A Noninvasive Genetic Insight into the Spatial and Social Organization of an Endangered Population of the Eurasian Otter (Lutra lutra, Mustelidae, Carnivora)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection and Storage
2.3. DNA Extraction and Analyses
3. Results
3.1. Spatial Organisation and Kinship
3.2. Population Size, Density, and Structure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roos, A.; Loy, A.; de Silva, P.; Hajkova, P.; Zemanová, B. Lutra lutra The IUCN Red List of Threatened Species. Version 2015.2. 2015. Available online: www.iucnredlist.org (accessed on 11 July 2015).
- Conroy, J.W.H.; Chanin, P.R.F. The Status of the Eurasian Otter (Lutra lutra) in Europe. A Review. In Proceedings of the First Otter Toxicology Conference, Isle of Skye, UK, September 2000; Conroy, J.W.H., Yoxon, P., Gutleb, A.C., Eds.; Journal of the International Otter Survival Fund: Broadford, UK, 2000; p. 28. [Google Scholar]
- Cianfrani, C.; Lay, G.L.; Maiorano, L.; Satizábal, H.F.; Loy, A.; Guisan, A. Adapting Global Conservation Strategies to Climate Change at the European Scale: The Otter as a Flagship Species. Biol. Conserv. 2011, 144, 2068–2080. [Google Scholar] [CrossRef]
- Prigioni, C.; Remonti, L.; Balestrieri, A.; Sgrosso, S.; Priore, G.; Mucci, N.; Randi, E. Estimation of European Otter (Lutra lutra) Population Size by Fecal DNA Typing in Southern Italy. J. Mammal. 2006, 87, 855–858. [Google Scholar] [CrossRef]
- Prigioni, C.; Balestrieri, A.; Remonti, L. Decline and Recovery in Otter Lutra lutra Populations in Italy. Mammal Rev. 2007, 37, 71–79. [Google Scholar] [CrossRef]
- Loy, A.; Boitani, L.; Bonesi, L.; Canu, A.; Di Croce, A.; Fiorentino, P.L.; Genovesi, P.; Mattei, L.; Panzacchi, M.; Prigioni, C.; et al. The Italian Action Plan for the Endangered Eurasian Otter Lutra Lutra. Hystrix Ital. J. Mammal. 2010, 21, 19–33. [Google Scholar]
- Loy, A.; Bucci, L.; Carranza, M.L.; De Castro, G.; Marzio, D.; Reggiani, P. Survey and Habitat Evaluation for a Peripheral Population of the Eurasian Otter in Italy. IUCN Otter Spec. Group 2004, 21, 1–9. [Google Scholar]
- Giovacchini, S.; Marrese, M.; Loy, A. Good News from the South: Filling the Gap between Two Otter Populations in Italy. IUCN Otter Spec. Group 2018, 35, 212–221. [Google Scholar]
- Lapini, L.; Bonesi, L. Evidence of the Natural Recovery of the Eurasian Otter in NE Italy. In Proceedings of the 29th European Mustelid Colloquium, Southampton, UK, 3–4 December 2011; pp. 3–4. [Google Scholar]
- Righetti, D. Return of the Otter in South Tyrol (NE Italy). In Proceedings of the 11th International Otter Colloquium, Pavia, Italy, 30 August–4 September 2011. [Google Scholar]
