Acidified Cow Dung-Assisted Phytoextraction of Heavy Metals by Ryegrass from Contaminated Soil as an Eco-Efficient Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Acidulated Cow Dung Slurry
2.2. Pot Trial
2.3. Plant Analyses
2.3.1. Digestion
2.3.2. Pb and Cd Determination
2.3.3. Chlorophyll Contents
2.3.4. Photosynthesis System
2.3.5. Antioxidant Determination in Leaves
Ascorbate Peroxidase (APX)
Catalase (CAT) Assay
Superoxide Dismutase (SOD) Assay
Lipid Peroxidation
Peroxidase (POD)
Determination of H2O2 Contents
2.3.6. Statistical Analyses
3. Results
3.1. Growth Attributes
3.2. Physiological Attributes
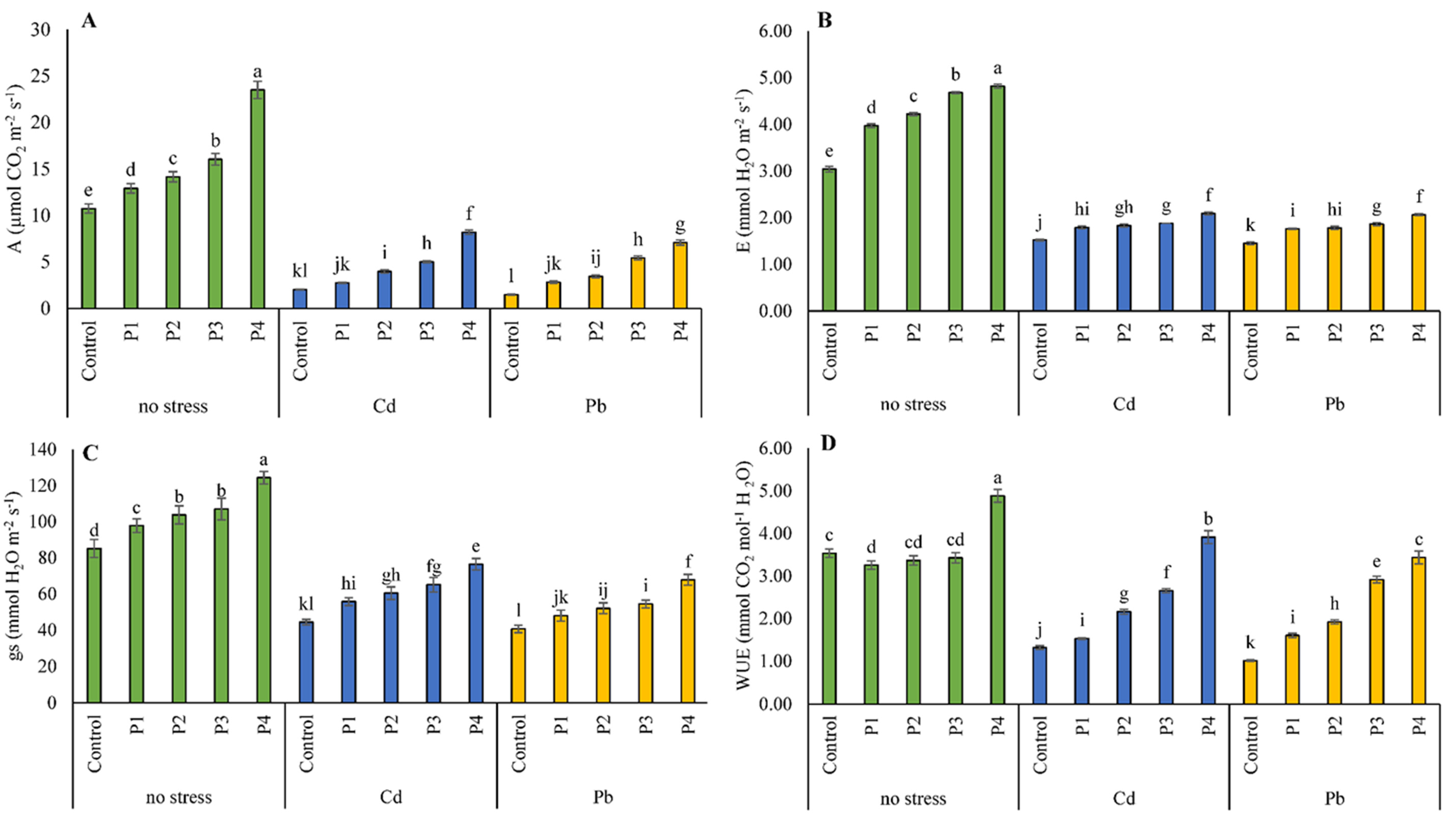
3.3. Gas Exchange Attributes
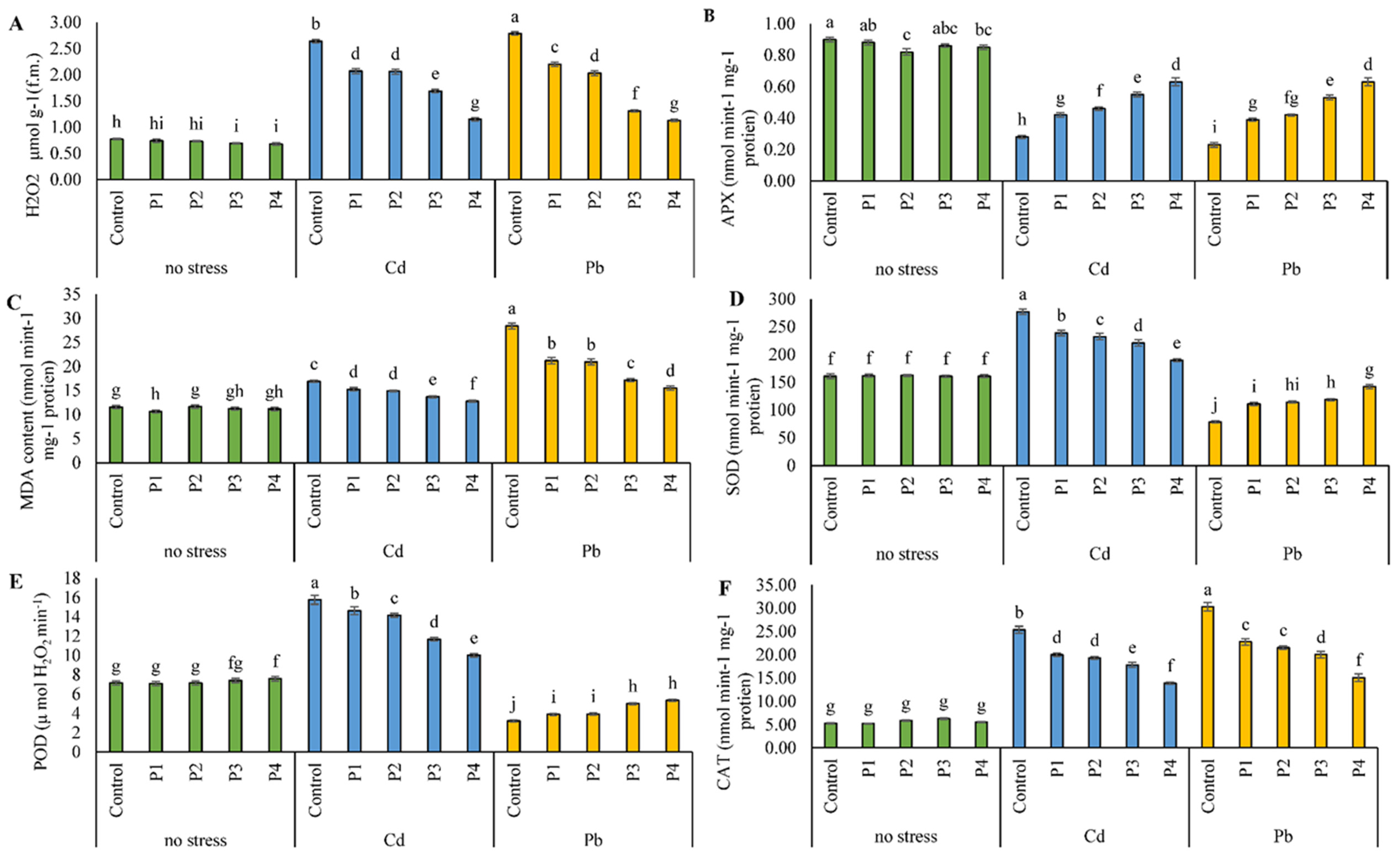
3.4. Antioxidant Activities
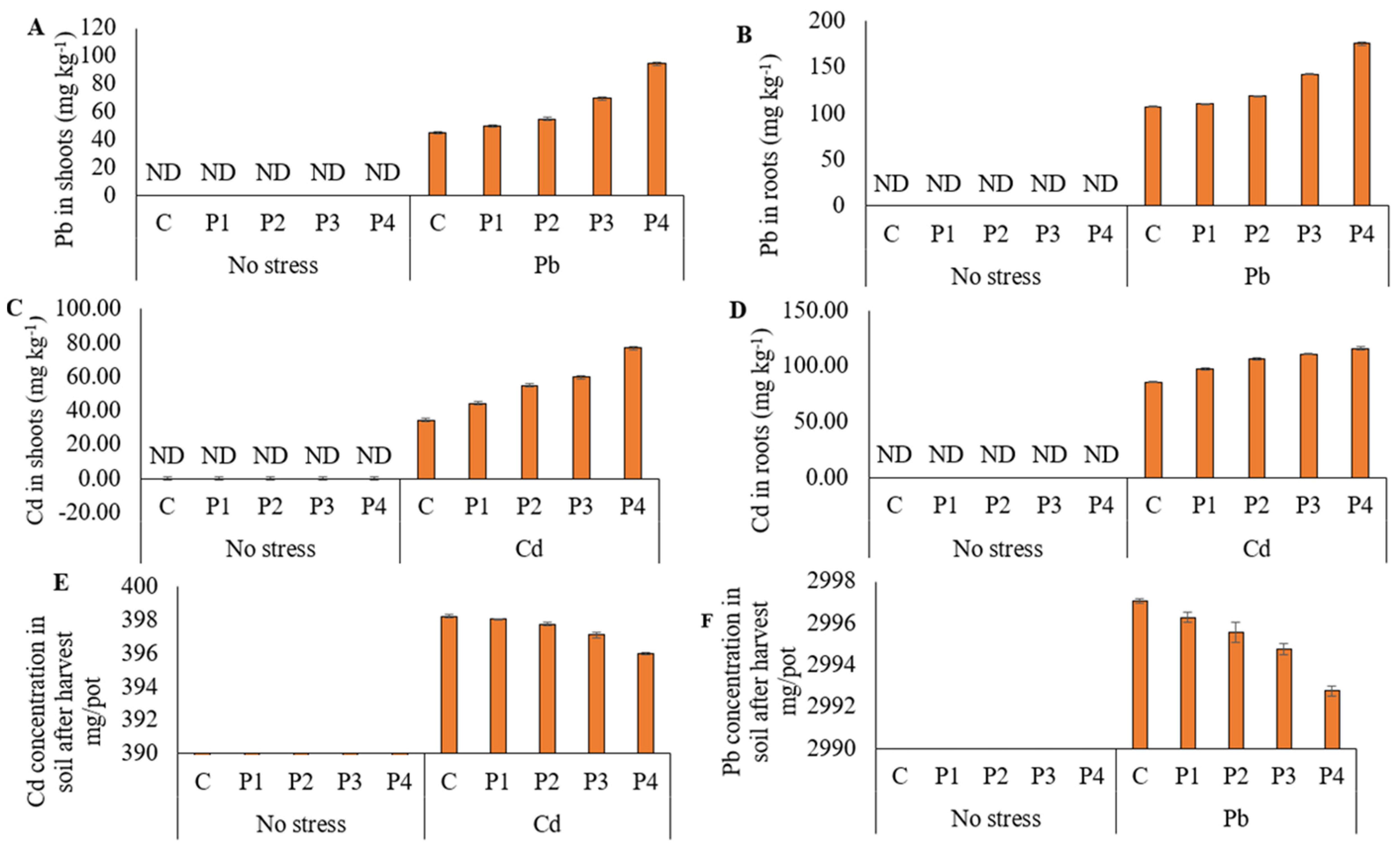
3.5. Heavy Metal (Pb and Cd) Concentration in Plant Parts and Soil after Harvest of Ryegrass
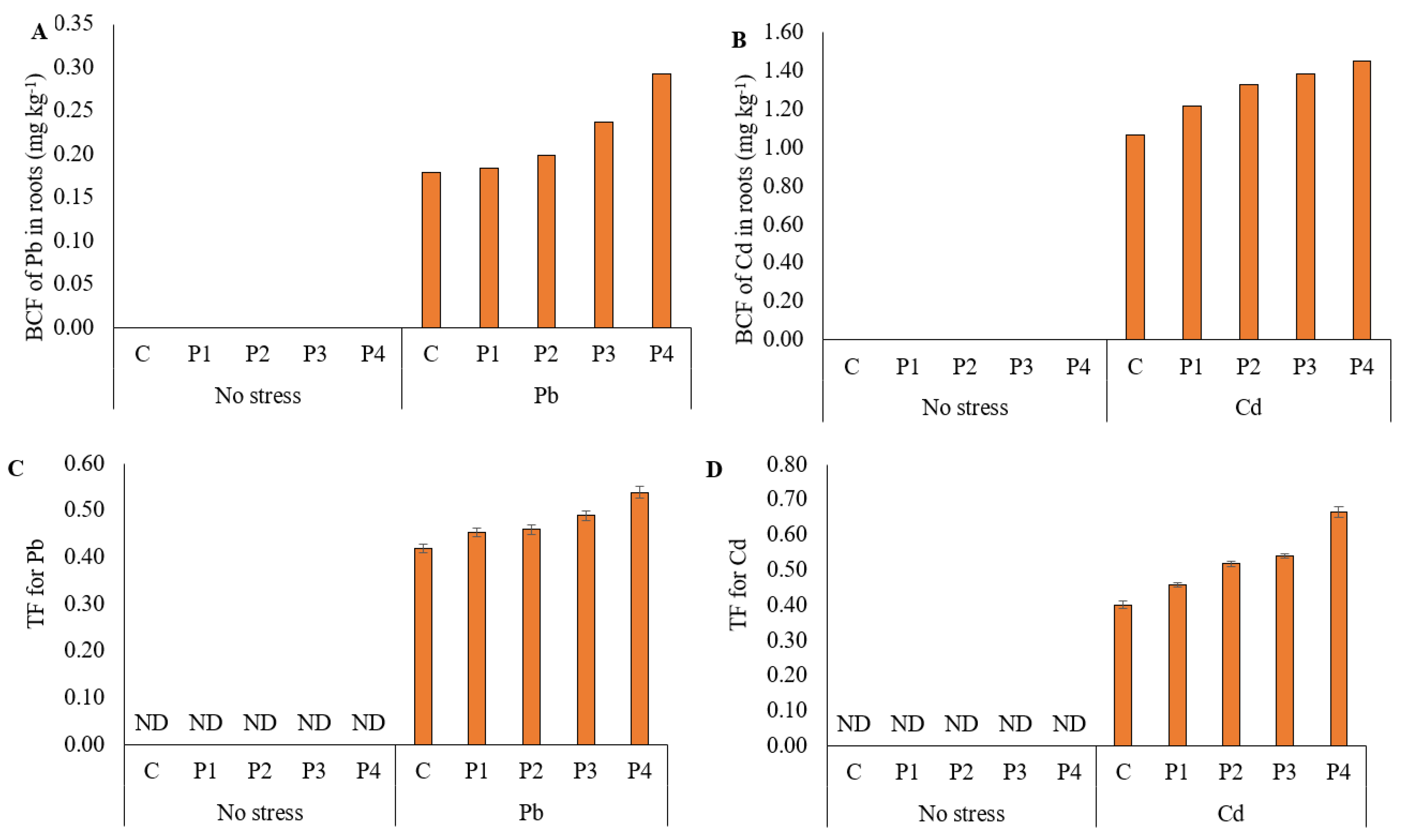
3.6. Bioconcentration and Translocation Factors of Ryegrass for Lead and Cadmium
4. Discussion
5. Conclusions
- a.
- Phytoextraction is the most efficient ecofriendly soil reclamation technique and application of acidulated cow dung slurry enhanced the efficiency of this technique by increasing the availability of Pb and Cd to ryegrass.
- b.
- Application of cow dung slurry not only enhanced the availability of Pb and Cd, but also promoted the growth of ryegrass.
- c.
- Acidulated cow dung slurry also improved the antioxidative defense mechanism of ryegrass.
- d.
- For future research work, phytoextraction of heavy metals may be done at the field level with the application of acidulated cow dung slurry by growing the same or some other plant species for environmental sustainability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brodin, M.; Vallejos, M.; Opedal, M.T.; Area, M.C.; Chinga-Carrasco, G. Lignocellulosics as sustainable resources for production of bioplastics–A review. J. Clean. Prod. 2017, 162, 646–664. [Google Scholar] [CrossRef]
