Assessment of Community Dynamics of Arbuscular Mycorrhizal Fungi in the Rice (Oryza sativa L.) Rhizosphere and Potential Application as Biofertilizer
Abstract
1. Introduction
2. Materials and Methods
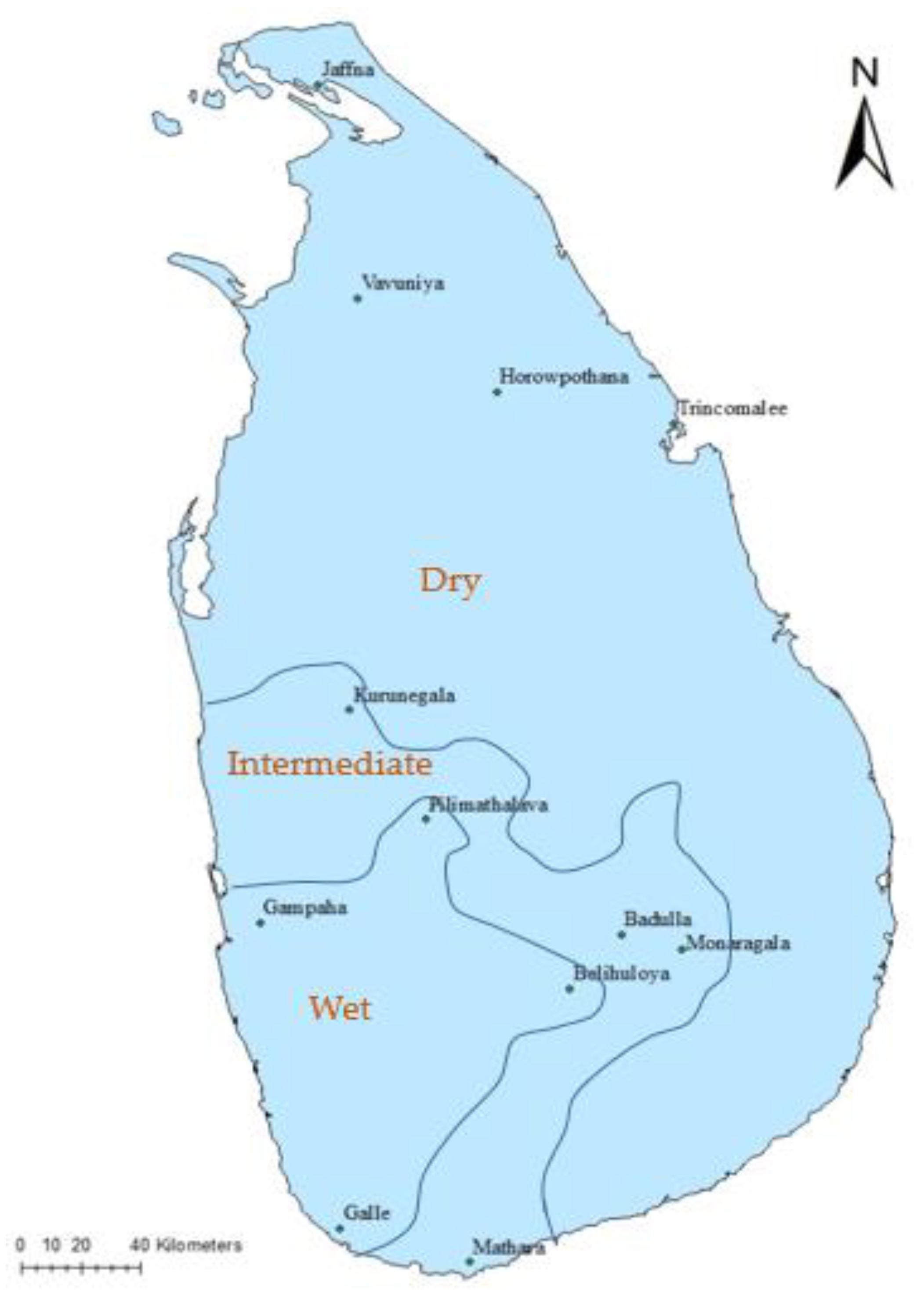
2.1. Sampling Sites and Sample Collection
2.2. AMF Spore Isolation, Identification, and Calculation of Spore Density
2.3. Assessment of AMF Colonization
2.4. Trap Culture Method for Increasing the Population of Specific AMF Species
2.5. Development of the Biofertilizer
2.6. Pot Experiment
2.7. Data Collection and Agronomic Study
2.8. Statistical Analysis
3. Results
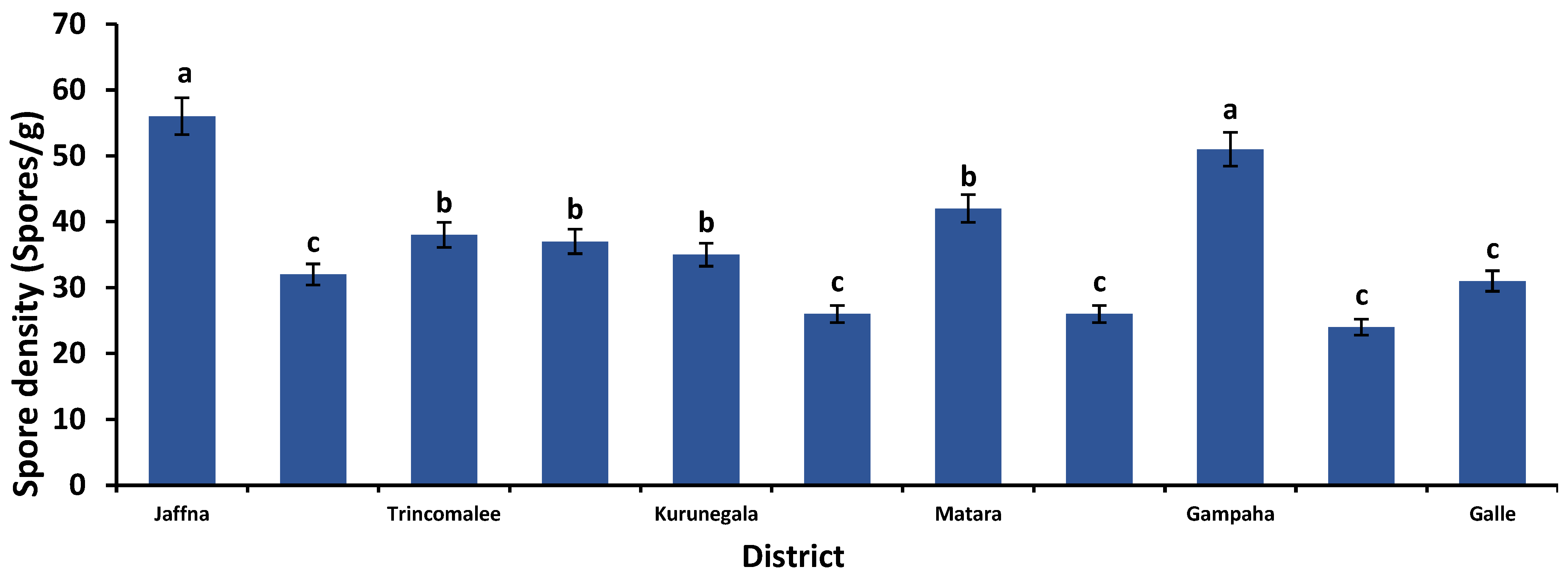
3.1. AMF Spore Density at Different Rice Cultivation Fields in Dry, Wet, and Intermediate Zones
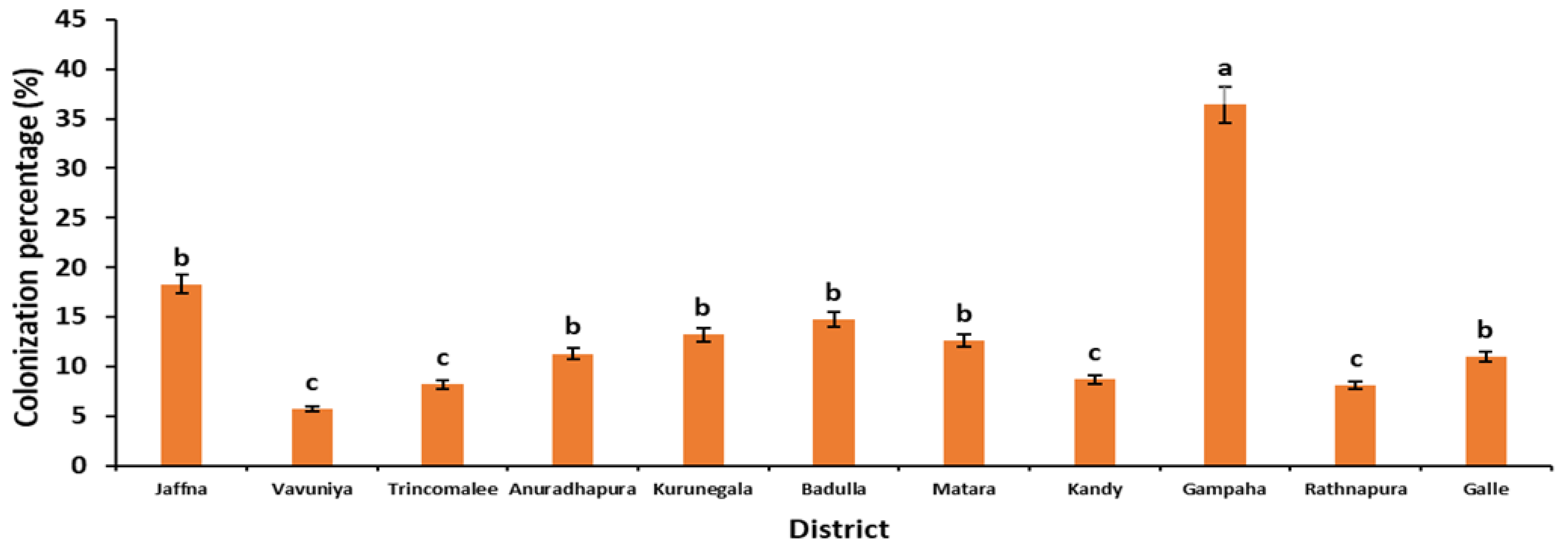
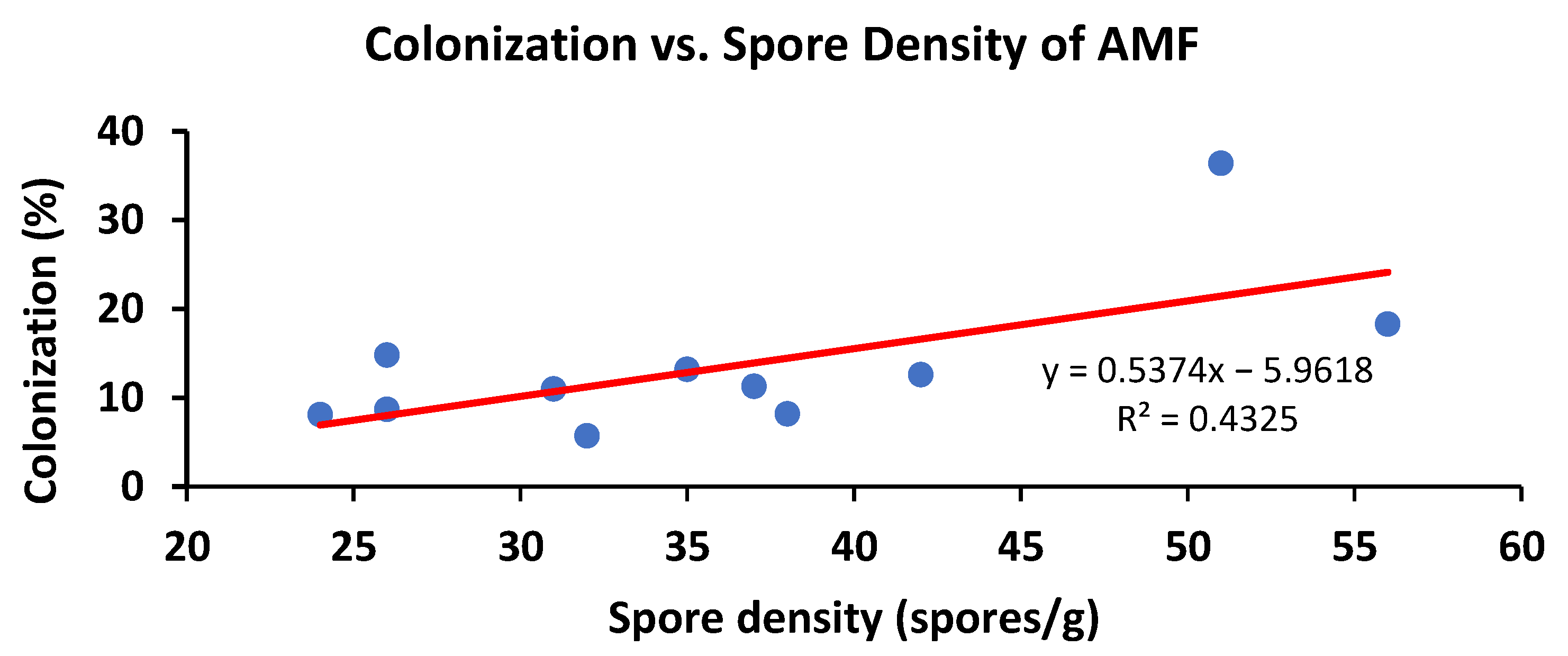
3.2. AMF Colonization
3.3. Identification of Adapted Indigenous AMF Species
3.4. AMF Colonization in Rice Roots under Different Fertilizer Applications and Soil Conditions
