Ecological Insight, Anatomical Features, and Fiber Characterization of Leptadenia pyrotechnica (Forrsk.) Decne. as a Promising Resource
Abstract
1. Introduction
2. Materials and Methods
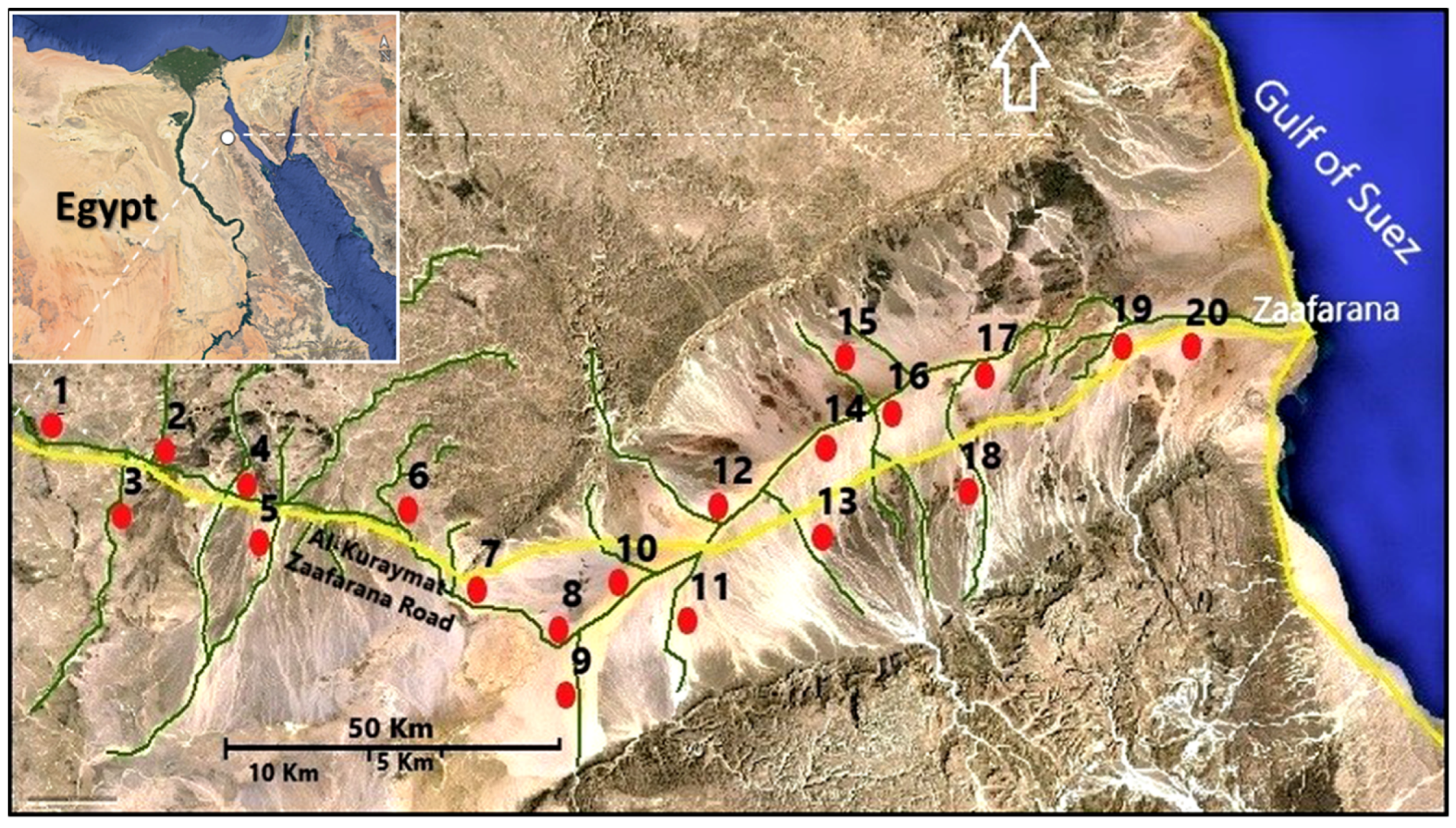
2.1. Vegetation Analysis
2.2. Soil Analysis
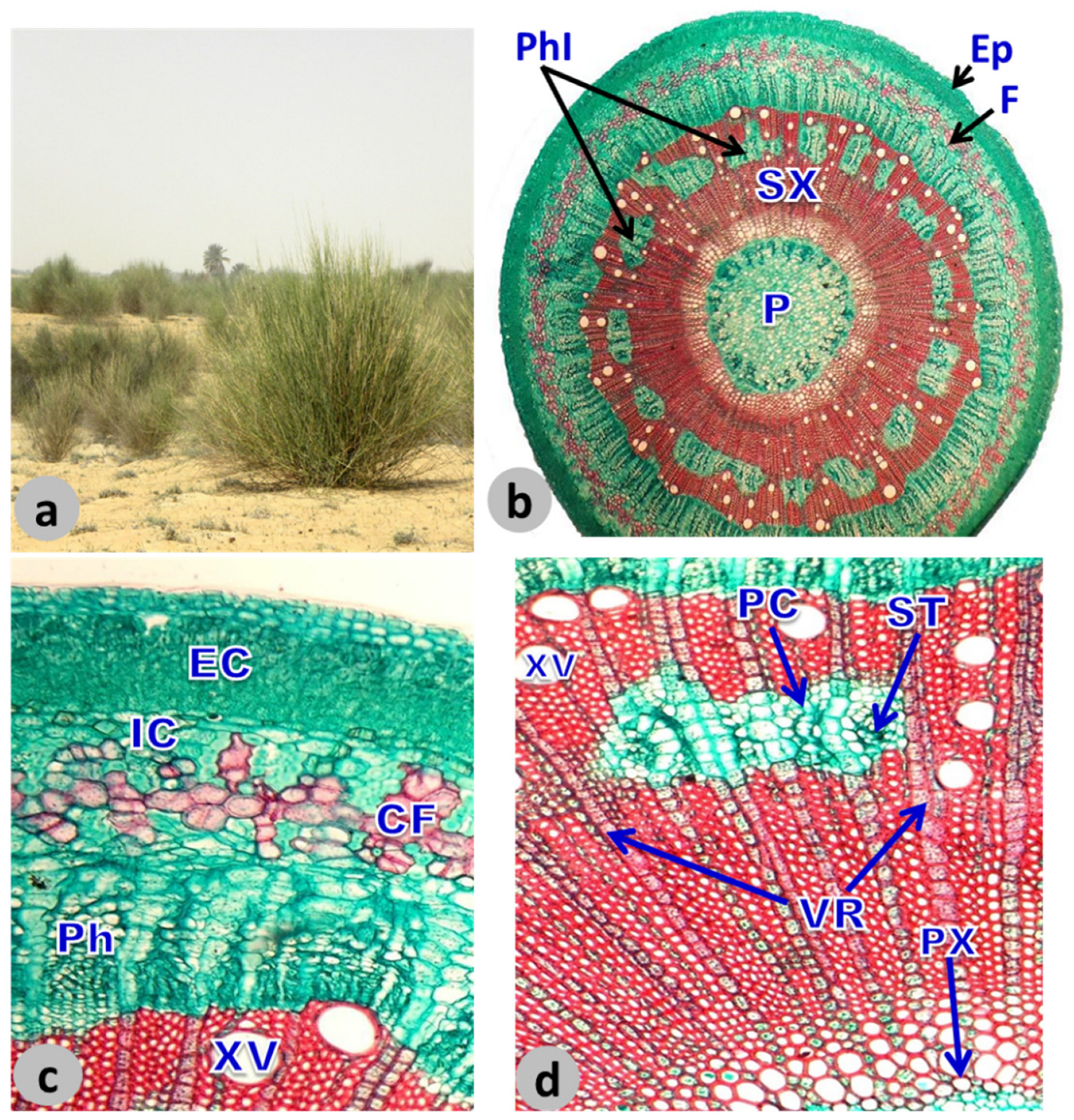
2.3. Anatomical Analysis
2.4. Fiber Characteristics
2.4.1. Chemical Properties
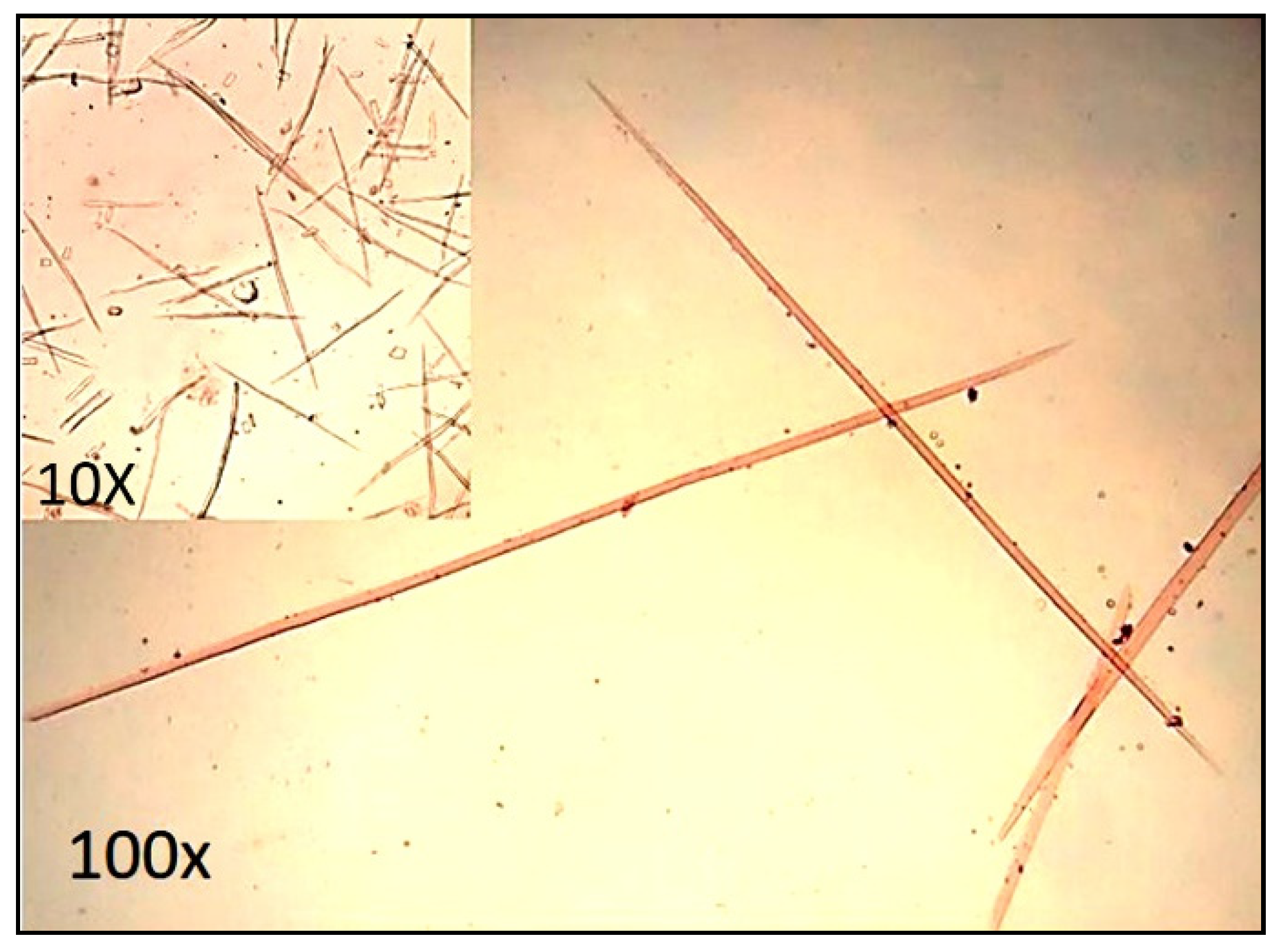
2.4.2. Biometry and Morphological Properties of Fiber
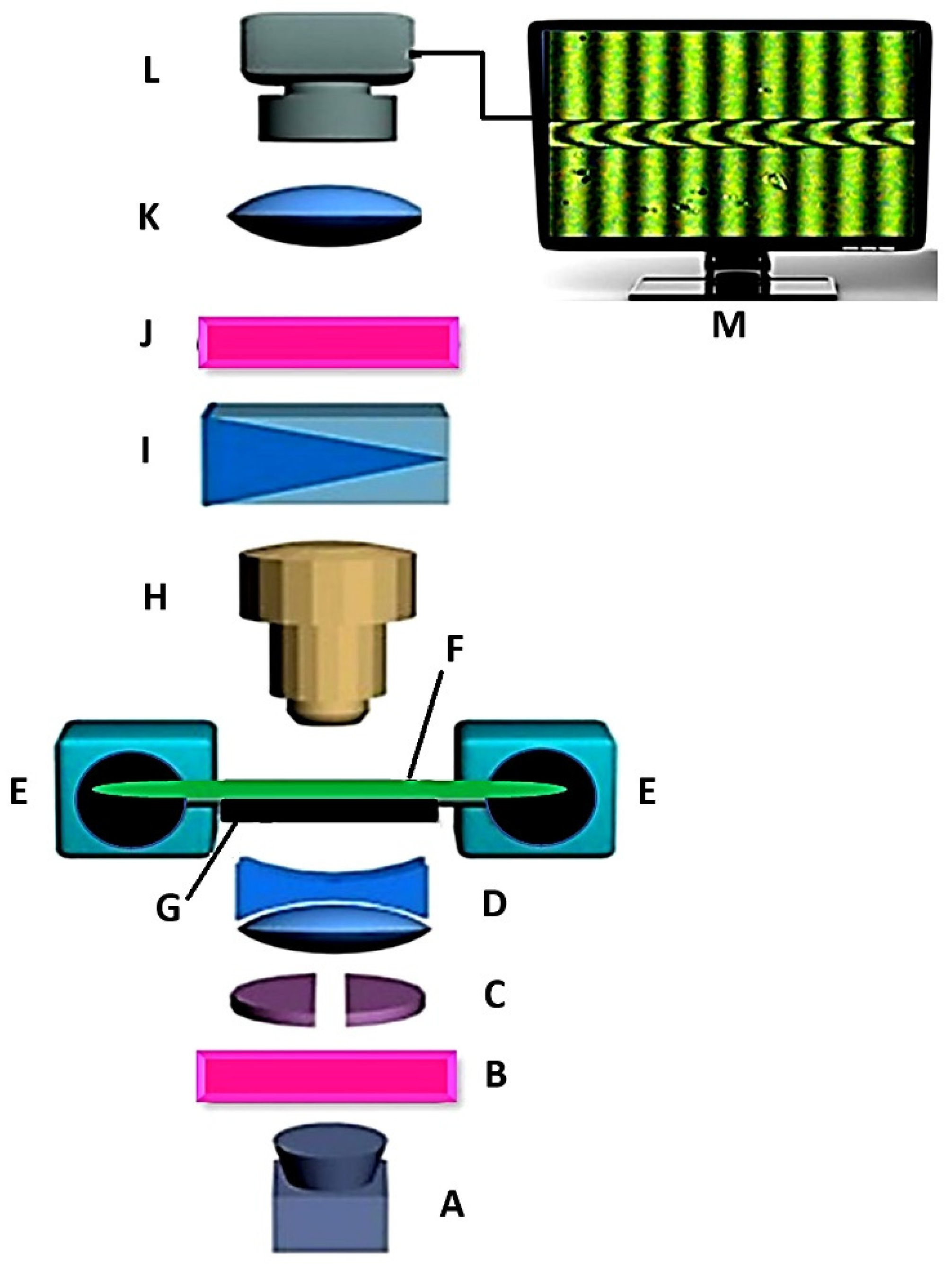
2.4.3. Optical Properties
2.5. Treatment of Data
3. Results and Discussion
3.1. Floristic Composition of the L. pyrotechnica Habitat
3.2. Vegetation Analysis of L. pyrotechnica Communities
3.3. Anatomical Investigation of L. pyrotechnica Stem
3.4. Fiber Characteristics of L. pyrotechnica
3.4.1. Chemical Properties
3.4.2. Biometry Properties
3.4.3. Morphological Properties
3.4.4. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zahran, M.A.; El-Amier, Y.A. Non-traditional fodders from the halophytic vegetation of the deltaic Mediterranean coastal desert, Egypt. J. Biol. Sci. 2013, 13, 226–233. [Google Scholar] [CrossRef]
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Plant fibre based bio-composites: Sustainable and renewable green materials. Renew. Sustain. Energy Rev. 2017, 79, 558–584. [Google Scholar] [CrossRef]
- Khan, A.; Rangappa, S.M.; Siengchin, S.; Asiri, A.M. Biofibers and Biopolymers for Biocomposites: Synthesis, Characterization and Properties; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Viotti, C.; Albrecht, K.; Amaducci, S.; Bardos, P.; Bertheau, C.; Blaudez, D.; Bothe, L.; Cazaux, D.; Ferrarini, A.; Govilas, J. Nettle, a long-known fiber plant with new perspectives. Materials 2022, 15, 4288. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-D.; Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors (2015–2019). Anal. Chem. 2019, 92, 397–430. [Google Scholar] [CrossRef]
- Boulos, L. Flora of Egypt; All Hadara Publishing: Cairo, Egypt, 1995; volume 1. [Google Scholar]
- Cioffi, G.; Sanogo, R.; Vassallo, A.; Dal Piaz, F.; Autore, G.; Marzocco, S.; De Tommasi, N. Pregnane Glycosides from Leptadenia pyrotechnica. J. Nat. Prod. 2006, 69, 625–635. [Google Scholar] [CrossRef]
- Partap, S.; Tewari, U.; Sharma, K.; Jha, K.K. Hepatoprotective activity of whole plant extract of Leptadenia pyrotechnica against paracetamol induced damage in rats. J. Drug Deliv. Ther. 2014, 4, 36–39. [Google Scholar] [CrossRef]
