Resource Recycling Utilization of Distillers Grains for Preparing Cationic Quaternary Ammonium—Ammonium Material and Adsorption of Acid Yellow 11
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Pre-treatment of DG and Preparation of CDG
2.3. Characterization of CDG
2.4. Batch Adsorption Experiment
2.5. Regeneration Performance
2.6. Adsorption Mechanism
2.6.1. Adsorption Kinetic Model
2.6.2. Adsorption Isotherm Model
2.6.3. Adsorption Thermodynamic Model
3. Results and Discussion
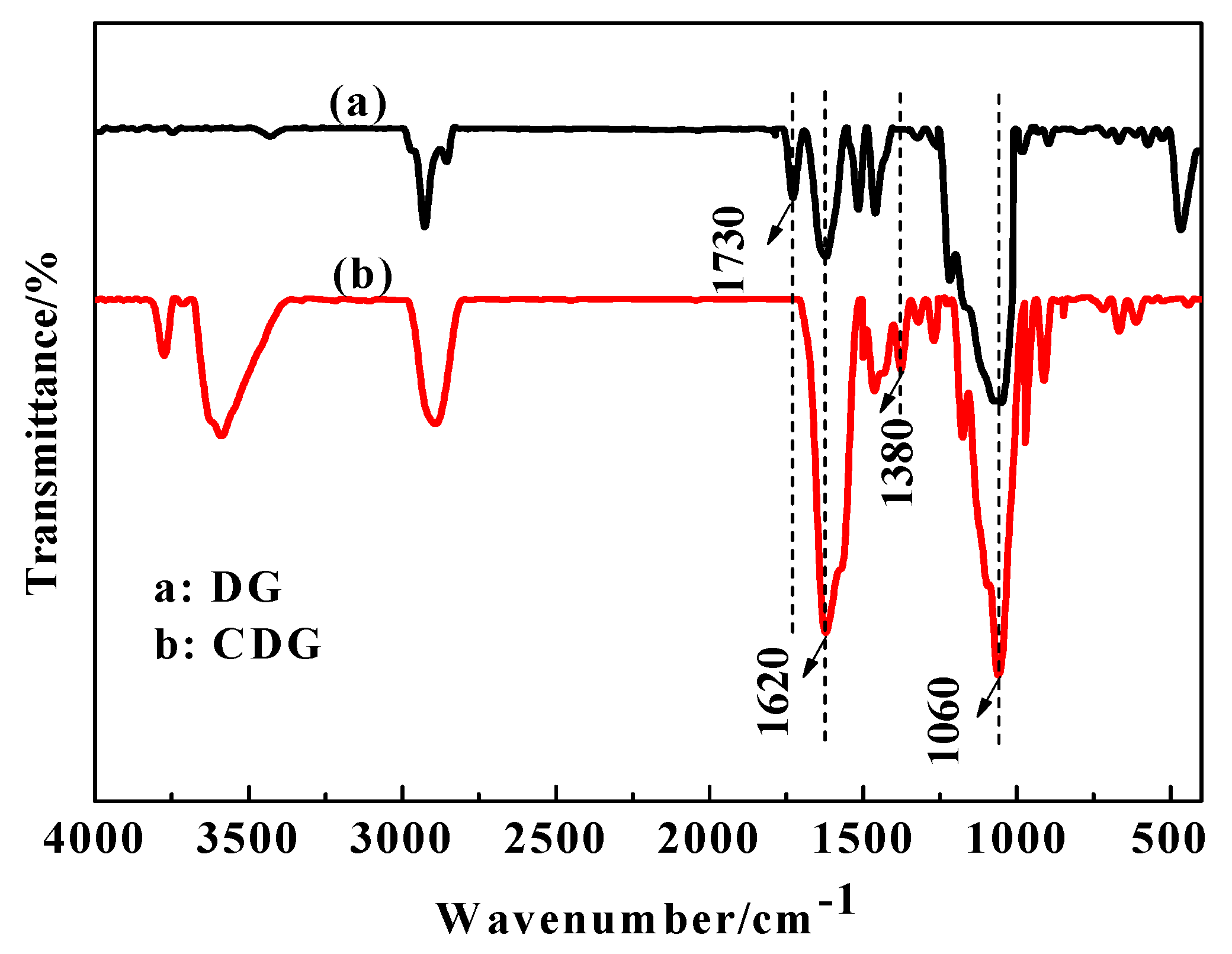
3.1. FTIR Analysis of DG and CDG
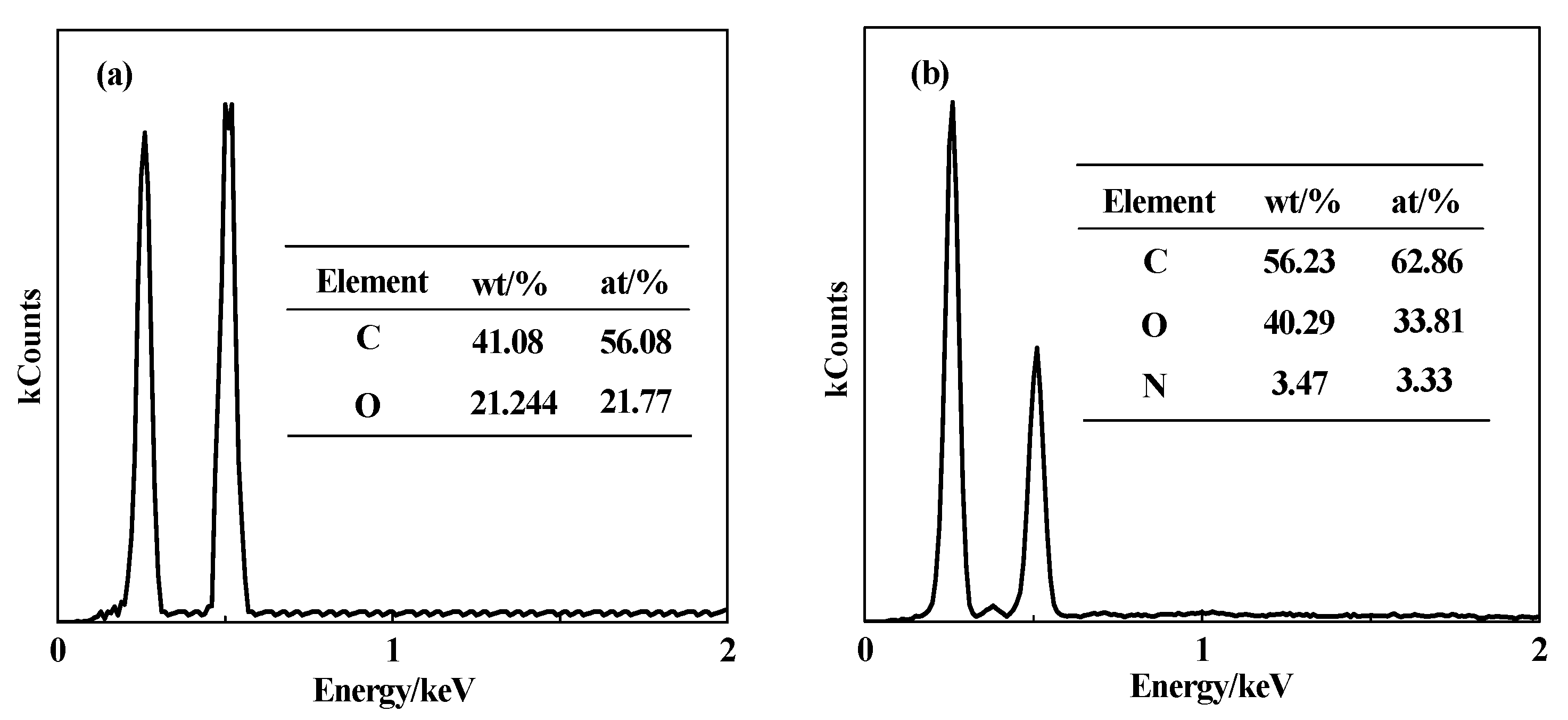
3.2. EDS Structure Characterization of DG and CDG
3.3. SEM Analysis of DG and CDG
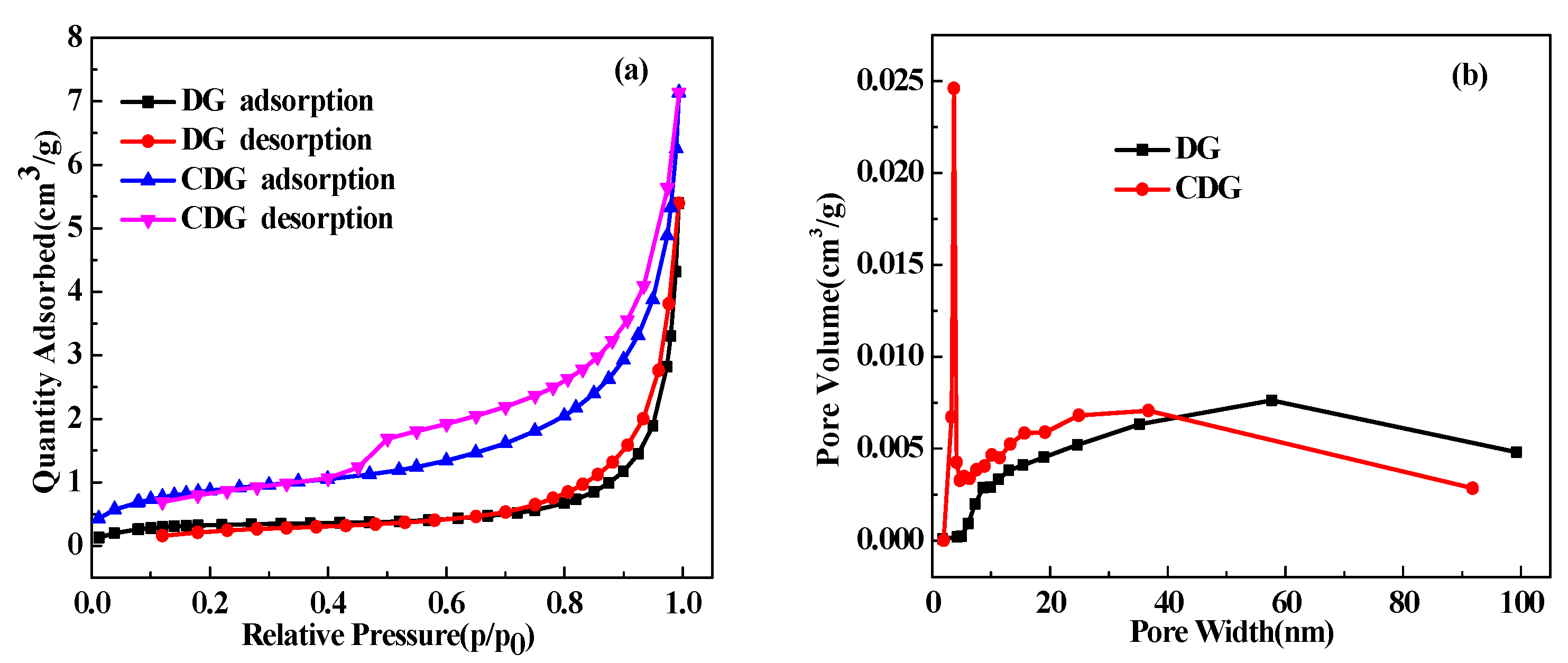
3.4. The BET Analysis of CDG
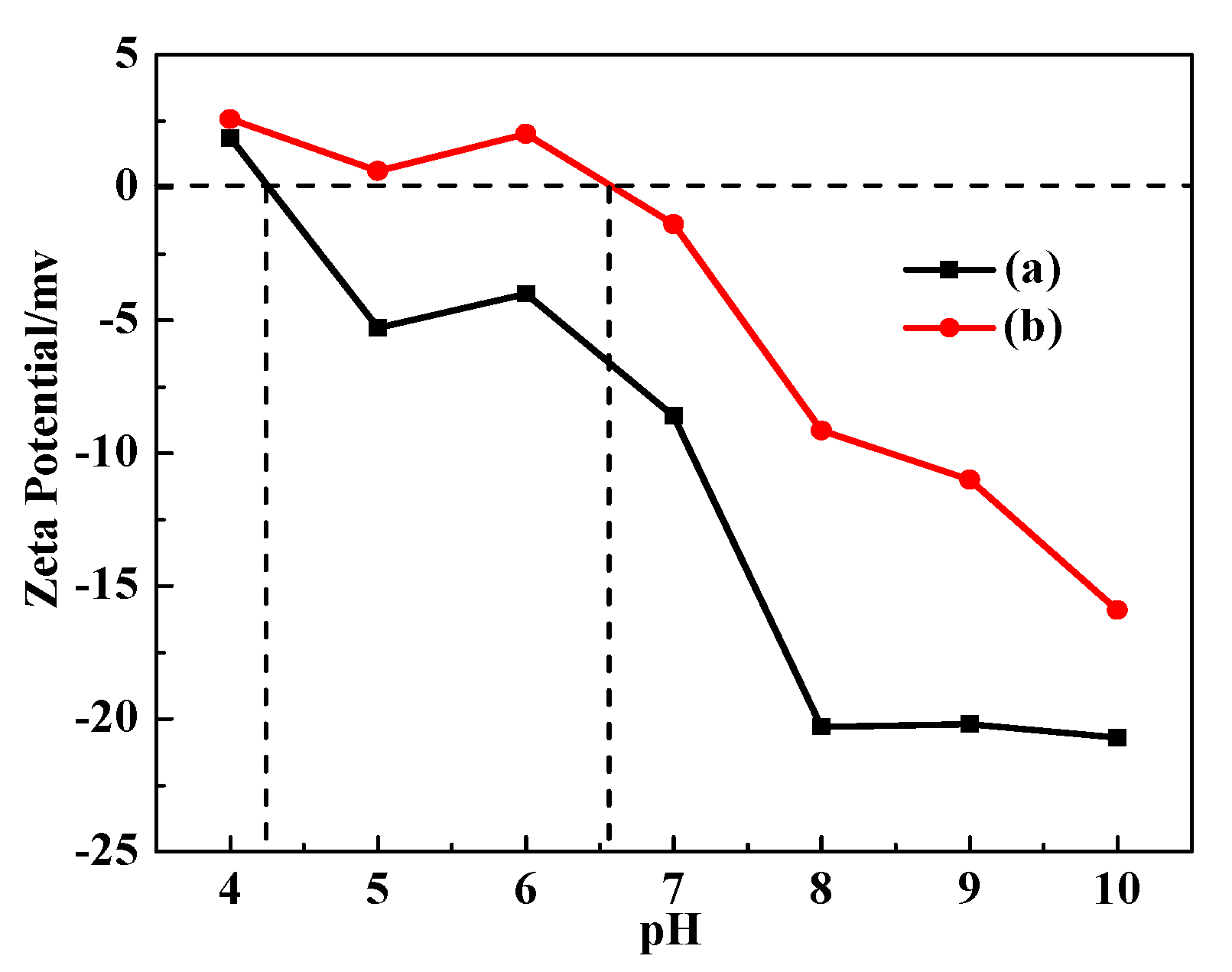
3.5. Zeta Potential Analysis of DG and CDG
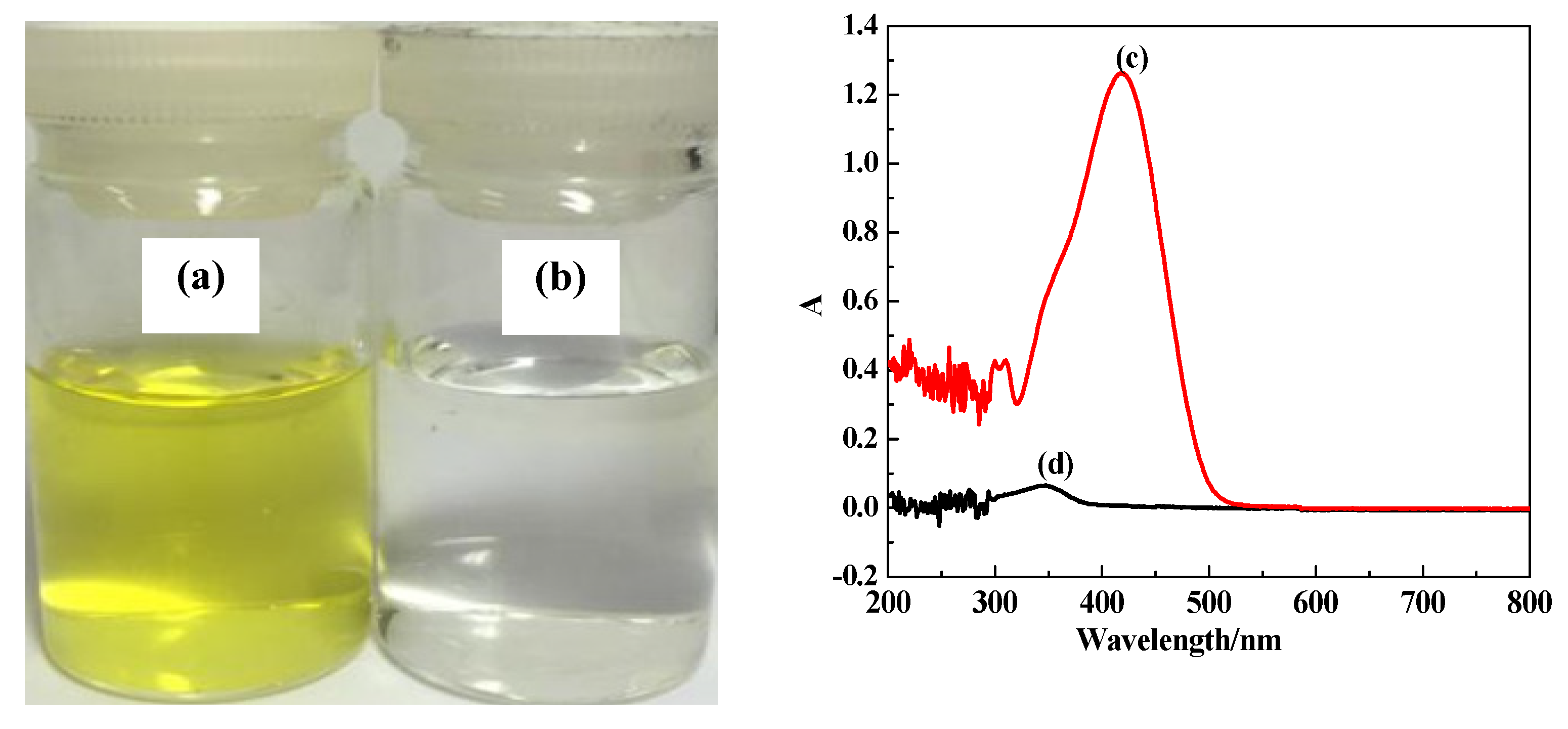
3.6. Adsorption of AY11 on CDG
3.7. The Effect of Adsorption Conditions on Adsorption Performance
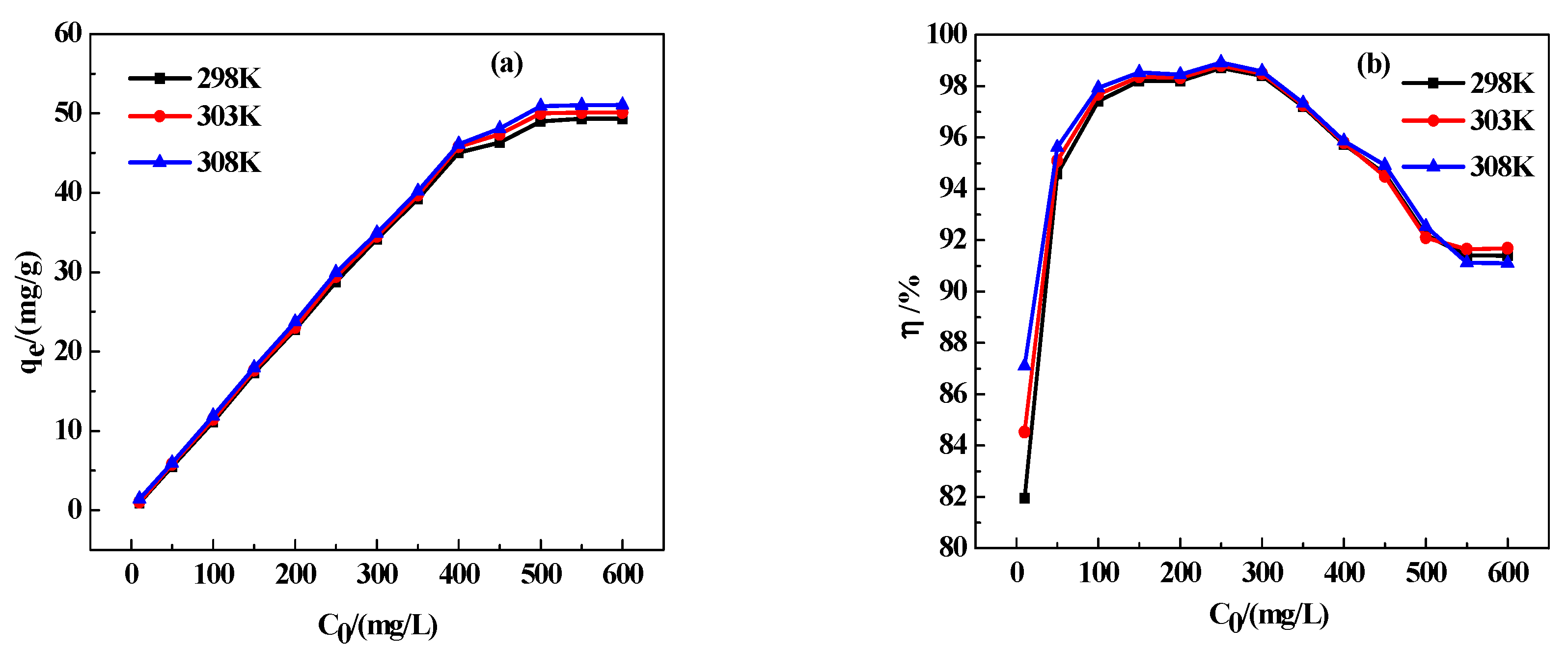
3.7.1. Adsorption Temperature
3.7.2. Adsorption Time
3.7.3. Mass of CDG
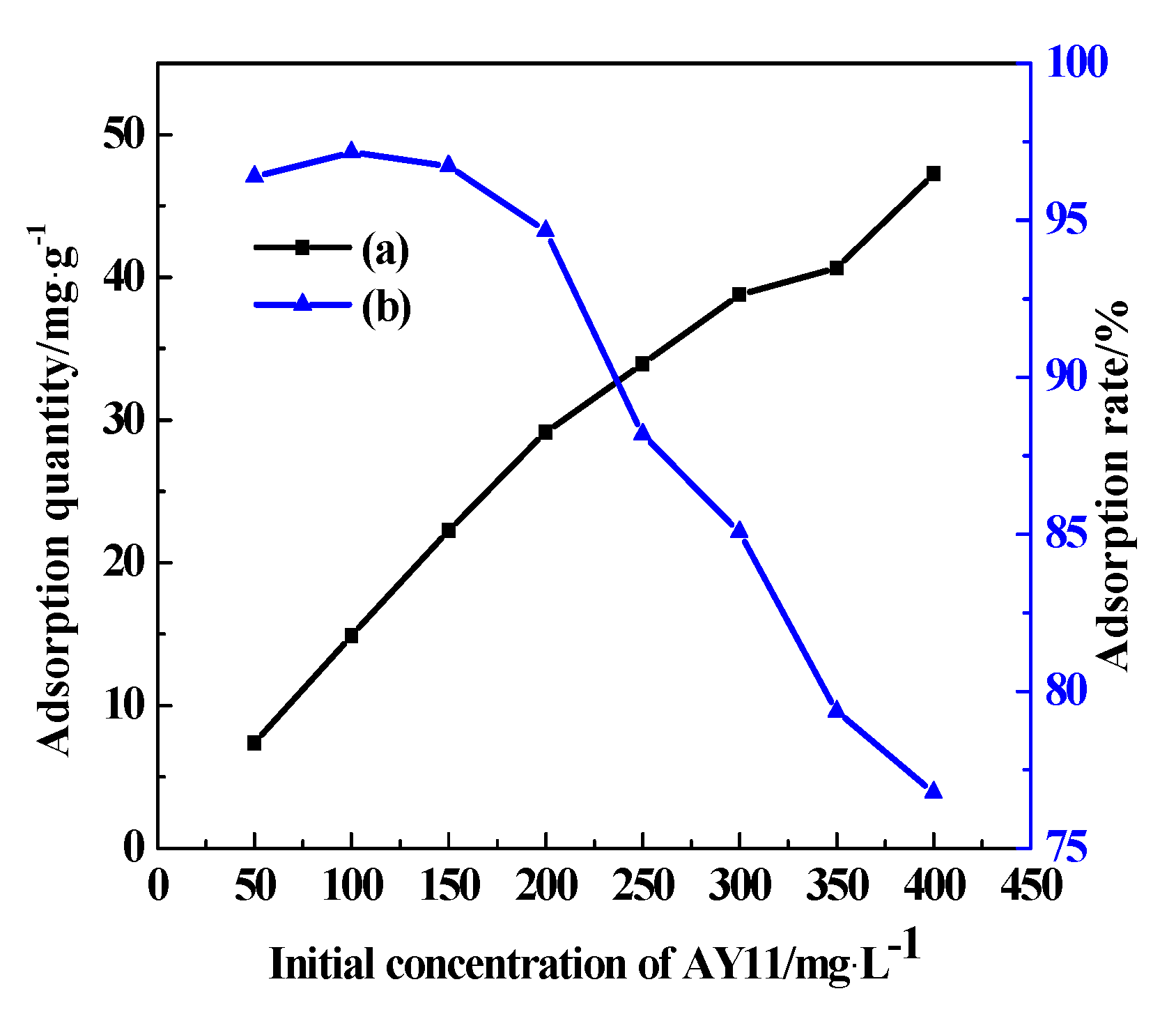
3.7.4. Initial Concentration of AY11
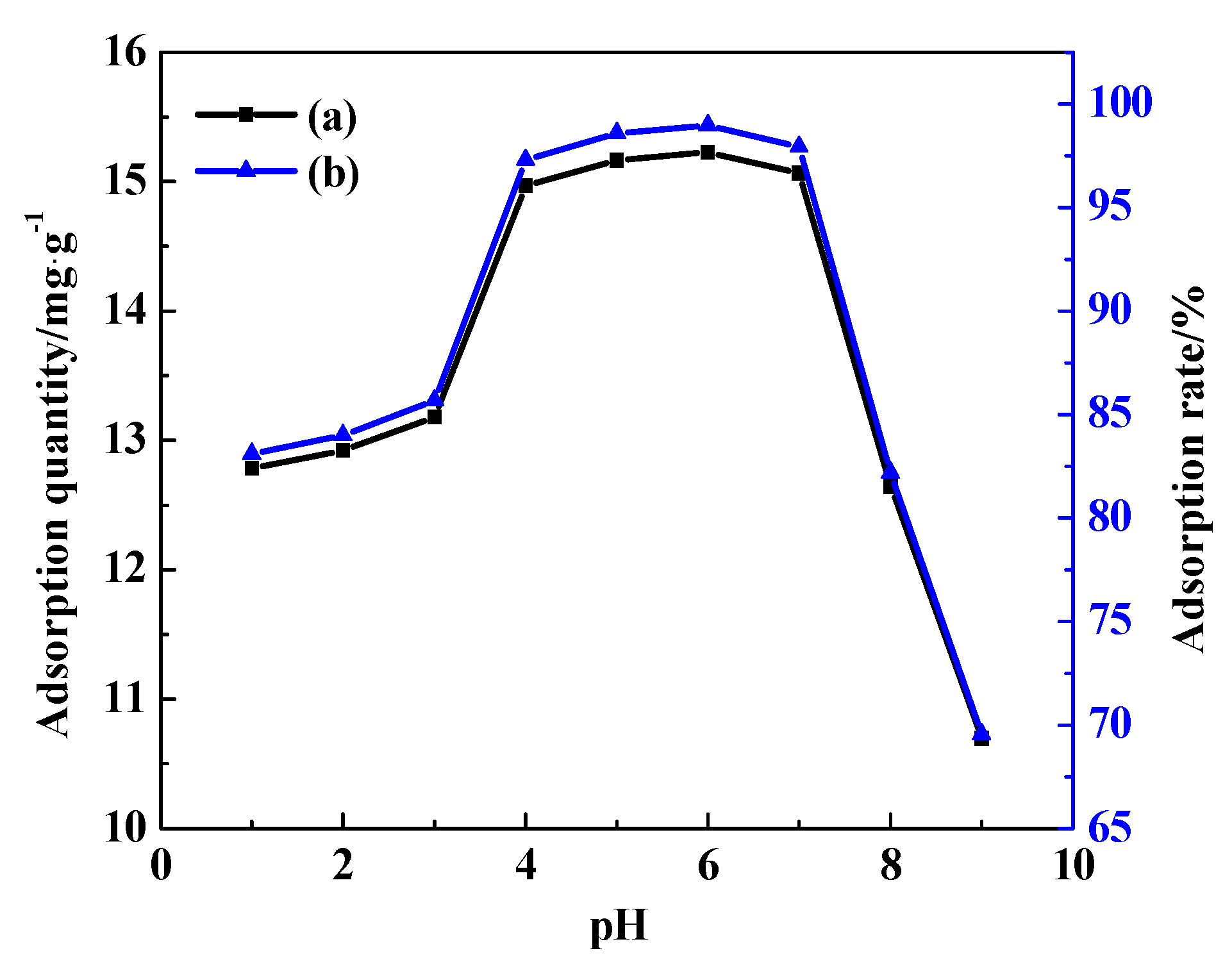
3.7.5. The Effect of pH
3.8. Regeneration Performance
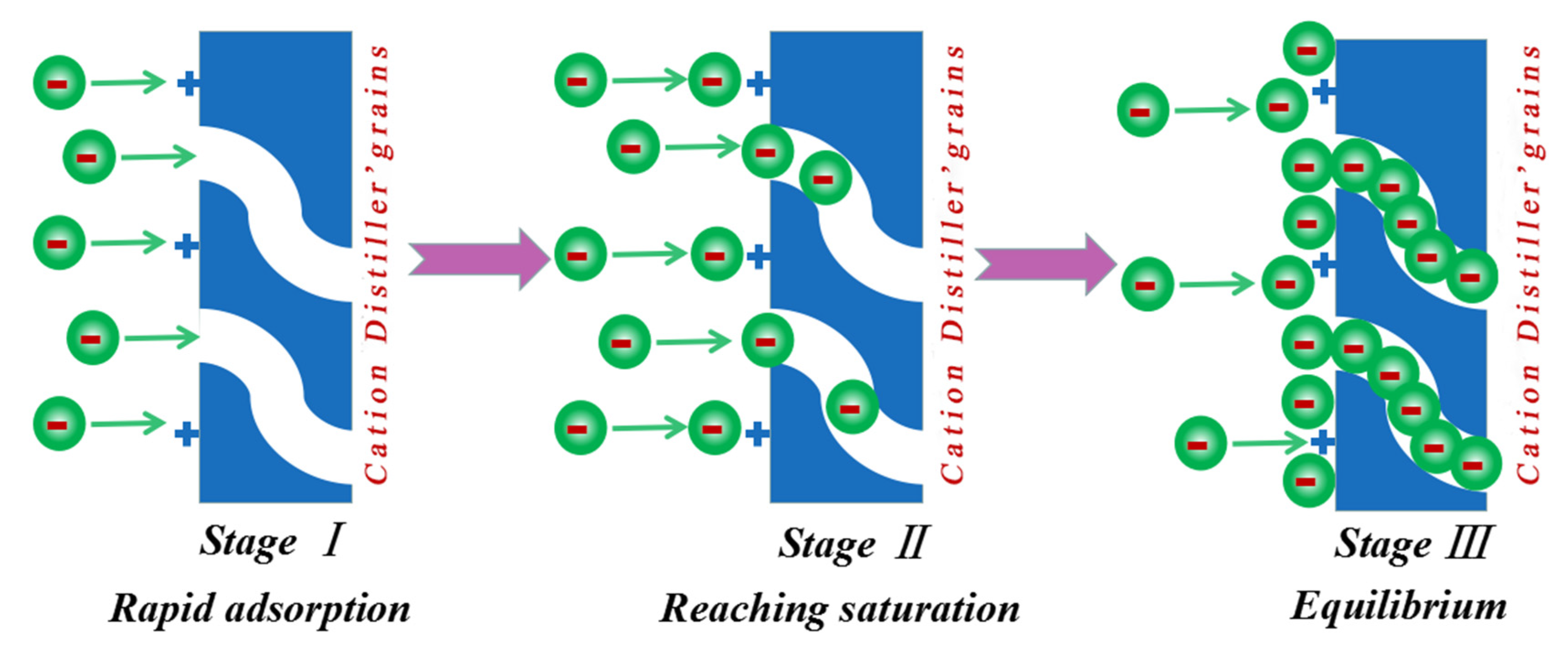
3.9. Investigations of the Adsorption Mechanisms
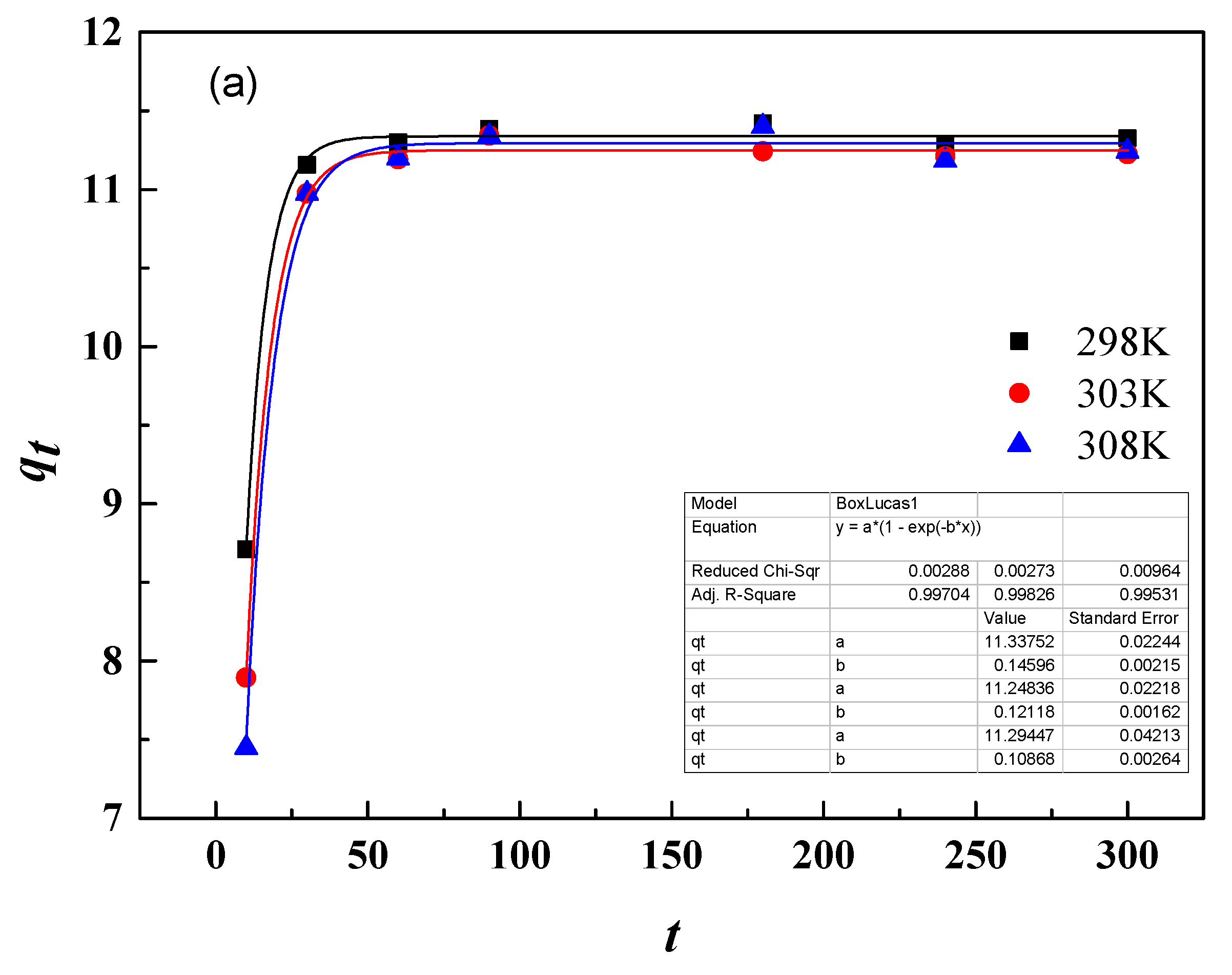
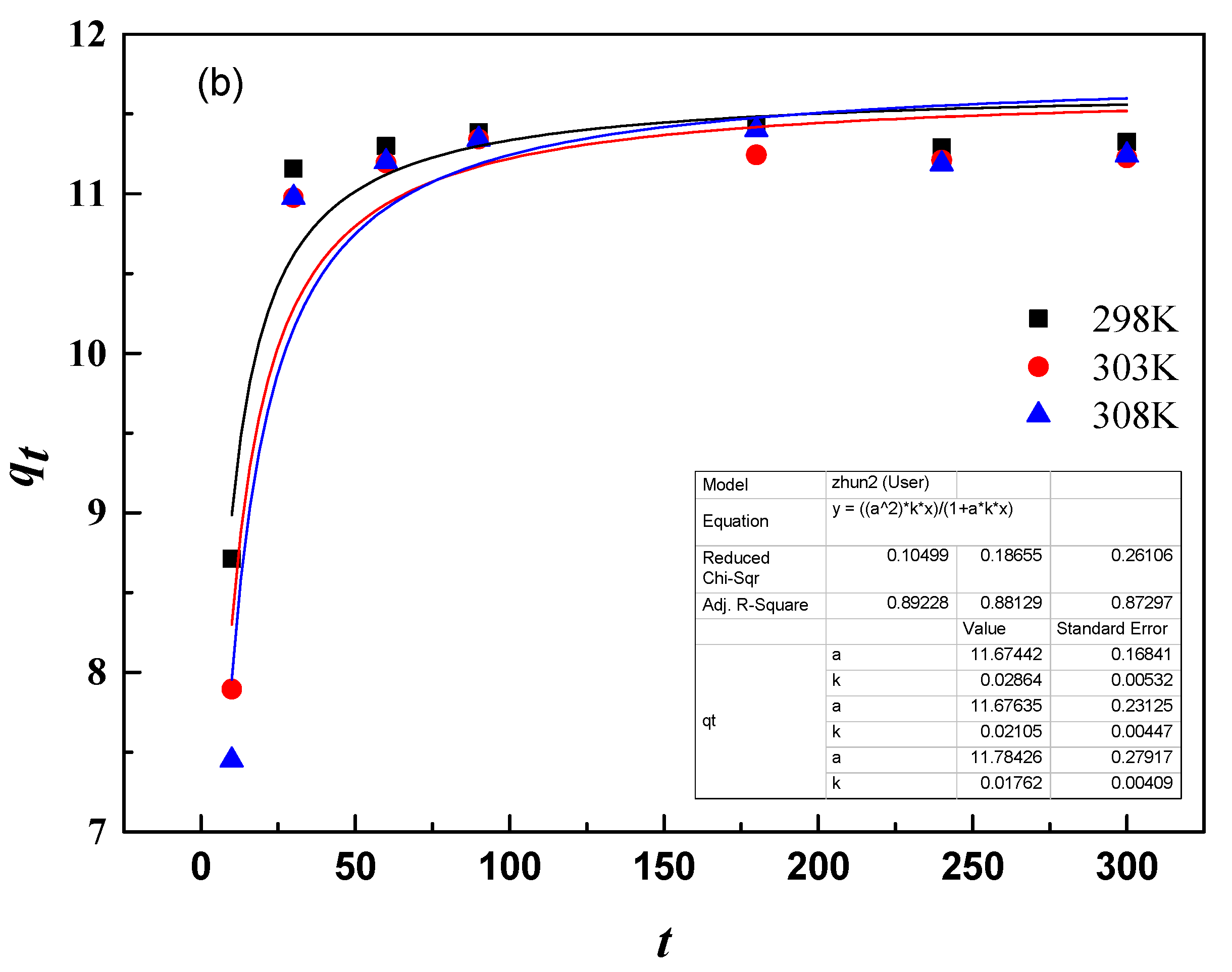
3.9.1. Adsorption Kinetics
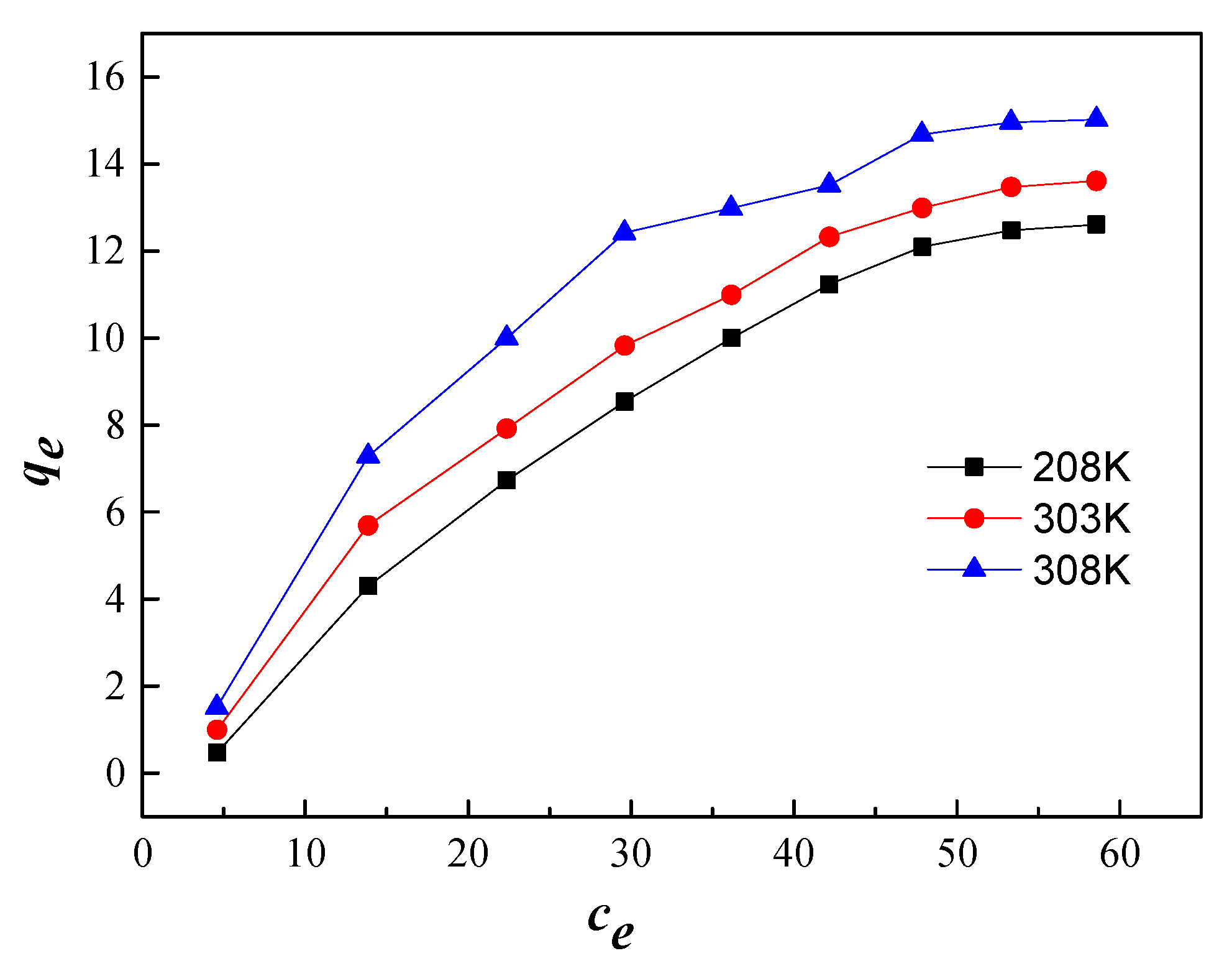
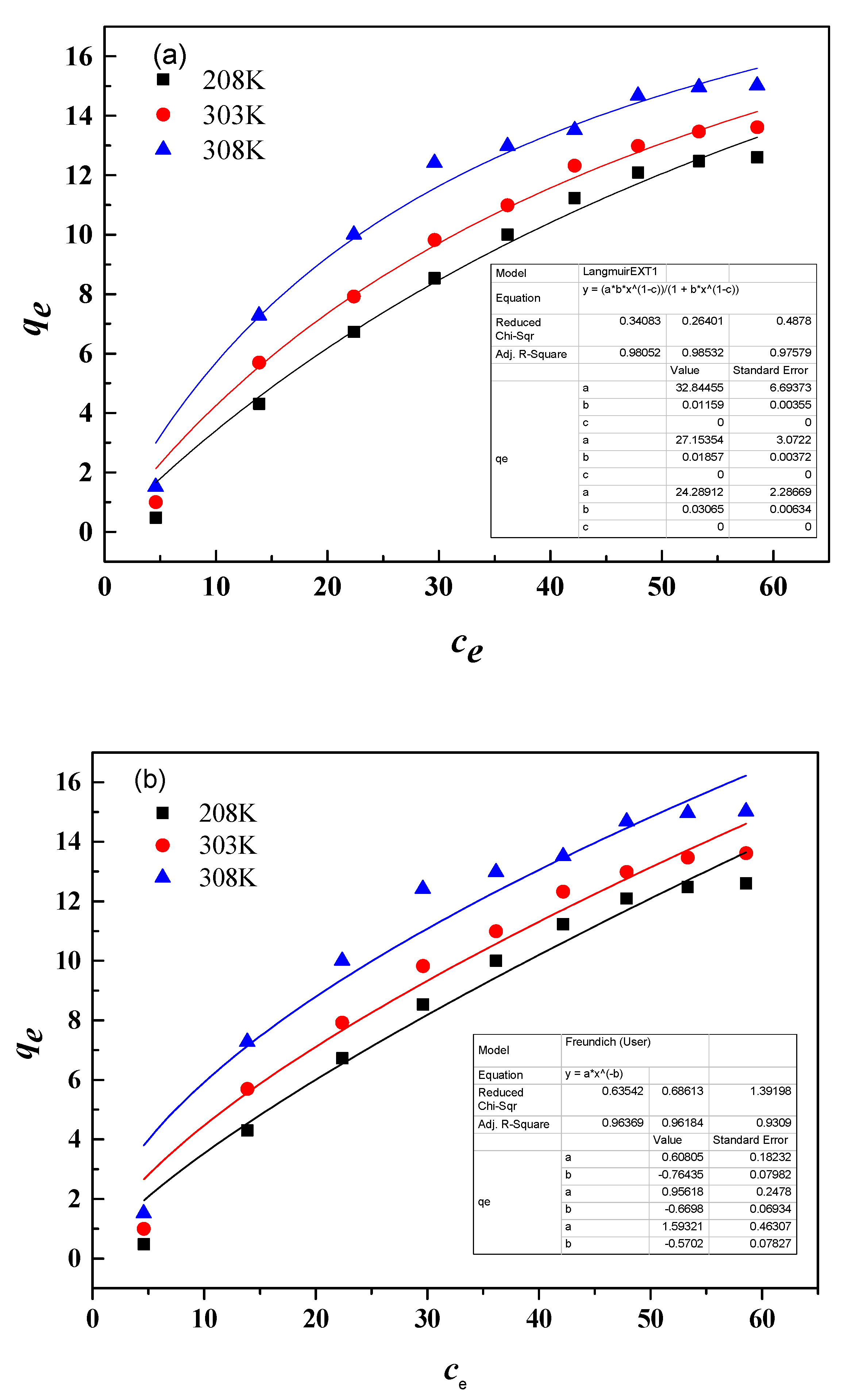
3.9.2. Adsorption Isotherm
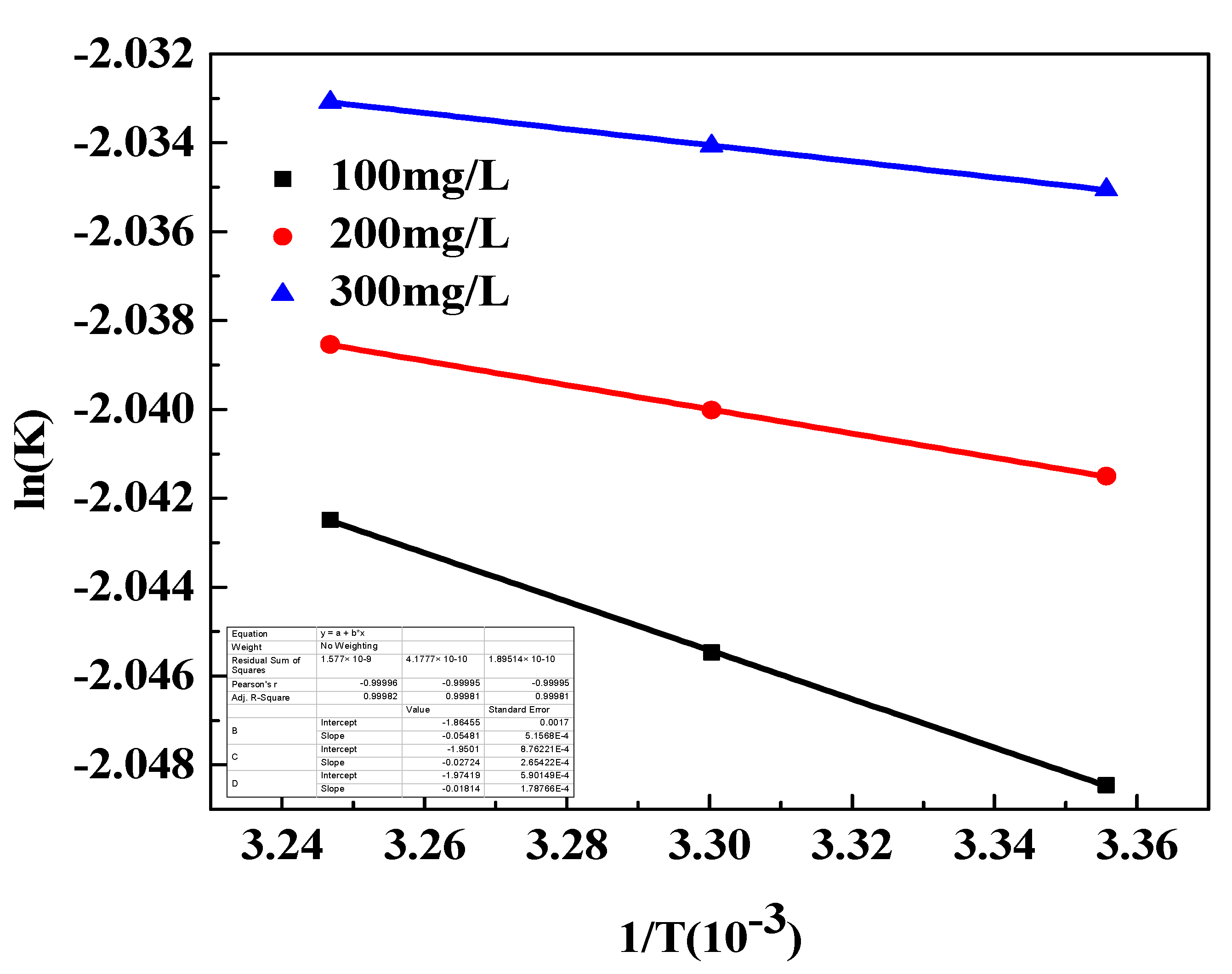
3.9.3. Adsorption Thermodynamics
3.9.4. Adsorption Model
3.10. Comparison of Various Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqbal, M.; Abbas, M.; Nisar, J.; Nazir, A. Bioassays based on higher plants as excellent dosimeters for ecotoxicity monitoring: A review. Chem. Int. 2019, 5, 1–80. [Google Scholar]
- Abbas, M.; Adil, M.; Ehtisham-Ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Tahir, M.A.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef]
- Iqbal, M. Vicia faba bioassay for environmental toxicity monitoring: A review. Chemosphere 2016, 144, 785–802. [Google Scholar] [CrossRef]
- Siddiqua, U.H.; Ali, S.; Iqbal, M.; Hussain, T. Relationship between structure and dyeing properties of reactive dyes for cotton dyeing. J. Mol. Liq. 2017, 241, 839–844. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Khan, Z.U.H.; Iqbal, J.; Arshad, S.; Junaid, M.; Khan, H.M. Synergistic effects of H2O2 and S2O82− in the gamma radiation induced degradation of congo-red dye: Kinetics and toxicities evaluation. Sep. Purif. Technol. 2020, 233, 115966. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Huang, H. Eco-friendly polyvinyl alcohol/carboxymethyl cellulose hydrogels reinforced with graphene oxide and bentonite for enhanced adsorption of methylene blue. Carbohydr. Polym. 2018, 185, 1–11. [Google Scholar]
