Co-Firing Zhundong Coal with Its Gangue: Combustion Performance, Sodium Retention and Ash Fusion Behaviors
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Combustion Tests
2.3. Chemical Composition and Morphology Analyses of Ashes
2.4. Prediction of Slagging and Fouling Tendency
3. Results and Discussion
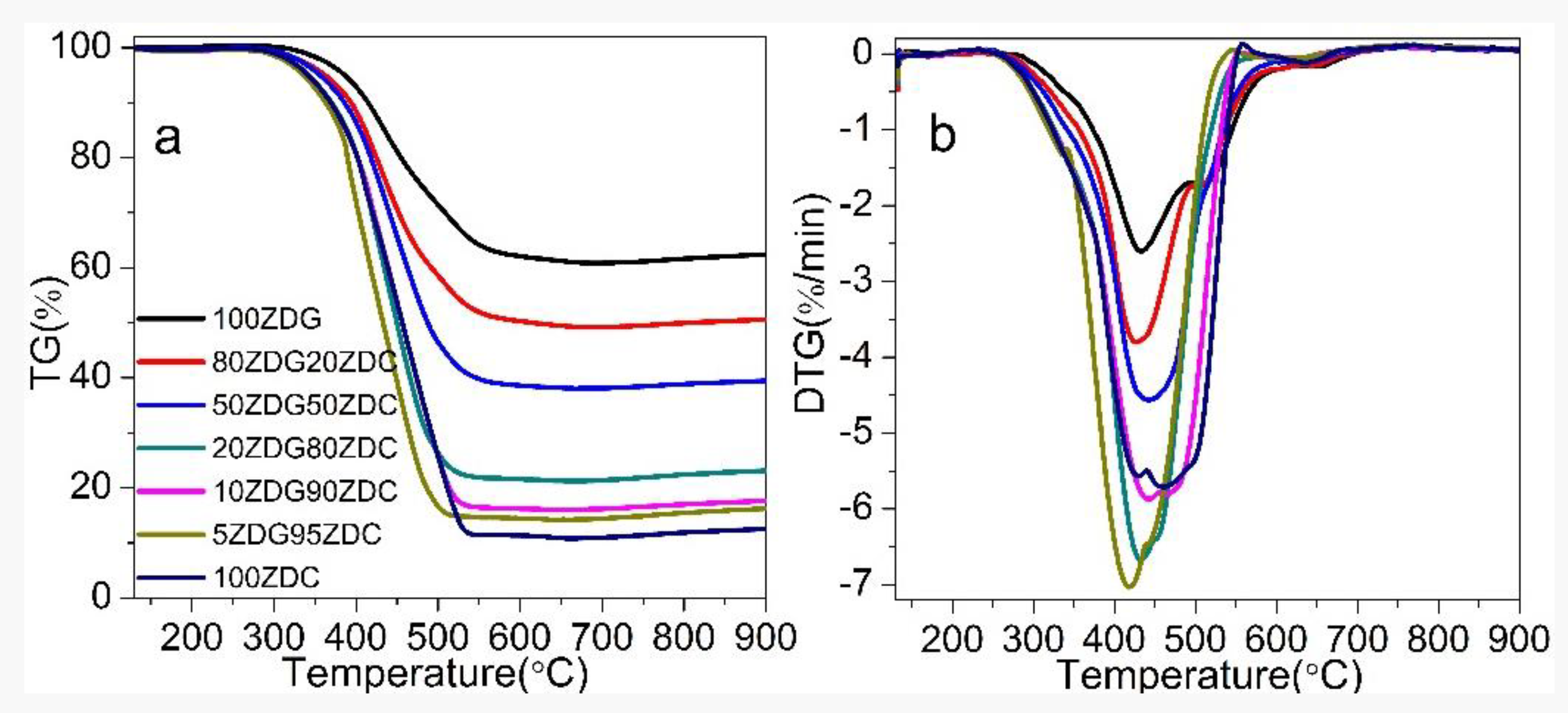
3.1. Co-Combustion Performance
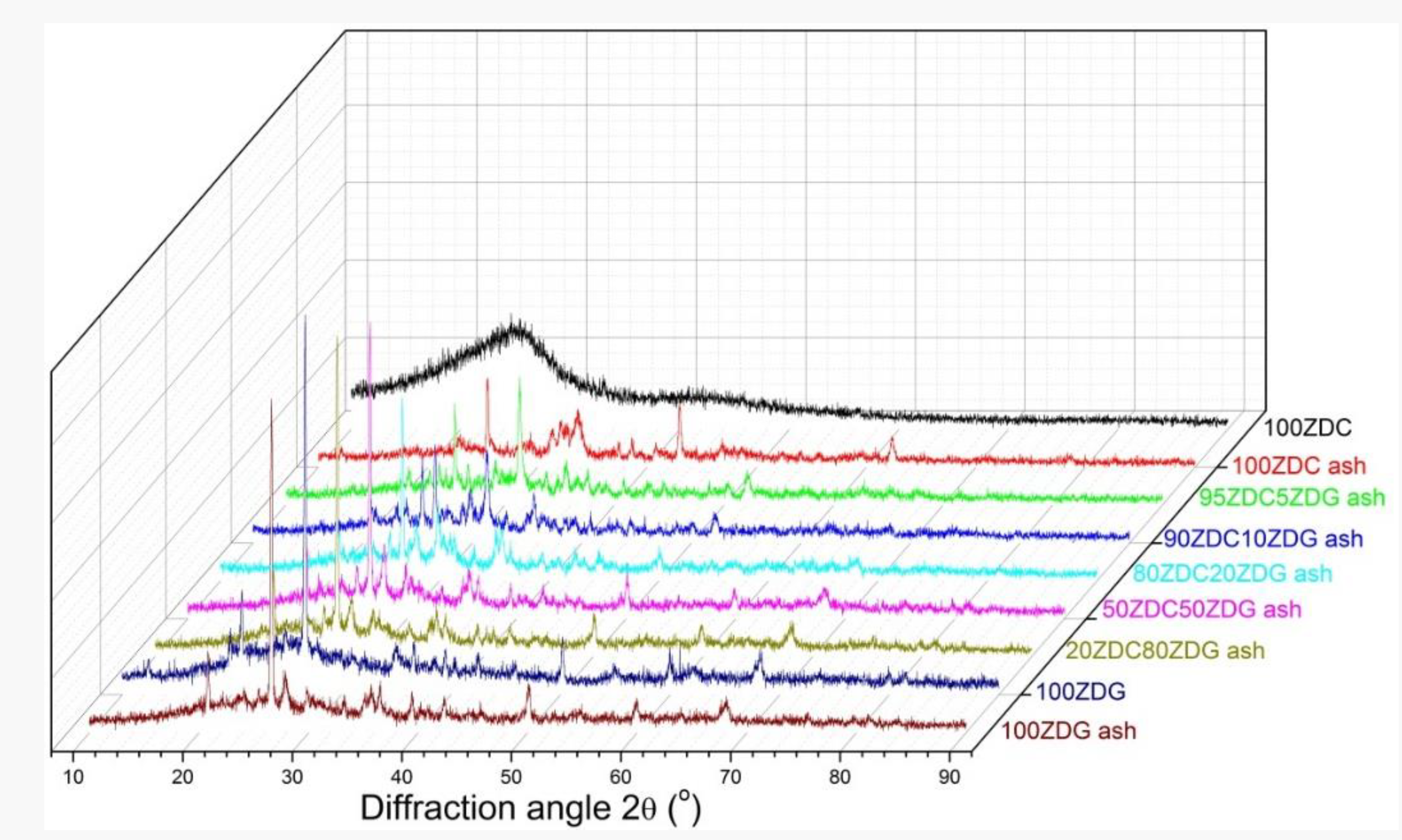
3.2. Ash Compositions and Mineralogy
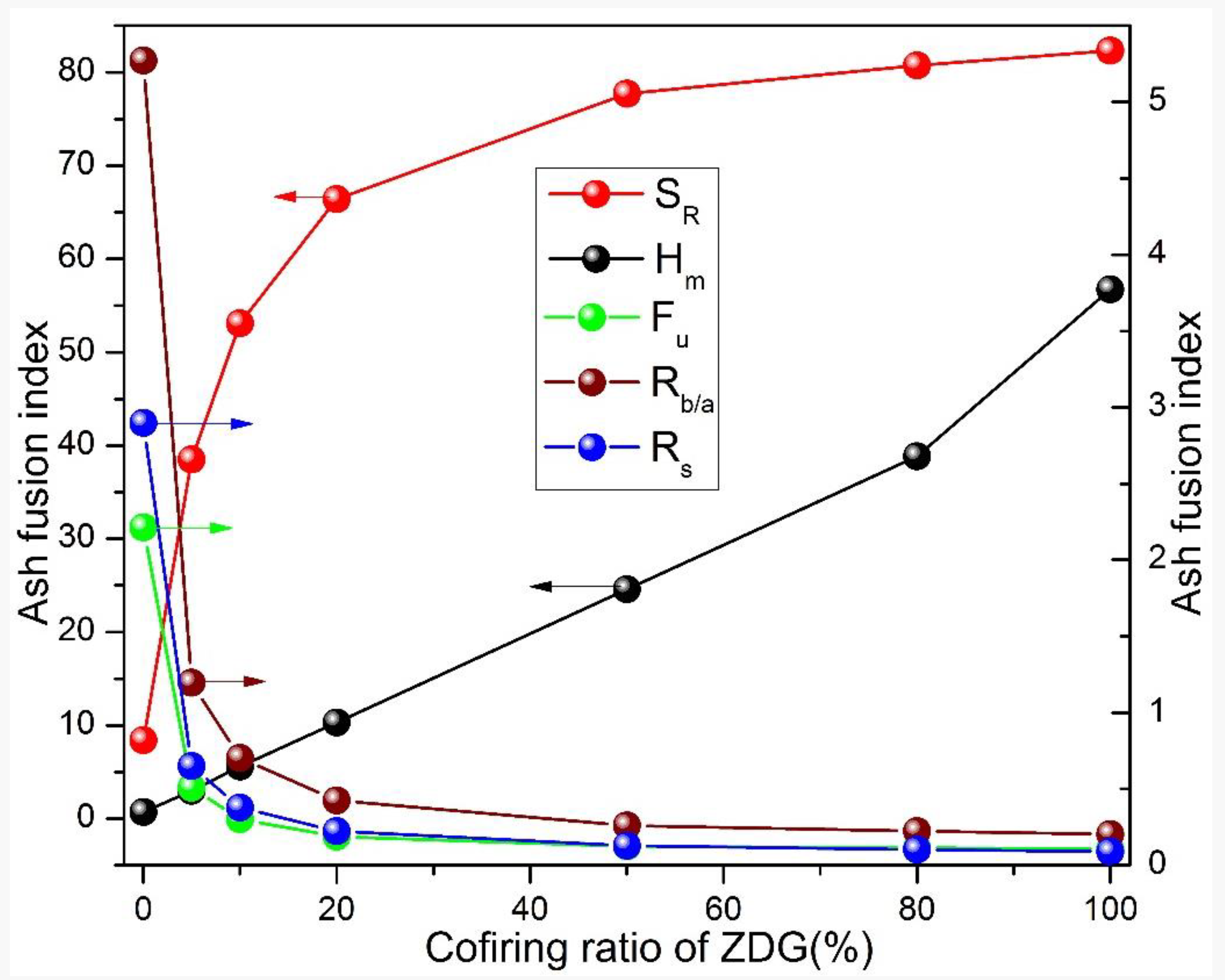
3.3. Slagging and Fouling Propensity
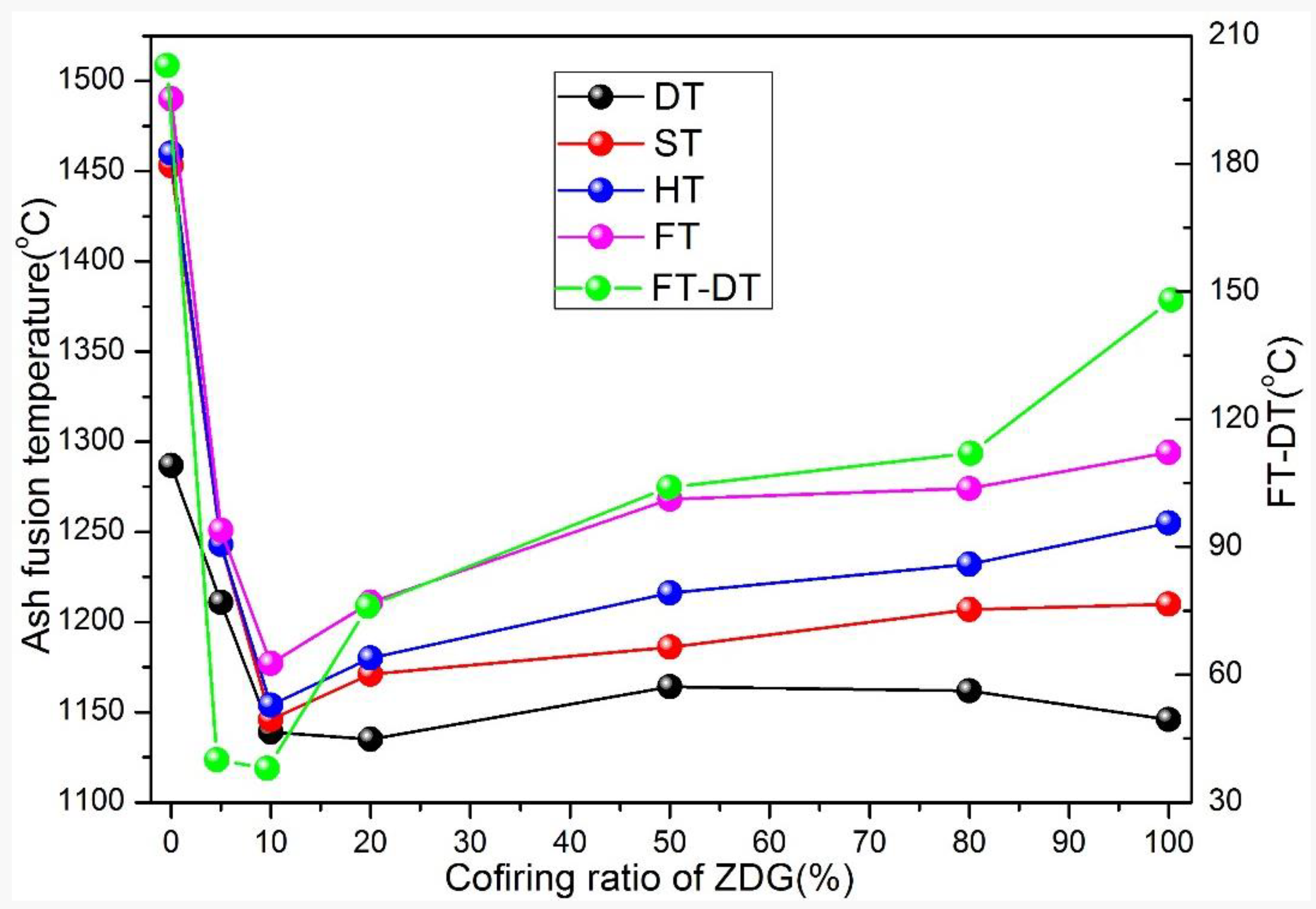
3.4. Ash Melting Temperatures
4. Conclusions
- (1)
- ZDC ignites more easily than ZDG due to its higher content of combustible substances. Co-firing ZDG does not hinder ignition but lowers the burning rate of ZDC. The comprehensive combustion index () gradually decreases with the reducing . However, the burning rate increases at proper co-firing ratios (e.g., 10% or 20%) of ZDG.
