Characterization of Biochar Derived from Crop Residues for Soil Amendment, Carbon Sequestration and Energy Use
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feed Stock and Biochar Preparation
2.2. Biochar Characterization
2.2.1. Proximate Analysis
2.2.2. Ultimate Analysis
2.2.3. Recovery of Total Carbon and Nitrogen in Biochar
2.2.4. Biochar Stability
2.2.5. Carbon Dioxide Reduction Potential
2.2.6. Fuel Properties
2.2.7. Statistical Analyses
3. Results and Discussion
3.1. Biochar Yield
3.2. Proximate Analysis
3.3. Ultimate Analysis
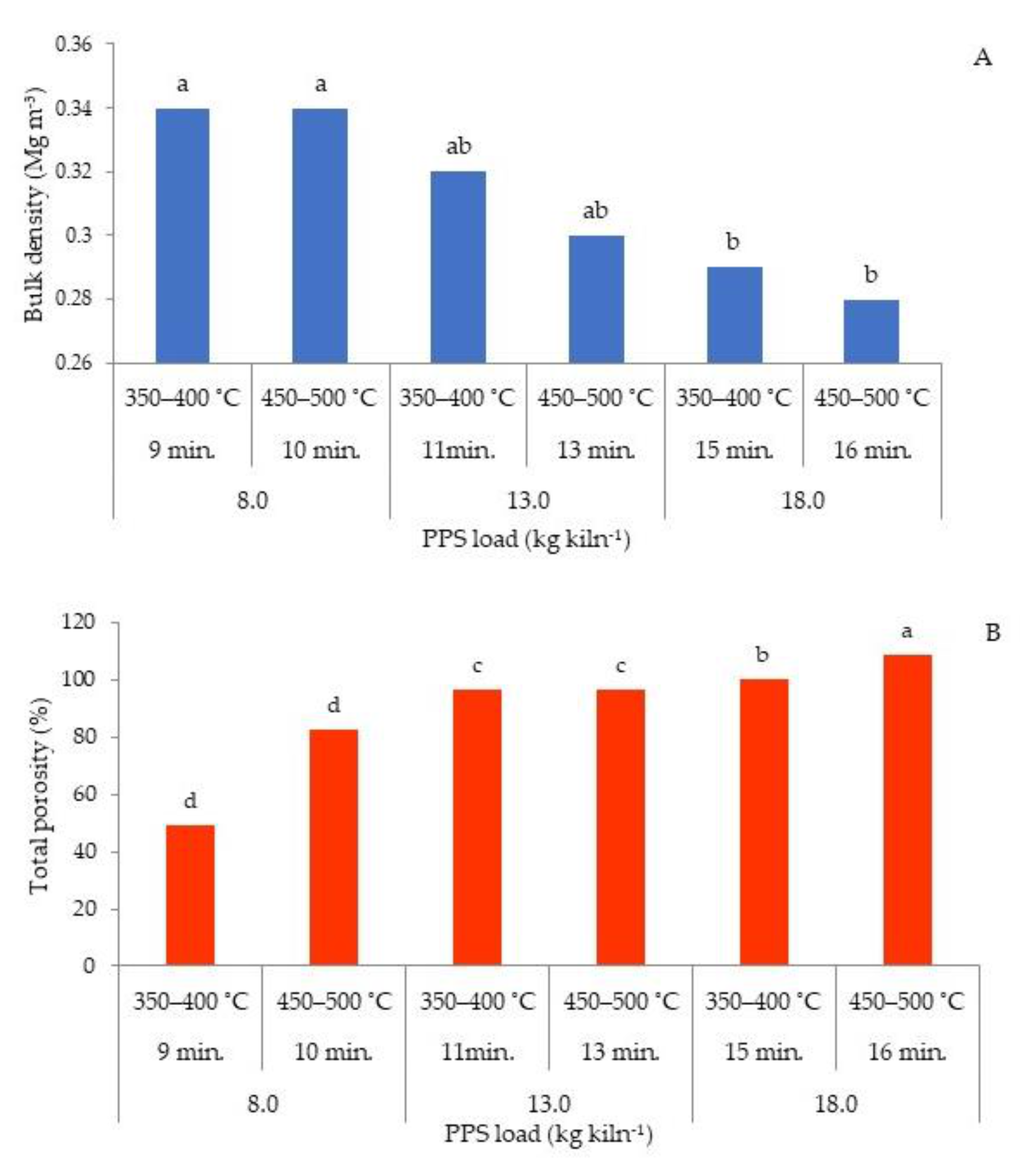
3.3.1. Bulk Density and Total Porosity
3.3.2. pH and Electrical Conductivity
3.3.3. Cation Exchange Capacity
3.3.4. Total Carbon (C), Nitrogen (N), Phosphorous (P) and Potassium (K)
3.3.5. Recovery of Total Carbon and Nitrogen
3.3.6. Biochar Stability
3.3.7. CO2 Reduction Potential
3.3.8. Energy Related Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar]
- Masto, R.E.; Kumar, S.; Rout, T.K.; Sarkar, P.; George, J.; Ram, L.C. Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. Catena 2013, 111, 64–71. [Google Scholar] [CrossRef]
- Sohi, S.; Krull, E.; Capel, E.L.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Geng, C.; Li, S.; Yue, C.; Ma, Y. Pyrolysis characteristics of bituminous coal. J. Energy Inst. 2015, 89, 725–730. [Google Scholar] [CrossRef]
- Valde´s, C.F.; Chejne, F.; Marrugo, G.; Macias, R.J.; Go´mez, C.A.; Montoya, J.I.; Londoño, C.A.; Cruz, J.D.L.; Arenas, E. Co-gasification of subbituminous coal with palm kernel shell in fluidized bed coupled to a ceramic industry process. Appl. Therm. Eng. 2016, 107, 1201–1209. [Google Scholar] [CrossRef]
- Ajayi, A.E.; Horn, R. Modification of chemical andhydro physical properties of two texturally differentiated soils due to varying magnitudes of added biochar. Soil Tillage Res. 2016, 164, 34–44. [Google Scholar] [CrossRef]