- Liu, H.; Guo, W.; Li, Y.; He, S.; He, C. Photocatalytic degradation of sixteen organic dyes by TiO2/WO3-coated magnetic nanoparticles under simulated visible light and solar light. J. Chem. Environ. Eng. 2018, 6, 59–67. [Google Scholar] [CrossRef]
- Sinha, A.; Lulu, S.; Vino, S.; Banerjee, S.; Acharjee, S.; Osborne, W.J. Degradation of reactive green dye and textile effluent by Candida sp. VITJASS isolated from wetland paddy rhizosphere soil. J. Environ. Chem. Eng. 2018, 6, 5150–5159. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Li, J.; Zhao, C.; He, X.; Yang, G. Fabrication of slag particle three-dimensional electrode system for methylene blue degradation: Characterization, performance and mechanism study. Chemosphere 2018, 213, 377–383. [Google Scholar] [CrossRef]
- Obiora-Okafo, I.A.; Onukwuli, O.D. Characterization and optimization of spectrophotometric colour removal from dye containing wastewater by Coagulation-Flocculation. Pol. J. Chem. Technol. 2018, 20, 49–59. [Google Scholar] [CrossRef]
- Wolski, L.; Walkowiak, A.; Ziolek, M. Formation of reactive oxygen species upon interaction of Au/ZnO with H2O2 and their activity in methylene blue degradation. Catal. Today 2019, 333, 54–62. [Google Scholar] [CrossRef]
- Criscuoli, A.; Zhong, J.; Figoli, A.; Carnevale, M.; Huang, R.; Drioli, E. Treatment of dye solutions by vacuum membrane distillation. Water Res. 2008, 42, 5031–5037. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, Z.; Sun, D.; Wu, T.; Li, Y. Enhanced adsorption capacity of dyes by surfactant-modified layered double hydroxides from aqueous solution. J. Ind. Eng. Chem. 2017, 49, 208–218. [Google Scholar] [CrossRef]
- Tian, S.; Xu, S.; Liu, J.; He, C.; Xiong, Y.; Feng, P. Highly efficient removal of both cationic and anionic dyes from wastewater with a water-stable and eco-friendly Fe-MOF via host-guest encapsulation. J. Clean. Prod. 2019, 239, 117767. [Google Scholar] [CrossRef]
- Shanmugam, M.; Alsalme, A.; Alghamdi, A.; Jayavel, R. Enhanced Photocatalytic Performance of the Graphene-V2O5 Nanocomposite in the Degradation of Methylene Blue Dye under Direct Sunlight. ACS Appl. Mater. Interfaces 2015, 7, 14905–14911. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Bilgin, A.; Tünay, O.; Kabdasli, I. Sulphate control by ettringite precipitation in textile industry wastewaters. Environ. Technol. 2015, 37, 446–451. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Wiśniewska, M.; Wołowicz, A.; Gun’Ko, V.M.; Zarko, V.I. Mixed silica-alumina oxide as sorbent for dyes and metal ions removal from aqueous solutions and wastewaters. Microporous Mesoporous Mater. 2017, 250, 128–147. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Su, K.; Fan, T.; Cao, L. Mussel-inspired modification of PPS membrane to separate and remove the dyes from the wastewater. Chem. Eng. J. 2018, 341, 371–382. