Bio-Mediated Zinc Oxide Nanoparticles through Tea Residue: Ecosynthesis, Characterizations, and Biological Efficiencies
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Gathering and Processing of Tea Residue Extracts
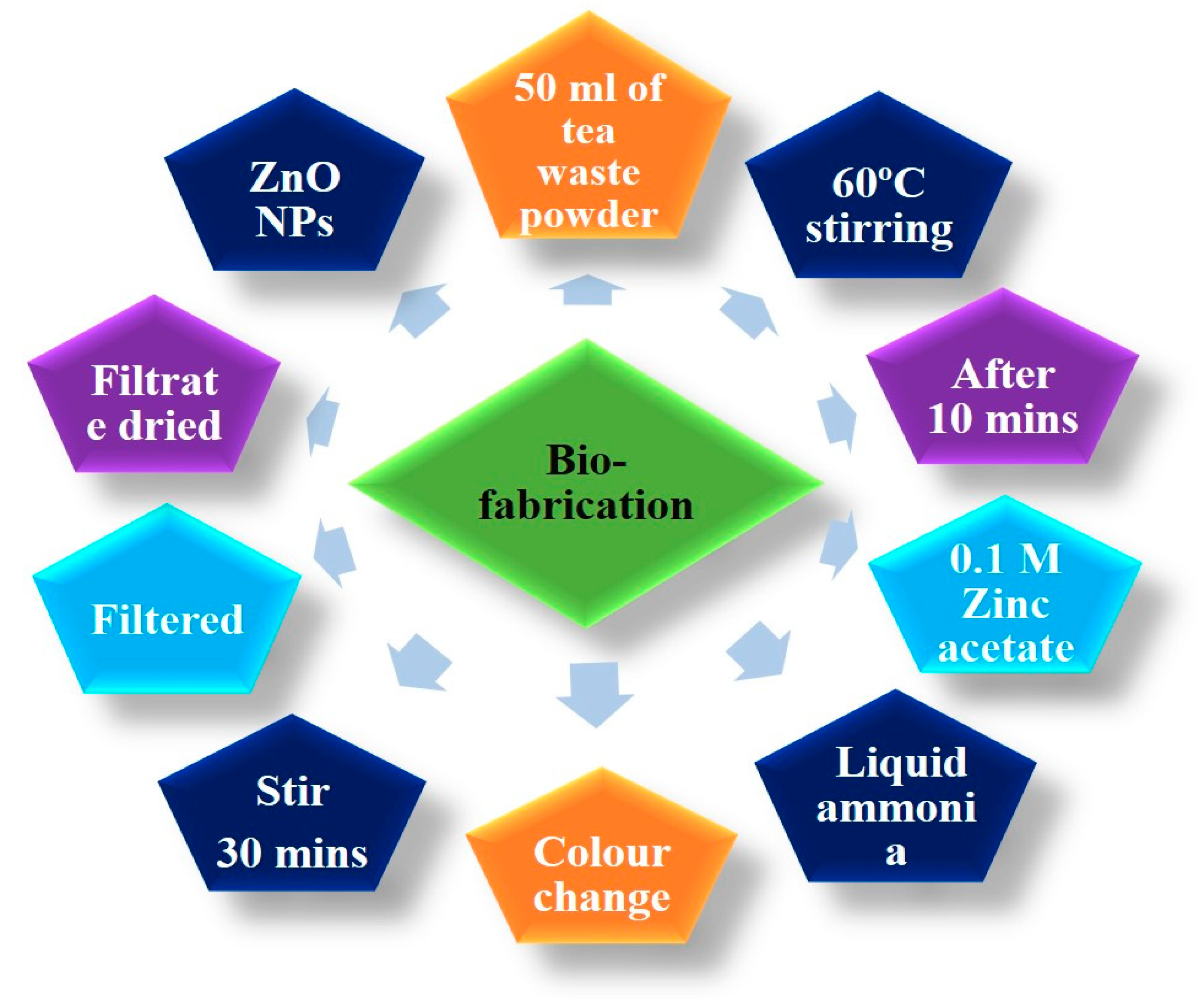