- Ferrey, M.L.; Hamilton, M.C.; Backe, W.J.; Anderson, K.E. Pharmaceuticals and other anthropogenic chemicals in atmospheric particulates and precipitation. Sci. Total Environ. 2018, 612, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Shabaan, M.; Asghar, H.N.; Akhtar, M.J.; Ali, Q.; Ejaz, M. Role of plant growth promoting rhizobacteria in the alleviation of lead toxicity to Pisum sativum L. Int. J. Phytorem. 2021, 23, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and environmental effects of heavy metals. J. King Saud. Univ.-Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Kumar, V.; Thakur, R.K.; Kumar, P. Assessment of heavy metals uptake by cauliflower (Brassica oleracea var. botrytis) grown in integrated industrial effluent irrigated soils: A prediction modeling study. Sci. Hortic. 2019, 257, 108682. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs)(arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monitor. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Tian, S.; Jingsong, L.; Xuewu, L.; Chuanwei, Z.; Hejie, Y. Improving the Corrosion Resistance of Aluminum Alloy by Creating a Superhydrophobic Surface Structure through a Two-Step Process of Etching Followed by Polymer Modification. Polymers 2022, 14, 4509. [Google Scholar]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Akram, R.; Fahad, S.; Masood, N.; Rasool, A.; Ijaz, M.; Ihsan, M.Z.; Maqbool, M.M.; Ahmad, S.; Hussain, S.; Ahmed, M.; et al. Plant growth and morphological changes in rice under abiotic stress. In Advances in Rice Research for Abiotic Stress Tolerance; Woodhead Publishing: Sawston, UK, 2019; pp. 69–85. [Google Scholar]
- Bali, S.; Jamwal, V.L.; Kohli, S.K.; Kaur, P.; Tejpal, R.; Bhalla, V.; Ohri, P.; Gandhi, S.G.; Bhardwaj, R.; Al-Huqail, A.A.; et al. Jasmonic acid application triggers detoxification of lead (Pb) toxicity in tomato through the modifications of secondary metabolites and gene expression. Chemosphere 2019, 235, 734–748. [Google Scholar] [CrossRef]
- Jan, S.; Parray, J.A. Heavy metal stress signalling in plants. In Approaches to Heavy Metal Tolerance in Plants; Springer: Singapore, 2016; pp. 33–55. [Google Scholar]
- Ekoa Bessa, A.Z.; Ngueutchoua, G.; Kwewouo Janpou, A.; El-Amier, Y.A.; Njike Njome Mbella Nguetnga, O.A.; Kankeu Kayou, U.R.; Bisse, S.B.; Ngo Mapuna, E.C.; Armstrong-Altrin, J.S. Heavy metal contamination and its ecological risks in the beach sediments along the Atlantic Ocean (Limbe coastal fringes, Cameroon). Earth Syst. Environ. 2021, 5, 433–444. [Google Scholar] [CrossRef]