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Waeijen, H.A.; Berry, R.M.; Tam, K.C. Continuous flow adsorption of methylene blue by cellulose nanocrystal-alginate hydrogel beads in fixed bed columns. Carbohydr. Polym. 2016, 136, 1194–1202. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Wiśniewska, M.; Gun’ko, V.M.; Zarko, V.I. Adsorptive removal of acid, reactive and direct dyes from aqueous solutions and wastewater using mixed silicaealumina oxide. Powder Technol. 2015, 278, 306–315. [Google Scholar] [CrossRef]
- Gładysz-Płaska, A.; Skwarek, E.; Budnyak, T.M.; Kołodyńska, D. Metal Ions Removal Using Nano Oxide Pyrolox™ Material. Nanoscale Res. Lett. 2017, 12, 95. [Google Scholar] [CrossRef]
- Du, H.; Cheng, J.; Wang, M.; Tian, M.; Yang, X.; Wang, Q. Red dye extracted sappan wood waste derived activated carbons characterization and dye adsorption properties. Diam. Relat. Mater. 2020, 102, 107646. [Google Scholar] [CrossRef]
- Panda, J.; Sahoo, J.K.; Panda, P.K.; Sahu, S.N.; Samal, M.; Pattanayak, S.K.; Sahu, R. Adsorptive behavior of zeolitic imidazolate framework-8 towards anionic dye in aqueous media: Combined experimental and molecular docking study. J. Mol. Liq. 2019, 278, 536–545. [Google Scholar] [CrossRef]
- Pisareva, A.S.; Tikhomirova, T.I. Sorption of Synthetic Anionic Amaranth Dye from an Aqueous Solution on Hydrophobized Silica and Alumina. Russ. J. Phys. Chem. A 2019, 93, 534–537. [Google Scholar] [CrossRef]
- Baliś, A.; Id, S.Z. Tailored synthesis of core-shell mesoporous silica particles—Optimization of dye sorption properties. Nanomaterials 2018, 8, 230. [Google Scholar] [CrossRef]
- Luensmann, D.; Jones, L. Impact of fluorescent probes on albumin sorption profiles to ophthalmic biomaterials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 327–336. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, W.-H.; Ren, Z.-G.; Lang, J.-P. A 1D anionic coordination polymer showing superior Congo Red sorption and its dye composite exhibiting remarkably enhanced photocurrent response. Chem. Commun. 2015, 51, 14893–14896. [Google Scholar] [CrossRef]
- Malik, P.K. Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: A case study of Acid Yellow 36. Dye. Pigment. 2003, 56, 239–249. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Hameed, B.H. Fixed-bed adsorption of reactive azo dye onto granular activated carbon prepared from waste. J. Hazard. Mater. 2010, 175, 298–303. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, B.; Rastogi, A.; Agarwal, S.; Nayak, A. A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye—Acid Blue 113. J. Hazard. Mater. 2011, 186, 891–901. [Google Scholar] [CrossRef]
- Ríos-Gómez, J.; Ferrer-Monteagudo, B.; López-Lorente, Á.I.; Lucena, R.; Luque, R.; Cárdenas, S. Efficient combined sorption/photobleaching of dyes promoted by cellulose/titania-based nanocomposite films. J. Clean. Prod. 2018, 194, 167–173. [Google Scholar] [CrossRef]
