Abstract
The effect of soil amendment with biochar has been widely evaluated for its effects in mitigating greenhouse gas emissions (GHG) and remediating polluted soils with metals; however, a synergic understanding of the system, including biochar, soil, and microbial activity, is lacking. In this study, a meta-analysis of 854 paired data from 73 studies demonstrate that biochar application in soil affects GHG emissions and soil metal availability. First, several properties of biochar, soil, and microbial activity were considered as parameters in the meta-analysis. Then, the size effect was evaluated using the percentage of change (Pc) as obtained by the meta-analyzed data. Several parameters were related as influencer factors in GHG emissions and soil metal availability. Notably, biochar addition in soil resulted in a significant CO2 increase in emissions, whereas N2O emissions decreased; these results were directly correlated with microbial activity. Although this trend, demonstrated by the data analysis, differs from results of other studies found in the literature, it also emphasized the need for a deep understanding of the effect of biochar addition to soil (properties, nutrients, gas exchange, etc.) and to microorganisms (activity, diversity, etc.). Furthermore, it was also proved, that soil metal concentration decreases significantly when biochar was added (Cd > Zn > Pb > Cu > Fe). According to the results, biochar addition in soils contaminated with Cd and Cu was related to an increase in the microbial activity; while, soils amended with biochar but polluted with Pb, Zn, and Fe presented a higher inhibition effect on microorganisms. To improve the interpretation of soil amendment with biochar, it would be necessary to standardize the form for reporting results, particularly of the microbial activity and GHG emissions, in order to be used for future comparative studies.
1. Introduction
The soil plays a crucial role in the biosphere due to the interaction between organic matter, gases, aqueous substances, minerals, plants, and microorganisms. Besides, soil is the source of food production and a sustainable environment. The overexploitation of soil for agricultural and industrial activities has caused environmental pollution and perturbations of ecosystems [1]. Thereby, the enforcement of strategies to enhance the quality of soil and mitigation of climate change caused by greenhouse gas (GHG) emissions has increased in recent years.
Biochar is a material rich in organic carbon, which is obtained from thermal decomposition of biomass and carbonaceous waste under limited oxygen conditions (pyrolysis) [2,3]. Biochar production can be performed at different temperatures and using different feedstock, which leads to different physicochemical properties to be used in multiple applications [4]. Soil amendment with biochar has been developed as a successful approach for mitigating GHG emissions and controlling soil pollution by potentially toxic elements such as metal(oid)s. Nevertheless, it is considered that soil amendment with biochar has been widely used during the last years without complete knowledge of the possible impact on the ecosystems. Some authors have questioned if biochar application could have collateral effects that have not yet been identified [5,6,7,8]. When biochar is incorporated into the soil, it creates a complex system constituted by soil–microorganisms–biochar. Interactions among these components are influenced by multiple variables, including biochar properties, i.e., type of feedstock, pyrolysis temperature, particle size, specific surface area (SSA), application rate, etc. [9]; and soil characteristics, i.e., texture, soil pH, soil organic matter (SOC), density, conductivity, etc. [10].
Several important processes occur in soils (for instance biogeochemical cycles among others), hence when biochar is added to this environmental matrix, it can cause changes in the natural processes. Microbial-driven processes, such as the capture of C, the emission or mitigation of GHG as CO2 by microbial respiration, and the emission of CH4 by methanogenesis or N2O by denitrification are of special relevance [11]. Hence, it is important to consider the mediated interactions of biochar and/with microorganisms and metal(oid)s or pollutants [8,12,13]. Studies have reported that biochar promotes the mitigation of CH4 and CO2 emissions [14,15,16], but it has also been proved that biochar can enhance CH4 production in bioprocess [13,17,18,19,20].
Soil amendment with biochar has also been used to control and remediate polluted soils. Several studies have reported the efficiency of biochar as an adsorbent and stabilizing material for metal(oid)s such as Cd, Cu, Pb, Zn, Ni, and As [21,22,23,24].
To date, several studies have been focused on evaluating biochar for bioremediation applications and on controlling soil GHG emissions. However, no systematic study has compared the effect of biochar addition on GHG emissions and soil metal availability, considering the complex system of soil–microorganisms–biochar. This is important for understanding the complete effect of this practice.
Meta-analysis is a useful statistical method to obtain an overall scenario and conclusions about the effect of different variables studied based on the available data in the literature [25]. Several meta-analyses have been reported to evaluate the effect of biochar application in soil on different responses. Notwithstanding, the effect of the most relevant responses such as GHG emissions, metal(oid)s concentration, microbial activity, crop yield, and organic carbon content, among others, have been studied individually. This practice has not considered that in a complex matrix such as soil, several interactions, and therefore responses, must occur at the same time. Within the meta-analysis reported, Li et al. [6] performed a study to meta-analyze data reported in the literature, evaluating the increase in soil microbial biomass caused by the soil amendment with biochar considering the microbial diversity; they found a significant increase in the total microbial diversity with biochar addition, particularly in acidic and sandy soils with low soil carbon content. Additionally, Yuan et al. [23] reported a meta-analysis to study the effect of biochar aging on heavy metal bioavailability in soils; their results showed that metal bioavailability decreased significatively in weakly acidic soils (pH 6–6.99), contrary to alkaline soils (pH > 8) that resulted in an increasing concentration. Additionally, they observed that heavy metals removal was higher when the biochar treatment was shorter than the longer treatment, due to the aging biochar process. Additionally, Arabi et al. [26] meta-analyzed the reported data to assess the effect of biochar on the immobilization of As, Cr, and Ni in soils; authors concluded that biochar effectively immobilizes Cr and Ni in soils, but the As immobilization by biochar was limited.
Moreover, Zhang et al. [7] performed a meta-analysis to measure the effect of biochar application on GHG emissions from agricultural soils; results revealed that CH4 and CO2 emissions showed a significant increase, and the opposite effect was observed in N2O, which showed a significant decrease. Alternatively, Shakoor et al. [5] evaluated the effect of biochar to accelerate the mitigation of GHG emissions from agricultural soils using a meta-analysis; they concluded that maize crop showed a significant mitigation effect for CO2, CH4 and N2O when biochar was added; results indicated that biochar conditions had a significant impact on the global warming potential (GWP) of total GHG emissions. As seen in the last-mentioned studies, the individuality to analyze merely one response in soil amendment with biochar has been developed. The present study pretends to elucidate the effect of biochar application in soil on GHG emissions, metals concentration, and microbial activity, in order to relate the influence of biochar and soil properties. To assess the responses of GHG emissions, metals concentration, and microbial activity to biochar application, this study conducted a meta-analysis focusing on clearing up the questions related to interactions between the different processes that occur in soil. In consequence, this study aims to statistically explain if (1) biochar application in soil decreases GHG emissions; (2) soil amendment with biochar reduces the concentration of metals; and, (3) the microorganisms induce the effect of biochar application on GHGs and metal concentrations.
2. Materials and Methods
2.1. Search Strategy and Data Compilation
A search of the literature was conducted using The Web of Science database (main collection), including articles published from January 2012 to January 2022. The search strategy consisted of three sets of keywords: (1) biochar AND soil AND metals AND microorganisms; (2) biochar AND soil AND metals AND greenhouse gases AND microorganisms; and (3) biochar AND metal AND greenhouse gases. The selection criteria were the following: (1) random experimental design applied; (2) at least triplicates used for each experiment; (3) the data should include the mean and standard deviation (SD) or standard error (SE); (4) the results of control experiments (without biochar) under the same experimental conditions should be given; (5) the method of determination of the different parameters was clearly defined. The Plot Digitizer 2.6.9 software was utilized per data extraction when data were presented in the figures. A total of 73 studies with 854 observations were selected for data extraction and meta-analysis. The detailed process of compilation and selection studies is shown in Figure S1.