2.3. Synthesis of Zinc Oxide Nanoparticles
2.4. Characterizations of ZnO Nanoparticles
2.5. Antimicrobial Efficiencies
2.6. Antiproliferation Efficiencies
2.7. Cell Morphology Examination
2.8. Flow-Cytometric Study of the Cell Cycle
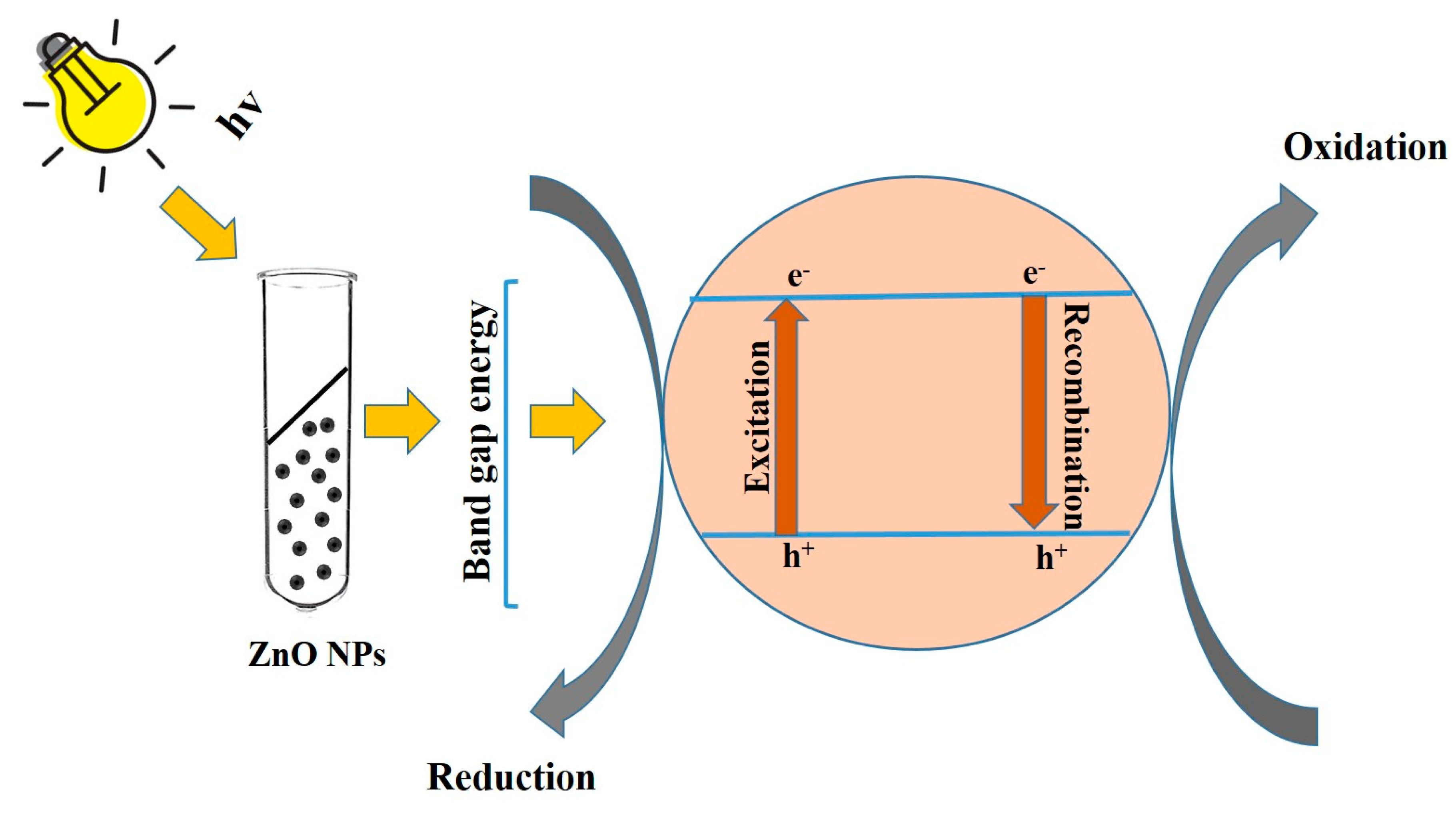
2.9. Photocatalytic Efficiencies