- Loy, A.; Balestrieri, A.; Bartolomei, R.; Bonesi, L.; Caldarella, M.; De Castro, G.; Salda, D.; Fulco, L.; Fusillo, E.; Gariano, R.; et al. The Eurasian Otter (Lutra lutra) in Italy: Distribution, Trend and Threats. In Proceedings of the European Otter Workshop, Stockholm, Sweden, 8–11 June 2015; IUCN Otter Specialist Group Bulletin. Volume 32, pp. 9–10. [Google Scholar]
- Pavanello, M.; Lapini, L.; Kranz, A.; Iordan, F. Rediscovering the Eurasian Otter (Lutra lutra L.) in Friuli Venezia Giulia and Notes on Its Possible Expansion in Northern Italy. IUCN/SCC Otter Spec. Group Bull. 2015, 32, 12–20. [Google Scholar]
- Randi, E.; Davoli, F.; Pierpaoli, M.; Pertoldi, C.; Madsen, A.B.; Loeschcke, V. Genetic Structure in Otter (Lutra lutra) Populations in Europe: Implications for Conservation. Anim. Conserv. 2003, 6, 93–100. [Google Scholar] [CrossRef]
- Rondinini, C.; Battistoni, A.; Peronace, V.; Teofili, C. Lista Rossa IUCN dei Vertebrati Italiani; Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Rome, Italy, 2013. [Google Scholar]
- Prigioni, C.; Balestrieri, A.; Remonti, L.; Gargaro, A.; Priore, G. Diet of the Eurasian Otter (Lutra lutra) in Relation to Freshwater Habitats and Alien Fish Species in Southern Italy. Ethol. Ecol. Evol. 2006, 18, 307–320. [Google Scholar] [CrossRef]
- Remonti, L.; Prigioni, C.; Balestrieri, A.; Sgrosso, S.; Priore, G. Trophic Flexibility of the Otter (Lutra lutra) in Southern Italy. Mamm. Biol. 2008, 73, 293–302. [Google Scholar] [CrossRef]
- Smiroldo, G.; Balestrieri, A.; Remonti, L.; Prigioni, C. Seasonal and Habitat-Related Variation of Otter Lutra lutra Diet in a Mediterranean River Catchment (Italy). Folia Zool. 2009, 58, 87–97. [Google Scholar]
- Prigioni, C.; Smiroldo, G.; Remonti, L.; Balestrieri, A. Distribution and Diet of Reintroduced Otters (Lutra lutra) on the River Ticino (Northern Italy). Hystrix Ital. J. Mammal. 2009, 20, 4432. [Google Scholar] [CrossRef]
- Marcolin, F.; Iordan, F.; Pizzul, E.; Pallavicini, A.; Torboli, V.; Manfrin, C.; Quaglietta, L. Otter Diet and Prey Selection in a Recently Recolonized Area Assessed Using Microscope Analysis and DNA barcoding. Hystrix Ital. J. Mammal. 2020, 31, 64–72. [Google Scholar]
- Scorpio, V.; Loy, A.; Di Febbraro, M.; Rizzo, A.; Aucelli, P. Hydromorphology Meets Mammal Ecology: River Morphological Quality, Recent Channel Adjustments and Otter Resilience. River Res. Appl. 2014, 32, 267–279. [Google Scholar] [CrossRef]
- Loy, A.; Carranza, M.L.; Cianfrani, C.; D’Alessandro, E.; Bonesi, L.; Di Marzio, P.; Minotti, M.; Reggiani, G. Otter Lutra lutra population expansion: Assessing habitat suitability and connectivity in south-central Italy. Folia Zool. 2009, 58, 309–326. [Google Scholar]
- Cianfrani, C.; Le Lay, G.; Hirzel, A.H.; Loy, A. Do habitat Suitability Models Reliably Predict the Recovery Areas of Threatened Species? J. Appl. Ecol. 2010, 47, 421–430. [Google Scholar] [CrossRef]
