Microbial Fuel Cells (MFC): A Potential Game-Changer in Renewable Energy Development
Abstract
1. Introduction
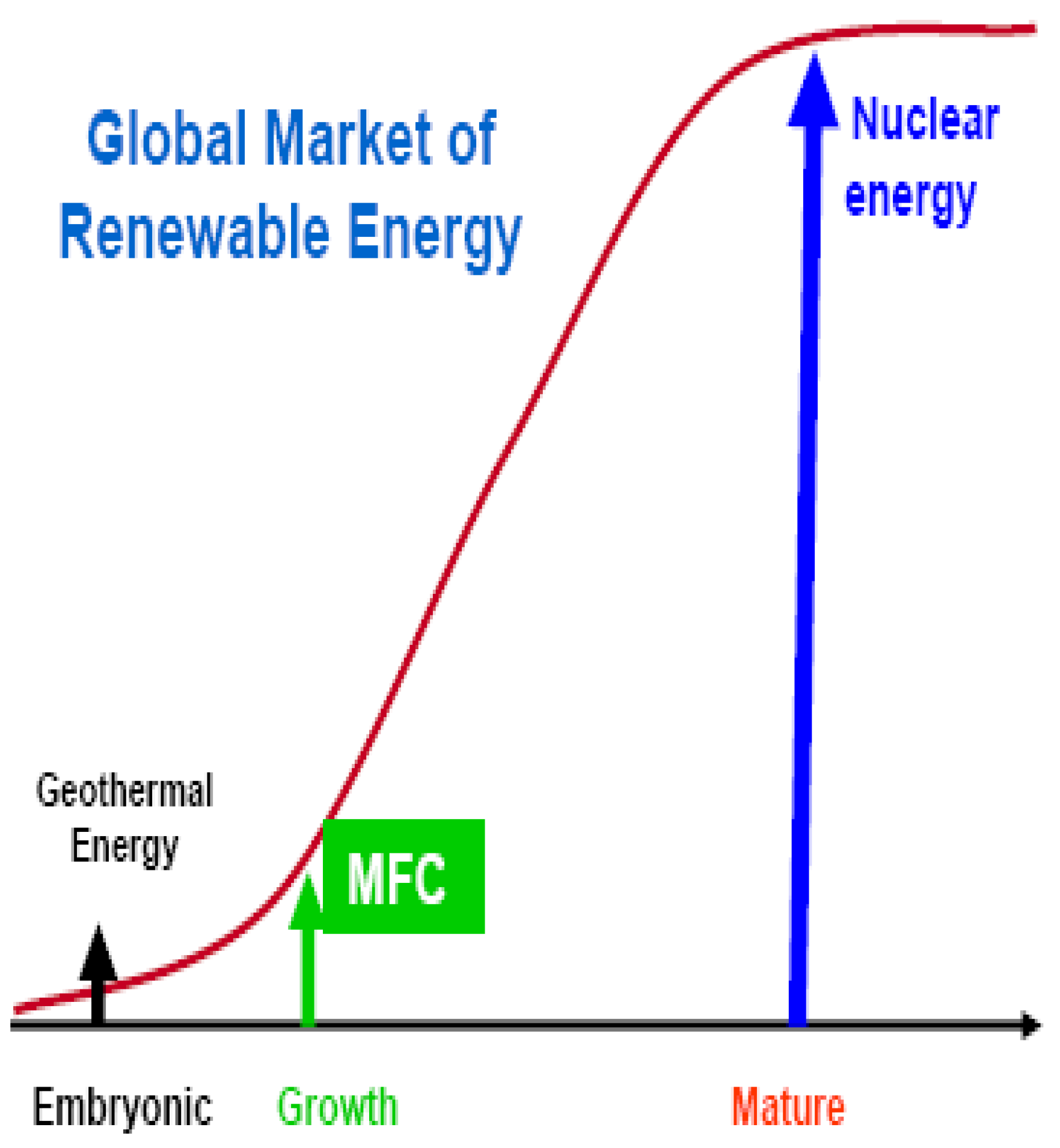
2. MFC: An Option in Renewable Energy Development
3. Mechanism of Electricity Generation by MFC
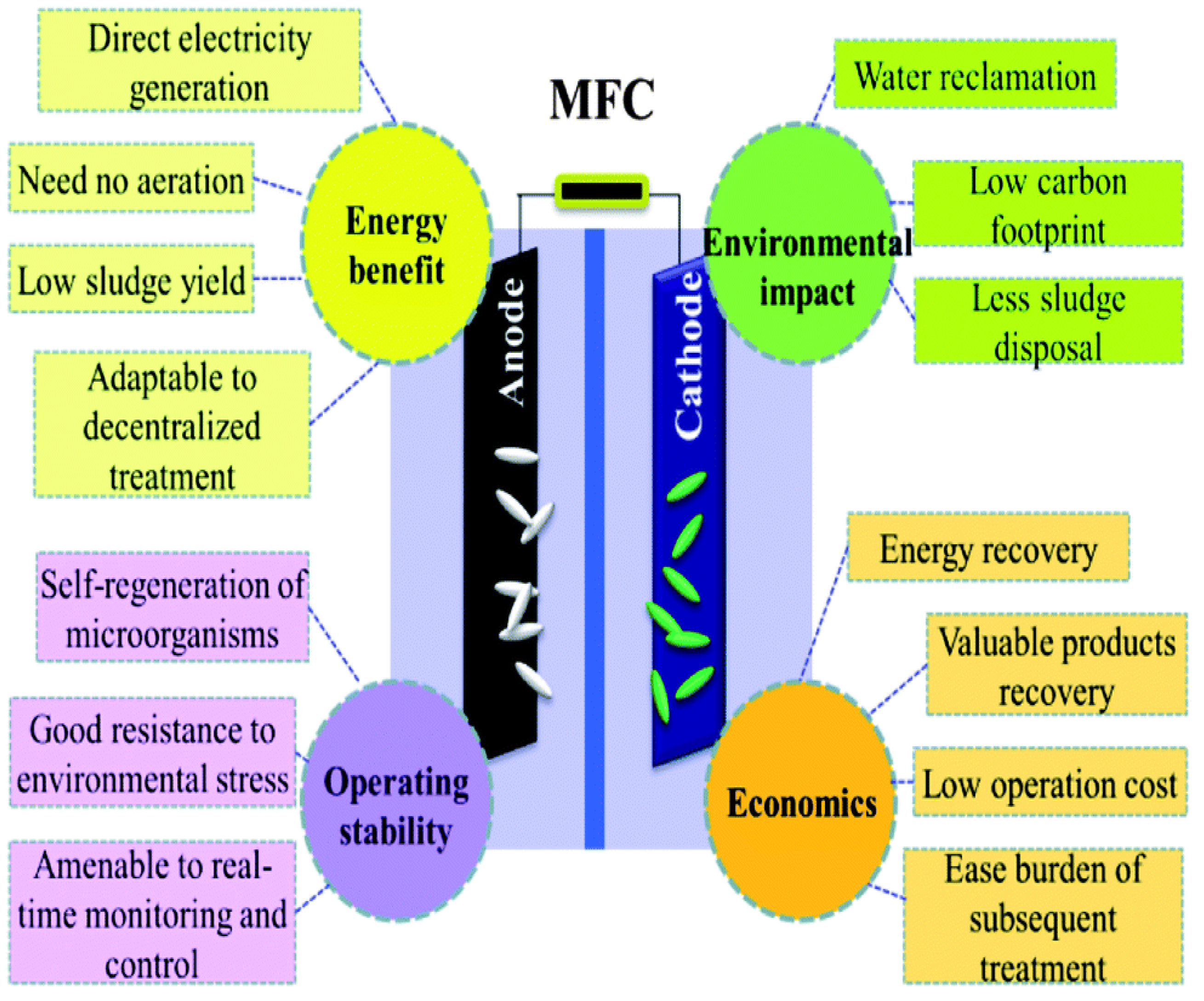
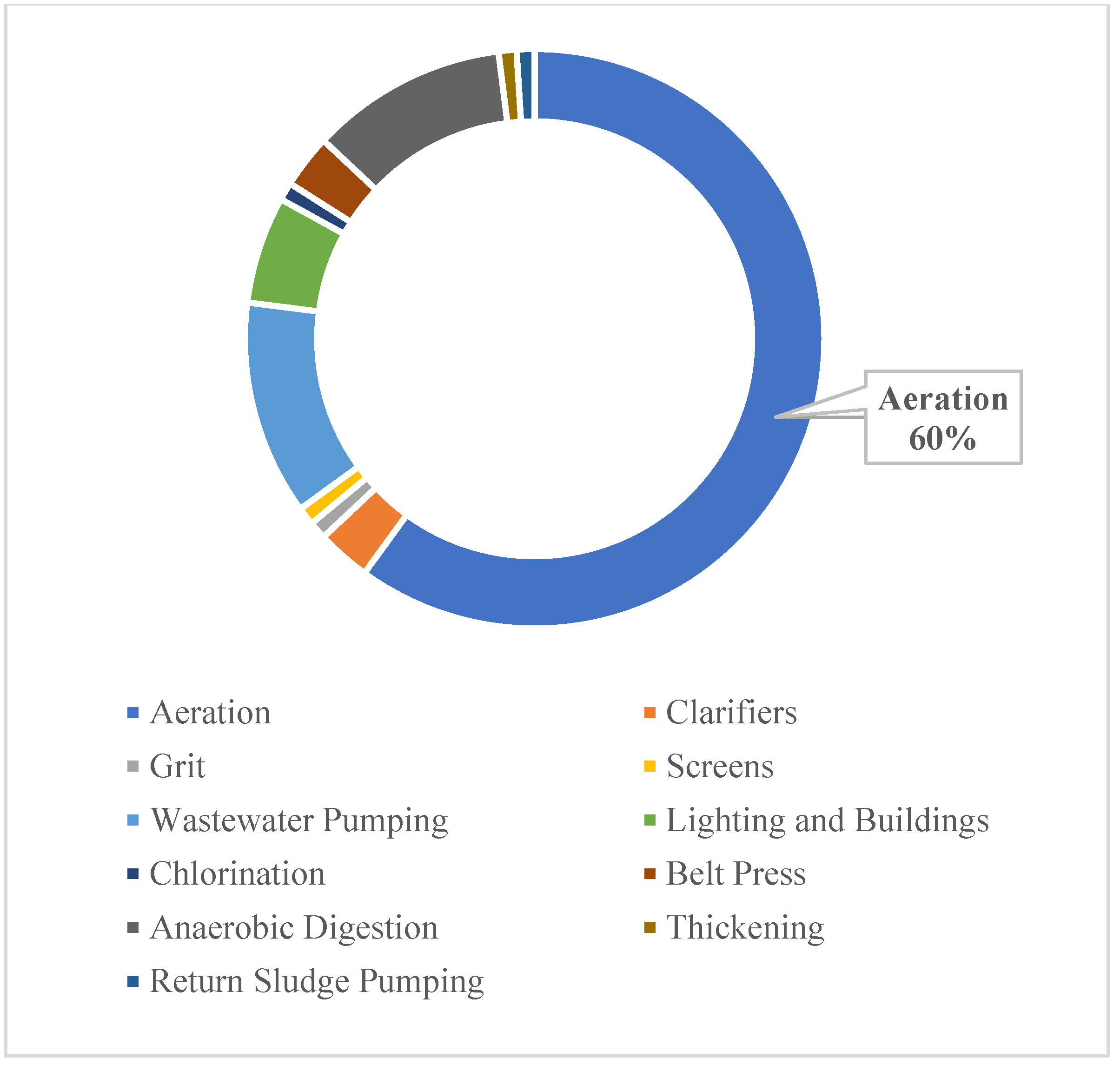
4. Technological Strengths of MFC
5. Bottlenecks of MFCs
6. Development Trends of MFC for Dual Functions
6.1. Electrode Development for Enhancing Power Output
6.2. Phosphorus (P) Removal Using MFC
6.3. Key Factors for P Removal
6.4. Feasibility of P Removal Using MFCs
7. Economic Feasibility Analysis
8. Online pH Monitoring in MFC Operations
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kurniawan, T.A.; Liang, X.; O’Callaghan, E.; Goh, H.; Othman, M.H.D.; Avtar, R.; Kusworo, T.D. Transformation of solid waste management in China: Moving towards sustainability through digitalization-based circular economy. Sustainability 2022, 14, 2374. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.; Singh, D.; Othman, M.H.D.; Avtar, R.; Hwang, G.H.; Albadarin, A.B.; Kern, A.O.; Shirazian, S. A societal transition of MSW management in Xiamen (China) toward a circular economy through integrated waste recycling and technological digitization. Environ. Pollut. 2021, 277, 116741. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Kurniawan, T.A.; Avtar, R.; Othman, M.H.D.; Ouyang, T.; Yujia, H.; Xueting, Z.; Setiadi, T.; Iswanto, I. Applicability of TiO2(B) nanosheets@hydrochar composites for adsorption of tetracycline (TC) from contaminated water. J. Hazard. Mater. 2021, 405, 123999. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Avtar, R.; Singh, D.; Xue, W.; Dzarfan Othman, M.H.; Hwang, G.H.; Iswanto, I.; Albadarin, A.B.; Kern, A.O. Reforming MSWM in Sukunan (Yogjakarta, Indonesia): A case-study of applying a zero-waste approach based on circular economy paradigm. J. Clean. Prod. 2021, 284, 124775. