A Hawaiian Tropical Dry Forest Regenerates: Natural Regeneration of Endangered Species under Biocultural Restoration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Monitoring Recruitment, Survival, and Growth
3. Results
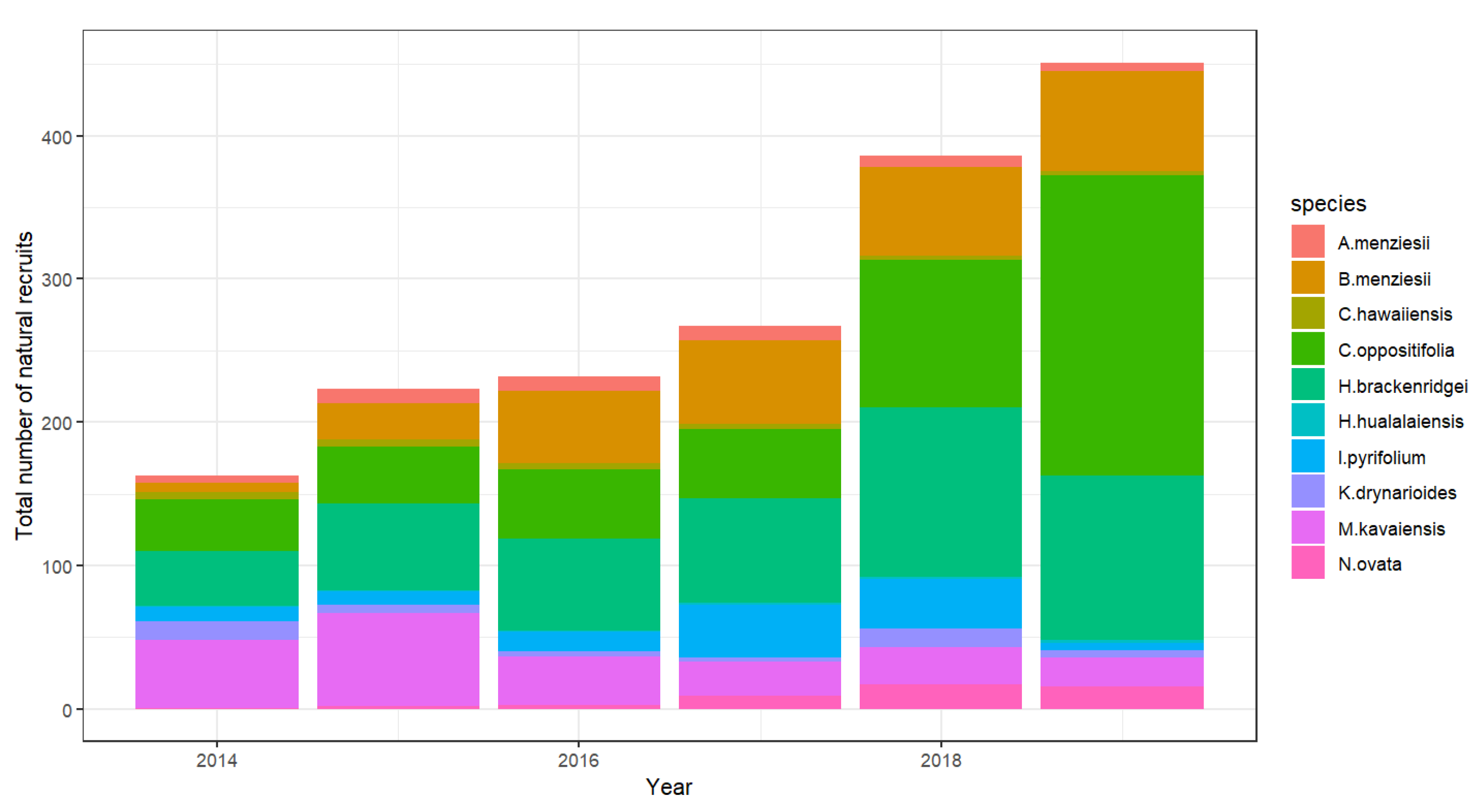
3.1. Natural Recruitment of Endangered Species
3.2. Survival of New Recruits
3.3. Growth of New Recruits
4. Discussion
4.1. Effects of Rainfall on Natural Regeneration
4.2. Bottlenecks in Natural Regeneration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miles, L.; Newton, A.C.; DeFries, R.S.; Ravilious, C.; May, I.; Blyth, S.; Kapos, V.; Gordon, J.E. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 2006, 33, 491–505. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Azofeifa, G.A.; Quesada, M.; Rodriguez, J.P.; Nassar, J.M.; Stoner, K.E.; Castillo, A.; Garvin, T.; Zent, E.L.; Calvo-Alvarado, J.C.; Kalacska, M.E.; et al. Research Priorities for Neotropical Dry Forests1. Biotropica 2005, 37, 477–485. [Google Scholar] [CrossRef]
- Rock, J.F. The Indigenous Trees of the Hawaiian Islands; T. H.: Honolulu, HI, USA, 1913. [Google Scholar]
- Bruegmann, M.M. Hawai’i’s dry forests. Endanger. Species Bull. 1996, 11, 26–27. [Google Scholar]
- Pau, S.; Gillespie, T.W.; Price, J.P. Natural history, biogeography, and endangerment of Hawaiian dry forest trees. Biodivers. Conserv. 2009, 18, 3167–3182. [Google Scholar] [CrossRef]
- Sakai, A.K.; Wagner, W.L.; Mehrhoff, L.A. Patterns of Endangerment in the Hawaiian Flora. Syst. Biol. 2002, 51, 276–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Fish and Wildlife Service (USFWS). Environmental Conservation Online. System: Species Search. Available online: https://ecos.fws.gov/ecp0/reports/ad-hoc-species-report-input (accessed on 11 February 2021).
- Mueller-Dombois, D. The Hawaiian ahupua ‘a land use system: Its biological resource zones and the challenge for sil-vicultural restoration. Bishop Mus. Bull. Cult. Environ. Stud. 2007, 3, 23–33. [Google Scholar]
- Winter, K.B.; Lincoln, N.K.; Berkes, F. The Social-Ecological Keystone Concept: A Quantifiable Metaphor for Understanding the Structure, Function, and Resilience of a Biocultural System. Sustainability 2018, 10, 3294. [Google Scholar] [CrossRef] [Green Version]