- Carranza, M.L.; D’Alessandro, E.; Saura, S.; Loy, A. Connectivity Providers for Semi-Aquatic Vertebrates: The Case of the Endangered Otter in Italy. Landsc. Ecol. 2012, 27, 281–290. [Google Scholar] [CrossRef]
- Carone, M.T.; Guisan, A.; Cianfrani, C.; Simoniello, T.; Loy, A.; Carranza, M.L. A Multi-Temporal Approach to Model Endangered Species Distribution in Europe: The Case of the Eurasian Otter in Italy. Ecol. Model. 2014, 274, 21–28. [Google Scholar]
- Marcelli, M.; Fusillo, R. Assessing Range Re-Expansion and Recolonization of Human-Impacted Landscapes by Threatened Species: A Case Study of the Otter (Lutra lutra) in Italy. Biodivers. Conserv. 2009, 18, 2941–2959. [Google Scholar] [CrossRef]
- Buglione, M.; Petrelli, S.; Troiano, C.; Notomista, T.; Petrella, A.; De Riso, L.; Poerio, L.; Cascini, V.; Bartolomei, R.; Fulgione, D. Spatial Genetic Structure in the Eurasian Otter (Lutra lutra) Meta-Population from its Core Range in Italy. Contrib. Zool. 2020, 90, 70–92. [Google Scholar] [CrossRef]
- Quaglietta, L.; Fusillo, R.; Marcelli, M.; Loy, A.; Boitani, L. First Telemetry Data on Wild Individuals from the Threatened, Isolated Italian Otter (Lutra lutra) Population. Mammalia 2019, 83, 447–452. [Google Scholar] [CrossRef]
- Johnson, D.H.; Boitani, L.; Fuller, T.K. Research Techniques in Animal Ecology: Controversies and Consequences. J. Wildl. Manag. 2001, 65, 599. [Google Scholar] [CrossRef]
- Fernández-Morán, J.; Saavedra, D.; Manteca-Vilanova, X. Reintroduction of the Eurasian Otter (Lutra lutra) in Northeastern Spain: Trapping, Handling, and Medical Management. J. Zoo Wildl. Med. 2002, 33, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Arrendal, J.; Walker, C.W.; Sundqvist, A.K.; Hellborg, L.; Vilà, C. Genetic Evaluation of an Otter Translocation Program. Conserv. Genet. 2004, 5, 79–88. [Google Scholar] [CrossRef]
- Tison, J.-L.; Blennow, V.; Palkopoulou, E.; Gustafsson, P.; Roos, A.; Dalén, L. Population Structure and Recent Temporal Changes in Genetic Variation in Eurasian Otters from Sweden. Conserv. Genet. 2015, 16, 371–384. [Google Scholar] [CrossRef]
- Hájková, P.; Zemanová, B.; Roche, K.; Hájek, B. An Evaluation of Field and Noninvasive Genetic Methods for Estimating Eurasian Otter Population Size. Conserv. Genet. 2009, 10, 1667–1681. [Google Scholar] [CrossRef]
- Stanton, D.; Hobbs, G.I.; Chadwick, E.; Slater, F.M.; Bruford, M.W. Mitochondrial Genetic Diversity and Structure of the European Otter (Lutra lutra) in Britain. Conserv. Genet. 2009, 10, 733–737. [Google Scholar] [CrossRef]
- Janssens, X.; Fontaine, M.C.; Michaux, J.R.; Libois, R.; De Kermabon, J.; Defourny, P.; Baret, P.V. Genetic Pattern of the Recent Recovery of European Otters in Southern France. Ecography 2008, 31, 176–186. [Google Scholar] [CrossRef]
- Aristizábal Duque, S.L.; Orozco-Jiménez, L.Y.; Zapata-Escobar, C.; Palacio-Baena, J.A. Conservation genetics of otters: Review about the use of non-invasive samples. Therya 2018, 9, 85–93. [Google Scholar] [CrossRef]