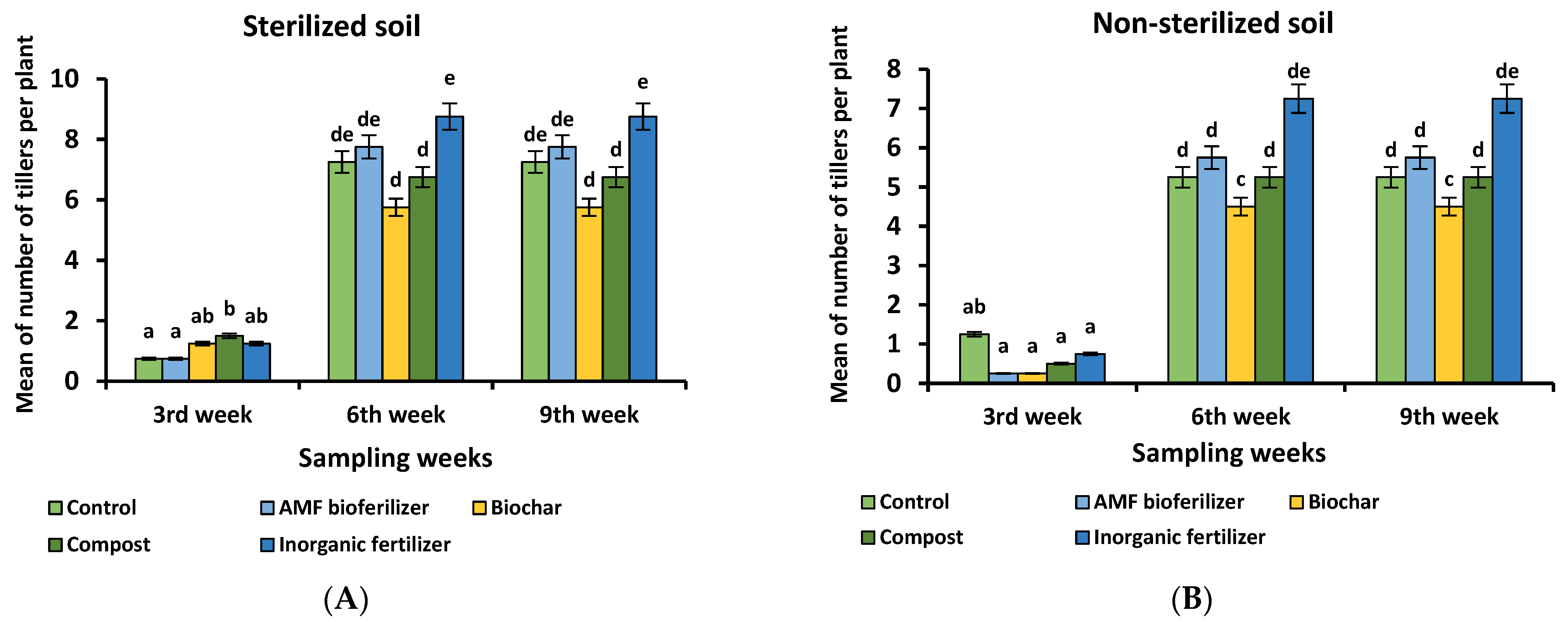
3.5. Microbial Community Arrangement at 9 Weeks of the Rice Plant According to the Different Treatments
3.6. Effectiveness of the Produced Biofertilizer
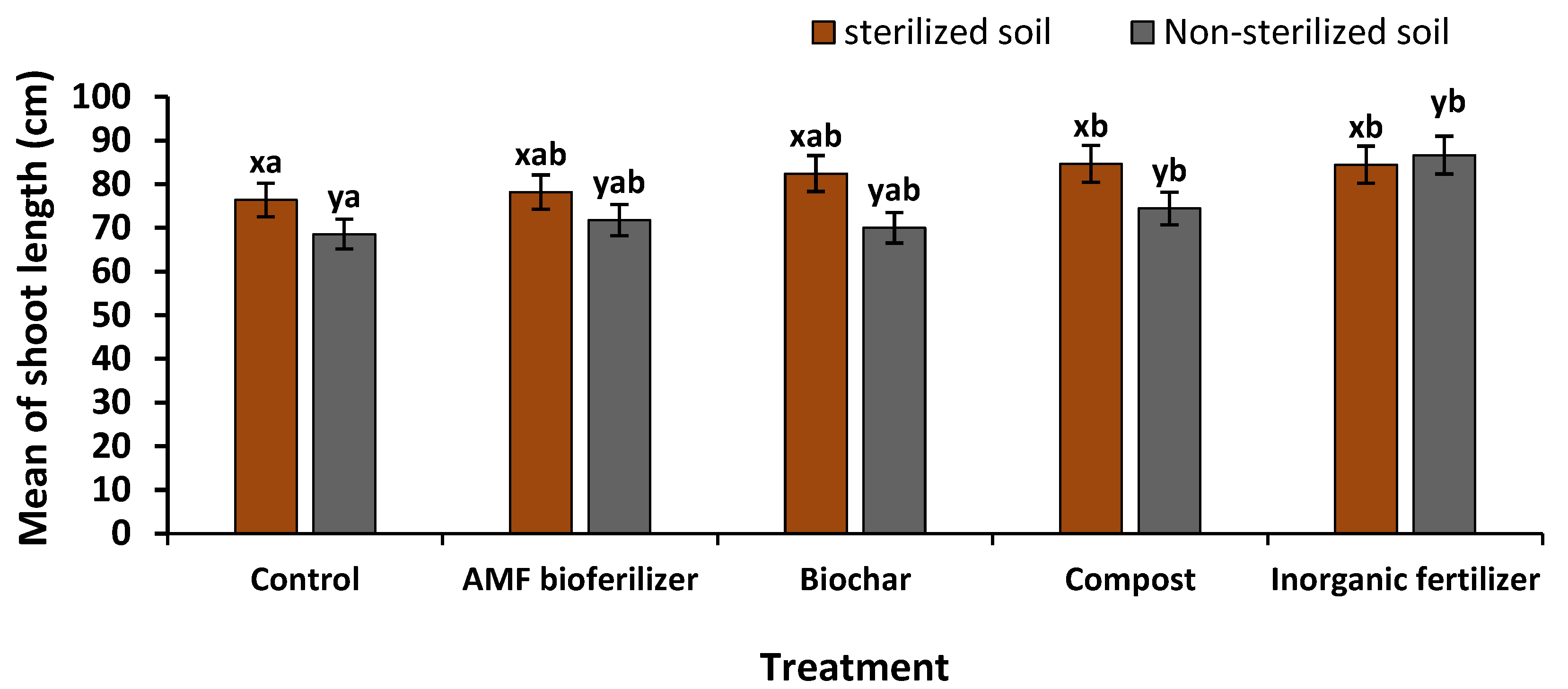
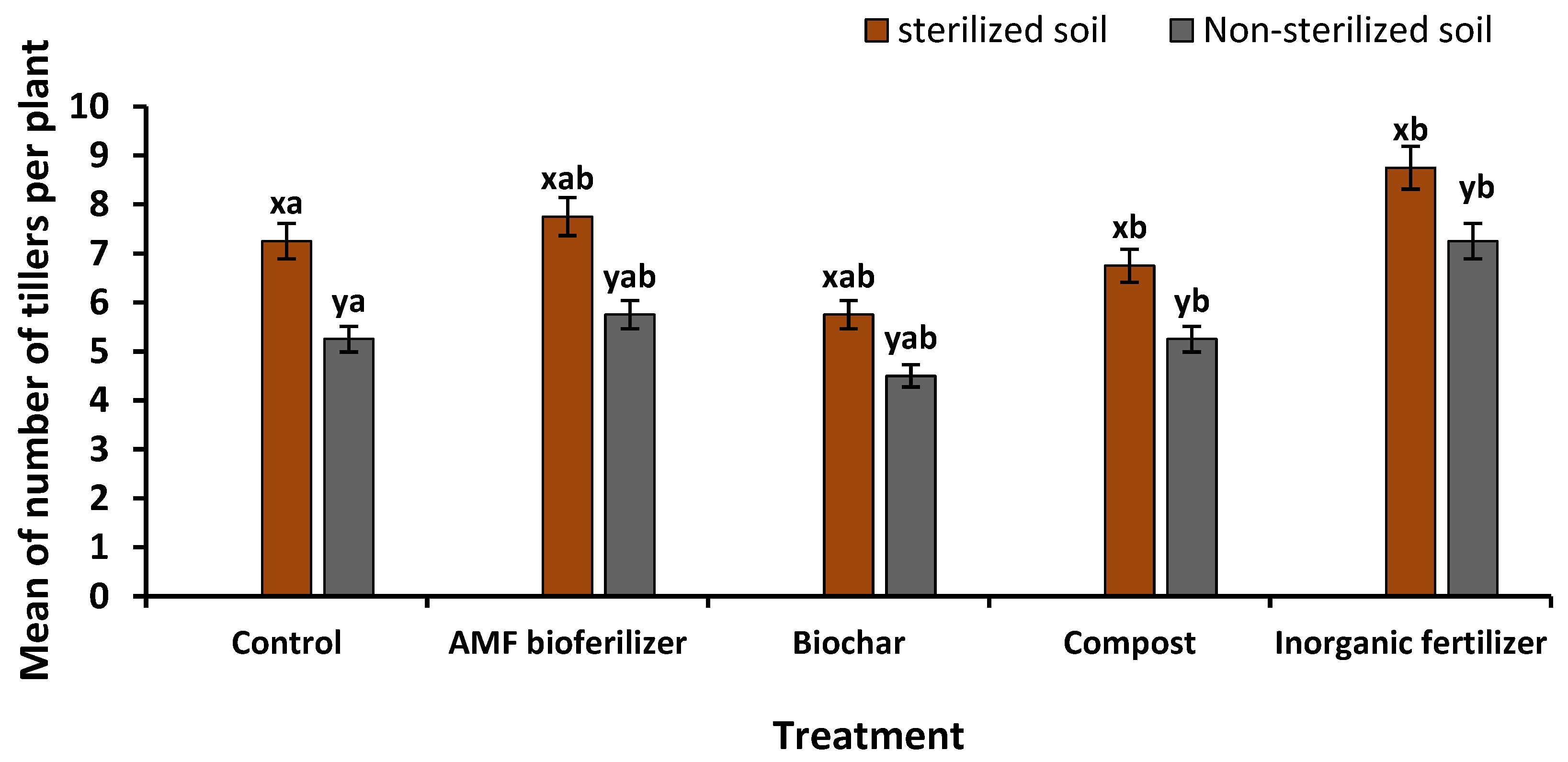
3.6.1. Biometric Characters
3.6.2. Rice Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velten, S.; Leventon, J.; Jager, N.; Newig, J. What is sustainable agriculture? A systematic review. Sustainability 2015, 7, 7833–7865. [Google Scholar]
- Ram Singh, S.; Singh, U.; Chaubey, A. Mycorrhizal fungi for sustainable agriculture—A review. Agric. Rev. 2010, 211, 93–104. [Google Scholar]
- Dahunsi, S.O.; Oranusi, S.; Efeovbokhan, V.E.; Adesulu-Dahunsi, A.T.; Ogunwole, J.O. Crop performance and soil fertility improvement using organic fertilizer produced from valorization of Carica papaya fruit peel. Sci. Rep. 2021, 11, 4696. [Google Scholar] [CrossRef] [PubMed]