- HFIA. Hawai’i’s Dryland Forests. Hawai’i Forest Industry Association. Web. 11 March 2011. 2007. Available online: http://www.Hawai’iforest.org/reports/dryland.html (accessed on 11 February 2021).
- Gon, S.M.; Tom, S.L.; Woodside, U. Āina Momona, Honua Au Loli—Productive Lands, Changing World: Using the Hawaiian Footprint to Inform Biocultural Restoration and Future Sustainability in Hawai‘i. Sustainability 2018, 10, 3420. [Google Scholar] [CrossRef] [Green Version]
- Trauernicht, C.; Pickett, E.; Giardina, C.P.; Litton, C.M.; Cordell, S.; Beavers, A. The Contemporary Scale and Context of Wildfire in Hawai‘i. Pac. Sci. 2015, 69, 427–444. [Google Scholar] [CrossRef]
- Chimera, C.G.; Drake, D.R. Could poor seed dispersal contribute to predation by introduced rodents in a Hawaiian dry forest? Biol. Invasions 2011, 13, 1029–1042. [Google Scholar] [CrossRef]
- Cabin, R.J.; Weller, S.G.; Lorence, D.H.; Flynn, T.W.; Sakai, A.K.; Sandquist, D.; Hadway, L.J. Effects of Long-Term Ungulate Exclusion and Recent Alien Species Control on the Preservation and Restoration of a Hawaiian Tropical Dry Forest. Conserv. Biol. 2000, 14, 439–453. [Google Scholar] [CrossRef]
- Medieros, A.C.; VonAllmen, E.I. Restoration of Native Hawaiian Dryland Forest at Auwahi, Maui; U.S. Geological Survey (USGS) Fact Sheet No. 2006-3035; USGS: Reston, VA, USA, 2006. [CrossRef]
- Ammondt, S.A.; Litton, C.M.; Ellsworth, L.M.; Leary, J.K. Restoration of native plant communities in a Hawaiian dry lowland ecosystem dominated by the invasive grass Megathyrsus maximus. Appl. Veg. Sci. 2013, 16, 22–39. [Google Scholar] [CrossRef]
- Wada, C.A.; Bremer, L.L.; Burnett, K.; Trauernicht, C.; Giambelluca, T.; Mandle, L.; Parsons, E.; Weil, C.; Kurashima, N.; Ticktin, T. Estimating Cost-Effectiveness of Hawaiian Dry Forest Restoration Using Spatial Changes in Water Yield and Landscape Flammability Under Climate Change. Pac. Sci. 2017, 71, 401–424. [Google Scholar] [CrossRef] [Green Version]
- Sato, A.Y.; Ticktin, T.; Alapai, L.; von Allmen, E.I.; Brawner, W.P.; Carter, Y.Y.; Carter, K.A.; Keakealani, R.K.; Medeiros, A.C.; Zahawi, R.A. Biocultural restoration of Hawaiian tropical dry forests. Pac. Conserv. Biol. 2021, 27, 362–375. [Google Scholar] [CrossRef]
- Sato, A.Y. Restoration of Hawaiian Tropical Dry Forests: A Biocultural Approach. Master’s Thesis, University of Hawai‘i at Manoa, Manoa, HI, USA, 2020. [Google Scholar]
