The Reuse of Municipal Solid Waste Fly Ash as Flame Retardant Filler: A Preliminary Study
Abstract
:1. Introduction
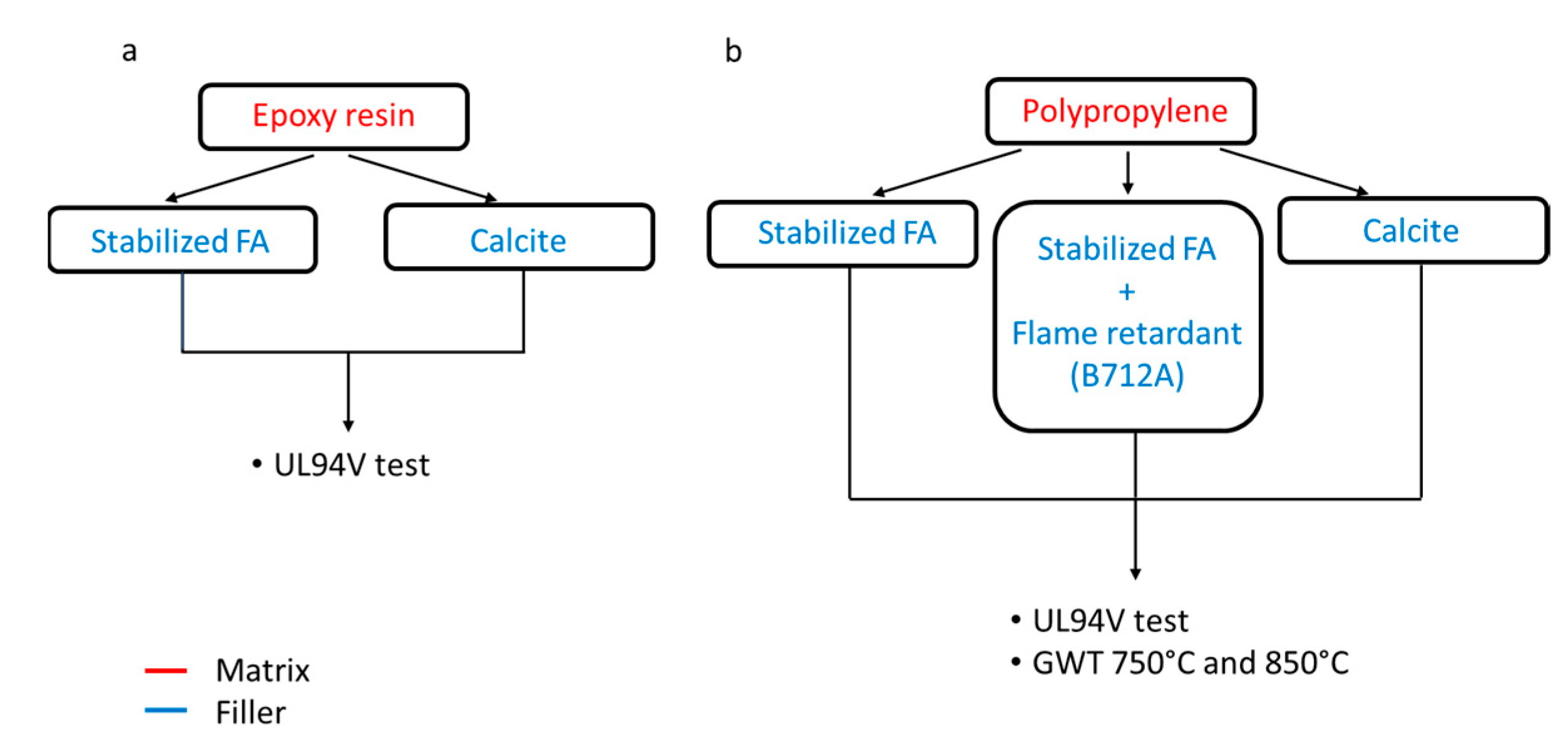
2. Experimental Section
2.1. Materials and Methods
2.2. Epoxy Resin Matrix
2.2.1. Sample Preparation
2.2.2. UL94-V Test
- The candle was positioned at 10 ± 1 mm from lower bound of the sample for at least 10 ± 0.5 s. After this time, the flame was removed and afterflame time (t1) was evaluated;
- As soon as the sample stopped burning, the flame was placed again under the sample at the same distance as before for 10 ± 0.5 s;
- After this period, the flame was removed, and it was possible to calculate afterflame (t2) and afterglow (t3).