- Dahunsi, S.O.; Ogunrinola, G.A. Improving soil fertility and performance of tomatoes plant using the anaerobic digestate of Tithonia diversifolia as biofertilizer. IOP Conf. Ser. Earth Environ. Sci. 2018, 210, 012014. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial services of arbuscular mycorrhizal fungi—From ecology to application. Front. Plant Sci. 2018, 9, 01270. [Google Scholar]
- Basalingappa, K.M.; Nataraj, R.; Thangaraj, G. Biofertilizer for crop production and soil fertility biofertilizer for crop production and soil fertility. Acad. J. Agric. Res. 2018, 6, 299–306. [Google Scholar]
- Willis, A.; Rodrigues, B.; Harris, P.J.C. The ecology of arbuscular mycorrhizal fungi critical reviews in plant sciences the ecology of arbuscular mycorrhizal fungi. CRC Crit. Rev. Plant Sci. 2013, 32, 1–20. [Google Scholar]
- Panneerselvam, P.; Kumar, U.; Sugitha, T.C.; Parameswaran, C.; Sahoo, S.; Binodh, A.K.; Jahan, A.; Anandan, A. Arbuscular Mycorrhizal Fungi (AMF) for Sustainable Rice Production. In Advances in Soil Microbiology: Recent Trends and Future Prospects; Adhya, T., Mishra, B., Annapurna, K., Verma, D., Kumar, U., Eds.; Springer: Singapore, 2017; Volume 4, pp. 99–126. [Google Scholar]
