Water Chemical Characteristics and Safety Assessment of Irrigation Water in the Northern Part of Hulunbeier City, Grassland Area in Eastern China
Abstract
1. Introduction
2. Materials and Methods
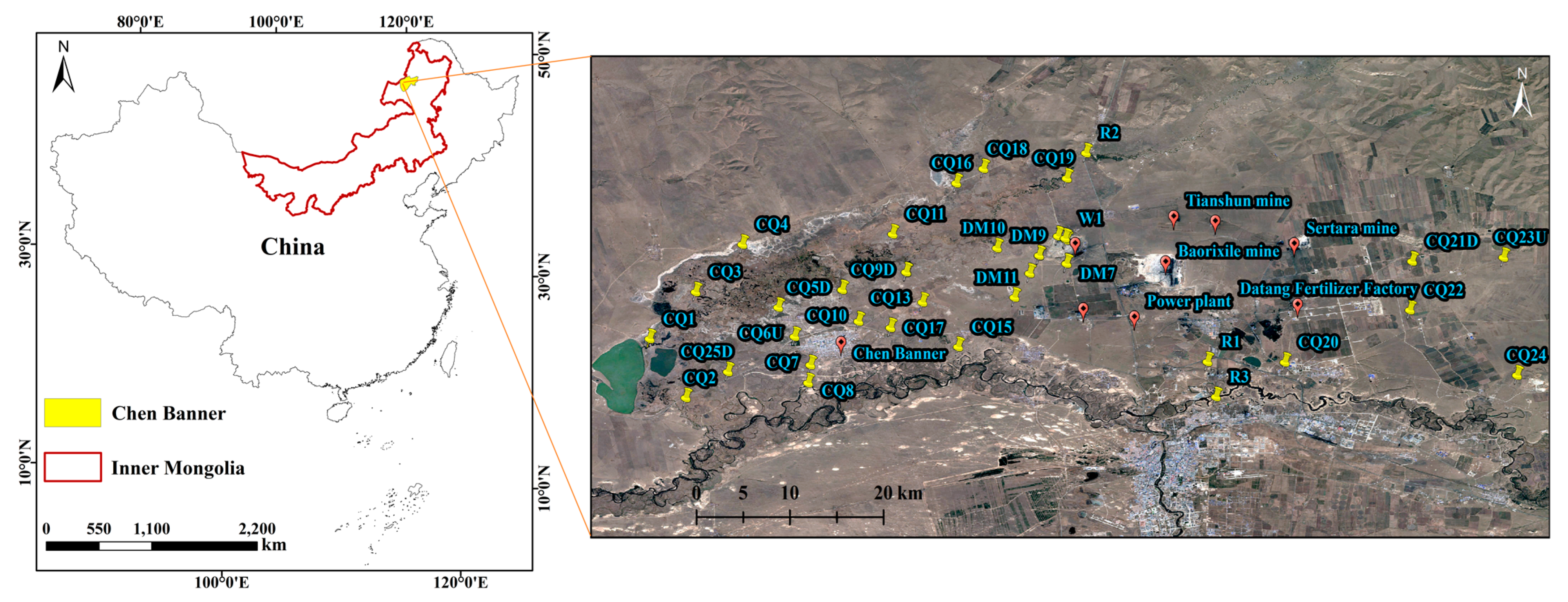
Survey Region
3. Results and Discussion
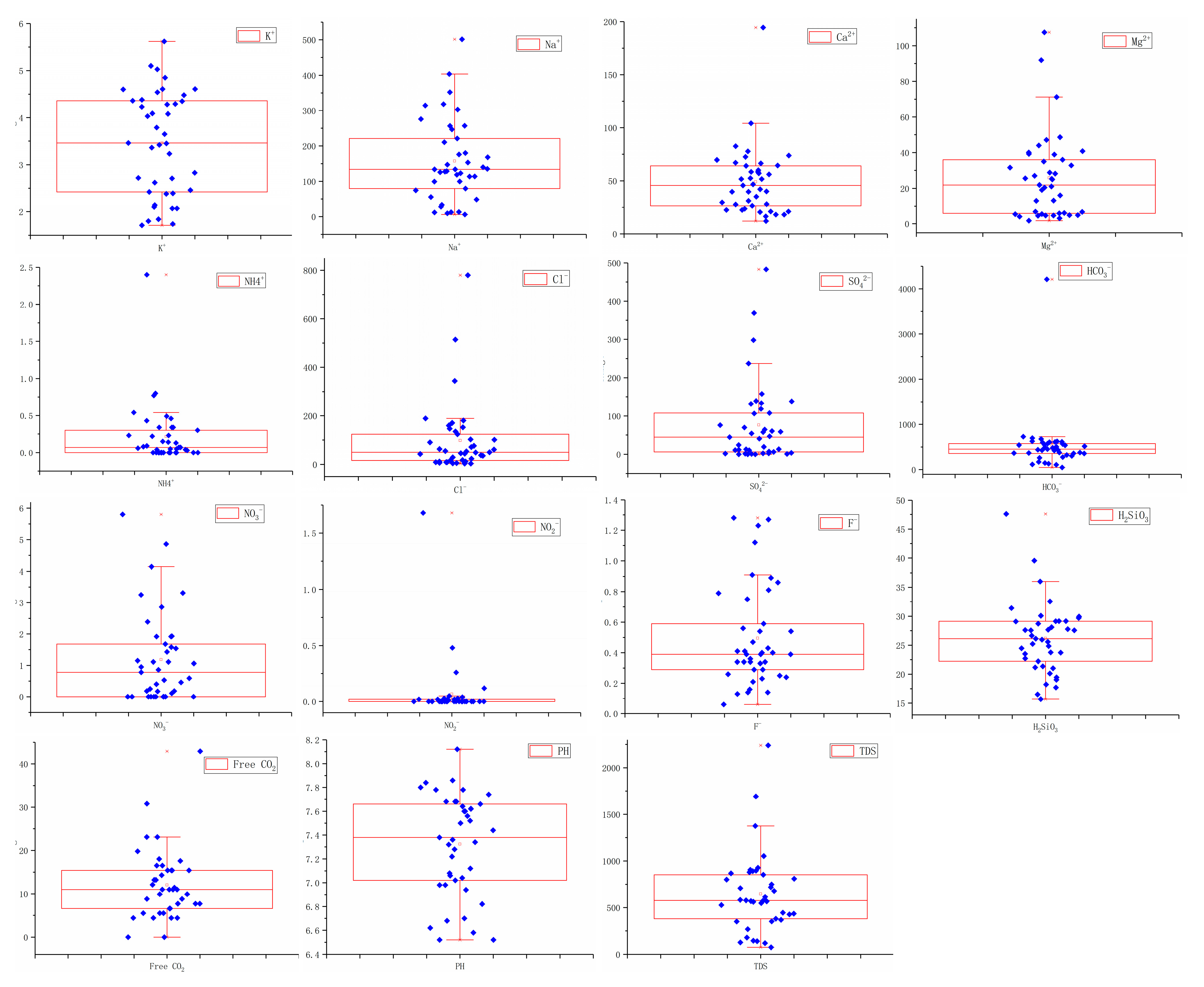
3.1. Descriptive Statistical Analysis of Groundwater Chemistry
3.2. Chemical Correlation Analysis of Groundwater
3.3. Influencing Factors of Hydrochemical Characteristics
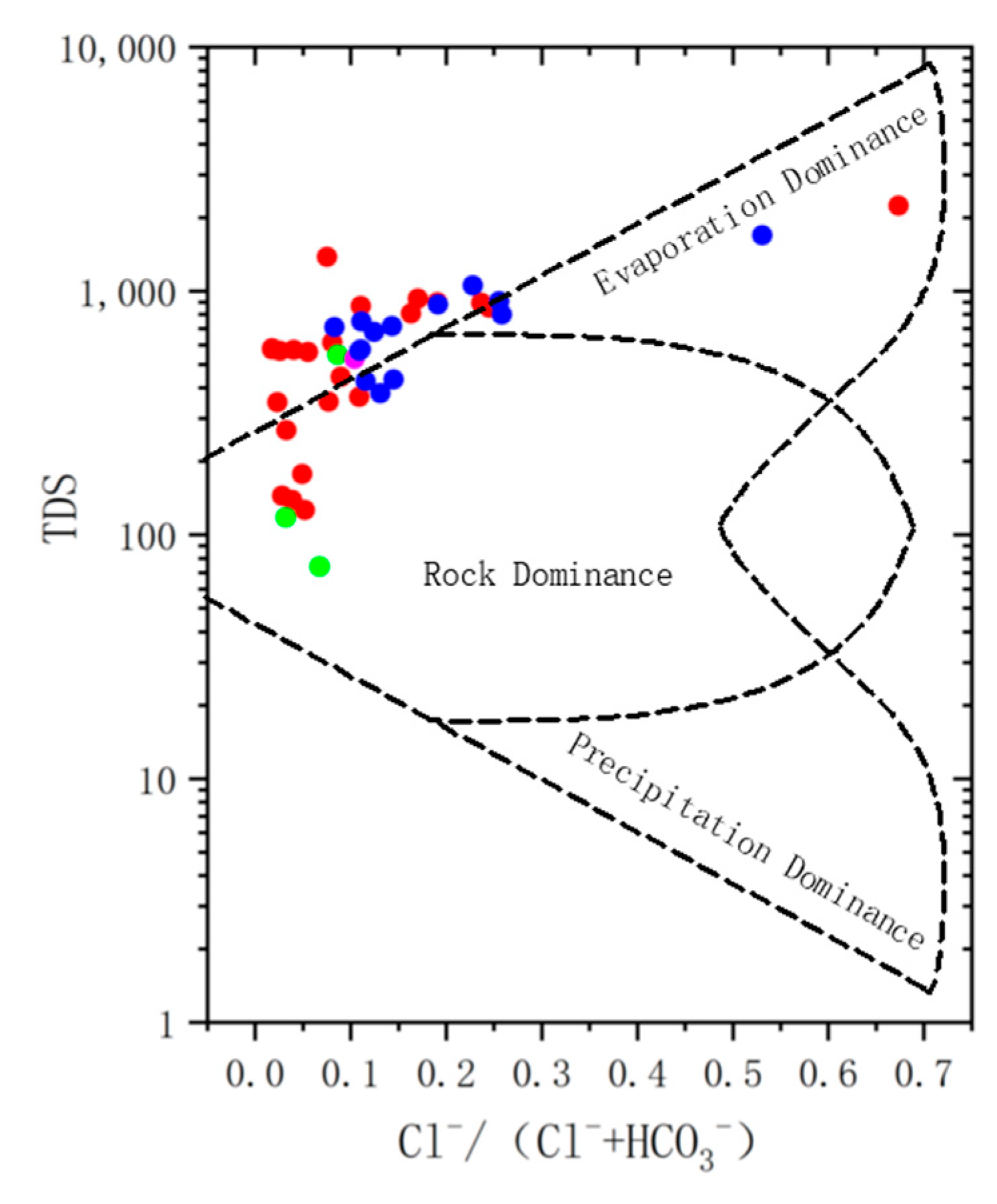
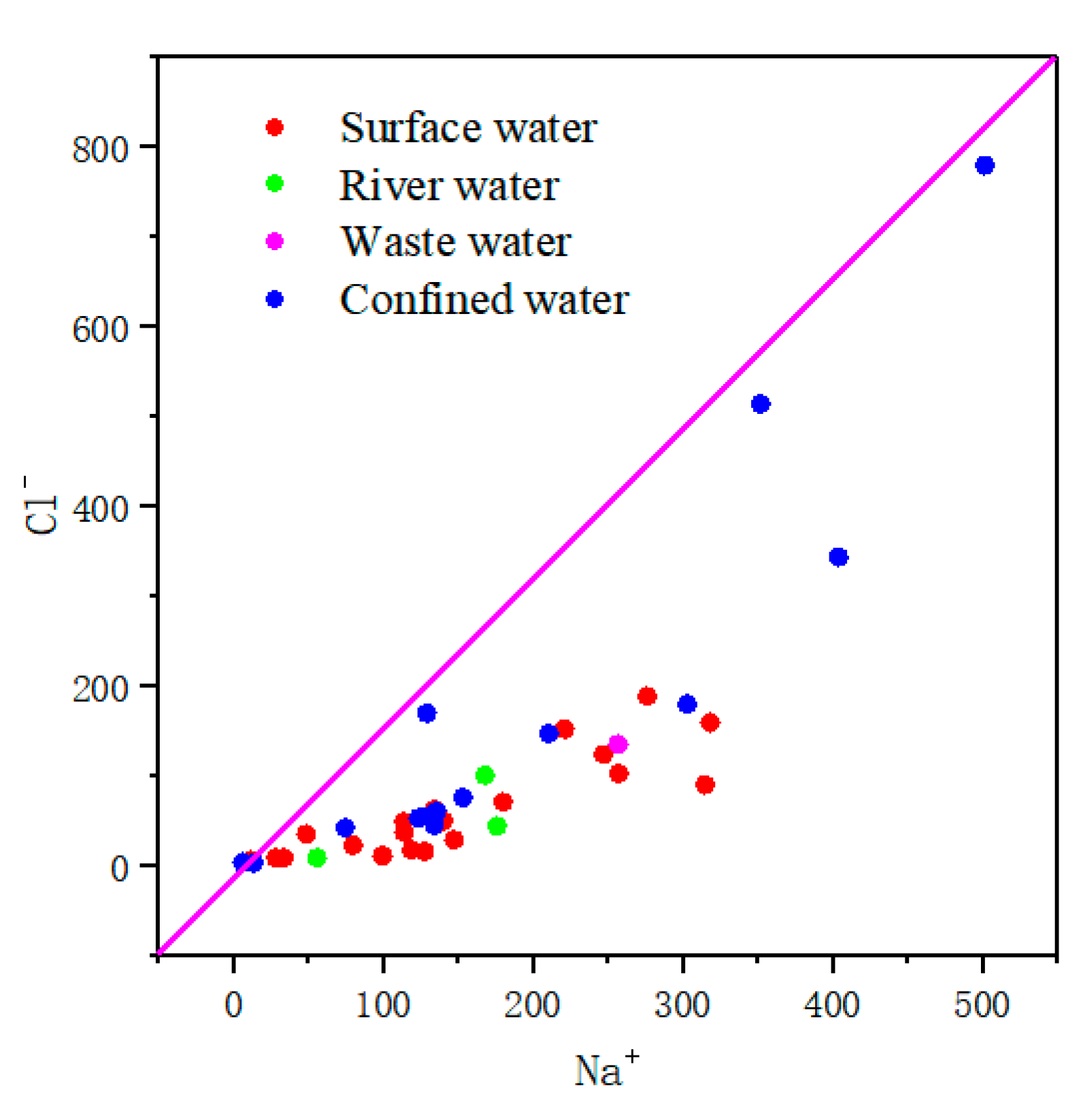
3.3.1. Rock Leaching and Evaporation Concentration
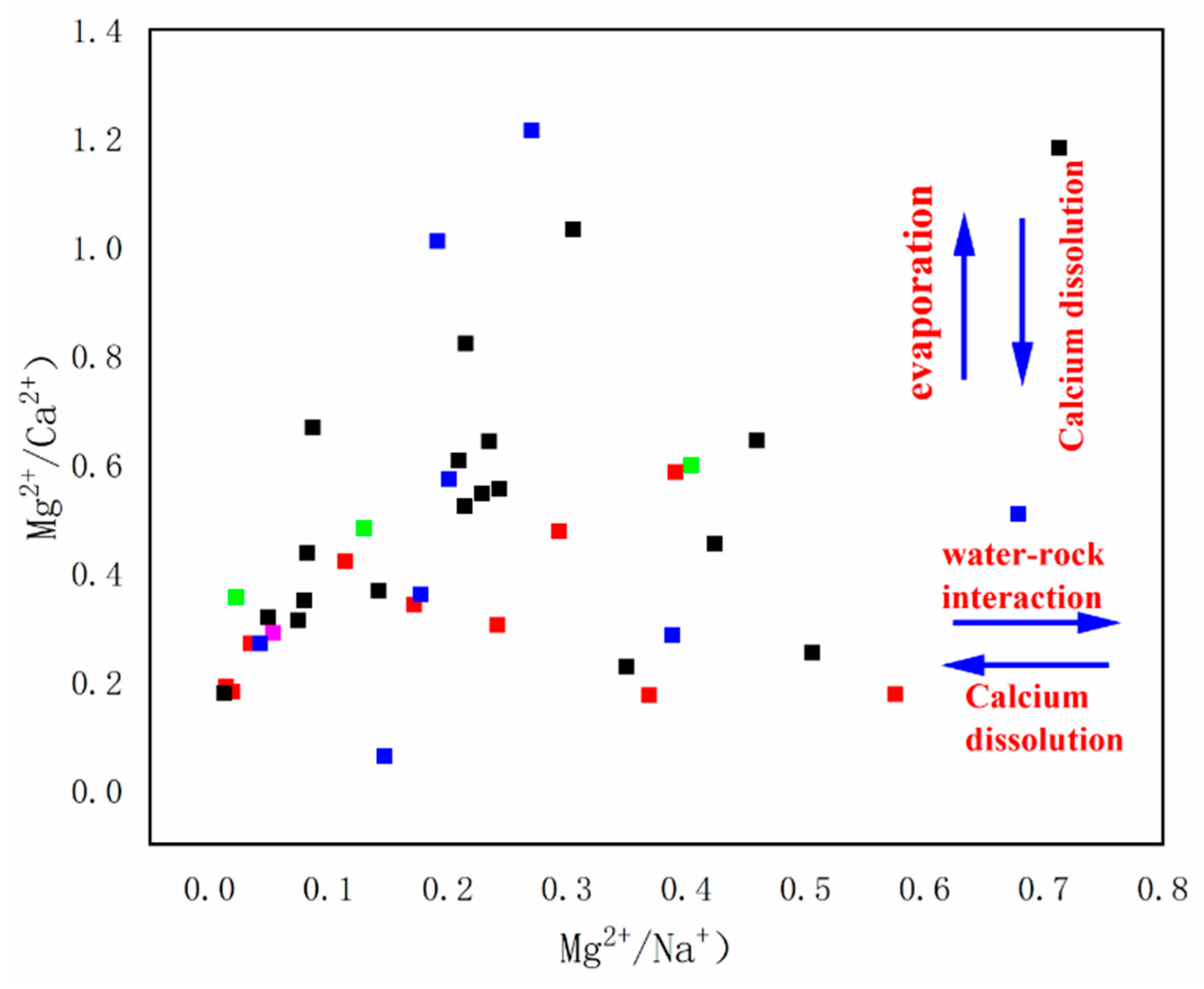
3.3.2. Ion Exchange
3.4. Grade Assessment of Groundwater Irrigation
4. Conclusions
- (a)
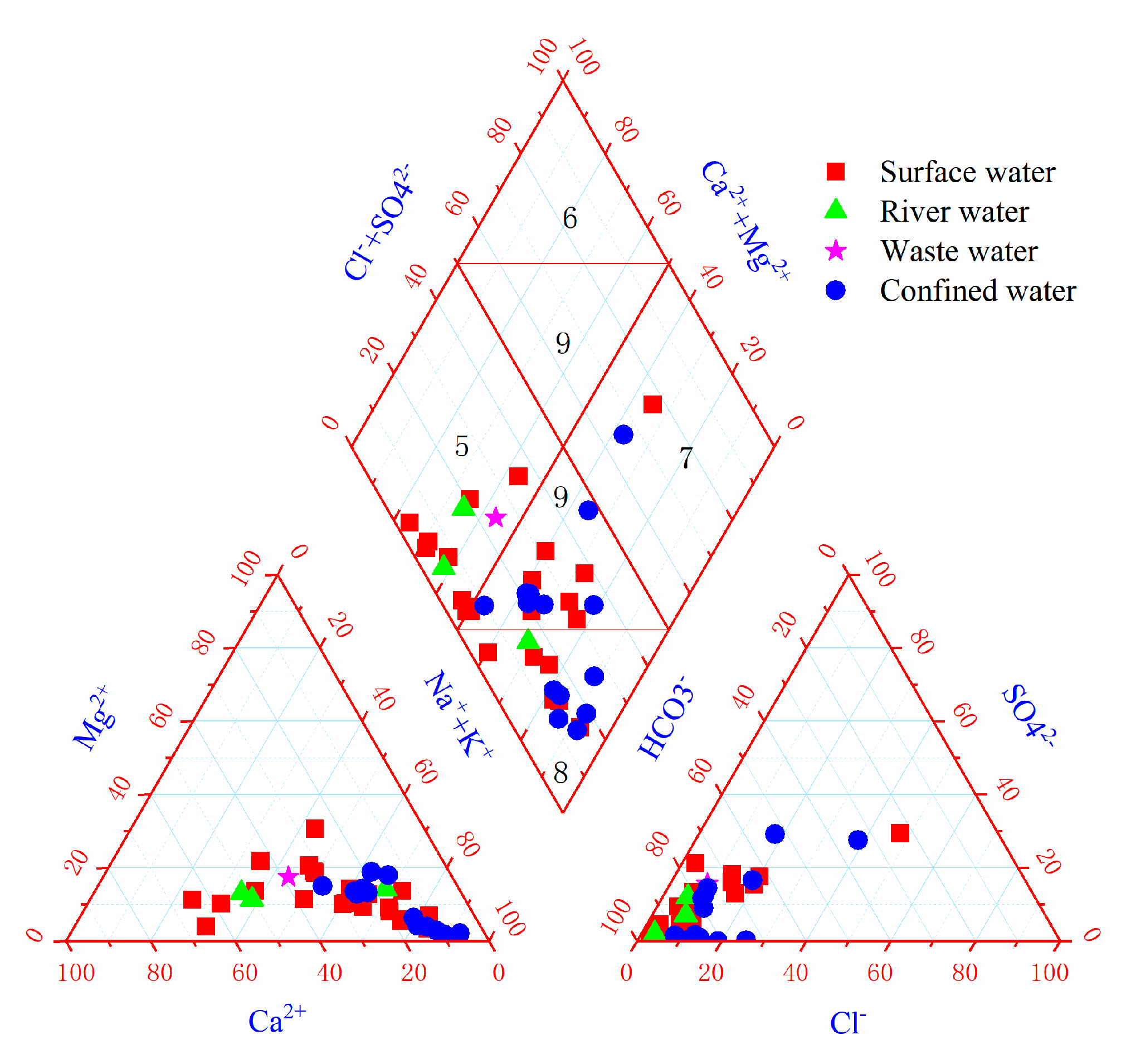
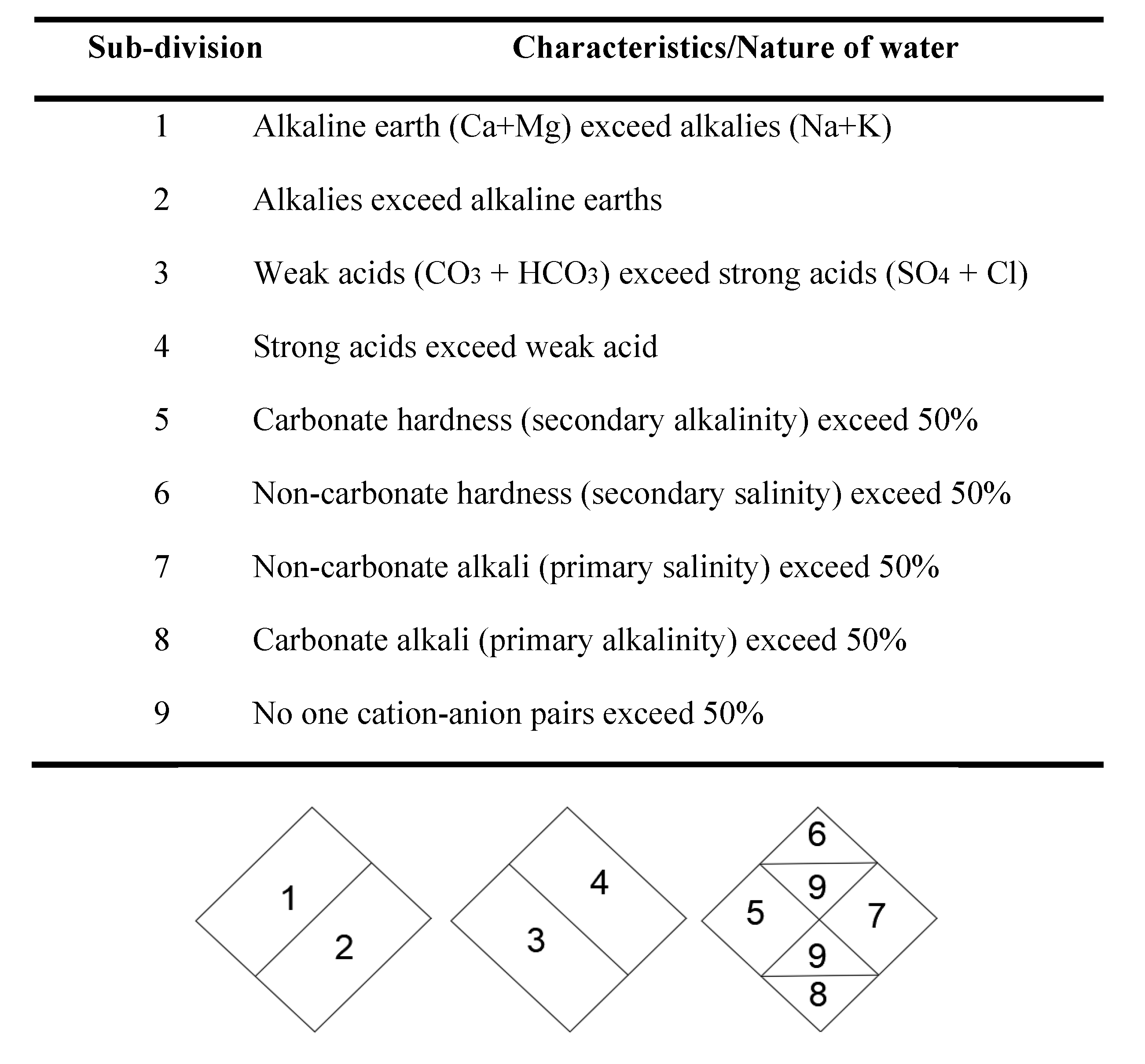
- The main chemical types of river water are HCO3−Na and HCO3−Ca·Na, the main chemical types of surface water are HCO3−Na and HCO3−Na·Ca, and the main chemical type of confined water is HCO3−Na. The salinity of water samples in the study area is generally low, showing bicarbonate;
- (b)
- Among the human factors affecting the change of hydrochemical characteristics, coal chemical plants and coal mining enterprises have limited influence on the change of groundwater chemical characteristics. The pollution of a large quantity of local cattle and sheep manure, industrial and domestic sewage and farmland fertilization are the main reasons for the increase of local underground NH4+ and NO2− mass concentrations, because the nitrate of groundwater is reduced to nitrite and ammonia under the action of anaerobic microorganisms;
- (c)
- The natural factors for the change of hydrochemical characteristics mainly include the internal influence of rocks and water, which is mainly manifested in the leaching of sodium and calcium salts in rocks, and the ion exchange between Na+ in aqueous media and Ca2+ and Mg2+ in groundwater;