The observations included control and soil amendment with biochar treatments. The mean, SD (or SE), and the number of repetitions (r) were registered for greenhouse gases (GHG) as accumulated emissions of CO2, CH4, and N2O and were converted to t/ha. Additionally, metal concentration was registered in soil (mg/Kg) after soil amendment with biochar, and the metals considered in this study were Cd, Pb, Cu, Fe, Zn, and Cr; Ni and metalloid As were discarded because of insufficient sample size. The SE was converted to SD using Equation (1). For data reported without SD values, 1/10 of the mean value was estimated as the SD as a valid approximation of real value [6].
2.2. Data Categorization
Additionally, biochar and soil properties were collected and categorized into different groups and subgroups (Table 1). The collected data of biochar properties include feedstock type, biochar pH, pyrolysis temperature (°C), biochar rate (%, and t/ha), and experimental period and experiment type. For soil properties, the compilated data include soil texture (USDA, 1987) [27], soil pH at the initial time, soil pH at the final time, and soil organic carbon (SOC,%). The data were converted to the same units for comparison. The biochar application rate was converted into the weight percentage using the bulk density of soil and soil depth to which biochar was applied [28]. However, when not enough data were provided by the authors the values were registered as t/ha. Soil pH values were standardized at 1:2.5 (soil: water, w:v) using the equations listed in the supporting information (Table S1) [29,30,31]. Besides, soil pH classification was performed according to the U.S. Department of Agriculture Handbook (USDA, 1993) [32]. For SOC values, the Van Bemmelen Factor (1.724) was applied to convert the data of SOM into SOC [33]; this factor assumes a 58% carbon content for the soil as a standard value, reported to homologate the values of soils to SOC as a soil biological parameter and as a component of SOM.

Table 1.
Factors group and subgroups as predictive variables.
The microbial activity was included in the meta-analysis; however, the extensive diversification of tests to evaluate the presence and activity of microorganisms by the effect of soil amended by biochar severely complicated the comparison in a quantitative form. Thereby, this study considered only quantitative reported data: enzymatic activity reported as the geometric mean of enzyme activities (Gmea) and urease, phospholipid fatty acids (PLFA) concentration, microbial biomass C, cell counting, Operational Taxonomic Units (OTU), and bacterial genes detection. The assessment was carried out for all data, which was compared with the control group as a percentage relative difference (Rd) following Equation (2):
where, Xt and Xc were the values of the treatment group and control group, respectively. If the Rd value is <−15%, the microbial activity was considered negative; if the Rd value is >15%, the microbial activity was considered positive; and if the Rd is between −15% and 15% the microbial activity was considered neutral.
2.3. Meta-Analysis
The natural log of response ratio (lnRR) was applied as the effect size caused by soil amendment by biochar compared to the control. OpenMEE [34] software, developed for meta-analysis in ecology and evolutionary biology, was used to perform this study. The lnRR was calculated according to Equation (3):
where, Xt and Xc were the values of the treatment group and control group, respectively. The negative values of lnRR are related to a decrease in greenhouse emissions gases (CO2, CH4, and N2O) or metal concentration (Cd, Pb, Cu, Fe, Zn, and Cr) in the soil of the treatments group compared to control group; whereas, positive values of lnRR are related to an increase in greenhouse emissions gases or metal concentration in soil of the treatments group compared to control group. The mean effect sizes and 95% confidence intervals (CI) were obtained by developing random effects models with maximum likelihood (RMML) in OpenMEE software. The effect size was converted into the percentage of change (Pc) following Equation (4) [35].
When the biochar application treatment group was significantly different from the control group, with 95% CI obtained by RMML in the statistical software, it did not overlap with zero in forest plot figures; meanwhile, no significant difference was observed between the biochar application treatment and control [11].
3. Results
The accumulated GHG and metal concentrations in soil were considered in order to to evaluate the effect of soil amendment by biochar, and in addition different properties of biochar and soil were contemplated in this study. Table 2 shows the overall effect of all observations (N). Concerning the accumulated GHG, the mean values of CO2 emissions (7.73 t/ha ± 0.41), and CH4 emissions (4.94 t/ha ± 0.52) were higher than those recorded without biochar application; conversely, N2O emission (1.58 t/ha ± 0.28) showed a decrease compared with the non-amendment treatments (7.25 t/ha, 2.77 t/ha, and 2.34 t/ha, respectively). Further, the concentration of different metals at the end of the biochar treatment application was evaluated. For almost all considered metals (Cd, Pb, Cu, and Zn), a decrease in the concentration compared to control experiments was observed. In contrast, Fe and Cr showed a slight increase in the concentration compared to non-amendment tests.

Table 2.
Overall statistics of selected studied variables.
The sum of all the variables, considering biochar and soil properties, impacting on the GHG emissions and metal concentrations by biochar application is represented by the “grand” mean percentage change (Table 2). According to the results, biochar application significantly increases soil CO2 emissions by 19.24%, CH4 emissions by 2.63%; and decreases soil N2O emissions by almost 22%. These data contradict with several reports in the literature and will be extensively discussed in the following section. In addition, it was observed that metal concentration in soil decreased by 42%, 23.5%, 6.95%, and 40.9% for Cd, Pb, Cu, and Zn, respectively, whereas it was slightly increased by 3.54% and 9.64% for Fe and Cr, respectively.
3.1. Effect of Biochar and Soil Properties on GHG Emissions
The GHG emissions were considered to undergo a significant change when the 95% confidence intervals did not overlap the zero line. The percentage of change in soil GHG accumulated emissions are presented by biochar (a) and soil (b) properties in Figure 1, Figure 2 and Figure 3. Figure 1a shows that properties of biochar which changed in response to soil CO2 accumulated emissions were feedstock, pH, pyrolysis temperature and rate, while the experimental type and experimental period showed great influence. The type of biochar feedstock appeared to significantly induce the CO2 emissions by materials like straw (27.7%) and manure (23.6%), while grass-derived biochars presented a decrease (~15%) in CO2 emissions, and residues and wood feedstock did not significant statistically. Although, in overall terms, the type of feedstock was a significant variable to increase the in CO2 emissions (19.2%). The CO2 emissions were also influenced by biochar pH, with an overall 19.36% increase, mainly by biochars with alkaline and strongly alkaline pH (>9), as well as by pyrolysis temperature. Soil CO2 emissions were increased by 19.24%, especially for biochars obtained between 300–500 °C.
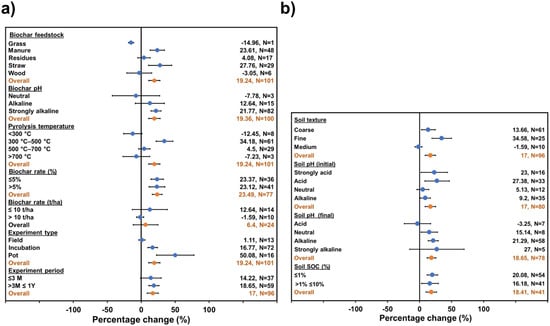
Figure 1.
Change induced by biochar (a) and soil (b) properties on CO2 accumulated emissions generated by biochar application. The overall mean represents results grouped across study change (95% confidence intervals (CI)). Biochar treatment was considered significant compared to control when 95% CI of change did not overlap with zero.
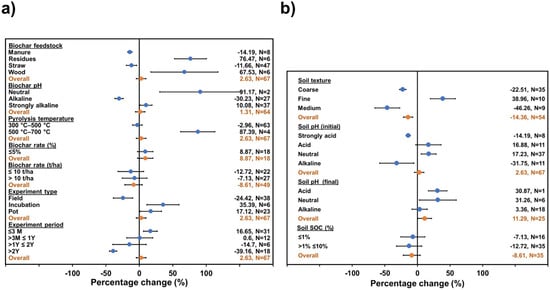
Figure 2.
