi.org/10.3390/su14052818
Chicago/Turabian StyleVíctor-Ortega, María Dolores, Ana S. Fajardo, and Diego Airado-Rodríguez. 2022. "Sustainable Development: Use of Agricultural Waste Materials for Vanillic Acid Recovery from Wastewater" Sustainability 14, no. 5: 2818. https://doi.org/10.3390/su14052818
APA StyleVíctor-Ortega, M. D., Fajardo, A. S., & Airado-Rodríguez, D. (2022). Sustainable Development: Use of Agricultural Waste Materials for Vanillic Acid Recovery from Wastewater. Sustainability, 14(5), 2818. https://doi.org/10.3390/su14052818