- Laghari, M.; Hu, Z.; Mirjat, M.S.; Xiao, B.; Tagar, A.A.; Hu, M. Fast pyrolysis biochar from sawdust improves the quality of desert soils and enhances plant growth. J. Sci. Food Agric. 2016, 96, 199–206. [Google Scholar]
- Phanphanich, M.; Sudhagar Mani, S. Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, S.; Zhu, M.; Song, J.; Peng, P.A. Comprehensive characterization of biochars produced from three major crop straws of China. Bioresources 2017, 12, 3316–3330. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Lou, L.; Luo, L.; Cao, R.; Duan, D.; Chen, Y. Effect of bamboo biochar on pentachlorophenol leachability and bioavailability in agricultural soil. Sci. Total Environ. 2012, 414, 727–731. [Google Scholar] [PubMed]
- Prabha, B.; Pugalendhi, S.; Subramanian, P. Design and development of semi-indirect non-electric pyrolytic reactor for biochar production from farm waste. Indian J. Agric. Sci. 2015, 85, 585–591. [Google Scholar]
- Purakayastha, T.J.; Kumari, S.; Pathak, H. Characterization, stability, and microbial effects of four biochars produced from crop residues. Geoderma 2015, 239–240, 293–303. [Google Scholar] [CrossRef]
- Jothiprakash, G.; Palaniappan, V. Development and optimization of pyrolysis unit for producing charcoal. Int. J. Agric. Environ. Biotechnol. 2014, 7, 863–868. [Google Scholar] [CrossRef]
- Munongo, M.E.; Nkeng, G.E.; Njukeng, J.N. Production and characterization of compost manure and biochar from cocoa pod husks. Int. J. Adv. Scient. Res. Manag. 2017, 2, 26–31. [Google Scholar]
- Ministry of New and Renewable Energy Resources. National Biomass Resource. Atlas; 2009. Available online: https://biomasspower.gov.in/biomass-info-asa-fuel-resources.php (accessed on 12 January 2020).