- (d)
- The results show that 46% of the water samples can be directly used for irrigation and 16% cannot be used for irrigation. Different water samples with different hydrochemical characteristics can be classified, and different measures can be taken to improve the use and management of groundwater chemistry characteristics. Regulating agricultural activities and sewage discharge is the main way to improve the water chemistry characteristics in the study area, to strengthen the monitoring of the groundwater environment, and to increase the investment in water source treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Şehnaz, Ş.; Erhan, Ş.; Ayşen, D. Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Sci. Total Environ. 2017, 584–585, 131–144. [Google Scholar]
- Adimalla, N.; Li, P.; Venkatayogi, S. Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environ. Process. 2018, 5, 363–383. [Google Scholar] [CrossRef]
- Selemani, J.R.; Zhang, J.; Muzuka, A.N.; Njau, K.N.; Zhang, G.; Maggid, A.; Mzuza, M.K.; Jin, J.; Pradhan, S. Seasonal water chemistry variability in the Pangani River basin, Tanzania. Environ. Sci. Pollut. Res. 2017, 24, 26092–26110. [Google Scholar] [CrossRef] [PubMed]
- Varol, S.; Davraz, A. Evaluation of the groundwater quality with WQI (Water Quality Index) and multivariate analysis: A case study of the Tefenni plain (Burdur/Turkey). Environ. Earth Sci. 2015, 73, 1725–1744. [Google Scholar] [CrossRef]
- Edet, A.; Ukpong, A.; Nganje, T. Hydrochemical studies of Cross River Basin (southeastern Nigeria) river systems using cross plots, statistics and water quality index. Environ. Earth Sci. 2013, 70, 3043–3056. [Google Scholar] [CrossRef]
- Arumugam, K.; Elangovan, K. Hydrochemical characteristics and groundwater quality assessment in Tirupur region, Coimbatore district, Tamil Nadu, India. Environ. Geol. 2009, 58, 1509. [Google Scholar] [CrossRef]