- Sun, X.; Yang, L.; Li, Q.; Zhao, J.; Li, X.; Wang, X.; Liu, H. Amino-functionalized magnetic cellulose nanocomposite as adsorbent for removal of Cr(VI): Synthesis and adsorption studies. Chem. Eng. J. 2014, 241, 175–183. [Google Scholar] [CrossRef]
- Sarsenov, A.; Bishimbaev, V.; Kapsalamov, B.; Lepesov, K.; Gapparova, K.; Grzesiak, P. Chemical modification of cellulose for boron sorption from water solutions. Pol. J. Chem. Technol. 2018, 20, 123–128. [Google Scholar] [CrossRef]
- Villegas-Torres, M.F.; Ward, J.M.; Lye, G.J. The protein fraction from wheat-based dried distiller’s grain with solubles (DDGS): Extraction and valorization. New Biotechnol. 2015, 32, 606–611. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tan, J.; Wei, X.H.; Wang, L.J. Removal of Remazol turquoise Blue G-133 from aqueous solution using modified waste newspaper fiber. Carbohydr. Polym. 2013, 92, 1497–1502. [Google Scholar] [CrossRef]
- Hashem, A.M.A.; El-Shishtawy, R.M. Preparation and Characterization of Cationized Cellulose for the Removal of Anionic Dyes. Adsorpt. Sci. Technol. 2001, 19, 197–210. [Google Scholar] [CrossRef]
- Qi, Y.; Li, J.; Wang, L.J. Removal of Remazol Turquoise Blue G-133 from aqueous medium using functionalized cellulose from recycled newspaper fiber. Ind. Crop. Prod. 2013, 50, 15–22. [Google Scholar] [CrossRef]
- Idan, I.J.; Abdullah, L.C.; Mahdi, D.S.; Obaid, M.K.; Jamil, S.N.A.B.M. Adsorption of Anionic Dye Using Cationic Surfactant-Modified Kenaf Core Fibers. Open Access Libr. J. 2017, 4, 1. [Google Scholar] [CrossRef]
- Khy, A.; Pj, A.; Kbr, B.; Sumant, A.V.; Skoog, S.A.; Narayan, R.J. Correlation of zeta potential and contact angle of oxygen and fluorine terminated nitrogen incorporated ultrananocrystalline diamond (N-UNCD) thin films. Mater. Lett. 2021, 295, 129823. [Google Scholar]
- Cheng, F.; Zhou, X.; Zhang, J.; Lu, L.; Ma, J. Effects of cations on the adsorption kinetics of bt protein on mineral surfaces. J. Agro-Environ. Sci. 2017, 9, 1844–1849. [Google Scholar]
- Lei, S.; Li, X. Adsorption and degradation of 1,2,4-trichlorobenzene by activated carbon-microorganisms in soil. J. Agro-Environ. Sci. 2015, 8, 1535–1541. [Google Scholar]
- Zou, W.; Zhao, L.; Han, R. Adsorption characteristics of uranyl ions by manganese oxide coated sand in batch mode. J. Radioanal. Nucl. Chem. 2011, 288, 239–249. [Google Scholar] [CrossRef]
- Aksu, Z.; Tezer, S. Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process Biochem. 2005, 40, 1347–1361. [Google Scholar] [CrossRef]
- Bergaoui, M.; Nakhli, A.; Benguerba, Y.; Khalfaoui, M.; Erto, A.; Soetaredjo, F.E.; Ismadji, S.; Ernst, B. Novel insights into the adsorption mechanism of methylene blue onto organo-bentonite: Adsorption isotherms modeling and molecular simulation. J. Mol. Liq. 2018, 272, 697–707. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Guibal, E. Arsenic(V) sorption using chitosan/Cu(OH)2 and chitosan/CuO composite sorbents. Carbohydr. Polym. 2015, 134, 190–204. [Google Scholar] [CrossRef]