- Kaur, A. Crop residue in Punjab agriculture- Status and constraints. J. Krishi Vigyan 2017, 5, 22–26. [Google Scholar] [CrossRef]
- IARI. Crop Residues Management with Conservation Agriculture: Potential, Constraints and Policy Needs; Indian Agricultural Research Institute: New Delhi, India, 2012; p. 32. [Google Scholar]
- Government of India. Agricultural Statistics at a Glance 2018. Directorate of Economics and Statistics, Ministry of Agriculture and Farmers Welfare, Department of Agriculture, Cooperation and Farmers Welfare, Government of India. 2019. Available online: https://eands.dacnet.nic.in/PDF/Agricultural%20Statistics%20at%20a%20Glance%202018.pdf (accessed on 1 February 2020).
- Venkatesh, G.; Gopinath, K.A.; Reddy, K.S.; Reddy, B.S.; Prasad, J.V.N.S.; Rao, G.R.; Pratibha, G.; Srinivasarao, C.; Chary, G.R.; Prabhakar, M.; et al. Biochar Production and its Use in Rainfed Agriculture: Experiences from CRIDA; CRIDA-NICRA Research Bulletin 02/2018; ICAR—Central Research Institute for Dryland Agriculture: Hyderabad, India, 2018; p. 50. [Google Scholar]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212. [Google Scholar]
- Gonzalez, M.E.; Cea, M.; Sangaletti, N.; Gonzalez, A.; Toro, C.; Diez, M.C.; Moreno, N.; Querol, X.; Navia, R. Biochar derived from agricultural and forestry residual biomass: Characterization and potential application for enzymes immobilization. J. Biobased Mater. Bioenerg. 2013, 7, 724–732. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of low temperature pyrolysis conditions on biochar for agricultural use. Tran. Am. Soc. Agric. Biol. Eng. 2008, 51, 2061–2069. [Google Scholar]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [PubMed]
- Hass, A.; Gonzalez, J.M.; Lima, I.M.; Godwin, H.W.; Halvorson, J.J.; Boyer, D.G. Chicken manure biochar as liming and nutrient source for acid Appalachian soil. J. Environ. Qual. 2012, 41, 1096–1106. [Google Scholar] [PubMed]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [PubMed]
- Uras, U.; Carrier, M.; Hardie, A.G.; Knoetze, J.H. Physico-chemical characterization of biochars from vacuum pyrolysis of South African agricultural wastes for application as soil amendments. J. Anal. Appl. Pyrol. 2012, 98, 207–213. [Google Scholar] [CrossRef]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenerg. 2012, 47, 268–276. [Google Scholar]
- Venkatesh, G.; Srinivasarao, C.; Venkateswarlu, B.; Gopinath, K.A.; Prasad, J.V.N.S.; Reddy, B.S.; Sasikala, C.; Rao, G.; Babu, P.V.R. Operational process for biochar preparation from castor bean stalk and its characterization for soil application. Indian J. Dryland Agric. Res. Dev. 2013, 28, 21–26. [Google Scholar]
- Venkatesh, G.; Venkateswarlu, B.; Gopinath, K.A.; Srinivasrao, C.; Korwar, G.R.; Reddy, B.S.; Prasad, J.V.N.S.; Grover, M.; Raju, B.M.K.; Sasikala, C.; et al. Biochar production technology for conversion of cotton stalk bioresidue into biochar and its characterization for soil amendment qualities. Indian J. Dryland Agric. Res. Dev. 2013, 28, 48–57. [Google Scholar]
- Naeem, M.A.; Khalid, M.; Arshad, M.; Ahmad, R. Yield and nutrient composition of biochar produced from different feedstocks at varying pyrolytic temperatures. Pak. J. Agric. Sci. 2014, 51, 75–82. [Google Scholar]