- Lanszki, J.; Hidas, A.; Szentes, K.; Révay, T.; Lehoczky, I.; Jeney, Z.; Weiss, S. Genetic Structure of Otter (Lutra lutra) Populations from Two Fishpond Systems in Hungary. Mamm. Biol. 2010, 75, 447–450. [Google Scholar] [CrossRef]
- Hájková, P.; Pertoldi, C.; Zemanová, B.; Roche, K.; Hájek, B.; Bryja, J.; Zima, J. Genetic structure and evidence for recent population decline in Eurasian otter populations in the Czech and Slovak Republics: Implications for conservation. J. Zool. 2007, 272, 1–9. [Google Scholar] [CrossRef]
- Kalz, B.; Jewgenow, K.; Fickel, J. Structure of an Otter (Lutra lutra) Population in Germany—Results of DNA and Hormone Analyses from Faecal Samples. Mamm. Biol. 2006, 71, 321–335. [Google Scholar] [CrossRef]
- Janssen, M.H.M.; Hayden, S.; Gilman, C.; Cox, C.L.; Dalton, R.; Niemuller, C.; Spindler, R. Fecal Steroid Analysis to Assess Reproduction and Adrenal Activity in the Spotted-Necked Otter (Lutra maculicollis). Biol. Reprod. 2006, 167. [Google Scholar]
- Koelewijn, H.P.; Pérez-Haro, M.; Jansman, H.A.H.; Boerwinkel, M.C.; Bovenschen, J.; Lammertsma, D.R.; Niewold, F.J.J.; Kuiters, A.T. The Reintroduction of the Eurasian Otter (Lutra lutra) into the Netherlands: Hidden Life Revealed by Noninvasive Genetic Monitoring. Conserv. Genet. 2010, 11, 601–614. [Google Scholar] [CrossRef]
- Arrendal, J.; Vilà, C.; Björklund, M. Reliability of Noninvasive Genetic Census of Otters Compared to Field Censuses. Conserv. Genet. 2007, 8, 1097–1107. [Google Scholar] [CrossRef]
- Ferrando, A.; Lecis, R.; Domingo-Roura, X.; Ponsà, M. Genetic Diversity and Individual Identification of Reintroduced Otters (Lutra lutra) in North-Eastern Spain by DNA Genotyping of Spraints. Conserv. Genet. 2008, 9, 129–139. [Google Scholar] [CrossRef]
- Quaglietta, L.; Fonseca, V.C.; Hájková, P.; Mira, A.; Boitani, L. Fine-Scale Population Genetic Structure and Short-Range Sex-Biased Dispersal in a Solitary Carnivore, Lutra lutra. J. Mammal. 2013, 94, 561–571. [Google Scholar] [CrossRef]
- Quaglietta, L.; Hájková, P.; Mira, A.; Boitani, L. Eurasian Otter (Lutra lutra) Density Estimate Based on Radio Tracking and Other Data Sources. Mamm. Res. 2015, 60, 127–137. [Google Scholar] [CrossRef][Green Version]
- Vergara, M.; Ruiz-González, A.; de Luzuriaga, J.L.; Gómez-Moliner, B.J. Individual Identification and Distribution Assessment of Otters (Lutra lutra) through Non-Invasive Genetic Sampling: Recovery of an endangered species in the Basque Country (Northern Spain). Mamm. Biol. 2014, 79, 259–267. [Google Scholar] [CrossRef]
- Trinca, C.S.; Jaeger, C.F.; Eizirik, E. Molecular Ecology of the Neotropical Otter (Lontra longicaudis): Non-Invasive Sampling Yields Insights into Local Population Dynamics. Biol. J. Linn. Soc. 2013, 109, 932–948. [Google Scholar] [CrossRef]