Change induced by biochar (a) and soil (b) properties on CH4 accumulated emissions generated by biochar application. The overall mean represents results grouped across study change (95% confidence intervals (CI)). Biochar treatment was considered significant compared to control when 95% CI of change did not overlap with zero.
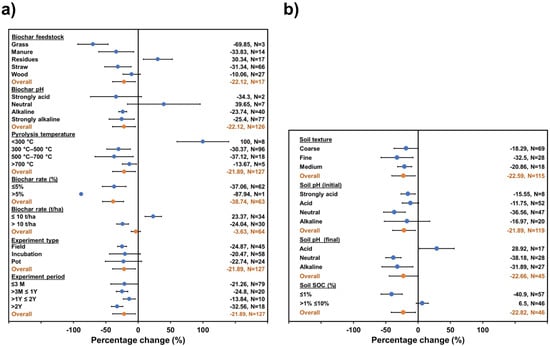
Figure 3.
Change induced by biochar (a) and soil (b) properties on N2O accumulated emissions generated by biochar application. The overall mean represents results grouped across study change (95% confidence intervals (CI)). Biochar treatment was considered significant compared to the control when 95% CI of change did not overlap with zero.
The biochar application rate appeared to significantly induce soil CO2 emissions at any value. The experimental type and experiment period were significant variables to increase the CO2 emissions. This was specifically the case for incubation (16.7%), pot size (50%), or at any studied time value (17%).
At the same time, soil properties (Figure 1b) such as soil texture, soil pH (initial and final of the treatment), and % SOC were considered to undergo meta-analysis (Figure 1, Figure 2 and Figure 3). Soil CO2 emissions were significantly induced by soil texture (17%), and fine-classified soils showed the highest increase (34.9%) followed by coarse texture soils (13.66%).
The pH of the soil was also related to the increase in the CO2 emissions; acidic soils (initial pH: 4.5–6.5) induced a 27.38% increase in CO2 emissions. The change in acidic soil (at the end of the experiment, after biochar addition) to neutral (pH: 6.6–7.5) and alkaline conditions (pH: 7.6–9) also increased the CO2 emissions by 15.14% and 21.29%, respectively. In terms of % of SOC, all reported concentrations significantly increased soil CO2 emissions (18.41%).
Regarding soil CH4 emissions (Figure 2), the biochar properties evaluated showed an insignificant effect on the response. However, for soil properties, only soil texture appeared to significantly decrease CH4 emissions, by 14.36%. This could be related to the lower number of observations (N = 67), and as consequence, the existence of less possible interaction data. The number of comparisons for CH4 emissions was minor since the reported data did not accomplish the criteria to be meta-analyzed.
On the other hand, soil N2O emissions showed a contrasting scenario due to a decrease in its response (Figure 3). According to the biochar properties (Figure 3a), the type of feedstock could inhibit 22.12% of N2O emissions. Specifically, materials like grass, manure, and straw appeared to significantly decrease emissions by 69.85%, 33.83%, and 31.34%, respectively. On the contrary, biochar from residues could significantly increase N2O emissions up to 30.34%. Biochar with alkaline (pH: 7.6–9) and strongly alkaline (pH > 9) pH significantly inhibits soil N2O emissions by 23.74% and 25.4%, respectively; the response of neutral and acid biochar (pH: <7.6) did not significantly affect soil N2O emissions. Pyrolysis temperature also demonstrated a significant impact on decreased N2O emissions (21.89%); specifically, temperatures higher than 300 °C induced an abatement in N2O emissions. According to meta-analyzed data, the biochar application rate can decrease N2O emissions when it is applied at lower concentrations (≤5%) up to 37.06%; but rate values <10 t/ha can induce an increase in N2O emissions of 23.37%. The experiment type was a significant variable only when field size was studied, inducing inhibition of 24.87% on N2O emissions. The experiment period had a significantly impact on an overall decline in N2O emissions of 21.89%.
Simultaneously, the soil characteristics were meta-analyzed and appeared to have a significant effect on diminishing the soil N2O accumulated emissions (Figure 3b). The soil texture dropped soil N2O emissions by 22.59% on average for coarse soils (18.29%), fine soils (32.5%), and medium soils (20.86%). The soil pH at initial conditions decreased the N2O emissions by 15.55% in strongly acidic soil (pH: <4.5) and 36.56% in a neutral soil (pH: 6.6–7.5), while acid and alkaline conditions were not significant. The soil pH at the end of the tests resulted in a diminishing of N2O emissions in neutral soil (pH: 6.6–7.5) of 38.18%, and in alkaline soils (pH: 7.6–9) of 31.89%, in contrast to acidic soil (pH: 4.5–6.5), the response increased the N2O emissions by 28.92%. Finally, the % of SOC reduced the soil N2O emissions for values <1% by 40.9%.
3.2. Effect of Biochar and Soil Properties on Metal Concentration
Metal concentrations in soil were considered undergo a significant change when the 95% confidence intervals did not overlap the zero line; the metals considered in this study were Cd, Pb, Cu, Fe, Zn, and Cr. The percentage change in metal concentrations in soil are presented by biochar and soil properties in Table 3. The characteristics considered in the meta-analysis for metal concentration were biochar properties: type of feedstock, biochar pH, pyrolysis temperature, biochar rate, and experiment period, whereas soil properties considered were soil texture, soil pH at initial and final conditions, and % SOC. In broad terms, most of the evaluated metals appeared to undergo a significant decrease in their final concentration with all the variables considered when soil was amended with biochar. However, Cr was the only metal that showed an increase in its final concentration when biochar was applied to soil, even though the statistical analysis showed that the data were not significant. This could have been because of the variation and the small number of observations.

Table 3.
Meta-analyzed data of metal concentration in soil considering biochar and soil properties.
Though the metal concentrations were lower at the final of the biochar treatment, the percentage change (Pc) was not the same for all evaluated metals (Table 3). Regarding biochar properties, the type of feedstock induced a more significant decrease in Cd (41.3%), Zn (41.1%), and Pb (23.5%, p value: >0.05) than in Cu (6.9%, p value: >0.05), and Fe (3.5%). Additionally, the pH of biochar induced a significant decline in Cd (42%) and Zn (41.5%) soil concentration, but the effect was lower in Fe (3.5%) concentration, while the effect was not significant for Pb and Cu. The same trend was observed for pyrolysis temperature. The biochar application rate promoted a higher decrease in metal concentrations in Cd (45.3%), Pb (43.9%), and Zn (48.8), compared to Cu (17.1%) and Fe (7.6%). The experiment period appeared to see a significant decrease in Cd and Zn concentration, 42%, and 40.9%, respectively, while there was only a 4.6% decrease in Fe concentration due to biochar treatment and Pb and Cu did not present a significant effect.
Regarding soil properties, the soil texture showed a significant effect on decreasing metal concentration. However, Cd (41.6%) and Zn (44.2%) presented a higher effect compared to Cu (9.2%) and Fe (3.9%), while Pb did not present a significant effect. The soil pH data for initial and final conditions demonstrated a significant impact on the reduction of metal concentrations, with Cd and Zn being the most influenced, followed by Pb, Cu, and Fe. Finally, the SOC was shown to promote a decrease in metals concentrations by 43.2% for Cd, 41.3% for Zn, 27.5% for Pb, 9.9% for Cu, and 6.4% for Fe.
