Optimization of a Textile Effluent Treatment System and Evaluation of the Feasibility to Be Reused as Influents in Textile Dyeing Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Description
2.2. Water Sample Collection
2.3. Physicochemical Parameters and Methods of Analysis
2.4. Fabrics Dyed Assessment
3. Results and Discussion
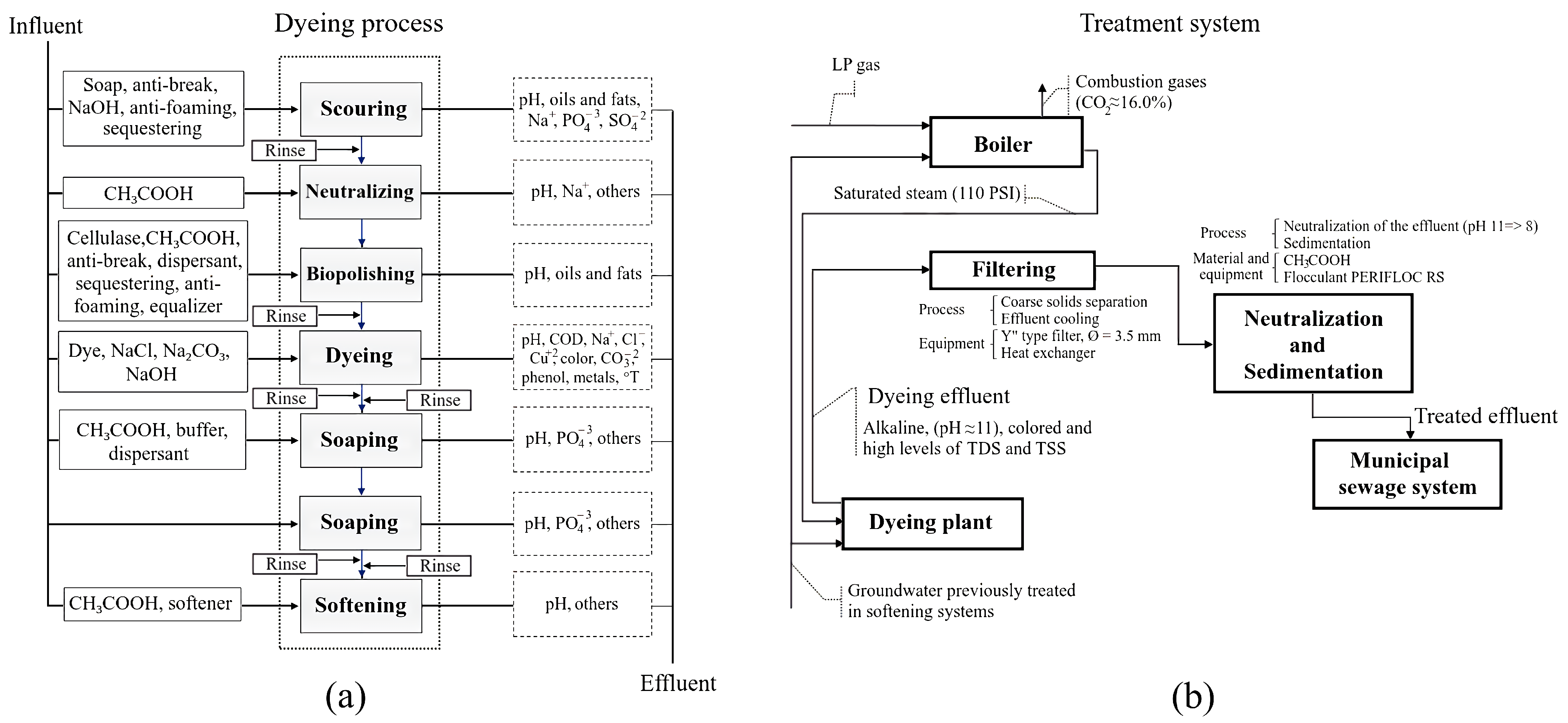
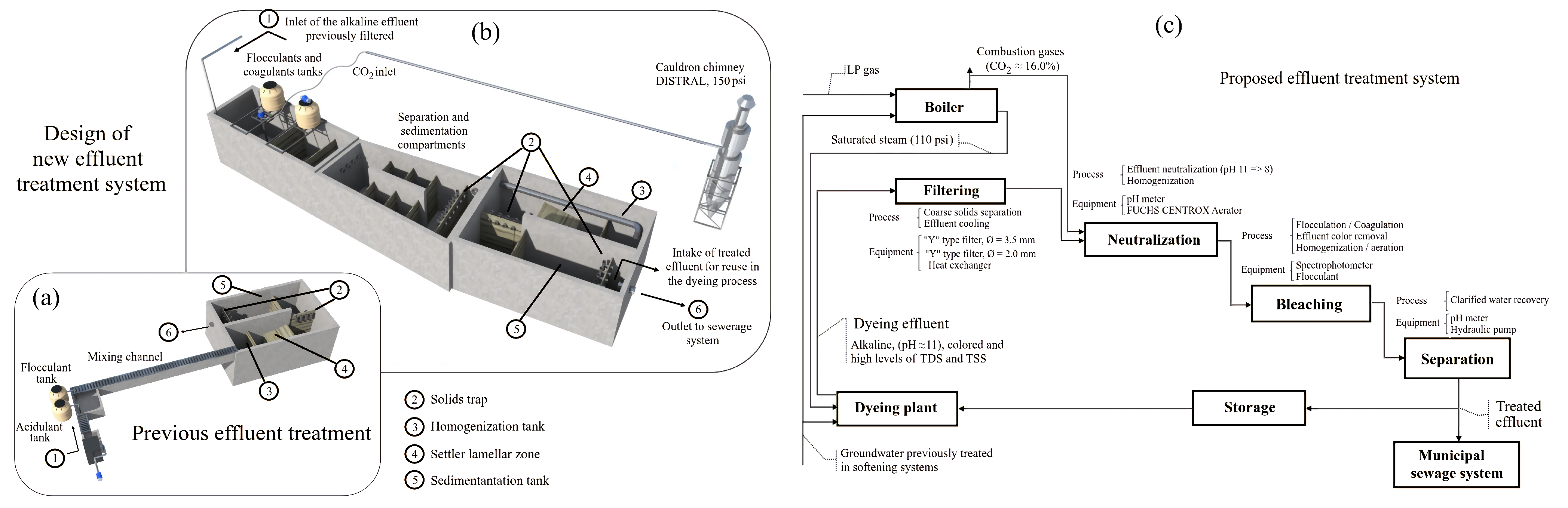
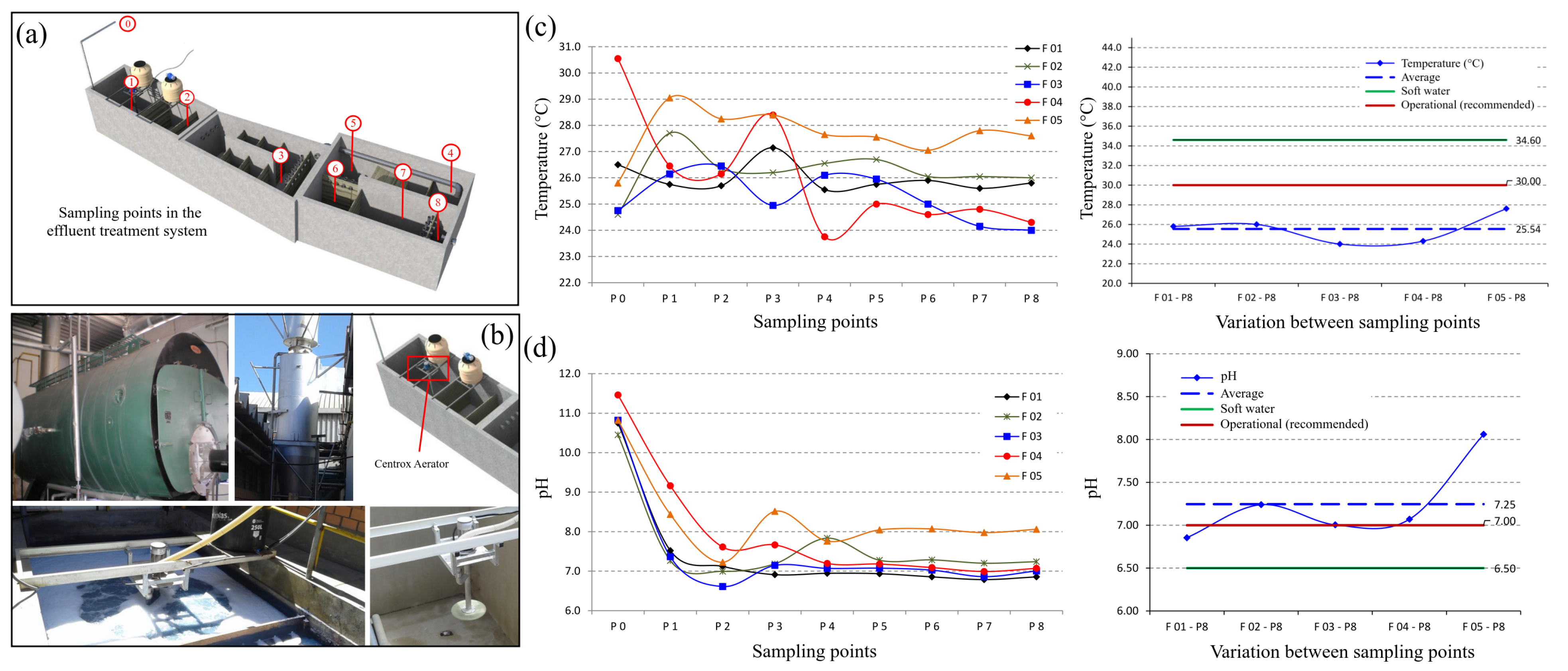
3.1. Determination of the Process Parameters to Be Optimized
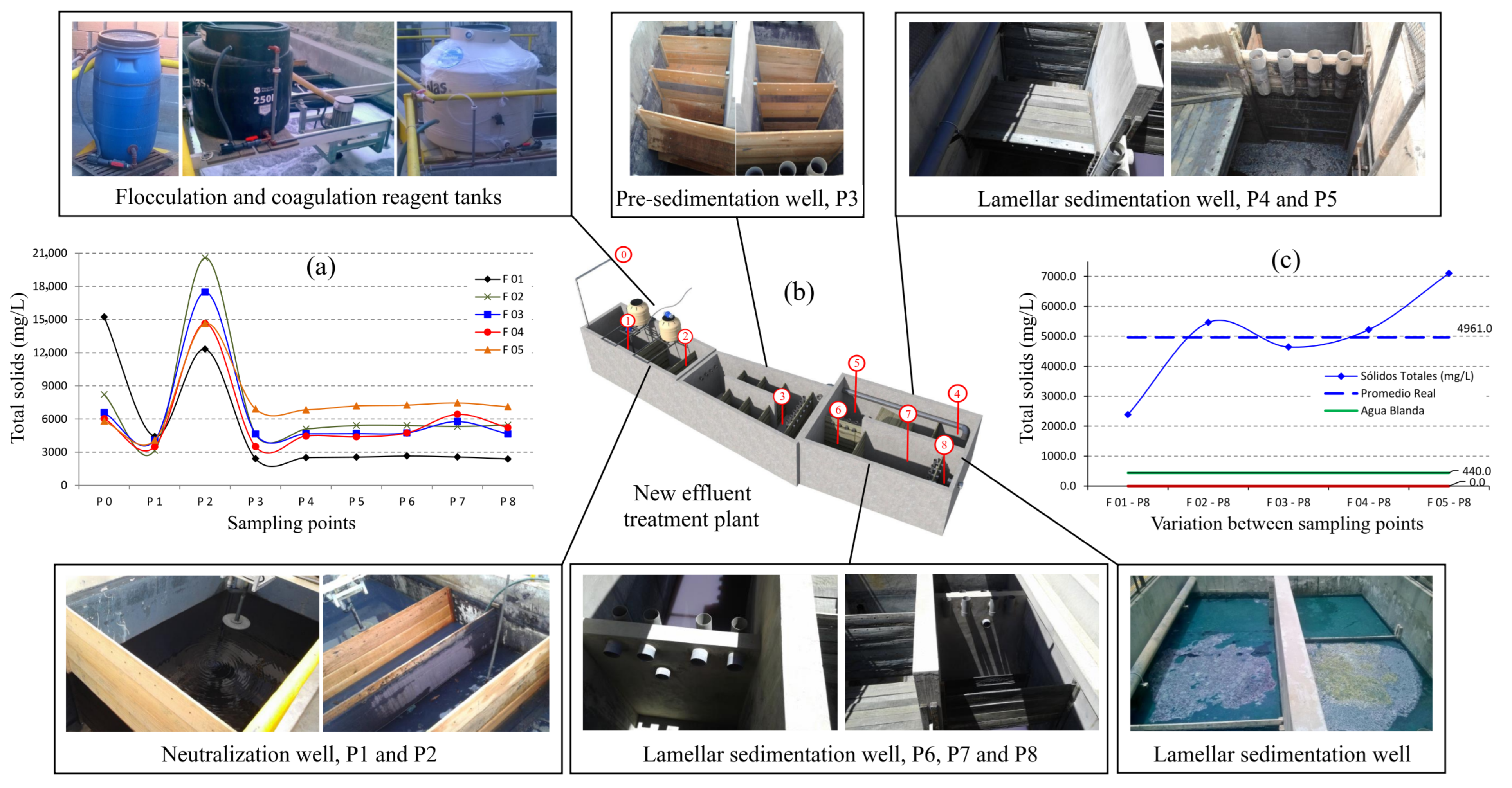