- Ribas, C.; Cunha, H.A.; Damasceno, G.; Magnusson, W.E.; Sole-Cava, A.; Mourão, G. More than meets the Eye: Kinship and Social Organization in Giant Otters (Pteronura brasiliensis). Behav. Ecol. Sociobiol. 2016, 70, 61–72. [Google Scholar] [CrossRef]
- Brzeski, K.E.; Gunther, M.S.; Black, J.M. Evaluating River Otter Demography Using Noninvasive Genetic Methods. J. Wildl. Manag. 2013, 77, 1523–1531. [Google Scholar] [CrossRef]
- Lampa, S.; Mihoub, J.B.; Gruber, B.; Klenke, R.; Henle, K. Non-Invasive Genetic Mark-Recapture as a Means to Study Population Sizes and Marking Behaviour of the Elusive Eurasian Otter (Lutra lutra). PLoS ONE 2015, 10, e0125684. [Google Scholar] [CrossRef] [PubMed]
- Kruuk, H. Otters: Ecology, Behaviour and Conservation; Oxford Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Lerone, L.; Mengoni, C.; Carpaneto, G.M.; Randi, E.; Loy, A. Procedures to Genotype Problematic Non-Invasive Otter (Lutra lutra) Samples. Acta Theriol. 2014, 59, 511–520. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Pullin, A.S.; Dolman, P.M.; Knight, T.M. The need for evidence-based conservation. Trends Ecol. Evol. 2004, 19, 305–308. [Google Scholar] [CrossRef]
- Ruiz-Olmo, J.; Jiménez, J.; López-Martín, J.M. Radio-Tracking of Otters Lutra lutra in North-Eastern Spain. Lutra 1995, 38, 11–21. [Google Scholar]
- Quaglietta, L.; Fonseca, V.C.; Mira, A.; Boitani, L. Sociospatial Organization of a Solitary Carnivore, the Eurasian Otter (Lutra lutra). J. Mammal. 2014, 95, 140–150. [Google Scholar] [CrossRef]
- De Castro, G.; Loy, A. Un Nuovo Censimento della Lontra (Lutra Lutra Carnivora Mammalia) nel Fiume Sangro (Abruzzo): Inizia la Ricolonizzazione dell’Italia Centrale? In Proceedings of the 68° Convegno Unione Zoologica Italiana, Lecce, Italy, 24–27 September 2007; p. 105. [Google Scholar]
- Hajkova, P.; Zemanova, B.; Bryja, J.; Hajek, B.; Roche, K.; Tkadlec, E.; Zima, J. Factors Affecting Success of PCR Amplification of Microsatellite Loci from Otter Faeces. Mol. Ecol. Notes 2006, 6, 559–562. [Google Scholar] [CrossRef]
- Valière, N. GIMLET: A Computer Program for Analysing Genetic Individual Identification Data. Mol. Ecol. Notes 2002, 2, 377–379. [Google Scholar] [CrossRef]
- Dallas, J.F.; Conroy, J.W.H.; Green, R.; Jefferies, D.J.; Marshall, F.; Bacon, P.J.; Carss, D.N.; Kruuk, H.; Piertney, S.B.; Racey, P.A. Genetic Diversity in the Eurasian Otter, Lutra lutra, in Scotland. Evidence from Microsatellite Polymorphism. Biol. J. Linn. Soc. 1999, 68, 73–86. [Google Scholar] [CrossRef]
- Huang, C.C.; Hsu, Y.C.; Lee, L.L.; Li, S.H. Isolation and Characterization of Tetramicrosatellite DNA Markers in the Eurasian Otter (Lutra lutra). Mol. Ecol. Notes 2005, 5, 314–316. [Google Scholar] [CrossRef]
