Abstract
Biosurfactant-producing microorganisms improve the efficacy of hydrocarbon biodegradation as the biosurfactant is essential in making hydrocarbons available for breakdown. The present study reports the isolation of biosurfactant-producing bacteria that can be used for crude oil remediation and to characterize the biosurfactant generated during the breakdown of crude oil. This study also reports evaluating the synergism and potentiality of biosurfactant-producing bacteria for simultaneous hydrocarbon biodegradation and power generation. Two bacterial strains (Bacillus subtilis strain B1 and Pseudomonas aeruginosa strain B2) were isolated from petroleum-contaminated soils, which are found effective in producing biosurfactants and degrading crude oil as the sole carbon source. B. subtilis B1 exhibited a higher potential for biosurfactant production and crude oil degradation than P. aeruginosa B2. The FTIR and GC-MS analysis were conducted for further characterization of the biosurfactant, which revealed that the surfactant produced by strain B1 and B2 was surfactin and rhamnolipid, respectively. The application of the B1 and B2 co-culture in microbial fuel cells (MFCs) showed synergism among them and resulted in a maximum power density production of 6.3 W/m3 with an open circuit voltage of 970 mV while degrading 2.5% v/v crude oil containing anolyte. The findings indicate that the co-culture of isolated crude oil-degrading strains has great potential for enhanced power generation and the bioremediation of hydrocarbon-contaminated environments. Moreover, the synergism of isolated strains in MFCs suggested their potent applicability in environmental, energy, and industrial sectors as an economical and feasible alternative to the existing technologies.
1. Introduction
Crude petroleum oil is one of the prevalent pollutants of the environment that causes negative impacts on the environment. With the continuous development of oil recovery technology and rising energy demand in the world, the utilization of crude oil and its exploitation is increasing day by day, and the accidental or routine release of petroleum oil into the environment during its production or transportation has caused a threat to the environment [1]. These releases of crude oils are a threat to the ecosystem, as they can be affected by the accumulation of sulfur metals, nitrogen, aromatics, and aliphatics into the food chain [2,3]. Polycyclic aromatic hydrocarbon is a hazardous pollutant because of its chemical stability and high resistance to several bioconversions [4,5]. Depending on the organism and the exposure, various toxic effects are caused by crude petroleum oil, such as acute fatal toxicity, sub-fatal chronic toxicity, or both [6,7]. These problems can be for the short term or long term. It is observed that this problem persists because of the higher cost of proper and safe disposal of petroleum waste. Hence, there is a need for the development of hydrocarbon remediation technology to remove and recover these crude oils from the environment.
The microbial remediation of crude petroleum is found to be more sustainable, effective, and cheap than other treatment methods [8]. After several studies, it was observed that pervasive and free-living microbes are predominant in hydrocarbon degradation [9,10]. Despite several hydrocarbon degraders in nature, we need to utilize external degraders as the growth of natural degraders is hindered by different factors such as the lack of organic compounds in the aquatic system, which prevent their utilization by microflora or substrate’s recalcitrant nature [11]. In search of any suitable method, the remediation of hydrocarbon-polluted sites with the addition of biosurfactants seems a better option [12,13]. Engine oil or motor oil has been found hazardous to the environment. Base lubricant oil, polyalphaolefins, additives, and ester are combined to make engine oil, which contains >75% cyclic alkanes and long-chain (C16–C36) saturated hydrocarbons. Engine oil is dispersed in the environment by dumping or incineration after use. Used engine oil is toxic as it contains PAHs, metals, etc. [14]. It contains potential threats to animals, humans, and the environment and therefore its necessary to eliminate it [15].
Microbial petroleum wastewater treatment has been gaining attention recently [16]. These microbes are found to produce biosurfactants with various chemical compositions such as neutral lipids, phospholipids, lipoproteins, lipopeptides, fatty acids, and glycolipids [17,18]. One of the most widely used biosurfactants is glycolipids, which have various structural variations and applications [19,20]. These biosurfactants reduce interfacial or surface tension depending on the interface (air/water or oil/water) [21]. The biosurfactant develops a new surface at the water/oil interface by producing a monolayer around the hydrocarbon particle directed by the surfactant, with the surfactant’s hydrophobic tail facing out to the liquid phase [22]. It causes elevations in the facilitation of emulsification and surface area. Through improved solubilization of water in hydrocarbons or hydrocarbons in water, the overall phenomenon increases the bioavailability of pollutants for microbial breakdown [23,24,25]. Microbial surfactants are considered best suited for environmental applications such as petroleum oil clean-up due to their biodegradable nature and reduced toxicity compared to chemical surfactants [26].
Bacteria that have the potential to break down petroleum waste are isolated and evaluated for their ability to do so in this study, which reviews the effective microbial degradation of crude petroleum oil. We found that Bacillus subtilis bacteria were engaged in the remediation of crude petroleum oil. Bacteria are favored over fungi because they proliferate at a pace that is 10 times faster than that of fungi. Oxidizing reactions are been observed in the degradation pathways of various petroleum hydrocarbons (e.g., aliphatics and polyaromatics); however, these pathways vary greatly due to the specific oxygenases found in various bacterial species. For instance, whereas certain bacteria can metabolize alkanes, others may break down aromatic or resinous subsets of hydrocarbons. The chemistry of petroleum hydrocarbons is linked to this occurrence. Table 1 summarizes the different species that are shown to metabolizepetroleum hydrocarbons and the specific petroleum components they degrade. Recent research has found that bacteria from more than 79 genera can break down petroleum hydrocarbons. Some of these bacteria include Achromobacter, Acinetobacter, Alkanindiges, Alteromonas, Arthrobacter, Burkholderia, Dietzia, Enterobacter, Kocuria, Marinobacter, Mycobacterium, Pandoraea, Pseudomonas, and Staphylococcus [27].
A microbial fuel cell (MFC) is a type of bio-electrochemical system for renewable energy that can generate electricity from a wide range of organic substrates catalyzed by electroactive bacteria [28,29]. The organic substrate is oxidized by microorganisms in these systems, releasing free electrons which are collected by anode and pass through an electric load before completing half-cell reduction reaction in the cathode [30]. Since MFCs rely on bacterial metabolism to generate energy, electrochemically active bacteria are the most favoured ones in MFCs. Inoculum from waste sources like, wastewater, soil sediment, and anaerobic sludge are frequently used in MFCs because of their habitat and physiological environments suitable for such group of bacteria [29] and co-metabolism in some [31].
In most studies reported, pure cultures generated electricity using simple substrates (glucose, acetate, carbon, etc.) [32]. However, pure cultures are ineffective inocula for waste matter-fed MFC because they cannot utilize complex substrates [33]. Mixed cultures are considered robust inocula due to their tolerant towards extreme environmental conditions, can accommodate a wide range of natural substrates, and may secrete hydrolytic enzymes and biosurfactants to degrade complex biomass [11]. Co-cultures, in which two or more cell populations grow in symbiosis, are studied to improve biotechnological processes. Co-culturing microorganisms have progressed and are now widely used in biomanufacturing, where they play a significant role in the mass production of pharmaceuticals, nutraceuticals, foods, and drinks. The approach is also widely used in bioremediation and bioenergy. Co-culture systems that have proven to be effective have been shown to have extensive potential in biotechnological applications due to their adaptability, sturdiness, and capacity to perform complex tasks [34].
Antagonistic interactions between bacteria may have a negative effect on MFC performance, while synergistic interactions within the microorganisms, especially those involved in the electron transfer, could favorably influence MFC performance [32]. Selective co-cultivation, also known as mixed-culture inoculum, has recently received fresh interest from scientists as a possible solution to the above-mentioned issues [35]. Examples include a 14-fold increase in current density in co-culture MFCs compared to monocultures [36], where it was found that a fermentation product (2,3-butanediol) produced by Enterobacter aerogens increased the production of mediator by P. aeruginosa. Higher power generation was achieved in co-culture MFC compared to monoculture MFC in another work by Islam et al. [37], who found that the metabolite promoted mutualistic interactions between P. aeruginous and Klebsiella. The success of selective co-cultivation strategies is based not only on the microorganisms used, but also several other variables. For instance, a recent report indicated that the electrolyte pH greatly affects the growth and physiology of bacteria cells and thus plays a crucial role in the energy production of MFCs [38]. Researchers have found that a reduction in anode pH might have a negative impact on both bacterial metabolism and MFC efficiency over time [39].