- Xiong, S.; Zhang, S.; Wu, Q.; Guo, X.; Dong, A.; Chen, C. Investigation on cotton stalk and bamboo sawdust carbonization for barbecue charcoal preparation. Bioresour. Technol. 2014, 152, 86–92. [Google Scholar] [PubMed]
- Anupam, K.; Sharma, A.K.; Lal, P.S.; Dutta, S.; Maity, S. Preparation, characterization and optimization for upgrading Leucaena leucocephala bark to biochar fuel with high energy yielding. Energy 2016, 106, 743–756. [Google Scholar]
- Narzari, R.; Bordoloi, N.; Chutia, R.S.; Borkotoki, B.; Gogoi, N.; Bora, A.; Kataki, R. Biochar: An Overview on its Production, Properties and Potential Benefits. In Biology, Biotechnology and Sustainable Development, Research; Choudhury, H., Ed.; India Publications: New Delhi, India, 2015; pp. 13–39. [Google Scholar]
- Kookana, R.S.; Sarmah, A.K.; Van Zwieten, L.; Krull, E.; Singh, B. Biochar application to soil: Agronomic and environmental benefits and unintended consequences. Adv. Agron. 2011, 112, 103–143. [Google Scholar]
- Lehmann, J.; Abiven, S.; Kleber, M.; Pan, G.; Singh, B.P.; Sohi, S.P.; Zimmerman, A.R. Persistence of biochar in soil. In Biochar for Environmental Management: Science, Technology, and Implementation, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 235–283. [Google Scholar]
- Usman, A.R.A.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Anal. Appl. Pyrol. 2015, 115, 392–400. [Google Scholar]
- Klasson, K.T. Biochar characterization and a method for estimating biochar quality from proximate analysis results. Biomass Bioenerg. 2017, 96, 50–58. [Google Scholar]
- Shen, Y.; Yu, S.; Ge, S.; Chen, X.; Ge, X.; Chen, M. Hydrothermal carbonization of medical wastes and lignocellulosic biomass for solid fuel production from lab-scale to pilot-scale. Energy 2017, 118, 312–323. [Google Scholar]
- Kaewtrakulchai, N.; Fuji, M.; Eiad-ua, A. Investigation of parametric effects on fuel characteristics of biochar obtained from agricultural wastes pyrolysis. J. Material. Sci. Appl. Energ. 2018, 7, 333–339. [Google Scholar]
- Wei, S.; Zhu, M.; Fan, X.; Song, J.; Peng, P.; Li, K.; Jia, W.; Song, H. Influence of pyrolysis temperature and feedstock on carbon fractions of biochar produced from pyrolysis of rice straw, pine wood, pig manure and sewage sludge. Chemosphere 2019, 218, 624–631. [Google Scholar] [PubMed]
- Wijitkosum, S.; Jiwnok, P. Elemental composition of biochar obtained from agricultural waste for soil amendment and carbon sequestration. Appl. Sci. 2019, 9, 3980. [Google Scholar] [CrossRef] [Green Version]
- Allyson, S. Biochar Production for Carbon Sequestration. Bachelor’s Thesis, Worcester Polytechnique Institute, Worcester, MA, USA, 2011. [Google Scholar]
- Gangil, S.; Wakudkar, H.M. Generation of bio-char from crop residues. Int. J. Emerging Technol. Adv. Eng. 2013, 3, 566–570. [Google Scholar]
- Tesfamichael, B.; Gesesse, N.; Jabasingh, S.A. Application of rice husk and maize straw biochar for carbon sequestration and nitrous oxide emission impedement. J. Sci. Ind. Res. 2018, 77, 587–591. [Google Scholar]
- Lu, X.; Pellechia, P.J.; Flora, J.R.V.; Berge, N.D. Influence of reaction time and temperature on product formation and characteristics associated with the hydrothermal carbonization of cellulose. Bioresour. Technol. 2013, 138, 180–190. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Upgrading the characteristics of biochar from cellulose, lignin, and xylan for solid biofuel production from biomass by hydrothermal carbonization. J Ind. Eng. Chem. 2016, 42, 95–100. [Google Scholar]