- Saeed, H.A.; Liu, Y.; Lucia, L.A.; Chen, H. Sudanese dicots as alternative fiber sources for pulp and papermaking. Drv. Ind. 2018, 69, 175–182. [Google Scholar] [CrossRef]
- Boulos, L. Flora of Egypt Checklist; Revised Annotated Edition; All Hadara Publishing: Cairo, Egypt, 2009; pp. 198–201. [Google Scholar]
- Chaudhary, S.A. Flora of the Kingdom of Saudi Arabia; Ministry of Agriculture and Water: Riyadh, Saudi Arabia, 1999; Volume 1. [Google Scholar]
- Qureshi, R.; Munazir, M.; Ahmed, A.A.A.-S.M.; Shabbir, G. In vitro callus induction protocol for Leptadenia pyrotechnica using various explants. J. Med. Plants Res. 2012, 6, 379–382. [Google Scholar]
- Bhatti, G.R.; Qureshi, R.; Shah, M. Ethnobotany of Qadanwari of Nara Desert. Pak. J. Bot. 2001, 33, 801–812. [Google Scholar]
- Usman, H.; Aji, A.; Ahmed, I.A.; Wiam, I.M.; Jambaima, M.A. Phytochemical and Antidiarrhoeal Screening of the Aerial Part of Leptadenia pyrotechnica (Forsk.) Decne. Experimental Animals 2017, 8, 9. [Google Scholar]
- Verma, N.; Jha, K.; Chaudhary, S.; Singh, O.; Kumar, A. Phytochemistry, pharmacology and traditional uses of Leptadenia pyrotechnica-An important medicinal plant. Indian J. Pharm. Biol. Res. 2014, 2, 128. [Google Scholar] [CrossRef]
- Youssef, M.A.M.; Khodair, A.I.; Saleh, M.A. Isolation, structural elucidation of flavonoid constituents from Leptadenia pyrotechnica and evaluation of their toxicity and antitumor activity. Pharm. Biol. 2009, 47, 539–552. [Google Scholar] [CrossRef]
- Zahran, M.A.; El-Amier, Y.A. Ecology and establishment of fiber producing taxa naturally growing in the Egyptian deserts. Egypt. J. Basic Appl. Sci. 2014, 1, 144–150. [Google Scholar] [CrossRef][Green Version]
- Canfield, R.H. Application of the line interception method in sampling range vegetation. J. For. 1941, 39, 388–394. [Google Scholar]
- Shukla, R.S.; Chandel, P.S. Plant Ecology and Soil Science; S. Cand. & Company LTD: New Delhi, India, 1989. [Google Scholar]
- Tackholm, V. Students’ Flora of Egypt, 2nd ed.; University Press: Cairo, Egypt, 1974. [Google Scholar]
- Raunkiaer, C. Plant Life Forms; Clarendon Press: Oxford, UK, 1937. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Scientific Publishers: New Delhi, India, 1947. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course; UW-Madison Libraries Parallel Press: Madison, WI, USA, 1962. [Google Scholar]
- Pierce, W.C.; Haenisch, E.L.; Sawyer, D.T. Quantitative Analysis; Wiley Toppen: Tokyo, Japan, 1958. [Google Scholar]