- Pawar, R.R.; Kim, M.; Kim, J.G.; Hong, S.M.; Sawant, S.Y.; Lee, S.M. Efficient removal of hazardous lead, cadmium, and arsenic from aqueous environment by iron oxide modified clay-activated carbon composite beads. Appl. Clay Sci. 2018, 162, 339–350. [Google Scholar] [CrossRef]
- Gottesfeld, P.; Were, F.H.; Adogame, L.; Gharbi, S.; San, D.; Nota, M.M.; Kuepouo, G. Soil contamination from lead battery manufacturing and recycling in seven African countries. Environ. Res. 2018, 161, 609–614. [Google Scholar] [CrossRef]
- Liu, S.; Li, X. Colorimetric detection of copper ions using gold nanorods in aquatic environment. Mater. Sci. Eng. B-Solid 2019, 240, 49–54. [Google Scholar] [CrossRef]
- Liu, C.; Ye, J.; Lin, Y.; Wu, J.; Price, G.W.; Burton, D.; Wang, Y. Removal of Cadmium (II) using water hyacinth (Eichhornia crassipes) biochar alginate beads in aqueous solutions. Environ. Pollut. 2020, 264, 114785. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Y.; Fu, J.; Yuan, L.; Li, Z.; Liu, C.; Zhao, D.; Wang, X. A novel magnetic biochar/Mg Fe-layered double hydroxides composite removing Pb2+ from aqueous solution: Isotherms, kinetics and thermodynamics. Colloid Surf. 2019, 567, 278–287. [Google Scholar] [CrossRef]
- Liu, T.; Lawluvy, Y.; Shi, Y.; Ighalo, J.O.; He, Y.; Zhang, Y.; Yap, P.S. Adsorption of cadmium and lead from aqueous solution using modified biochar: A review. J. Environ. Chem. Eng. 2022, 10, 106502. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Ahmad, S.; Ullah, F.; Sadiq, A.; Ayaz, M.; Imran, M.; Ali, I.; Zeb, A.; Ullah, F.; Shah, M.R. Chemical composition, antioxidant and anticholinesterase potentials of essential oil of Rumex hastatus D. Don collected from the North West of Pakistan. BMC Complement. Altern. Med. 2016, 16, 29. [Google Scholar] [CrossRef]
- Li, X.; Lan, X.; Liu, W.; Cui, X.; Cui, Z. Toxicity, migration and transformation characteristics of lead in soil-plant system: Effect of lead species. J. Hazard. Mater. 2020, 395, 122676. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Choudhary, S.; Laskar, R.A.; Naaz, N.; Sharma, N. Comparative study of cadmium nitrate and lead nitrate [Cd (NO3)2 and Pb (NO3)2] stress in cyto-physiological parameters of Capsicum annuum L. Hortic. Environ. Biotechnol. 2022, 25, 627–641. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, D.; Wang, Q. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Res. 2018, 147, 440–460. [Google Scholar] [CrossRef] [PubMed]
