Is the Potential for Multi-Functional Use of Industrial Hemp Greater than Maize under Saline Conditions?
Abstract
1. Introduction
1.1. Sustainable Development vs. Multifunctional Crops
1.2. Problem Statement and Specific Objective
1.3. Outline of the Study
2. Hemp and Maize in Industrial Applications: State-of-the-Art
2.1. A Short History of Maize and Hemp Use for Industrial Purposes
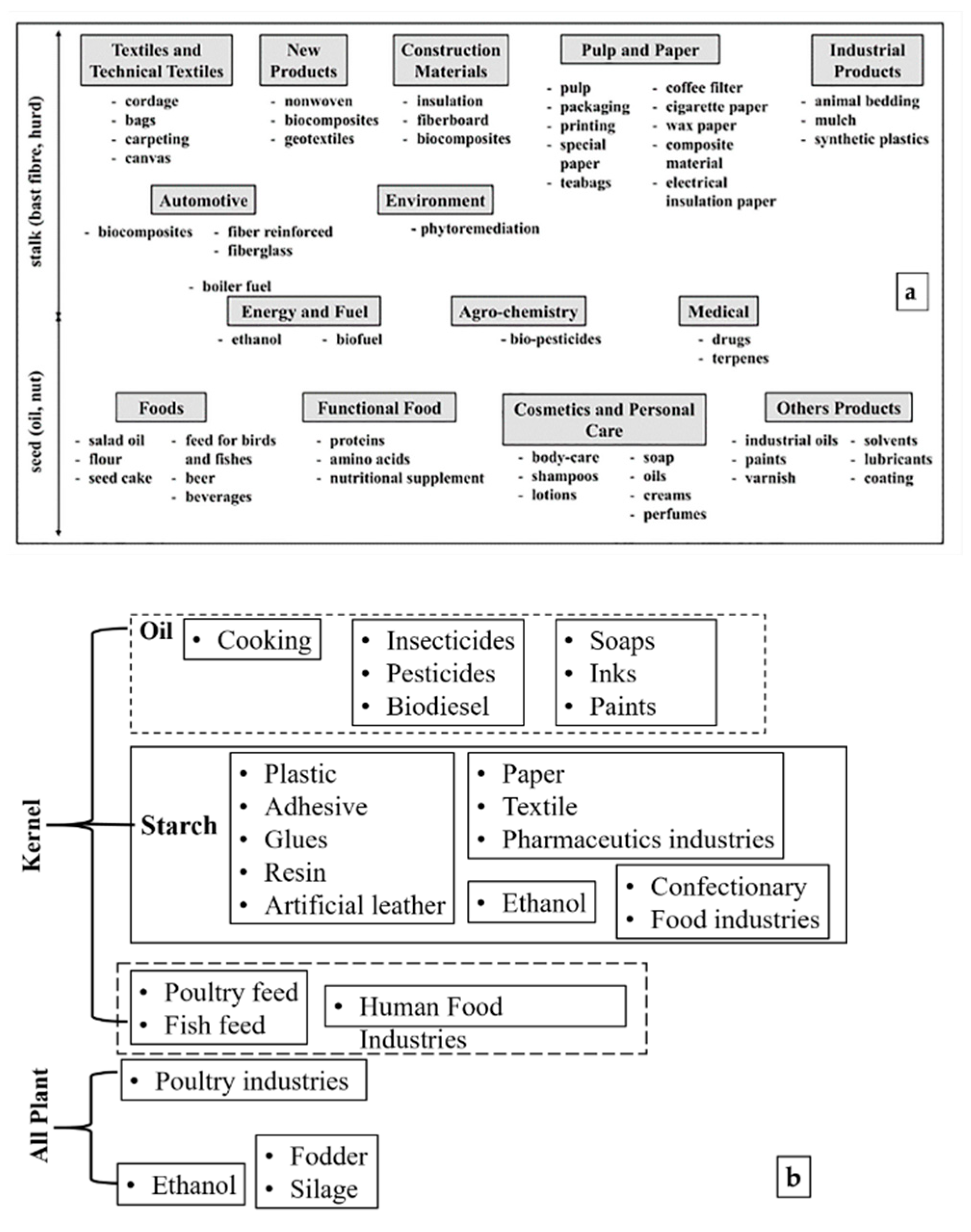
2.2. Marketable Products
2.3. Market Value of Raw Plant Materials
2.4. Product-Specific Plant Materials
3. The Hemp and Maize: Plants, Thermal and Water Requirements in Relation to the Response to Abiotic Stresses
- (a).
- the response of maize and hemp biomass under water and salinity stress;
- (b).
- maize and hemp allometry under stress to estimate the dry matter partition.
4. Case Study: Response of Hemp and Maize to Saline Stress
4.1. Motivation and Description of the Case Study