- Mucci, N.; Randi, E. Sex Identification of Eurasian Otter (Lutra lutra) Non-Invasive DNA Samples Using ZFX/ZFY Sequences. Conserv. Genet. 2007, 8, 1479–1482. [Google Scholar] [CrossRef]
- Taberlet, P.; Waits, L.P.; Luikart, G. Noninvasive Genetic Sampling: Look Before you Leap. Trends Ecol. Evol. 1999, 14, 323–327. [Google Scholar] [CrossRef]
- Miller, C.R.; Joyce, P.; Waits, L.P. Assessing Allelic Dropout and Genotype Reliability Using Maximum Likelihood. Genetics 2002, 160, 357–366. [Google Scholar] [CrossRef]
- Peakall, R.O.D.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population Genetic Software for Teaching And Research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Sokal, R.R. Statistical Tables; Macmillan Publishers: New York, NY, USA, 1995. [Google Scholar]
- Nei, M. Estimation of Average Heterozygosity and Genetic Distance from a Small Number of Individuals. Genetics 1978, 89, 583–590. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A New Series of Programs to Perform Population Genetics Analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 567. [Google Scholar] [CrossRef]
- Miller, C.R.; Joyce, P.; Waits, L.P.A. New Method for Estimating the Size of Small Populations from Genetic Mark-Recapture Data: Genetic Mark-Recapture Estimation. Mol. Ecol. 2005, 14, 1991–2005. [Google Scholar] [CrossRef]
- Pennell, M.W.; Stansbury, C.R.; Waits, L.P.; Miller, C.R. CAPWIRE: A R Package for Estimating Population Census Size from Non-Invasive Genetic Sampling. Mol. Ecol. Resour. 2012, 13, 154–157. [Google Scholar] [CrossRef]
- Blouin, M.S. DNA-Based Methods for Pedigree Reconstruction and Kinship Analysis in Natural Populations. Trends Ecol. Evol. 2003, 18, 503–511. [Google Scholar] [CrossRef]
- Wang, J. A New Method for Estimating Effective Population Sizes from a Single Sample of Multilocus Genotypes. Mol. Ecol. 2009, 18, 2148–2164. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.R.; Wang, J. Colony: A Program for Parentage and Sibship Inference from Multilocus Genotype Data. Mol. Ecol. Resour. 2010, 10, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Kruuk, H.; Moorhouse, A. The Spatial Organization of Otters (Lutra lutra) in Shetland. J. Zool. 1991, 224, 41–57. [Google Scholar] [CrossRef]
- Kruuk, H.; Moorhouse, A.; Conroy, J.W.H.; Durbin, L.; Frears, S. An Estimate of Numbers and Habitat Preferences of Otters Lutra lutra in Shetland, UK. Biol. Conserv. 1989, 49, 241–254. [Google Scholar] [CrossRef]
- Ansorge, H. Zur Situation Des Eurasischen Fischotters Lutra lutra Linné, 1758 Im Raum Oberlausitz-Sachsen. Säugetierkd. Inf. 1994, 3, 617–622. [Google Scholar]
- Hauer, S.; Ansorge, H.; Zinke, O. Mortality Patterns of Otters (Lutra lutra) from Eastern Germany. J. Zool. 2002, 256, 361–368. [Google Scholar] [CrossRef]