- Guérin, T.; Ghinet, A.; Waterlot, C. The phytoextraction power of Cichorium intybus L. on metal-contaminated soil: Focus on time-and cultivar-depending accumulation and distribution of cadmium, lead and zinc. Chemosphere 2022, 287, 132122. [Google Scholar] [CrossRef]
- Alzahrani, A.Y. Enhancing the Phytoextraction Capacity of Ornamental Plants by Organic Fertilizer: Review. Sch. Acad. J. Biosci. 2022, 6, 122–125. [Google Scholar] [CrossRef]
- Karalija, E.; Selović, A.; Bešta-Gajević, R.; Šamec, D. Thinking for the future: Phytoextraction of cadmium using primed plants for sustainable soil clean-up. Physiol. Plant 2022, 174, e13739. [Google Scholar] [CrossRef]
- Pandey, B.; Singh, S.; Roy, L.B.; Shekhar, S.; Singh, R.K.; Prasad, B.; Singh, K.K. Phytostabilization of coal mine overburden waste, exploiting the phytoremedial efficacy of lemongrass under varying level of cow dung manure. Ecotoxicol. Environ. Safe 2021, 208, 111757. [Google Scholar] [CrossRef]
- Turgut, C.; Pepe, M.K.; Cutright, T.J. The effect of EDTA and citric acid on phytoremediation of Cd, Cr, and Ni from soil using Helianthus annuus. Environ. Pollut. 2004, 131, 147–154. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef]
- Wei, Q.; Li, Y.; Zu, Y.Q. Advances in researching cultivation techniques on hyperaccumulators phytore-mediating the heavy metal contaminated soil. J. Yunnan Agric. Univ. 2008, 23, 104–108. [Google Scholar]
- Kaplan, M.; Orman, S.; Kadar, I.; Koncz, J. Heavy metal accumulation in calcareous soil and sorghum plants after addition of sulphur-containing waste as a soil amendment in Turkey. Agric. Ecosyst. Environ. 2005, 111, 41–46. [Google Scholar] [CrossRef]
- Kayser, A.; Wenger, K.; Keller, A.; Attinger, W.; Felix, H.R.; Gupta, S.K. Enhancement of phytoextraction of Zn, Cd, and Cu from calcareous soil: The use of NTA and sulphur amendments. Environ. Sci. Technol. 2000, 34, 1778–1783. [Google Scholar] [CrossRef]