- Bao, S.Y.; Tang, L.H.; Li, K.; Ning, P.; Peng, J.H.; Guo, H.B.; Zhu, T.T.; Liu, Y. Highly selective removal of Zn(II) ion from hot-dip galvanizing pickling waste with amino-functionalized Fe3O4@SiO2, magnetic nano-adsorbent. J. Colloid Interface Sci. 2016, 462, 235–242. [Google Scholar] [CrossRef]
- Ren, Y.; Abbood, H.A.; He, F.; Peng, H.; Huang, K. Magnetic EDTA-modified chitosan/SiO2/Fe3O4, adsorbent: Preparation, characterization, and application in heavy metal adsorption. Chem. Eng. J. 2013, 226, 300–311. [Google Scholar] [CrossRef]
- Bajaj, K.; Sakhuja, R. Benzotriazole: Much More than Just Synthetic Heterocyclic Chemistry; The Chemistry of Benzotriazole Derivatives; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Lonappan, L.; Rouissi, T.; Brar, S.K.; Verma, M.; Surampalli, R.Y. An insight into the adsorption of diclofenac on different biochars: Mechanisms, surface chemistry, and thermodynamics. Bioresour. Technol. 2018, 249, 386–394. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpaa, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Park, K.-H.; Yu, S.-H.; Kim, H.-S.; Park, H.-D. Inhibition of biofouling by modification of forward osmosis membrane using quaternary ammonium cation. Water Sci. Technol. 2015, 72, 738–745. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, C.; Jin, J.; Sun, Y.; Yao, J.; Zhao, G.; Liu, Y. In-situ synthesis and ultrasound enhanced adsorption properties of MoS2/graphene quantum dot nanocomposite. Chem. Eng. J. 2017, 327, 774–782. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, K.; Li, J.; Yue, X. Simultaneously enhancing hydrophilicity, chlorine resistance and anti-biofouling of APA-TFC membrane surface by densely grafting quaternary ammonium cations and salicylaldimines. J. Membr. Sci. 2017, 528, 296–302. [Google Scholar] [CrossRef]
- Choi, A.E.S.; Roces, S.; Dugos, N.; Arcega, A.; Wan, M.W. Adsorptive removal of dibenzothiophene sulfone from fuel oil using clay material adsorbents. J. Clean. Prod. 2017, 161, 267–276. [Google Scholar] [CrossRef]
- Değermenci, G.D.; Değermenci, N.; Ayvaoğlu, V.; Durmaz, E.; Çakır, D.; Akan, E. Adsorption of reactive dyes on lignocellulosic waste; characterization, equilibrium, kinetic and thermodynamic studies. J. Clean. Prod. 2019, 225, 1220–1229. [Google Scholar] [CrossRef]
- Li, C.; Wu, N.; Zhang, M.; Wei, C.; Yan, F.; Wang, Z. Effects of E44 and KH560 modifiers on properties of distillers grains poly(butylene succinate) composites. Polym. Compos. 2018, 40, 1499–1509. [Google Scholar] [CrossRef]
- Wang, B.; Lian, G.; Lee, X.; Gao, B.; Li, L.; Liu, T.; Zhang, X.; Zheng, Y. Phosphogypsum as a novel modifier for distillers grains biochar removal of phosphate from water. Chemosphere 2020, 238, 124684. [Google Scholar] [CrossRef]