4.2. Germination Test
4.3. Field Trial
5. Results
5.1. Germination Test
5.2. Root Water Uptake
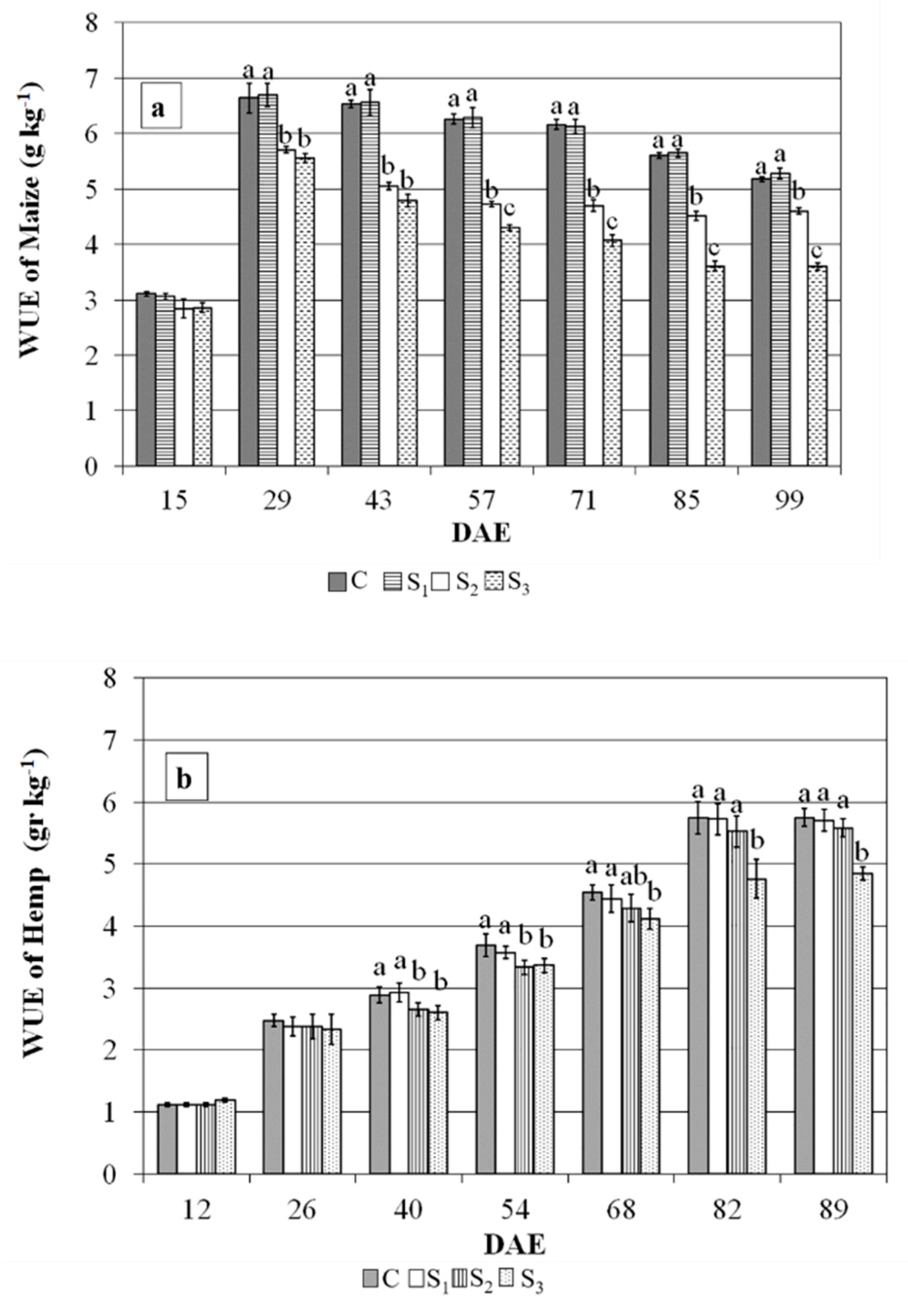
5.3. Crop Water Use, Leaf Area Duration, Dry Matter Accumulation and WUE
5.4. Response to Water and Salt Stress, and Salt Tolerance
5.5. Crop Yield and Crop Water Productivity
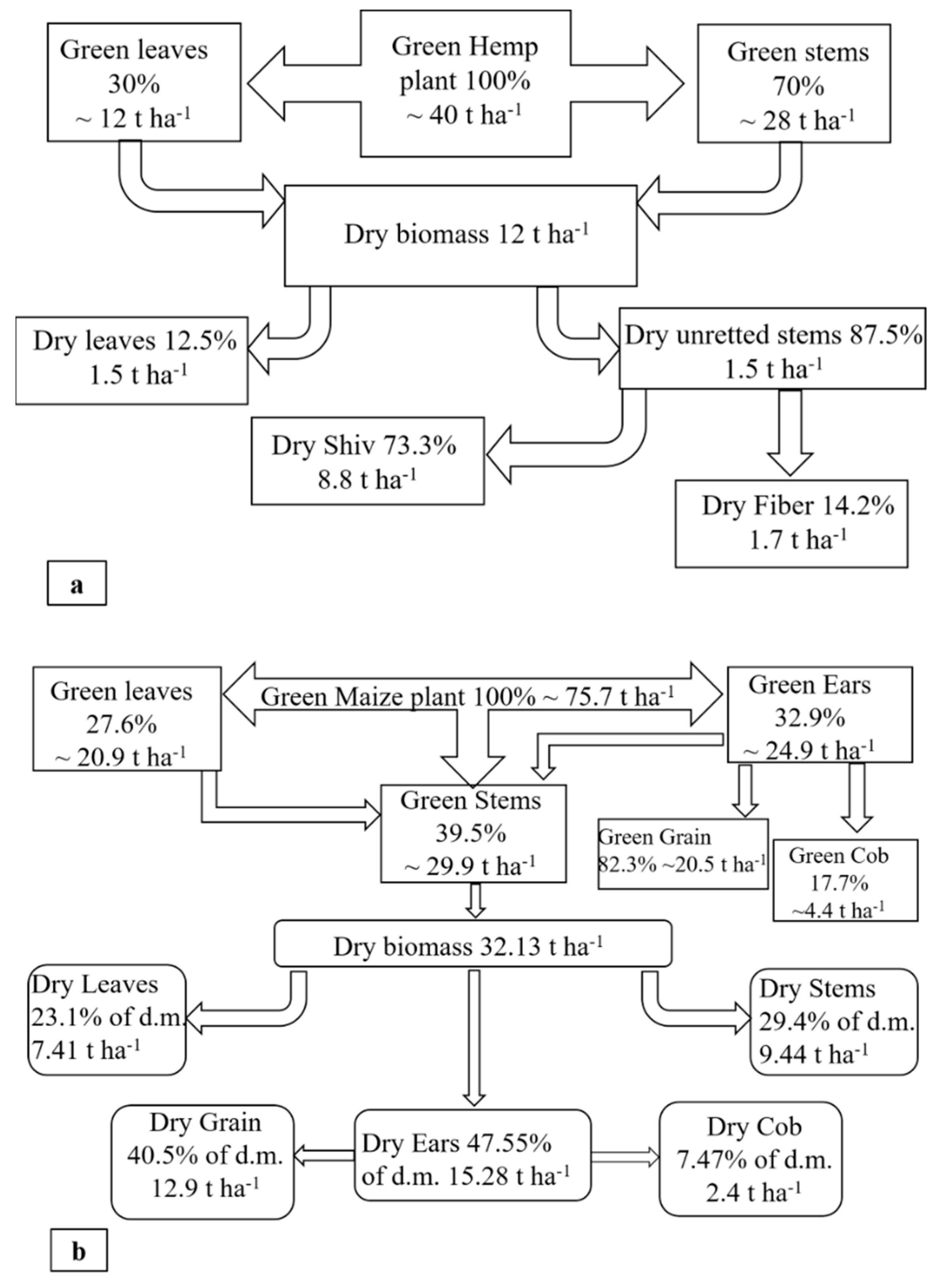
5.6. Biomass Partition
6. Discussion
- (a).
- Is the multifunctional potential of hemp and maize documented in detail, including the relation of consumer products with plant raw materials?
- (b).
- Is the current body-of-knowledge on the response of hemp yield and allometry to water and salinity stress sufficient to assess the multifunctional potential of hemp in a given environment?
- (c).
- Is multifunctional use of maize and hemp an attractive option to farmers, notwithstanding environmental constraints, which may lead to sub-optimal yield?