- Kusale, S.P.; Attar, Y.C.; Sayyed, R.Z.; Malek, R.A.; Ilyas, N.; Suriani, N.L.; Khan, N.; El Enshasy, H.A. Production of plant beneficial and antioxidants metabolites by Klebsiella variicola under salinity stress. Molecule 2021, 6, 1894. [Google Scholar] [CrossRef]
- Malik, K.M.; Khan, K.S.; Billah, M.; Akhtar, M.S.; Rukh, S.; Alam, S.; Munir, A.; Mahmood Aulakh, A.; Rahim, M.; Qaisrani, M.M.; et al. Organic Amendments and Elemental Sulfur Stimulate Microbial Biomass and Sulfur Oxidation in Alkaline Subtropical Soils. Agronomy 2021, 11, 2514. [Google Scholar] [CrossRef]
- Khan, A.U.; Ullah, F.; Khan, N.; Mehmood, S.; Fahad, S.; Datta, R.; Irshad, I.; Danish, S.; Saud, S.; Alaraidh, I.A.; et al. Production of organic fertilizers from rocket seed (Eruca sativa L.), chicken peat and Moringa oleifera leaves for growing linseed under water deficit stress. Sustainability 2021, 13, 59. [Google Scholar] [CrossRef]
- Ikeura, H.; Ozawa, S.; Tamaki, M. Varietal differences in Zinnia hybrida for remediation in oil-contaminated soil. Ecol. Safety 2019, 10, 265–272. [Google Scholar]
- Ashraf, S.; Zahir, Z.A.; Asghar, H.N.; Asghar, M. Isolation, screening and identification of lead and cadmium resistant sulfur oxidizing bacteria. Pak. J. Agric. Sci. 2018, 55, 349–359. [Google Scholar]
- Ryan, J.; Estefan, G.; Rashid, A. Soil and Plant Analysis Laboratory Manual; ICARDA: Beirut, Lebanon, 2001. [Google Scholar]
- Wolf, B.A. Comprehensive system of leaf analysis and its use for diagnosing crop nutrient status. Commun. Soil Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Ben-Asher, J.; Tsuyuki, I.; Bravdo, B.; Sagih, M. Irrigation of grapevines with saline water: I. leaf area index, stomatal conductance, transpiration and photosynthesis. Agric. Water Manag. 2006, 83, 13–21. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.F.J.; Gilbert, H.S. Pyrogallol assay for SOD: Absence of a glutathione artifact. Anal. Biochem. 1984, 137, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. In Plant Stress Tolerance; Methods in Molecular Biology; Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; Volume 639, pp. 291–297. [Google Scholar]