- Martin, E.A.; Heurich, M.; Müller, J.; Bufka, L.; Bubliy, O.; Fickel, J. Genetic Variability and Size Estimates of the Eurasian Otter (Lutra lutra) Population in the Bohemian Forest Ecosystem. Mamm. Biol. 2017, 86, 42–47. [Google Scholar] [CrossRef]
- Loy, A.; Diamente, S.; Di Febbraro, M. Population Viability Analysis of the Italian Otter Population. In Book of Abstracts, Proceedings of the 8th European Congress of Mammalogy, Warsaw, Poland, 23–27 September 2019; Borowski, Z., Ed.; Convention+ Spółka z Ograniczoną Odpowiedzialnością: Warsaw, Poland, 2019; Volume 175. [Google Scholar]
- Vander Wal, E.; Laforge, M.P.; McLoughlin, P.D. Density Dependence in Social Behaviour: Home Range Overlap and Density Interacts to Affect Conspecific Encounter Rates in a Gregarious Ungulate. Behav. Ecol. Sociobiol. 2014, 68, 383–390. [Google Scholar] [CrossRef]
- Hinde, R.A. Interactions, Relationships and Social Structure. Man 1976, 11, 1–17. [Google Scholar] [CrossRef]
- Whitehead, H. Analysing animal social structure. Anim. Behav. 1997, 53, 1053–1067. [Google Scholar] [CrossRef]
- Wronski, T.; Apio, A. Home-Range Overlap, Social Vicinity and Agonistic Interactions Denoting Matrilineal Organisation in Bushbuck, Tragelaphus scriptus. Behav. Ecol. Sociobiol. 2006, 59, 819–828. [Google Scholar] [CrossRef]
- Green, J.; Green, R. A Radio-Tracking Survey of Otters Lutra lutra on a Perthshire River System. Lutra 1984, 27, 85–145. [Google Scholar]
- Kruuk, H. Hunter and Hunted: Relationships between Carnivores and People; Cambridge University Press: New York, NY, USA, 2002. [Google Scholar]
- Johnson, D.D.P.; Kays, R.; Blackwell, P.G.; Macdonald, D.W. Does the Resource Dispersion Hypothesis Explain Group Living? Trends Ecol. Evol. 2002, 17, 563–570. [Google Scholar] [CrossRef]
- Askeyev, A.; Askeyev, O.; Yanybaev, N.; Askeyev, I.; Monakhov, S.; Marić, S.; Hulsman, K. River Fish Assemblages along an Elevation Gradient in the Eastern Extremity of Europe. Environ. Biol. Fishes 2017, 100, 585–596. [Google Scholar] [CrossRef]
- Durbin, L.S. Some Changes in the Habitat Use of a Free-ranging Female Otter Lutra lutra during Breeding. J. Zool. 1996, 240, 761–764. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H. Review Lecture: Mammalian mating systems. Proc. R. Soc. Lond. B Boil. Sci. 1989, 236, 339–372. [Google Scholar]
- Macdonald, D.W. The Ecology of Carnivore Social Behaviour. Nature 1983, 301, 379–384. [Google Scholar] [CrossRef]
- Sandell, M. The Mating Tactics and Spacing Patterns of Solitary Carnivores. In Carnivore Behavior, Ecology and Evolution; Gittleman, J.L., Ed.; Springer: Boston, MA, USA, 1989; pp. 164–182. [Google Scholar]
- Saavedra, D. Reintroduction of the Eurasian Otter (Lutra Lutra) in Muga and Fluvià Basins (North-Eastern Spain): Viability, Development, Monitoring and Trends of the New Population. Ph.D. Thesis, University of Girona, Girona, Spain, 2002. [Google Scholar]