The results of regression analysis are shown in Figure 4 and Figure 5 by biochar and soil properties, respectively. For the experiment period, the biochar pH and biochar rate were considered to be related to the lnRR for metal concentration in soil (Figure 4). The experiment period presented a slight increase in the effect size when the time was longer for Cd, Pb, Cu, and Zn. Conversely, for Fe and Cr, the increase in the effect size was minimum. Moreover, the biochar pH exhibited a limited decrease in the effect size when the pH values are higher, being more remarkable for Fe, Zn, and Cr. The biochar application rate presented a minimum increase in the effect size when the rate is higher for all evaluated metals. Besides, the soil pH at initial and final conditions and the SOC (%) were assessed as related to the lnRR for metal concentration in soil (Figure 5). For the soil pH at initial conditions, the effect size was lower when pH turned to alkaline values in Cd, Pb, and Zn; on the contrary, the effect size was higher when pH values were higher in Cu, Fe, and Cr. Likewise, the soil pH at final conditions demonstrated a decrease in effect size when pH turned to alkaline values, with that exception of Cu, which showed the opposite effect. Lastly, the SOC (%) presented an almost null correlation with the effect size in Cd, Cu, Fe, and Zn; however, a positive correlation was observed with Pb and Cr when SOC turned to higher values.
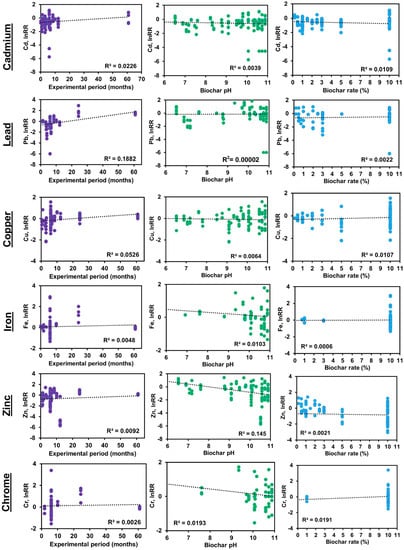
Figure 4.
Scatter plot with linear regression graph of the natural log of response ratio (lnRR) of metal concentration for biochar properties.
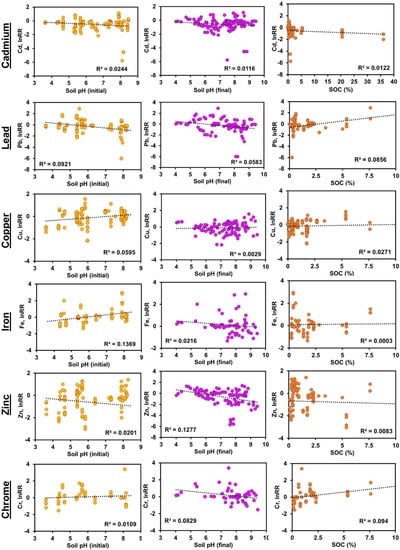
Figure 5.
Scatter plot with linear regression graph of the natural log of response ratio (lnRR) of metal concentration for biochar properties.
3.3. Influence of Microbial Activity on GHG Emissions and Metal Concentration
The GHG emissions and metal concentration were considered to undergo significant change when the 95% confidence intervals did not overlap the zero line. The percentage of change in soil metal concentration (Figure 6a) and soil GHG accumulated emissions (Figure 6b) were evaluated, considering the microbial activity as the response to biochar application treatments (Figure 6). In this study, the microbial activity was evaluated based on the quantitative data reported by the authors and then was transformed into a percentage relative difference. The microbial performance was classified into negative, neutral, and positive activity. The neutral group showed no difference in the microbial activity against the control experiment, whereas the positive group was thought to increase the microbial activity against the control experiment. The negative group was thought to be inhibiting the microbial activity against the control experiment. The overall cohort was contemplated as indicating the global effect of the microbial activity.
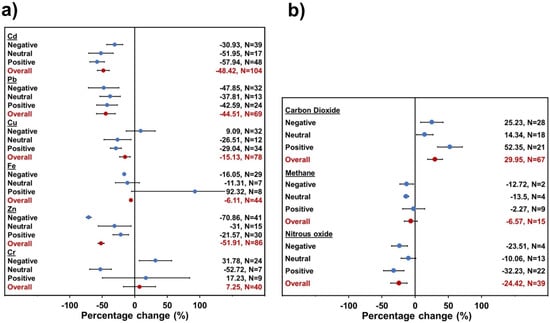
Figure 6.
Change induced by metals concentration in soil (a) and GHG accumulated emissions by soil (b) on microbial activity by biochar application. Overall mean represents results grouped across study change (95% confidence intervals (CI)). Biochar treatment was considered significant compared to control when 95% CI of change did not overlap with zero.
In general, the metal concentration decreased significantly in the treatments with biochar application (Figure 6a), with the exception of Cr, which did not present significant data. Moreover, microbial activity played an important role in the interaction between soil and biochar. In the case of Cd, it was possible to observe a significative decline in its concentration at the end of biochar treatment. The positive group (57.94%) of microorganisms had a higher decrease than the negative group (30.93%). This could be related to a larger increase in the metabolic response of microorganisms than the effect of inhibition by the toxicity of the metal. Cu showed similar results to Cd; conversely, Pb and Zn presented a significant increase in the negative group (47.85% and 70.86%, respectively) compared to the positive group (42.52% and 21.57%, respectively) and Fe appeared to decrease its concentration, however no discernible impact on microbial activity was detected.
According to the GHG emission meta-analysis about the microbial activity (Figure 6b), soil CO2 accumulated emissions significantly increased when using biochar treatment. However, with the presence of microbial activity (positive group, 52.35%) the CO2 emissions were more than twice higher than the inhibited treatments (negative group, 25.23%). In contrast, the soil N2O accumulated emissions significantly decreased with the existence of microorganisms (positive group, 32.23%) more than with the inhibited treatments (negative group, 23.51%). For soil CH4 accumulated emissions, the data did not present a significant effect to be considered.
4. Discussion
4.1. Effect of Biochar Properties on Soil GHG Emissions
The percentage of change in soil amendment with biochar demonstrated different scenarios for GHG accumulated emissions. The effects of biochar amendment on GHG emissions could vary with soil and site conditions as well as biochar properties. The findings in this study are based on statistics of the data of compiled research that met the criteria for selection, making them a part of the universe of published data that is already available. According to the meta-analysis, soil CO2 emissions showed a tendency of increasing when biochar is applied with almost all biochar properties. Specifically, biochar rate, pH, type of feedstock, and pyrolysis temperature were the properties with a significant positive effect size for biochar application. The type of experiment and experiment period showed a significant positive effect size for biochar application. The meta-analyzed data were consistent with other reports that demonstrated that biochar application induces soil CO2 emissions [36,37,38,39]. Based on the results of the meta-analysis, the pyrolysis temperature influences CO2 emissions, in particular, when the temperature was at 300 °C to 500 °C. Deng et al. [36] also reported an increase in CO2 emissions by biochar obtained at lower pyrolysis temperatures (300°C and 450 °C) using spent mushroom as feedstock. Recently, Sial et al. [37] demonstrated an increase in CO2 emissions when walnut shell biochar was used. Emissions increased by 73% and 69% for biochar obtained at 300 °C and 450 °C, respectively; while CO2 emissions were minor (51%) for biochar produced at 600 °C. Besides, Yang et al. [40] reported that the age of the biochar (fresh or aged biochar) was not significative for increased soil CO2 emissions. Likewise, their results found that biochar obtained at lower pyrolysis temperatures (300 °C) induced CO2 emissions because of the increase in soil-dissolved organic matter (DOM) content and enrichment of copiotrophic bacteria.