- Yang, W.; Wang, H.; Zhang, M.; Zhu, J.; Zhou, J.; Wu, S. Fuel properties and combustion kinetics of hydrochar prepared by hydrothermal carbonization of bamboo. Bioresour. Technol. 2016, 205, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar]
- Nakason, K.; Pathomrotsakun, J.; Kraithong, W.; Khemthong, P.; Panyapinyopol, B. Torrefaction of Agricultural Wastes: Influence of Lignocellulosic Types and Treatment Temperature on Fuel Properties of Biochar. Int. Energy J. 2019, 19, 253–266. [Google Scholar]
- Parikh, J.; Channiwala, S.A.; Ghosal, G.K. A correlation for calculating elemental composition from proximate analysis of biomass materials. Fuel 2007, 86, 1710–1719. [Google Scholar]
- Tillman, D.; Rossi, A.J.; William, D.K. Wood Combustion: Principles, Processes, and Economics; Academic Press: New York, NY, USA, 1981; p. 280. [Google Scholar]
- Lee, Y.; Park, J.; Ryu, C.; Gang, K.S.; Yang, W.; Park, Y.K.; Jung, J.; Hyun, S. Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 °C. Bioresour. Technol. 2013, 148, 196–201. [Google Scholar] [PubMed]
- Antal, M.J.; Gronli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar]
- ASTM. Standard Test Method for Chemical Analysis of Wood Charcoal D 1762-84; American Society for Testing and Material: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Baruah, T.C.; Barthakur, H.P. A Text Book of Soil Analysis; Vikas Publishing House Pvt. Ltd.: New Delhi, India, 1997; p. 234. [Google Scholar]
- Miller, R. Nitric-per Chloric Acid Wet Digestion in an Open Vessel. In Hand Book of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: New York, NY, USA, 1998; pp. 57–61. [Google Scholar]
- Streubel, J.D.; Collins, H.P.; Perez, M.G.; Tarara, J.; Granatstein, D.; Kruger, C.E. Influence of contrasting biochar types on five soils at increasing rates of application. Soil Sci. Soc. Am. J. 2011, 75, 1402–1413. [Google Scholar]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis. 2004, 72, 243–248. [Google Scholar]
- Downie, A.; Crosky, A.; Munroe, P. Physical Properties of Biochar. In Biochar for Environmental Management: Science, Technology, and Implementation, 1st ed.; Lehmann, J., Joseph, S., Eds.; Earth Scan: London, UK, 2009; pp. 12–13. [Google Scholar]
- Singh, B.; Singh, B.P.; Cowie, A.L. Characterization and evaluation of biochars and their application as a soil amendment. Aust. J. Soil. Res. 2010, 48, 516–525. [Google Scholar]
- Harvey, O.R.; Herbert, B.E.; Kuo, L.J.; Louchouarn, P. Generalized two- dimensional perturbation correlation infrared spectroscopy reveals mechanisms for the development of surface charge and recalcitrance in plant-derived biochars. Environ. Sci. Technol. 2012, 46, 10641–10650. [Google Scholar] [CrossRef] [PubMed]
- Spokas, K. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar]
- Kimetu, J.M.; Lehmann, J.; Ngoze, O.S.; Mugendi, N.D.; Kinyangi, M.J.; Riha, S.; Verchot, L.; Recha, W.J.; Pell, N.A. Reversibility of soil productivity decline with organic matter of differing quality along a degradation gradient. Ecosystems 2008, 11, 726–739. [Google Scholar]
- Knoepp, J.D.; Debano, L.F.; Neary, D.G. Soil Chemistry; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2005.