2.3. Polypropylene Matrix
2.3.1. Samples Preparation
2.3.2. GWT Test
2.4. Life Cycle Assessment (LCA) Modelling
3. Results and Discussion
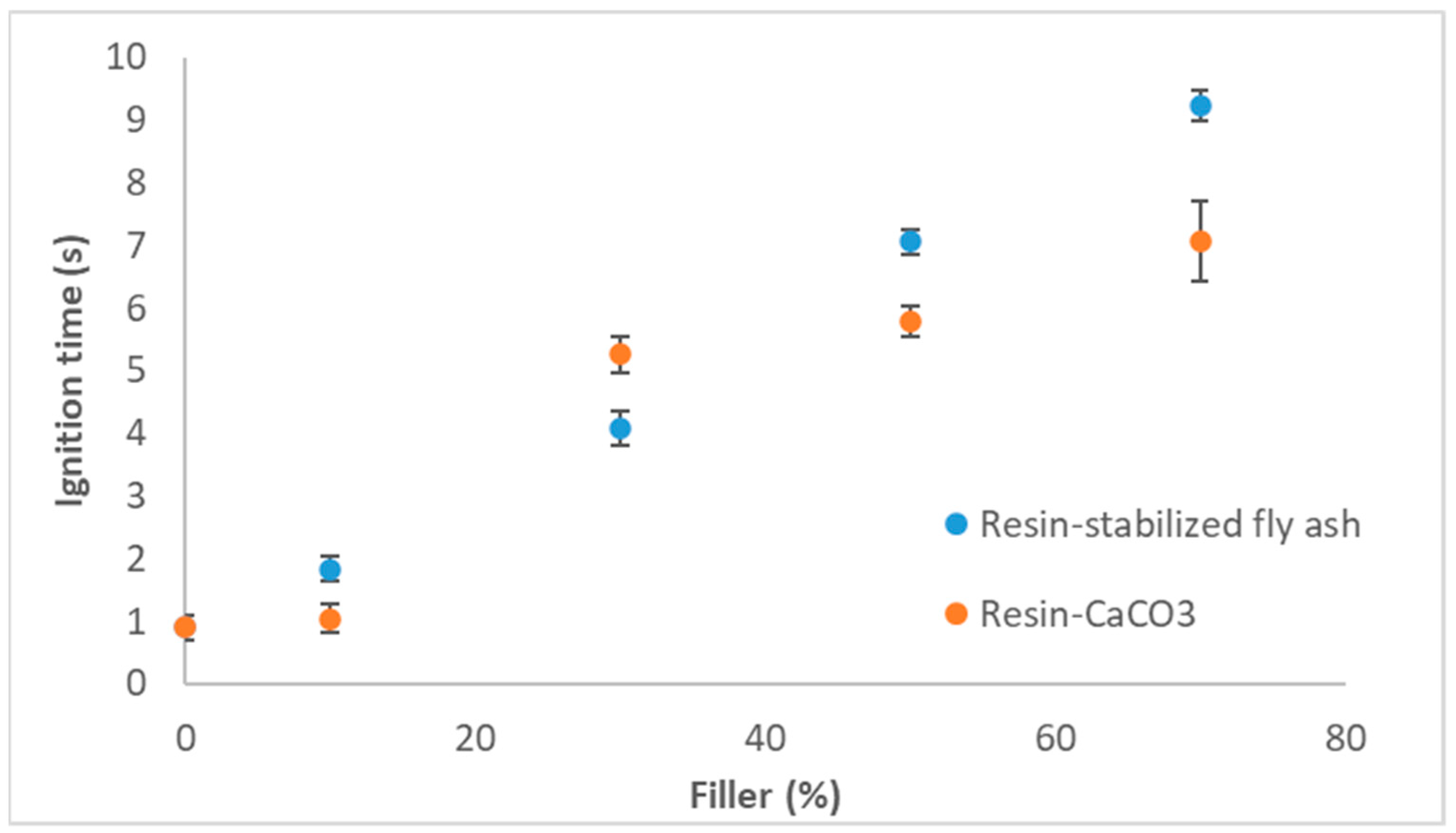
3.1. Epoxy Resin
3.2. Polypropylene
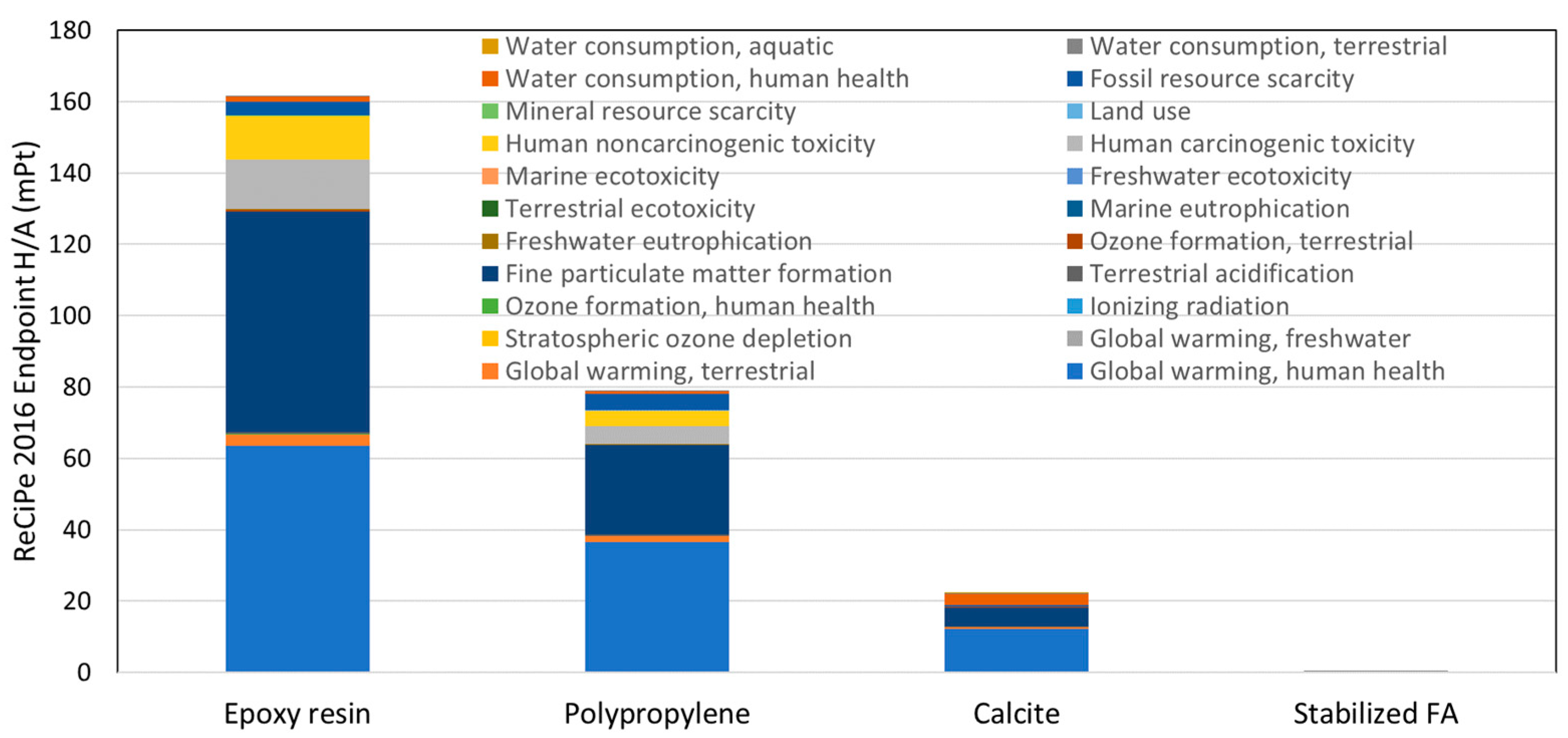
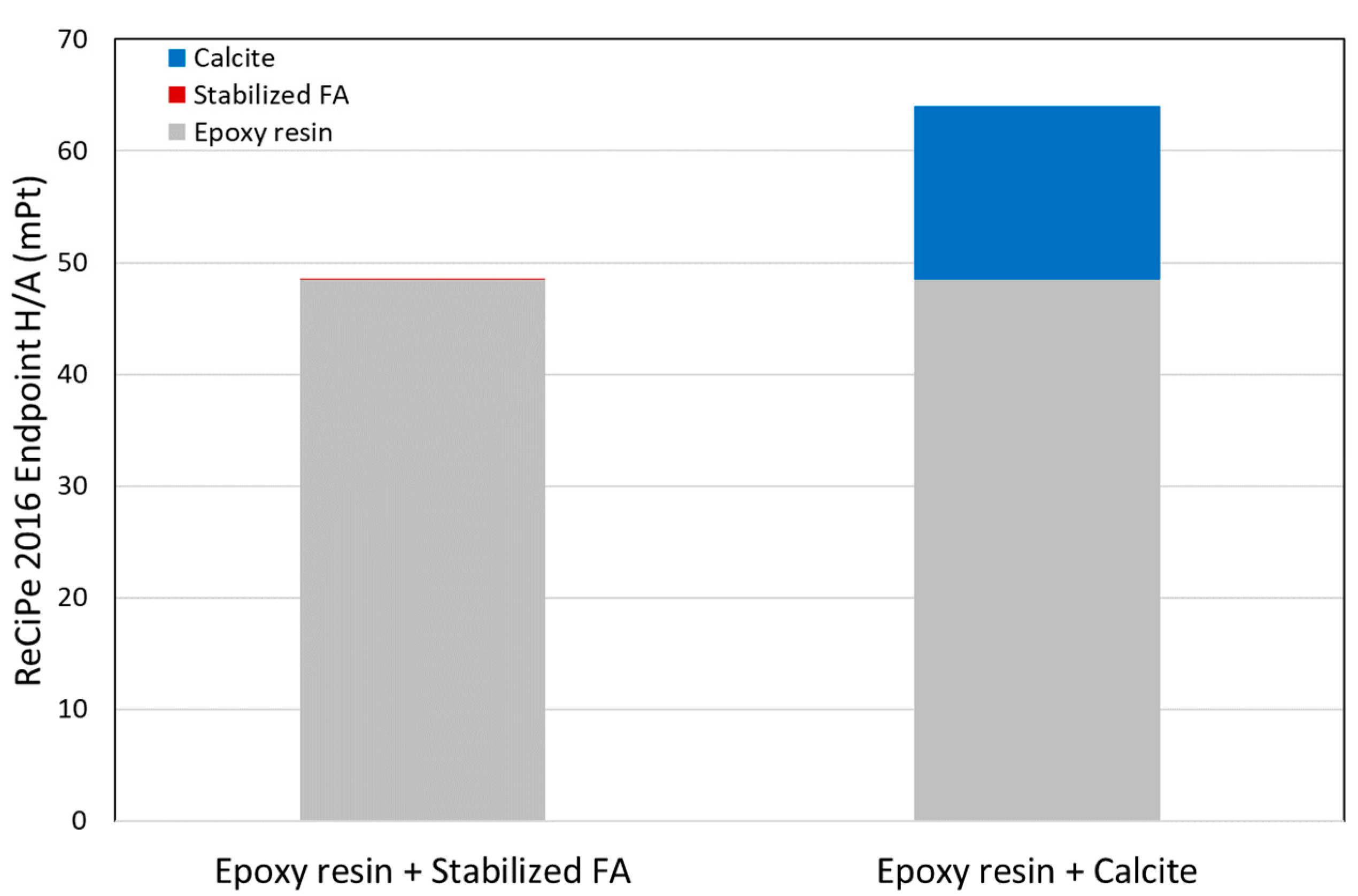
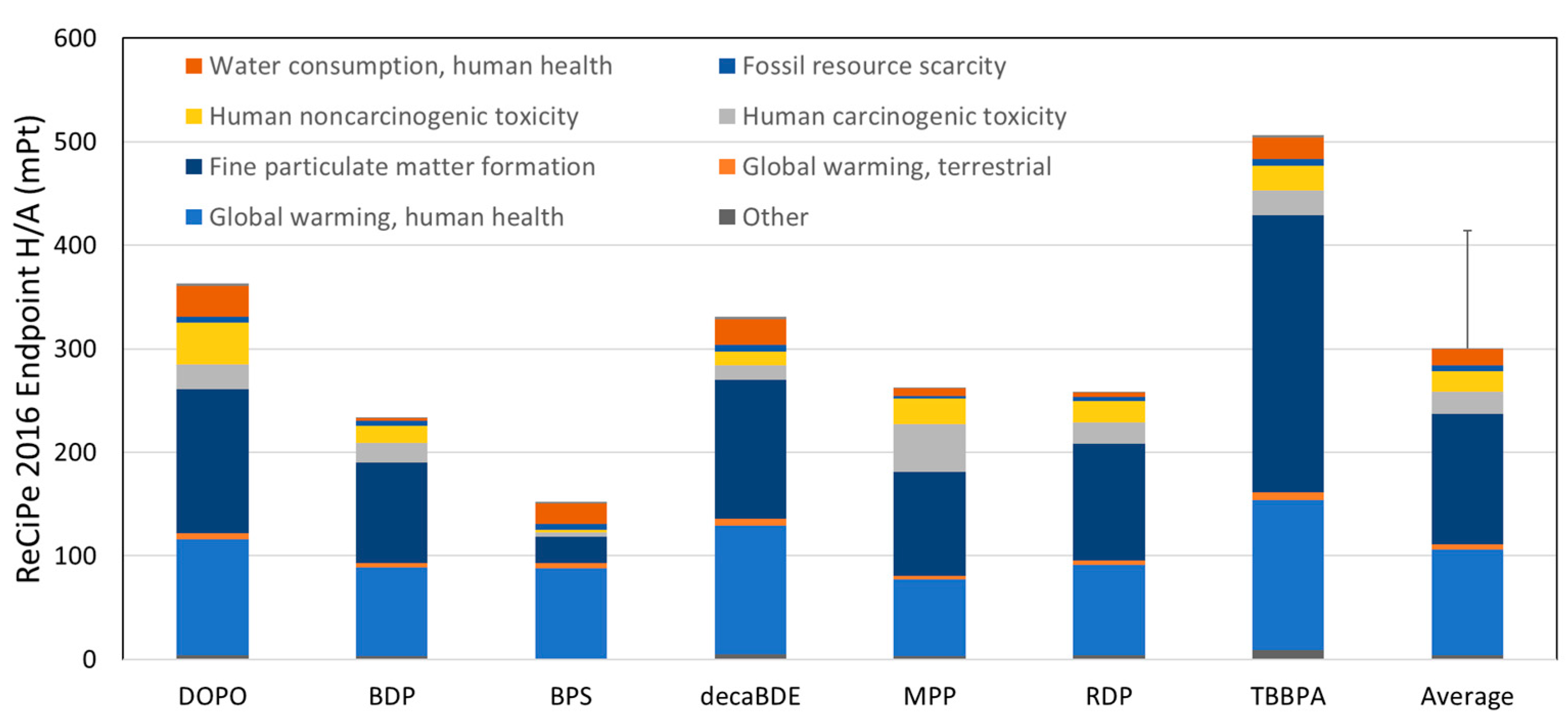
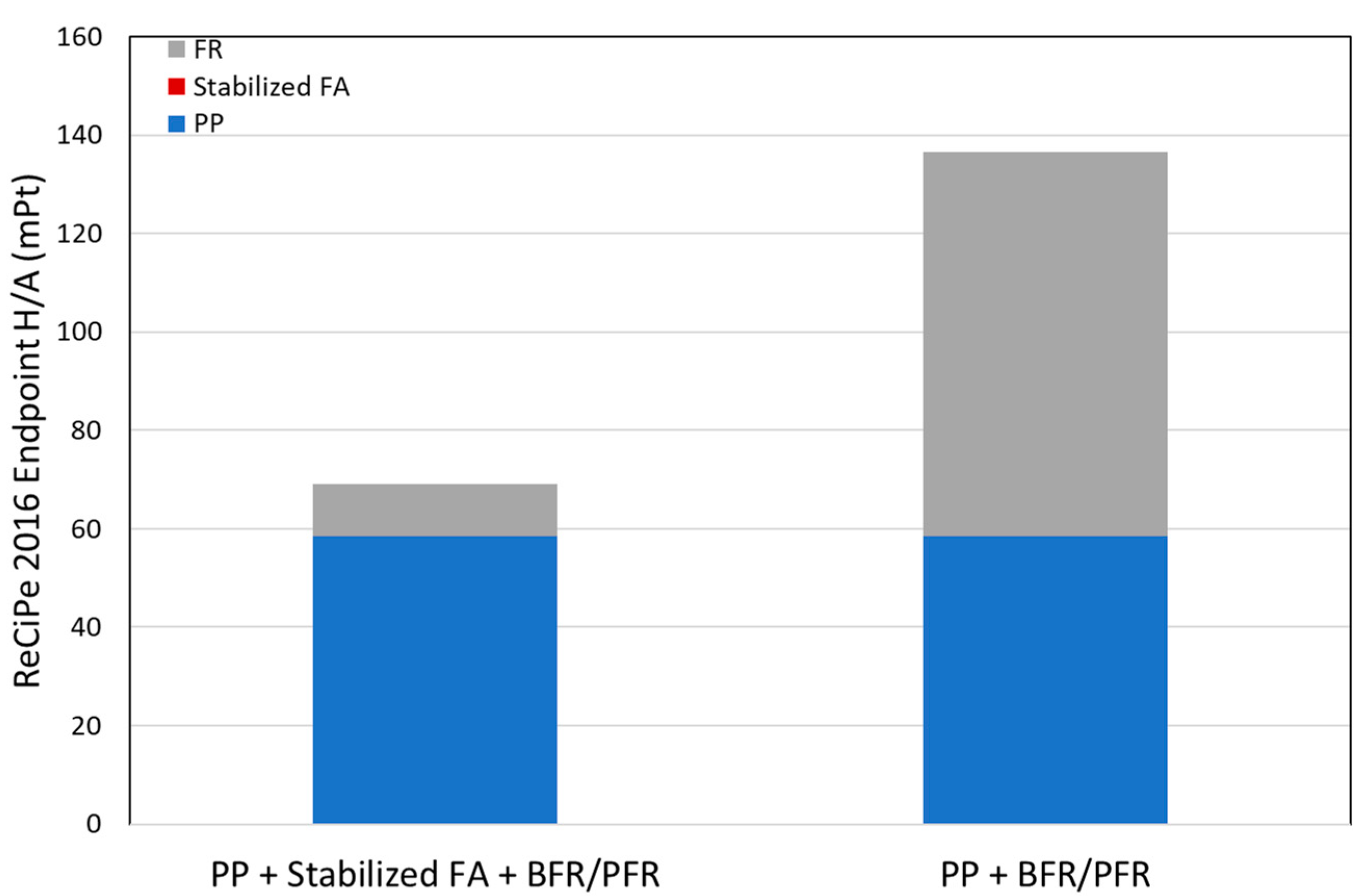
3.3. Screening LCA Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vilardi, G.; Verdone, N. Exergy analysis of municipal solid waste incineration processes: The use of O2-enriched air and the oxy-combustion process. Energy 2022, 239, 122147. [Google Scholar] [CrossRef]