- Liu, W.; Wang, G.L. Plant innate immunity in rice: A defense against pathogen infection. Natl. Sci. Rev. 2016, 3, 295–308. [Google Scholar] [CrossRef]
- Vallino, M.; Fiorilli, V.; Paola, B. Rice flooding negatively impacts root branching and arbuscular mycorrhizal colonization, but not fungal viability. Plant Cell Environ. 2014, 37, 557–572. [Google Scholar] [CrossRef]
- Chareesri, A.; De Deyn, G.B.; Sergeeva, L.; Polthanee, A.; Kuyper, T.W. Increased arbuscular mycorrhizal fungal colonization reduces yield loss of rice (Oryza sativa L.) under drought. Mycorrhiza 2020, 30, 315–328. [Google Scholar]
- Wang, Y.; Li, T.; Li, Y.; Björn, O.; Rosendahl, S.; Olsson, A.; Li, S.; Fu, X. Community dynamics of arbuscular mycorrhizal fungi in high-input and intensively irrigated rice cultivation systems. Appl. Environ. Microbiol. 2015, 81, 2958–2965. [Google Scholar] [CrossRef] [PubMed]
- Lumini, E.; Vallino, M.; Alguacil, M.M.; Romani, M.; Bianciotto, V. Different farming and water regimes in Italian rice fields affect arbuscular mycorrhizal fungal soil communities. Ecol. Appl. 2011, 21, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Watanarojanaporn, N.; Boonkerd, N.; Tittabutr, P.; Longtonglang, A.; Young, J.P.W.; Teaumroong, N. Effect of rice cultivation systems on indigenous arbuscular mycorrhizal fungal community structure. Microbes Environ. 2013, 28, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar]
- Brundrett, M.; Bougher, N.; Grove, T.; Malajczuk, N. Examining mycorrhizal Association. In Working with Mycorrhizas in Forestry and Agriculture; Brundrett, M., Bougher, N., Dell, B., Grove, T., Malajczuk, N., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 1982; pp. 141–171. [Google Scholar]
- INVAM International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. Available online: https://invam.wvu.edu/ (accessed on 12 January 2022).