- Allen, S.E.; Grimshaw, H.M.; Parkinson, J.A.; Quarmby, C. Chemical Analysis of Ecological Materials, 2nd ed.; Blackwell Scientific Publications: Hoboken, NJ, USA, 1989. [Google Scholar]
- Jensen, W.A. Botanical Histochemistry—Principles and Practice; W. H. Freeman and Company: San Francisco, CA, USA, 1962. [Google Scholar]
- Peacock, P.; Bradbury, S. Peacock’s Elementary Microtechnique, 4th ed.; Edward Arnold: London, UK, 1973. [Google Scholar]
- Esau, K. Anatomy of Seed Plants, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1977; Volume 445, p. 448. [Google Scholar]
- Fahn, A. Plant Anatomy, 3rd ed.; Pergamon Press: Oxford, UK, 1982. [Google Scholar]
- El-Amier, Y.A.; Abd El-Gawad, A.M. Anatomical investigation of three emergent Cyperus species growing naturally on the canal banks of the Nile delta, Egypt. J. Sci. Agric. 2017, 294–299. [Google Scholar] [CrossRef]
- Yuan, Z.; Kapu, N.S.; Beatson, R.; Chang, X.F.; Martinez, D.M. Effect of alkaline pre-extraction of hemicelluloses and silica on kraft pulping of bamboo (Neosinocalamus affinis Keng). Ind. Crops Prod. 2016, 91, 66–75. [Google Scholar] [CrossRef]
- 11885:2007; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICPOES). International Organization for Standardization (ISO): Geneva, Switzerland, 2007.
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Company, Inc.: London, UK, 1940; 530p. [Google Scholar]
- Rafat, M. Chemical and biometry properties of Iranian cultivated paulownia wood (Paulownia fortunei). Middle East J. Sci. Res. 2011, 10, 604–607. [Google Scholar]
- Albert, S.; Padhiar, A.; Gandhi, D. Fiber properties of Sorghum halepense and its suitability for paper production. J. Nat. Fibers 2011, 8, 263–271. [Google Scholar] [CrossRef]
- Pirralho, M.; Flores, D.; Sousa, V.B.; Quilhó, T.; Knapic, S.; Pereira, H. Evaluation on paper making potential of nine Eucalyptus species based on wood anatomical features. Ind. Crops Prod. 2014, 54, 327–334. [Google Scholar] [CrossRef]
- Kljun, A.; El-Dessouky, H.M.; Benians, T.A.; Goubet, F.; Meulewaeter, F.; Knox, J.P.; Blackburn, R.S. Analysis of the physical properties of developing cotton fibres. Eur. Polym. J. 2014, 51, 57–68. [Google Scholar] [CrossRef]
- Pluta, M. Interference microscopy of polymer fibres. J. Microsc. 1972, 96, 309–332. [Google Scholar] [CrossRef]
- Sokkar, T.; El-Tonsy, M.; El-Morsy, M.; El-Khateep, S.; Raslan, M. Multi-mode opto-thermo-mechanical stretching system for determination of 3D refractive index along the axis of stretched and/or heated fibres. Opt. Laser Technol. 2011, 43, 1054–1060. [Google Scholar] [CrossRef]