7. Final Considerations
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodland, R.; Daly, H. Environmental sustainability: Universal and non-negotiable. Ecol. Appl. 1996, 6, 1002–1017. [Google Scholar] [CrossRef]
- Kanter, D.R.; Musumba, M.; Wood, S.L.R.; Palm, C.; Antle, J.; Balvanera, P.; Dale, V.H.; Havlik, P.; Kline, K.L.; Scholes, R.J.; et al. Evaluating agricultural trade-offs in the age of sustainable development. Agric. Syst. 2018, 163, 73–88. [Google Scholar] [CrossRef]
- Young, E.M. Revival of Industrial Hemp: A Systematic Analysis of the Current Global Industry to Determine Limitations and Identify Future Potentials within the Concept of Sustainability. Master’s Thesis, International Environmental Science Lund University, Lund, Sweden, 2005. [Google Scholar]
- Fike, J. Industrial Hemp: Renewed Opportunities for an Ancient Crop. Crit. Rev. Plant Sci. 2016, 35, 406–424. [Google Scholar] [CrossRef]
- Schluttenhofer, C.; Yuan, L. Challenges towards revitalizing Hemp: A multifaceted crop. Trends Plant Sci. 2017, 22, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Struik, P.C.; Yin, X.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Amaducci, S. Comparing hemp (Cannabis sativa L.) cultivars for dual-purpose production under contrasting environments. Ind. Crops Prod. 2016, 87, 33–44. [Google Scholar] [CrossRef]
- Tedeschi, A.; Tedeschi, P. The potential of hemp to produce bioenergy. In Proceedings of the 2nd World Conference and Technology Exhibition on Biomass for Energy, Industry and Climate Protection, Rome, Italy, 10–14 May 2004; ISBN 88-89407-03-4. [Google Scholar]
- Salentijn, E.M.J.; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New developments in fiber hemp (Cannabis sativa L.) breeding. Ind. Crops Prod. 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Viswanathan, M.B.; Cheng, M.-H.; Clemente, T.E.; Dweikat, I.; Singh, V. Economic perspective of ethanol and biodiesel coproduction from industrial hemp. J. Clean. Prod. 2021, 299, 126875. [Google Scholar] [CrossRef]
- Warman, A. Corn & Capitalism: How a Botanical Bastard Grew to Global Dominance; The University of North Carolina Press: Chapel Hill, NC, USA; London, UK, 2003. [Google Scholar]
- Marconi, P. Usi industriali. In Il Mais. Coltura & Cultura; Angelini, R., Maggiore, T., Ponti, I., Eds.; HRE Edizioni: Milan, Italy, 2008; pp. 362–379. [Google Scholar]
- Setti, C.; Suarato, G.; Perotto, G.; Athanassiou, A.; Bayer, I.S. Investigation of in vitro hydrophilic and hydrophobic dual drug release from polymeric films produced by sodium alginate-MaterBi drying emulsions. Eur. J. Pharm. Biopharm. 2018, 130, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.; Gerin, P.A.; Noo, A.; Foucart, G.; Flammang, J.; Lemaigre, S.; Sinnaeve, G.; Dardenne, P.; Delfosse, P. Assessment of factors influencing the biomethane yield of maize silages. Bioresour. Technol. 2014, 153, 260–268. [Google Scholar] [CrossRef]
- Ranum, P.; Pena-Rosas, J.P.; García-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- Negri, M.; Bacenetti, J.; Manfredini, A.; Lovarelli, D.; Fiala, M.; Maggiore, T.M.; Bocchi, S. Evaluation of methane production from maize silage by harvest of different plant portions. Biomass Bioenergy 2014, 67, 339–346. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Durán-Zuazo, V.H.; Pérez-Álvarez, R.; Hernández, A.; Casano, S.; Morón, M.; Muriel-Fernández, J.L. Impact of plant density and irrigation on yield of hemp (Cannabis sativa L.) in a mediterranean semi-arid environment. J. Agric. Sci. Technol. 2014, 16, 887–895. [Google Scholar]
- Johnson, R. Hemp as an Agricultural Commodity. Congressional Research Service 7-5700. 2018. Available online: https://crsreports.congress.gov/product/details?prodcode=RL32725 (accessed on 1 January 2020).
- Trenda, E. Agricultural Area Dedicated to Hemp Cultivation in Europe in 2019, by Country. 2021. Available online: https://www.statista.com/statistics/1204146/area-for-hemp-cultivation-by-country-europe/ (accessed on 15 April 2021).
- Welcome to, I. Stat, the Complete Data Warehouse for Experts. Available online: http://dati.istat.it/index.aspx?queryid=37850 (accessed on 1 August 2022).
- FAO. FAO Stat. 2022. Available online: https://www.fao.org/faostat (accessed on 1 August 2022).
- Industrial Hemp Licensing Statistics. 2020. Available online: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/producing-selling-hemp/about-hemp-canada-hemp-industry/statistics-reports-fact-sheets-hemp.html#_2020 (accessed on 15 June 2021).
- Overview of the Global Hemp Market: CBD to Fiber. MJBizDaily Staff. Available online: https://mjbizdaily.com/global-hemp-potential (accessed on 15 April 2021).
- The U.S. Hemp Market Landscape. Cannabinoids, Grain and Fiber. In Partnership with Hemp Benchmarks, Sponsored by Blue Sky. Available online: http://info.newfrontierdata.com/u.s.-hemp-market-landscape (accessed on 15 June 2021).
- Di Candilo, M.; Ranalli, P.; Liberalato, D. Gli interventi necessari per la reintroduzione della canapa in Italia. Agroindustria 2003, 2, 27–36. [Google Scholar]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Williams & Marshall Strategy. Available online: https://www.wm-strategy.com/ (accessed on 10 August 2022).
- Smith, P.; Bustamante, M.; Ahammad, H.; Clark, H.; Dong, H.; Elsiddig, E.A.; Haberl, H.; Harper, R.; House, J.; Jafari, M.; et al. Chapter 11—Agriculture, forestry and other land use (AFOLU). In Climate Change; Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 811–922. [Google Scholar]
- Vermeulen, S.J.; Campbell, B.M.; Ingram, J.S. Climate change and food systems. Annu. Rev. Environ. Resour. 2012, 37, 195. [Google Scholar] [CrossRef]
- Garnett, T.; Godfray, C. Sustainable Intensification in Agriculture. Navigating a Course Through Competing Food System Priorities. Food Climate Research Network and the Oxford Martin Programme on the Future of Food; University of Oxford: Oxford, UK, 2012; p. 51. [Google Scholar]
- Morin-Crini, N.; Loiacono, S.; Placet, V.; Torri, G.; Bradu, C.; Kostić, M.; Cosentino, C.; Chanet, G.; Martel, B.; Lichtfouse, E.; et al. Hemp-based adsorbents for sequestration of metals: A review. Environ. Chem. Lett. 2019, 17, 393–408. [Google Scholar] [CrossRef]
- Dell’Orto, V.; Corino, C.; Savoini, G. Usi zootecnici. In Il Mais. Coltura & Cultura; Angelini, R., Maggiore, T., Ponti, I., Eds.; HRE Edizioni: Milan, Italy, 2008; pp. 328–361. [Google Scholar]
- Maize Market by End-User and Geography-Forecast and Analysis 2022–2026. Available online: https://www.technavio.com/report/maize-market-industry-analysis (accessed on 10 August 2022).
- Market Overview. Available online: https://www.hempalta.com/market-overview (accessed on 15 June 2021).
- Hǔtnan, M.; Špalková, V.; Bodík, I. Biogas production from maize grains and maize silage. Pol. J. Environ. Stud. 2010, 19, 323–329. [Google Scholar]
- Mazurkiewicz, J.; Marczuk, A.; Pochwatka, P.; Kujawa, S. Maize straw as a valuable energetic material for biogas plant feeding. Materials 2019, 12, 3848. [Google Scholar] [CrossRef]
- Koca, Y.O.; Erekel, O. Changes of dry matter, biomass and relative growth rate with different phenological stages of corn. Agric. Agric. Sci. Procedia 2016, 10, 67–75. [Google Scholar] [CrossRef][Green Version]
- Wasaya, A.; Tahir, M.; Ali, H.; Hussain, M.; Yasir, T.A.; Sher, A.; Ijaz, M.; Sattar, A. Influence of varying tillage systems and nitrogen application on crop allometry, chlorophyll contents, biomass production and net returns of maize (Zea mays L.). Soil Tillage Res. 2017, 170, 18–26. [Google Scholar] [CrossRef]
- Angelini, R.; Maggiore, T.; Ponti, I. Il Mais. Coltura&Cultura; HRE Edizioni: Milan, Italy, 2008; p. 425. ISBN 978-8896301-03-6. [Google Scholar]
- Available online: https://ministryofhemp.com/hemp/sustainability (accessed on 15 June 2021).