- Chenini, I.; Khmiri, S. Evaluation of ground water quality using multiple linear regression and structural equation modeling. Int. J. Environ. Sci. Technol. 2009, 6, 509–519. [Google Scholar] [CrossRef]
- Galbraith, L.M.; Burns, C.W. Linking land-use, water body type and water quality in southern New Zealand. Landsc. Ecol. 2007, 22, 231–241. [Google Scholar] [CrossRef]
- Rao, Y.S.; Reddy, T.; Nayudu, P. Groundwater quality in the Niva river basin, Chittoor district, Andhra Pradesh, India. Environ. Geol. 1997, 32, 56–63. [Google Scholar]
- Fauriel, S.; Laloui, L. A bio-chemo-hydro-mechanical model for microbially induced calcite precipitation in soils. Comput. Geotech. 2012, 46, 104–120. [Google Scholar] [CrossRef]
- Choo, J.; Sun, W. Cracking and damage from crystallization in pores: Coupled chemo-hydro-mechanics and phase-field modeling, Comput. Methods Appl. Mech. Eng. 2018, 335, 347–379. [Google Scholar] [CrossRef]
- Ghorbani, J.; El-Zein, A.; Airey, D.W. Thermo-elasto-plastic analysis of geosynthetic clay liners exposed to thermal dehydration. Environ. Geotech. 2018, 8, 566–580. [Google Scholar] [CrossRef]
- Penga, S.; Fenga, F.; Dua, W.; Hea, Y.; Chonga, S.; Xinga, Z. Analysis of water chemical characteristics and application around large opencast coal mines in grassland: A case study of the North Power Shengli coal mine. Desalination Water Treat. 2019, 141, 149–162. [Google Scholar] [CrossRef]
- Tang, K.; Hou, J.; Tang, K. Assessment of groundwater quality in China: Ⅰ. Hydrochemical characteristics of groundwater in plain area. Water Resour. Prot. 2006, 22, 1–5. [Google Scholar]
- He, X.; Wu, J.; He, S. Hydrochemical characteristics and quality evaluation of groundwater in terms of health risks in Luohe aquifer in Wuqi County of the Chinese Loess Plateau, northwest China. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 32–51. [Google Scholar] [CrossRef]
- Cao, G.Y.; Yang, H.T.; Ren, Y.J. Hydrogeochemical characteristics and causes of groundwater in Chengjiaying Basin, Inner Mongolia. Geol. Chem. Miner. 2019, 41, 285–290. [Google Scholar]