- Simmens, S. Birefringence determination in objects of irregular cross-sectional shape and constant weight per unit length. Nature 1958, 181, 1260–1261. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El-Amier, Y.A.; Assaeed, A.M.; Al-Rowaily, S.L. Interspecific variations in the habitats of Reichardia tingitana (L.) Roth leading to changes in its bioactive constituents and allelopathic activity. Saudi J. Biol. Sci. 2020, 27, 489–499. [Google Scholar] [CrossRef]
- Zahran, M.; Willis, A.J. The Vegetation of Egypt; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; Volume 2. [Google Scholar]
- Funk, V.; Susanna, A.; Stuessy, T.; Bayer, R. Systematics, Evolution, and Biogeography of Compositae; International Association for Plant Taxonomy: Bratislava, Slovakia, 2009. [Google Scholar]
- El-Amier, Y.; Abdul-Kader, O. Vegetation and species diversity in the northern sector of Eastern Desert, Egypt. West Afr. J. Appl. Ecol. 2015, 23, 75–95. [Google Scholar]
- Danin, A. Plants of Desert Dunes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Danin, A.; Plitmann, U. Revision of the plant geographical territories of Israel and Sinai. Plant Syst. Evol. 1987, 156, 43–53. [Google Scholar] [CrossRef]
- Schweingruber, F.H.; Börner, A.; Schulze, E.-D. Atlas of Stem Anatomy in Herbs, Shrubs and Trees; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 1. [Google Scholar]
- Saeed, H.A.; Liu, Y.; Lucia, L.A.; Chen, H. Sudanese agro-residue as a novel furnish for pulp and paper manufacturing. BioResources 2017, 12, 4166–4176. [Google Scholar] [CrossRef]
- Kiaei, M.; Samariha, A.; Kasmani, J.E. Characterization of biometry and the chemical and morphological properties of fibers from bagasse, corn, sunflower, rice and rapeseed residues in Iran. Afr. J. Agric. Res. 2011, 6, 3762–3767. [Google Scholar]
- Hemmasi, A.H.; Samariha, A.; Tabei, A.; Nemati, M.; Khakifirooz, A. Study of morphological and chemical composition of fibers from Iranian sugarcane bagasse. Am.-Eurasian J. Agric. Environ. Sci. 2011, 11, 478–481. [Google Scholar]
- De Carvalho, D.M.; Moser, C.; Lindström, M.E.; Sevastyanova, O. Impact of the chemical composition of cellulosic materials on the nanofibrillation process and nanopaper properties. Ind. Crops Prod. 2019, 127, 203–211. [Google Scholar] [CrossRef]
- Syed, N.F.N.; Zakaria, M.H.; Bujang, J.S. Fiber characteristics and papermaking of seagrass using hand-beaten and blended pulp. BioResources 2016, 11, 5358–5380. [Google Scholar] [CrossRef]