- Kage, H.; Kochler, M.; Stützel, H. Root growth and dry matter partitioning of cauliflower under drought stress conditions: Measurement and simulation. Eur. J. Agron. 2004, 20, 379–394. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Testa, G.; Scordia, D.; Copani, V. Sowing time and prediction of flowering of different hemp (Cannabis sativa L.) genotypes in southern Europe. Ind. Crops Prod. 2012, 37, 20–23. [Google Scholar] [CrossRef]
- Faux, A.M.; Draye, X.; Lambert, R.; d’Andrimont, R.; Raulier, P.; Bertin, P. The relationship of stem and seed yields to flowering phenology and sex expression in monoecious hemp (Cannabis sativa L.). Eur. J. Agron. 2013, 47, 11–22. [Google Scholar] [CrossRef]
- Hall, J.; Surya, P.; Bhattarai, S.P.; Midmore, D.J. The Effects of Photoperiod on phenological Development and Yields of Industrial Hemp. J. Nat. Fibers 2014, 11, 87–106. [Google Scholar] [CrossRef]
- USDA. Industrial Hemp in United States: Status and Market Potential (Rep. No. ERS AGES No. 001); USDA Economic Research Service: Washington, DC, USA, 2000.
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key cultivation techniques for hemp in Europe and China. Ind. Crops Prods. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Ehrensing, D.T. Feasibility of Industrial Hemp Production in United State Pacific Northwest; Oregon State University Extension Service Station Bulletin: Corvallis, OR, USA, 1998; p. 681. Available online: https://catalog.extension.oregonstate.edu/sb681 (accessed on 10 August 2022).
- Van der Werf, H.M.G.; Haasken, H.J.; Wijlhuizen, M. The effect of day length on yield and quality of hemp (Cannabis sativa L.). Eur. J. Agron. 1994, 3, 117–123. [Google Scholar] [CrossRef]
- Struik, P.C.; Amaducci, S.; Bullard, M.J.; Stutterheim, N.C.; Venturi, G.; Cromack, H.T.H. Agronomy of fibre hemp (Cannabis sativa L.) in Europe. Ind. Crops Prod. 2000, 11, 107–118. [Google Scholar] [CrossRef]
- Van der Werf, H.M.G.; Mathijssen, E.W.J.M.; Haeverkort, A.J. The potential of hemp (Cannabis sativa L) for sustainable fiber production: A crop physiological appraisal. Ann. Appl. Biol. 1996, 129, 109–123. [Google Scholar] [CrossRef]
- Bouloc, P.; Werf, H.M.G. The role of hemp in sustainable development. In Hemp: Industrial Production and Uses; Bouloc, P., Ed.; CPi Group (UK) Ltd: Croydon, UK, 2013; pp. 278–289. [Google Scholar]
- Stickland, D. The suitability of hemp for ecological agriculture. In Proceedings of the Biorohstoff Hanf Symposium, Frankfurt am Main, Germany, 2–5 March 1995; pp. 255–258. [Google Scholar]
- Ranalli, P.; Di Candilo, M.; Mandolino, G.; Grassi, G.; Carboni, A. Hemp for sustainable agricultural systems. Agro-Food-Ind. Hi-Tech. 1999, 2, 33–38. [Google Scholar]
- Baldini, M.; Ferfuia, C.; Zuliani, F.; Danuso, F. Suitability assessment of different hemp (Cannabis sativa L.) varieties to the cultivation environment. Ind. Crops Prod. 2020, 143, 111860. [Google Scholar] [CrossRef]
- Cheng, X.; Deng, G.; Su, Y.; Liu, J.J.; Yang, Y.; Du, G.H. Protein mechanisms in response to NaCl-stress of salt-tolerant and salt-sensitive industrial hemp based on iTRAQ technology. Ind. Crops Prod. 2016, 83, 444–452. [Google Scholar] [CrossRef]
- Przemyslaw, B.; Grabowska, L.; Mankowski, J. Recultivation of degraded areas through cultivation of hemp. In Proceedings of the Biorohstoff Hanf Symposium, Frankfurt am Main, Germany, 2–5 March 1995. [Google Scholar]
- Lixandru, G.; Filipov, F.; Dumbravă, I. Plant tolerance to soil salinity in the conception of the Iaşi School of Soil Science. Cercet. Agron. Mold. 2007, 40, 15–31. [Google Scholar]
- Cosentino, S.L.; Riggi, E.; Testa, G.; Scordia, D.; Copani, V. Evaluation of European developed fibre hemp genotypes (Cannabis sativa L.) in semi-arid Mediterranean environment. Ind. Crops Prod. 2013, 50, 312–324. [Google Scholar] [CrossRef]
- Maggiore, T.; Mariani, L.; Verderio, A. Tecnica colturale. In Il Mais. Coltura&Cultura; Angelini, R., Maggiore, T., Ponti, I., Eds.; HRE Edizioni: Milan, Italy, 2008; pp. 142–177. [Google Scholar]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance. Current assessment. ASCE J. Irrig. Drain. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Elazab, A.; Ordóñez, R.A.; Savin, R.; Slafer, G.A.; Araus, J.L. Detecting interactive effects of N fertilization and heat stress on maize productivity by remote sensing techniques. Eur. J. Agron. 2016, 73, 11–24. [Google Scholar] [CrossRef]
- Krishnamurthy, L.; Zaman-Allah, M.; Purushothaman, R.; Irshad Ahmed, M.; Vadez, V. Plant Biomass Productivity Under Abiotic Stresses in SAT Agriculture, Biomass—Detection, Production and Usage; Matovic, D., Ed.; InTech: London, UK, 2011; p. 504. ISBN 978-953-307-492-4. Available online: http://www.intechopen.com/books/biomass-detection-production-and-usage/plant-biomass-productivity-under-abiotic-stresses-in-sat-agriculture (accessed on 1 January 2021).