Co-cultures are advantageous to the symbiotic connections among microbial species that are the drivers of MFC operation and offer possibilities for enhancing technical applications, as well as the capability of providing researchers with a larger in vivo investigation on a laboratory scale. This facilitates a greater rate of throughput testing and offers a more extraordinary perspective for tracking the causes and consequences of interactions among cells and between cells and their substrates. Leading a complex community of microorganisms toward specific biotechnological applications is tough. Because of these, researchers have started using defined co-cultures [40].
The present study dealt with the screening and isolation of oil-degrading bacteria, the characterization of biosurfactants produced by isolates, and their co-culture application as biocatalysts in the anode chamber of MFC using oil as a substrate to evaluate bacterial synergism and performance. The performance was measured in terms of COD removal, substrate consumption, and power generation.
2. Materials and Methods
2.1. Soil Samples
Soil samples for the isolation of bacterial strains were collected from three different hydrocarbon-contaminated areas around petrol pumps and the petrochemical industry. Crude oil (engine oil) was used as a hydrocarbon contaminant. The chemicals and media used in this study were of AR grade purchased from Sigma-Aldrich (Banglore, India), Himedia (Mumbai, India), and SRL (New Delhi, India).
2.2. Isolation, Screening, and Identification of Bacterial Strains
For the isolation of bacteria, 2 g of soil contaminated with hydrocarbon was used as an inocula in a mineral salt medium (MSM) that contained crude oil in 2% (v/v) as the sole carbon source [19,41]. The MSM used was as reported by Patowary et al. [41]. The medium had an altered pH of 7.0 ± 0.2. The inoculated media was incubated at 37 °C for 7 days at 150 rpm. After 7 days of incubation, 2 mL of inoculum was added to 100 mL fresh MSM media and incubated for 7 days under identical conditions to decrease undesirable microorganisms. Later, 1 mL of the cultured media was utilized for 10-fold serial dilution, and 100 µL of the diluted media was spread on nutrient agar plates followed by incubation at 37 °C for 18–24 h. Different bacterial colonies based on their morphological differences were then picked and streaked on a nutrient agar plate. The isolates were also preserved in 30% glycerol at −80° and nutrient agar slants. Then, 24 h fresh liquid inoculum was prepared in nutrient broth from all of the bacterial isolates followed by transferring of 10 mL of the inoculum into a 500 mL Erlenmeyer flask containing 200 mL MSM with glucose (2% (w/v)) as the carbon source. The flask was then incubated at 37 °C in a rotary incubator at 200 rpm. Bacterial growth rates and their biosurfactant production were studied. A very effective crude oil degrader strain was chosen from all of the biosurfactant-producing isolates based on their increased cell mass in crude oil-containing conditions. The assay was performed according to the modified procedure described by previous studies [42,43,44]. Abiotic controls were also prepared to maintain similar conditions except for bacterial inoculum. The growth of the bacteria in each flask was observed from day 0 to 7 by reading the optical density at 600 nm in a UV-Vis spectrophotometer. Lastly, the isolates that showed maximum growth in media containing crude oil as the sole carbon source were separated for further analysis. The standard biochemical test was performed to identify the most effective microbial strain in the same way reported byPatowary et al. [19]. The initial characterization of the strains was carried out using a methyl red test, indole test, glucose test, Voges Proskauer’s test, adonitol test, arabinose test, lactose test, citrate utilization test, sorbitol test, mannitol test, rhamnose test, and sucrose test following the standard procedures. The 16S rRNA was used for molecular identification [44].
2.3. Assay for the Biosurfactant Production (Drop Collapse Assay)
A drop collapse assay with hydrocarbon substrate (crude oil) was performed [45]. The experiment aimed to break down crude oil by microbes; hence crude oil was used as a substrate [46]. To perform this assay, a drop of crude oil was placed on a glass slide which was followed by a drop of 48 h broth culture and finally allowed it to react at room temperature.
2.4. Crude Oil Degradation
The bacterial strains selected for crude oil degradation were added to a flask and cultured in a shaker incubator at 37 °C. The strains were then inoculated in a freshly prepared 100 mL MSM containing 2% crude oil as a carbon source and incubated at 37 °C for 4 weeks at 200 rpm. The bacterial growth was observed through optical density at 600 nm before extracting the crude oil residual every week from each flask by evaporating the sample through dichloromethane and then storing in falcon tubes. Then, all tubes were enumerated according to their week of collection. The test samples were compared with extracted crude oil samples from the abiotic control. Both crude oil samples extracted were used to analyze the degradation of different hydrocarbon fractions. Crude oil samples were extracted from the abiotic control for the comparison and analysis of degradation by both strains.
The crude oil samples extracted through dichloromethane were scrutinized using a single quadruple gas chromatograph–mass spectrometer (Shimadzu QP-2010 Plus with Thermal Desorption system TD 20). The gas chromatograph program was optimized for different petroleum hydrocarbon detection. All of the analyses were carried out at a 20:1 ratio. The GC-MS used helium as the carrier gas at a 1.0 mL/min flow rate with a constant 300 °C injection temperature. The column oven temperature was initially adjusted at 60 °C with a 5 min hold time, and then it was raised to 280 °C with an 8 °C/min ramp with 37 min of final hold [47]. The mass spectrometry data were obtained in electron ionization mode (70 eV). The interface and ion source temperatures were 310 and 230 °C, respectively. The complete analysis was carried out in the 45–600 m/z mass range. The GC-MS solution software version 4 was used to analyze the chromatograms, and the NIST 11 library database was used to identify the compounds.
2.5. Biosurfactant Extraction
After the treatment of crude oil for 4 weeks, the oil was extracted from the flask, and the highest degradation of total petroleum hydrocarbon (TPH) was measured through gravimetric assay. For biosurfactant extraction, the cell-free supernatant was made through the centrifugation of broth culture at 10,000 rpm for 10 min at 15–20 °C. 6N HCl was added into the supernatant to adjust the pH to 2 and then stored at 4 °C. The following day, ethyl acetate was used for biosurfactant extraction at room temperature, added at a 1:1 ratio, shaken vigorously on a vortex machine, and left at a stable position for phase separation. The biosurfactant was collected as an organic phase and a viscous product in a dark honey color obtained through evaporation using a rotary evaporator after evaporation of the solvent at 40 °C [48,49]. A gravimetric assay was carried out for the quantification of the crude biosurfactant.
2.6. Biosurfactant Purification and Calculation of Critical Micelle Concentration
Crude biosurfactant purification was conducted according to the method followed by earlier research [19,50]. A chromatographic column (26 × 3.3 cm2) was used for the purpose, and the chromatographic column contained about 50 g of activated silica gel and a slurry of mesh chloroform. A total of 1 g of crude biosurfactant was mixed with 5 mL of CHCl3 to prepare the sample and loaded onto the column, which was further washed with chloroform for the complete elution of natural lipids. Different ratios of CHCl3/CH3OH as the mobile phase were applied in sequence, i.e., 50:50 v/v (100 mL), 50:5 v/v (200 mL), and 50:3 v/v (250 mL) at a flow rate of 1 mL/min. Then, 20 mL from each fraction was collected separately, and the surface tension was measured to detect biosurfactants. The column was washed with 50:50 methanol/chloroform to elute the leftover biosurfactant. Finally, all of the biosurfactant fractions were mixed thoroughly and dried in a rotary evaporator to obtain a pure product. The amount of an amphiphilic unit in a solution at which micelle production begins refers to critical micelle concentration (CMC). For the CMC calculation, a concentration gradient was prepared from the range of 0.1 to 200 mg L−1 by mixing the purified biosurfactants from each organism with distilled water. The critical micelle concentration was calculated by plotting biosurfactant concentration against surface tension as its function [51].
2.7. Biosurfactant Characterization
The characterization of produced biosurfactant was achieved using FTIR analysis [47]. FTIR was performed for the chemical characterization of the produced biosurfactant using a NICOLET 6700 FTIR-Spectrophotometer at 500 to 4000 cm−1 in ATR mode for the present functional groups and bond type detection [52].