- Peng, X.; Nie, S.; Li, X.; Huang, X.; Li, Q. Characteristics of the water-and alkali-soluble hemicelluloses fractionated by sequential acidification and graded-ethanol from sweet maize stems. Molecules 2019, 24, 212. [Google Scholar] [CrossRef] [PubMed]
- Mattos, B.D.; Lourençon, T.V.; Gatto, D.A.; Serrano, L.; Labidi, J. Chemical characterization of wood and extractives of fast-growing Schizolobium parahyba and Pinus taeda. Wood Mater. Sci. Eng. 2016, 11, 209–216. [Google Scholar] [CrossRef]
- Kaur, D.; Bhardwaj, N.K.; Lohchab, R.K. Prospects of rice straw as a raw material for paper making. Waste Manag. 2017, 60, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Plazonić, I.; Barbarić-Mikočević, Ž.; Antonović, A. Chemical composition of straw as an alternative material to wood raw material in fibre isolation. Drv. Ind. 2016, 67, 119–125. [Google Scholar] [CrossRef]
- Sotannde, O.; Oluwadare, A. Fibre and elemental contents of Thaumatococcus daniellii stalk and its implications as a non-wood fibre source. Int. J. Appl. 2014, 4, 178–185. [Google Scholar]
- Machmud, M.N.; Fadi, F.; Fuadi, Z.; Kokarkin, C. Alternative fiber sources from Gracilaria Sp and Eucheuma Cottonii for papermaking. Int. J. Sci. Eng. 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Bakar, N.; Chin, S.; Siregar, J.; Ngien, S. A review on physical, mechanical, thermal properties and chemical composition of plant fibers. MS&E 2020, 736, 052017. [Google Scholar]
- Carrillo-Varela, I.; Valenzuela, P.; Gacitúa, W.; Mendonca, R.T. An Evaluation of Fiber Biometry and Nanomechanical Properties of Different Eucalyptus Species. BioResources 2019, 14, 6433–6446. [Google Scholar]
- Comlekcioglu, N.; Tutus, A.; Cicekler, M.; Canak, A.; Zengin, G. Investigation of Isatis tinctoria and Isatis buschiana stalks as raw materials for pulp and paper production. Drv. Ind. 2016, 67, 249–255. [Google Scholar] [CrossRef]
- Adi, D.S.; Risanto, L.; Damayanti, R.; Rullyati, S.; Dewi, L.M.; Susanti, R.; Dwianto, W.; Hermiati, E.; Watanabe, T. Exploration of unutilized fast growing wood species from secondary forest in Central Kalimantan: Study on the fiber characteristic and wood density. Procedia Environ. Sci. 2014, 20, 321–327. [Google Scholar] [CrossRef]
- Amode, N.S.; Jeetah, P. Paper Production from Mauritian Hemp Fibres. Waste Biomass Valorization 2020, 12, 1781–1802. [Google Scholar] [CrossRef]
- Nasser, R.A.; Hiziroglu, S.; Abdel-Aal, M.A.; Al-Mefarrej, H.A.; Shetta, N.D.; Aref, I.M. Measurement of some properties of pulp and paper made from date palm midribs and wheat straw by soda-AQ pulping process. Measurement 2015, 62, 179–186. [Google Scholar] [CrossRef]