- Di Bari, V.; Tedeschi, P.; Colucci, R.; Mastrorilli, M.; Carone, F. Influenza dell’epoca di semina e del regime irriguo sulla canapa nel sud d’Italia. Agroindustria 2002, 2, 32–36. [Google Scholar]
- Hu, H.; Liu, H.; Liu, F. Seed germination of hemp (Cannabis sativa L.) cultivars responds differently to the stress of salt type and concentration. Indust. Crops Prod. 2018, 123, 254–261. [Google Scholar] [CrossRef]
- Akram, M.; Asghar Malik, M.; Yasin Ashraf, M.; Farruk Saleem, M.; Hussain, M. Competitive seedling growth and K/Na ratio in different Maize (Zea mays L.) hybrids under salinity stress. Pak. J. Bot. 2007, 39, 2553–2563. [Google Scholar]
- Hussein, M.M.; Balbaa, L.K.; Gaballah, M.S. Salicylic acid and salinity effects on growth of maize plants. Res. J. Agric. Biol. Sci. 2007, 3, 321–328. [Google Scholar]
- Amer, K.H. Corn crop response under managing different irrigation and salinity levels. Agric. Water Manag. 2010, 97, 1553–1563. [Google Scholar] [CrossRef]
- Hussain, M.; Bashir, W.; Farooq, S.; Rehim, A. Root development, allometry and productivity of Maize hybrid under terminal drought sown by varying method. Inter. J. Agric. Biol. 2013, 15, 1243–1250. [Google Scholar]
- Yi, L.; Shenjiao, Y.; Shiqing, L.; Xinping, C.; Fang, C. Growth and development of maize (Zea mays L.) in response to different field water management practice: Resource capture and use efficiency. Agric. For. Meteor. 2010, 150, 606–613. [Google Scholar] [CrossRef]
- Eziz, A.; Yan, Z.; Tian, D.; Han, W.; Tang, Z.; Fang, J. Drought effect on plant biomass allocation: A meta- analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Tedeschi, A.; Dell’Aquila, R. Effects of irrigation with saline waters, at different concentrations, on soil physical and chemical characteristics. Agric. Water Manag. 2005, 77, 308–322. [Google Scholar] [CrossRef]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis. Part 3. Chemical Methods; Spark, D.L., Ed.; SSSA Book Series no. 5. ASA and SSSA; American Society of Agronomy: Madison, WI, USA, 1996. [Google Scholar]
- Hunt, R. Plant Growth Curves. An Introduction to the Functional Approach to Plant Growth Analysis; Edward Arnold: London, UK, 1982. [Google Scholar]
- Doorenbos, J.; Kassam, A.H. Yield Response to Water; U.N. Food and Agriculture Organization Irrigation and Drainage Paper No. 33; FAO: Rome, Italy, 1979. [Google Scholar]
- Hoffman, G.J.; Maas, E.V.; Prichard, T.L.; Mayer, J.L. Salt tolerance of corn in the Sacramento-San joaquin Delta of California. Irrig. Sci. 1983, 4, 31–44. [Google Scholar] [CrossRef]
- Gomiero, T. Are biofuels an effective and viable energy strategy for industrialized societies? A reasoned overview of potentials and limits. Sustainability 2015, 7, 8491–8521. [Google Scholar] [CrossRef]
- Lark, T.J.; Hendricks, N.P.; Smith, A.; Pates, N.; Spawn-Lee, S.A.; Bougie, M.; Booth, E.G.; Kucharik, C.J.; Gibbs, H.K. Environmental outcomes of the US Renewable Fuel Standard. Proc. Natl. Acad. Sci. USA 2022, 119, e2101084119. [Google Scholar] [CrossRef]
- Giupponi, L.; Leoni, V.; Matteo Carrer, M.; Ceciliani, G.; Sala, S.; Panseri, S.; Pavlovic, R.; Giorgi, A. Overview on Italian hemp production chain, related productive and commercial activities and legislative framework. Ital. J. Agron. 2020, 15, 1552. [Google Scholar] [CrossRef]
- Katerji, N.; van Hoorn, J.W.; Hamdy, A.; Karam, F.; Mastrorilli, M. Effect of salinity on emergence and water stress and early seedling growth of sunflower and maize. Agric. Water Manag. 1994, 26, 81–91. [Google Scholar] [CrossRef]
- Steduto, P. Water use efficiency. In Sustainability of Irrigated Agriculture NATO ASI Series E: Applied Science; Pereira, L.S., Feddes, R.A., Gilley, J.R., Lesaffre, B., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 193–209. [Google Scholar]
- Steduto, P.; Hsiao, T.C.; Fereres, E.; Raes, D. Crop Yield Response to Water; Food and Agriculture Organization of the United Nations, No 66; FAO: Rome, Italy, 2012; p. 7. [Google Scholar]
- Jiang, J.; Feng, S.; Huo, Z.; Wang, Y.; Sun, Z. Effect of irrigation with saline water on soil water–salt dynamics and maize yield in arid Northwest China. Wuhan Univ. J. Nat. Sci. 2010, 15, 85–92. [Google Scholar] [CrossRef]
- Shenker, M.; Ben-Gal, A.; Shani, U. Sweet corn response to combined nitrogen and salinity environmental stresses. Plant Soil 2003, 256, 139–147. [Google Scholar] [CrossRef]

| Country | Area (ha) |
|---|---|
| Canada [21] | 22,243 |
| China [22] | 65,927 |
| U.S. [23] | 63,535 |
| Europe [18] | 56,196 |
| France [18] | 17,900 |
| Lithuania [18] | 9182 |
| Estonia [18] | 4555 |
| Italy [18] | 4.000 |
| The Netherlands [18] | 3833 |
| Romania [18] | 3400 |
| Germany [18] | 3114 |
| Total | 207,901 |
| Country | Products | |||||||
|---|---|---|---|---|---|---|---|---|
| CBD | Fiber | Seeds | Food | Cosmetic | ||||