- Litton, C.M.; Sandquist, D.R.; Cordell, S. Effects of non-native grass invasion on aboveground carbon pools and tree population structure in a tropical dry forest of Hawaii. For. Ecol. Manag. 2006, 231, 105–113. [Google Scholar] [CrossRef]
- Thaxton, J.M.; Cordell, S.; Cabin, R.J.; Sandquist, D.R. Non-Native Grass Removal and Shade Increase Soil Moisture and Seedling Performance during Hawaiian Dry Forest Restoration. Restor. Ecol. 2012, 20, 475–482. [Google Scholar] [CrossRef]
- Thaxton, J.M.; Cole, T.C.; Cordell, S.; Cabin, R.J.; Sandquist, D.R.; Litton, C.M. Native Species Regeneration Following Ungulate Exclusion and Nonnative Grass Removal in a Remnant Hawaiian Dry Forest. Pac. Sci. 2010, 64, 533–544. [Google Scholar] [CrossRef]
- Cabin, R.J.; Weller, S.G.; Lorence, D.H.; Cordell, S.; Hadway, L.J.; Montgomery, R.; Goo, D.; Urakami, A. Effects of Light, Alien Grass, and Native Species Additions on Hawaiian Dry Forest Restoration. Ecol. Appl. 2002, 12, 1595–1610. [Google Scholar] [CrossRef]
- Cordell, S.; McClellan, M.; Carter, Y.Y.; Hadway, L.J. Towards restoration of Hawaiian tropical dry forests: The Kaupulehu outplanting programme. Pac. Conserv. Biol. 2008, 14, 279–284. [Google Scholar] [CrossRef]
- Burney, D.A.; Burney, L.P. Monitoring results from a decade of native plant translocations at Makauwahi Cave Reserve, Kaua‘i. Plant Ecol. 2016, 217, 139–153. [Google Scholar] [CrossRef]
- Dimson, M.; Gillespie, T.W. Trends in active restoration of tropical dry forest: Methods, metrics, and outcomes. For. Ecol. Manag. 2020, 467, 118150. [Google Scholar] [CrossRef]
- Khurana, E.; Singh, J.S. Ecology of seed and seedling growth for conservation and restoration of tropical dry forest: A review. Environ. Conserv. 2001, 28, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Maza-Villalobos, S.; Balvanera, P.; Martínez-Ramos, M. Early Regeneration of Tropical Dry Forest from Abandoned Pastures: Contrasting Chronosequence and Dynamic Approaches. Biotropica 2013, 43, 666–675. [Google Scholar] [CrossRef]
- Martínez-Ramos, M.; Balvanera, P.; Villa, F.A.; Mora, F.; Maass, J.M.; Méndez, S.M. Effects of long-term inter-annual rainfall variation on the dynamics of regenerative communities during the old-field succession of a neotropical dry forest. For. Ecol. Manag. 2018, 15, 91–100. [Google Scholar] [CrossRef]