2.8. Emulsification Assay
The emulsification assay of the biosurfactant produced by both the strains in a medium containing crude oil was assessed according to the protocol mentioned by Patil and Saima [53] against crude oil. In total, 1 mL of crude oil and 1 mL of cell-free supernatant containing biosurfactant-producing culture was added in an Eppendorf tube, vortexed vigorously for 5 min, and left to stand for 30 min at 35 °C followed by determining emulsification activity.
2.9. Determination of Chemical Oxygen Demand Reduction
This test was performed with both strains (B1 and B2). Both organisms were cultured in nutrient broth media and utilized for the assay. The experiments were carried out in 100 mL Erlenmeyer flasks, and the samples were taken after incubating them for 0, 24, 48, 72, and 120 h at 30 °C and 150 rpm. A total of eight tests were carried out in which 10 µL of each strain was initially added to 50 mL of the crude oil. Secondly, 5 µL of each strain was added simultaneously to 50 mL of the crude oil. Next, 0.25 g of each biosurfactant produced by strains B1 and B2 was separately added to each 50 mL of the crude oil. Finally, 0.125 g of each biosurfactant produced by the strains was added simultaneously to 50 mL of the crude oil. The COD determination was performed in a COD reactor using potassium dichromate as an oxidant agent [54].
2.10. Microbial Fuel Cell Design, Inoculation, and Electricity Generation
Five single-chambered air cathode MFCs were made using earthen pots to treat wastewater with different concentrations of hydrocarbons. Each chamber had a total working capacity of 300 mL. Anode was made of 6 × 6 cm carbon felt. The cathode was exposed to open air, and MFCs were operated on closed circuit mode connected to the external resistance (100 Ω) [55]. The inoculum was a 1:1 mix of each isolated bacterial culture, B. subtilis B1 and P. aeruginosa B2. The cathode, anode, reference electrode (Ag/AgCl, saturated KCl; +197 mV, Equiptronics, Mumbai, India) and sampling ports were located on the top of the anode chamber [56]. The isolated strains (B1 and B2) were co-cultured and incorporated with MSM with glucose as a carbon source in the anode chambers of various MFCs to analyze the biodegradation of hydrocarbons at different concentrations. The next step was to test whether these bacteria could degrade hydrocarbons and produce current while degrading hydrocarbons. Once the OCV was stable, an electrochemical investigation of the MFC was carried out in the form of polarization and power density curves using the variable resistor method (10–10k Ohms) [57]. The stainless-steel mesh employed in this research had 50 squares per inch of holes and was of the SS-304 variety. The SS mesh was made with wire that was 0.17 mm in diameter. A concealed copper wire served as the cathode termination and completed the connection. All tests had an inter-electrode spacing of roughly 2.5 cm. A digital multimeter was employed to monitor the current flow (HTC 830 L). The polarization experiment employed a data recorder and a variable external resistance box. We tested the corresponding volumetric power density using the following equations. The volumetric power density, Pd (W/m3 and mW/m2) = EI/Vand, where E and I are voltage and current, respectively, linked to exactly imposed loads, and Vand is the expected anolyte volume, was used to calculate the power density normalized to the expected anode surface area. The MFC’s internal resistance was calculated using the slope of the linear region of the voltage vs. current graph. When conducting electrochemical tests on MFCs, the platinum rod and anode were used as the counter and working electrodes, respectively [54]. The current and voltage were measured against Ag/AgCl electrode (+197 mV vs. SHE). EIS was achieved by applying alternating current (AC) at a frequency of 100 kHz to 100 MHz and a voltage intensity of 5 mV. The analogous network model was used to determine the solution resistance (Rs), charge transfer resistance (Rct), and the solution resistance from the EIS spectra [57]. Using a COD measurement instrument set, the anolyte’s COD levels were determined [54]. The MFCs were ready to be studied for polarization 48 h after they were inoculated. Once the OCV was stable, different resistor values were found to record the MFC polarization and power density curves. Ohm’s Law (I = V/R) was used to determine the current (I), and the resistance was optimized and used to obtain the most current possible.
3. Results
3.1. Screening, Selection and Identification of Biosurfactant-Producing Bacteria
Colonies of five different contrasting morphologies were isolated from three different soils contaminated by hydrocarbon or petroleum oil, and they were screened for biosurfactant production. Among the five isolates, the culture B1 and B2 showed positive drop collapse assay results indicating biosurfactant production in the liquid culture media. The crude oil metabolzed within a few minutes of the addition of the broth culture no furthere metabolisms was observed thereafter (Figure 1). These isolates exhibited maximum growth (OD600 = 1.13) in a medium containing crude oil as the sole carbon source after day 7 of inoculation and produced biosurfactant in a higher amount than the strain B2 in MSM containing glucose in 2% (w/v). The optical density of different bacterial isolates at 600 nm and their produced amount of biosurfactant are presented in Figure 1.
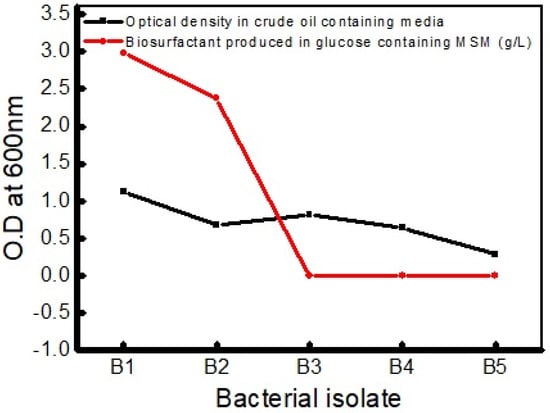
Figure 1.
Measured optical density of bacterial isolates and biosurfactant production in glucose-containing MSM (g/L).
3.2. Characterization of Hydrocarbon-Degrading and Biosurfactant-Producing Bacteria
Physiological and morphological characteristics of the B1 isolate resemble B. subtilis with a circular white colony, jagged edges, and rough texture. Table 1 shows the biochemical test for the isolated B. subtilis strain B1. The morphology of strain B1 was checked by Gram-staining, and the strain showed positive results with a rod-shaped structure. The 16S rRNA gene sequencing of strain B1 showed 99.68% sequence similarity to its closest relative, B. subtilis strain MT3T. On the other hand, the physiological and morphological characteristics of strain B2 resemble P. aeruginosa with a small, smooth surface colony. The morphology of strain B2 was also checked by Gram-staining, and the strain exhibited negative results with rod-shaped structures. The biochemical test confirmed the isolate to be P. aeruginosa (Table 1). The 16S rRNA gene sequencing of strain B2 showed 100% sequence similarity to its closest relative, P. aeruginosa strain E167. A phylogenetic analysis was performed to find the species most closely related to the query sequences (Figure 2a,b).


Table 1.
Biochemical test result for strain B1 (B. subtilis) and B2 (P. aeruginosa).
Table 1.
Biochemical test result for strain B1 (B. subtilis) and B2 (P. aeruginosa).
| Biochemical Test | B1 (B. subtilis) | B2 (P. aeruginosa) |
|---|---|---|
| Citrate utilization | + | + |
| Lysine utilization | − | − |
| Ornithine utilization | − | − |
| Urease | − | − |
| Phenylalanine deamination | − | − |
| Nitrate reduction | + | + |
| H2S production | − | − |
| Glucose | + | − |
| Adonitol | − | − |
| Lactose | − | − |
| Arabinose | + | + |
| Sorbitol | + | − |
Note: + indicates a positive reaction and − indicates a negative reaction.
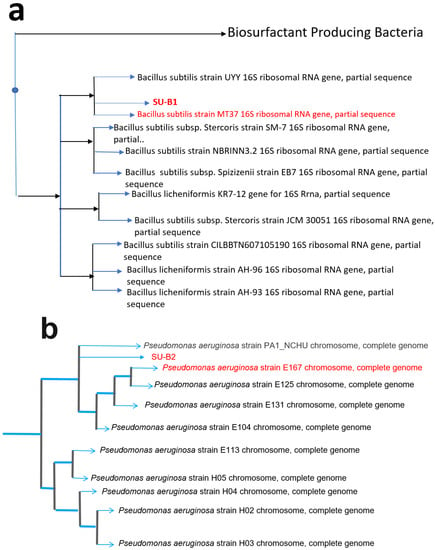
Figure 2.