- (2)
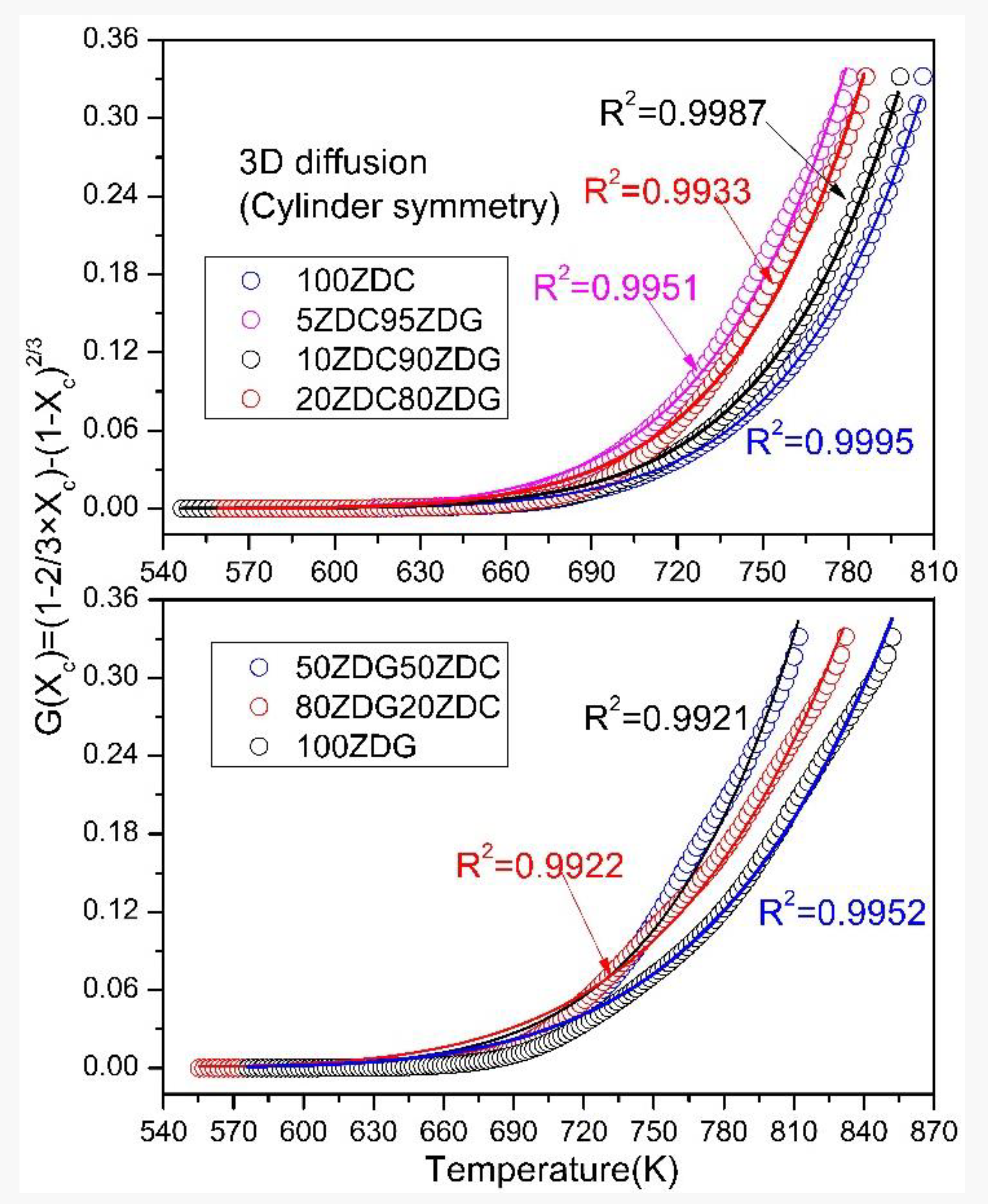
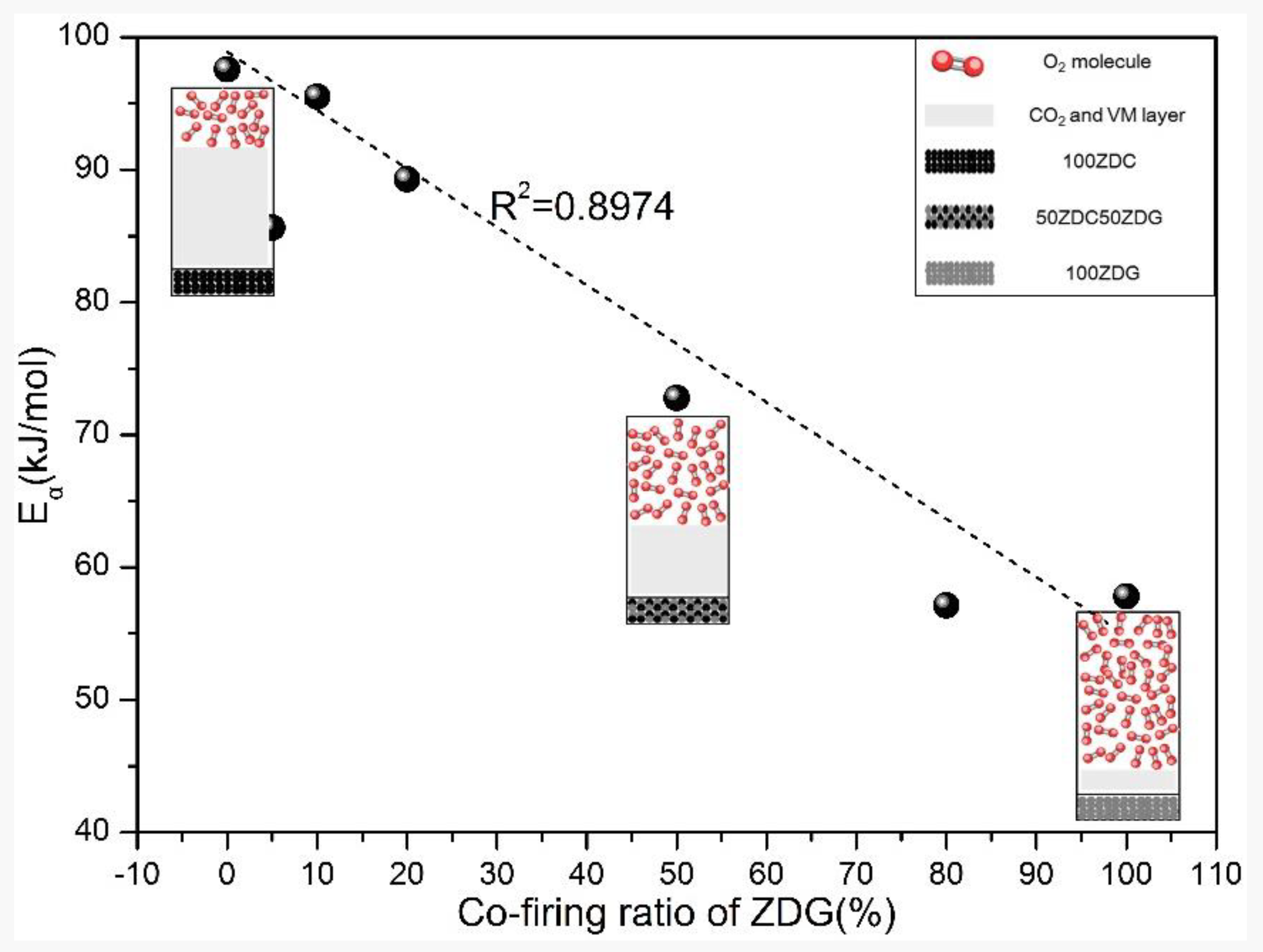
- Co-firing ZDC and ZDG follows the mechanism of a 3D diffusion model (cylinder symmetry) during non-isothermal combustion in TGA. The activation energy obtained from the mathematical fitting process with the model probably reflects the diffusion resistance of oxygen molecules to the carbon matrix of the fuels.
- (3)
- The enriched silica and alumina in ZDG can react with the calcium, magnesium and sodium in ZDC to form complex minerals. The sodium retention in ashes is remarkably enhanced. The varying indexes including , , , and indicate the effectiveness of co-firing ZDG in reducing the propensity of ash-fouling and slagging.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Liang, H.; Zhu, S. Mercury emission from spontaneously ignited coal gangue hill in Wuda coalfield, Inner Mongolia, China. Fuel 2016, 182, 525–530. [Google Scholar] [CrossRef]
- Chuncai, Z.; Guijian, L.; Dun, W.; Ting, F.; Ruwei, W.; Xiang, F. Mobility behavior and environmental implications of trace elements associated with coal gangue: A case study at the Huainan Coalfield in China. Chemosphere 2014, 95, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, J.; Chou, C.-L.; Li, Y.; Wang, Z.; Ge, Y.; Zheng, C. Trace element emissions from spontaneous combustion of gob piles in coal mines, Shanxi, China. Int. J. Coal Geol. 2008, 73, 52–62. [Google Scholar] [CrossRef]
- Querol, X.; Izquierdo, M.; Monfort, E.; Alvarez, E.; Font, O.; Moreno, T.; Alastuey, A.; Zhuang, X.; Lu, W.; Wang, Y. Environmental characterization of burnt coal gangue banks at Yangquan, Shanxi Province, China. Int. J. Coal Geol. 2008, 75, 93–104. [Google Scholar] [CrossRef]
- Dai, R.-W.; Zhao, R.-D.; Wang, Z.-Q.; Qin, J.-G.; Chen, T.-J.; Wu, J.-H. Study on the oxy-fuel co-combustion of coal gangue and semicoke and the pollutants emission characteristics. J. Fuel Chem. Technol. 2022, 50, 152–159. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, G.; Fang, T.; Lam, P.K.S. Investigation on thermal and trace element characteristics during co-combustion biomass with coal gangue. Bioresour. Technol. 2015, 175, 454–462. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Liu, L.; Wang, X.; Zhang, Z. Environmental investigation on co-combustion of sewage sludge and coal gangue: SO2, NOx and trace elements emissions. Waste Manag. 2016, 50, 213–221. [Google Scholar] [CrossRef]
- Deng, S.; Tan, H.; Wei, B.; Wang, X.; Yang, F.; Xiong, X. Investigation on combustion performance and ash fusion characteristics of Zhundong coal co-combustion with coal gangue. Fuel 2021, 294, 120555. [Google Scholar] [CrossRef]
- Si, J.; Liu, X.; Xu, M.; Sheng, L.; Zhou, Z.; Wang, C.; Zhang, Y.; Seo, Y.-C. Effect of kaolin additive on PM2.5 reduction during pulverized coal combustion: Importance of sodium and its occurrence in coal. Appl. Energy 2014, 114, 434–444. [Google Scholar] [CrossRef]
- Kosminski, A.; Ross, D.P.; Agnew, J.B. Reactions between sodium and silica during gasification of a low-rank coal. Fuel Proc. Technol. 2006, 87, 1037–1049. [Google Scholar] [CrossRef]