- Kormanik, P.P.; McGraw, A.C. Quantification of vesicular-arbuscular mycorrhizae in plant roots. In Methods and Principles of Mycorrhizal Research; Schenck, N.C., Ed.; The American Phytopathological Society: St. Paul, MN, USA, 1982; pp. 37–45. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedure for cleaning roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorhhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Gopal, S.; Kim, K.; Walitang, D.; Korea, S.; Chanratana, M. Trap culture technique for propagation of arbuscular mycorrhizal fungi using trap culture technique for propagation of arbuscular mycorrhizal fungi using different host plants. Korean J. Soil Sci. Fertil. 2016, 49, 608–613. [Google Scholar]
- Pathirana, B.K.W.; Yapa, N. Evaluation of different carrier substances for the development of an effective pelleted biofertilizer for rice (Oryza sativa L.) using co-inoculated bacteria and arbuscular mycorrhizal fungi. Asian J. Biotechnol. Bioresour. Technol. 2020, 6, 53857. [Google Scholar] [CrossRef]
- Sarkodee-Addo, E.; Yasuda, M.; Lee, C.G.; Kanasugi, M.; Fujii, Y.; Omari, R.A.; Oppong Abebrese, S.; Bam, R.; Asuming-Brempong, S.; Mohammad Golam Dastogeer, K.; et al. Arbuscular mycorrhizal fungi associated with rice (Oryza sativa L.) in Ghana: Effect of regional locations and soil factors on diversity and community assembly. Agronomy 2020, 10, 559. [Google Scholar] [CrossRef]
- Bernaola, L.; Cange, G.; Way, M.O.; Gore, J.; Hardke, J.; Stout, M. Natural colonization of rice by arbuscular mycorrhizal fungi in different production areas. Rice Sci. 2018, 25, 169–174. [Google Scholar]
- Surendirakumar, K.; Pandey, R.R.; Muthukumar, T. Arbuscular mycorrhizal fungi in roots and rhizosphere of black rice in terrace fields of North-East India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 277–287. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Z.; Yang, M.; Lu, S.; Cao, L.; Wang, X. Molecular diversity and distribution of arbuscular mycorrhizal fungi at different elevations in Mt. Taibai of Qinling Mountain. Front. Microbiol. 2021, 12, 609386. [Google Scholar] [CrossRef] [PubMed]
- Olubodea, A.; Babalolaa, O.; Darea, M.; Adeyemib, N.O.; Aderibigbeb, S.; Okonjic, C.; Sakariyawo, O.S. Diversity of indigenous arbuscular mycorrhizal fungi in rhizosphere of upland rice (Oryza sativa L.) varieties in Southwest Nigeria. Acta Fytotech. Zootech. 2020, 23, 42–48. [Google Scholar]
- Baki, M.Z.I.; Suzuki, K.; Takashashi, K.; Chowdhury, S.A.; Asiloglu, R.; Harada, N. Moloecular genetic characterization of arbuscular mycorrhizal fungi associated with upland rice in Bangladesh. Rhizosphere 2021, 18, 100357. [Google Scholar] [CrossRef]
- Karmakar, A.; Mandal, P.; Adhikary, R.; Mandal, V. Assessment of rhizospheric arbuscular mycorrhizae spores in relation to soil characters in the rice fields of Malda District, India. Russ. Agric. Sci. 2020, 46, 48–55. [Google Scholar] [CrossRef]
- Chowdhury, F.R.; Shihab, Q.; Ibrahim, U.; Bari, S.; Alam, J.; Dunachie, S.J.; Rodriguez-Morales, A.J.; Patwary, M.I. The association between temperature, rainfall and humidity with common climate-sensitive infectious diseases in Bangladesh. PLoS ONE 2018, 13, e0199579. [Google Scholar] [CrossRef]
- Nadjilom, Y.; Toukam, S.T.; Issa, M.; Ngakou, A. Field evaluation of growth and yield of two local rice varieties (Tox-728-1 and Madjitolngar) in response to indogenous mycorrhizal inoculation in South-Chad. Am. J. Plant Sci. 2020, 11, 1175–1192. [Google Scholar]
- Sooksa-Nguan, T.; Gypmantasiri, P.; Boonkerd, N.; Thies, J.E.; Teaumroong, N. Changes in bacterial community composition in the system of rice intensification (SRI) in Chiang Mai, Thailand. Microbes Environ. 2010, 25, 224–227. [Google Scholar] [CrossRef]