2.10. Statistical Analysis
3. Results and Discussion
3.1. Optical Study
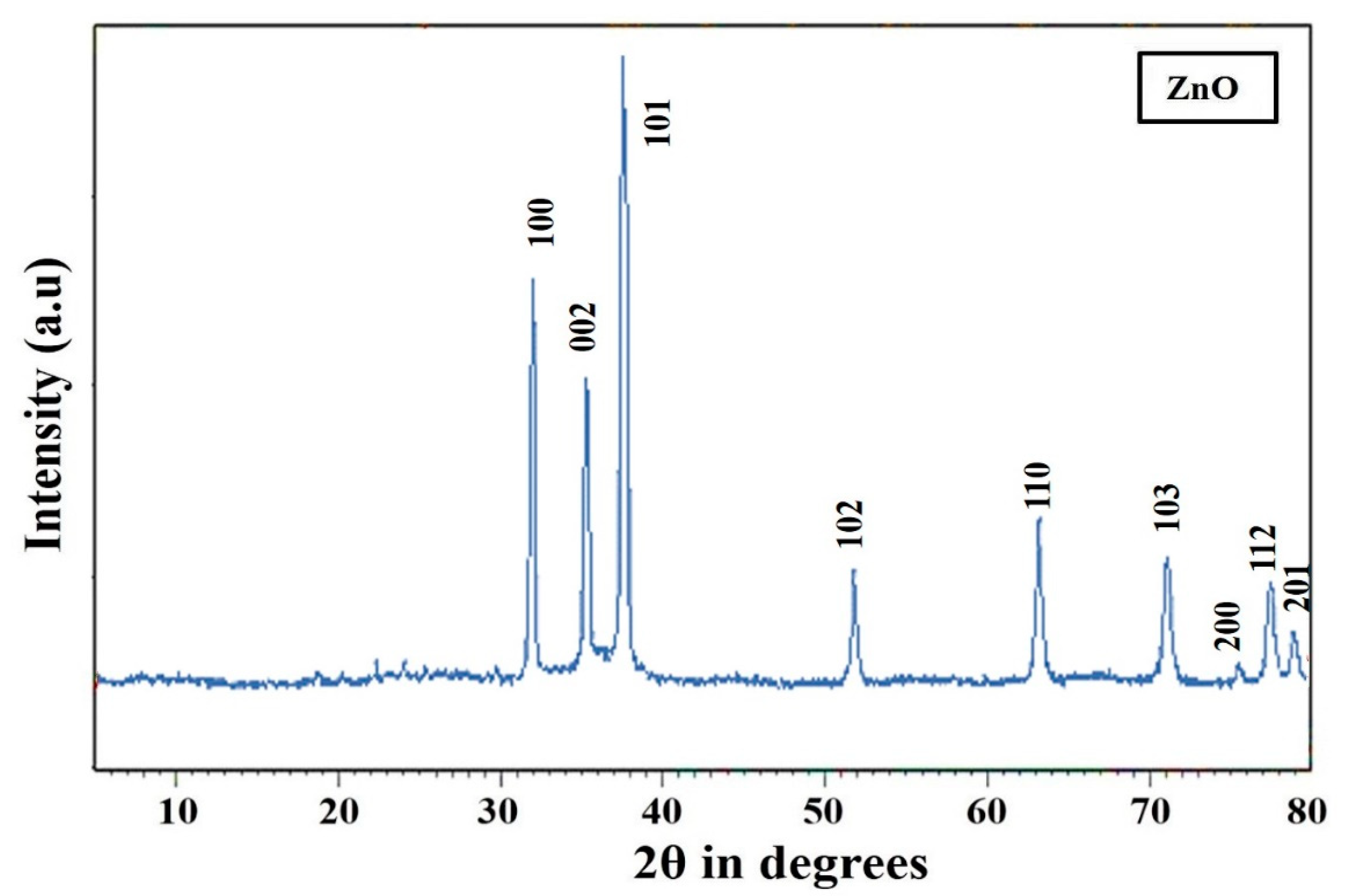
3.2. XRD
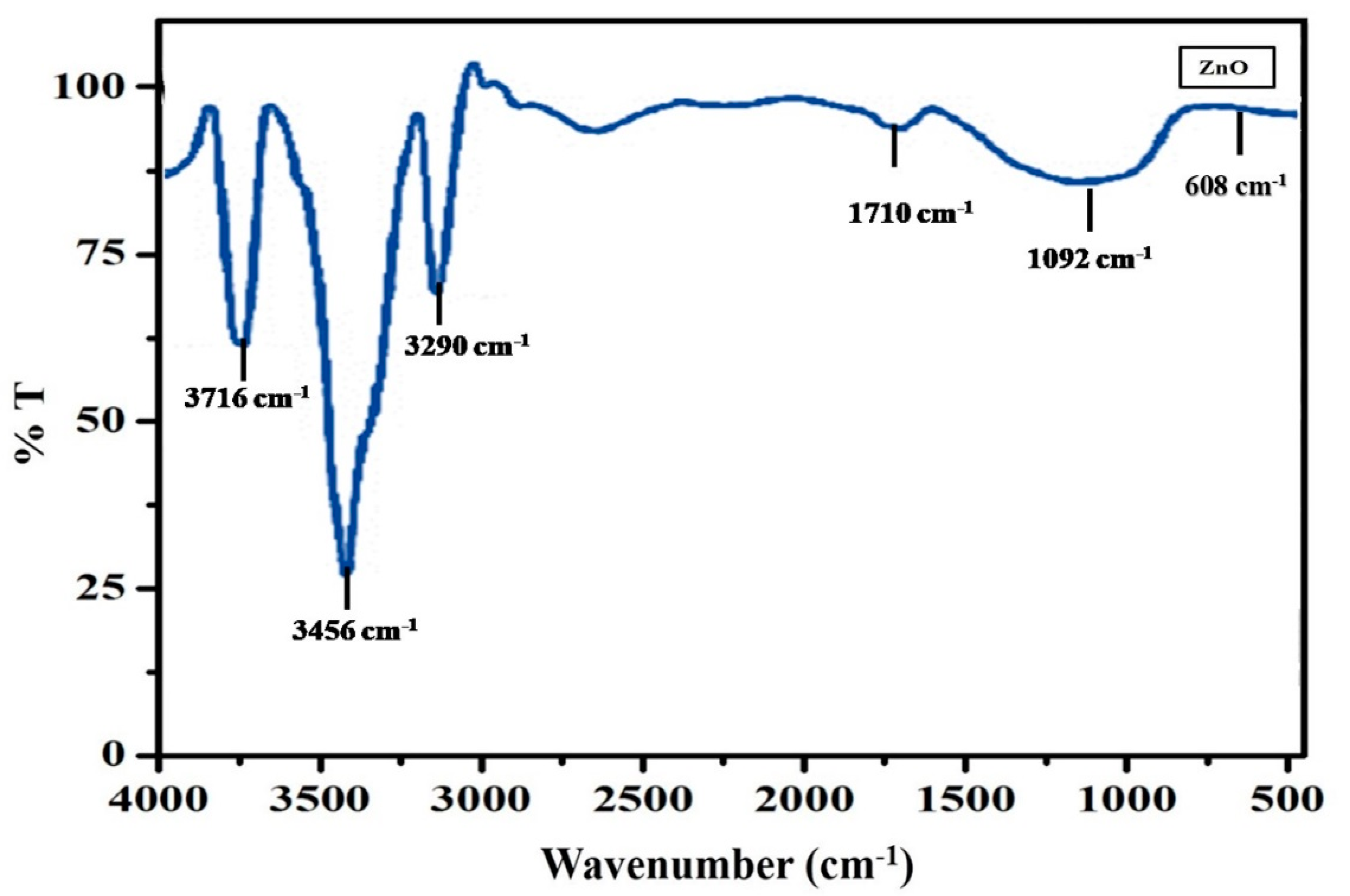
3.3. FT-IR Analysis
3.4. FE-SEM with EDX
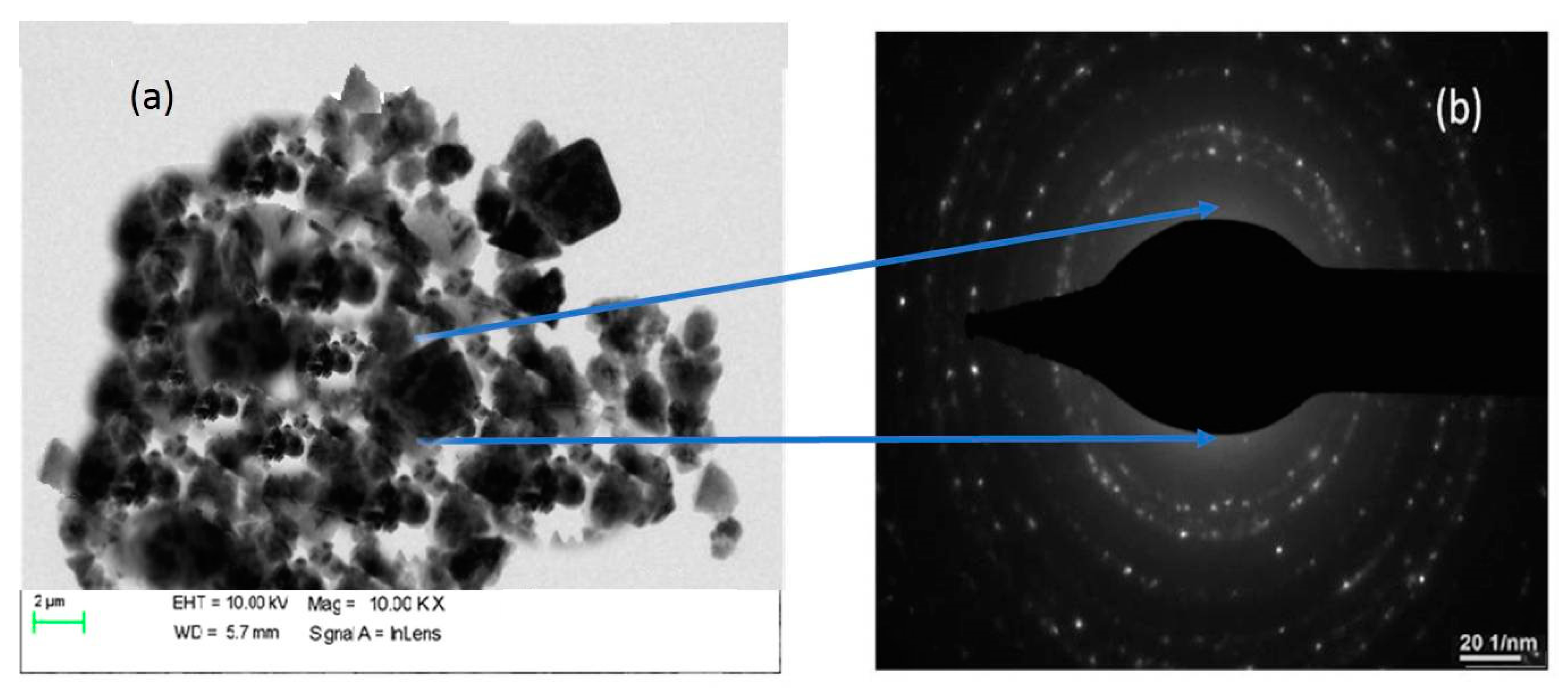
3.5. TEM