- Oliveira, K.R.; Segura, J.G.; Oliveira, B.A.; Medeiros, A.C.L.; Zimba, R.D.; Viegas, E.M. Distillers’ dried grains with soluble in diets for Pacu, Piaractus mesopotamicus juveniles:Growth performance, feed utilization, economic viability, and phosphorus release. Anim. Feed. Sci. Technol. 2020, 262, 114393. [Google Scholar] [CrossRef]
- Lee, C.; Morris, D.L.; Lefever, K.M.; Dieter, P.A. Feeding a diet with corn distillers grain with solubles to dairy cows alters manure characteristics and ammonia and hydrogen sulfide emissions from manure. J. Dairy Sci. 2020, 103, 2363–2372. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Li, L.; Xu, G. Pyrolysis characteristics, kinetics and evolved volatiles determination of rice-husk-based distiller’s grains. Biomass Bioenergy 2020, 135, 105525. [Google Scholar] [CrossRef]
- Thommes, M.; Guillet-Nicolas, R.; Cychosz, K.A. Physical Adsorption Characterization of Mesoporous Zeolites. Mesoporous Zeolites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 349–384. [Google Scholar]
- Sadeek, S.A.; Negm, N.; Hefni, H.; Wahab, M.M.A. Metal adsorption by agricultural biosorbents: Adsorption isotherm, kinetic and biosorbents chemical structures. Int. J. Biol. Macromol. 2015, 81, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Verma, R.; Asthana, A.; Vidya, S.S.; Singh, A.K. Adsorption of hazardous chromium (VI) ions from aqueous solutions using modified sawdust: Kinetics, isotherm and thermodynamic modelling. Int. J. Environ. Anal. Chem. 2019, 101, 911–928. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Zhao, L.; Meng, G.; Wu, J.; Liu, Z. Cellulose-based porous adsorbents with high capacity for methylene blue adsorption from aqueous solutions. Fibers Polym. 2017, 8, 891–899. [Google Scholar] [CrossRef]
- Battirola, L.C.; Zanata, D.M.; Gon Alves, M.C. Improvement of cellulose acetate dimensional stability by chemical crosslinking with cellulose nanocrystals. Compos. Part A Appl. Sci. Manuf. 2018, 113, 105–113. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. MWH’s Water Treatment: Principles and Design, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Firdaous, L.; Fertin, B.; Khelissa, O.; Dhainaut, M.; Nedjar, N.; Chataigné, G.; Ouhoud, L.; Lutin, F.; Dhulster, P. Adsorptive removal of polyphenols from an alfalfa white proteins concentrate: Adsorbent screening, adsorption kinetics and equilibrium study. Sep. Purif. Technol. 2017, 178, 29–39. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Mangwandi, C.; Al-Muhtaseb, A.H.; Walker, G.M.; Allen, S.J.; Ahmad, M.N. Kinetic and thermodynamics of chromium ions adsorption onto low-cost dolomite adsorbent. Chem. Eng. J. 2012, 179, 193–202. [Google Scholar] [CrossRef]
- Wu, Z.; Zhong, H.; Yuan, X.; Wang, H.; Wang, L.; Chen, X.; Zeng, G.; Wu, Y. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef]
- Heibati, B.; Rodriguez-Couto, S.; Turan, N.G.; Ozgonenel, O.; Albadarin, A.B.; Asif, M.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Removal of noxious dye—Acid Orange 7 from aqueous solution using natural pumice and Fe-coated pumice stone. J. Ind. Eng. Chem. 2015, 31, 124–131. [Google Scholar] [CrossRef]