3.2. Optimization of the Effluent Treatment System
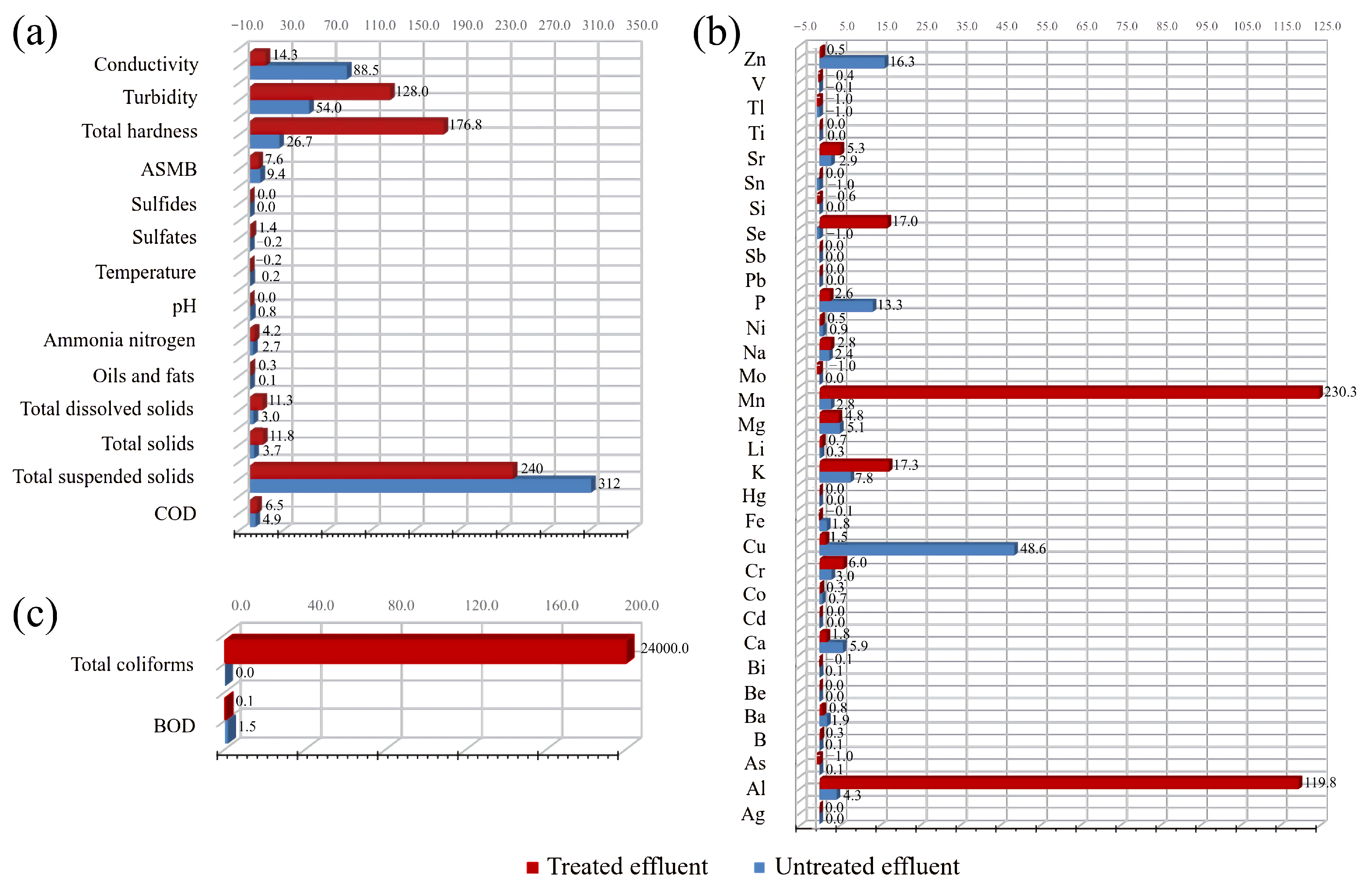
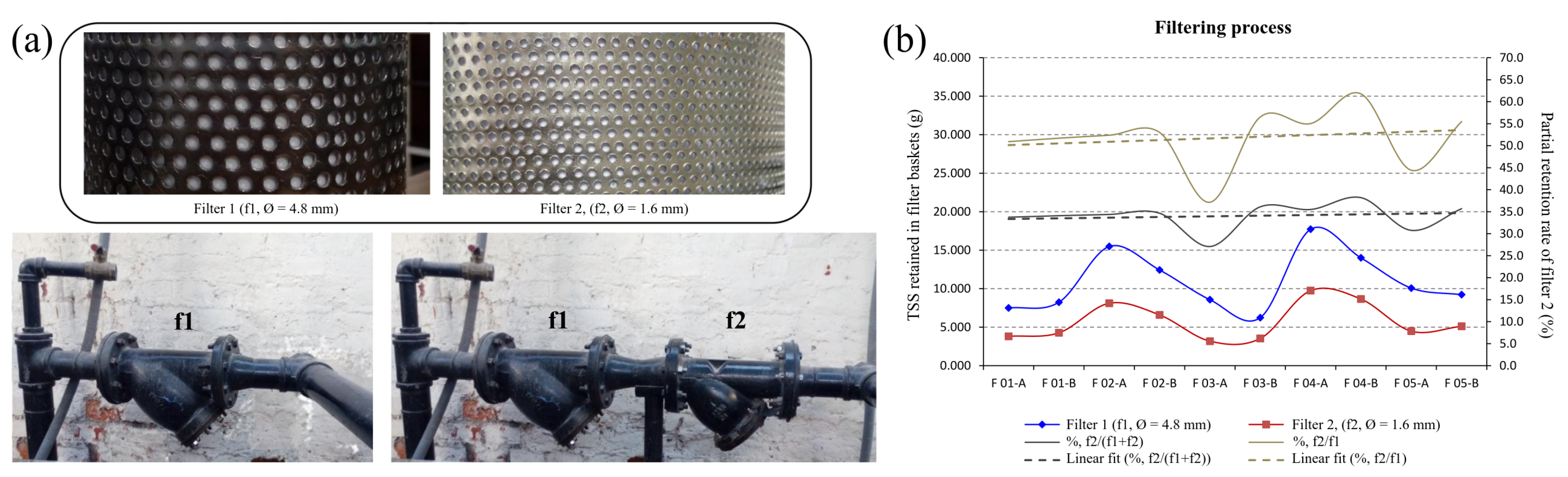
3.2.1. Suspended Solids
3.2.2. Temperature and pH
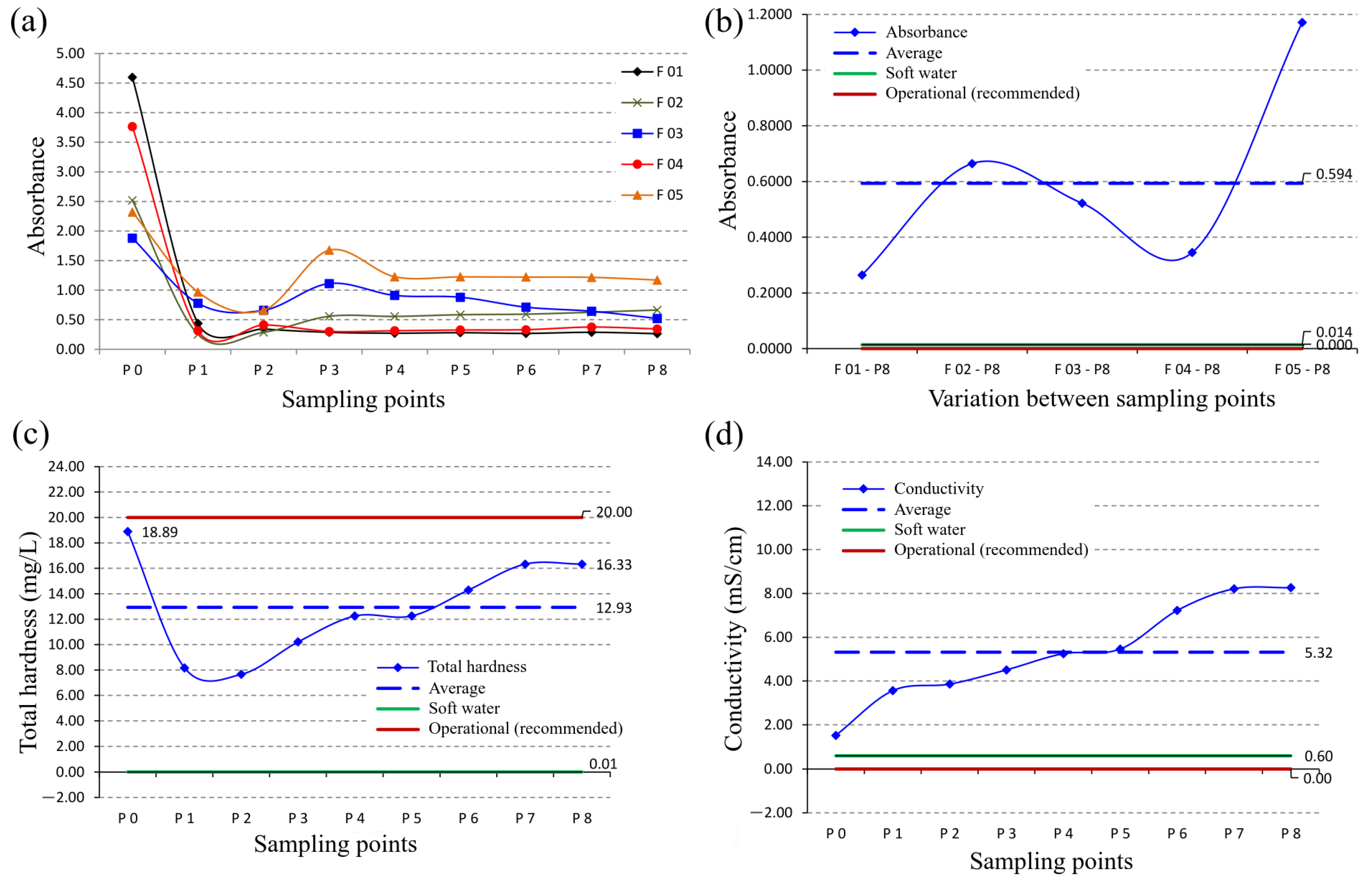
3.3. Effluent Decolorization Process (Total Solids and Turbidity)
3.4. Total Hardness and Conductivity