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Liang, X.; Singh, D.; Othman, M.H.D.; Goh, H.H.; Gikas, P.; Kern, A.O.; Kusworo, T.D.; Shoqeir, J.A. Harnessing landfill gas (LFG) for electricity: A strategy to mitigate greenhouse gas (GHG) emissions in Jakarta (Indonesia). J. Environ. Manag. 2022, 301, 113882. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Singh, D.; Avtar, R.; Goh, H.H.; Setiadi, T.; Lo, W.H. Technological solutions for long-term management of partially used nuclear fuel: A critical review. Ann. Nucl. Energy 2022, 166, 108736. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Babel, S. Insights on microbial fuel cells for sustainable biological nitrogen removal from wastewater: A review. Environ. Res. 2022, 204, 112095. [Google Scholar] [CrossRef]
- Sniatala, B.; Kurniawan, T.A.; Sobotka, D.; Makinia, J.; Othman, M.H.D. Macro-nutrients recovery from wastewater as a sustainable resource for synthetic fertilizer: Uncovering alternative options to promote global food security cost-effectively. Sci. Total Environ. 2022, 856, 159283. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.H.; Sillanpää, M. Treatment of contaminated water laden with 4-chlorophenol using coconut shell waste-based activated carbon modified with chemical agents. Sep. Sci. Technol. 2011, 46, 460–472. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Maiurova, A.; Kustikova, M.; Bykovskaia, E.; Othman, M.H.D.; Goh, H.H. Accelerating sustainability transition in St. Petersburg (Russia) through digitalization-based circular economy in waste recycling industry: A strategy to promote carbon neutrality in era of Industry 4.0. J. Clean. Prod. 2022, 363, 132452. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Hwang, G.H.; Gikas, P. Unlocking digital technology in waste recycling industry in Industry 4.0 era: A transformation towards digitalization-based circular economy in Indonesia. J. Clean. Prod. 2022, 357, 131911. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Adam, M.R.; Goh, H.H.; Mohyudin, A.; Avtar, R.; Kusworo, T.D. Treatment of pulping whitewater using membrane filtrations. Chem. Pap. 2022, 76, 5001–5010. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Singh, D.; Avtar, R.; Othman, M.H.D.; Hwang, G.H.; Albadarin, A.B.; Rezakazemi, M.; Setiadi, T.; Shirazian, S. Resource recovery from landfill leachate: An experimental investigation and perspectives. Chemosphere 2021, 274, 129986. [Google Scholar] [CrossRef]
- Goh, H.H.; Zong, L.; Zhang, D.; Liu, H.; Dai, W.; Lim, C.S.; Kurniawan, T.A.; Teo, K.T.K.; Goh, K.C. Mid-and long-term strategy based on electric vehicle charging unpredictability and ownership estimation. Int. J. Electr. Power Energy Syst. 2022, 142, 108240. [Google Scholar] [CrossRef]
- Goh, H.H.; Li, C.; Zhang, D.; Dai, W.; Lim, C.S.; Kurniawan, T.A.; Goh, K.C. Application of choosing by advantages to determine the optimal site for solar power plants. Sci. Rep. 2022, 12, 4113. [Google Scholar] [CrossRef]
- Goh, H.H.; Zong, L.; Zhang, D.; Dai, W.; Lim, C.S.; Kurniawan, T.A.; Goh, K.C. Orderly charging strategy based on optimal time of use price demand response of electric vehicles in distribution network. Energies 2022, 15, 1869. [Google Scholar] [CrossRef]
- Fu, D.; Kurniawan, T.A.; Li, H.; Wang, H.; Wang, Y.; Li, Q. Co-oxidative removal of arsenite and tetracycline based on a heterogeneous Fenton-like reaction using iron nanoparticles-impregnated biochar. Environ. Pollut. 2021, 290, 118062. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). World Energy Outlook; IEA: Paris, France, 2019; Available online: https://www.iea.org/reports/world-energy-outlook-2019 (accessed on 30 August 2020).