| Oil | Seed | Seedcake | ||||||
| U.S. [23] | 3830 | 5.03 | 57.43 | 4.67 | 67.1 | * 120 | ||
| China [22] | 800 | 1200 | ||||||
| CBD | Bast Fiber | Hurd | Infused beverage | Cosmetic | ||||
| Concrete | Composite | Textiles | Pet litter | Potting mix | ||||
| Canada [33] | 22,000 | 3000 | 116,000 | 12,000 | 5000 | 1800 | 4600 | 430,000 |
| Application | Hemp | Maize | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Seeds | Fiber | Leaves | Flower | Shivs | Grain | Stems | Spathes | Cobs | |
| % | |||||||||
| Oil Food | 15 | 3 | |||||||
| Oil Feed | 0.5 | ||||||||
| 1 Flour | 70 | ||||||||
| Cosmetics | 0.5 | ||||||||
| Whole seeds: food | 5 | ||||||||
| Whole seeds: feed | 67 | ||||||||
| Dehulled seeds: Feed | 2% | ||||||||
| Dehulled seeds: Food | 9.5 | ||||||||
| pharmaceutical | X | X | 10 | ||||||
| Feed | X | 1 | |||||||
| Food | 27 | ||||||||
| Tea/infusion | X | ||||||||
| paper | 55 | 28 | |||||||
| Moulding (automotive) | 14 | ||||||||
| Mulch & other | 5 | ||||||||
| Insulation material | 26 | ||||||||
| Construction | 15 | ||||||||
| Bedding material | 63 | X | X | X | |||||
| Other | 22 | 4 | |||||||
| Ethanol | X | 30 | X | X | X | ||||
| Confectionary | 30 | ||||||||
| Treatment | H cm | Green Biomass | % dm Biomass | Total dm Biomass | Partition in % of dm | HI | CWF | WUE | CWS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | Stems | Hurd or Shive | Fiber | |||||||||
| % | ||||||||||||
| % FC-mm | Drought stress-Di Bari et al. [62]—Biomass (t ha−1) | |||||||||||
| 100–612 | 231 | 42.5 | 39.1 | 16.6 | 21.7 | 78.3 | 60.3 | 18.0 | 0.18 | 0.5 | 2.7 | 2.12 |
| 68–416 | 237 | 39.0 | 37.7 | 14.7 | 18.4 | 81.6 | 61.5 | 20.1 | 0.20 | 0.7 | 3.5 | 2.88 |
| 34–282 | 207 | 33.6 | 37.2 | 12.5 | 20.0 | 80.0 | 61.5 | 18.5 | 0.18 | 0.8 | 4.4 | 3.55 |
| 0–182 | 212 | 27.6 | 35.1 | 9.7 | 20.6 | 79.4 | 62.5 | 16.9 | 0.17 | 0.9 | 5.3 | 4.23 |
| Harvest 23/6 | Cosentino et al. [41]—Biomass (t ha−1) | |||||||||||
| ETm % | ||||||||||||
| 100 | 102 | 6.24 | 33.8 | 66.2 | 1.91 | 1.26 | ||||||
| 50 | 98 | 7.06 | 33.7 | 66.3 | 2.37 | 1.57 | ||||||
| 25 | 98 | 6.91 | 30.7 | 69.3 | 2.43 | 1.69 | ||||||
| - | 95 | 6.62 | 31.9 | 68.1 | 2.48 | 1.69 | ||||||
| Harvest 13/7 | ||||||||||||
| 100 | 12.02 | 27.6 | 72.4 | 2.73 | 1.97 | |||||||
| 50 | 11.09 | 26.2 | 73.8 | 3.13 | 2.31 | |||||||
| 25 | 9.84 | 29.5 | 70.5 | 3.15 | 2.22 | |||||||
| - | 9.77 | 27.0 | 73.0 | 3.63 | 2.65 | |||||||
| Treatment | H cm | Green Biomass | % dm Biomass | Total dm Biomass | Partition in % of dm | HI | CWP | WUE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | Stems | Grain | Cob | Ear | ||||||||
| % | ||||||||||||
| ECw (dSm−1) | Salinity stress-Akram et al. [67]—Biomass (g pt−1) | |||||||||||
| 3.0 | 79 | 33.44 | 10.5 | 3.52 | ||||||||
| 8.8 | 58 | 29.36 | 10.3 | 3.01 | ||||||||
| 13.0 | 44 | 16.18 | 10.0 | 1.62 | ||||||||
| Salt concentration in ppm of +Na ion | Hussein et al. [68]—Biomass (g pt−1) | |||||||||||
| 250 | 70 | 39.01 | 54.45 | 45.55 | ||||||||
| 2000 | 57 | 27.14 | 57.89 | 42.11 | ||||||||
| 4000 | 48 | 21.43 | 53.34 | 46.66 | ||||||||
| ECw-ECs (dSm−1)-(mm) | Amer [64]—Biomass (t ha−1) | |||||||||||
| 0.89–2.68–453 | 27.1 | 32.25 | 0.32 | 1.93 | 5.98 | |||||||
| 2.81–5.38–423 | 24.6 | 30.28 | 0.30 | 1.76 | 5.81 | |||||||
| 5.73–7.25–380 | 19.3 | 28.50 | 0.29 | 1.45 | 5.08 | |||||||
| ETm-(mm) | Drought stress-Amer [64]—Biomass (t ha−1) | |||||||||||
| 1.4–634 | 25.2 | 29.4 | 0.29 | 1.17 | 3.97 | |||||||
| 1.2–544 | 25.5 | 32.2 | 0.32 | 1.51 | 4.69 | |||||||
| 1.0–453 | 26.2 | 33.4 | 0.33 | 1.93 | 5.78 | |||||||
| 0.8–362 | 22.2 | 32.2 | 0.32 | 1.98 | 6.13 | |||||||
| 0.6–272 | 16.2 | 27.8 | 0.28 | 1.65 | 5.96 | |||||||
| %FC-mm | Hussain et al. [65]—Biomass (t ha−1) | |||||||||||
| 75–516 | 153 | 18.74 | 30.74 | 0.31 | 1.12 | 3.63 | ||||||
| 50–440 | 148 | 14.95 | 29.16 | 0.29 | 0.99 | 3.40 | ||||||
| %FC-mm | Yi et al. [66]—Biomass (t ha−1) | |||||||||||
| SI-467 | 25.6 | 60.2 | 0.60 | 3.30 | 5.48 | |||||||
| RF-371 | 19.9 | 59.3 | 0.59 | 3.18 | 5.36 | |||||||
| FM-365 | 24.0 | 60.0 | 0.60 | 3.95 | 6.58 | |||||||
| Soil Layer (m) | (dS m−1) | Water Extracted % of the Total ETa | ||
|---|---|---|---|---|
| Maize | Hemp | Maize | Hemp | |
| 0.0–0.3 | 2.65 (±1.62) c | 3.24 (±2.05) c | 52.7 (±1.1) a | 50.9 (±2.8) a |
| 0.3–0.6 | 3.46 (±2.13) bc | 3.85 (±2.29) ab | 41.9 (±2.2) a | 35.6 (±1.3) b |
| 0.6–0.9 | 4.12 (±2.73) a | 4.20 (±2.36) a | 5.4 (±6.1) d | 13.5 (±4.8) c |
| 0.0–0.9 | 3.41 (±2.08) | 3.76 (±2.19) | 100.0 | 100.0 |
| Treatment | Maize | ||||
|---|---|---|---|---|---|
| ( dS m−1) | Irrigation Water Retained (mm) | Rainfall (mm) | Change in Soil Water Storage (mm) | ETa (mm) | ETa/ET0 |
| C = 0.95 | 200.7 | 123.6 | 75.7 | 400.0 (±14.8) a | 0.81 |
| S1 = 2.25 | 192.0 | 123.6 | 57.4 | 373.0 (±15.6) ab | 0.76 |