- Xiao, M.; Han, Z.; Xu, S.; Wang, Z. Temporal Variations of Water Chemistry in the Wet Season in a Typical Urban Karst Groundwater System in Southwest China. Int. J. Environ. Res. Public Health 2020, 17, 2520. [Google Scholar] [CrossRef]
- Howladar, M.F.; Deb, P.K.; Muzemder, A.S.H.; Ahmed, M. Evaluation of water resources around Barapukuria coal mine industrial area, Dinajpur, Bangladesh. Appl. Water Sci. 2014, 4, 203–222. [Google Scholar] [CrossRef]
- JKim, G.; Ko, K.-S.; Kim, T.H.; Lee, G.H.; Song, Y.; Chon, C.-M.; Lee, J.-S. Effect of mining and geology on the chemistry of stream water and sediment in a small watershed. Geosci. J. 2007, 11, 175–183. [Google Scholar]
- Lin, D.; Qiang, W.; Zhang, R.; Song, Y.; Chen, S.; Pei, L.; Liu, S.; Bi, C.; Lv, Z.; Huang, S. Environmental characteristics of groundwater: An application of PCA to water chemistry analysis in Yulin. J. China Univ. Min. Technol. 2007, 17, 73–77. [Google Scholar]
- Zhang, B.; Zhao, D.; Zhou, P.; Qu, S.; Liao, F.; Wang, G. Hydrochemical Characteristics of Groundwater and Dominant Water–Rock Interactions in the Delingha Area, Qaidam Basin, Northwest China. Water 2020, 12, 836. [Google Scholar] [CrossRef]
- Cario, G.; Casavola, A.; Gjanci, P.; Lupia, M.; Petrioli, C.; Spaccini, D. Long lasting underwater wireless sensors network for water quality monitoring in fish farms. In Proceedings of the OCEANS 2017-Aberdeen, Aberdeen, UK, 19–22 June 2017; pp. 1–6. [Google Scholar]
- Mahmud, M.A.; Hussain, K.A.; Hassan, M.; Jewel, A.R.; Shamsad, S.Z. Water quality assessment using physiochemical parameters and heavy metal concentrations of circular rivers in and around Dhaka city, Bangladesh. Int. J. Water Res. 2017, 7, 23–29. [Google Scholar]
- Hou, H.; Zhou, J.; Liu, G.; Kong, J.; Enyao, M.; Luo, W. Study on the geographical origin identification of american ginseng based on multi-element analysis and statistical methods. Hubei Agric. Sci. 2020, 59, 151–154. [Google Scholar]