- Fu, D.; Huang, Y.; Zhang, X.; Kurniawan, T.A.; Ouyang, T. Uncovering potentials of integrated TiO2(B) nanosheets and H2O2 for removal of tetracycline from aqueous solution. J. Mol. Liq. 2017, 248, 112–120. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.; Othman, M.H.D.; Goh, H.H.; Chong, K.K. Biosorption and adsorption modeling of Cu(II), Zn(II), Pb(II) and Cd(II) from synthetic wastewater by using activated sludge, Aeromas hydrophyla and/or Branhemella sp. Environ. Res. 2022, 214, 114070. [Google Scholar] [CrossRef]
- Fu, D.; Kurniawan, T.A.; Li, H.; Wang, L.; Chen, Z.; Li, W.; Wang, Y.; Wang, H.; Li, Q. Applicability of HDPC-supported Cu nanoparticles composite synthesized from anaerobically digested wheat straw for octocrylene degradation in aqueous solutions. Chem. Eng. J. 2019, 355, 650–660. [Google Scholar] [CrossRef]
- Goh, H.H.; Lan, Z.; Zhang, D.; Dai, W.; Kurniawan, T.A.; Goh, K.C. Estimation of the state of health (SOH) of batteries using discrete curvature feature extraction. J. Energy Storage 2022, 50, 104646. [Google Scholar] [CrossRef]
- Goh, H.H.; Huang, Y.; Lim, C.S.; Zhang, D.; Liu, H.; Dai, W.; Kurniawan, T.A.; Rahman, S. An assessment of multi-stage reward function design for deep reinforcement learning-based microgrid energy management. IEEE Trans. Smart Grid 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Liang, X.; Goh, H.H.; Kurniawan, T.A.; Zhang, D.; Dai, W.; Liu, H.; Liu, J.; Goh, K.C. Utilizing landfill gas (LFG) for promoting digitalization of data center in China to facilitate sustainable energy transition in era of Industrial Revolution 4.0. J. Clean. Prod. 2022, 369, 133297. [Google Scholar] [CrossRef]
- Fu, D.; Kurniawan, T.A.; Avtar, R.; Xu, P.; Othman, M.H.D. Recovering heavy metals from electroplating wastewater and their conversion into Zn2Cr-layered double hydroxide (LDH) for pyrophosphate removal from industrial wastewater. Chemosphere 2021, 271, 129861. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Singh, D.; Xue, W.; Avtar, R.; Othman, M.H.D.; Hwang, G.H.; Setiadi, T.; Albadarin, A.B.; Shirazian, S. Resource recovery toward sustainability through nutrient removal from landfill leachate. J. Environ. Manag. 2021, 287, 112265. [Google Scholar] [CrossRef]
- Goh, H.H.; He, B.; Liu, H.; Zhang, D.; Dai, W.; Kurniawan, T.A.; Goh, K.C. Multi-convolution feature extraction and recurrent neural network dependent model for short-term load forecasting. IEEE Access 2021, 9, 118528–118540. [Google Scholar] [CrossRef]
- Fu, D.; Kurniawan, T.A.; Lin, L.; Li, Y.; Avtar, R.; Dzarfan Othman, M.H.; Li, F. Arsenic removal in aqueous solutions using FeS2. J. Environ. Manag. 2021, 286, 112246. [Google Scholar] [CrossRef]
- Goh, H.H.; Luo, Q.; Zhang, D.; Liu, H.; Dai, W.; Lim, C.S.; Kurniawan, T.A.; Goh, K.C. A hybrid SDS and WPT-IBBO-DNM based model for ultra-short term photovoltaic prediction. CSEE J. Power Energy Syst. 2022. [Google Scholar] [CrossRef]
- Goh, H.H.; Peng, G.; Zhang, D.; Dai, W.; Kurniawan, T.A.; Goh, K.C.; Cham, C.L. A new wind speed scenario generation method based on principal component and R-Vine copula theories. Energies 2022, 15, 2698. [Google Scholar] [CrossRef]
- Fu, D.; Kurniawan, T.A.; Gui, H.; Li, H.; Wang, Y.; Li, Q. Role of CuxO-anchored pyrolyzed hydrochars on H2O2-activated degradation of tetracycline: Effects of pyrolysis temperature and pH. Ind. Eng. Chem. Res. 2022, 61, 8847–8857. [Google Scholar] [CrossRef]
- Zhu, M.; Kurniawan, T.A.; Liang, D.; Song, Y.; Hermanowicz, S.W.; Othman, M.H.D. Advances in BiOX-based ternary photocatalysts for energy storage applications: A critical review. Rev. Environ. Sci. Bio/Technol. 2022, 21, 331–370. [Google Scholar] [CrossRef]
- Fu, D.; Kurniawan, T.A.; Gui, H.; Feng, S.; Li, Q.; Othman, M.H.D. Treatment of As(III)-laden contaminated water using iron-coated carbon fiber. Materials 2022, 15, 4365. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.L.; Liu, G.; Motelica-Wagenaar, A.M.; van der Hoek, J.P. Toward carbon-neutral water systems: Insights from global cities. Engineering 2022, 14, 77–85. [Google Scholar] [CrossRef]
- Maiurova, A.; Kurniawan, T.A.; Kustikova, M.; Bykovskaia, E.; Othman, M.H.D.; Sing, D.; Goh, H.H. Promoting digital transformation in waste collection service and waste recycling in a smart city: Applying circular economy in Moscow (Russia) to mitigate climate change effects on the environment. J. Clean. Prod. 2022, 354, 131604. [Google Scholar] [CrossRef]