Phylogenetic trees of (a) B. subtilis B1 and (b) P. aeruginosa B2 were isolated from soil samples.
3.3. Biodegradation of Crude Oil by Isolated Bacteria
The crude oil degradation pattern of P. aeruginosa B2 showed an elevation in degradation in each successive week, recorded from first (40.36%) to fifth (80.92%). The strain B. subtilis B1 also showed a degradation pattern of 45.4% to 87.3% in the first and fifth weeks, respectively (Figure 3).
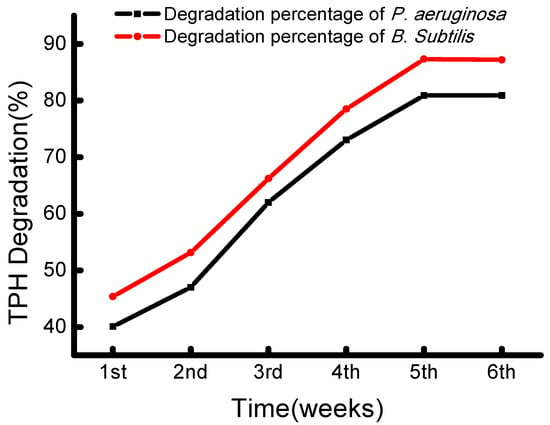
Figure 3.
Percentage of crude oil degradation by the strain B1 (B. subtilis) and B2 (P. aeruginosa) at weekly intervals and incubation for 6 weeks.
GCMS was analyzed for the extracted hydrocarbon residue after 4 weeks with B1 (B. subtilis) and B2 (P. aeruginosa) and compared with an abiotic control under identical working conditions. Figure 4 shows the obtained chromatograms. It is seen from the chromatograms that there is a reduction in the total petroleum hydrocarbon in samples treated with strains B1 and B2 when compared with the abiotic control. This indicates that both strains are highly effective in various crude oil component degradation and confirms the gravimetric results. Bacteria break down alkaline hydrocarbons by first oxidizing the terminal methyl group to generate primary alcohol, which is converted into the equivalent aldehyde and finally into fatty acid derivatives, while either anaerobic or aerobic peripheral routes are used for the microbial breakdown of aromatic hydrocarbons. Oxygen can oxidize and split aromatic rings under aerobic conditions. This activation of O2, which mostly produces protocatechuate, homogentisate, gentisate, and catechol, requires the enzyme oxygenases. These core intermediates have ortho- or para-located electron-rich substituents that serve as “activators” for oxidative ring breakage. In contrast, alternative central intermediates are used in anaerobic conditions to facilitate the transport of electrons to the ring because O2 is not present. The typical intermediate in the anaerobic breakdown of aromatic compounds is benzoyl-CoA, with the carboxy-thioester group serving as the electron-withdrawing substituent. Various alkanes, including long-chain and short-chain alkanes (C36 to C8), namely, n-Hexatriacontane (C36), n-Tritriacontane (C33), 3-methyl-octacosane (C29), didecyleicosane (C20), hexadecane (C16), n-undecane (C11), and n-octane (C8), were detected in the abiotic control. These were degraded by both bacterial strains B1 and B2. Other aromatic hydrocarbons and six different PAHs were also present in the abiotic control. Among the six detected PAHs, both strains can almost degrade all (>55%). Among the six PAHs, strain B2 was able to degrade 1H-Indene completely. The rest of the hydrocarbons are also degraded to some extent (>55%) (Table 2).
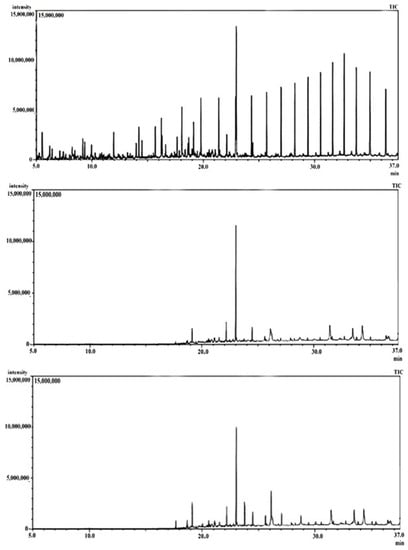
Figure 4.
GCMS chromatographs: abiotic control (top), crude oil treated for 4 weeks by B. subtilis B1 (middle), and crude oil treated for 4 weeks by P. aeruginosa B2 (below).

Table 2.
Comparison of the PAHs detected in the untreated abiotic control crude oil sample and the crude oil samples treated by strains B1 and B2 after 4 weeks.
3.4. Extraction and CMC Determination of Crude Biosurfactant
The quantity of crude biosurfactant produced by strain B1 extracted during crude oil degradation was 3.4 g/L, whereas the quantity extracted by strain B2 was 2.8 g/L. The value of CMC of the extracted biosurfactants from both strains was evaluated. The reduction in the surface tension is majorly dependent on CMC. Surfactant molecules in a polar or aqueous environment bind and form micelles at CMC. It was found that surface tension rapidly decreases with the increase in biosurfactant concentration. The lowest surface tension obtained by the biosurfactant produced by strain B1 was 29.4 mN/m at 55 mg/L and 27.5 mN/m at 57 mg/L by the biosurfactant produced by strain B2.
Further, the concentration of each biosurfactant was increased, but no decrease in surface tension was observed beyond 55 mg/L for strain B1 and 57 mg/L for strain B2. Hence, the value of CMC of crude biosurfactant was evaluated to be ~55 mg/L for B. subtilis B1 and ~57 mg/L for P. aeruginosa B2 from the surface tension breakpoint vs. its concentration curve log. The results showed that the crude biosurfactant has excellent surface tension properties and a decreased CMC value.
3.5. Biosurfactant Characterization
The biosurfactant-purified column of the FTIR spectrum showed the important bands at 3376, 1637, 1071, and 691 cm−1 for strain B1, which indicates that the components are similar to surfactin biosurfactant [58]. On the other hand, for strain B2, the important bands are at 3398, 3417, 1633, 1100, and 691 cm−1, indicating that the components are like rhamnolipids (Figure 5) [59]. In the case of strain B1, the formation of a broad band at 3376 cm−1 confirms the presence of a hydroxyl group. The band peak at 1637 cm−1 shows the presence of the COO− group. Absorption at 1071 cm−1 indicates the presence of a glycosidic bond, whereas absorption at 690 cm−1 shows the presence of the C-H group. In the case of strain B2, the formation of broad band at 3398 and 3417 cm−1 indicates the presence of a hydroxyl group. The band at 1633 cm−1 asserted the presence of the carboxyl group (COOˉ), whereas the absorption at 1100 cm−1 showed the presence of a glycosidic bond (C-O-C). The absorption at 690 cm−1 shows the presence of the C-H group. Therefore, from the discussion above, the biosurfactant produced by strain B1 was confirmed as surfactin, while the biosurfactant produced by strain B2 was confirmed as rhamnolipid. For the interpretation of the results, the spectrum was compared with Pornsunthorntawee et al. [60].
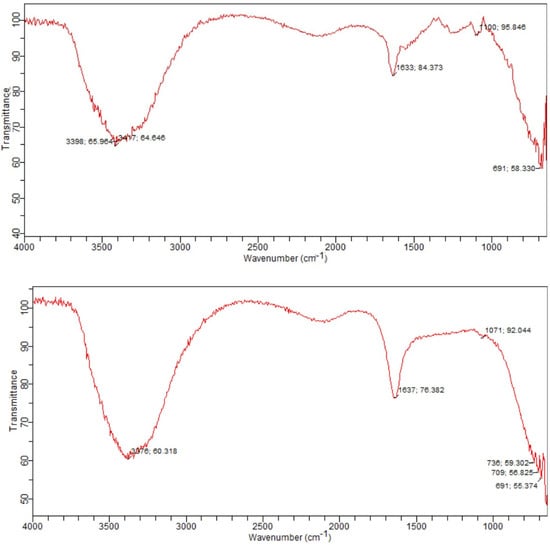
Figure 5.
FTIR spectrum of biosurfactant produced by the strain B1 (above) and B2 (below).