- Scarlat, N.; Fahl, F.; Dallemand, J.F. Status and Opportunities for Energy Recovery from Municipal Solid Waste in Europe. Waste Biomass Valoriz. 2019, 10, 2425–2444. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Tian, A.; Zhang, J.; Tang, Y.; Shi, P.; Tang, Q.; Huang, Y. Physical and chemical properties, pretreatment, and recycling of municipal solid waste incineration fly ash and bottom ash for highway engineering: A literature review. Adv. Civ. Eng. 2020, 2020, 8886134. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; Spina, R.L.; Depero, L.E.; Bontempi, E. Review of the reuse possibilities concerning ash residues from thermal process in a medium-sized urban system in Northern Italy. Sustainability 2020, 12, 4193. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.; Choi, J.; Lee, J.; Yu, K.; Baeck, S.H.; Shim, S.E.; Qian, Y. Valorization of fly ash as a harmless flame retardant via carbonation treatment for enhanced fire-proofing performance and mechanical properties of silicone composites. J. Hazard. Mater. 2021, 404, 124202. [Google Scholar] [CrossRef]
- Fahimi, A.; Zanoletti, A.; Federici, S.; Assi, A.; Bilo, F.; Depero, L.E.; Bontempi, E. New eco-materials derived fromwaste for emerging pollutants adsorption: The case of diclofenac. Materials 2020, 13, 3964. [Google Scholar] [CrossRef]
- Assi, A.; Federici, S.; Bilo, F.; Zacco, A.; Depero, L.E.; Bontempi, E. Increased sustainability of carbon dioxide mineral sequestration by a technology involving fly ash stabilization. Materials 2019, 12, 2714. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, C.; Ribeiro, A.; Ottosen, L. Possible applications for municipal solid waste fly ash. J. Hazard. Mater. 