On the contrary, they found that biochar produced at higher pyrolysis temperatures (600 °C) caused a decrease in CO2 emissions and decline in DOM content. Moreover, the type of feedstock has a direct impact on the biochar properties since biomass varies in carbon content, lignin and cellulose content, moisture, etc. These variations determine the pyrolysis temperature required and the surface chemistry of pyrolytic charcoals [4]. Based on the results, biochar feedstock from manure and straw increased CO2 emissions, while biochar feedstock from grass showed a decrease in CO2 emissions of almost 15%. Moreover, it has been reported that biochar with large cellulose contents in their feedstocks could increase CO2 emissions more than biochar with large lignin contents [41]. The biomass rich in lignin, such as wood or some agricultural residues, requires higher temperatures to be pyrolyzed than biomass rich in cellulose such as grass. Therefore, CO2 emissions are expected to be higher in the pyrolytic process of woody feedstock [19]. Another factor to consider is that the moisture content of biomass could also increase the energy required to pyrolyze the biomass and the net balance of CO2 emissions could be affected, and hence a deep understanding of feedstock is necessary [42]. Biochar application rate is an influential parameter in GHG emissions. Other meta-analyses have demonstrated that lower rates of biochar application developed strong effects on CO2 and CH4 emissions, while N2O emissions were affected by higher biochar rates.
The soil N2O emissions showed a decrease when biochar was applied. Almost all biochar properties, types of experiment, and experiment periods exhibited a significant negative effect size on biochar application, something which meant a decrease in soil N2O accumulated emissions. According to meta-analyzed results, lower pyrolysis temperatures (<300 °C) induced the largest decrease in N2O emissions. These results were similar to the report by Sial et al. [37]; they proved that biochar derived from walnut shells reduces N2O emissions by up to 65% when the pyrolysis temperature oscillates between 300–600 °C. Deng et al. [36] demonstrated similar relation between pyrolysis temperature and N2O emissions, as the authors denoted the change in biochar nitrogen concentrations due to pyrolysis, something which impacted on the nitrogen availability for N2O emissions and influenced microbial community composition by modifying the carbon and nitrogen balance indirectly. The nitrogen loss and N-functional groups of biochar are directly impacted by the pyrolysis temperature; particularly, the nitrogen content of biochar tends to decrease as the pyrolysis temperature rises [43]. Additionally, the type of feedstock could affect the nitrogen content of biochar. For example, the N-content in biochar from sludge is much greater than that in wood sawdust-derived biochar. Alternatively, feedstocks such as microalgae have been reported with higher nitrogen content [43].
On the contrary, according to the no-significative meta-analyzed data of soil CH4 emissions arising because of the limited number of observations, it is difficult to assume that biochar application causes an increase or decrease in the CH4 emissions. The number of comparisons was restricted because the results shown by the majority of the evaluated studies did not accomplish the selection criteria required to be statistically comparable, and thus did not obtain reliable results for the evaluation of the effect. For this reason, the premises of how soil amendment with biochar could affect CH4 emissions must not be discussed. However, several studies have reported a mitigation effect of CH4 emissions when biochar is incorporated into soil. The principal mechanisms reported are related to the change in soil properties, such as increasing pH, and reduction of bulk density, that generates changes in the oxygen content and decreasing the redox potential. These effects suppressed methanogenic activity and enhanced methanotrophs, resulting in a decline in CH4 production [5,19]. The biochar feedstock has been related to CH4 mitigation response. This was particularly the case when methanogen and methanotrophic activities developed an affinity for CH4 metabolism. The type of biochar feedstock has an influence on physicochemical properties of biochar. Particularly, the pH of straw and grass biochar are higher than wood biochar; this fact could increase the soil pH, increase the abundance of methanotrophs and promote methane oxidation [44].
4.2. Effect of Soil Properties on Soil GHG Emissions
A similar situation was observed concerning the effect size of soil properties on GHG emissions with the biochar application. The meta-analyzed data were consistent with other reports that demonstrated that biochar application induces the generation of soil CO2 emissions [36,37,38,39]. Specifically, pH is an important soil property that can relate to biochar. Sheng et al. [39] showed that biochar induced high CO2 emissions in acidic soils, the pH was the dominant factor affecting CO2 emissions which worked by rising the bioavailability of organic carbon and abundance of copiotrophic bacteria. The biochar application improves soil permeability, pH, and water content. This then modifies the biota of soil, more significantly in upload soils, increasing soil microbial biomass and CO2 emissions. Additionally, the decomposition of unstable carbon in biochar directly increases the CO2 production [19].
On the contrary, the meta-analysis demonstrates that biochar application in soil could cause a decrease in N2O emissions. These results show a similar trend to that reported in the literature [37,38,45]. The considered soil properties significantly influence the reduction in N2O emissions. In general, biochar addition changes soil properties; specifically, N2O flux tends to decline as a consequence of the enhanced soil aeration that inhibits denitrification as a result of the presence of oxygen. Besides, labile C in the biochar induces complete denitrification (N2 formation); also, alkaline pH of biochar promotes N2O reductase activity, and NH4+ or NO3− adsorption to biochar reduces the inorganic -N available to nitrify or denitrify and generate N2O [43,46]. Soil texture and soil pH are crucial factors in N2O emissions; Wu et al. [45] reported that alkaline biochar interaction with acidic sandy soils resulted in suppressed N2O emissions due to soil NO3− adsorption. Therefore, the liming potential (fraction of alkaline oxides and carbonates) of biochar allows soils a pH-buffering capacity; this fact is also related to the diminishing N2O/N2 ratio and hence N2O emissions [47,48].
Results of CH4 emissions related to soil properties were not significant in the meta-analysis because of the limited number of observations. For this reason, the assumptions of how soil amendment with biochar could affect the CH4 emissions must not be discussed. Although it has been suggested that biochar may have an impact on soil properties and also have a direct influence on microbial activity (methanogenic-methanotrophs ratio) [7,49], not all types of soils may be affected in the same manner, which could have an effect on CH4 emissions.
4.3. Effect of Biochar Properties on Soil Metal Availability
Many studies have reported that biochar is a good alternative to remove or immobilize metals in soil [23,24,26] and this study statistically corroborates this fact. Biochar can act as sorbent/adsorbent of heavy metals; however, the properties of biochar can alter the efficiency of this biomaterial to immobilize metals. Pyrolysis temperature is a factor that affects properties of biochar such as specific superficial area, volume pore, surface functional groups, etc. [50]. Uchimiya et al. [51] examined the biochar properties (pyrolysis temperature and biochar pH) such as a heavy metal sorbent for Ni, Cu, Pb, and Cd in an acidic sandy loam soil, concluding that lower pyrolytic temperatures were more effective in diminishing soluble concentrations for all studied metals. Besides, the higher values of biochar pH had a wide interaction with the acidic soil. Therefore, electrostatic interactions between the cationic metal species and the negatively charged surface of biochar are stronger.
This study showed different size effects on declining metal concentration after biochar addition depending on the metal, e.g., Cd > Zn > Pb > Cu > Fe; this fact could be related to many factors, and depends on metals solubility, mobility, conductivity, electronegativity, speciation, etc. When Dai et al. [52] investigated the use of biochar to remove various metals, they found that Cd and Cu were strongly associated with the reducible fraction that can convert towards acid species that, under certain circumstances, can be utilized by microbes or plants. These results revealed that Zn and As in soil are mostly present in a stable state with even less risk to the environment, whereas Cd and Cu are more soluble and mobile. Zn and As were mostly associated with the residual fraction that is fixed in soil lattices.