- Zhou, J.; Zhuang, X.; Alastuey, A.; Querol, X.; Li, J. Geochemistry and mineralogy of coal in the recently explored Zhundong large coal field in the Junggar basin, Xinjiang province, China. Int. J. Coal Geol. 2010, 82, 51–67. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wu, G.-G.; Bai, Z.-Q.; Li, W. Clean and efficient utilization of sodium-rich Zhundong coals in China: Behaviors of sodium species during thermal conversion processes. Fuel 2018, 218, 162–173. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, B.; Li, L.; Zhang, H. Experimental Measurement of the Effective Thermal Conductivity of Ash Deposit for High Sodium Coal (Zhun Dong Coal) in a 300 KW Test Furnace. Energy Fuels 2013, 27, 7008–7022. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, J.; Hou, F.; Zhang, Y.; Wang, G.; Liu, B.; Zhai, Z. Understanding effect of phosphorus-based additive on ash deposition characteristics during high-sodium and high-calcium Zhundong coal combustion in drop-tube furnace. Fuel 2021, 287, 119462. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Z.; Wei, B.; Zhang, L.; Tan, H.; Yang, T.; Mikulčić, H.; Duić, N. The ash deposition mechanism in boilers burning Zhundong coal with high contents of sodium and calcium: A study from ash evaporating to condensing. Appl. Therm. Eng. 2015, 80, 150–159. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, L.; Zhao, Y.; Ji, J.; Wang, Q.; Luo, Z.; Bai, Y. Transformation behavior of alkali metals in high-alkali coals. Fuel Proc. Technol. 2018, 169, 288–294. [Google Scholar] [CrossRef]
- Yang, S.; Song, G.; Na, Y.; Yang, Z. Alkali metal transformation and ash deposition performance of high alkali content Zhundong coal and its gasification fly ash under circulating fluidized bed combustion. Appl. Therm. Eng. 2018, 141, 29–41. [Google Scholar] [CrossRef]
- Wang, C.a.; Li, G.; Du, Y.; Yan, Y.; Li, H.; Che, D. Ash deposition and sodium migration behaviors during combustion of Zhundong coals in a drop tube furnace. J. Energy Inst. 2018, 91, 251–261. [Google Scholar] [CrossRef]
- Ji, H.; Wu, X.; Dai, B.; Zhang, L. Xinjiang lignite ash slagging and flow under the weak reducing environment at 1300 °C—Release of sodium out of slag and its modelling from the mass transfer perspective. Fuel Proc. Technol. 2018, 170, 32–43. [Google Scholar] [CrossRef]
- Li, J.; Zhu, M.; Zhang, Z.; Zhang, K.; Shen, G.; Zhang, D. Characterisation of ash deposits on a probe at different temperatures during combustion of a Zhundong lignite in a drop tube furnace. Fuel Proc. Technol. 2016, 144, 155–163. [Google Scholar] [CrossRef]
- Zi, J.; Ma, D.; Wang, X.; Rahman, Z.u.; Li, H.; Liao, S. Slagging behavior and mechanism of high-sodium–chlorine coal combustion in a full-scale circulating fluidized bed boiler. J. Energy Inst. 2020, 93, 2264–2270. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Q.; Ma, P.; Li, S. Mechanistic studies on the slagging propensity in low-rank coal combustion. Combust Flame 2022, 238, 111956. [Google Scholar] [CrossRef]
- Song, G.; Song, W.; Qi, X.; Lu, Q. Transformation Characteristics of Sodium of Zhundong Coal Combustion/Gasification in Circulating Fluidized Bed. Energy Fuels 2016, 30, 3473–3478. [Google Scholar] [CrossRef]
- Li, G.; Wang, C.a.; Yan, Y.; Jin, X.; Liu, Y.; Che, D. Release and transformation of sodium during combustion of Zhundong coals. J. Energy Inst. 2016, 89, 48–56. [Google Scholar] [CrossRef]