- Husband, R.; Herre, E.A.; Young, J.P.W. Temporal variation in the arbuscular mycorrhizal communities colonising seedlings in a tropical forest. FEMS Microbiol. Ecol. 2002, 42, 131–136. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Khan, M.Y.; Waqas, M.R.; Binyamin, R.; Akhtar, S.; Zahir, Z.A. Arbuscular Mycorrhizas: An Overview. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Wu, Q.S., Ed.; Springer Nature: Berlin/Heidelberg, Germany, 2017; pp. 1–327. [Google Scholar]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity. 2020, 12, 370. [Google Scholar] [CrossRef]
- Sagar, A.; Rathore, P.; Ramteke, P.W.; Ramakrishna, W.; Reddy, M.S.; Pecoraro, L. Plant growth promoting rhizobacteria, arbuscular mycorrhizal fungi and their synergistic interactions to counteract the negative effects of saline soil on agriculture: Key macromolecules and mechanisms. Microorganisms 2021, 9, 1491. [Google Scholar] [CrossRef] [PubMed]
- Campo, S.; Martín-cardoso, H.; Olivé, M.; Pla, E.; Catala-forner, M.; Martínez-eixarch, M.; Segundo, B.S. Effect of root colonization by arbuscular mycorrhizal fungi on growth, productivity and blast resistance in rice. Rice 2020, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, L.; Ma, F.; Bloomfield, K.J.; Yang, J.; Atkin, O.K. Is resource allocation and grain yield of rice altered by inoculation with arbuscular mycorrhizal fungi? J. Plant Ecol. 2013, 8, 436–448. [Google Scholar] [CrossRef]





| Compound | Amount (g/L) | Stock Solution |
|---|---|---|
| KH2PO4 | 10.80 | A |
| K2SO4 | 45.00 | A |
| NH4NO3/2 weeks | 15.00 | A |
| CaCl2·2H2O | 45.00 | B |
| MgSO4·7H2O | 6.00 | C |
| MnSO4·H2O | 3.00 | C |
| ZnSO4·7H2O | 3.00 | C |
| CuSO4·5H2O | 1.50 | C |
| H3BO3 | 0.24 | C |
| CoSO4·7H2O | 0.12 | C |
| Na2MoO4·2H2O | 0.09 | C |
| Treatment | Amount of Soil (g) | Amount of Fertilizer (g) | Time of Fertilizer Application | ||
|---|---|---|---|---|---|
| Control (T1) | 3000 | - | - | - | - |
| Biofertilizer (T2) | 3000 | 30 | Applied | - | Applied |
| Biochar (T3) | 2750 | 250 | Applied | - | Applied |
| Compost (T4) | 2750 | 250 | Applied | - | Applied |
| Inorganic fertilizer (T5) | 3000 | Ammonium sulphate—0.6 | - | Applied | Applied |
| Monocalcium phosphate—0.3 | Applied | - | - | ||
| Potassium chloride/muriate—0.3 | - | - | Applied | ||
| Zone | Sample Site | GPS Coordinate | Annual Temperature (°C) | Relative Humidity (%) | Soil Type | Soil pH | Electric Conductivity of Soil (µS/cm) |
|---|---|---|---|---|---|---|---|
| Dry | Jaffna | 9°42′10″ N 80°01′14″ E | 28–29 | 78 | Grumusols | 6.28 | 550.60 |
| Vavuniya | 8°45′43″ N 80°30′08″ E | 29–30 | 84 | Alluvial | 5.57 | 88.96 | |
| Trincomalee | 8°21′26″ N 81°00′29″ E | 28–29 | 84 | Alluvial | 5.23 | 332.80 | |
| Anuradhapura | 8°16′56″ N 80°42′49″ E | 29–30 | 84 | Reddish brown earth | 7.10 | 44.58 | |
| Intermediate | Kurunegala | 7°39′09″ N 80°22′18″ E | 30–31 | 83 | Red-yellow latosols | 6.54 | 70.30 |
| Badulla | 6°59′37″ N 81°03′12″ E | 27–28 | 88 | Red-yellow podzolic | 5.59 | 247.80 | |
| Matara | 5°57′55″ N 80°31′53″ E | 30–31 | 80 | Alluvial | 5.25 | 124.60 | |
| Wet | Kandy | 7°16′02″ N 80°32′56″ E | 28–29 | 84 | Reddish brown lateritic | 5.61 | 32.58 |
| Gampaha | 7°05′59″ N 79°59′46″ E | 31–32 | 79 | Alluvial | 5.99 | 46.36 | |
| Rathnapura | 6°43′00″ N 80°46′22″ E | 30–31 | 85 | Alluvial | 5.42 | 22.03 | |
| Galle | 6°20′48″ N 80°14′35″ E | 29–30 | 79 | Alluvial | 5.92 | 123.3 |
| Treatment | AMF Colonization in the 9 Week (%) | |
|---|---|---|
| Non-Sterilized | Control | 10.425 c |
| AMF-biofertilizer | 26.675 a | |
| Biochar | 8.300 c | |
| Compost | 11.125 c | |
| Inorganic fertilizer | 7.950 c | |
| Sterilized | Control | 0.000 d |
| AMF-biofertilizer | 21.275 b | |
| Biochar | 0.000 d | |
| Compost | 0.125 d | |
| Inorganic fertilizer | 0.000 d | |
| Treatment | Total Culturable Bacteria (×1010) | Total Culturable Fungi (×105) | AMF Spore Density in Soil (spores/g) | |
|---|---|---|---|---|
| Non-sterilized soil | Control | 0.23 ± 0.10 d | 15.00 ± 3.16 b | 26.00 ± 2.38 b |