- Watson, A.; Dadswell, H. Influence of fibre morphology on paper properties. Appita Technol. Innov. Manuf. Environ. 2017, 70, 271. [Google Scholar]
- Tutus, A.; Ezici, A.C.; Ates, S. Chemical, morphological and anatomical properties and evaluation of cotton stalks (Gossypium hirsutum L.) in pulp industry. Sci. Res. Essays 2010, 5, 1553–1560. [Google Scholar]
- Akgül, M.; Tozluoğlu, A. Some chemical and morphological properties of juvenile woods from beech (Fagus orientalis L.) and pine (Pinus nigra A.) plantations. Trends Appl. Sci. Res. 2009, 4, 116–125. [Google Scholar] [CrossRef]
- Anoop, E.; Ajayghosh, V.; Nijil, J.; Jijeesh, C. Evaluation of pulp wood quality of selected tropical pines raised in the high ranges of Idukki District, Kerala. J. Trop. Agric. 2014, 52, 59–66. [Google Scholar]
- Riki, J.; Sotannde, O.; Oluwadare, A. Anatomical and chemical properties of wood and their practical implications in pulp and paper production: A review. J. Res. For. Wildl. Environ. 2019, 11, 358–368. [Google Scholar]
- Sharma, M.; Sharma, C.; Lama, D. Anatomical and fibre characteristics of some agrowaste materials for pulp and paper making. Int. J. Agric. Sci. Res. 2015, 5, 155–162. [Google Scholar]
- Kiaei, M.; Tajik, M.; Vaysi, R. Chemical and biometrical properties of plum wood and its application in pulp and paper production. Maderas Cienc. Tecnol. 2014, 16, 313–322. [Google Scholar] [CrossRef]
| Group | Stands | Total Species | Shannon Evenness | Simpson Diversity | Dominant Species | Other Important Species |
|---|---|---|---|---|---|---|
| I | 5, 6, 7, 20 | 29 | 0.84 | 0.96 | Leptadenia pyrotechnica (31.45 ± 2.13) Haloxylon salicornicum (29.52 ± 1.82) * | Diplotaxis harra (20.21 ± 2.47) * Senecio glaucus (16.09 ± 0.87) Rumex vesicarius (12.85 ± 1.37) Zygophyllum coccineum (11.80 ± 2.09) Bassia muricata (9.89 ± 0.43) Astragalus spinosus (9.54 ± 0.47) Fagonia mollis (7.93 ± 0.56) |
| II | 13, 17, 18, 19 | 33 | 0.87 | 0.98 | Zilla spinosa (26.60 ± 2.48) Retama raetam (25.98 ± 1.39) | Leptadenia pyrotechnica (24.97 ± 3.10) Emex spinosa (17.62 ± 2.54) Zygophyllum coccineum (9.41 ± 1.77) Zygophyllum simplex (9.05 ± 0.86) Launaea nudicaulis (8.93 ± 0.78) Fagonia mollis (8.10 ± 0.46) |
| III | 1, 3, 4, 9, 10, 12, 15 | 23 | 0.85 | 0.94 | Leptadenia pyrotechnica (40.84 ± 2.88) | Ochradenus baccatus (21.15 ± 1.27) Retama raetam (17.71 ± 1.69) Zygophyllum coccineum (15.83 ± 0.49) Pulicaria undulata (14.62 ± 0.83) Lavandula coronopifolia (12.99 ± 0.76) Zilla spinosa (12.52 ± 0.88) Launaea spinosa (10.14 ± 0.37) |