- Arkoc, O.; Ucar, S.; Ozcan, C. Assessment of impact of coal mining on ground and surface waters in Tozaklı coal field, Kırklareli, northeast of Thrace, Turkey. Environ. Earth Sci. 2016, 75, 514. [Google Scholar] [CrossRef]
- Zhang, H.; Su, L.; Wang, J.; Yang, L.; Wang, D.; Hu, X.; Xiong, L. Study on LA-ICP-MS Determination of Trace Elements in Sulfide Minerals. Hans J. Chem. Eng. Technol. 2019, 9, 401–409. [Google Scholar] [CrossRef]
- Jiang, Q.; Han, Y.; Sun, X.; Gong, H.; Qian, W.; Guoxing, L.U. Study on the Determination and Its Difference Analysis of Chloride and Sulfate in Different Soils by Ion Chromatography and Capillary Electrophoresis. Soils 2016, 48, 343–348. [Google Scholar]
- Durowoju, O.S.; Ekosse GI, E.; Odiyo, J.O. Occurrence and Health-Risk Assessment of Trace Metals in Geothermal Springs within Soutpansberg, Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2020, 17, 4438. [Google Scholar] [CrossRef]
- Lai, Z.; Lin, F.; Qiu, L.; Wang, Y.; Chen, X.; Hu, H. Development of a sequential injection analysis device and its application for the determination of Mn(II) in water. Talanta 2020, 211, 120752. [Google Scholar] [CrossRef]
- General Administration of Quality Supervision I.a.Q.; China S.A.o. Standard for Groundwater Quality; China Environmental Science Press: Beijing, China, 2017; p. 20. [Google Scholar]
- Choi, H.; Poythress, J.C.; Park, C.; Jeon, J.J.; Park, C. Regularized boxplot via convex clustering. J. Stat. Comput. Simul. 2019, 89, 1227–1247. [Google Scholar] [CrossRef]
- Khare, P. A large-scale investigation of the quality of groundwater in six major districts of Central India during the 2010–2011 sampling campaign. Environ. Monit. Assess. 2017, 189, 429. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Tewari, G.; Kumar, S. Evaluation of Groundwater Quality for Suitability of Irrigation Purposes: A Case Study in the Udham Singh Nagar, Uttarakhand. J. Chem. 2020, 2020, 6924026. [Google Scholar] [CrossRef]