- Nicell, J.A.; Wright, H. A model of peroxidase activity with inhibition by hydrogen peroxide. Enzym. Microb. Technol. 1997, 21, 302–310. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics. A Biometrical Approach, 3rd ed.; McGraw Hill Book Co., Inc.: New York, NY, USA, 1997. [Google Scholar]
- Tanwir, K.; Javed, M.T.; Abbas, S.; Shahid, M.; Akram, M.S.; Chaudhary, H.J.; Iqbal, M. Serratia sp. CP-13 alleviates Cd toxicity by morpho-physio-biochemical improvements, antioxidative potential and diminished Cd uptake in Zea mays L. cultivars differing in Cd tolerance. Ecotoxicol. Environ. Safe 2021, 208, 111584. [Google Scholar] [CrossRef]
- Chi, Y.; You, Y.; Wang, J.; Chen, X.; Chu, S.; Wang, R.; Zhang, X.; Yin, S.; Zhang, D.; Zhou, P. Two plant growth-promoting bacterial Bacillus strains possess different mechanisms in affecting cadmium uptake and detoxification of Solanum nigrum L. Chemosphere 2022, 305, 135488. [Google Scholar] [CrossRef]
- Jacob, J.N.; Okuo, J.M.; Archibong, U.D.; Imafidon, M.I.; Emeribe, O.F.; Anegbe, B. Comparative Studies on The Effects of Biochar and Cow Dung Amendments on the Mobility, Bioavailability and Toxicity of Heavy Metals in Lead-Acid Contaminated Soil. Dutse J. Pure Appl. Sci. 2022, 8, 171–186. [Google Scholar] [CrossRef]
- Baghaie, A.H. Effect of EDTA chelate, cow manure, elemental sulfur application and Thiobacillus inoculant on Cd, Zn and Fe phytoremediation efficiency in a Cd-polluted soil. Iran J. Health Sci. 2021, 9, 35–45. [Google Scholar] [CrossRef]
- Besharati, H. Effects of sulfur application and Thiobacillus inoculation on soil nutrient availability, wheat yield and plant nutrient concentration in calcareous soils with different calcium carbonate content. J. Plant Nutr. 2017, 40, 447–456. [Google Scholar] [CrossRef]
- Sangwan, P.; Kumar, V.; Khatri, R.S.; Joshi, U.N. Chromium (VI) induced biochemical changes and gum content in cluster bean (Cyamopsis tetragonoloba L.) at different developmental stages. J. Bot. 2013, 2013, 578627. [Google Scholar]
- Bashir, M.A.; Naveed, M.; Ahmad, Z.; Gao, B.; Mustafa, A.; Núñez-Delgado, A. Combined application of biochar and sulfur regulated growth, physiological, antioxidant responses and Cr removal capacity of maize (Zea mays L.) in tannery polluted soils. J. Environ. Manag. 2020, 259, 110051. [Google Scholar] [CrossRef]