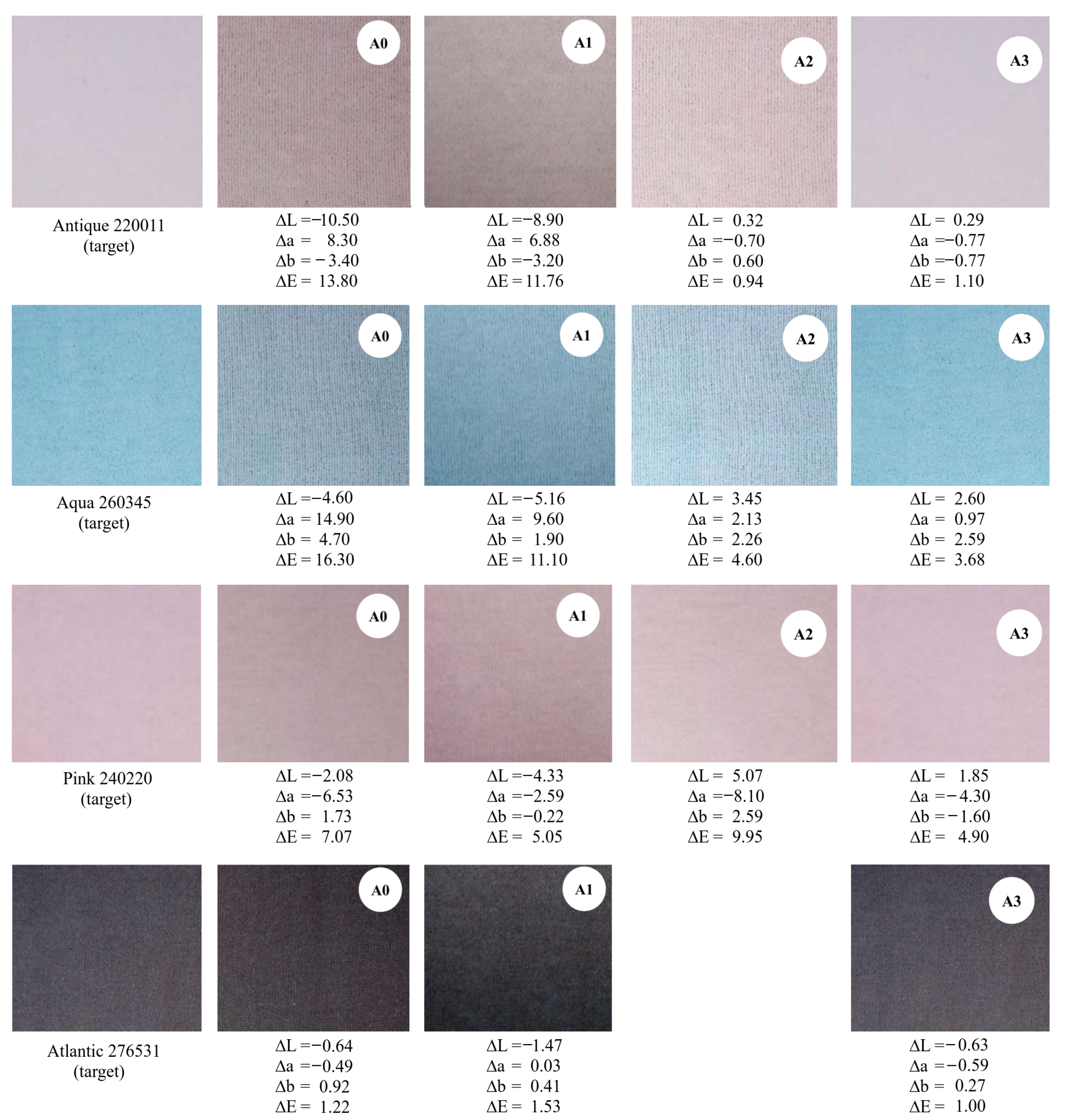
3.5. Fabric Dyeing Using the Treated Effluent as Influent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Water.org. The Water Crisis. 2022. Available online: https://water.org/our-impact/water-crisis/ (accessed on 20 August 2022).