- Kumar, P.S.; Balamurugan, P. Evaluation of groundwater quality for irrigation purpose in Attur taluk, Salem, Tamilnadu, India. Water Energy Int. 2018, 61, 59–64. [Google Scholar]
- Haile, E.; Fryar, A.E. Chemical evolution of groundwater in the Wilcox aquifer of the northern Gulf Coastal Plain, USA. Hydrogeol. J. 2017, 25, 2403–2418. [Google Scholar] [CrossRef]
- Davis, A.; Heatwole, K.; Greer, B.; Ditmars, R.; Clarke, R. Discriminating between background and mine-impacted groundwater at the Phoenix mine, Nevada USA. Appl. Geochem. 2010, 25, 400–417. [Google Scholar] [CrossRef]
- Sefati, Z.; Khalilimoghadam, B.; Nadian, H. Assessing urban soil quality by improving the method for soil environmental quality evaluation in a saline groundwater area of Iran. Catena 2019, 173, 471–480. [Google Scholar] [CrossRef]
- Shakerkhatibi, M.; Mosaferi, M.; Pourakbar, M.; Ahmadnejad, M.; Safavi, N.; Banitorab, F. Comprehensive investigation of groundwater quality in the north-west of Iran: Physicochemical and heavy metal analysis. Groundw. Sustain. Dev. 2019, 8, 156–168. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, W.; Liu, H. Soil moisture characteristic curve and prediction of available water content of overburden in Xilinhot Mining Area. Coal Sci. Technol. 2020, 48, 169–177. [Google Scholar]
- Liu, H.; Huang, H.; Shuai, B.; Feng, Y. Study on Stability of East Side Slope of Shengli East No. 2 Mine Based on Geo-Studio Numerical Software. Adv. Geosci. 2020, 10, 622–628. [Google Scholar] [CrossRef]
- Alhamed, M. The hydrological and the hydrogeological framework of the Lottenbachtal, Bochum, Germany. Appl. Water Sci. 2017, 7, 315–328. [Google Scholar] [CrossRef][Green Version]
- Arslan, B.; Akün, E. Management, contamination and quality evaluation of groundwater in North Cyprus. Agric. Water Manag. 2019, 222, 1–11. [Google Scholar] [CrossRef]