- Ida, T.K.; Mandal, B. Microbial fuel cell design, application, and performance: A review. Mater. Today Proc. 2022. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Ray, S.G.; Ghangrekar, M.M. Third generation in bio-electrochemical system research–A systematic review on mechanisms for recovery of valuable by-products from wastewater. Renew. Sust. Energy Rev. 2017, 76, 1022–1031. [Google Scholar] [CrossRef]
- Heidari, F.; Øverland, M.; Hansen, J.Ø.; Mydland, L.T.; Urriola, P.E.; Chen, C.; Shurson, C.G.; Hu, B. Solid-state fermentation of Pleurotus ostreatus to improve the nutritional profile of mechanically-fractionated canola meal. Biochem. Eng. J. 2022, 187, 108591. [Google Scholar] [CrossRef]
- Sun, M.; Zhai, L.F.; Li, W.W.; Yu, H.Q. Harvest and utilization of chemical energy in wastes by microbial fuel cells. Chem. Soc. Rev. 2016, 45, 2847–2870. [Google Scholar] [CrossRef]
- Hasany, M.; Mardanpour, M.M.; Yaghmaei, S. Biocatalysts in microbial electrolysis cells: A review. Int. J. Hydr. Energy 2016, 41, 1477–1493. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Y.; Liu, H. Hibernations of electroactive bacteria provide insights into the flexible and robust BOD detection using microbial fuel cell-based biosensors. Sci. Total Environ. 2021, 753, 142244. [Google Scholar] [CrossRef]
- Kannan, N.; Donnellan, P. Algae-assisted microbial fuel cells: A practical overview. Bioresour. Technol. Rep. 2021, 15, 100747. [Google Scholar] [CrossRef]
- Wu, T.; Yang, S.; Zhong, L.; Pang, J.; Zhang, L.; Xia, X.; Yang, F.; Xie, G.; Liu, B.; Ren, N. Simultaneous nitrification, denitrification and phosphorus removal: What have we done so far and how do we need to do in the future? Sci. Total Environ. 2022, 856, 158977. [Google Scholar] [CrossRef] [PubMed]
- De Vela, R.J. A review of the factors affecting the performance of anaerobic membrane bioreactor and strategies to control membrane fouling. Rev. Environ. Sci. Biotechnol. 2021, 20, 607–644. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; de los Ríos, A.P.; Mateo-Ramírez, F.; Juarez, M.D.; Lozano-Blanco, L.J.; Godínez, C. New application of polymer inclusion membrane based on ionic liquids as proton exchange membrane in microbial fuel cell. Separ. Purif. Technol. 2016, 160, 51–58. [Google Scholar] [CrossRef]
- Vijay, A.; Sonawane, J.M.; Chhabra, M. Denitrification process in microbial fuel cell: A comprehensive review. Bioresour. Technol. Rep. 2022, 17, 100991. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, W. China’s energy transition pathway in a carbon neutral vision. Engineering 2022, 14, 64–76. [Google Scholar] [CrossRef]
- Acién, F.G.; Gómez-Serrano, C.; Morales-Amaral, M.M. Wastewater treatment using microalgae: How realistic a contribution might it be to significant urban wastewater treatment? Appl. Microbiol. Biotechnol. 2016, 100, 9013–9022. [Google Scholar] [CrossRef]
- Gajda, I.; Greenman, J.; Melhuish, C.; Ieropoulos, I. Self-sustainable electricity production from algae grown in a microbial fuel cell system. Biomass Bioenergy 2015, 82, 87–93. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Balaji, R.; Ramakrishnan, S. Recent developments in hydrogen fuel cells: Strengths and weaknesses. In Sustainable Fuel Technologies Handbook; Elsevier: London, UK, 2021; pp. 431–456. [Google Scholar] [CrossRef]
- Kokabian, B.; Gude, V.G. Photosynthetic microbial desalination cells (PMDCs) for clean energy, water and biomass production. Environ. Sci. Process. Impacts 2013, 15, 2178–2185. [Google Scholar] [CrossRef]
- Goh, H.H.; Liao, L.; Zhang, D.; Dai, W.; Lim, C.S.; Kurniawan, T.A.; Goh, K.C.; Cham, C.L. Denoising transient power quality disturbances using an improved adaptive wavelet threshold method based on energy optimization. Energies 2022, 15, 3081. [Google Scholar] [CrossRef]
- Hinkley, J.T.; Heenan, A.R.; Low, A.C.; Watson, M. Hydrogen as an export commodity–Capital expenditure and energy evaluation of hydrogen carriers. Int. J. Hydrogen Energy 2022, 47, 35959–35975. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Molognoni, D.; Dallago, E.; Liberale, A.; Cella, R.; Longoni, P.; Pantaleoni, L.; Marceta Kaninski, M.P.; Pei, P.; Morosuk, T. Microbial fuel cells for direct electrical energy recovery from urban wastewaters. Sci. World J. 2013, 2013, 634738. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Artyushkova, K.; Babanova, S.; Atanassov, P.; Ieropoulos, I.; Grattieri, M.; Cristiani, P.; Trasatti, S.; Li, B.; Schuler, A.J. Parameters characterization and optimization of activated carbon (AC) cathodes for microbial fuel cell application. Bioresour. Technol. 2014, 163, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.H.; Samhan, F.A.; Ali, G.H.; Ibrahim, M.K.; Hassan, R.Y. Assisting the biofilm formation of exoelectrogens using nanostructured microbial fuel cells. J. Electroanal. Chem. 2018, 824, 128–135. [Google Scholar] [CrossRef]