- Stanton, K.M.; Mickelbart, M.V. Maintenance of water uptake and reduced water loss contribute to water stress tolerance of Spiraea alba Du Roi and Spiraea tomentosa L. Hortic. Res. 2014, 1, 14033. [Google Scholar] [CrossRef][Green Version]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2019, 21, 148. [Google Scholar] [CrossRef]
- Rana, M.; Bhantana, P.; Sun, X.C.; Imran, M.; Shaaban, M.; Moussa, M.; Hamzah Saleem, M.; Elyamine, A.; Binyamin, R.; Alam, M.; et al. Molybdenum as an essential element for crops: An overview. Int. J. Sci. Res. Growth 2020, 24, 18535. [Google Scholar]
| Treatments | Plant Height (cm) | Shoot Fresh Biomass (g) | Shoot Dry Biomass (g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Cd (mg kg−1) | Pb (mg kg−1) | Control | Cd (mg kg−1) | Pb (mg kg−1) | Control | Cd (mg kg−1) | Pb (mg kg−1) | |
| Control | 57.36 ef | 50.57 k | 50.21 i | 47.07 d | 26.81 h | 30.23 g | 3.65 fg | 2.20 h | 2.30 h |
| P1 | 61.29 cd | 52.02 j | 52.20 h | 50.55 c | 33.33 fg | 40.62 e | 5.17 bc | 3.58 g | 3.76 fg |
| P2 | 62.45 bc | 54.38 ij | 53.17 gh | 51.56 bc | 35.18 f | 44.28 d | 5.58 b | 4.49 de | 4.15 ef |
| P3 | 65.22 ab | 59.83 i | 60.38 fg | 54.11 ab | 39.50 e | 45.51 d | 5.65 b | 4.15 ef | 4.98 cd |
| P4 | 67.06 a | 62.77 h | 63.05 de | 55.08 a | 40.70 e | 52.00 a–c | 6.28 a | 5.1 bc | 5.48 bc |
| HSD value | 3.4727 | 3.1196 | 0.54 | ||||||
| Treatments | Root Length (cm) | Root Fresh Biomass (g) | Root Dry Biomass (g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Cd (mg kg−1) | Pb (mg kg−1) | Control | Cd (mg kg−1) | Pb (mg kg−1) | Control | Cd (mg kg−1) | Pb (mg kg−1) | |
| Control | 47.71 ef | 28.79 k | 23.87 l | 6.72 de | 3.37 g | 4.05 g | 3.16 ef | 1.46 i | 1.53 i |
| P1 | 51.43 cd | 32.26 j | 40.49 h | 8.20 b | 3.74 g | 5.25 f | 3.29 de | 2.60 h | 2.80 gh |
| P2 | 53.85 bc | 34.41 ij | 42.49 gh | 8.33 b | 4.02 g | 5.36 f | 3.48 d | 2.72 h | 2.98 fg |
| P3 | 55.84 ab | 36.50 i | 45.59 fg | 8.69 b | 5.26 f | 7.28 cd | 3.77 c | 3.73 c | 3.46 d |
| P4 | 58.73 a | 41.25h | 50.15 de | 10.10 a | 6.3 e | 7.93 bc | 4.74 a | 4.35 b | 4.65 a |
| HSD value | 3.4727 | 0.7724 | 0.2102 | ||||||
| Treatments | SPAD Value | Chlorophyll ‘a’ (mg g−1 Fresh Weight) | ||||
|---|---|---|---|---|---|---|