3.6. Emulsification Assay
The emulsification assay of the supernatant cultures of both strains was evaluated against crude oil. Initially, the test was performed and left for incubation for 24 h, which showed the E24 indices as 87.3% for strain B1 and 84.92% for strain B2. Furthermore, the incubation time of the test was increased, which showed little increase in the E24 index, i.e., by 3–5%. The strain B1 showed maximum emulsification after 48–72 h, i.e., 92%, while strain B2 was also close enough to the emulsification of the B1 strain, i.e., 89%. For the biodegradation of hydrocarbon, the strong emulsification action of a hydrocarbon-degrading microorganism is always regarded as a highly important component.
3.7. Determination of COD Reduction
Initially, the value of the COD of crude oil was observed to be 4200 mg/L (Figure 6). The highest results in COD deduction came from bioremediation studies that utilized individual bacterial strains. About 75% COD reduction was observed with strain B1, while strain B2 resulted in a 71% reduction. In 24 h of incubation, the reduction in COD value was achieved with the interaction of biosurfactants that facilitated the initiation of oil-in-water and water-in-oil emulsions above 50%.
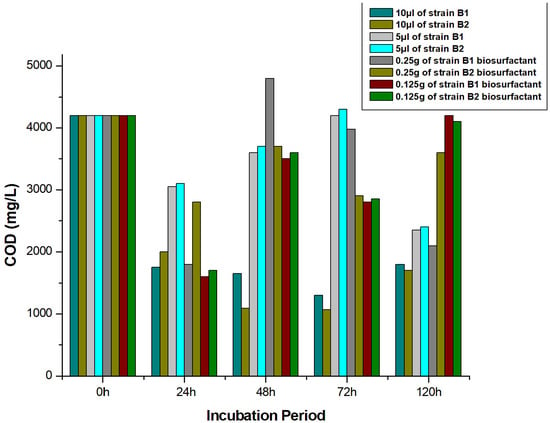
Figure 6.
Reduction of COD of crude oil by strains B1 and B2.
3.8. Application of Hydrocarbon-Degrading Bacteria in Bioremediation
MFCs may generate power from various carbon sources, but contaminated soil with petroleum hydrocarbon could be a viable substrate for MFCs. Compared to alternative methods that use costly and hazardous chemical dispersants, bioremediation methods based on MFCs systems have shown considerable potential benefits, such as being environmentally friendly. Multiple studies have utilized the P. aeruginosa strain for electricity production [56,57]. Therefore, we used co-cultures of the isolated hydrocarbon-degrading strains B. subtilis B1 and P. aeruginosa B2 in the MFC for hydrocarbon degradation and electricity generation through microbial respiration. Efforts were also made to use the strain B. subtilis B1 only for the same, but the obtained current density was not useful enough. The MFC was filled with hydrocarbon-contaminated water and inoculated with a culture of strains B1 and B2. The produced electricity was measured through the polarization study.
The polarization study was carried out with a co-culture of B1 and B2 using a 1:1 ratio in the MFC to determine how much power was produced during the biodegradation of hydrocarbons. Figure 7 shows the relationship between the anode potential (Ea) and the current density measured in MFCs tested with varying concentrations of hydrocarbon in their anode chambers. A drop in anode half-cell potential relative to current density is shown in the Figure 7, with a corresponding increase in the OCV. The drop in anode potentials is caused by a reduction in the accumulation of electrons produced by the exoelectrogenic microbe because of a lower concentration of hydrocarbons as a carbon source. The half-cell potentials at the anode against 10k Ohms external resistance were −280 mV, −297 mV, −310 mV, −370 mV, and −340 mV for 1%, 1.5%, 2%, 2.5%, and 3% v/v, respectively.
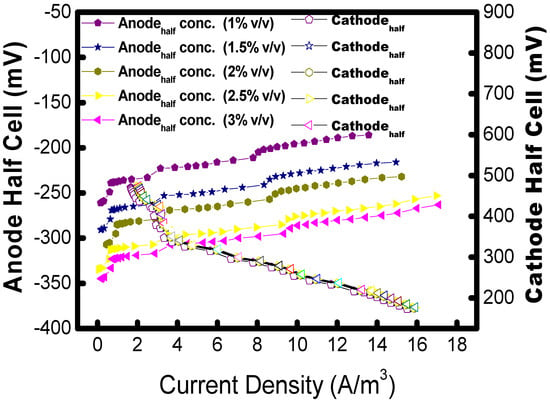
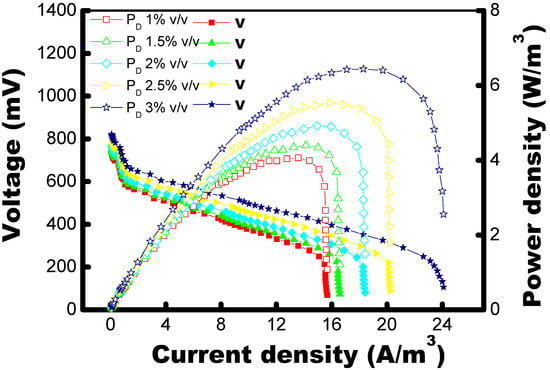
Figure 7.
Analysis of anode half-cell (above) and full-cell (below) with respect to volumetric current density. The voltage and power density data points are presented as solid and open symbols.
Each MFC was operated for almost 8 weeks, proving its ability to supply a constant current over extended periods. After the fifth cycle, a polarization study was conducted utilizing a co-culture of B. subtilis B1 and P. aeruginosa B2 to evaluate the variation in power output among the five MFCs. Power densities observed from MFCs vary with crude oil concentrations in the anode chamber. As seen in Figure 7, the altered electrodes exhibited a notable increase in energy output. The maximum power generated was 6.3 W/m3 in the case of 2.5% in 100 mL oil concentration, compared to the other crude oil concentrations tested. The power density was measured to be 4.0, 4.4, 4.8, and 5.2 W/m3 at oil concentrations of 1% v/v, 1.5% v/v, 2% v/v, and 3% v/v, respectively. The power density determined after inoculation of co-culture of hydrocarbon-degrading bacteria (B. subtilis and P. aeruginosa) was 6.3 W/m3, and open circuit voltage was recorded as −370 mV. Figure 8 shows an equivalent circuit of MFCs.
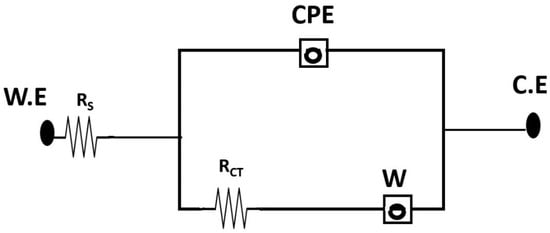
Figure 8.
Various resistances simulated by equivalent circuit for single-chambered MFC (sMFC).
Electrochemical impedance spectroscopy (EIS) is a well-known way to determine how an anode electrode’s surface behaves at the interface. Figure 9 shows the Nyquist plots for the anode on the 8 weeks of operations with different co-culture inocula. The Nyquist plot has a semicircle and a linear portion, which represents charge-transfer resistance and diffusion resistance, respectively. This implies that the electrochemical reaction on the biofilm is mix-controlled by the charge transfer and diffusion step. The experimental spectra were fit into equivalent circuits to evaluate the impedance data. A potentiostat was used to record the EIS data. The working electrode was the anode, and the counter electrode was the cathode. During the electrochemical measurements, the saturated Ag/AgCl (1 M KCl) electrode was used as a reference electrode. This electrode was placed close to the anode electrode. From the fitting data of the above-mentioned equivalent circuit, it was possible to determine a few of the most important resistances, such as charge transfer resistance (Rct) and solution resistance (Rs). When testing the anode chamber with varying concentrations of crude oil (1.0% v/v, 1.5% v/v, 2% v/v, 2.5% v/v, and 3% v/v), the 2.5% concentration showed an internal resistance of 85 Ω. The internal resistance measured at different concentrations of crude oil wwere 273.5 Ω, 117.5 Ω, 103.5 Ω, and 90 Ω at 1, 1.5, 2 v/v, and 3% v/v, respectively.