- Carter, S.K.; Rosas, F.C.W. Biology and Conservation of the Giant Otter Pteronura brasiliensis. Mammal Rev. 1997, 27, 1–26. [Google Scholar] [CrossRef]
- Ornocker, M.G.; Messick, J.P.; Melquist, W.E. Spatial Strategies in Three Species of Mustelidae. Acta Zool. Fenn. 1983, 174, 185–188. [Google Scholar]
- Reid, D.G.; Code, T.E.; Reid, A.C.H.; Herrero, S.M. Spacing, Movements, and Habitat Selection of the River Otter in Boreal Alberta. Can. J. Zool. 1994, 72, 1314–1324. [Google Scholar] [CrossRef]
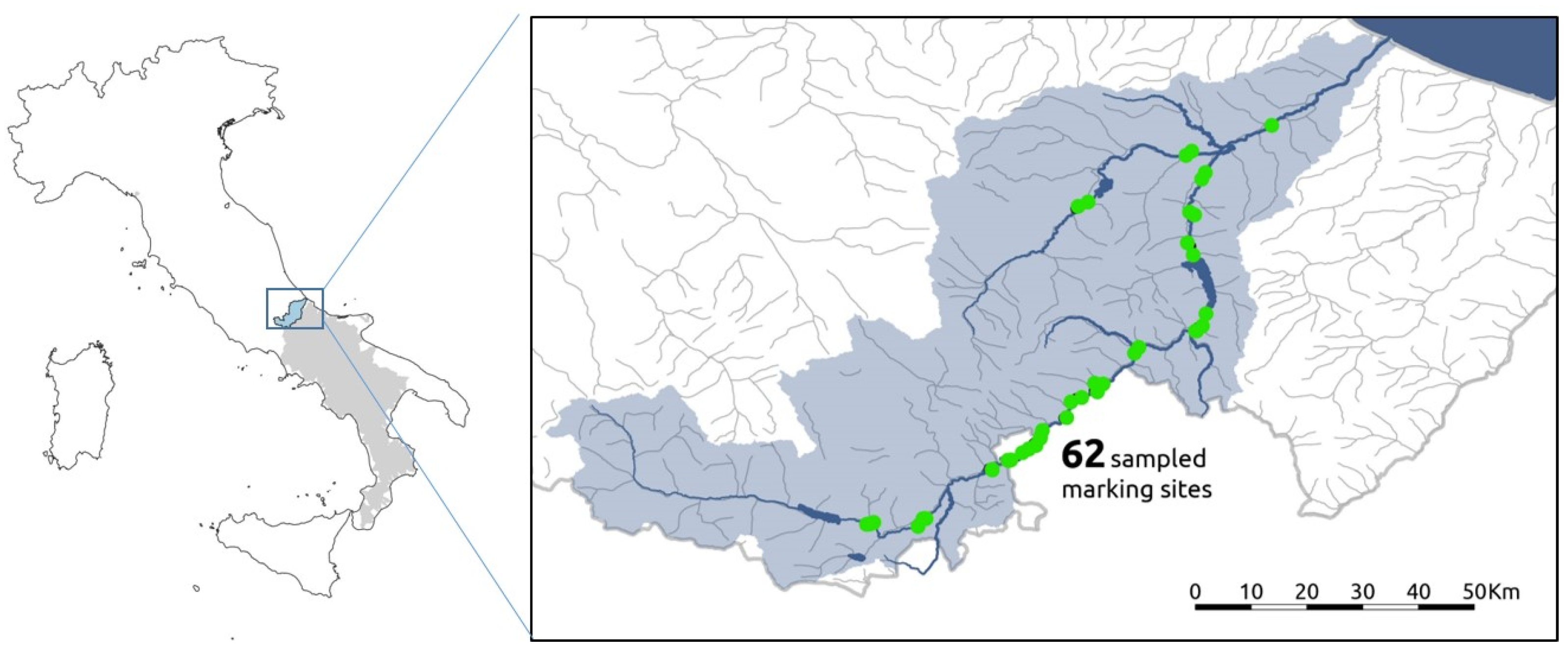
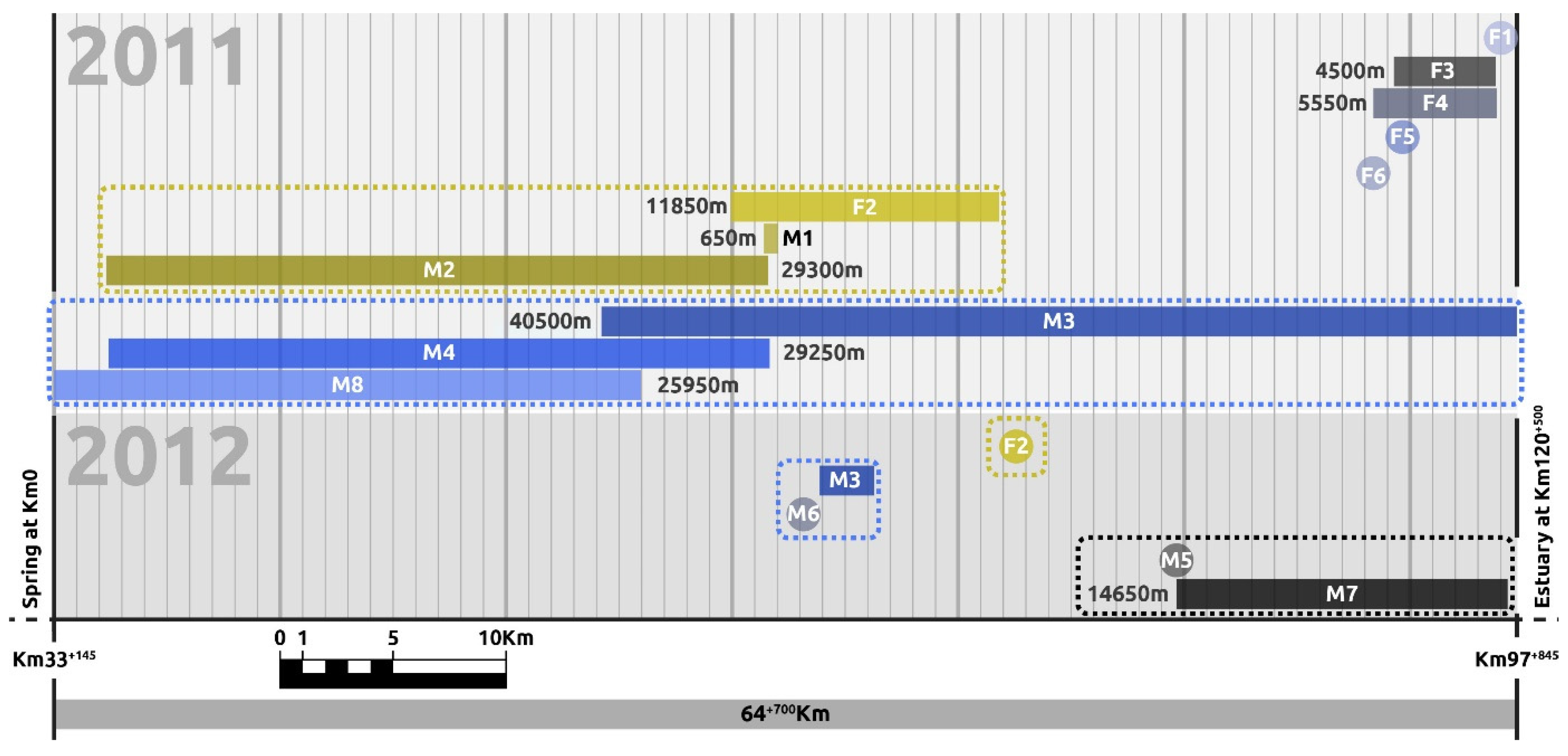
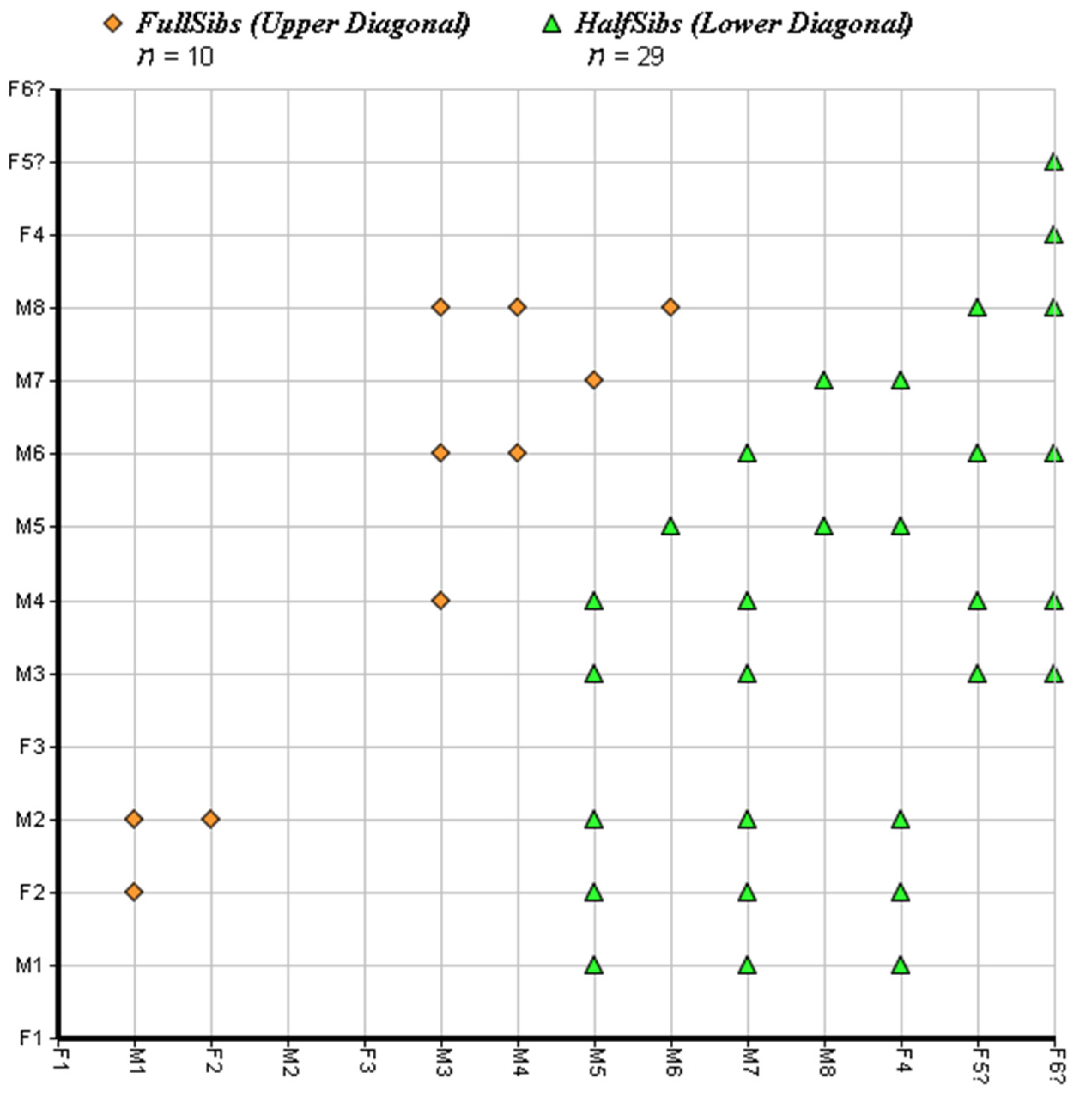
| Code | First Capture | Last Recapture | N. Captures/Recaptures | River Length Covered (M) |
|---|---|---|---|---|
| M1 | 3 June 2011 | 5 August 2011 | 2 | 650 (7650 corrected) |
| M2 | 3 June 2011 | 16 September 2011 | 7 | 29,300 |
| M3 | 5 April 2011 | 23 September 2011 | 14 | 40,500 |
| M4 | 15 September 2011 | 23 September 2011 | 2 | 29,250 |
| M8 | 18 September 2011 | 21 September 2011 | 2 | 25,950 |
| F1 | 2 May 2011 | 6 May 2011 | 2 | 25 (7025 corrected) |
| F2 | 3 June 2011 | 21 September 2011 | 7 | 11,850 |
| F3 | 28 April 2011 | 15 July 2011 | 4 | 4500 |
| F4 | 28 April 2011 | 21 August 2011 | 2 | 5550 |
| F5 likely female | 22 July 2011 | - | 1 | 7000 (corrected) |
| F6 likely female | 24 September 2011 | - | 1 | 7000 (corrected) |
| FullSibIndex | Prob (Inc.) | Prob (Exc.) | Member 1 | Member 2 | Member 3 | Member 4 |
|---|---|---|---|---|---|---|
| 2 | 0.9846 | 0.983 | M1 | F2 | M2 | |
| 4 | 0.9986 | 0.9986 | M3 | M4 | M6 | M8 |
| 5 | 0.995 | 0.995 | M5 | M7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lerone, L.; Mengoni, C.; Di Febbraro, M.; Krupa, H.; Loy, A. A Noninvasive Genetic Insight into the Spatial and Social Organization of an Endangered Population of the Eurasian Otter (Lutra lutra, Mustelidae, Carnivora). Sustainability 2022, 14, 1943. https://doi.org/10.3390/su14041943
Lerone L, Mengoni C, Di Febbraro M, Krupa H, Loy A. A Noninvasive Genetic Insight into the Spatial and Social Organization of an Endangered Population of the Eurasian Otter (Lutra lutra, Mustelidae, Carnivora). Sustainability. 2022; 14(4):1943. https://doi.org/10.3390/su14041943
Chicago/Turabian StyleLerone, Laura, Chiara Mengoni, Mirko Di Febbraro, Hannah Krupa, and Anna Loy. 2022. "A Noninvasive Genetic Insight into the Spatial and Social Organization of an Endangered Population of the Eurasian Otter (Lutra lutra, Mustelidae, Carnivora)" Sustainability 14, no. 4: 1943. https://doi.org/10.3390/su14041943
APA StyleLerone, L., Mengoni, C., Di Febbraro, M., Krupa, H., & Loy, A. (2022). A Noninvasive Genetic Insight into the Spatial and Social Organization of an Endangered Population of the Eurasian Otter (Lutra lutra, Mustelidae, Carnivora). Sustainability, 14(4), 1943. https://doi.org/10.3390/su14041943