- Water, United Nations. 2018 UN World Water Development Report, Nature-Based Solutions for Water, 1st ed.; UNESCO: Paris, France, 2018. [Google Scholar]
- United Nations World Water Assessment Programme (WWAP). The United Nations World Water Development Report 2014: Water and Energy, 1st ed.; UNESCO: Paris, France, 2014. [Google Scholar]
- Organización para la Cooperación y el Desarrollo Económicos (OCDE). OECD Environmental Outlook to 2050. The Consequences of Inaction, 1st ed.; Ediciones de la OCDE: Paris, France, 2012. [Google Scholar]
- Safe Drinking Water Foundation (SDWF). Industrial Waste. Industrial Waste Fact Sheet. 2020. Available online: https://www.safewater.org/fact-sheets-1/2017/1/23/industrial-waste (accessed on 15 July 2022).
- Ahmed, J.; Thakur, A.; Goyal, A. Biological Treatment of Industrial Wastewater. In Industrial Wastewater and Its Toxic Effects; The Royal Society of Chemistry: London, UK, 2021; Chapter I; pp. 1–14. [Google Scholar]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Brañez, M.; Gutierrez, R.; Perez, R.; Uribe, C.; Valle, P. Pollution of aquatic environments generated by textile industry. Esc. Univ. Posgrado UNFV Lima-Perú 2018, 23, 129–143. [Google Scholar]
- AquaTech. Industrial Water: Our Essential Guide to Pollution, Treatment & Solutions. 2019. Available online: https://www.aquatechtrade.com/news/industrial-water/industrial-water-essential-guide (accessed on 22 July 2022).
- Kant, R. Textile dyeing industry an environmental hazard. Nat. Sci. 2011, 4, 17027. [Google Scholar] [CrossRef]
- World Bank, W.B. How Much Do Our Wardrobes Cost to the Environment? 2019. Available online: https://www.worldbank.org/en/news/feature/2019/09/23/costo-moda-medio-ambiente (accessed on 20 July 2022).
- Ntuli, F.; Omoregbe, D.I.; Kuipa, P.K.; Muzenda, E.; Belaid, M. Characterization of effluent from textile wet finishing operations. In Proceedings of the World Congress on Engineering and Computer Science 2009, London, UK, 1–3 July 2009; pp. 69–74. [Google Scholar]
- Varadarajan, G.; Venkatachalam, P. Sustainable textile dyeing processes. Environ. Chem. Lett. 2016, 14, 113–122. [Google Scholar] [CrossRef]
- Heredia-R, M.; Layedra-Almeida, A.P.; Torres, Y.; Toulkeridis, T. Evaluation of a Microbial Consortium and Selection of a Support in an Anaerobic Reactor Directed to the Bio-Treatment of Wastewater of the Textile Industry. Sustainability 2022, 14, 8889. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Militky, J. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry. In Sustainability in Textile Dyeing: Recent Developments; Springer International Publishing: London, UK, 2020; pp. 37–79. [Google Scholar]
- Shaikh, M.A. Water conservation in textile industry. Pak. Text. J. 2009, 58, 48–51. [Google Scholar]
- Khan, S.; Malik, A. Environmental and health effects of textile industry wastewater. In Environmental Deterioration and Human Health; Springer: Dordrecht, The Netherlands, 2014; pp. 55–71. [Google Scholar]
- Palamutcu, S. Sustainable textile technologies. In Textiles and Clothing Sustainability; Springer: Singapore, 2017; pp. 1–22. [Google Scholar]
- Dos Santos, R.F.; Ramlow, H.; Dolzan, N.; Machado, R.A.F.; de Aguiar, C.R.L.; Marangoni, C. Influence of different textile fibers on characterization of dyeing wastewater and final effluent. Environ. Monit. Assess. 2018, 190, 1–12. [Google Scholar] [CrossRef]
- Uddin, F. Introductory chapter: Textile manufacturing processes. In Textile Manufacturing Processes; IntechOpen: London, UK, 2019. [Google Scholar]
- Değermenci, G.D.; Değermenci, N.; Ayvaoğlu, V.; Durmaz, E.; Çakır, D.; Akan, E. Adsorption of reactive dyes on lignocellulosic waste; characterization, equilibrium, kinetic and thermodynamic studies. J. Clean. Prod. 2019, 225, 1220–1229. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, M.; Huang, K.; Liu, Z. Textile dyeing wastewater treatment. Adv. Treat. Text. Effl. 2011, 5, 91–116. [Google Scholar]
- Vajnhandl, S.; Valh, J.V. The status of water reuse in European textile sector. J. Environ. Manag. 2014, 141, 29–35. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef] [PubMed]
- Abuhatab, S.; El-Qanni, A.; Marei, N.N.; Hmoudah, M.; El-Hamouz, A. Sustainable competitive adsorption of methylene blue and acid red 88 from synthetic wastewater using NiO and/or MgO silicate based nanosorbcats: Experimental and computational modeling studies. RSC Adv. 2019, 9, 35483–35498. [Google Scholar] [CrossRef] [PubMed]
- Hmoudah, M.; El-Qanni, A.; Abuhatab, S.; Marei, N.N.; El-Hamouz, A.; Tarboush, B.J.A.; Alsurakji, I.H.; Baniowda, H.M.; Russo, V.; Di Serio, M. Competitive adsorption of Alizarin Red S and Bromocresol Green from aqueous solutions using brookite TiO2 nanoparticles: Experimental and molecular dynamics simulation. Environ. Sci. Pollut. Res. 2022, 29, 77992–78008. [Google Scholar] [CrossRef]
- Bakar, N.A.; Othman, N.; Yunus, Z.; Daud, Z.; Norisman, N.S.; Hisham, M.H. Physico-chemical water quality parameters analysis on textile. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 498, p. 012077. [Google Scholar]
- Zhang, Y.; Shaad, K.; Vollmer, D.; Ma, C. Treatment of Textile Wastewater Using Advanced Oxidation Processes—A Critical Review. Water 2021, 13, 3515. [Google Scholar] [CrossRef]
- Pinheiro, L.R.S.; Gradíssimo, D.G.; Xavier, L.P.; Santos, A.V. Degradation of Azo Dyes: Bacterial potential for bioremediation. Sustainability 2022, 14, 1510. [Google Scholar] [CrossRef]
- Xiao, H.; Zhao, T.; Li, C.H.; Li, M.Y. Eco-friendly approaches for dyeing multiple type of fabrics with cationic reactive dyes. J. Clean. Prod. 2017, 165, 1499–1507. [Google Scholar] [CrossRef]