- Jin, X.; Yu, B.; Chen, Z.; Arocena, J.M.; Thring, R.W. Adsorption of Orange II dye in aqueous solution onto surfactant-coated zeolite: Characterization, kinetic and thermodynamic studies. J. Colloid Interface Sci. 2014, 435, 15–20. [Google Scholar] [CrossRef]
- Patra, B.N.; Majhi, D. Removal of Anionic Dyes from Water by Potash Alum Doped Polyaniline: Investigation of Kinetics and Thermodynamic Parameters of Adsorption. J. Phys. Chem. B 2015, 119, 8154–8164. [Google Scholar] [CrossRef]
- Guo, X.; Wei, Q.; Du, B.; Zhang, Y.; Xin, X.; Yan, L.; Yu, H. Removal of Metanil Yellow from water environment by amino functionalized graphenes (NH2-G)—Influence of surface chemistry of NH2-G. Appl. Surf. Sci. 2013, 284, 862–869. [Google Scholar] [CrossRef]
- Meng, F.; Wang, L.; Pei, M.; Guo, W.; Liu, G. Adsorption of metanil yellow from aqueous solution using polyaniline-bentonite composite. Colloid Polym. Sci. 2017, 295, 1165–1175. [Google Scholar] [CrossRef]
- El-Bindary, A.A.; Hussien, M.A.; Diab, M.A.; Eessa, A.M. Adsorption of Acid Yellow 99 by polyacrylonitrile/activated carbon composite: Kinetics, thermodynamics and isotherm studies. J. Mol. Liq. 2014, 197, 236–242. [Google Scholar] [CrossRef]
- Amooey, A.A.; Alinejad-Mir, A.; Ghasemi, S.; Marzbali, M.H. The removal of Direct Yellow 12 from synthetic aqueous solution by a novel magnetic nanobioadsorbent. Int. J. Environ. Sci. Technol. 2019, 16, 8343–8354. [Google Scholar] [CrossRef]
- El-Sharkawy, R.; El-Ghamry, H.A. Multi-walled carbon nanotubes decorated with Cu(II) triazole Schiff base complex for adsorptive removal of synthetic dyes. J. Mol. Liq. 2019, 282, 515–526. [Google Scholar] [CrossRef]



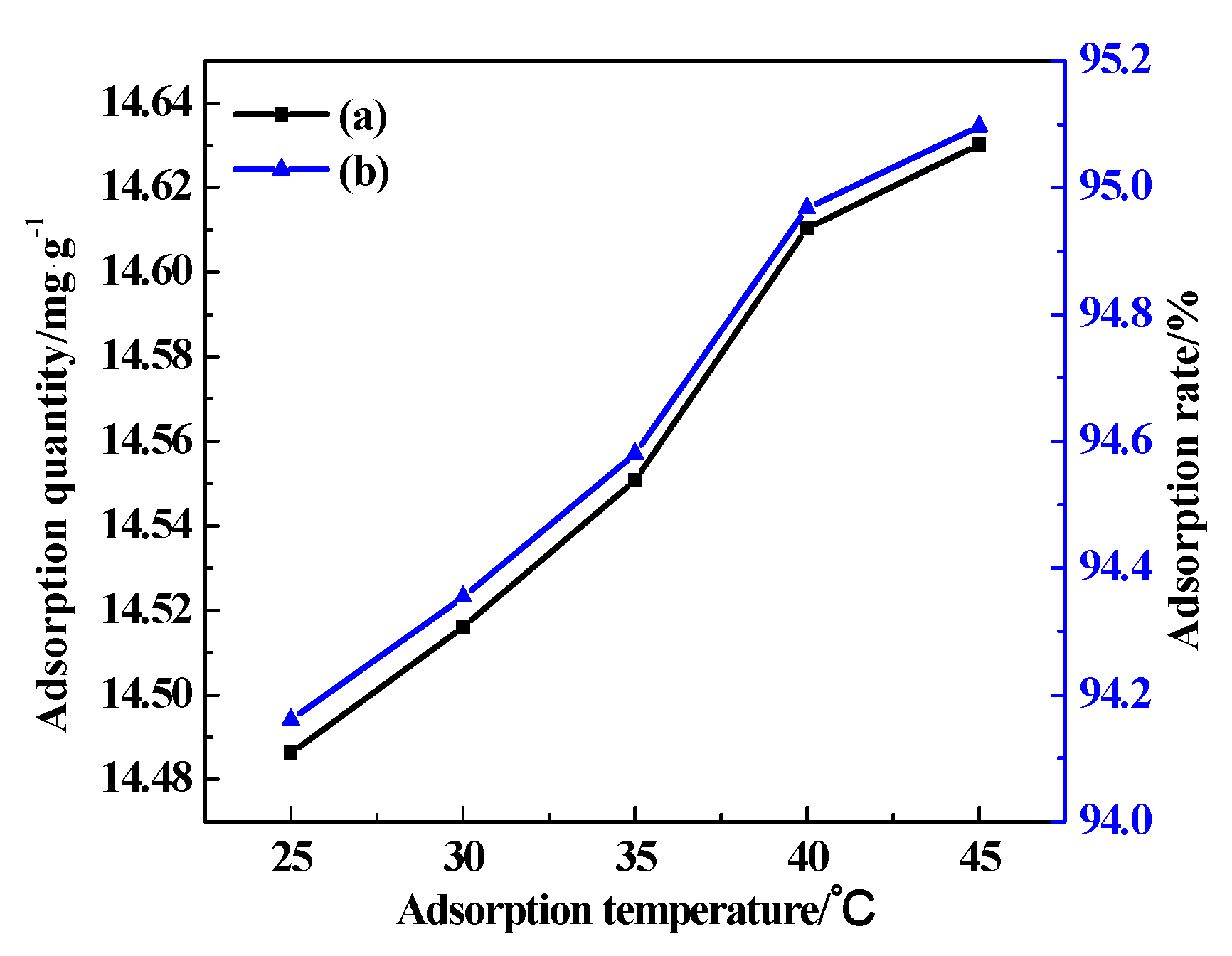
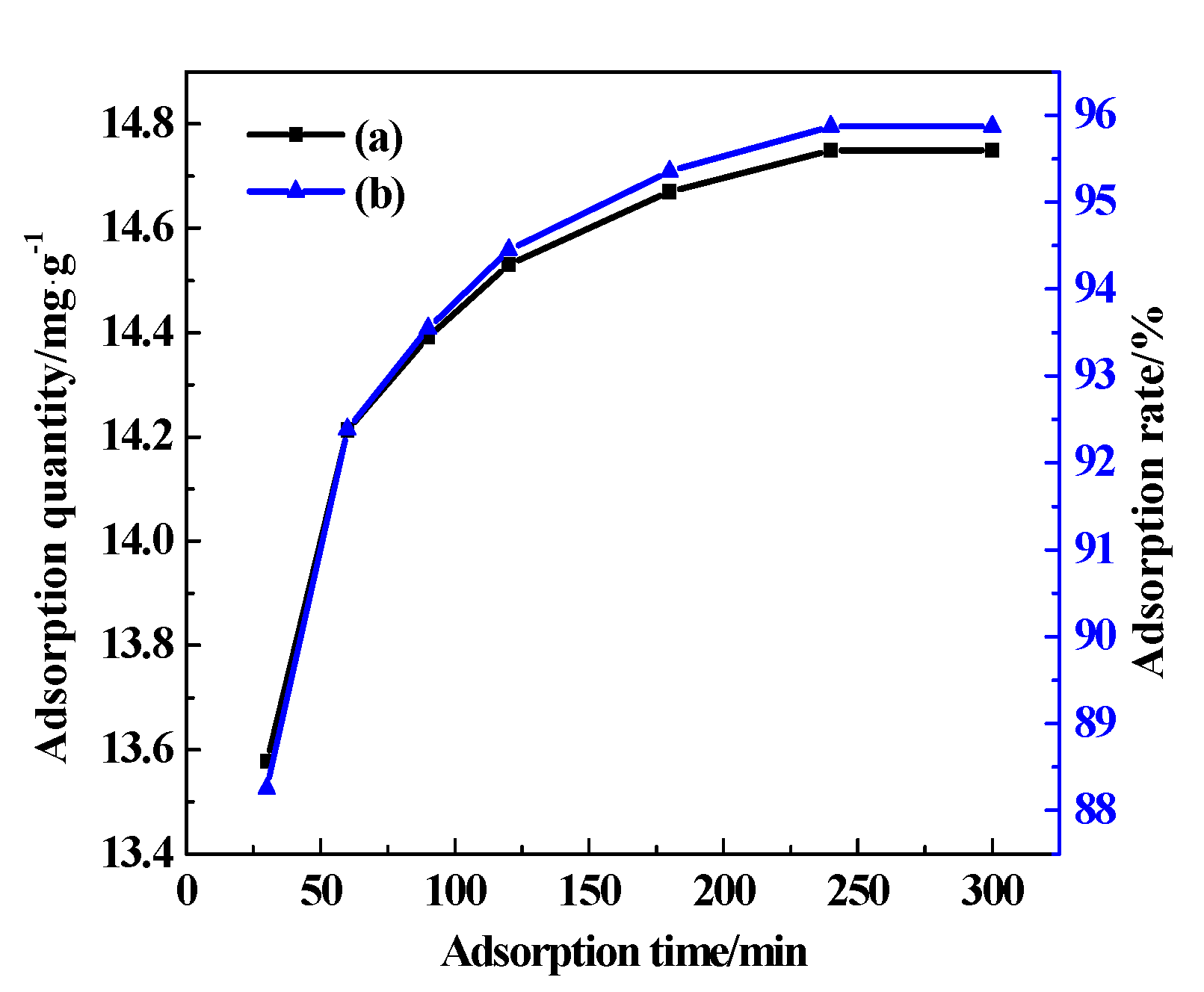
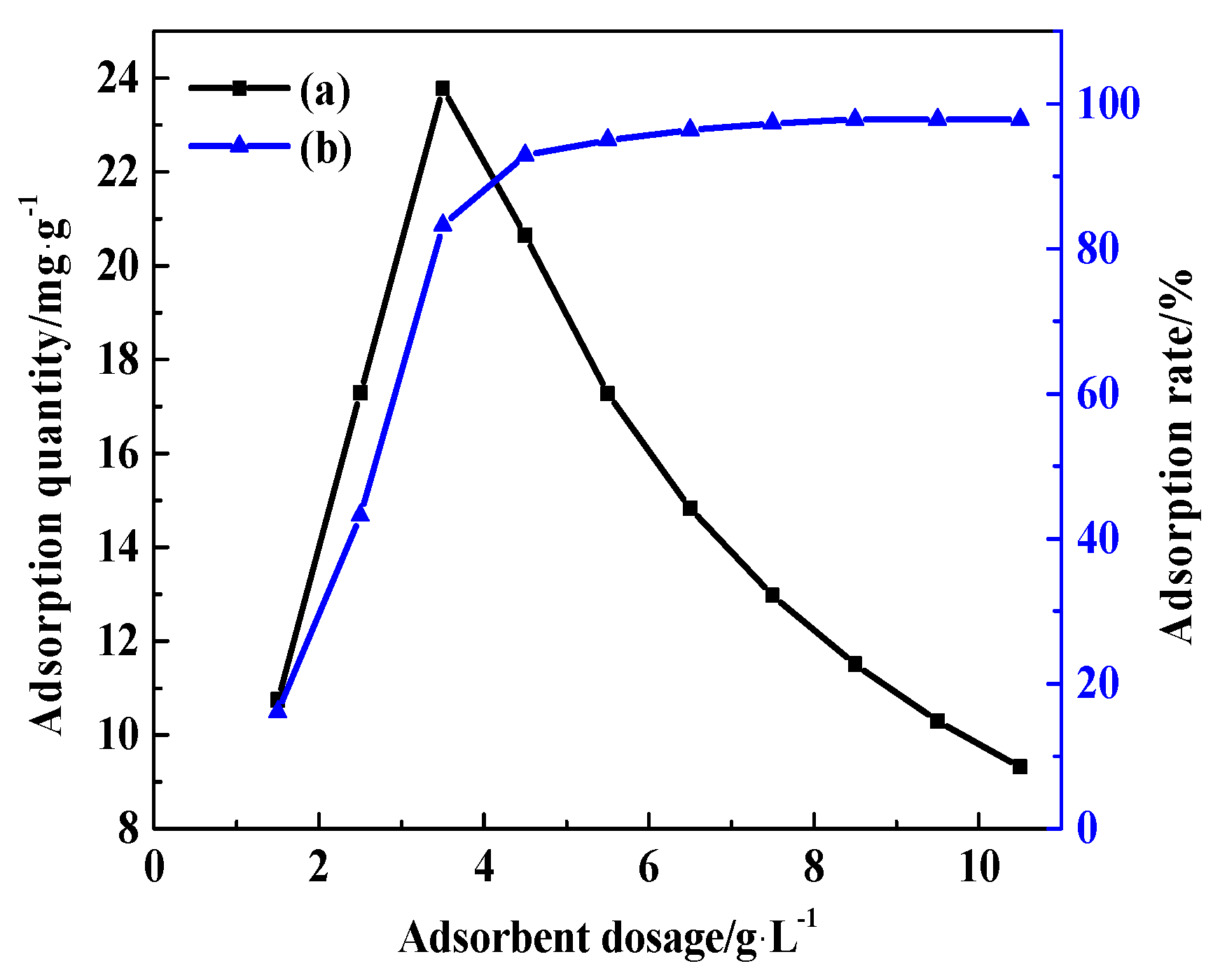

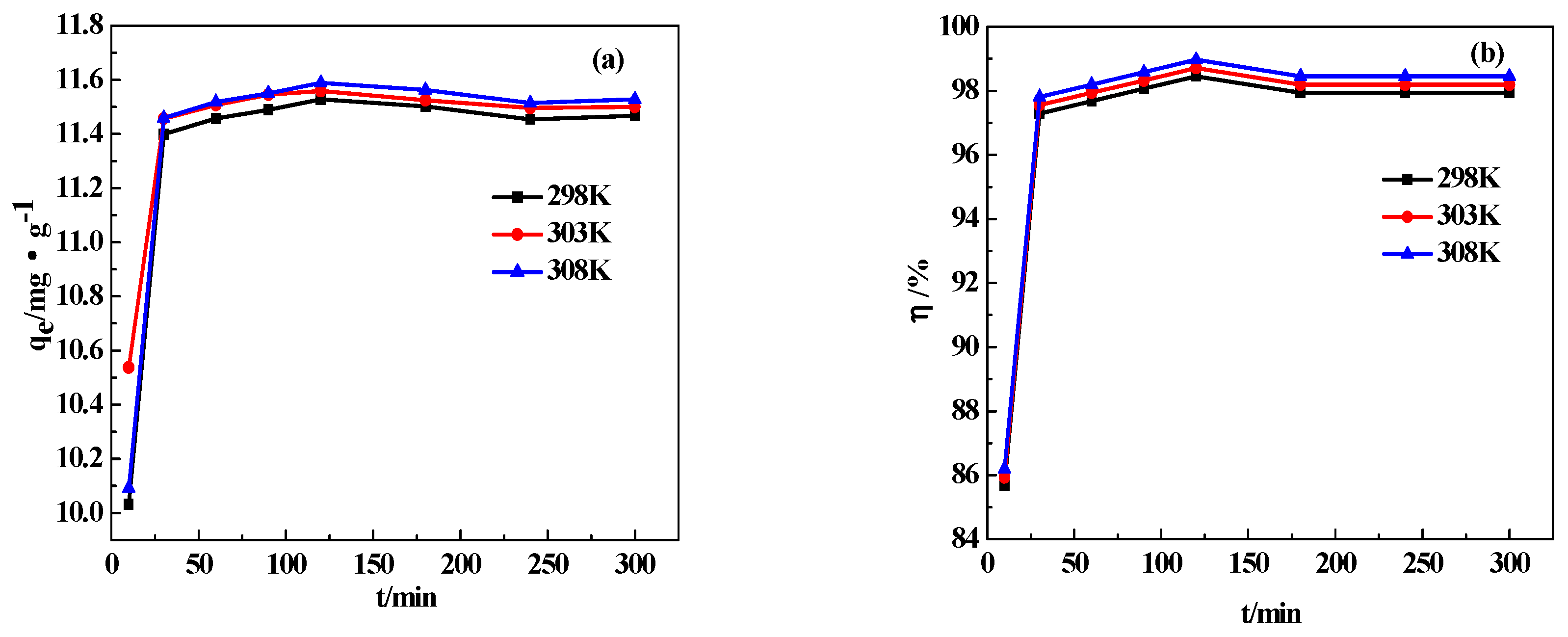

| The Effect of Temperature | The Effect of Time | The Effect of Mass of CDG | The Effect of Initial Concentration of AY11 | The Effect of pH | |
|---|---|---|---|---|---|
| temperature/°C | 25, 30, 35, 40, 45 | 25 | 25 | 25 | 25 |
| time/min | 180 | 30, 60, 90, 120, 180, 240, 300 | 180 | 180 | 180 |
| the mass of CDG/ g/L | 6.5 | 6.5 | 1.5, 2.5, 3.5, 4.5, 5.5, 6.5, 7.5, 8.5, 9.5, 10.5 | 6.5 | 6.5 |
| the initial concentration of AY11/mg/L | 200 | 200 | 200 | 50, 100, 150, 200, 250, 300, 350, 400 | 200 |
| pH | 7 | 7 | 7 | 7 | 1, 2, 3, 4, 5, 6, 7, 8, 9 |
| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| DG | 1.09 | 6.3 × 10−5 | 29.42 |
| CDG | 3.51 | 1.19 × 10−4 | 20.93 |
| T/K | qe,exp/(mg/g) | First-Order Kinetic Model | Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| k1/min−1 | qe,cal/(mg/g) | R2 | k2/[g·(mg·min)−1] | qe,cal/(mg/g) | R2 | ||
| 298 K | 11.33 | 0.146 | 11.34 | 0.997 | 0.029 | 11.67 | 0.892 |