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci. Total Environ. 2021, 754, 142429. [Google Scholar] [CrossRef]
- Vilve, M.; Vilhunen, S.; Vepsäläinen, M.; Kurniawan, T.A.; Lehtonen, N.; Isomäki, H.; Sillanpää, M. Degradation of 1,2-dichloroethane from contaminated water laden with ion-exchange resin using Fenton’s oxidation. Environ. Sci. Pollut. Res. 2010, 17, 875–884. [Google Scholar] [CrossRef]
- Li, M.; Zhou, M.; Tian, X.; Tan, C.; Gu, T. Enhanced bioenergy recovery and nutrient removal from swine wastewater using an airlift-type photosynthetic microbial fuel cell. Energy 2021, 226, 120422. [Google Scholar] [CrossRef]
- Samrat, M.V.V.N.; Rao, K.K.; Ruggeri, B.; Tommasi, T. Denitrification of water in a microbial fuel cell (MFC) using seawater bacteria. J. Clean. Prod. 2018, 178, 449–456. [Google Scholar] [CrossRef]
- Khandelwal, G.; Joseph Raj, N.P.M.; Alluri, N.R.; Kim, S.J. Enhancing hydrophobicity of starch for biodegradable material-based triboelectric nanogenerators. ACS Sustain. Chem. Eng. 2021, 9, 9011–9017. [Google Scholar] [CrossRef]
- Liu, N.; Xu, Z.; Morrin, A.; Luo, X. Low fouling strategies for electrochemical biosensors targeting disease biomarkers. Anal. Methods 2019, 11, 702–711. [Google Scholar] [CrossRef]
- Ramya, M.; Kumar, P.S. A review on recent advancements in bioenergy production using microbial fuel cells. Chemosphere 2022, 288, 132512. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, G.; Jung, H.Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.H. A comprehensive review on microbial fuel cell technologies: Process, utilization, and advanced developments in electrode and membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Al-Daghistani, H.I.; Mohammad, B.T.; Kurniawan, T.A.; Singh, D.; Rabadi, A.D.; Xue, W.; Avtar, R.; Othman, M.H.D.; Shirazian, S. Characterization and applications of Thermomonas hydrothermalis isolated from Jordanian hot springs for biotechnological and medical purposes. Process Biochem. 2021, 104, 171–181. [Google Scholar] [CrossRef]
- Mohan, S.V.; Velvizhi, G.; Modestra, J.A.; Srikanth, S. Microbial fuel cell: Critical factors regulating bio-catalyzed electrochemical process and recent advancements. Renew. Sust. Energy Rev. 2014, 40, 779–797. [Google Scholar] [CrossRef]
- Mangwandi, C.; Kurniawan, T.A.; Albadarin, A.B. Comparative biosorption of chromium (VI) using chemically modified date pits (CM-DP) and olive stone (CM-OS): Kinetics, isotherms and influence of co-existing ions. Chem. Eng. Res. Des. 2020, 156, 251–262. [Google Scholar] [CrossRef]
- Xue, W.; He, Y.; Yumunthama, S.; Udomkittayachai, N.; Hu, Y.; Tabucanon, A.S.; Zhang, X.; Kurniawan, T.A. Membrane cleaning and operating conditions on performance of an osmotic microbial fuel cell. Chemosphere 2021, 285, 131549. [Google Scholar] [CrossRef]
- Hou, H.; Li, Z.; Liu, B.; Liang, S.; Xiao, K.; Zhu, Q.; Hu, S.; Yang, J.; Hu, J. Biogas and phosphorus recovery from waste activated sludge with protocatechuic acid enhanced Fenton pretreatment, anaerobic digestion and microbial electrolysis cell. Sci. Total Environ. 2020, 704, 135274. [Google Scholar] [CrossRef]
- Wresta, A.; Sintawardani, N.; Adisasmito, S.; Kurniawan, T.A.; Setiadi, T. Characteristics of tofu whey degradation during self-sustaining batch anaerobic process for methane production. J. Environ. Chem. Eng. 2021, 9, 106359. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Zhang, S.; Xing, X.H.; Su, Z. Microbial fuel cell based biosensor for in situ monitoring of anaerobic digestion process. Bioresour. Technol. 2011, 102, 10221–10229. [Google Scholar] [CrossRef]
- Desmidt, E.; Karel, G.; Yang, Z.; Luc, P.; Bruggen, B.; Verstraete, W.; Rabaey, K.; Meesschaert, B. Global phosphorus scarcity and full-scale P-recovery techniques: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Ichihashi, O.; Hirooka, K. Removal and recovery of phosphorus as struvite from swine wastewater using microbial fuel cell. Bioresour. Technol. 2012, 114, 303–307. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency. Global Water Consumption in the Energy Sector by Fuel Type in the Sustainable Development Scenario, 2016–2030. 2022. Available online: https://www.iea.org/data-and-statistics/charts/global-water-consumption-in-the-energy-sector-by-fuel-type-in-the-sustainable-development-scenario-2016-2030# (accessed on 3 September 2022).