- Lewis, D.M. Developments in the chemistry of reactive dyes and their application processes. Color. Technol. 2014, 130, 382–412. [Google Scholar] [CrossRef]
- Mosbah, A.; Chouchane, H.; Abdelwahed, S.; Redissi, A.; Hamdi, M.; Kouidhi, S.; Neifar, M.; Slaheddine Masmoudi, A.; Cherif, A.; Mnif, W. Peptides fixing industrial textile dyes: A new biochemical method in wastewater treatment. J. Chem. 2019, 2019, 5081807. [Google Scholar] [CrossRef]
- Khattab, T.A.; Abdelrahman, M.S.; Rehan, M. Textile dyeing industry: Environmental impacts and remediation. Environ. Sci. Pollut. Res. 2020, 27, 3803–3818. [Google Scholar] [CrossRef]
- Ahmad, A.; Hameed, B. Fixed-bed adsorption of reactive azo dye onto granular activated carbon prepared from waste. J. Hazard. Mater. 2010, 175, 298–303. [Google Scholar] [CrossRef]
- Wang, S.; Kong, F.; Fatehi, P.; Hou, Q. Cationic high molecular weight lignin polymer: A flocculant for the removal of anionic azo-dyes from simulated wastewater. Molecules 2018, 23, 2005. [Google Scholar] [CrossRef] [PubMed]
- Tutak, M.; Oktay Özdemir, A. Reactive dyeing of cationized cotton: Effects on the dyeing yield and the fastness properties. J. Appl. Polym. Sci. 2011, 119, 500–504. [Google Scholar] [CrossRef]
- Xia, L.; Wang, A.; Zhang, C.; Liu, Y.; Guo, H.; Ding, C.; Wang, Y.; Xu, W. Environmentally friendly dyeing of cotton in an ethanol–water mixture with excellent exhaustion. Green Chem. 2018, 20, 4473–4483. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef]
- Siddique, K.; Rizwan, M.; Shahid, M.J.; Ali, S.; Ahmad, R.; Rizvi, H. Textile wastewater treatment options: A critical review. Enhancing Cleanup Environ. Pollut. 2017, 2, 183–207. [Google Scholar]
- Aziz, K.H.H.; Mahyar, A.; Miessner, H.; Mueller, S.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Application of a planar falling film reactor for decomposition and mineralization of methylene blue in the aqueous media via ozonation, Fenton, photocatalysis and non-thermal plasma: A comparative study. Process. Saf. Environ. Prot. 2018, 113, 319–329. [Google Scholar] [CrossRef]
- Hien, N.; Nguyen, L.H.; Van, H.T.; Nguyen, T.D.; Nguyen, T.H.V.; Chu, T.H.H.; Nguyen, T.V.; Vu, X.H.; Aziz, K.H.H. Heterogeneous catalyst ozonation of Direct Black 22 from aqueous solution in the presence of metal slags originating from industrial solid wastes. Sep. Purif. Technol. 2020, 233, 115961. [Google Scholar] [CrossRef]
- Karim, M.; Aziz, K.; Omer, K.; Salih, Y.; Mustafa, F.; Rahman, K.; Mohammad, Y. Degradation of aqueous organic dye pollutants by heterogeneous photo-assisted Fenton-like process using natural mineral activator: Parameter optimization and degradation kinetics. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 958, p. 012011. [Google Scholar]
- Chenna, M.; Chemlal, R.; Drouiche, N.; Messaoudi, K.; Lounici, H. Effectiveness of a physicochemical coagulation/flocculation process for the pretreatment of polluted water containing Hydron Blue Dye. Desalin. Water Treat. 2016, 57, 27003–27014. [Google Scholar] [CrossRef]
- Yin, H.; Qiu, P.; Qian, Y.; Kong, Z.; Zheng, X.; Tang, Z.; Guo, H. Textile wastewater treatment for water reuse: A case study. Processes 2019, 7, 34. [Google Scholar] [CrossRef]
- Chakraborty, R.; Ahmad, F. Economical use of water in cotton knit dyeing industries of Bangladesh. J. Clean. Prod. 2022, 340, 130825. [Google Scholar] [CrossRef]
- Ministerio de Vivienda, C.y.S. Decreto Supremo N° 010-2019-VIVIENDA. 2019. Available online: https://busquedas.elperuano.pe/normaslegales/decreto-supremo-que-aprueba-el-reglamento-de-valores-maximos-decreto-supremo-n-010-2019-vivienda-1748339-3/ (accessed on 5 February 2022).
- Kumar, P.S.; Saravanan, A. Sustainable wastewater treatments in textile sector. In Sustainable Fibres and Textiles; Elsevier: Amsterdam, The Netherlands, 2017; pp. 323–346. [Google Scholar]
- Ubale, M.A.; Salkar, V.D. Experimental study on electrocoagulation of textile wastewater by continuous horizontal flow through aluminum baffles. Korean J. Chem. Eng. 2017, 34, 1044–1050. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Kanwar, R.; Singh, J. Physicochemical assessment of industrial textile effluents of Punjab (India). Appl. Water Sci. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Wei, F.; Shahid, M.J.; Alnusairi, G.S.; Afzal, M.; Khan, A.; El-Esawi, M.A.; Abbas, Z.; Wei, K.; Zaheer, I.E.; Rizwan, M.; et al. Implementation of floating treatment wetlands for textile wastewater management: A review. Sustainability 2020, 12, 5801. [Google Scholar] [CrossRef]
- Dwivedi, P.; Tomar, R.S. Bioremediation of textile effluent for degradation and decolourization of synthetic dyes: A review. Int. J. Curr. Res. Life Sci. 2018, 7, 1948–1951. [Google Scholar]
- Manogaran, M.; Yasid, N.A.; Othman, A.R.; Gunasekaran, B.; Halmi, M.I.E.; Shukor, M.Y.A. Biodecolourisation of Reactive Red 120 as a sole carbon source by a bacterial consortium—Toxicity assessment and statistical optimisation. Int. J. Environ. Res. Public Health 2021, 18, 2424. [Google Scholar] [CrossRef]
- Mubashar, M.; Naveed, M.; Mustafa, A.; Ashraf, S.; Shehzad Baig, K.; Alamri, S.; Siddiqui, M.H.; Zabochnicka-Świątek, M.; Szota, M.; Kalaji, H.M. Experimental investigation of Chlorella vulgaris and Enterobacter sp. MN17 for decolorization and removal of heavy metals from textile wastewater. Water 2020, 12, 3034. [Google Scholar] [CrossRef]
- Ahmed, S.; Aktar, S.; Zaman, S.; Jahan, R.A.; Bari, M. Use of natural bio-sorbent in removing dye, heavy metal and antibiotic-resistant bacteria from industrial wastewater. Appl. Water Sci. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Fernandez-F, F.; Lopez-C, P.; Febres-Molina, C.; Gamero-Begazo, P.L.; Gómez, B.; Bernabe-Ortiz, J.C.; Cáceres-Huambo, A.; Aguilar-Pineda, J.A. Identification and Characterization of Peruvian Native Bacterial Strains as Bioremediation of Hg-Polluted Water and Soils Due to Artisanal and Small-Scale Gold Mining in the Secocha Annex, Arequipa. Sustainability 2022, 14, 2669. [Google Scholar] [CrossRef]