- Benmoussa, Y.; Remini, B.; Remaoun, M. Quality assessment and hydrogeochemical characteristics of groundwater in Kerzaz and Beni Abbes along Saoura valley, southwest of Algeria. Appl. Water Sci. 2020, 10, 170. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jin, D.; Wang, T.; Gao, M.; Yang, J.; Wang, Q. Hydrogeochemical processes and quality assessment of shallow groundwater in Chenqi coalfield, Inner Mongolia, China. Environ. Earth Sci. 2019, 78, 347. [Google Scholar] [CrossRef]
- Mahato, M.K.; Singh, P.K.; Tiwari, A.K. Hydrogeochemical evaluation of groundwater quality and seasonal variation in East Bokaro coalfield region, Jharkhand. J. Geol. Soc. India 2016, 88, 173–184. [Google Scholar] [CrossRef]
- Mahato, M.K.; Singh, P.K.; Singh, A.K.; Tiwari, A.K. Assessment of hydrogeochemical processes and mine water suitability for domestic, irrigation, and industrial purposes in East Bokaro Coalfield, India. Mine Water Environ. 2018, 37, 493–504. [Google Scholar] [CrossRef]
- Wilcox, L. Classification and Use of Irrigation Waters; US Department of Agriculture: Washington, DC, USA, 1955. [Google Scholar]
- Handa, B. Studies on US Salinity Laboratory Diagram for Classification of Irrigation Waters. J. Indian Soc. Soil Sci. 1965, 13, 227–232. [Google Scholar]




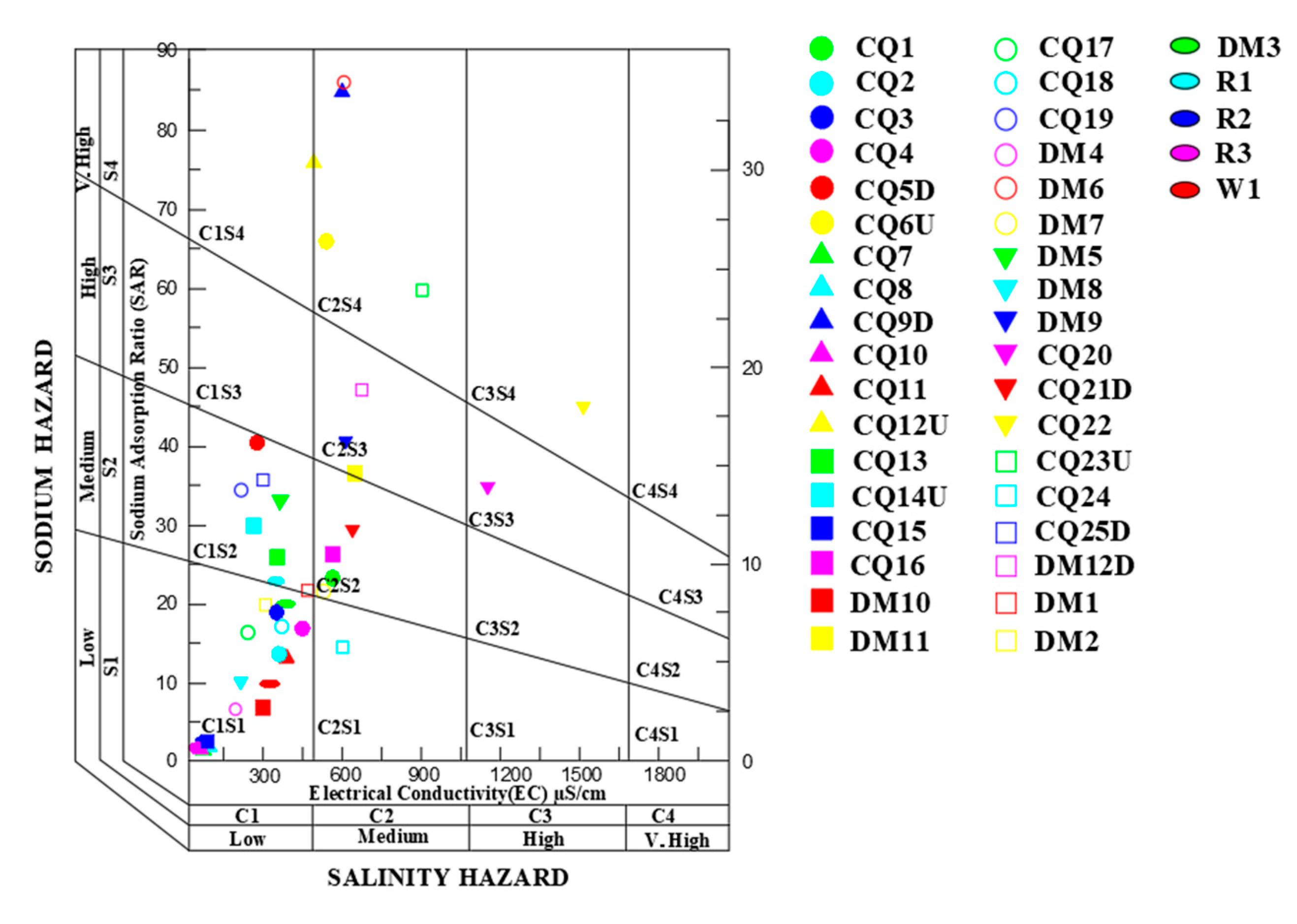
| Water Sample Number | SAR | EC | %Na |
|---|---|---|---|
| CQ1 | 33.79 | 579.77 | 0.72 |
| CQ2 | 13.71 | 390.92 | 0.50 |
| CQ3 | 0.72 | 386.41 | 0.60 |
| CQ4 | 17.16 | 481.59 | 0.53 |
| CQ5D | 41.38 | 290.47 | 0.86 |
| CQ6U | 65.92 | 544.35 | 0.89 |
| CQ7 | 2.22 | 98.26 | 0.24 |
| CQ8 | 7.37 | 120.75 | 0.51 |
| CQ9D | 84.77 | 598.27 | 0.92 |
| CQ10 | 3.17 | 94.34 | 0.32 |
| CQ11 | 13.56 | 395.03 | 0.49 |
| CQ12U | 76.97 | 488.41 | 0.92 |
| CQ13 | 27.13 | 381.29 | 0.72 |
| CQ14U | 30.87 | 258.38 | 0.81 |
| CQ15 | 3.02 | 85.78 | 0.31 |
| CQ16 | 26.98 | 508.52 | 0.67 |
| DM10 | 6.93 | 302.34 | 0.35 |