2003, 96, 201–216. [Google Scholar] [CrossRef]
- Porąbka, A.; Jurkowski, K.; Laska, J. Fly ash used as a reinforcing and flame-retardant filler in low-density polyethylene. Polimery 2015, 60, 251–257. [Google Scholar] [CrossRef]
- Segev, O.; Kushmaro, A.; Brenner, A. Environmental impact of flame retardants (persistence and biodegradability). Int. J. Environ. Res. Public Health 2009, 6, 478–491. [Google Scholar] [CrossRef] [Green Version]
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.M.; Gras, E. Recent developments in the chemistry of lithiated epoxides. Synthesis (Stuttg) 2002, 27, 1625–1642. [Google Scholar] [CrossRef]
- van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Bai, L.; Song, B.; Ma, X.; Hou, M.; Fu, J.; Shi, Y.; Wang, Y.; Jiang, G. Presence of organophosphate flame retardants (OPEs) in different functional areas in residential homes in Beijing, China. J. Environ. Sci. (China) 2022, 115, 277–285. [Google Scholar] [CrossRef]
- Samani, P.; van der Meer, Y. Life cycle assessment (LCA) studies on flame retardants: A systematic review. J. Clean. Prod. 2020, 274, 123259. [Google Scholar] [CrossRef]
- Jonkers, N.; Krop, H.; van Ewijk, H.; Leonards, P.E.G. Life cycle assessment of flame retardants in an electronics application. Int. J. Life Cycle Assess. 2016, 21, 146–161. [Google Scholar] [CrossRef] [Green Version]
- Broeren, M.L.M.; Molenveld, K.; van den Oever, M.J.A.; Patel, M.K.; Worrell, E.; Shen, L. Early-stage sustainability assessment to assist with material selection: A case study for biobased printer panels. J. Clean. Prod. 2016, 135, 30–41. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ducoli, S.; Ramorino, G.; Gobetti, A.; Zacco, A.; Federici, S.; Depero, L.E.; Bontempi, E. A circular economy virtuous example-use of a stabilized waste material instead of calcite to produce sustainable composites. Appl. Sci. 2020, 10, 754. [Google Scholar] [CrossRef] [Green Version]
- ISO 14040:2006/A1:2020; Environmental Management—Life Cycle Assessment—Principles and Framework; ISO: Geneva, Switzerland, 2020.
- ISO 14044:2006/A2:2020; Environmental Management, Life Cycle Assessment, Requirements and Guidelines; ISO: Geneva, Switzerland, 2020.