4.4. Effect of Soil Properties on Soil Metal Availability
As mentioned before, several studies have reported that biochar addition can modify soil properties. These changes could result in higher or lower soil functionality depending on the physicochemical or biological process involved [52,53,54]. The meta-analyzed data show that soil pH and SOC content were the factors with a stronger effect size on decreasing metal concentrations in soils amended with biochar. Ahmad et al. [52] observed that in alkaline soils, metals such as Pb and Cu significantly decrease by biochar addition. They attributed this fact to the alkaline shooting range soil induced via surface complexation with carbonyl groups, Fe-/Al minerals of biochar, and metal–phosphates precipitation. Moreover, Pb and Zn mobility was affected by the modification of pH in acidic soils since biochar application increases the soil pH, resulting in metal–hydroxides precipitation. According to the meta-analyzed data, the size effect of soil pH after the biochar addition is higher than at the beginning of the treatment, specifically for Cd, Pb, and Zn. Additionally, Wang et al. [55] reported changes in soil properties when biochar was applied. Specifically, they observed a decrease in pH, an increase in the CEC and a decrease in DOM; also, they reported a decline in Cd, and Cu concentration after biochar addition.
4.5. Effect of Biochar Application on Soil GHG Emissions and Soil Metal Availability According to Microbial Activity
The role of the microbial community in multiple processes that occur in soil has been studied for some time. The interaction between microorganisms, soil, and biochar is highly complex, and these three elements play different functions in GHG emissions and metal availability. In this study, the microbial activity was evaluated by a relative percentage of change against the control assay (without biochar); the meta-analysis considered only quantitative results from the studies evaluated. Different studies have reported the positive influence of microbial activity on GHG emissions when biochar is applied to soils [39,45]. Particularly, the meta-analyzed data showed a higher size effect on CO2 emissions for the positive group, which means greater influence of the microbial activity than the inhibition effect induced by biochar application. Various authors demonstrated a higher microbial activity associated with CO2 emissions when biochar was added to soil (or after biochar addition to soil) [36,38,39,45]. This meta-analysis considered several studies that evaluated the microbial activity according to the enzymatic activity. Therefore, if the effect size for the positive group showed a positive result, this could be related to a higher enzymatic activity for the microorganisms. Sial et al. [38] and Wu et al. [45] have observed a direct correlation between higher specific CO2 emissions and an increase in the C-acquisition enzyme activity, N-acquisition enzymes, and P-acquisition enzymes, in particular in cellobiohydrolase, chitinase, β-glucosidase, urease, and alkaline phosphatase, respectively. Additionally, Deng et al. [36] found that biochar application can increase C substrate content available for microorganisms, stimulating mineralization of SOC, oxidizing biochar-C, and increasing CO2 emissions. Furthermore, Sheng et al. [39] observed that biochar amendment can increase OTU richness and bacterial diversity, a fact that was related to CO2 emissions, and significantly increase the pH levels in acidic soil.
By contrast, the results indicated stronger microbial activity than that produced by the inhibitory effect (negative group) and showed a decrease in N2O emissions from soils modified with biochar. The findings are similar to those reported by Deng et al. [36] that observed a decline in N2O emission; it was assumed that biochar addition increased the pH of soil hence N2O emissions since N2O production generally occurs at specific soil pH ranges (pH 6–9, with a maximum level at pH 7–7.5) [56]. The pH is also related to functional microbial communities such as nitrifiers and denitrifiers; moreover, these groups of bacteria need available NO3−N, and biochar addition alters the relation of nitrogen in the soil. Alkaline pH induces the nitrification of N2O to N2 and activates the expression of nosZ genes that encode N2O reductase; also, this fact is related to the pyrolysis temperature which modifies the N content of biochar and indirectly changes the N/C ratio which is associated with microbial activity.
At the same time, the meta-analyzed reports showed different size effects on soil metal concentration and microbial activity by biochar addition. In the case of Cd and Cu, microbial activity exhibited an improved positive effect versus the inhibition effect. Similar results were observed by Liu et al. [57], and they reported higher soil biological activity when biochar was added to metal-polluted soil, suggesting that biochar could act as an ameliorant to immobilize heavy metals and improve soil properties. On the contrary, Pb and Zn demonstrate a higher inhibition of microbial activity by biochar application. Similar to the results reported in this study, Tang et al. [50] observed a decreasing trend of enzymatic activity even when heavy metal-containing soil was amended with biochar. Authors reported affectations in enzymatic activities in soil including dehydrogenase, catalase, and urease activity. Metals such as Cr and Fe did not show a significant size effect related to microbial activity. On the other hand, the bioavailability of metals to be bio-absorbed or toxic organisms is related to a combination of physicochemical, biological, and environmental factors. Metals can interact with biochar and, in most cases, decrease their concentrations in soil, this could be attributed to the formation of metal carbonate species and the interaction with biochar surface functional groups. Furthermore, biochar affects the amount of organic carbon, increasing the content in soil. As result, microbes are forced to utilize metals in their metabolism, which induces stabilization [58,59].
5. Conclusions
The soil GHG emissions and soil metal availability exhibit a relation to biochar addition. The effect of biochar on soil, added as an amendment, depends on both biochar and soil properties. Biochar application induced the CO2 emissions but decreased the N2O emissions. The soil CH4 emissions cannot be evaluated because of the most of the compilated data did not meet the specific criteria required for consideration. Specifically, biochar properties such as type of feedstock, pyrolysis temperature, pH, and biochar rate were associated with an increase in CO2 emissions. Soil properties such as texture, pH, and SOC (%) were related to an increase in CO2. Soil N2O emissions demonstrated a significant diminution in all biochar and soil properties considered, except for acidic soil, which corroborates the possible interaction between biochar and functional microbial communities, such as that of nitrifiers and denitrifiers, because of increased pH. The microbial activity was be an important in GHG emissions and soil metal availability, because of microorganisms-soil and microorganisms-biochar interactions. CO2 emissions showed an increase associated with a higher microbial activity. On the other hand, the N2O emissions were a side effect of increased microbial activity, which resulted in a decrease in N2O emissions.
Furthermore, biochar application decreased metal availability in soils following the order Cd > Zn > Pb > Cu > Fe, while Cr showed no significant effect for all parameters. The pyrolytic temperature, biochar pH, and soil pH were factors with higher effects for the evaluated metals. This fact could be related to the physicochemical and biological interactions between soil, microorganisms, and biochar. In general, the soil metal availability was lower, and the microbial activity was higher, for Cd and Cu, whereas Pb, Zn, and Fe demonstrated higher inhibition of microbial activity.
This study concludes that properties of soil and biochar are correlated with the generation of changes in the soil environment and directly affect the GHG emissions and soil metal availability. The role of microorganisms should be considered as an important parameter to elucidate these interactions. Future studies should consider homologizing the way to report the microbial activity and GHG emissions. This would be particularly useful to enhance the interpretation of CH4 emissions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su142315648/s1. Figure S1. Selection path for meta-analysis included in the systematic study. Table S1. Equations for the standardization of soil pH values.
Author Contributions
M.M.A.-C.: Investigation, methodology, formal analysis, and writing—original draft preparation; A.P.C.L.: Investigation, and data-acquisition; A.M.O.J.: Investigation, and data-acquisition; A.K.V.C.: Investigation, and data-acquisition; A.M.P.-E.: Conceptualization, writing—review and editing, funding acquisition, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Council for Science and Technology from Mexico (CONACyT), Fund FOP16, grant number 319750.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented in this study are available in this manuscript.