- Xiao, H.; Qi, C.; Cheng, Q.; Dou, C.; Ru, Y.; Kang, Z.; Sun, B. Effect of Sodium-Containing Sulfates on Ash Fusibility. Energy Fuels 2018, 32, 9908–9915. [Google Scholar] [CrossRef]
- Wei, B.; Tan, H.; Wang, Y.; Wang, X.; Yang, T.; Ruan, R. Investigation of characteristics and formation mechanisms of deposits on different positions in full-scale boiler burning high alkali coal. Appl. Therm. Eng. 2017, 119, 449–458. [Google Scholar] [CrossRef]
- Shi, H.; Wu, Y.; Zhang, M.; Zhang, Y.; Lyu, J. Ash deposition of Zhundong coal in a 350 MW pulverized coal furnace: Influence of sulfation. Fuel 2020, 260, 116317. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, X.; Yan, K.; Chen, N.; Zhang, J.; Xu, X.; Dai, B.; Zhang, J.; Zhang, L. Ash deposition and slagging behavior of Chinese Xinjiang high-alkali coal in 3MWth pilot-scale combustion test. Fuel 2016, 181, 1191–1202. [Google Scholar] [CrossRef]
- Hu, X.; Wu, X.; Zhang, Z.; Fan, H.; Fan, J.; Chen, S.; Lan, D. Sodium retention behavior of Xinjiang high-alkali coal in a 20 kW slag-tapping combustor test. Fuel 2022, 317, 123298. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Xu, Y.; Li, Y.; Hao, W.; Zhang, Y. Investigation of ash fusion characteristics and migration of sodium during co-combustion of Zhundong coal and oil shale. Appl. Therm. Eng. 2017, 121, 224–233. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, L.; Li, X.; Wang, W.; Xue, Y.; Zhu, X.; Hu, H.; Luo, G.; Naruse, I.; Bai, Z.; et al. Na&Ca removal from Zhundong coal by a novel CO2-water leaching method and the ashing behavior of the leached coal. Fuel 2017, 210, 8–14. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Yang, Y.; Shen, F.; Zhang, Z. Theoretical investigation of sodium capture mechanism on kaolinite surfaces. Fuel 2018, 234, 318–325. [Google Scholar] [CrossRef]
- Yao, Y.; Jin, J.; Liu, D.; Wang, Y.; Kou, X.; Lin, Y. Evaluation of Vermiculite in Reducing Ash Deposition during the Combustion of High-Calcium and High-Sodium Zhundong Coal in a Drop-Tube Furnace. Energy Fuels 2016, 30, 3488–3494. [Google Scholar] [CrossRef]
- Yu, K.; Chen, X.; Cai, T.; Ma, J.; Liu, D.; Liang, C. Release and migration characteristics of sodium and potassium in high alkali coal under oxy-fuel fluidized bed combustion condition. Fuel 2020, 262, 116413. [Google Scholar] [CrossRef]
- Dai, B.-Q.; Wu, X.; De Girolamo, A.; Zhang, L. Inhibition of lignite ash slagging and fouling upon the use of a silica-based additive in an industrial pulverised coal-fired boiler. Part 1. Changes on the properties of ash deposits along the furnace. Fuel 2015, 139, 720–732. [Google Scholar] [CrossRef]
- Zeng, X.; Yu, D.; Liu, F.; Fan, B.; Wen, C.; Yu, X.; Xu, M. Scavenging of refractory elements (Ca, Mg, Fe) by kaolin during low rank coal combustion. Fuel 2018, 223, 198–210. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, Y.; Zhang, Y.; Xiong, Z.; Zhang, J.; Zhao, Y. Effect of kaolinite additive on water-soluble sodium release and particle matter formation during Zhundong coal combustion. Fuel 2023, 333, 126422. [Google Scholar] [CrossRef]