- López-Casaperalta, P.; Febres-Molina, C.; Aguilar-Pineda, J.A.; Bernabe-Ortiz, J.C.; Fernandez-F, F. Peruvian Native Bacterial Strains as Potential Bioremediation Agents in Hg-Polluted Soils by Artisanal Mining Activities in Southern Peru. Sustainability 2022, 14, 10272. [Google Scholar] [CrossRef]
- Islam, A.; Guha, A.K. Removal of pH, TDS and color from textile effluent by using coagulants and aquatic/non aquatic plants as adsorbents. Resour. Environ. 2013, 3, 101–114. [Google Scholar]
- Park, Y.J.; Lee, H.C. A Study on the Field Application of Alkaline Tunnel Wastewater to Neutralization Using CO2. J. Korean Geotech. Soc. 2020, 36, 27–34. [Google Scholar]
- Choi, S.; Ko, K.; Chun, H.; Kim, J. Utilization of Carbon Dioxide for Neutralization of Alkaline Wastewater. In Proceedings of the Greenhouse Gas Control Technologies-6th International Conference; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1871–1874. [Google Scholar]
- Son, M.K.; Sung, H.J.; Lee, J.K. Neutralization of Synthetic Alkaline Wastewater with CO2 in a Semi-batch Jet Loop Reactor. J. Korean Soc. Combust. 2013, 18, 17–22. [Google Scholar] [CrossRef]
- Glazyrin, S.A.; Zhumagulov, M.G.; Aydimbaeva, Z.A.; Dostiyarov, A.M. Universal Installation for the integrated utilization of flue gases and wastewater from thermal power plants. In Proceedings of the E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2020; Volume 178, p. 01062. [Google Scholar]
- Zheng, H.; Xu, J.; Ren, Z.; Li, M.; Zhong, G.; Zhao, D.; Hu, X.; Cheng, X.; Guo, J.; Yao, G.; et al. High-alkali lime method combined with flue gas neutralization for the removal of low-concentration chloride ions in dye wastewater. In Advances in Applied Chemistry and Industrial Catalysis; CRC Press: Boca Raton, FL, USA, 2022; pp. 454–459. [Google Scholar]
- Colina Andrade, G.J.; Vilca Quilla, J.M.; Terán Hilares, R.; Tejada Meza, K.; Mogrovejo Valdivia, A.C.; Aguilar-Pineda, J.A.; Cárdenas García, J.D.; Pacheco Tanaka, D.A. Enhanced Removal of Bordeaux B and Red G Dyes Used in Alpaca Wool Dying from Water Using Iron-Modified Activated Carbon. Water 2022, 14, 2321. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef]
- Dotto, J.; Fagundes-Klen, M.R.; Veit, M.T.; Palacio, S.M.; Bergamasco, R. Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 2019, 208, 656–665. [Google Scholar] [CrossRef]
- Berradi, M.; El Harfi, A. Optimization the treatment of the wastewater industrial from the hot-dip galvanizing of steel by the method of experiment design. Moroc. J. Chem. 2015, 3, 142–146. [Google Scholar]
- Pinto, A. Evaluación y Comparación de la Efectividad del Uso de Floculantes Naturales Aloe Vera (Sábila) y Opuntia Ficus-Indica (Nopal/Tuna) y Orgánicos (Ferrocryl y Chemlok 2040) en el Tratamiento de Aguas Residuales del Proceso de teñido de la Empresa Franky y Ricky S.A. Bachelor’s Thesis, Universidad Católica de Santa María, Arequipa, Peru, 2017. [Google Scholar]
- hanane Arroub, H.; El Harfi, A. Modeling and optimization of experimental parameters in the treatment of effluents by coagulation-flocculation processes: Methodology of experimental design. Mediterr. J. Chem. 2018, 7, 183–197. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, J.A.; Vargas-Villalobos, S.; Aparicio-Mora, C.; Nova-Bustos, N.; Pinnock-Branford, M. Tratamiento físico, químico y biológico de residuos químicos de los laboratorios de docencia de la Universidad Nacional, Costa Rica. Uniciencia 2020, 34, 82–94. [Google Scholar] [CrossRef]
- Gilpavas, E.; Arbeláez-Castaño, P.E.; Medina-Arroyave, J.D.; Gómez-Atehortua, C.M. Tratamiento de aguas residuales de la industria textil mediante coagulación química acoplada a procesos fenton intensificados con ultrasonido de baja frecuencia. Rev. Int. Contam. Ambient. 2018, 34, 157–167. [Google Scholar] [CrossRef]
- Goudjil, S.; Guergazi, S.; Masmoudi, T.; Achour, S. Effect of reactional parameters on the elimination of Congo Red by the combination of coagulation–floculation with aluminum sulfate. Desalin. Water Treat 2021, 209, 429–436. [Google Scholar] [CrossRef]
- Costa, M.R. Las Fibras Textiles y su Tintura; CONCYTEC: Lima, Peru, 1990. [Google Scholar]
- Zotesso, J.P.; Honorio, J.F.; Cossich, E.S.; Tavares, C.R.G. Tratamento de efluente de lavanderia hospitalar por coagulação/floculação seguida de adsorção visando ao seu reuso. Blucher Chem. Eng. Proc. 2015, 2, 1097–1103. [Google Scholar]
- Shaki, H. Evaluation of removal of acid dyes using PAM-FeSO4 hybrid polymer as flocculant. Pigment. Resin Technol. 2022. [Google Scholar] [CrossRef]
- Ali, A.; Ejaz, N.; Nasreen, S.; Nasir, A.; Qureshi, L.A.; Al-Sakkaf, B.M. Enhanced degradation of dyes present in textile effluent by ultrasound assisted electrochemical reactor. Civ. Eng. J. 2019, 5, 2131–2142. [Google Scholar] [CrossRef]
- Sethulekshmi, S.; Chakraborty, S. Textile wastewater treatment using horizontal flow constructed wetland and effect of length of flow in operation efficiency. J. Environ. Chem. Eng. 2021, 9, 106379. [Google Scholar] [CrossRef]
- LennTech. Water Conductivity. 2022. Available online: https://www.lenntech.com/applications/ultrapure/conductivity/water-conductivity.htm (accessed on 3 March 2022).
- Granville, M. Guía para Entender la Comunicación del Color. 2002. Available online: https://www.xrite.com/-/media/xrite/files/whitepaper_pdfs/l10-001_a_guide_to_understanding_color_communication/l10-001_understand_color_es.pdf (accessed on 25 February 2022).