| S2 = 3.96 | 185.1 | 123.6 | 20.3 | 329.0 (±7.2) bc | 0.67 |
| S3 = 6.48 | 179.6 | 123.6 | 1.8 | 305.0 (±12.0) c | 0.62 |
| Hemp | |||||
| C = 0.95 | 178.5 | 112.2 | 58.9 | 349.6 (±10.0) a | 0.76 |
| S1 = 2.88 | 179.5 | 112.2 | 41.6 | 333.3 (±9.6) ab | 0.72 |
| S2 = 4.18 | 170.0 | 112.2 | 23.3 | 305.5 (±11.4) bc | 0.66 |
| S3 = 7.03 | 163.0 | 112.2 | 7.6 | 282.8 (±10.8) c | 0.61 |
| Maize | |||
|---|---|---|---|
| Treatments | LAI (%) | LAD (%) | W (%) |
| S1 | −5 (±1.7) | −6 (±0.7) | −5 (±9.6) |
| S2 | −20 (±4.0) b | −26 (±3.5) a | −27 (±1.4) a |
| S3 | −31 (±3.8) c | −38 (±0.5) b | −47 (±0.7) a |
| Hemp | |||
| S1 | −3 (±4.8) | −3 (±1.3) | −5 (±2.0) |
| S2 | −12 (±5.8) | −18 (±0.8) | −15 (±1.2) |
| S3 | −28 (±6.2) a | −28 (±1.4) a | −32 (±1.7) a |
| Stress Indicators | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Maize | Hemp | ||||||||
| Ky | M & H | WUE | Ky | M & H | |||||
| KW | KLAD | a (dS m−1) | b% | (g kg−1) | KW | KLAD | a (dS m−1) | b% | WUE (g kg−1) |
| 1.79 | 1.41 | 1.38 | 9.1% | 5.13 | 1.47 | 1.47 | 2.34 | 6.2% | 4.98 |
| Crops | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Maize | Hemp | ||||||||
| Paramters | Unit | C | S1 | S2 | S3 | C | S1 | S2 | S3 |
| dS m−1 | 0.95 (±0.14) d | 2.25 (±0.18) c | 3.96 (±0.36) b | 6.48 (±0.36) a | 0.95 (±0.08) d | 2.88 (±0.32) c | 4.18 (±0.30) b | 7.03 (±0.35) a | |
| dm biomass | t ha−1 | 20.7 (±0.29) a | 19.7 (±0.26) a | 15.1 (±0.23) b | 10.97 (±0.18) c | 20.1 (±0.51) a | 19.0 (±0.53) a | 17.1 (±0.47) b | 13.7 (±0.56) c |
| dm grain | t ha−1 | 9.2 (±0.25) a | 8.5 (±0.12) a | 7.1 (±0.31) b | 4.9 (±0.15) c | - | - | - | - |
| Kernels per spike | n. | 502 (±4.0) a | 492 (±16.0) a | 489 (±22.0) a | 394 (±12.0) b | - | - | - | - |
| Weight of 1000 seeds | gr | 305 (±15) a | 288 (±18) a | 242 (±11) b | 207 (±10) c | - | - | - | - |
| dm stems | t ha−1 | - | - | - | - | 17.8 (±0.56) a | 17.1 (±0.58) a | 15.4 (±0.36) b | 12.5 (±0.51) c |
| Fiber/Stems | % | - | - | - | - | 22.5 (±0.52) | 22.8 (±0.46) | 22.7 (±0.56) | 22.4 (±0.37) |
| dm fiber | t ha−1 | - | - | - | - | 4.0 (±0.16) a | 3.9 (±0.12) a | 3.5 (±0.15) ab | 2.8 (±0.04) b |
| WUE of dm Biomass | kg m−3 | 5.18 (±0.04) a | 5.28 (±0.1) a | 4.59 (±0.07) b | 3.60 (±0.09) c | 5.75 (±0.15) a | 5.71 (±0.17) a | 5.61 (±0.15) a | 4.84 (±0.1) b |
| Maize | |||||||||||
| dm | Partition in % of dm | ||||||||||
| Treatment | ETa | Biomass | Leaves | Stems | Ears | Grain | Leaves | Stems | Cob | Grain | Ears |
| (dS m−1) | (mm) | t ha−1 | % | ||||||||
| C = 0.95 | 400 | 20.7 (±0.29) a | 1.17 (±0.05) a | 7.2 (±0.4) a | 12.4 (±0.1) a | 9.2 (±0.1) a | 5.6 (±0.1) | 34.7 (±2.2) | 14.9 (±3.2) | 44.8 (±1.1) | 59.7 (±2.3) |
| S1 = 2.25 | 373 | 19.7 (±0.26) a | 1.14 (±0.16) a | 7.6 (±0.6) a | 11.0 (±0.8) b | 8.5 (±0.7) a | 5.8 (±0.7) | 38.5 (±3.6) | 12.7 (±1.1) | 43.0 (±2.3) | 55.7 (±3.3) |
| S2 = 3.96 | 329 | 15.1 (±0.23) b | 0.66 (±0.05) b | 5.5 (±0.5) b | 8.9 (±0.6) c | 7.1 (±0.4) b | 4.4 (±0.5) | 36.5 (±3.2) | 12.0 (±2.9) | 47.1 (±2.0) | 59.1 (±3.3) |
| S3 = 6.48 | 305 | 11.0 (±0.18) c | 0.54 (±0.03) b | 3.8 (±0.1) c | 6.7 (±0.2) d | 4.9 (±0.1)c | 4.9 (±0.2) | 34.4 (±1.3) | 15.4 (±1.5) | 45.3 (±0.4) | 60.7 (±1.2) |
| Hemp | |||||||||||
| dm | Partition in % of dm | ||||||||||
| Treatment | ETa | Biomass | Leaves | Stems | Fiber | Fiber/Stem | Leaves | Shiv | Fiber | Stems | |
| (dS m−1) | (mm) | t ha−1 | % | % | |||||||
| C = 0.95 | 350 | 20.1 (±0.51) a | 2.5 (±0.6) A | 17.6 (±0.56) a | 4.0 (±0.16) a | 22.5 (±0.25) | 12.4 (±3.8) | 67.7 (±3.0) | 19.9 (±0.7) | 87.6 (±3.8) | |
| S1 = 2.88 | 333 | 19.0 (±053) a | 1.9 (±0.3) AB | 17.1 (±0.58) a | 3.9 (±0.12) a | 22.8 (±0.12) | 10.0 (±1.2) | 69.5 (±1.1) | 20.5 (±0.5) | 90.0 (±1.1) | |
| S2 = 4.18 | 305 | 17.1 (±0.47) b | 1.7 (±0.3) BC | 15.4 (±0.36) b | 3.5 (±0.15) ab | 22.7 (±0.31) | 10.0 (±1.5) | 69.5 (±1.5) | 20.5 (±0.7) | 90.0 (±1.5) | |
| S3 = 7.03 | 283 | 13.7 (±0.56) c | 1.2 (±0.5) C | 12.5 (±0.51) c | 2.8 (±0.04) b | 22.4 (±0.15) | 8.8 (±0.8) | 70.8 (±1.1) | 20.4 (±0.6) | 91.2 (±0.8) | |
| Maize | |||||||
| Treatment | |||||||
| (dSm−1) | ETa (mm) | Biomass % | Leaves % | Stems % | Grain % | Cob % | Average |
| C = 0.95 | 400 | - | |||||
| S1 = 2.25 | 373 | 4.8 (±0.9) D | 3.1 (±9.8) D | 0.0 | 8.7 (±6.0) D | 17.5 (±9.9) C | 6.7 (±7.8) c |
| S2 = 3.96 | 329 | 27.3 (±1.2) C | 43.6 (±2.6) AB | 23.7 (±4.2) C | 23.5 (±3.7) C | 37.9 (±11.1) B | 31.8 (±10.8) b |
| S3 = 6.48 | 305 | 47.1 (±0.6) A | 53.8 (±2.1) A | 47.3 (±4.4) A | 46.1 (±0.7) A | 43.5 (±10.7) AB | 47.5(±11.1) a |
| Average | 26.4 (±17.1) b | 33.8 (±19.1) a | 23.7 (±18.1) b | 26.1 (±16.5) b | 33.0 (±15.3) a | ||
| Hemp | |||||||
| Treatment | |||||||
| (dSm−1) | ETa (mm) | Biomass % | Leaves % | Stems % | Fiber % | ||
| C = 0.95 | 350 | - | |||||
| S1 = 2.88 | 333 | 5.5 (±3.5) | 17.8 (±16.0) | 3.9 (±2.7) | 2.5 (±9.6) | 7.4 (±10.2) c | |