| DM11 | 36.00 | 630.75 | 0.73 |
| CQ17 | 16.99 | 239.05 | 0.65 |
| CQ18 | 18.81 | 388.72 | 0.61 |
| CQ19 | 33.62 | 250.13 | 0.84 |
| DM4 | 6.18 | 181.97 | 0.38 |
| DM6 | 86.46 | 590.15 | 0.92 |
| DM7 | 22.17 | 550.70 | 0.60 |
| DM5 | 33.45 | 417.29 | 0.76 |
| DM8 | 9.73 | 237.54 | 0.47 |
| DM9 | 40.83 | 609.40 | 0.77 |
| CQ20 | 34.20 | 1150.38 | 0.63 |
| CQ21D | 27.55 | 615.94 | 0.65 |
| CQ22 | 43.49 | 1523.43 | 0.66 |
| CQ23U | 61.02 | 935.52 | 0.82 |
| CQ24 | 13.99 | 605.84 | 0.44 |
| CQ25D | 36.38 | 294.98 | 0.83 |
| DM12D | 47.26 | 715.57 | 0.79 |
| DM1 | 22.58 | 461.30 | 0.63 |
| DM2 | 19.50 | 384.87 | 0.61 |
| DM3 | 20.06 | 391.51 | 0.62 |
| R1 | 23.69 | 372.37 | 0.68 |
| R2 | 3.73 | 79.93 | 0.38 |
| R3 | 2.19 | 50.36 | 0.35 |
| W1 | 10.47 | 357.94 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.; Feng, F.; Yang, K.; Zhou, Y.; Zhang, J.; Sun, J. Water Chemical Characteristics and Safety Assessment of Irrigation Water in the Northern Part of Hulunbeier City, Grassland Area in Eastern China. Sustainability 2022, 14, 16068. https://doi.org/10.3390/su142316068
Su W, Feng F, Yang K, Zhou Y, Zhang J, Sun J. Water Chemical Characteristics and Safety Assessment of Irrigation Water in the Northern Part of Hulunbeier City, Grassland Area in Eastern China. Sustainability. 2022; 14(23):16068. https://doi.org/10.3390/su142316068
Chicago/Turabian StyleSu, Wanli, Feisheng Feng, Ke Yang, Yong Zhou, Jiqiang Zhang, and Jie Sun. 2022. "Water Chemical Characteristics and Safety Assessment of Irrigation Water in the Northern Part of Hulunbeier City, Grassland Area in Eastern China" Sustainability 14, no. 23: 16068. https://doi.org/10.3390/su142316068
APA StyleSu, W., Feng, F., Yang, K., Zhou, Y., Zhang, J., & Sun, J. (2022). Water Chemical Characteristics and Safety Assessment of Irrigation Water in the Northern Part of Hulunbeier City, Grassland Area in Eastern China. Sustainability, 14(23), 16068. https://doi.org/10.3390/su142316068