| Control | Cd (mg kg−1) | Pb (mg kg−1) | Control | Cd (mg kg−1) | Pb (mg kg−1) | |
| Control | 38.00 de | 23.60 h | 21.90 h | 2.4033 b | 1.2667 i | 1.4533 h |
| P1 | 41.83 c | 30.70 g | 28.27 g | 2.5133 b | 1.6200 e–g | 1.4600 h |
| P2 | 43.00 bc | 33.97 f | 29.30 g | 2.4767 b | 1.7033 d–f | 1.5300 gh |
| P3 | 44.53 b | 36.51 e | 33.90 f | 2.7067 a | 1.7800 d | 1.6100 fg |
| P4 | 48.00 a | 38.97 de | 39.10 d | 2.8167 a | 1.9833 c | 1.7333 de |
| HSD value | 2.4744 | 0.0901 | ||||
| Treatments | Chlorophyll ‘b’ (mg g−1 Fresh Weight) | Carotenoid Contents (mg g−1 Fresh Weight) | ||||
| Control | Cd (mg kg−1) | Pb (mg kg−1) | Control | Cd (mg kg−1) | Pb (mg kg−1) | |
| Control | 0.6900 de | 0.4333 j | 0.5267 hi | 0.4200 de | 0.3000 jk | 0.2167 m |
| P1 | 0.7300 cd | 0.5033 i | 0.5867 fg | 0.4433 cd | 0.3200 ij | 0.2467 lm |
| P2 | 0.7633 bc | 0.5267 hi | 0.6100 fg | 0.4633 cd | 0.3500 g–i | 0.2700 kl |
| P3 | 0.8033 ab | 0.5767 gh | 0.6400 ef | 0.5267 b | 0.3600 gh | 0.3300 h–j |
| P4 | 0.8433 a | 0.7000 d | 0.7100 cd | 0.5867 a | 0.4000 ef | 0.3800 fg |
| HSD value | 0.0136 | 0.0112 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, S.; Ahmad, S.R.; Ali, Q.; Ashraf, S.; Majid, M.; Zahir, Z.A. Acidified Cow Dung-Assisted Phytoextraction of Heavy Metals by Ryegrass from Contaminated Soil as an Eco-Efficient Technique. Sustainability 2022, 14, 15879. https://doi.org/10.3390/su142315879
Ashraf S, Ahmad SR, Ali Q, Ashraf S, Majid M, Zahir ZA. Acidified Cow Dung-Assisted Phytoextraction of Heavy Metals by Ryegrass from Contaminated Soil as an Eco-Efficient Technique. Sustainability. 2022; 14(23):15879. https://doi.org/10.3390/su142315879
Chicago/Turabian StyleAshraf, Sana, Sajid Rashid Ahmad, Qasim Ali, Sobia Ashraf, Muzaffar Majid, and Zahir Ahmad Zahir. 2022. "Acidified Cow Dung-Assisted Phytoextraction of Heavy Metals by Ryegrass from Contaminated Soil as an Eco-Efficient Technique" Sustainability 14, no. 23: 15879. https://doi.org/10.3390/su142315879
APA StyleAshraf, S., Ahmad, S. R., Ali, Q., Ashraf, S., Majid, M., & Zahir, Z. A. (2022). Acidified Cow Dung-Assisted Phytoextraction of Heavy Metals by Ryegrass from Contaminated Soil as an Eco-Efficient Technique. Sustainability, 14(23), 15879. https://doi.org/10.3390/su142315879