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Akanbi, M.N. Effects of Compatibilization and Filler Content on the Flameability of Polypropylene/Calcium Diphosphate Composite and Its Modelling. J. Inst. Polym. Eng. 2017, 1, 107–116. [Google Scholar]
- Xu, Q.; Wu, L.; Yan, X.; Zhang, S.; Dong, L.; Su, Z.; Zhong, T.; Jiang, C.; Chen, Y.; Jiang, M.; et al. Halogen-free flame retardant polypropylene fibers with modified intumescent flame retardant: Preparation, characterization, properties and mode of action. Polymers 2021, 13, 2553. [Google Scholar] [CrossRef]
- Levchik, S. Phosphorus-based FRs. In Non-Halogenated Flame Retardant Hanbook; Scrivener Publishing LLC: Beverly, MA, USA, 2014. [Google Scholar]
- De Boer, J.; Stapleton, H. Toward fire safety without chemical risk. Science 2019, 625, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Yasin, S.; Behary, N.; Perwuelz, A.; Guan, J. Life cycle assessment of flame retardant cotton textiles with optimized end-of-life phase. J. Clean. Prod. 2018, 172, 1080–1088. [Google Scholar] [CrossRef]
- Cenci, M.P.; Scarazzato, T.; Munchen, D.D.; Dartora, P.C.; Veit, H.M.; Bernardes, A.M.; Dias, P.R. Eco-Friendly Electronics—A Comprehensive Review. Adv. Mater. Technol. 2021, 7, 2001263. [Google Scholar] [CrossRef]
- Maia, M.L.; Sousa, S.; Pestana, D.; Faria, A.; Teixeira, D.; Delerue-Matos, C.; Domingues, V.F.; Calhau, C. Impact of brominated flame retardants on lipid metabolism: An in vitro approach. Environ. Pollut. 2022, 294, 118639. [Google Scholar] [CrossRef] [PubMed]
- Usenko, C.Y.; Abel, E.L.; Hopkins, A.; Martinez, G.; Tijerina, J.; Kudela, M.; Norris, N.; Joudeh, L.; Bruce, E.D. Evaluation of common use Brominated Flame Retardant (BFR) toxicity using a Zebrafish embryo model. Toxics 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Composites | Epoxy Resin (%) | Stabilized FA (%) | CaCO3 (%) |
|---|---|---|---|
| 1 | 100 | - | - |
| 2 | 90 | 10 | - |
| 3 | 70 | 30 | - |
| 4 | 50 | 50 | - |
| 5 | 30 | 70 | - |
| 6 | 90 | - | 10 |
| 7 | 70 | - | 30 |
| 8 | 50 | - | 50 |
| 9 | 30 | - | 70 |
| Composites | PP (%) | Stabilized FA (%) | CaCO3 (%) | Flame Retardant (%) |
|---|---|---|---|---|
| A | 70 | 30 | - | - |
| B | 94 | 6 | - | - |
| C | 70 | - | 30 | - |
| D | 94 | - | 6 | - |
| E | 74 | 22.50 | - | 3.50 |
| Composites | Specimen Thickness | Results |
|---|---|---|
| A | 4 mm | Negative |
| B | 4 mm | Positive |
| C | 4 mm | Positive |
| D | 4 mm | Positive |
| E | 4 mm | Positive |
| Composites | Specimen Thickness | Results |
|---|---|---|
| A | 4 mm | Negative |
| C | 4 mm | Negative |
| E | 4 mm | Positive |
| E | 2 mm | Negative |
| Composites | Specimen Thickness | Results |
|---|---|---|
| A | 3.2 mm | Negative |
| C | 3.2 mm | Negative |
| E | 3.2 mm | Negative |
| E | 1.6 mm | Positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanoletti, A.; Ciacci, L. The Reuse of Municipal Solid Waste Fly Ash as Flame Retardant Filler: A Preliminary Study. Sustainability 2022, 14, 2038. https://doi.org/10.3390/su14042038
Zanoletti A, Ciacci L. The Reuse of Municipal Solid Waste Fly Ash as Flame Retardant Filler: A Preliminary Study. Sustainability. 2022; 14(4):2038. https://doi.org/10.3390/su14042038
Chicago/Turabian StyleZanoletti, Alessandra, and Luca Ciacci. 2022. "The Reuse of Municipal Solid Waste Fly Ash as Flame Retardant Filler: A Preliminary Study" Sustainability 14, no. 4: 2038. https://doi.org/10.3390/su14042038
APA StyleZanoletti, A., & Ciacci, L. (2022). The Reuse of Municipal Solid Waste Fly Ash as Flame Retardant Filler: A Preliminary Study. Sustainability, 14(4), 2038. https://doi.org/10.3390/su14042038