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 85, 91–118. [Google Scholar]
- Qian, K.; Kumar, A.; Patil, K.; Bellme, D.; Wang, D.; Yuan, W.; Huhnke, R.L. Effects of Biomass Feedstocks and Gasification Conditions on the Physiochemical Properties of Char. Energies 2013, 6, 3972–3986. [Google Scholar] [CrossRef]
- IBI Guidelines. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. 2012. Available online: http://www.biocharnternational.org/sites/default/files/Guidelines_for_Biochar_That_Is_Used_in_Soil_Final.pdf (accessed on 11 October 2015).
- EBC. European Biochar Certificate—Guidelines for a Sustainable Production of Biochar. European Biochar Foundation (EBC): Arbaz, Switzerland, 2012. Available online: http://www.europeanbiochar.org/en/download (accessed on 1 January 2020).
- Guo, J.; Chen, B. Insights on the molecular mechanism for the recalcitrance of biochar: Interactive effects of carbon and silicon components. Environ. Sci. Technol. 2014, 48, 9103–9112. [Google Scholar]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.M.; Fuhner, C.; Bens, O.; Jurgen, K.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 89–124. [Google Scholar]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Ghugare, S.B.; Tiwary, S.; Elangovan, V.; Tambe, S.S. Prediction of higher heating value of solid biomass fuels using artificial intelligence formalisms. Bioenergy Res. 2013, 7, 681–692. [Google Scholar]
- An, Y.; Tahmasebi, A.; Yu, J. Mechanism of synergy effect during microwave co-pyrolysis of biomass and lignite. J. Anal. Appl. Pyrol. 2017, 128, 75–82. [Google Scholar] [CrossRef]
- Abdullah, H.; Wu, H. Biochar as a fuel: 1. Properties and grindability of biochars produced from the pyrolysis of mallee wood under slow-heating conditions. Energy Fuels 2009, 23, 4174–4181. [Google Scholar]
| Load (kgkiln−1) | Reaction Time (min) | Color Phase Development | Corresponding Temperature Range (°C) |
|---|---|---|---|
| 8 | 9 | Grey | 350–400 |
| 8 | 10 | Blue | 450–500 |
| 13 | 11 | Grey | 350–400 |
| 13 | 13 | Blue | 450–500 |
| 18 | 15 | Grey | 350–400 |
| 18 | 16 | Blue | 450–500 |
| Load (kgkiln−1) | Reaction Time (min) | Temp. Range (°C) | Biochar Yield 1 (%) | Ash 2 (%) | VM 2 (%) | FC 2 (%) |
|---|---|---|---|---|---|---|
| 8 | 9 | 350–400 | 21.3 ± 1.0 b | 32.9 ± 0.9 b | 26.0 ± 2.0 a | 41.1 ± 0.9 d |
| 8 | 10 | 450–500 | 18.5 ± 0.3 c | 40.1 ± 0.9 a | 16.7 ± 0.5 b | 43.1 ± 0.6 d |
| 13 | 11 | 350–400 | 21.6 ± 1.0 b | 40.4 ± 0.4 a | 15.8 ± 0.4 b | 43.8 ± 0.7 d |