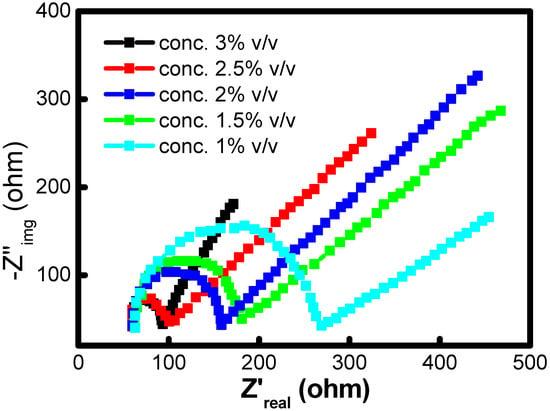
Figure 9.
Nyquist plot of MFCs with different crude oil concentrations in the anode.
4. Discussion
The above study reported the isolation and utilization of hydrocarbon-degrading bacteria. Two hydrocarbon-degrading strains were isolated and identified as, B. subtilis and P. aeruginosa, of which B. subtilis produced a higher amount of biosurfactant. Both strains are effective in the degradation process. The biosurfactant produced by B. subtilis was around 3.4 g surfactant in one liter of glucose-containing MSM, while P. aeruginosa produced 2.8 g surfactant in one liter of glucose-containing MSM. Patowary et al. [19] reported that P. aeruginosa, produced around 2.2 g of biosurfactant while another study by Kazemzadeh et al. [61] on crude oil metabolism and isolation by Achromobacter kerstersii LMG3441, demonstrated around 7 g of biosurfactant. Antoniou et al. [62] reported multiple hydrocarbon-degrading bacteria, producing around 20 mg/L of biosurfactants. Mandalenaki et al. [63] showed around 46 g/L biosurfactants from P. aeruginosa UFPEDA 614 by adding extra carbon sources such as soybean and sunflower seed meal. Additionally, this study performed the emulsification test with 24 h fresh culture, which exhibited an emulsification index of 85–88%. Lee et al. [64] performed an emulsification test with crude oil, which gave around 65% of the E24 index. Mandalenaki et al. [63] with the Alcanivorax borkumensis SK2 strain for heavy oil degradation showied an oil-degrading efficiency of 52%. Hence, if the incubation time of the culture can increase with additional carbon sources such as sugarcane bagasse, sunflower seed meal, and soybean, the result can improve with higher biosurfactant production. In the above study, the initial COD value was 4200 mg/L, and around 75% reduction in COD is seen by B. subtilis and 71% by P. aeruginosa. Another study showed a 76% decrease in COD after 72 h of incubation by the Planococcus citreus strain and a 70% reduction by Pantoea agglomerans with an initial COD value of 4400 mg/L [65]. Multiple studies have also tested different hydrocarbon-degrading strains for the antimicrobial activity of the biosurfactant, which showed a positive result; hence, both of these strains can be further tested to increase their application in bioremediation [66,67,68]. Our isolated strains (P. aeruginosa + B. subtilis) used in a 1:1 ratio for power generation were capable enough to enhance power production. So, this can be an additional application of hydrocarbon-degrading bacteria apart from bioremediation. B. subtilis B1 and P. aeruginosa B2 were shown to have a synergistic relationship in a co-culture system, leading the MFC to generate power more efficiently. Evidence was presented in this study that “inter-species ecological communication” can electrochemically activate MFCs, which plays a crucial role in power enhancement. However, a more thorough analysis of the mutualistic interaction of bacteria in co-culture or mixed-culture inoculated MFCs is warranted.
5. Conclusions
In this study, two hydrocarbon-degrading bacteria were isolated and identified as B. subtilis strain B1 and P. aeruginosa strain B2. These strains were primarily checked for morphology using the Gram-staining method followed by biochemical tests. Bacteria that break down hydrocarbons were found and used, namely, B. subtilis and P. aeruginosa. B. subtilis produced more bio-surfactant than P. aeruginosa. Both strains were initially tested for biosurfactant production using an oil-collapsed assay, which showed positive results by collapsing the oil within a few seconds. Moreover, their biosurfactant production was estimated, which showed that B. subtilis B1 and P. aeruginosa B2 could produce 3.4 and 2.8 g/L of biosurfactant, respectively. The FTIR spectrum further characterized the biosurfactant, which showed that the surfactants produced by strains B1 and B2 were surfactin and rhamnolipid, respectively. Finally, the co-culture of the isolated strains was applied in a 1:1 ratio in a microbial fuel cell for energy production, which gave the power density of 6.3 W/m3 with an open circuit voltage of 370 mV. It has been shown that the combined application of these strains can also be utilized to enhance power production as well as clean up hydrocarbon contamination owing to their synergism in the co-culture system; it was discovered that when B. subtilis B1 and P. aeruginosa B2 were co-cultured, a synergistic interaction emerged that increased the MFC’s efficiency at producing electricity. This research shows that MFCs can be electrochemically activated by “inter-species ecological communication”, which is a key factor in power augmentation, suggesting their potent applicability in environmental, energy, and industrial sectors as an economical and feasible alternative to the existing technologies.
Author Contributions
Conceptualization, K.S., S.P., B.S.T. and T.R.T.; methodology, K.S., S.P., B.S.T., K.P. and T.R.T.; investigation, K.S., V.S. and S.P.; writing—original draft preparation, K.S. and S.P.; writing—review and editing, S.P., B.S.T. and T.R.T.; visualization, K.S., S.P. and K.P.; supervision, S.P. and T.R.T.; project administration, S.P. and B.S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thakur, A.; Koul, B. Chapter 7—Impact of Oil Exploration and Spillage on Marine Environments. In Advances in Oil-Water Separation; Das, P., Manna, S., Pandey, J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 115–135. ISBN 978-0-323-89978-9. [Google Scholar]
- Haider, F.U.; Ejaz, M.; Cheema, S.A.; Khan, M.I.; Zhao, B.; Liqun, C.; Salim, M.A.; Naveed, M.; Khan, N.; Núñez-Delgado, A.; et al. Phytotoxicity of Petroleum Hydrocarbons: Sources, Impacts and Remediation Strategies. Environ. Res. 2021, 197, 111031. [Google Scholar] [CrossRef] [PubMed]
- Stephen, E. Comprehensive Perspectives in Bioremediation of Crude Oil Contaminated Environments. In Introduction to Enhanced Oil Recovery (EOR) Processes and Bioremediation of Oil-Contaminated Sites; Intech: Singapore, 2012. [Google Scholar]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Analytical Methods for Polycyclic Aromatic Hydrocarbons and Their Global Trend of Distribution in Water and Sediment: A Review; IntechOpen: London, UK, 2017; ISBN 978-953-51-3810-5. [Google Scholar]
- Sakshi; Singh, S.K.; Haritash, A.K. Polycyclic Aromatic Hydrocarbons: Soil Pollution and Remediation. Int. J. Environ. Sci. Technol. 2019, 16, 6489–6512. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Rajendran, P. Petroleum Hydrocarbon and Living Organisms; IntechOpen: London, UK, 2019; ISBN 978-1-78984-421-4. [Google Scholar]
- Bender, M.L.; Giebichenstein, J.; Teisrud, R.N.; Laurent, J.; Frantzen, M.; Meador, J.P.; Sørensen, L.; Hansen, B.H.; Reinardy, H.C.; Laurel, B.; et al. Combined Effects of Crude Oil Exposure and Warming on Eggs and Larvae of an Arctic Forage Fish. Sci. Rep. 2021, 11, 8410. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Nag, S. Chapter 6—Microbial Remediation of Petroleum Hydrocarbons in Liquid Wastes. In Development in Wastewater Treatment Research and Processes; Rodriguez-Couto, S., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 117–129. ISBN 978-0-323-85839-7. [Google Scholar]