| 303 K | 11.56 | 0.121 | 11.25 | 0.998 | 0.021 | 11.68 | 0.881 |
| 308 K | 11.59 | 0.109 | 11.29 | 0.995 | 0.018 | 11.78 | 0.873 |
| T(K) | Langmuir Equation | Freundlich Equation | ||||
|---|---|---|---|---|---|---|
| qm/(mg/g) | KL/(mL/g) | R2 | KF/(mg/g) | 1/n | R2 | |
| 298 | 32.84 | 0.0116 | 0.9805 | 0.6081 | 0.76 | 0.9637 |
| 303 | 27.15 | 0.0186 | 0.9853 | 0.9562 | 0.67 | 0.9618 |
| 308 | 24.30 | 0.0307 | 0.9758 | 1.5932 | 0.57 | 0.9309 |
| C0/(mg/L) | ΔH0/(kJ·mol−1) | ΔS0/(J·mol·K) | ΔG0/(kJ·mol−1) | ||
|---|---|---|---|---|---|
| 298 K | 303 K | 308 K | |||
| 100 | 0.46 | −15.50 | −5.08 | −5.06 | −5.04 |
| 200 | 0.23 | −16.21 | −5.15 | −5.14 | −5.12 |
| 300 | 0.15 | −16.41 | −5.23 | −5.22 | −5.21 |
| Adsorbent | Dye | Optimum Adsorption Conditions | MG Adsorption qmax (mg/g) | Reference |
|---|---|---|---|---|
| Fe-coated pumice stone | Acid orange 7 | 3.2 g/L; 120 min; pH = 7; 24 °C | 27.68 | [71] |
| Surfactant-coated zeolite | Acid orange 7 | 4.0 g/L; 60 min; pH = 1; 30 °C | 38.96 | [72] |
| Potash alum doped polyaniline | Acid orange 7 | 1.6 g/L; 100 min; pH = 7; 25 °C | 99 | [73] |
| Metanil yellow | 1.6 g/L; 100 min; pH = 7; 25 °C | 142 | ||
| Amino functionalized graphenes | Metanil yellow | 0.4 g/L; 120 min; pH = 7; 25 °C | 71.62 | [74] |
| Polyaniline-bentonite composite | Metanil yellow | 1.0 g/L; 240 min; pH = 7; 25 °C | 444.4 | [75] |
| Polyacrylonitrile/activated carbon composite | Acid yellow 99 | 0.4 g/L; 60 min; pH = 1; 50 °C | 217.39 | [76] |
| Magnetic gelatin-biocomposite | Direct Yellow 12 | 0.1 g/L; 240 min; pH = 3; 30 °C | 476.2 | [77] |
| [Cu2-L]@MWCNT | Sunset yellow | 0.01 g/L; 120 min; pH = 7; 30 °C | 26.63 | [78] |
| Cationic distiller’s grains (CDG) | Acid yellow 11 | 8.5 g/L; 180 min; pH = 7; 25 °C | 636.94 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Kong, D.; Yao, X.; Ma, X.; Wei, C.; Wang, H. Resource Recycling Utilization of Distillers Grains for Preparing Cationic Quaternary Ammonium—Ammonium Material and Adsorption of Acid Yellow 11. Sustainability 2022, 14, 2469. https://doi.org/10.3390/su14042469
Li C, Kong D, Yao X, Ma X, Wei C, Wang H. Resource Recycling Utilization of Distillers Grains for Preparing Cationic Quaternary Ammonium—Ammonium Material and Adsorption of Acid Yellow 11. Sustainability. 2022; 14(4):2469. https://doi.org/10.3390/su14042469
Chicago/Turabian StyleLi, Chengtao, Deyi Kong, Xiaolong Yao, Xiaotao Ma, Chunhui Wei, and Hong Wang. 2022. "Resource Recycling Utilization of Distillers Grains for Preparing Cationic Quaternary Ammonium—Ammonium Material and Adsorption of Acid Yellow 11" Sustainability 14, no. 4: 2469. https://doi.org/10.3390/su14042469
APA StyleLi, C., Kong, D., Yao, X., Ma, X., Wei, C., & Wang, H. (2022). Resource Recycling Utilization of Distillers Grains for Preparing Cationic Quaternary Ammonium—Ammonium Material and Adsorption of Acid Yellow 11. Sustainability, 14(4), 2469. https://doi.org/10.3390/su14042469