- Shabri, H.A.; Othman, M.H.D.; Mohamed, M.A.; Kurniawan, T.A.; Jamil, S.M. Recent progress in metal-ceramic anode of solid oxide fuel cell for direct hydrocarbon fuel utilization: A review. Fuel Process. Technol. 2021, 212, 106626. [Google Scholar] [CrossRef]
- Tao, Q.; Luo, J.; Zhou, J.; Zhou, S.; Liu, G.; Zhang, R. Effect of dissolved oxygen on nitrogen and phosphorus removal and electricity production in microbial fuel cell. Bioresour. Technol. 2014, 164, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, K.; Ichihashi, O. Phosphorus recovery from artificial wastewater by microbial fuel cell and its effect on power generation. Bioresour. Technol. 2013, 137, 368–375. [Google Scholar] [CrossRef]
- Lei, Y.; Du, M.; Kuntke, P.; Saakes, M.; Weijden, R.; Buisman, C.J.N. Energy efficient phosphorus recovery by microbial electrolysis cell induced calcium phosphate precipitation. ACS Sust. Chem. Eng. 2019, 7, 8860–8867. [Google Scholar] [CrossRef]
- Wang, Z.; Mei, X.; Ma, J.; Wu, Z. Recent advances in microbial fuel cells integrated with sludge treatment. Chem. Eng. Technol. 2012, 35, 1733–1743. [Google Scholar] [CrossRef]
- Ichihashi, O.; Yamamoto, N.; Hirooka, K. Power generation by microbial fuel cell and microbial community structure in microbial fuel cell treating animal wastewater. J. Jpn. Soc. Water Environ. 2012, 35, 19–26. [Google Scholar] [CrossRef]
- Almatouq, A.; Babatunde, A.O. Identifying optimized conditions for concurrent electricity production and phosphorus recovery in a mediator-less dual chamber microbial fuel cell. Appl. Energ. 2018, 230, 122–134. [Google Scholar] [CrossRef]
- Paucar, N.E.; Sato, C. Microbial fuel cell for energy production, nutrient removal and recovery from wastewater: A review. Processes 2021, 9, 1318. [Google Scholar] [CrossRef]
- Fischer, F.; Christèle, B.; Happe, M.; Mabillard, E.; Schmidt, N. Microbial fuel cell enables phosphate recovery from digested sewage sludge as struvite. Bioresour. Technol. 2011, 102, 5824–5830. [Google Scholar] [CrossRef]
- Xie, B.; Liu, B.; Yi, Y.; Yang, L.; Liang, D.; Zhu, Y.; Liu, H. Microbiological mechanism of the improved nitrogen and phosphorus removal by embedding microbial fuel cell in Anaerobic–Anoxic–oxic wastewater treatment process. Bioresour. Technol. 2016, 207, 109–117. [Google Scholar] [CrossRef]
- Ge, X.; Cao, X.; Song, X.; Wang, Y.; Si, Z.; Zhao, Y.; Wang, W.; Tesfahunegn, A.A. Bioenergy generation and simultaneous nitrate and phosphorus removal in a pyrite-based constructed wetland-microbial fuel cell. Bioresour. Technol. 2020, 296, 122350. [Google Scholar] [CrossRef]
- Marti, N.; Bouzas, A.; Seco, A.; Ferrer, J. Struvite precipitation assessment in anaerobic digestion processes. Chem. Eng. J. 2008, 141, 67–74. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Akhter, F.; Mazari, S.A.; Sabzoi, N.; Aziz, S.; Soomro, S.A.; Mubarak, N.M.; Baloch, H.; Memon, A.Q.; Ahmed, S. Advanced microbial fuel cell for wastewater treatment—A Review. Environ. Sci. Pollut. Res. 2021, 28, 5005–5019. [Google Scholar] [CrossRef]
- Corbella, C.; Jaume, P. Improving domestic wastewater treatment efficiency with constructed wetland microbial fuel cells: Influence of anode material and external resistance. Sci. Total Environ. 2018, 631, 1406–1414. [Google Scholar] [CrossRef]
- Tamta, P.; Neetu, R.; Yadav, A.K. Enhanced wastewater treatment and electricity generation using stacked constructed wetland–Microbial fuel cells. Environ. Chem. Lett. 2020, 18, 871–879. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Liu, R.; Morgan, D. Global development of emerged substrates utilized in constructed wetlands. Bioresour. Technol. 2018, 261, 441–452. [Google Scholar] [CrossRef]
- Cusick, R.D.; Logan, B.E. Phosphate recovery as struvite within a single chamber microbial electrolysis cell. Bioresour. Technol. 2021, 107, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.A.; Zaghloul, M.S.; Iorhemen, O.T.; Sheng, Z.; Tay, J.H. Optimization of organics to nutrients (COD: N: P) ratio for aerobic granular sludge treating high-strength organic wastewater. Sci. Total Environ. 2019, 650, 3168–3179. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, K.S.; Eugenia, V.J.; Phil, H.; Simon, A.P. Phosphorus recovery from wastewater by struvite crystallization: A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef] [PubMed]
- Durrant, A.E.; Scrimshaw, M.D.; Stratful, I.; Lester, J.N. Review of the feasibility of recovering phosphate from wastewater for use as a raw material by the phosphate industry. Environ. Technol. 1999, 20, 749–758. [Google Scholar] [CrossRef]