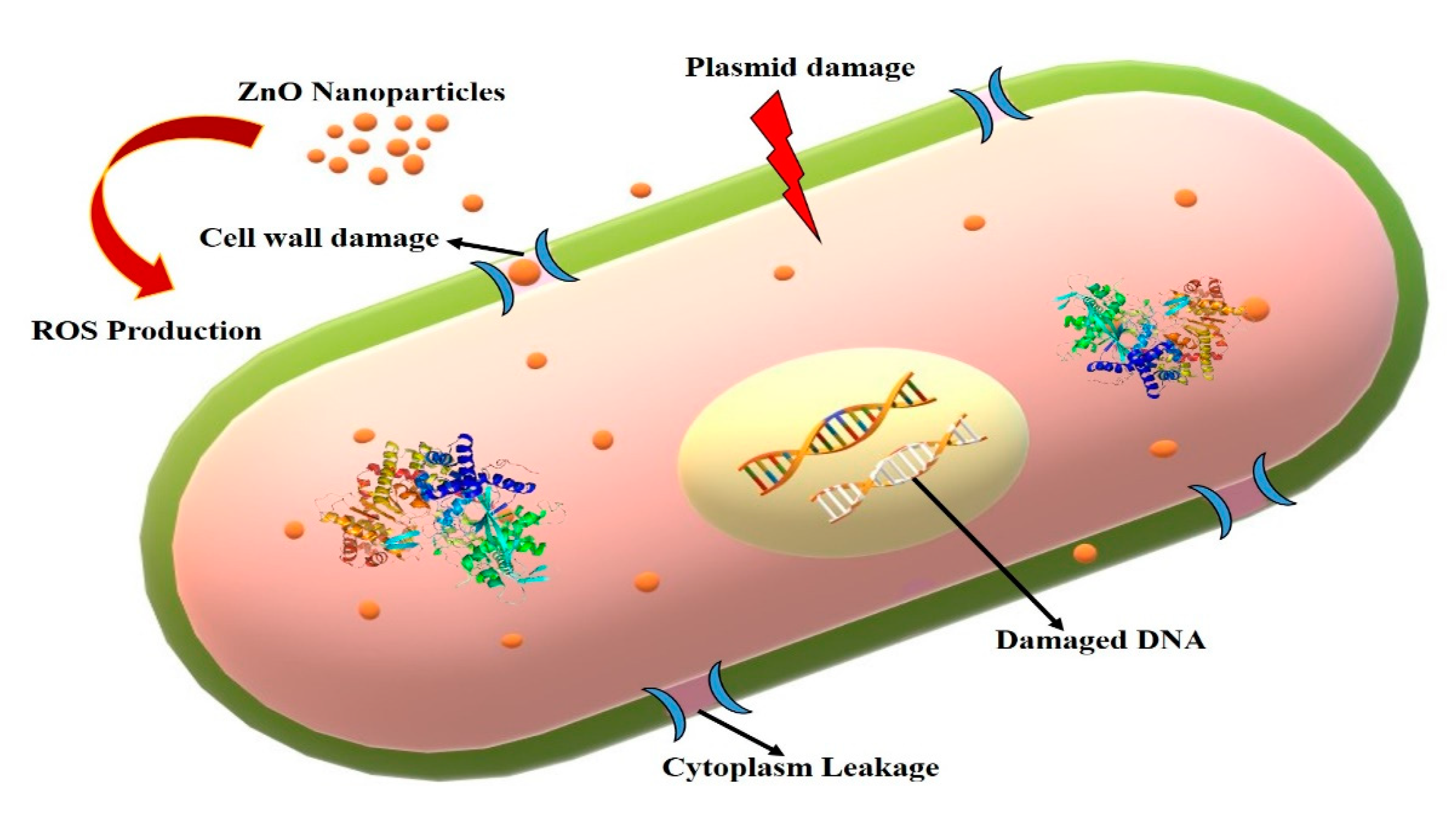
3.6. Antimicrobial Efficiencies
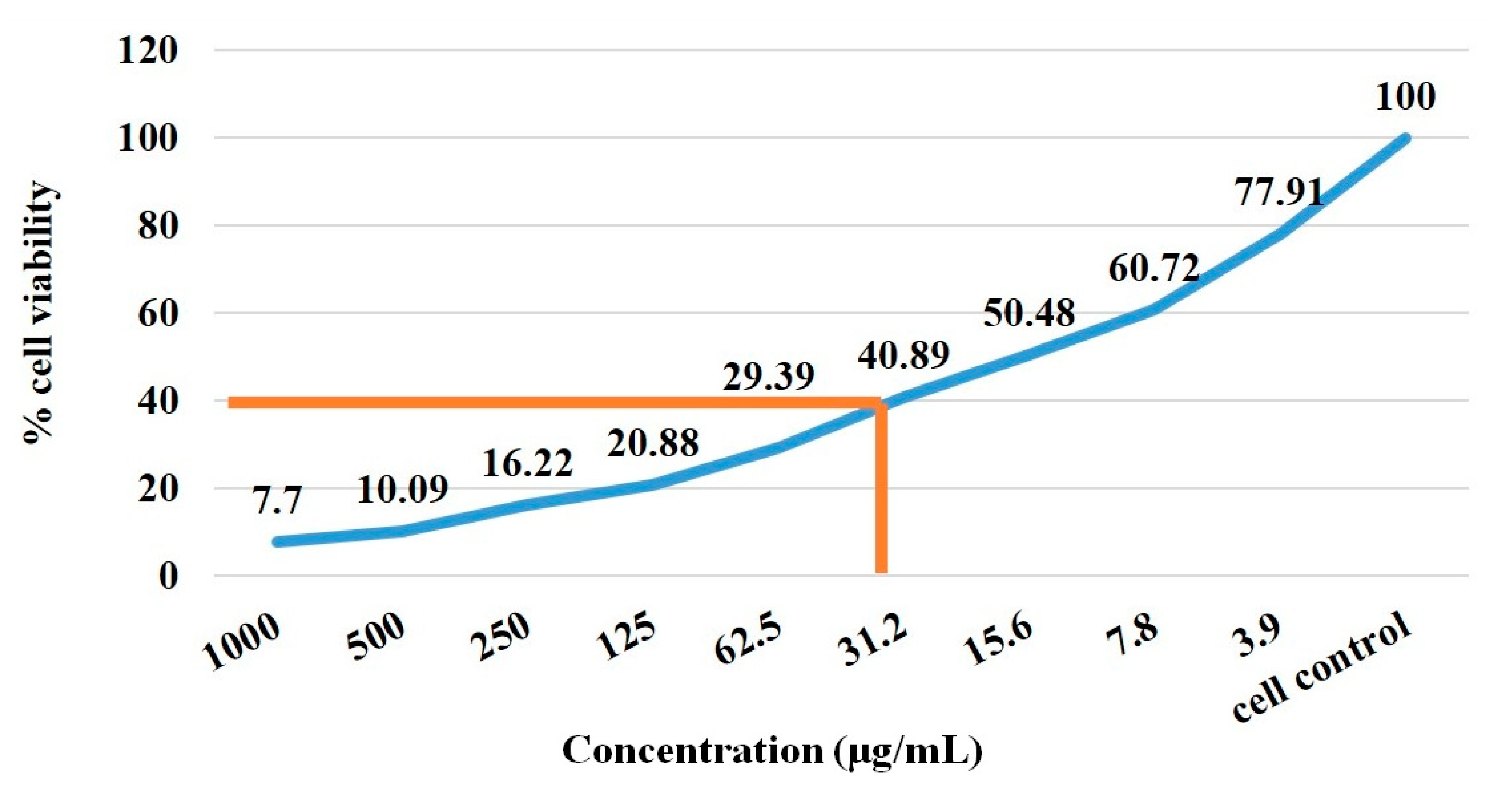
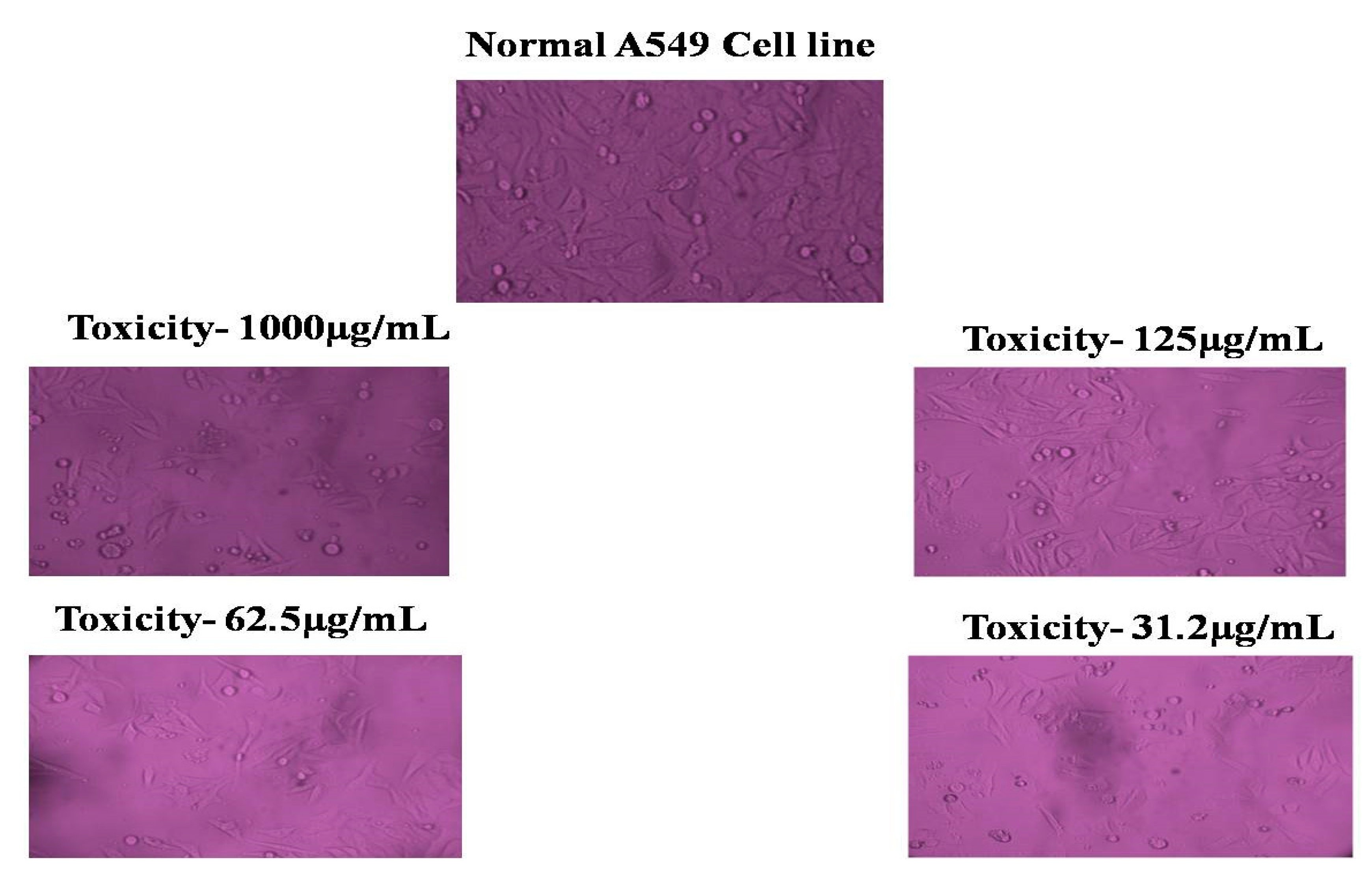
3.7. MTT Assay
3.8. Analysis of Cell Cycle by Flow Cytometry
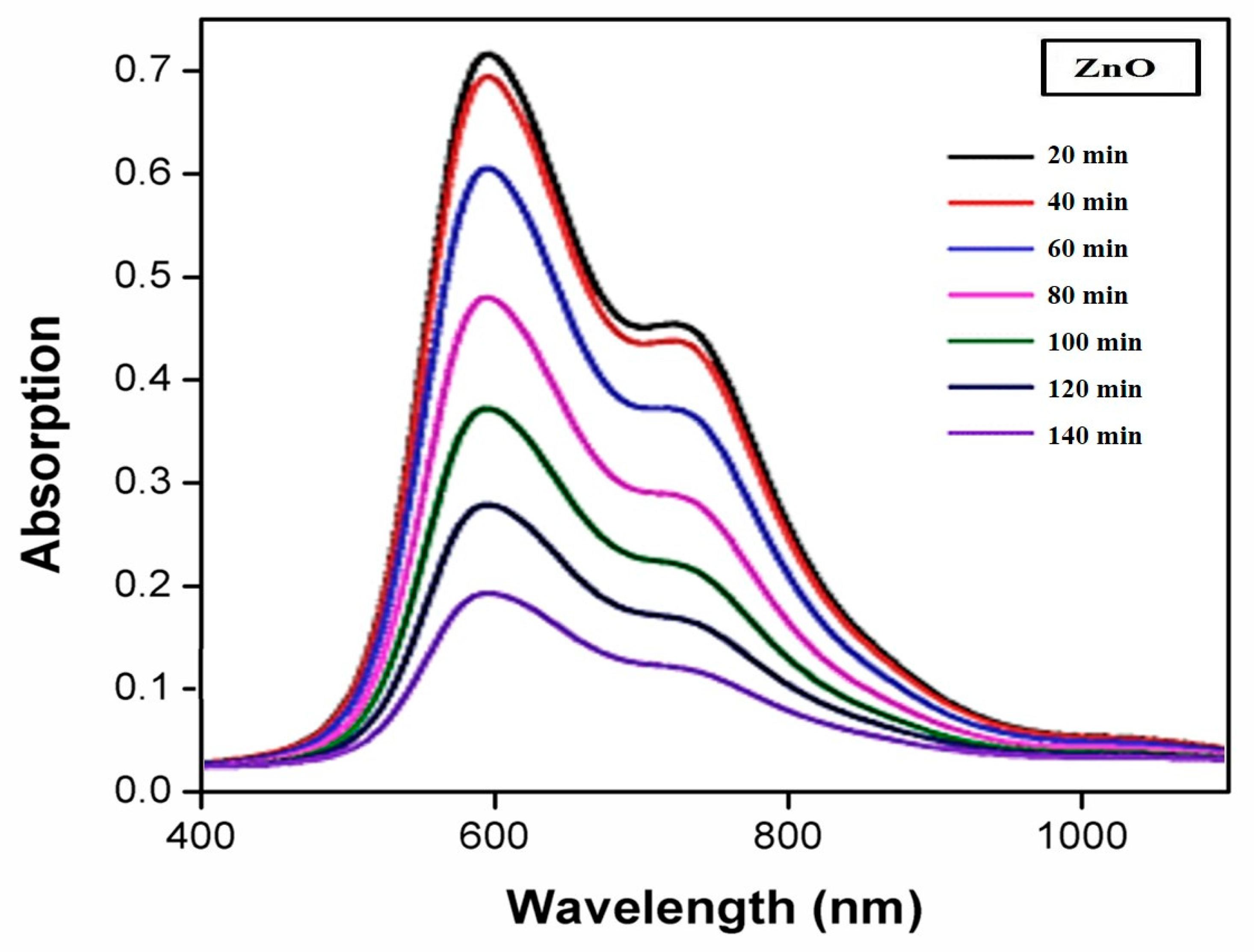
3.9. Photocatalytic Efficiencies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samer, B.; Muhammad, A.; Tiziano, T.; Marco, C.; Flavio, R. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef]
- Joseph, R.R.; Venkatraman, S.S. Drug delivery to the eye: What benefits do nanocarriers offer? Nanomedicine 2017, 12, 683–702. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Ashwini, J.; Aswathy, T.R.; Achuthsankar, S.N. Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef]
- National Nanotechnology Initiative (NNI). Available online: www.nano.gov (accessed on 22 July 2019).