Acknowledgments
The authors would like to thank to National Council for Science and Technology from Mexico (CONACyT) for the financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ansari, A.A.; Gill, S.S.; Aftab, T.; Parwez, R.; Gill, R.; Naeem, M. An Overview of the Hazardous and Trace Materials in Soil and Plants; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780323916325. [Google Scholar]
- Zhang, M.; Riaz, M.; Zhang, L.; Zeinab El-desouki, J.C. Biochar Induces Changes to Basic Soil Properties and Bacterial Communities of Different Soils to Varying Degrees at 25 Mm Rainfall: More Effective on Acidic Soils. Front. Microbiol. 2019, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Johaness Lehmann, S.J. Biochar for Environmental Management; Earthscan: London, UK, 2009; ISBN 9780415704151. [Google Scholar]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Shakoor, A.; Arif, M.S.; Shahzad, S.M.; Farooq, T.H.; Ashraf, F.; Altaf, M.M.; Ahmed, W.; Tufail, M.A.; Ashraf, M. Does Biochar Accelerate the Mitigation of Greenhouse Gaseous Emissions from Agricultural Soil?—A Global Meta-Analysis. Environ. Res. 2021, 202, 111789. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Chang, S.X.; Jiang, X.; Song, Y. Biochar Increases Soil Microbial Biomass but Has Variable Effects on Microbial Diversity: A Meta-Analysis. Sci. Total Environ. 2020, 749, 141593. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, J.; Xue, J.; Zhang, L. Quantifying the Effects of Biochar Application on Greenhouse Gas Emissions from Agricultural Soils: A Global Meta-Analysis. Sustainability 2020, 12, 3436. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. The Biochar Dilemma. Soil Res. 2014, 52, 217–230. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Garbowski, T.; Bar-Michalczyk, D.; Charazińska, S.; Grabowska-Polanowska, B.; Kowalczyk, A.; Lochyński, P. An Overview of Natural Soil Amendments in Agriculture. Soil Tillage Res. 2023, 225, 105462. [Google Scholar] [CrossRef]
- Albert, H.A.; Li, X.X.; Jeyakumar, P.; Wei, L.; Huang, L.; Huang, Q.; Kamran, M.; Shaheen, S.M.; Hou, D.; Rinklebe, J.; et al. Influence of Biochar and Soil Properties on Soil and Plant Tissue Concentrations of Cd and Pb: A Meta-Analysis. Sci. Total Environ. 2021, 755, 142582. [Google Scholar] [CrossRef]
- Abhishek, K.; Shrivastava, A.; Vimal, V.; Kumar, A.; Krushna, S. Science of the Total Environment Biochar Application for Greenhouse Gas Mitigation, Contaminants Immobilization and Soil Fertility Enhancement: A State-of-the-Art Review. Sci. Total Environ. 2022, 853, 158562. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.; Huang, D.; Lai, C.; Chen, M.; Cheng, M.; Tang, W.; Tang, L.; Dong, H.; Huang, B.; et al. Biochar for Environmental Management: Mitigating Greenhouse Gas Emissions, Contaminant Treatment, and Potential Negative Impacts. Chem. Eng. J. 2019, 373, 902–922. [Google Scholar] [CrossRef]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in Climate Change Mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Han, X.; Sun, X.; Wang, C.; Wu, M.; Dong, D.; Zhong, T.; Thies, J.E.; Wu, W. Mitigating Methane Emission from Paddy Soil with Rice-Straw Biochar Amendment under Projected Climate Change. Sci. Rep. 2016, 6, 24731. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable Biochar to Mitigate Global Climate Change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ma, J.; Zhai, L.; Liu, H. Enhanced Methane Production and Syntrophic Connection between Microorganisms during Semi-Continuous Anaerobic Digestion of Chicken Manure by Adding Biochar. J. Clean. Prod. 2019, 240, 118178. [Google Scholar] [CrossRef]
- Reddy, K.R.; Yargicoglu, E.N.; Yue, D.; Yaghoubi, P. Enhanced Microbial Methane Oxidation in Landfill Cover Soil Amended with Biochar. J. Geotech. Geoenvironmental Eng. 2014, 140, 1–11. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, H.; Chu, M.; Zhang, C.; Tang, J.; Chang, S.X.; Mašek, O.; Ok, Y.S. Biochar Affects Greenhouse Gas Emissions in Various Environments: A Critical Review. L. Degrad. Dev. 2022, 33, 3327–3342. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, J.; Zhang, D.; Cheng, K.; Zhou, H.; Zhang, A.; Li, L.; Joseph, S.; Smith, P.; Crowley, D.; et al. Biochar Has No Effect on Soil Respiration across Chinese Agricultural Soils. Sci. Total Environ. 2016, 554–555, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, M.; Hu, X. Removal of Heavy Metals from Soil with Biochar Composite: A Critical Review of the Mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Bogusz, A.; Oleszczuk, P.; Dobrowolski, R. Adsorption and Desorption of Heavy Metals by the Sewage Sludge and Biochar-Amended Soil. Environ. Geochem. Health 2019, 41, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Gao, B.; Peng, Y.; Gao, X.; Fan, B.; Chen, Q. A Meta-Analysis of Heavy Metal Bioavailability Response to Biochar Aging: Importance of Soil and Biochar Properties. Sci. Total Environ. 2021, 756, 144058. [Google Scholar] [CrossRef]
- Trakal, L.; Komárek, M.; Száková, J.; Zemanová, V.; Tlustoš, P. Biochar Application to Metal-Contaminated Soil: Evaluating of Cd, Cu, Pb and Zn Sorption Behavior Using Single- and Multi-Element Sorption Experiment. Plant Soil Environ. 2011, 57, 372–380. [Google Scholar] [CrossRef]
- Mikolajewicz, N.; Komarova, S.V. Meta-Analytic Methodology for Basic Research: A Practical Guide. Front. Physiol. 2019, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Z.; Rinklebe, J.; El-Naggar, A.; Hou, D.; Sarmah, A.K.; Moreno-Jiménez, E. (Im)Mobilization of Arsenic, Chromium, and Nickel in Soils via Biochar: A Meta-Analysis. Environ. Pollut. 2021, 286, 117199. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. USDA Textural Soil Classification. In Soil Mechanics Level 1 Module 3 USDA Soil Textural Classification Study Guide; USDA Soil Conservation Service: Washington, DC, USA, 1987; pp. 1–53. [Google Scholar]
- Nguyen, T.T.N.; Xu, C.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Helen, M.; Wallace, S.H.B. Effects of Biochar on Soil Available Inorganic Nitrogen: A Review and Meta-Analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Conyers, M.K.; Davey, B.G. Observations on Some Routine Methods for Soil PH Determination. Soil Sci. 1988, 145, 29–36. [Google Scholar] [CrossRef]
- Kabała, C.; Musztyfaga, E.; Gałka, B.; Łabuńska, D.; Mańczyńska, P. Conversion of Soil PH 1:2.5 KCl and 1:2.5 H2O to 1:5 H2O: Conclusions for Soil Management, Environmental Monitoring, and International Soil Databases. Pol. J. Environ. Stud. 2016, 25, 647–653. [Google Scholar] [CrossRef]
- Rambwawasvika, H.; Size, P.; Mutatu, W. Relationship between different soil pH measurement methods in the Zimbabwe sugar industry. Proc. S. Afr. Sug. Technol. Ass. 2021, 193–207. [Google Scholar]
- USDA. USDA Soil Survey Manual. Department of Agriculture Handbook; Soil Conservation Service: Washington, DC, USA, 1993; p. 18. [Google Scholar]