| S2 = 4.18 | 305 | 14.9 (±7.0) | 26.4 (±12.5) | 13.2 (±4.6) | 12.3 (±5.1) | 16.7 (±10.9) b | |
| S3 = 7.03 | 283 | 31.8 (±8.0) | 43.7 (±15.2) | 29.4 (±4.7) | 29.4 (±2.3) | 33.6 (±4.3) a | |
| Average | 17.4 (±10.7) b | 29.3 (±18.2) a | 15.5 (±11.0) b | 14.7 (±11.2) b | |||
| Hemp | |||||
| Cost of produced total biomass (€ ha−1) | 840 ÷ 1340 | ||||
| Fraction end-products | flour | oil | fiber | hurd | |
| Fraction of total market value | 20% | 22% | 15% | 42% | |
| Cost of biomass € ha−1 | 168 ÷ 268 | 185 ÷ 295 | 126 ÷ 201 | 353 ÷ 563 | |
| Fraction of seed biomass | 70% | 20% | |||
| Milling cost 60 € q−1 | Oil extraction cost 80 € q−1 | ||||
| Flour packaging cost 0.9 €/250 g | Oil packaging cost 0.9 € / 250 mL | ||||
| Market value | 10 € kg−1 | 38 € L−1 | 60 € q−1 | 50 € q−1 | |
| Fraction end-products | flour | oil | fiber | hurd | |
| Maize | |||||
| cost of produced total biomass (€ ha−1) | 1530 | ||||
| Fraction end-products | flour | oil | |||
| Fraction of total market value | 85 ÷ 95% * | 4% | |||
| Cost of biomass € ha−1 | 1300 ÷ 1453 | 61 | |||
| Fraction of seed biomass | 65 ÷ 100% # | 8% | |||
| Industrial mills extraction cost 0.07 € kg−1 Stone mills extraction cost 0.25 € kg−1 | Importation cost including packaging cost (1.47 € lt−1) | ||||
| Flour packaging cost (0.25 € kg−1) | |||||
| Market value | ◆ 1.5 ÷ 4.0 € kg−1 | 2.2 € lt−1 | |||
| Maize | |||||
|---|---|---|---|---|---|
| C | S1 | S2 | S3 | ||
| Seeds | q ha−1 | 92 | 85 | 71 | 49 |
| Market value seeds (30 € q−1) | 2760 | 2550 | 2130 | 1470 | |
| Flour | Market value industrial extraction and wholemeal flour (€ ha−1) | 36,800 | 34,000 | 28,400 | 19,600 |
| Market value stone mills extraction standard flour (€ ha−1) | 8970 | 8288 | 6923 | 4778 | |
| Industrial mills extraction € ha−1 | 644 | 595 | 1154 | 796 | |
| Stone mills extraction € ha−1 | 1495 | 1381 | 1775 | 1225 | |
| Flour packaging € ha−1 | 2300 ÷ 1495 ◊ | 2125 ÷ 1381 | 1775 ÷ 1154 | 1225 ÷ 796 | |
| Net benefit chain industrial exctraction wholemeal flour € ha−1 | 32,403 | 29,827 | 24,675 | 16,579 | |
| Net benefit chain stone mills exctraction flour € ha−1 | 4680 | 4226 | 3315 | 1886 | |
| Oil | Market value € ha−1 | 1619 | 1496 | 1250 | 862 |
| Importation cost plus packaging cost € ha−1 | 1082 | 1000 | 835 | 576 | |
| Net benefit oil € ha−1 | 476 | 435 | 354 | 225 | |
| Total net benefit (flour and oil) € ha−1 | 32,878 ÷ 5155 ♣ | 30,262 ÷ 4661 | 25,029 ÷ 3669 | 16,804 ÷ 2111 | |
| Hemp | |||||
|---|---|---|---|---|---|
| C | S1 | S2 | S3 | ||
| Seeds | q ha−1 | 4.8 | 4.6 | 4.1 | 3.4 |
| Market value seeds (180 € q−1) | 864 | 828 | 738 | 612 | |
| Market value oil € ha−1 | 3648 | 3496 | 3116 | 2584 | |
| Market value flour € ha−1 | 3360 | 3220 | 2870 | 2380 | |
| Cost for oil extraction € ha−1 | 384 | 368 | 328 | 272 | |
| Cost for milling € ha−1 | 202 | 193 | 172 | 143 | |
| Cost Oil packaging € ha−1 | 346 | 332 | 295 | 245 | |
| Cost Flour packaging € ha−1 | 1210 | 1159 | 1033 | 857 | |
| Net benefit oil (range) retail without contractor/with contractor (€ ha−1) | 2733 ÷ 2624 | 2612 ÷ 2502 | 2308 ÷ 2198 | 1892 ÷ 1782 | |
| Net benefit flour (range) retail without contractor/ with contractor (€ ha−1) | 1780 ÷ 1680 | 1699 ÷ 1599 | 1497 ÷ 1397 | 1212 ÷ 1112 | |
| Fiber | q ha−1 | 40 | 39 | 35 | 28 |
| Market value € ha−1 | 2400 | 2340 | 2100 | 1680 | |
| Net benefit fiber (range) retail without contractor/with contractor (€ ha−1) | 2274 ÷ 2199 | 2214 ÷ 2139 | 1974 ÷ 1899 | 1554 ÷ 1479 | |
| Hurd | q ha−1 | 138 | 132 | 119 | 97 |
| Market value € ha−1 | 6900 | 6600 | 5950 | 4850 | |
| Net benefit hurd (range) retail without contractor/with contractor (€ ha−1) | 6548 ÷ 6337 | 6248 ÷ 6037 | 5598 ÷ 5387 | 4498 ÷ 4287 | |
| Total net benefit (range) without contractor/with contractor (€ ha−1) | 13,335 ÷ 12,840 | 127,73 ÷ 12,277 | 11,377 ÷ 10,881 | 9156 ÷ 8660 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedeschi, A.; Cerrato, D.; Menenti, M. Is the Potential for Multi-Functional Use of Industrial Hemp Greater than Maize under Saline Conditions? Sustainability 2022, 14, 15646. https://doi.org/10.3390/su142315646
Tedeschi A, Cerrato D, Menenti M. Is the Potential for Multi-Functional Use of Industrial Hemp Greater than Maize under Saline Conditions? Sustainability. 2022; 14(23):15646. https://doi.org/10.3390/su142315646
Chicago/Turabian StyleTedeschi, Anna, Domenico Cerrato, and Massimo Menenti. 2022. "Is the Potential for Multi-Functional Use of Industrial Hemp Greater than Maize under Saline Conditions?" Sustainability 14, no. 23: 15646. https://doi.org/10.3390/su142315646
APA StyleTedeschi, A., Cerrato, D., & Menenti, M. (2022). Is the Potential for Multi-Functional Use of Industrial Hemp Greater than Maize under Saline Conditions? Sustainability, 14(23), 15646. https://doi.org/10.3390/su142315646