| 13 | 13 | 450–500 | 13.5 ± 0.3 d | 41.7 ± 0.5 a | 11.8 ± 0.5 c | 46.7 ± 1.0 c |
| 18 | 15 | 350–400 | 36.1 ± 0.8 a | 31.2 ± 0.7 b | 16.9 ± 0.9 b | 51.9 ± 0.7 b |
| 18 | 16 | 450–500 | 35.0 ± 0.6 a | 21.3 ± 1.0 c | 13.4 ± 0.9 c | 65.3 ± 3.0 a |
| Load (kg kiln−1) | Reaction Time (min.) | Temp. Range (°C) | pH | EC (dS m−1) | CEC (cmol kg−1) | Total Concentration (g kg−1) | C/N Ratio | |||
|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | C | |||||||
| 8 | 9 | 350–400 | 7.4 ± 0.22 a | 0.01 ± 0.0 b | 17.0 ± 1.0 c | 11.1 ± 0.7 d | 3.3 ± 0.2 e | 4.0 ± 0.6 a | 665.7 ± 0.8 e | 60.5 |
| 8 | 10 | 450–500 | 7.4 ± 0.08 a | 0.02 ± 0.01 ab | 14.0 ± 3.0 c | 13.0 ± 1.0 bc | 4.0 ± 0.2 de | 4.0 ± 0.6 a | 666.4 ± 6.0 e | 51.3 |
| 13 | 11 | 350–400 | 7.5 ± 0.08 a | 0.03 ± 0.01 ab | 39.0 ± 3.0 a | 11.0 ± 0.5 d | 5.0 ± 0.2 c | 4.1 ± 0.3 a | 719.0 ± 4.0 d | 65.4 |
| 13 | 13 | 450–500 | 7.7 ± 0.08 a | 0.04 ± 0.01 ab | 33.0 ± 0.9 b | 14.0 ± 1.0 b | 6.0 ± 0.2 b | 4.0 ± 0.3 a | 730.0 ± 4.0 c | 52.1 |
| 18 | 15 | 350–400 | 7.6 ± 0.22 a | 0.05 ± 0.01 a | 43.0 ± 1.0 a | 11.3 ± 0.3 cd | 3.8 ± 0.3 de | 4.1 ± 0.2 a | 740.0 ± 2.0 b | 65.8 |
| 18 | 16 | 450–500 | 7.8 ± 0.08 a | 0.05 ± 0.01 a | 40.0 ± 1.0 a | 17.0 ± 0.6 a | 8.0 ± 0.3 a | 4.0 ± 0.6 a | 756.5 ± 1.0 a | 44.5 |
| Load (kg kiln−1) | Reaction Time (min.) | Temp. Range (°C) | Biochar Yield (kg) | Total C in PPS (kg) | Total C in Biochar (kg) | C Recovery in Biochar # (%) | Total N in PPS (kg) | Total N in Biochar (kg) | N Recovery in Biochar # (%) |
|---|---|---|---|---|---|---|---|---|---|
| 8 | 9 | 350–400 | 1.7 | 3.7 | 1.1 | 30.8 ± 0.04 a | 0.10 | 0.02 | 20.7 ± 1.3 a |
| 8 | 10 | 450–500 | 1.5 | 3.7 | 1.0 | 26.8 ± 0.23 b | 0.10 | 0.02 | 21.2 ± 1.6 b |
| 13 | 11 | 350–400 | 2.8 | 6.0 | 2.0 | 33.8 ± 0.20 c | 0.10 | 0.03 | 21.0 ± 1.0 c |
| 13 | 13 | 450–500 | 1.8 | 6.0 | 1.3 | 21.4 ± 0.11 d | 0.10 | 0.02 | 16.7 ± 1.1 c |
| 18 | 15 | 350–400 | 6.5 | 8.3 | 4.8 | 58.1 ± 0.12 e | 0.20 | 0.07 | 36.0 ± 0.9 c |
| 18 | 16 | 450–500 | 6.3 | 8.3 | 4.8 | 57.6 ± 0.10 f | 0.20 | 0.11 | 52.5 ± 1.8 d |
| Parameters | Biochar Production Conditions | |||||
|---|---|---|---|---|---|---|
| Load (kg kiln−1) | 8 | 13 | 18 | |||
| Reaction time (min.) | 9 | 10 | 11 | 13 | 15 | 16 |
| Temp. range (°C) | 350–400 | 450–500 | 350–400 | 450–500 | 350–400 | 450–500 |
| Biochar stability | ||||||
| H | 2.38 | 1.73 | 1.68 | 1.42 | 1.86 | 1.76 |
| O | 10.33 | 5.50 | 5.06 | 3.12 | 6.29 | 5.47 |
| O/C atomic ratio | 0.15 | 0.11 | 0.10 | 0.08 | 0.10 | 0.07 |