- Yang, X.; López-Grimau, V.; Vilaseca, M.; Crespi, M.; Ribera-Pi, J.; Calderer, M.; Martínez-Lladó, X. Reuse of textile wastewater treated by moving bed biofilm reactor coupled with membrane bioreactor. Color. Technol. 2021, 137, 484–492. [Google Scholar] [CrossRef]
- Bezerra, K.C.H.; Fiaschitello, T.R.; Labuto, G.; Freeman, H.S.; Fragoso, W.D.; da Costa, S.M.; da Costa, S.A. Reuse of water from real reactive monochromic and trichromic wastewater for new cotton dyes after efficient treatment using H2O2 catalyzed by UV light. J. Environ. Chem. Eng. 2021, 9, 105731. [Google Scholar] [CrossRef]
- Pinto, C.; Fernandes, A.; Lopes, A.; Nunes, M.J.; Baía, A.; Ciríaco, L.; Pacheco, M.J. Reuse of Textile Dyeing Wastewater Treated by Electrooxidation. Water 2022, 14, 1084. [Google Scholar] [CrossRef]
- Rosa, J.M.; Fileti, A.M.; Tambourgi, E.B.; Santana, J.C. Dyeing of cotton with reactive dyestuffs: The continuous reuse of textile wastewater effluent treated by Ultraviolet/Hydrogen peroxide homogeneous photocatalysis. J. Clean. Prod. 2015, 90, 60–65. [Google Scholar] [CrossRef]

| Parameter | Methodology and Equipment | Sampling Requirements | ||||||
|---|---|---|---|---|---|---|---|---|
| Norm | Method | Equipment | Unit | Container | Sample vol. | Preservation/ Concentration | Max. SL | |
| physicochemical characteristics | ||||||||
| Temperature | - | Direct measuring | Multimeter | C | P or G | 25 mL | Inmediatly | 15 min |
| pH | - | Direct measuring | Multimeter | - | P or G | 100 mL | Inmediatly | 15 min |
| Conductivity | - | Direct measuring | Multimeter | s/cm | P or G | 500 mL | Cool at 4 C | 28 days |
| Total solids | AOAC Official Method 920.193 | Gravimetric, Dried at 110 C | Analytical balance | mg/L | P or G | 100 mL | Cool at 4 C | 2–7 days |
| Dissolved Solids | AOAC Official Method 920.19 | Gravimetric, Dried at 180 C | Analytical balance | mg/L | P or G | 100 mL | Cool at 4 C | 2–7 days |
| Suspended solids | NMX-F-527-1992 | Gravimetric, Dried at 110 C | Analytical balance | mg/L | P or G | 100 mL | Cool at 4 C | 2–7 days |
| Turbidity | APHA-AWWA-WEF part 2000 method 2130-b | Nephelometric | Turbidimeter | NTU | P or G | 100 mL | Cool at 4 C | 24 h |
| COD | NMX-AA-030-SCFI-2001 | Open Reflux, Colorimetric | Laboratory, Spectrophotometer | mg/L | P or G | 100 mL | Cool at 4 C, HSO, pH < 2 | 28 days |
| Ammonia nitrogen | NMX-AA-026-SCFI-2001 | Distillation, Titration | Analytical or Granataria balance, Potentiometer | mg/L | P | 2000 mL | Cool at 4 C, HSO, 1.5 < pH < 2 | 7 days |
| Sulfates | NTP 214.023.2000 | Turbidimeter | Turbidimeter | mg/L | P or G | 100 mL | Cool at 4 C | 28 days |
| Sulfides | EPA 376.1 | Volumetric | Laboratory | mg/L | P or G | 100 mL | Cool at 4 C | 7 days |
| Oil/Grease | Method SM 5520 E | Gravimetric-Extraction | Laboratory, Soxhlet extractor system | mg/L | G | 500 mL | Cool at 4 C, HSO, pH < 2 | 28 days |
| Total hardness | NTP 214.018.1999 | EDTA complexometric method | Laboratory | mg/L | P or G | 500 mL | Cool at 4 C, HNO, pH < 2 | 6 months |
| ASMB | NMX-AA-039-SCFI-2001 | Colorimetric | Laboratory, Spectrophotometer | mg/L | P | 600 mL | Cool at 4 C, HSO, pH < 2 | 7 days |
| Metal traces | ||||||||
| Metals | EPA 200.7 | ICP | ICP-AES | mg/L | G | 200 mL | Cool at 4 C, HNO (for P, HSO), pH < 2 | 28 days |
| Microbial activity | ||||||||
| BOD | NMX-AA-028-SCFI-2001 | DBO at 20 C) | Incubator | mg/L | P or G | 1000 mL | Cool at 4 °C | 48 h |
| Total Coliforms | APHA, AWWA, WPCF, 9221 Method B | Technique, Fermentation | Laboratory | NMP/100 mL (35 C) | P or G | 100–500 mL | Cool at 4 °C | 24 h |
| Parameter | Unity | Influent | Effluents | ||
|---|---|---|---|---|---|
| Soft Water | Untreated | Previous | Optimized | ||
| T. Suspend Solids | mg/L | 0.0 | 312.0 | 240.0 | - |
| Temperature | C | 34.6 | 39.9 | 26.9 | 26.2 ± 1.4 |
| pH | - | 6.5 | 10.5 | 6.5 | 7.3 ± 0.44 |
| Total Solids | mg/L | 440.0 | 2080.0 | 5644.0 | 4900.0 ± 1560.0 |
| Absorbance | - | 0.014 | 3.02 | 1.5 | 0.6 ± 0.4 |
| Total Hardness | mg/L | <0.01 | 26.7 | 176.8 | 12.9 ± 3.9 |
| Conductivity | mS/cm | 0.6 | 54.0 | 9.2 | 5.3 ± 2.3 |
| Reagent | Quantity (kg) | Volume (L) | Dosage (L/h) | Stirring Speed (rpm) | Contact Time (min) |
|---|---|---|---|---|---|
| FERROCRYL®7276 | 9.0 | 60.0 | 1.9 | 100 | 5 |
| Al(SO) | 6.8 | 225.0 | 28.1 | 100 | 10 |
| CHEMLOK 2040 | 0.19 | 375.0 | 46.9 | 30 | 20 |
| Process Conditions | Process Bath | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Scouring | Rinse | Neutralizing | Biopolishing | Rinse | Dyeing | Rinse | Rinse | Soaping | Soaping | Rinse | Rinse | Softening | |
| A0 | |||||||||||||
| A1 | |||||||||||||
| A2 | |||||||||||||
| A3 | |||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Casaperalta, P.; Molina-Rodríguez, F.N.; Fernandez-F, F.; Díaz-Quintanilla, J.F.; Barreda-Del-Carpio, J.E.; Bernabe-Ortiz, J.C.; Aguilar-Pineda, J.A. Optimization of a Textile Effluent Treatment System and Evaluation of the Feasibility to Be Reused as Influents in Textile Dyeing Processes. Sustainability 2022, 14, 15588. https://doi.org/10.3390/su142315588
López-Casaperalta P, Molina-Rodríguez FN, Fernandez-F F, Díaz-Quintanilla JF, Barreda-Del-Carpio JE, Bernabe-Ortiz JC, Aguilar-Pineda JA. Optimization of a Textile Effluent Treatment System and Evaluation of the Feasibility to Be Reused as Influents in Textile Dyeing Processes. Sustainability. 2022; 14(23):15588. https://doi.org/10.3390/su142315588
Chicago/Turabian StyleLópez-Casaperalta, Patricia, Fredy Nicolás Molina-Rodríguez, Fernando Fernandez-F, Jeanette Fabiola Díaz-Quintanilla, Jaime E. Barreda-Del-Carpio, Julio Cesar Bernabe-Ortiz, and Jorge Alberto Aguilar-Pineda. 2022. "Optimization of a Textile Effluent Treatment System and Evaluation of the Feasibility to Be Reused as Influents in Textile Dyeing Processes" Sustainability 14, no. 23: 15588. https://doi.org/10.3390/su142315588
APA StyleLópez-Casaperalta, P., Molina-Rodríguez, F. N., Fernandez-F, F., Díaz-Quintanilla, J. F., Barreda-Del-Carpio, J. E., Bernabe-Ortiz, J. C., & Aguilar-Pineda, J. A. (2022). Optimization of a Textile Effluent Treatment System and Evaluation of the Feasibility to Be Reused as Influents in Textile Dyeing Processes. Sustainability, 14(23), 15588. https://doi.org/10.3390/su142315588