- Marquis, R.J.; Diniz, I.R.; Morais, H.C. Patterns and correlates of interspecific variation in foliar insect herbivory and pathogen attack in Brazilian cerrado. J. Trop. Ecol. 2001, 17, 127–148. [Google Scholar] [CrossRef]
- Cuevas-Reyes, P.; Quesada, M.; Hanson, P.; Dirzo, R.; Oyama, K. Diversity of gall-inducing insects in a Mexican tropical dry forest: The importance of plant species richness, life-forms, host plant age and plant density. J. Ecol. 2004, 92, 707–716. [Google Scholar] [CrossRef]
- Sloan, S.A.; Zimmerman, J.K.; Sabat, A.M. Phenology of Plumeria alba and its Herbivores in a Tropical Dry Forest. Biotropica 2007, 39, 195–201. [Google Scholar] [CrossRef]
- Frazier, A.G.; Giambelluca, T.W. Spatial trend analysis of Hawaiian rainfall from 1920 to 2012. Int. J. Clim. 2017, 37, 2522–2531. [Google Scholar] [CrossRef]
- Timm, O.E.; Giambelluca, T.W.; Diaz, H.F. Statistical downscaling of rainfall changes in Hawai‘i based on the CMIP5 global model projections. J. Geophys. Res. Atmos. 2015, 120, 92–112. [Google Scholar] [CrossRef]
- Kimmerer, R.W. Braiding Sweetgrass: Indigenous Wisdom, Scientific Knowledge and the Teachings of Plants; Milkweed Editions: Minneapolis, MN, USA, 2013. [Google Scholar]
- Marks-Block, T.; Lake, F.K.; Bird, R.B.; Curran, L.M. Revitalized Karuk and Yurok cultural burning to enhance California hazelnut for basketweaving in northwestern California, USA. Fire Ecol. 2021, 17, 1–20. [Google Scholar] [CrossRef]
- Kurashima, N.; Jeremiah, J.; Ticktin, T. I Ka Wā Ma Mua: The Value of a Historical Ecology Approach to Ecological Restoration in Hawai‘i. Pac. Sci. 2017, 71, 437–456. [Google Scholar] [CrossRef]
- Memorandum on Indigenous Traditional Ecological Knoweldge and Federal Decision Making (15 November 2021). Available online: https://www.whitehouse.gov/ceq/news-updates/2021/11/15/white-house-commits-to-elevating-indigenous-knowledge-in-federal-policy-decisions/ (accessed on 11 January 2021).
- Lyver, P.O.B.; Akins, A.; Phipps, H.; Kahui, V.; Towns, D.R.; Moller, H. Key biocultural values to guide restoration action and planning in New Zealand. Restor. Ecol. 2016, 24, 314–323. [Google Scholar] [CrossRef]
- Reeves, M.K.; Amidon, F. Habitat Status Assessment Methods—Hawai‘i, Current Condition Summaries; Technical Report from United States Fish and Wildlife Service; Pacific Islands Fish and Wildlife Office: Honolulu, HI, USA, 2018; p. 439.