| IV | 2, 8, 11, 14, 16 | 20 | 0.79 | 0.93 | Retama raetam (35.20 ± 2.09) Ochradenus baccatus (34.95 ± 2.17) | Leptadenia pyrotechnica (15.43 ± 0.98) Launaea spinosa (15.24 ± 0.67) Echinops spinosus (14.96 ± 1.85) Astragalus spinosus (14.23 ± 0.36) Haloxylon salicornicum (13.43 ± 0.67) Deverra tortuosa (8.55 ± 0.51) |
| Soil Variables | Plant Group | F-Value | |||
|---|---|---|---|---|---|
| A (n = 4) | B (n = 4) | C (n = 7) | D (n = 5) | ||
| pH | 8.03 ± 0.06 | 8.03 ± 0.07 | 8.09 ± 0.03 | 7.95 ± 0.03 | 0.21 |
| EC (mS cm−1) | 0.83 ± 0.16 | 0.82 ± 0.08 | 0.74 ± 0.04 | 0.84 ± 0.11 | 0.05 |
| Sand (%) | 86.74 ± 1.54 | 85.45 ± 1.92 | 86.02 ± 0.92 | 82.01 ± 0.99 | 0.65 |
| Silt (%) | 10.88 ± 1.44 | 12.15 ± 1.74 | 12.16 ± 0.84 | 15.42 ± 0.98 | 0.64 |
| Clay (%) | 2.38 ± 0.24 | 2.40 ± 0.22 | 1.83 ± 0.11 | 2.57 ± 0.08 | 1.29 |
| Porosity (%) | 34.51 ± 1.68 | 31.48 ± 0.51 | 33.34 ± 0.23 | 29.95 ± 0.61 | 1.09 |
| WHC (%) | 24.26 ± 0.67 | 29.79 ± 1.94 | 27.80 ± 0.87 | 26.48 ± 0.40 | 0.85 |
| CaCO3 (%) | 18.75 ± 1.45 | 20.84 ± 1.11 | 17.29 ± 1.09 | 17.84 ± 1.08 | 0.08 |
| OC (%) | 0.26 ± 0.01 | 0.25 ± 0.01 | 0.24 ± 0.00 | 0.26 ± 0.01 | 0.02 |
| Cl− (%) | 0.08 ± 0.00 | 0.12 ± 0.02 | 0.09 ± 0.00 | 0.14 ± 0.01 | 1.45 |
| SO42− (%) | 0.26 ± 0.02 | 0.45 ± 0.05 | 0.29 ± 0.02 | 0.34 ± 0.01 | 1.25 |
| HCO3− (%) | 1.33 ± 0.07 | 1.37 ± 0.08 | 1.16 ± 0.03 | 1.53 ± 0.07 | 1.02 |
| Na+ (mg g−1) | 353.75 ± 110.97 | 352.85 ± 53.89 | 286.60 ± 30.10 | 369.03 ± 74.88 | 0.05 |
| K+ (mg g−1) | 50.37 ± 11.57 | 45.97 ± 4.79 | 42.26 ± 3.18 | 50.79 ± 7.81 | 0.08 |
| Ca2+ (mg g−1) | 114.30 ± 31.67 | 113.05 ± 15.82 | 95.65 ± 8.63 | 115.46 ± 21.44 | 0.04 |
| Mg2+ (mg g−1) | 60.94 ± 15.13 | 54.89 ± 6.17 | 50.76 ± 4.19 | 61.46 ± 10.34 | 0.09 |
| Plant Species | Chemical Compositions | Elemental Analysis | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Holocellulose (%) | Cellulose (%) | Hemicelluloses (%) | Lignin (%) | Ash (%) | N (%) | Na (%) | K (%) | Ca (%) | Mg (%) | Al (%) | Si (%) | ||
| L. pyrotechnica | 67.20 | 48.61 | 18.59 | 15.77 | 2.26 | 1.48 | 0.61 | 0.41 | 0.24 | 0.09 | 0.06 | 0.05 | Current study |
| L. pyrotechnica | 66.40 | 44.30 | 22.10 | 21.70 | 2.40 | 1.19 | N/A | N/A | 0.13 | 0.05 | 0.12 | 0.07 | [9] |
| Date palm leaves | 61.63 | 39.00 | 22.63 | 15.34 | 2.06 | N/A | 1.86 | N/A | 0.68 | 0.26 | 0.30 | 0.37 | [48] |
| Rice straw | 71.66 | 50.33 | 21.33 | N/A | 15.73 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | [49] |