- Heaton, L.; Fullen, M.A.; Bhattacharyya, R. Critical Analysis of the van Bemmelen Conversion Factor Used to Convert Soil Organic Matter Data to Soil Organic Carbon Data: Comparative Analyses in a UK Loamy Sand Soil Análise Crítica Do Fator de Conversão van Bemmelen Usado Para Converter Dados de Mat. Espaço Aberto 2016, 6, 35–44. [Google Scholar] [CrossRef]
- Wallace, B.C.; Lajeunesse, M.J.; Dietz, C.; Dahabreh, I.J.; Trikalinos, T.A.; Schmid, C.H.; Gurevitch, J. OpenMEE: Intuitive, Open-Source Software for Meta-Analysis in Ecology and Evolutionary Biology. Methods Ecol. Evol. 2017, 8, 941–947. [Google Scholar] [CrossRef]
- Luo, G.; Sun, B.; Li, L.; Li, M.; Liu, M.; Zhu, Y.; Guo, S.; Ling, N.; Shen, Q. Understanding How Long-Term Organic Amendments Increase Soil Phosphatase Activities: Insight into PhoD- and PhoC-Harboring Functional Microbial Populations. Soil Biol. Biochem. 2019, 139, 107632. [Google Scholar] [CrossRef]
- Deng, B.; Shi, Y.; Zhang, L.; Fang, H.; Gao, Y.; Luo, L.; Feng, W.; Hu, X.; Wan, S.; Huang, W.; et al. Effects of Spent Mushroom Substrate-Derived Biochar on Soil CO2 and N2O Emissions Depend on Pyrolysis Temperature. Chemosphere 2020, 246, 125608. [Google Scholar] [CrossRef]
- Sial, T.A.; Shaheen, S.M.; Lan, Z.; Korai, P.K.; Ghani, M.I.; Khan, M.N.; Syed, A.-u.A.; Ali, M.N.H.A.; Rajpar, I.; Memon, M.; et al. Addition of Walnut Shells Biochar to Alkaline Arable Soil Caused Contradictory Effects on CO2 and N2O Emissions, Nutrients Availability, and Enzymes Activity. Chemosphere 2022, 293, 133476. [Google Scholar] [CrossRef] [PubMed]
- Sial, T.A.; Khan, M.N.; Lan, Z.; Kumbhar, F.; Ying, Z.; Zhang, J.; Sun, D.; Li, X. Contrasting Effects of Banana Peels Waste and Its Biochar on Greenhouse Gas Emissions and Soil Biochemical Properties. Process. Saf. Environ. Prot. 2019, 122, 366–377. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhu, L. Biochar Alters Microbial Community and Carbon Sequestration Potential across Different Soil PH. Sci. Total Environ. 2018, 622–623, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, K.; Liu, J.; Chen, Y.; Han, L. Changes in Soil Properties and CO2 Emissions after Biochar Addition: Role of Pyrolysis Temperature and Aging. Sci. Total Environ. 2022, 839, 156333. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, L.; Pan, S.; Li, Y.; Kuzyakov, Y.; Xu, J.; Brookes, P.C.; Luo, Y. Feedstock Determines Biochar-Induced Soil Priming Effects by Stimulating the Activity of Specific Microorganisms. Eur. J. Soil Sci. 2018, 69, 521–534. [Google Scholar] [CrossRef]
- Xu, H.; Cai, A.; Wu, D.; Liang, G.; Xiao, J.; Xu, M.; Colinet, G.; Zhang, W. Effects of Biochar Application on Crop Productivity, Soil Carbon Sequestration, and Global Warming Potential Controlled by Biochar C:N Ratio and Soil PH: A Global Meta-Analysis. Soil Tillage Res. 2021, 213, 105125. [Google Scholar] [CrossRef]
- Leng, L.; Xu, S.; Liu, R.; Yu, T.; Zhuo, X.; Leng, S.; Xiong, Q.; Huang, H. Nitrogen Containing Functional Groups of Biochar: An Overview. Bioresour. Technol. 2020, 298, 122286. [Google Scholar] [CrossRef]
- Dong, D.; Yang, M.; Wang, C.; Wang, H.; Li, Y.; Luo, J.; Wu, W. Responses of Methane Emissions and Rice Yield to Applications of Biochar and Straw in a Paddy Field. J. Soils Sediments 2013, 13, 1450–1460. [Google Scholar] [CrossRef]
- Wu, D.; Senbayram, M.; Zang, H.; Ugurlar, F.; Aydemir, S.; Brüggemann, N.; Kuzyakov, Y.; Bol, R.; Blagodatskaya, E. Effect of Biochar Origin and Soil PH on Greenhouse Gas Emissions from Sandy and Clay Soils. Appl. Soil Ecol. 2018, 129, 121–127. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. A Review of Biochar and Soil Nitrogen Dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Downie, A.; Morris, S.; Petty, S.; Rust, J.; Chan, K.Y. A Glasshouse Study on the Interaction of Low Mineral Ash Biochar with Nitrogen in a Sandy Soil. Aust. J. Soil Res. 2010, 48, 569–576. [Google Scholar] [CrossRef]
- Chen, H.; Mothapo, N.V.; Shi, W. Soil Moisture and PH Control Relative Contributions of Fungi and Bacteria to N2O Production. Microb. Ecol. 2015, 69, 180–191. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Trinh, N.N.; Bach, Q.V. Methane Emissions and Associated Microbial Activities from Paddy Salt-Affected Soil as Influenced by Biochar and Cow Manure Addition. Appl. Soil Ecol. 2020, 152, 103531. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical Features, Metal Availability and Enzyme Activity in Heavy Metal-Polluted Soil Remediated by Biochar and Compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of Pyrolysis Temperature on Biochar Property and Function as a Heavy Metal Sorbent in Soil. J. Agric. Food Chem. 2011, 59, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lee, S.S.; Lee, S.E.; Al-Wabel, M.I.; Tsang, D.C.W.; Ok, Y.S. Biochar-Induced Changes in Soil Properties Affected Immobilization/Mobilization of Metals/Metalloids in Contaminated Soils. J. Soils Sediments 2017, 17, 717–730. [Google Scholar] [CrossRef]
- Chagas, J.K.M.; de Figueiredo, C.C.; Ramos, M.L.G. Biochar Increases Soil Carbon Pools: Evidence from a Global Meta-Analysis. J. Environ. Manag. 2022, 305, 114403. [Google Scholar] [CrossRef]
- Issaka, E.; Fapohunda, F.O.; Amu-Darko, J.N.O.; Yeboah, L.; Yakubu, S.; Varjani, S.; Ali, N.; Bilal, M. Biochar-Based Composites for Remediation of Polluted Wastewater and Soil Environments: Challenges and Prospects. Chemosphere 2022, 297, 134163. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, K.; Zhan, W.; Huang, L.; Liu, Y.; Li, T. Highly Effective Stabilization of Cd and Cu in Two Different Soils and Improvement of Soil Properties by Multiple-Modified Biochar. Ecotoxicol. Environ. Saf. 2021, 207, 111294. [Google Scholar] [CrossRef]
- Blum, J.M.; Su, Q.; Ma, Y.; Valverde-Pérez, B.; Domingo-Félez, C.; Jensen, M.M.; Smets, B.F. The PH Dependency of N-Converting Enzymatic Processes, Pathways and Microbes: Effect on Net N2O Production. Environ. Microbiol. 2018, 20, 1623–1640. [Google Scholar] [CrossRef]
- Liu, H.; Xu, F.; Xie, Y.; Wang, C.; Zhang, A.; Li, L.; Xu, H. Effect of Modified Coconut Shell Biochar on Availability of Heavy Metals and Biochemical Characteristics of Soil in Multiple Heavy Metals Contaminated Soil. Sci. Total Environ. 2018, 645, 702–709. [Google Scholar] [CrossRef]
- Dai, S.; Li, H.; Yang, Z.; Dai, M.; Dong, X.; Ge, X.; Sun, M.; Shi, L. Effects of Biochar Amendments on Speciation and Bioavailability of Heavy Metals in Coal-Mine-Contaminated Soil. Hum. Ecol. Risk Assess. 2018, 24, 1887–1900. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Ajibade, F.O.; Atakpa, E.O.; Baquy, M.A.-A.; Mia, S.; Odii, E.C.; Xu, R. Reduction of Heavy Metal Uptake from Polluted Soils and Associated Health Risks through Biochar Amendment: A Critical Synthesis. J. Hazard. Mater. Adv. 2022, 6, 100086. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).