- Font, O.; Córdoba, P.; Leiva, C.; Romeo, L.M.; Bolea, I.; Guedea, I.; Moreno, N.; Querol, X.; Fernandez, C.; Díez, L.I. Fate and abatement of mercury and other trace elements in a coal fluidised bed oxy combustion pilot plant. Fuel 2012, 95, 272–281. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Fu, C.; Tang, L.; Chen, J.; Wu, T.; Lin, C.-J.; Feng, X.; Fu, X. Fate of mercury in two CFB utility boilers with different fueled coals and air pollution control devices. Fuel 2019, 251, 651–659. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiu, X.; Wang, Y.; Duan, Y.; Li, L.; Lin, H.; Luo, Y.; Sun, Z.; Duan, L. Particulate matter formation during shoe manufacturing waste combustion in a full-scale CFB boiler. Fuel Proc. Technol. 2021, 221, 106914. [Google Scholar] [CrossRef]
- Ma, B.-G.; Li, X.-G.; Xu, L.; Wang, K.; Wang, X.-G. Investigation on catalyzed combustion of high ash coal by thermogravimetric analysis. Acta 2006, 445, 19–22. [Google Scholar] [CrossRef]
- Niu, S.-l.; Han, K.-h.; Lu, C.-m. Characteristic of coal combustion in oxygen/carbon dioxide atmosphere and nitric oxide release during this process. Energy Convers. Manag. 2011, 52, 532–537. [Google Scholar] [CrossRef]
- Wang, L.; Skjevrak, G.; Hustad, J.E.; Grønli, M.G. Sintering characteristics of sewage sludge ashes at elevated temperatures. Fuel Proc. Technol. 2012, 96, 88–97. [Google Scholar] [CrossRef]
- Pronobis, M. Evaluation of the influence of biomass co-combustion on boiler furnace slagging by means of fusibility correlations. Biomass Bioenergy 2005, 28, 375–383. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Zhu, M.; Cheng, F.; Zhang, D. Interactions of coal gangue and pine sawdust during combustion of their blends studied using differential thermogravimetric analysis. Bioresour. Technol. 2016, 214, 396–403. [Google Scholar] [CrossRef]
- Bagchi, T.P.; SEN, P.K. Combined differential and integral method for analysis of non-isothermal kinetic data. Acta 1982, 51, 175–189. [Google Scholar] [CrossRef]
- Yu, K.; Tang, H.; Cai, T.; Chen, X.; Zan, H.; Ma, J.; Liang, C. Mechanism of kaolinite’s influence on sodium release characteristics of high-sodium coal under oxy-steam combustion conditions. Fuel 2021, 290, 119812. [Google Scholar] [CrossRef]
- Yu, K.; Chen, X.; Cai, T.; Tang, H.; Ma, J.; Liu, D.; Liang, C. The effect of Kaolinite’s structure on migration and release characteristics of sodium under oxy-fuel combustion condition. Fuel 2020, 277, 118154. [Google Scholar] [CrossRef]



| Sample | Proximate Analysis/(wt.%) | Ultimate Analysis/(wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Ashad | VMad | FCad | Cdaf | Hdaf | Ndaf | Odaf * | Sdaf | |
| ZDC | 13.4 | 3.6 | 29.5 | 53.6 | 79.07 | 3.76 | 0.61 | 15.99 | 0.57 |
| ZDG | 1.9 | 66.9 | 10.6 | 20.6 | 79.77 | 3.64 | 1.09 | 14.14 | 1.35 |
| Sample | Mass Fraction (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | SO3 | Na2O | MgO | Fe2O3 | P2O5 | TiO2 | K2O | Others | |
| ZDC | 38.71 | 5.66 | 7.31 | 15.32 | 8.35 | 15.81 | 7.24 | 0.03 | 0.36 | 0.13 | 1.08 |
| ZDG | 4.86 | 57.76 | 22.99 | 0.67 | 2.48 | 1.94 | 5.60 | 0.00 | 0.97 | 1.88 | 0.85 |
| Sample | Minerals | Characteristic Peak Position (°) | Relative Content (%) |
|---|---|---|---|
| 100ZDC ash | Aluminum oxide (Al2O3) | 25.61, 37.60, 52.19 | 0.2 |
| Lime (CaO) | 32.31, 37.51, 54.03, 64.43 | 8.5 | |