- Kauahikaua, J.; Cashman, K.; Clague, D.; Champion, D.; Hagstrum, J. Emplacement of the most recent lava flows on Hualalai Volcano, Hawai‘i. Bull. Volcanol. 2002, 64, 229–253. [Google Scholar] [CrossRef]
- Wright, T.L.; Chun, J.Y.F.; Esposo, J.; Heliker, C.; Hodge, J.; Lockwood, J.P.; Vogt, S.M. Map Showing Lava Flow Hazard Zones, Island of Hawai‘i; Miscellaneous Field Studies Map MF-2193; US Geological Service: Reston, VA, USA, 1992.
- Giambelluca, T.W.; Chen, Q.; Frazier, A.G.; Price, J.P.; Chen, Y.-L.; Chu, P.-S.; Eischeid, J.K.; Delparte, D.M. Online Rainfall Atlas of Hawai‘i. Bull. Am. Meteorol. Soc. 2013, 94, 313–316. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. Available online: https://journal.r-project.org/archive/2017/RJ-2017-066/index.html (accessed on 6 September 2021). [CrossRef] [Green Version]
- Crouzeilles, R.; Curran, M.; Ferreira, M.S.; Lindenmayer, D.B.; Grelle, C.E.V.; Benayas, J.R. A global meta-analysis on the ecological drivers of forest restoration success. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Albrecht, M.A.; Osazuwa-Peters, O.L.; Maschinski, J.; Bell, T.J.; Bowles, M.L.; Brumback, W.E.; Duquesnel, J.; Kunz, M.; Lange, J.; McCue, K.A.; et al. Effects of life history and reproduction on recruitment time lags in reintroductions of rare plants. Conserv. Biol. 2019, 33, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Cabin, R.J.; Weller, S.G.; Lorence, D.H.; Cordell, S.; Hadway, L.J. Effects of microsite, water, weeding, and direct seeding on the regeneration of native and alien species within a Hawaiian dry forest preserve. Biol. Conserv. 2002, 104, 181–190. [Google Scholar] [CrossRef]
- Vieira, D.L.; Scariot, A. Principles of natural regeneration of tropical dry forests for restoration. Restor. Ecol. 2006, 14, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Boyer, A.G. Extinction patterns in the avifauna of the Hawaiian islands. Divers. Distrib. 2008, 14, 509–517. [Google Scholar] [CrossRef]
- Chimera, C.G.; Drake, D.R. Patterns of Seed Dispersal and Dispersal Failure in a Hawaiian Dry Forest Having Only Introduced Birds. Biotropica 2010, 42, 493–502. [Google Scholar] [CrossRef]
- Elliott, C.H.; Gillett, C.P.D.T.; Parsons, E.; Rubinoff, D. Conservation conundrum: Endangered species persists on noxious weed. Biotropica 2021, 53, 1265–1269. [Google Scholar] [CrossRef]
- Gregg, R.M. Hawaiian Islands Climate Vulnerability and Adaptation Synthesis; EcoAdapt: Bainbridge Island, WA, USA, 2018. [Google Scholar]
- Frazier, A.G.; Deenik, J.L.; Fujii, N.D.; Funderburk, G.R.; Giambelluca, T.W.; Giardina, C.P.; Helweg, D.A.; Keener, V.W.; Mair, A.; Marra, J.J.; et al. Managing Effects of Drought in Hawai’i and US-Affiliated Pacific Islands. In Effects of Drought on Forests and Rangelands in the United States; Translating Science into Management Responses; Vose, J.M., Peterson, D.L., Luce, C.H., Patel-Weynand, T., Eds.; Gen. Tech. Rep. WO-98; Department of Agriculture Forest Service, Washington Office: Washington, DC, USA, 2019; Chapter 5; pp. 95–121. [Google Scholar]
- Javar-Salas, C.M.; Pe’a, R.; Amidon, F.; Reeves, M.K.; Miller, S.E. Hawaiian Islands Dry Forest. In Encyclopedia of the World’s Biomes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 295–327. [Google Scholar] [CrossRef]