- Dasgupta, D.; Ghosh, R.; Sengupta, T.K. Biofilm-Mediated Enhanced Crude Oil Degradation by Newly Isolated Pseudomonas Species. ISRN Biotechnol. 2013, 2013, e250749. [Google Scholar] [CrossRef]
- Unimke, A.; Mmuoegbulam, A.; Anika, O. Microbial Degradation of Petroleum Hydrocarbons: Realities, Challenges and Prospects. Biotechnol. J. Int. 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of Soil and Water Contaminated with Petroleum Hydrocarbon: A Review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Hussain, I.; Puschenreiter, M.; Gerhard, S.; Schöftner, P.; Yousaf, S.; Wang, A.; Syed, J.H.; Reichenauer, T.G. Rhizoremediation of Petroleum Hydrocarbon-Contaminated Soils: Improvement Opportunities and Field Applications. Environ. Exp. Bot. 2018, 147, 202–219. [Google Scholar] [CrossRef]
- Mambwe, M.; Johnson, T. Remediation Technologies for Oil Contaminated Soil. Glob. J. Environ. Sci. Manag. 2021, 7, 419–438. [Google Scholar]
- Akintunde, W.O.; Olugbenga, O.A.; Olufemi, O.O. Some Adverse Effects of Used Engine Oil (Common Waste Pollutant) On Reproduction of Male Sprague Dawley Rats. Open Access Maced. J. Med. Sci. 2015, 3, 46–51. [Google Scholar] [CrossRef]
- Saimmai, A.; Kaewrueng, J.; Maneerat, S. Used Lubricating Oil Degradation and Biosurfactant Production by SC-9 Consortia Obtained from Oil-Contaminated Soil. Ann. Microbiol. 2012, 62, 1757–1767. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Biodegradation of Used Engine Oil by Novel Strains of Ochrobactrum Anthropi HM-1 and Citrobacter Freundii HM-2 Isolated from Oil-Contaminated Soil. 3 Biotech 2016, 6, 226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bjerk, T.R.; Severino, P.; Jain, S.; Marques, C.; Silva, A.M.; Pashirova, T.; Souto, E.B. Biosurfactants: Properties and Applications in Drug Delivery, Biotechnology and Ecotoxicology. Bioengineering 2021, 8, 115. [Google Scholar] [CrossRef]
- Saha, P. Biosurfactants—A Current Perspective on Production and Applications. Nat. Environ. Pollut. Technol. 2017, 16, 181–188. [Google Scholar]
- Patowary, K.; Patowary, R.; Kalita, M.C.; Deka, S. Characterization of Biosurfactant Produced during Degradation of Hydrocarbons Using Crude Oil as Sole Source of Carbon. Front. Microbiol. 2017, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Lou, H.; Wei, T.; Liu, X.; Chen, Q. Contributions of Glycolipid Biosurfactants and Glycolipid-Modified Materials to Antimicrobial Strategy: A Review. Pharmaceutics 2021, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.V.; Treichel, H.; Santos, L.O.; Martins, V.G. Chapter 16—Solid-State Fermentation for the Production of Biosurfactants and Their Applications. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 357–372. ISBN 978-0-444-63990-5. [Google Scholar]
- Sharma, P.; Sangwan, S.; Singh, S.; Kaur, H. Chapter 14—Microbial Biosurfactants: An Eco-Friendly Perspective for Soil Health Management and Environmental Remediation. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Vaishnav, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 277–298. ISBN 978-0-323-85579-2. [Google Scholar]
- Ławniczak, Ł.; Woźniak-Karczewska, M.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, Ł. Microbial Degradation of Hydrocarbons—Basic Principles for Bioremediation: A Review. Molecules 2020, 25, 856. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Truskewycz, A.; Gundry, T.D.; Khudur, L.S.; Kolobaric, A.; Taha, M.; Aburto-Medina, A.; Ball, A.S.; Shahsavari, E. Petroleum Hydrocarbon Contamination in Terrestrial Ecosystems—Fate and Microbial Responses. Molecules 2019, 24, 3400. [Google Scholar] [CrossRef]
- Mulugeta, K.; Murugesan, K.; Gemeda, M.; Aravind, J. A Review on Production, Properties, and Applications of Microbial Surfactants as a Promising Biomolecule for Environmental Applications. In Strategies and Tools for Pollutant Mitigation; Springer: Cham, Switzerland, 2021; pp. 3–28. ISBN 978-3-030-63574-9. [Google Scholar]
- Varjani, S.J. Microbial Degradation of Petroleum Hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Pandit, S.; Chandrasekhar, K.; Kakarla, R.; Kadier, A.; Jeevitha, V. Basic Principles of Microbial Fuel Cell: Technical Challenges and Economic Feasibility. In Microbial Applications Vol.1: Bioremediation and Bioenergy; Kalia, V.C., Kumar, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 165–188. ISBN 978-3-319-52666-9. [Google Scholar]
- Thapa, B.S.; Kim, T.; Pandit, S.; Song, Y.E.; Afsharian, Y.P.; Rahimnejad, M.; Kim, J.R.; Oh, S.-E. Overview of Electroactive Microorganisms and Electron Transfer Mechanisms in Microbial Electrochemistry. Bioresour. Technol. 2022, 347, 126579. [Google Scholar] [CrossRef]
- Thapa, B.S.; Pandit, S.; Patwardhan, S.B.; Tripathi, S.; Mathuriya, A.S.; Gupta, P.K.; Lal, R.B.; Tusher, T.R. Application of Microbial Fuel Cell (MFC) for Pharmaceutical Wastewater Treatment: An Overview and Future Perspectives. Sustainability 2022, 14, 8379. [Google Scholar] [CrossRef]
- Mathuriya, A.S.; Kaur, A.; Gupta, P.K.; Pandit, S.; Jadhav, D.A. Chapter 5—Potential of Microbial Fuel Cells for Wastewater Treatment. In Bioremediation, Nutrients, and Other Valuable Product Recovery; Singh, L., Mahapatra, D.M., Thakur, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 115–124. ISBN 978-0-12-821729-0. [Google Scholar]
- Islam, M.A.; Karim, A.; Mishra, P.; Dubowski, J.J.; Yousuf, A.; Sarmin, S.; Khan, M.M.R. Microbial Synergistic Interactions Enhanced Power Generation in Co-Culture Driven Microbial Fuel Cell. Sci. Total Environ. 2020, 738, 140138. [Google Scholar] [CrossRef] [PubMed]
- Rezk, H.; Sayed, E.T.; Abdelkareem, M.A.; Olabi, A.G. Performance Improvement of Co-Culture Inoculated Microbial Fuel Cell Using Fuzzy Modelling and Harris Hawks Optimization. Int. J. Energy Res. 2022, 46, 14396–14407. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Padmaperuma, G.; Maneein, S.; Vaidyanathan, S. Co-Culturing Microbial Consortia: Approaches for Applications in Biomanufacturing and Bioprocessing. Crit. Rev. Biotechnol. 2022, 42, 46–72. [Google Scholar] [CrossRef]
- Saraiva, J.P.; Worrich, A.; Karakoç, C.; Kallies, R.; Chatzinotas, A.; Centler, F.; Nunes da Rocha, U. Mining Synergistic Microbial Interactions: A Roadmap on How to Integrate Multi-Omics Data. Microorganisms 2021, 9, 840. [Google Scholar] [CrossRef]
- Islam, M.A.; Ong, H.R.; Ethiraj, B.; Cheng, C.K.; Rahman Khan, M.M. Optimization of Co-Culture Inoculated Microbial Fuel Cell Performance Using Response Surface Methodology. J. Environ. Manag. 2018, 225, 242–251. [Google Scholar] [CrossRef]
- Islam, M.A.; Ethiraj, B.; Cheng, C.K.; Yousuf, A.; Khan, M.M.R. An Insight of Synergy between Pseudomonas Aeruginosa and Klebsiella Variicola in a Microbial Fuel Cell. ACS Sustain. Chem. Eng. 2018, 6, 4130–4137. [Google Scholar] [CrossRef]
- Wang, A.; Liu, W.; Ren, N.; Zhou, J.; Cheng, S. Key Factors Affecting Microbial Anode Potential in a Microbial Electrolysis Cell for H2 Production. Int. J. Hydrog. Energy 2010, 35, 13481–13487. [Google Scholar] [CrossRef]