- Zhuang, L.; Zheng, Y.; Zhou, S.; Yuan, Y.; Yuan, H.; Chen, Y. Scalable microbial fuel cell stack for continuous real wastewater treatment. Bioresour. Technol. 2012, 106, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Falk, H.; Uwe, S.; Scholz, F.; Bogdanoff, P.; Herrmann, I. Challenges and constraints of using oxygen cathodes in microbial fuel cells. Environ. Sci. Technol. 2006, 40, 5193–5199. [Google Scholar] [CrossRef]
- Nawaz, A.; Ul Haq, I.; Qaisar, K.; Gunes, B.; Raja, S.I.; Mohyuddin, K.; Amin, H. Microbial fuel cells: Insight into simultaneous wastewater treatment and bioelectricity generation. Process Saf. Environ. 2022, 161, 357–373. [Google Scholar] [CrossRef]
- Aelterman, P.; Korneel, R.; Hai, T.P.; Nico, B.; Willy, V. Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ. Sci. Technol. 2006, 40, 3388–3394. [Google Scholar] [CrossRef]
- Lanas, V.; Logan, B.E. Evaluation of multi-brush anode systems in microbial fuel cells. Bioresour. Technol. 2013, 148, 379–385. [Google Scholar] [CrossRef]
- Daud, S.M.; Daud, W.R.M.; Bakar, M.H.A.; Kim, B.H.; Somalu, M.R.; Jahim, J.M.; Muchtar, A.; Ghasemi, M. A comparison of long-term fouling performance by zirconia ceramic filter and cation exchange in microbial fuel cells. Int. Biodeter. Biodegrad. 2019, 136, 63–70. [Google Scholar] [CrossRef]
- Do, M.H.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Nghiem, L.D.; Ni, B.J. Challenges in the application of microbial fuel cells to wastewater treatment and energy production: A mini review. Sci. Total Environ. 2018, 639, 910–920. [Google Scholar] [CrossRef]
- Futamata, H.; Bretschger, O.; Cheung, A.; Kan, J.; Owen, R.; Nealson, K.H. Adaptation of soil microbes during establishment of microbial fuel cell consortium fed with lactate. J. Biosci. Bioeng. 2013, 115, 58–63. [Google Scholar] [CrossRef]
- Premakumara, D.G.J.; Canete, A.L.M.L.; Nagaishi, M.; Kurniawan, T.A. Policy implementation of the Republic Act (RA) No. 9003 in the Philippines on MSW management: A case study of Cebu City. Waste Manag. 2014, 34, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Arun Prakash, V.R.; Xavier, J.F.; Ramesh, G.; Maridurai, T.; Kumar, K.S.; Raj, R. Mechanical, thermal and fatigue behavior of surface-treated novel Caryota urens fibre–reinforced epoxy composite. Biomass Conv. Bioref. 2020, 12, 5451–5461. [Google Scholar] [CrossRef]
- Bora, A.; Mohanrasu, K.; Angelin Swetha, T.; Ananthi, V.; Sindhu, R.; Chi, N.T.L.; Pugazhendhi, A.; Arun, A.; Mathimani, T. Microbial electrolysis cell (MEC): Reactor configurations, recent advances and strategies in biohydrogen production. Fuel 2022, 328, 125269. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Verma, P. Microbial fuel cell: A green approach for the utilization of waste for the generation of bioelectricity. Bioresour. Bioproc. 2016, 3, 1–14. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Meidiana, C.; Othman, M.H.D.; Goh, H.H.; Chew, K.W. Strengthening waste recycling industry in Malang (Indonesia): Lessons from waste management in the era of Industry 4.0. J. Clean. Prod. 2022, 382, 135296. [Google Scholar] [CrossRef]


| Type of MFC | Influent Used | Operational Time (Days) | P Removal (%) | Current Density (A/m2) | Reference |
|---|---|---|---|---|---|
| Air–cathode single chamber MFC | Swine wastewater | 76 | 82 | 6.0–7.0 | [73] |
| Air–cathode single chamber MFC | Artificial wastewater | 108 | 27 | 4.3 | [77] |
| Microbial electrolysis cell | domestic wastewater | 3 | 74 | 6.6 | [78] |
| Single-chamber MEC | Simulated wastewater | 23 | 66 | 20.7 | [74] |
| Pyrite-based wetland MFC | Simulated wastewater | 180 | 91 | 4.8 | [85] |
| Anaerobic–anoxic–oxic MFC | Sewage waste | 110 | 67 | - | [84] |
| Two-chamber MFC | Synthetic wastewater | 20 | 80 | - | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurniawan, T.A.; Othman, M.H.D.; Liang, X.; Ayub, M.; Goh, H.H.; Kusworo, T.D.; Mohyuddin, A.; Chew, K.W. Microbial Fuel Cells (MFC): A Potential Game-Changer in Renewable Energy Development. Sustainability 2022, 14, 16847. https://doi.org/10.3390/su142416847
Kurniawan TA, Othman MHD, Liang X, Ayub M, Goh HH, Kusworo TD, Mohyuddin A, Chew KW. Microbial Fuel Cells (MFC): A Potential Game-Changer in Renewable Energy Development. Sustainability. 2022; 14(24):16847. https://doi.org/10.3390/su142416847
Chicago/Turabian StyleKurniawan, Tonni Agustiono, Mohd Hafiz Dzarfan Othman, Xue Liang, Muhammad Ayub, Hui Hwang Goh, Tutuk Djoko Kusworo, Ayesha Mohyuddin, and Kit Wayne Chew. 2022. "Microbial Fuel Cells (MFC): A Potential Game-Changer in Renewable Energy Development" Sustainability 14, no. 24: 16847. https://doi.org/10.3390/su142416847
APA StyleKurniawan, T. A., Othman, M. H. D., Liang, X., Ayub, M., Goh, H. H., Kusworo, T. D., Mohyuddin, A., & Chew, K. W. (2022). Microbial Fuel Cells (MFC): A Potential Game-Changer in Renewable Energy Development. Sustainability, 14(24), 16847. https://doi.org/10.3390/su142416847