| H/C atomic ratio | 0.50 | 0.40 | 0.39 | 0.35 | 0.38 | 0.33 |
| Mean resident time (years) | 903.60 | 1231.66 | 1274.78 | 1462.75 | 1333.41 | 1553.58 |
| BC+100 (%) | 74.08 | 80.05 | 80.72 | 83.36 | 81.58 | 84.52 |
| CO2 reduction potential | ||||||
| Total potential carbon (g kg−1) | 87.56 | 79.58 | 94.37 | 62.93 | 187.07 | 228.66 |
| CO2 reduction potential (CO2 eq kg−1) | 25.69 | 23.34 | 27.68 | 18.46 | 54.87 | 67.07 |
| Parameters | Biochar Production Conditions | |||||
|---|---|---|---|---|---|---|
| Load (kgkiln−1) | 8 | 13 | 18 | |||
| Reaction time (min.) | 9 | 10 | 11 | 13 | 15 | 16 |
| Temp. range (°C) | 350–400 | 450–500 | 350–400 | 450–500 | 350–400 | 450–500 |
| Fuel Properties | ||||||
| Fuel ratio | 1.6 | 2.6 | 2.8 | 3.96 | 3.08 | 4.88 |
| Energetic retention efficiency (%) | 23.6 | 19.6 | 23.0 | 14.7 | 45.2 | 53.0 |
| Energy densification | 1.1 | 1.06 | 1.1 | 1.09 | 1.25 | 1.51 |
| HHV (MJ kg−1) | 18.3 | 17.53 | 17.6 | 18.02 | 20.74 | 25.01 |
| HHV improvement | 0.1 | 0.06 | 0.1 | 0.09 | 0.25 | 0.51 |
| FC densification | 2.42 | 2.53 | 2.58 | 2.75 | 3.05 | 3.84 |
| FC recovery efficiency (%) | 51.51 | 46.81 | 55.51 | 37.02 | 110.04 | 134.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesh, G.; Gopinath, K.A.; Reddy, K.S.; Reddy, B.S.; Prabhakar, M.; Srinivasarao, C.; Visha Kumari, V.; Singh, V.K. Characterization of Biochar Derived from Crop Residues for Soil Amendment, Carbon Sequestration and Energy Use. Sustainability 2022, 14, 2295. https://doi.org/10.3390/su14042295
Venkatesh G, Gopinath KA, Reddy KS, Reddy BS, Prabhakar M, Srinivasarao C, Visha Kumari V, Singh VK. Characterization of Biochar Derived from Crop Residues for Soil Amendment, Carbon Sequestration and Energy Use. Sustainability. 2022; 14(4):2295. https://doi.org/10.3390/su14042295
Chicago/Turabian StyleVenkatesh, Govindarajan, Kodigal A. Gopinath, Kotha Sammi Reddy, Baddigam Sanjeeva Reddy, Mathyam Prabhakar, Cherukumalli Srinivasarao, Venugopalan Visha Kumari, and Vinod Kumar Singh. 2022. "Characterization of Biochar Derived from Crop Residues for Soil Amendment, Carbon Sequestration and Energy Use" Sustainability 14, no. 4: 2295. https://doi.org/10.3390/su14042295
APA StyleVenkatesh, G., Gopinath, K. A., Reddy, K. S., Reddy, B. S., Prabhakar, M., Srinivasarao, C., Visha Kumari, V., & Singh, V. K. (2022). Characterization of Biochar Derived from Crop Residues for Soil Amendment, Carbon Sequestration and Energy Use. Sustainability, 14(4), 2295. https://doi.org/10.3390/su14042295