- Ren, J.; Li, N.; Du, M.; Zhang, Y.; Hao, C.; Hu, R. Study on the Effect of Synergy Effect between the Mixed Cultures on the Power Generation of Microbial Fuel Cells. Bioengineered 2021, 12, 844–854. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electricigens in the Anode of Microbial Fuel Cells: Pure Cultures versus Mixed Communities. Microb. Cell Factories 2019, 18, 39. [Google Scholar] [CrossRef]
- Patowary, K.; Saikia, R.R.; Kalita, M.C.; Deka, S. Degradation of Polyaromatic Hydrocarbons Employing Biosurfactant-Producing Bacillus Pumilus KS2. Ann. Microbiol. 2015, 65, 225–234. [Google Scholar] [CrossRef]
- Rahman, K.S.M.; Thahira-Rahman, J.; Lakshmanaperumalsamy, P.; Banat, I.M. Towards Efficient Crude Oil Degradation by a Mixed Bacterial Consortium. Bioresour. Technol. 2002, 85, 257–261. [Google Scholar] [CrossRef]
- Ray, M.; Kumar, V.; Banerjee, C.; Gupta, P.; Singh, S.; Singh, A. Investigation of Biosurfactants Produced by Three Indigenous Bacterial Strains, Their Growth Kinetics and Their Anthracene and Fluorene Tolerance. Ecotoxicol. Environ. Saf. 2021, 208, 111621. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.K.; Collins-Thompson, D.L.; Lee, H.; Trevors, J.T. A Drop-Collapsing Test for Screening Surfactant-Producing Microorganisms. J. Microbiol. Methods 1991, 13, 271–279. [Google Scholar] [CrossRef]
- Nayarisseri, A.; Singh, P.; Singh, S.K. Screening, Isolation and Characterization of Biosurfactant Producing Bacillus subtilis Strain ANSKLAB03. Bioinformation 2018, 14, 304–314. [Google Scholar] [CrossRef]
- Yuan, W.; Lu, Z.; Wang, H.; Li, C.M. Stimuli-Free Reversible and Controllable Loading and Release of Proteins under Physiological Conditions by Exponentially Growing Nanoporous Multilayered Structure. Adv. Funct. Mater. 2012, 22, 1932–1939. [Google Scholar] [CrossRef]
- George, S.; Jayachandran, K. Analysis of Rhamnolipid Biosurfactants Produced Through Submerged Fermentation Using Orange Fruit Peelings as Sole Carbon Source. Appl. Biochem. Biotechnol. 2009, 158, 694–705. [Google Scholar] [CrossRef]
- Rani, K.; Sangwan, S.; Singh, S.; Sharma, P.; Kaur, H.; Sindhu, M. Biosurfactant Production by Bacteria Retrieved from Hydrocarbon Polluted Environment. Int. J. Chem. Stud. 2020, 8, 1059–1065. [Google Scholar] [CrossRef]
- Banerjee, S.; Ghosh, U. Production, Purification and Characterization of Biosurfactant Isolated from Bacillus oceanisediminis H2. Mater. Today Proc. 2021; in press. [Google Scholar] [CrossRef]
- Deodhar, S.; Rohilla, P.; Muniyandi, M.; Thampi, S.; Madivala Gurappa, B. A Robust Method to Determine Critical Micelle Concentration via Spreading Oil Drops on Surfactant Solutions. Langmuir 2020, 36, 8100–8110. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yuan, W.; Lu, Z.; Li, C.M. Polymer/Nanosilver Composite Coatings for Antibacterial Applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 69–83. [Google Scholar] [CrossRef]
- Raghunath, N.; Patil, R.N.; Saima, A. Biosurfactant Production and Its Role in Candida albicans Biofilm Inhibition. J. Pure Appl. Microbiol. 2020, 14, 1337–1343. [Google Scholar]
- Sinha, A.; Zhao, H.; Chen, J.; Mugo, S. Dhanjai Determination of Chemical Oxygen Demand: An Analytical Approach. In Encyclopedia of Analytical Sciences; Elsevier: Amsterdam, The Netherlands, 2018; pp. 258–270. ISBN 978-0-12-409547-2. [Google Scholar]
- Ghadge, A.; Ghangrekar, M.M.; Pandit, S.; Das, D. Performance of Air Cathode Earthen Pot Microbial Fuel Cell for Simultaneous Wastewater Treatment with Bioelectricity Generation. Int. J. Environ. Technol. Manag. 2014, 17, 143–153. [Google Scholar] [CrossRef]
- Tripathi, B.; Pandit, S.; Sharma, A.; Chauhan, S.; Mathuriya, A.S.; Dikshit, P.K.; Gupta, P.K.; Singh, R.C.; Sahni, M.; Pant, K.; et al. Modification of Graphite Sheet Anode with Iron (II, III) Oxide-Carbon Dots for Enhancing the Performance of Microbial Fuel Cell. Catalysts 2022, 12, 1040. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, Y.; Shen, P.K.; Li, C.M.; Jiang, S.P. Significance of Wall Number on the Carbon Nanotube Support-Promoted Electrocatalytic Activity of Pt NPs towards Methanol/Formic Acid Oxidation Reactions in Direct Alcohol Fuel Cells. J. Mater. Chem. A 2015, 3, 1961–1971. [Google Scholar] [CrossRef]
- Phulpoto, I.A.; Yu, Z.; Hu, B.; Wang, Y.; Ndayisenga, F.; Li, J.; Liang, H.; Qazi, M.A. Production and Characterization of Surfactin-like Biosurfactant Produced by Novel Strain Bacillus nealsonii S2MT and It’s Potential for Oil Contaminated Soil Remediation. Microb. Cell Factories 2020, 19, 145. [Google Scholar] [CrossRef]
- Leite, G.G.F.; Figueirôa, J.V.; Almeida, T.C.M.; Valões, J.L.; Marques, W.F.; Duarte, M.D.D.C.; Gorlach-Lira, K. Production of Rhamnolipids and Diesel Oil Degradation by Bacteria Isolated from Soil Contaminated by Petroleum. Biotechnol. Prog. 2016, 32, 262–270. [Google Scholar] [CrossRef]
- Pornsunthorntawee, O.; Wongpanit, P.; Chavadej, S.; Abe, M.; Rujiravanit, R. Structural and Physicochemical Characterization of Crude Biosurfactant Produced by Pseudomonas Aeruginosa SP4 Isolated from Petroleum-Contaminated Soil. Bioresour. Technol. 2008, 99, 1589–1595. [Google Scholar] [CrossRef]
- Kazemzadeh, S.; Naghavi, N.S.; Emami-Karvani, Z.; Emtiazi, G.; Fouladgar, M. Production of Glycolipid Biosurfactant during Crude Oil Degradation by the Novel Indigenous Isolated Achromobacter kerstersii LMG3441. Water Sci. Technol. 2020, 82, 2134–2147. [Google Scholar] [CrossRef]
- Antoniou, E.; Fodelianakis, S.; Korkakaki, E.; Kalogerakis, N. Biosurfactant Production from Marine Hydrocarbon-Degrading Consortia and Pure Bacterial Strains Using Crude Oil as Carbon Source. Front. Microbiol. 2015, 6, 274. [Google Scholar] [CrossRef] [PubMed]
- Mandalenaki, A.; Kalogerakis, N.; Antoniou, E. Production of High Purity Biosurfactants Using Heavy Oil Residues as Carbon Source. Energies 2021, 14, 3557. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, H.; Kwon, B.-O.; Khim, J.S.; Yim, U.H.; Kim, B.S.; Kim, J.-J. Biosurfactant-Assisted Bioremediation of Crude Oil by Indigenous Bacteria Isolated from Taean Beach Sediment. Environ. Pollut. 2018, 241, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.; Oriani, M.; Durrant, L. Reducing COD Level on Oily Effluent by Utilizing Biosurfactant-Producing Bacteria. Braz. Arch. Biol. Technol. 2009, 52, 1037–1042. [Google Scholar] [CrossRef]
- De Giani, A.; Zampolli, J.; Di Gennaro, P. Recent Trends on Biosurfactants with Antimicrobial Activity Produced by Bacteria Associated with Human Health: Different Perspectives on Their Properties, Challenges, and Potential Applications. Front. Microbiol. 2021, 12, 655150. [Google Scholar] [CrossRef]
- Satapute, P.; Jogaiah, S. A Biogenic Microbial Biosurfactin That Degrades Difenoconazole Fungicide with Potential Antimicrobial and Oil Displacement Properties. Chemosphere 2022, 286, 131694. [Google Scholar] [CrossRef]
- Yuliani, H.; Perdani, M.S.; Savitri, I.; Manurung, M.; Sahlan, M.; Wijanarko, A.; Hermansyah, H. Antimicrobial Activity of Biosurfactant Derived from Bacillus subtilis C19. Energy Procedia 2018, 153, 274–278. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).