| Sugarcane bagasse | N/A | 55.75 | N/A | 20.50 | 1.85 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | [50] |
| Sunflower | 67.00 | 46.00 | 21.00 | N/A | 7.60 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | [49] |
| Properties | L. pyrotechnica | L. pyrotechnica | Rice Straw | Sunflower | Sugarcane Bagasse | ||
|---|---|---|---|---|---|---|---|
| Range | Mean | ±SD | |||||
| Biometry properties | |||||||
| Fiber length (mm) | 0.61–0.87 | 0.72 | 0.03 | 0.70 | 0.83 | 0.96 | 1.5 |
| Fiber width (μm) | 19.14–21.32 | 20.46 | 2.32 | 18.20 | 10.89 | 22.84 | 20.96 |
| Lumen diameter (μm) | 10.01–11.84 | 10.87 | 1.41 | 11.40 | 4.57 | 11.12 | 9.72 |
| Cell wall thickness (μm) | 5.81–7.22 | 6.48 | 1.83 | 7.00 | 3.16 | 5.85 | 5.64 |
| Morphological properties | |||||||
| Felting power | 31.87–40.81 | 35.19 | 1.61 | 38.46 | 76.58 | 42.03 | 75.86 |
| Rigidity coefficient | 30.4–33.9 | 31.67 | 0.92 | 38.46 | N/A | N/A | N/A |
| Slenderness ratio | 31.87–40.81 | 35.19 | 1.64 | 38.46 | 76.58 | 42.03 | 75.86 |
| Flexibility ratio | 52.30–55.53 | 53.13 | 2.41 | 62.64 | 41.96 | 48.68 | 46.37 |
| Runkel ratio | 1.16–1.22 | 1.19 | 0.11 | 1.23 | 1.38 | 1.05 | 1.16 |
| Optical properties | |||||||
| Birefringence (Ana) | 0.0018 | N/A | N/A | N/A | N/A | ||
| Reference | Current study | [9] | [49] | [49] | [50] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-ElGawad, A.M.; Assaeed, A.M.; Bonanomi, G.; El-Amier, Y.A. Ecological Insight, Anatomical Features, and Fiber Characterization of Leptadenia pyrotechnica (Forrsk.) Decne. as a Promising Resource. Sustainability 2022, 14, 16895. https://doi.org/10.3390/su142416895
Abd-ElGawad AM, Assaeed AM, Bonanomi G, El-Amier YA. Ecological Insight, Anatomical Features, and Fiber Characterization of Leptadenia pyrotechnica (Forrsk.) Decne. as a Promising Resource. Sustainability. 2022; 14(24):16895. https://doi.org/10.3390/su142416895
Chicago/Turabian StyleAbd-ElGawad, Ahmed M., Abdulaziz M. Assaeed, Giuliano Bonanomi, and Yasser A. El-Amier. 2022. "Ecological Insight, Anatomical Features, and Fiber Characterization of Leptadenia pyrotechnica (Forrsk.) Decne. as a Promising Resource" Sustainability 14, no. 24: 16895. https://doi.org/10.3390/su142416895
APA StyleAbd-ElGawad, A. M., Assaeed, A. M., Bonanomi, G., & El-Amier, Y. A. (2022). Ecological Insight, Anatomical Features, and Fiber Characterization of Leptadenia pyrotechnica (Forrsk.) Decne. as a Promising Resource. Sustainability, 14(24), 16895. https://doi.org/10.3390/su142416895