| AMF-biofertilizer | 45.50 ± 3.23 a | 26.75 ± 2.66 a | 51.50 ± 3.31 a | |
| Biochar | 7.50 ± 1.71 c | 4.50 ± 1.00 c | 16.25 ± 1.38 c | |
| Compost | 32.25 ± 4.59 b | 7.25 ± 1.70 b c | 27.75 ± 2.46 b | |
| Inorganic fertilizer | 3.00 ± 1.08 c | 4.50 ± 0.65 c | 29.50 ± 2.90 b | |
| Sterilized soil | Control | 4.25 ± 1.49 d | 0.30 ± 0.30 b | 0.15 ± 0.30 b |
| AMF-biofertilizer | 0.08 ± 0.20 a | 0.14 ± 0.30 a | 46.00 ± 6.49 a | |
| Biochar | 5.50 ± 1.04 c | 4.75 ± 1.03 c | 4.675 ± 0.31 c | |
| Compost | 5.00 ± 0.82 b | 4.75 ± 0.75 b c | 6.50 ± 1.26 b | |
| Inorganic fertilizer | 9.50 ± 1.71 c | 5.25 ± 1.11 c | 0.29 ± 0.05 b | |
| Treatment | Number of Tillers | Number of Effective Tillers | Panicle Length (cm) | Number of Grains/Panicle | Plant Fresh Weight | Plant Dry Weight | 100 Seeds Weight | |
|---|---|---|---|---|---|---|---|---|
| Non-sterilized soil | Control | 7.25 ± 0.63 xab | 1.00 ± 0.41 xc | 13.50 ± 4.57 xc | 66.00 ± 22.23 xc | 5.73 ± 0.23 xd | 5.08 ± 0.37 xc | 1.08 ± 0.21 xb |
| AMF-Biofertilizer | 7.75 ± 0.75 xab | 2.00 ± 0.41 xab | 23.90 ± 0.99 xab | 119.75 ± 7.39 xa | 8.53 ± 0.31 xc | 7.1 ± 0.14 xb | 2.51 ± 0.12 xa | |
| Biochar | 5.75 ± 0.48 xb | 1.00 ± 0.41 xc | 15.00 ± 5.05 xbc | 77.00 ± 25.77 xbc | 10.78 ± 0.76 xbc | 9.75 ± 0.61 xab | 0.94 ± 0.19 xb | |
| Compost | 6.75 ± 1.11 xb | 1.25 ± 0.25 xbc | 21.30 ± 0.49 xabc | 109.75 ± 4.87 xab | 10.85 ± 0.56 xab | 9.08 ± 0.55 xab | 2.25 ± 0.21 xa | |
| Inorganic fertilizer | 8.75 ± 0.95 xa | 3.25 ± 0.25 xa | 26.10 ± 0.85 xa | 130.25 ± 9.07 xa | 13.3 ± 0.44 xa | 10.55 ± 0.78 xa | 3.26 ± 0.11 xa | |
| Sterilized soil | Control | 5.25 ± 0.48 yab | 1.75 ± 0.25 yc | 18.60 ± 0.48 yc | 114.25 ± 2.52 yc | 6.45 ± 0.33 yd | 5.53 ± 0.25 yc | 1.30 ± 0.00 yb |
| AMF-biofertilizer | 5.75 ± 0.63 yab | 3.75 ± 0.48 yab | 24.00 ± 0.54 yab | 150.50 ± 10.12 ya | 10.20 ± 0.16 yc | 8.93 ± 2.23 yb | 3.11 ± 0.00 ya | |
| Biochar | 4.50 ± 0.50 yb | 2.00 ± 0.41 yc | 21.60 ± 0.96 yabc | 117.75 ± 5.18 ybc | 11.85 ± 0.32 ybc | 10.18 ± 2.55 yab | 1.80 ± 0.09 yb | |
| Compost | 5.25 ± 0.48 yb | 2.50 ± 0.5 ybc | 21.00 ± 0.82 yc | 155.25 ± 5.12 yab | 12.6 ± 0.31 yab | 11.45 ± 2.86 yab | 3.50 ± 0.20 ya | |
| Inorganic fertilizer | 7.25 ± 0.48 ya | 4.25 ± 0.48 ya | 27.70 ± 0.91 yab | 172.75 ± 2.56 ya | 14.25 ± 0.2 ya | 12.5 ± 3.13 ya | 3.54 ± 0.89 ya | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalamulla, R.; Sandaruwan, D.; Karunarathna, S.C.; Stephenson, S.L.; Tibpromma, S.; Elgorban, A.M.; Al-Rejaie, S.; Yapa, P.N.; Suwannarach, N. Assessment of Community Dynamics of Arbuscular Mycorrhizal Fungi in the Rice (Oryza sativa L.) Rhizosphere and Potential Application as Biofertilizer. Sustainability 2022, 14, 16537. https://doi.org/10.3390/su142416537
Kalamulla R, Sandaruwan D, Karunarathna SC, Stephenson SL, Tibpromma S, Elgorban AM, Al-Rejaie S, Yapa PN, Suwannarach N. Assessment of Community Dynamics of Arbuscular Mycorrhizal Fungi in the Rice (Oryza sativa L.) Rhizosphere and Potential Application as Biofertilizer. Sustainability. 2022; 14(24):16537. https://doi.org/10.3390/su142416537
Chicago/Turabian StyleKalamulla, Ruwanthika, Dhanushka Sandaruwan, Samantha C. Karunarathna, Steven L. Stephenson, Saowaluck Tibpromma, Abdallah M. Elgorban, Salim Al-Rejaie, Pinnaduwage Neelamanie Yapa, and Nakarin Suwannarach. 2022. "Assessment of Community Dynamics of Arbuscular Mycorrhizal Fungi in the Rice (Oryza sativa L.) Rhizosphere and Potential Application as Biofertilizer" Sustainability 14, no. 24: 16537. https://doi.org/10.3390/su142416537
APA StyleKalamulla, R., Sandaruwan, D., Karunarathna, S. C., Stephenson, S. L., Tibpromma, S., Elgorban, A. M., Al-Rejaie, S., Yapa, P. N., & Suwannarach, N. (2022). Assessment of Community Dynamics of Arbuscular Mycorrhizal Fungi in the Rice (Oryza sativa L.) Rhizosphere and Potential Application as Biofertilizer. Sustainability, 14(24), 16537. https://doi.org/10.3390/su142416537