| Anhydrite (CaSO4) | 38.78, 40.97, 48.83 | 30.2 | |
| Calcium ferrate (CaFeO3) | 33.69, 48.37, 52.99, 62.81 | 61.1 | |
| 5ZDG 95ZDC ash | Gehlenite (4CaO·2(Al2O3)·2(SiO2)) | 24.14, 29.29, 31.40, 36.95, 52.40 | 98.3 |
| Silicon diphosphate (SiO2·P2O5) | 20.67, 23.27, 26.70, 33.95 | 1.7 | |
| 10ZDG 90ZDC ash | Akermanite (4CaO·MgO·Al2O3·3(SiO2)) | 22.97, 23.99, 29.16, 31.37, 35.63 | 28.4% |
| Quartz (SiO2) | 20.79, 26.65, 36.65, 42.36, 45.79, 50.025, 59.92, 67.98 | 3.5% | |
| Augite (4CaO·FeO·3(MgO)·8(SiO2)) | 27.71, 29.95, 35.89 | 68.0% | |
| 20ZDG 80ZDC ash | Quartz (SiO2) | 20.79, 26.65, 36.65, 42.36, 45.79, 50.025, 59.92, 67.98 | 21.4% |
| Diopside (CaO·MgO·2(SiO2)) | 29.86, 30.87, 35.63 | 60.0% | |
| Enstatite (12MgO·2(Fe2O3)·16(SiO2)) | 27.95, 29.96, 35.07 | 18.6% | |
| 50ZDG 50ZDC ash | Quartz (SiO2) | 20.79, 26.65, 36.65, 42.36, 45.79, 50.025, 59.92, 67.98 | 52.0% |
| Anorthite sodian (0.5(Na2O·CaO·1.5(Al2O3)·5(SiO2)) | 21.10, 27.88, 42.35 | 48.0% | |
| 80ZDG 20ZDC ash | Quartz (SiO2) | 20.79, 26.65, 36.65, 42.36, 45.79, 50.025, 59.92, 67.98 | 28.7% |
| Anorthite sodian (0.5(Na2O·CaO·1.5(Al2O3)·5(SiO2)) | 21.10, 27.88, 42.35 | 71.3% | |
| 100ZDG ash | Quartz (SiO2) | 20.79, 26.65, 36.65, 42.36, 45.79, 50.025, 59.92, 67.98 | 65.5% |
| Diopside (CaO·MgO·2(SiO2)) | 29.86, 30.87, 35.63 | 34.5% |
| Sample | Mass Fraction (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | SO3 | Na2O | MgO | Fe2O3 | P2O5 | TiO2 | K2O | Others | |
| 100ZDG | 4.86 | 57.76 | 22.99 | 0.67 | 2.48 | 1.94 | 5.60 | 0.00 | 0.97 | 1.88 | 0.85 |
| 80ZDG20ZDC | 5.39 | 56.83 | 22.68 | 0.93 | 2.62 | 2.26 | 5.92 | 0.24 | 0.96 | 1.82 | 0.35 |
| 50ZDG50ZDC | 6.96 | 54.52 | 22.23 | 1.58 | 2.82 | 2.89 | 5.80 | 0.22 | 0.91 | 1.72 | 0.35 |
| 20ZDG80ZDC | 12.28 | 46.19 | 19.93 | 3.98 | 3.70 | 5.12 | 5.96 | 0.20 | 0.85 | 1.38 | 0.41 |
| 10ZDG90ZDC | 18.05 | 36.36 | 17.06 | 7.13 | 4.91 | 7.74 | 6.32 | 0.16 | 0.75 | 1.04 | 0.48 |
| 5ZDG95ZDC | 24.20 | 25.78 | 13.70 | 11.23 | 6.02 | 10.73 | 6.24 | 0.11 | 0.64 | 0.76 | 0.59 |
| 100ZDC | 38.71 | 5.66 | 7.31 | 15.32 | 8.35 | 15.81 | 7.24 | 0.03 | 0.36 | 0.13 | 1.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Yan, J.; Yang, Q.; Lei, Z.; Lei, Z.; Li, Z.; Ren, S.; Wang, Z.; Shui, H. Co-Firing Zhundong Coal with Its Gangue: Combustion Performance, Sodium Retention and Ash Fusion Behaviors. Sustainability 2022, 14, 16451. https://doi.org/10.3390/su142416451
Zhang L, Yan J, Yang Q, Lei Z, Lei Z, Li Z, Ren S, Wang Z, Shui H. Co-Firing Zhundong Coal with Its Gangue: Combustion Performance, Sodium Retention and Ash Fusion Behaviors. Sustainability. 2022; 14(24):16451. https://doi.org/10.3390/su142416451
Chicago/Turabian StyleZhang, Li, Jingchong Yan, Qitong Yang, Zhiping Lei, Zhao Lei, Zhanku Li, Shibiao Ren, Zhicai Wang, and Hengfu Shui. 2022. "Co-Firing Zhundong Coal with Its Gangue: Combustion Performance, Sodium Retention and Ash Fusion Behaviors" Sustainability 14, no. 24: 16451. https://doi.org/10.3390/su142416451
APA StyleZhang, L., Yan, J., Yang, Q., Lei, Z., Lei, Z., Li, Z., Ren, S., Wang, Z., & Shui, H. (2022). Co-Firing Zhundong Coal with Its Gangue: Combustion Performance, Sodium Retention and Ash Fusion Behaviors. Sustainability, 14(24), 16451. https://doi.org/10.3390/su142416451