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity, and anti-diabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Krishnakumar, C.; Arulmozhi, P.; Mahadevan, S.; Parameshwari, N. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Glycosmis pentaphylla (Retz.) DC. Microbpathog 2018, 116, 44–48. [Google Scholar] [CrossRef]
- Matinise, N.; Fuku, X.; Kaviyarasu, G.; Mayedwa, K.; Maaza, N. ZnO nanoparticles via Moringa oleifera green synthesis: Physical properties & mechanism of formation. Appl. Surf. Sci. 2017, 406, 339–347. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef]
- Sankapal, B.R.; Gajare, H.B.; Karade, S.S.; Salunkhe, R.R.; Dubal, D.P. Zinc Oxide Encapsulated Carbon Nanotube Thin Films for Energy Storage Applications. Electrochim. Acta 2016, 192, 377–384. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-Sensitive ZnO Quantum Dots-Doxorubicin Nanoparticles for Lung Cancer Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2016, 31, 22442–22450. [Google Scholar] [CrossRef]
- Vidhya, E.; Vijayakumar, S.; Prathipkumar, S.; Praseetha, P.K. Green way biosynthesis: Characterization, antimicrobial and anticancer activity of ZnO nanoparticles. Gene Rep. 2020, 20, 100688. [Google Scholar] [CrossRef]
- Datta, A.; Patra, C.; Bharadwaj, H.; Kaur, S.; Dimri, N.; Khajuria, R. Green synthesis of zinc oxide nanoparticles using Parthenium hysterophorus leaf extract and evaluation of their antibacterial properties. J. Biotechnol. Biomater. 2017, 7, 271–275. [Google Scholar] [CrossRef]
- Saravanakkumar, D.; Sivaranjani, S.; Umamaheswari, M.; Pandiarajan, S.; Ravikumar, B. Green synthesis of ZnO nanoparticles using Trachyspermum ammi seed extract for antibacterial investigation. Der. Pharma. Chemica 2016, 8, 173–180. [Google Scholar]
- Santhoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour. Effic. Technol. 2017, 3, 459–465. [Google Scholar] [CrossRef]
- Irshad, S.; Salamat, A.; Anjum, A.A.; Sana, S.; Saleem, R.S.Z.; Naheed, A.; Iqbal, A.; Yang, J. Green tea leaves mediated ZnO nanoparticles and its antimicrobial activity. Cogent Chem. 2018, 4, 1469207. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; RajeshKumar, S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour.-Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Moniri, M.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Saad, W.Z.; Namvar, F.; Navaderi, M.; Mohamad, R. Biosynthesis of ZnO Nanoparticles by a New Pichia kudriavzevii Yeast Strain and Evaluation of Their Antimicrobial and Antioxidant Activities. Molecules 2017, 22, 872. [Google Scholar] [CrossRef]
- Najma, B.; Khan Kasi, A.; Khan Kasi, J.; Akbar, A.; Bokhari, S.M.A.; Stroe, I.R. ZnO/AAO Photocatalytic Membranes for Efficient Water Disinfection: Synthesis, Characterization and Antibacterial Assay. Appl. Surf. Sci. 2018, 448, 104–114. [Google Scholar] [CrossRef]
- Anbuvannana, M.; Ramesh, M.; Manikandan, E.; Srinivasan, R. Vitex negundo leaf extract mediated synthesis of ZnO nanoplates and its antibacterial and photocatalytic activities. Asian. J. Nanosci. Mater. 2018, 2, 99–110. [Google Scholar] [CrossRef]
- Singh, Y.; Kumar, S.N.; Sharma, A.; Singla, A.; Singh, D.; Abd Rahim, E. Effect of ZnO nanoparticles concentration as additives to the epoxidized Euphorbia Lathyris oil and their tribological characterization. Fuel 2021, 285, 119148. [Google Scholar] [CrossRef]
- Ali, H.A.; Iliadis, A.A.; Mulligan, R.F.; Cresce, A.V.W.; Kofinas, P.; Lee, U. Properties of self-assembled ZnO nanostructures. Solid-State Electron. 2002, 46, 1639–1642. [Google Scholar] [CrossRef]
- Yaqoob, K.; Durrani, S.K.; Mazhar, M.; Jamil, A.; Riaz Khan, M.; Shamraz, F. Low-temperature synthesis of fluorescent ZnO nanoparticles. Appl. Surf. Sci. 2010, 257, 1756–1761. [Google Scholar] [CrossRef]
- Mebrahtu, H.K. Synthesis and characterization of ZnO nanoparticles using aqueous extract of Becium grand forum for antimicrobial activity and adsorption of methylene blue. Appl. Water Sci. 2021, 11, 45. [Google Scholar] [CrossRef]
- Ahmed, A.T.; El-Tras, W.F.; Shaaban, M.; El-Baz, A.F.; Hoda, M.; Mohammed, F.S.; Leon, B. Antibacterial Action of Zinc Oxide Nanoparticles Against Foodborne Pathogens. J. Food Saf. 2011, 31, 211–218. [Google Scholar] [CrossRef]