- Libby, R. Factors That Affect Natural Regeneration, Growth, and Survival Rates of Threatened and Endangered Species in Dryland Forests in Hawai’i. Master’s Thesis, University of Hawaiʻi at Mānoa, Mānoa, HI, USA, 2018. [Google Scholar]
- Parker, J.E.; Snyder, W.E.; Hamilton, G.C.; Rodriguez-Saona, C. Companion planting and insect pest control. Weed Pest Control. Conv. New Chall. 2013, 10, 55044. [Google Scholar]
- Chock, M.K.; Hoyt, B.; Amend, A.S. Mycobiome Transplant Increases Resistance to Austropuccinia psidii in an Endangered Hawaiian Plant. Phytobiomes J. 2021, 5, 326–334. [Google Scholar] [CrossRef]






| Scientific Name | Common Name | Family | Life Form | Presence of Wild Adults in Reserve | Year First Outplanted | No. of Adults (Reproductive–Sized Plants) in Reserve (2016) |
|---|---|---|---|---|---|---|
| Colubrina oppositifolia | Kauila | Rhamnaceae | Tree | Yes | 1999 | 144 |
| Mezoneuron kavaiensis * | Uhiuhi | Fabaceae | Tree | Yes | 1999 | 5 |
| Nothocestrum breviflorum | Aiea | Solanaceae | Tree | Yes | 1999 | 4 |
| Chrysodracon hawaiiensis | Halapepe | Asparagaceae | Tree | Yes | 2000 | 199 |
| Kokia drynarioides * | Hau Hele ʻUla | Malvaceae | Small tree/Shrub | Yes | 1999 | 62 |
| Hibiscus brackenridgei subsp. Brackenridgei * | Maʻo Hau Hele | Malvaceae | Small tree/Shrub | Yes | 2001 | 44 |
| Abutilon menziesii | Koʻoloaʻula | Malvaceae | Shrub | No | 2002 | 12 |
| Hibiscus hualalaiensis * | Hau Kuahiwi | Malvaceae | Shrub | Yes | 2003 | 11 |
| Bidens micrantha subsp. Ctenophylla | Koʻokoʻolau | Asteraceae | Shrub | No | 2002 | NA |
| Neraudia ovata | Maʻaloa | Urticaceae | Shrub | No | 2011 | NA |
| Isodendrion pyrifolium * | Aupaka | Violaceae | Small shrub | Yes | 2010 | 4 |
| Bonamia menziesii | NA | Convolvulaceae | Woody vine | No | 1999 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Libby, R.; Sato, A.Y.; Alapai, L.; Brawner, W.P.; Carter, Y.Y.; Carter, K.A.; Tomich, K.; Ticktin, T. A Hawaiian Tropical Dry Forest Regenerates: Natural Regeneration of Endangered Species under Biocultural Restoration. Sustainability 2022, 14, 1159. https://doi.org/10.3390/su14031159
Libby R, Sato AY, Alapai L, Brawner WP, Carter YY, Carter KA, Tomich K, Ticktin T. A Hawaiian Tropical Dry Forest Regenerates: Natural Regeneration of Endangered Species under Biocultural Restoration. Sustainability. 2022; 14(3):1159. https://doi.org/10.3390/su14031159
Chicago/Turabian StyleLibby, Reko, Aimee Y. Sato, Lehua Alapai, Wilds Pihanui Brawner, Yvonne Yarber Carter, Keoki Apokolani Carter, Kekaulike Tomich, and Tamara Ticktin. 2022. "A Hawaiian Tropical Dry Forest Regenerates: Natural Regeneration of Endangered Species under Biocultural Restoration" Sustainability 14, no. 3: 1159. https://doi.org/10.3390/su14031159
APA StyleLibby, R., Sato, A. Y., Alapai, L., Brawner, W. P., Carter, Y. Y., Carter, K. A., Tomich, K., & Ticktin, T. (2022). A Hawaiian Tropical Dry Forest Regenerates: Natural Regeneration of Endangered Species under Biocultural Restoration. Sustainability, 14(3), 1159. https://doi.org/10.3390/su14031159