- Getie, S.; Belay, A.; Reddy, A.R.; Chandra, Z.B. Synthesis and Characterizations of Zinc Oxide Nanoparticles for Antibacterial Applications. J. Nanomed. Nanotechnol. 2017, s8, 1–8. [Google Scholar] [CrossRef]
- Demissie, M.G.; Sabir, F.K.; Edossa, G.D.; Gonfa, B.A.; Gawdzik, B. Synthesis of Zinc Oxide Nanoparticles Using Leaf Extract of Lippia adoensis (Koseret) and Evaluation of Its Antibacterial Activity. J. Chem. 2020, 2020, 7459042. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Abdul Rahuman, A.; Vishnu Kirthi, A.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Bhaskara Rao, K.V. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta 2012, 90, 78–84. [Google Scholar] [CrossRef]
- Insoo, K.; Karthika, V.; Gopinath, K.; Sarinthip, T.; Kambiz, S.; Jongchul, S. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2022, 38, 537–565. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Nilavukkarasi, M.; Sakthivel, B. Bio-synthesized zinc oxide nanoparticles for anti-tuberculosis agent: Scientifically unexplored. Gene Rep. 2020, 20, 100764. [Google Scholar] [CrossRef]
- Namvar, F.; Baharara, J.; Mahdi, A.A. Antioxidant and anticancer activities of selected Persian Gulf algae. Ind. J. Clinic. Biochem. 2014, 29, 13–20. [Google Scholar] [CrossRef]
- Xu, W.; Shen, G.; Li, T.; Zhang, Y.; Zhang, T.; Xue, H.; Zuo, W.; Li, Y.; Zhang, D.; Jin, C. Isoorientin induces the apoptosis and cell cycle arrest of A549 human lung cancer cells via the ROS-regulated MAPK, STAT3 and NF-κB signalling pathways. Int. J. Oncol. 2020, 57, 550–561. [Google Scholar] [CrossRef]
- Gavade, N.L.; Kadam, A.N.; Gaikwad, Y.B.; Dhanavade, M.J.; Garadkar, K.M. Decoration of biogenic AgNPs on template free ZnO nanorods for sunlight driven photocatalytic detoxification of dyes and inhibition of bacteria. J. Mater. Sci. Mater. Electron. 2016, 27, 11080–11091. [Google Scholar] [CrossRef]
- Minha, R.; Ajda, K.; Lev, M.; Sonja, S.M.; Martin, S.; Andraz, S. Controlled growth of ZnO nanoparticles using ethanolic root extract of Japanese knotweed: Photocatalytic and antimicrobial properties. RSC Adv. 2022, 12, 31235–31245. [Google Scholar] [CrossRef]
- Gawade, V.V.; Gavade, N.L.; Shinde, H.M.; Babar, S.B.; Kadam, A.N.; Garadkar, K.M. Green synthesis of ZnO nanoparticles by using Calotropis procera leaves for the photodegradation of methyl orange. J. Mater. Sci. Mater. Electron. 2017, 28, 14033–14039. [Google Scholar] [CrossRef]



| S. No. | Name of the Organisms | Zone of Inhibition (µg/mL−1) a | |||
|---|---|---|---|---|---|
| Tea Waste Extract | Zinc Acetate | ZnO NPs | Control | ||
| 1. | Staphylococcus epidermidis | 13 ± 1.18 | 14 ± 1.80 | 25 ± 2.01 | 12 ± 1.08 |
| 2. | Enterococcus faecalis | 12 ± 0.99 | 13 ± 1.89 | 22 ± 1.90 | 11 ± 1.07 |
| 3. | Shigella dysenteriae | 10 ± 0.86 | 11 ± 0.75 | 19 ± 0.69 | 10 ± 1.12 |
| 4. | Salmonella paratyphi | 11 ± 1.02 | 12 ± 1.57 | 20 ± 1.11 | 13 ± 0.80 |
| 5. | Candida albicans | 9 ± 0.55 | 10 ± 1.94 | 18 ± 1.20 | 14 ± 0.76 |
| 6. | Aspergillus niger | 8 ± 0.66 | 9 ± 0.44 | 16 ± 0.99 | 10 ± 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathizhagan, T.E.; Subramaniyan, V.; Renganathan, S.; Elavarasan, V.; Subramaniyan, P.; Vijayakumar, S. Bio-Mediated Zinc Oxide Nanoparticles through Tea Residue: Ecosynthesis, Characterizations, and Biological Efficiencies. Sustainability 2022, 14, 15572. https://doi.org/10.3390/su142315572
Mathizhagan TE, Subramaniyan V, Renganathan S, Elavarasan V, Subramaniyan P, Vijayakumar S. Bio-Mediated Zinc Oxide Nanoparticles through Tea Residue: Ecosynthesis, Characterizations, and Biological Efficiencies. Sustainability. 2022; 14(23):15572. https://doi.org/10.3390/su142315572
Chicago/Turabian StyleMathizhagan, Tamil Elakkiya, Vijayakumar Subramaniyan, Sangeetha Renganathan, Vidhya Elavarasan, Prathipkumar Subramaniyan, and Sekar Vijayakumar. 2022. "Bio-Mediated Zinc Oxide Nanoparticles through Tea Residue: Ecosynthesis, Characterizations, and Biological Efficiencies" Sustainability 14, no. 23: 15572. https://doi.org/10.3390/su142315572
APA StyleMathizhagan, T. E., Subramaniyan, V., Renganathan, S., Elavarasan, V., Subramaniyan, P., & Vijayakumar, S. (2022). Bio-Mediated Zinc Oxide Nanoparticles through Tea Residue: Ecosynthesis, Characterizations, and Biological Efficiencies. Sustainability, 14(23), 15572. https://doi.org/10.3390/su142315572








