Analysis of Distribution and Structures of Heteroatom Compounds in Asphaltene of Medium/Low Temperature Coal Tar by Negative Anion Mode ESI FT-ICR MS
Abstract
:1. Introduction
2. Experimental Section
2.1. Precipitation of Asphaltenes
2.2. Analytical Methods
2.2.1. Elemental Analysis
2.2.2. Relative Molecular Mass Analysis
2.2.3. ESI FT-ICR MS
2.3. Data Analysis Procedure
3. Results and Discussions
3.1. Negative Ion ESI FT-ICR Mass Spectra
3.2. Heteroatom-Containing Compounds Distribution
3.3. The Molecular Structure of Heteroato-Containing Compounds
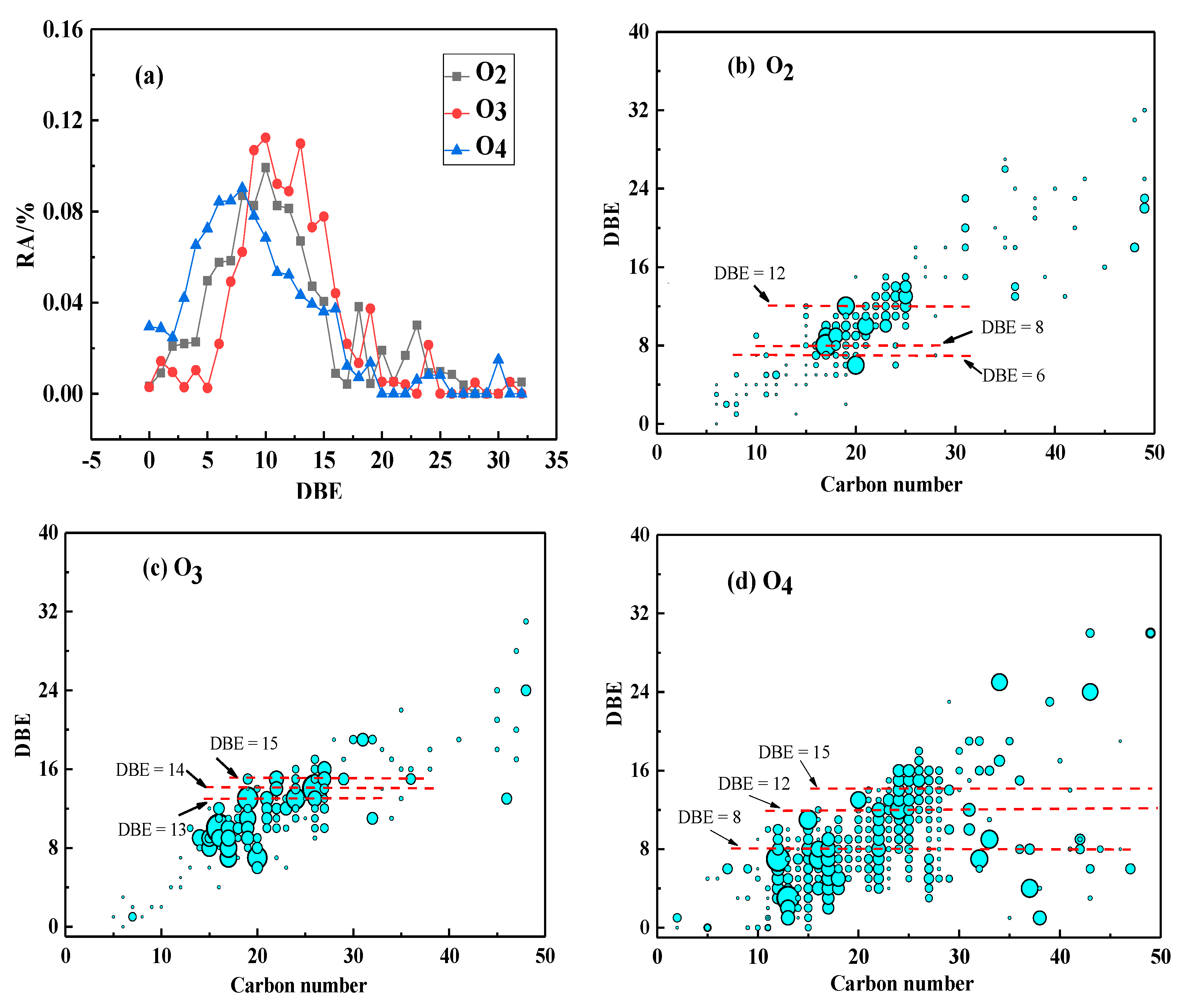
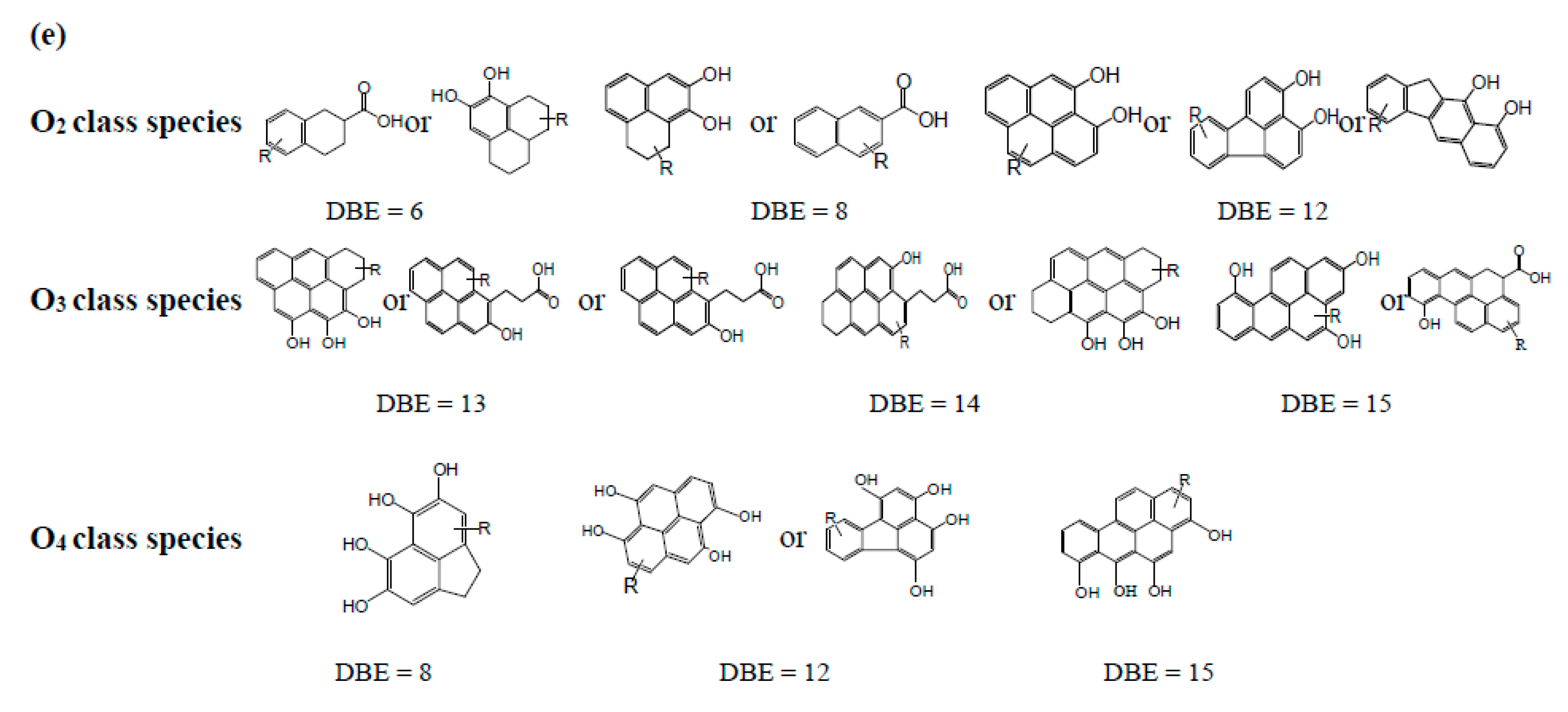
3.3.1. OX(x = 2–4) Class Species in M/LTCT Asphaltenes
O2 Class Species
O3 Class Species
O4 Class Species
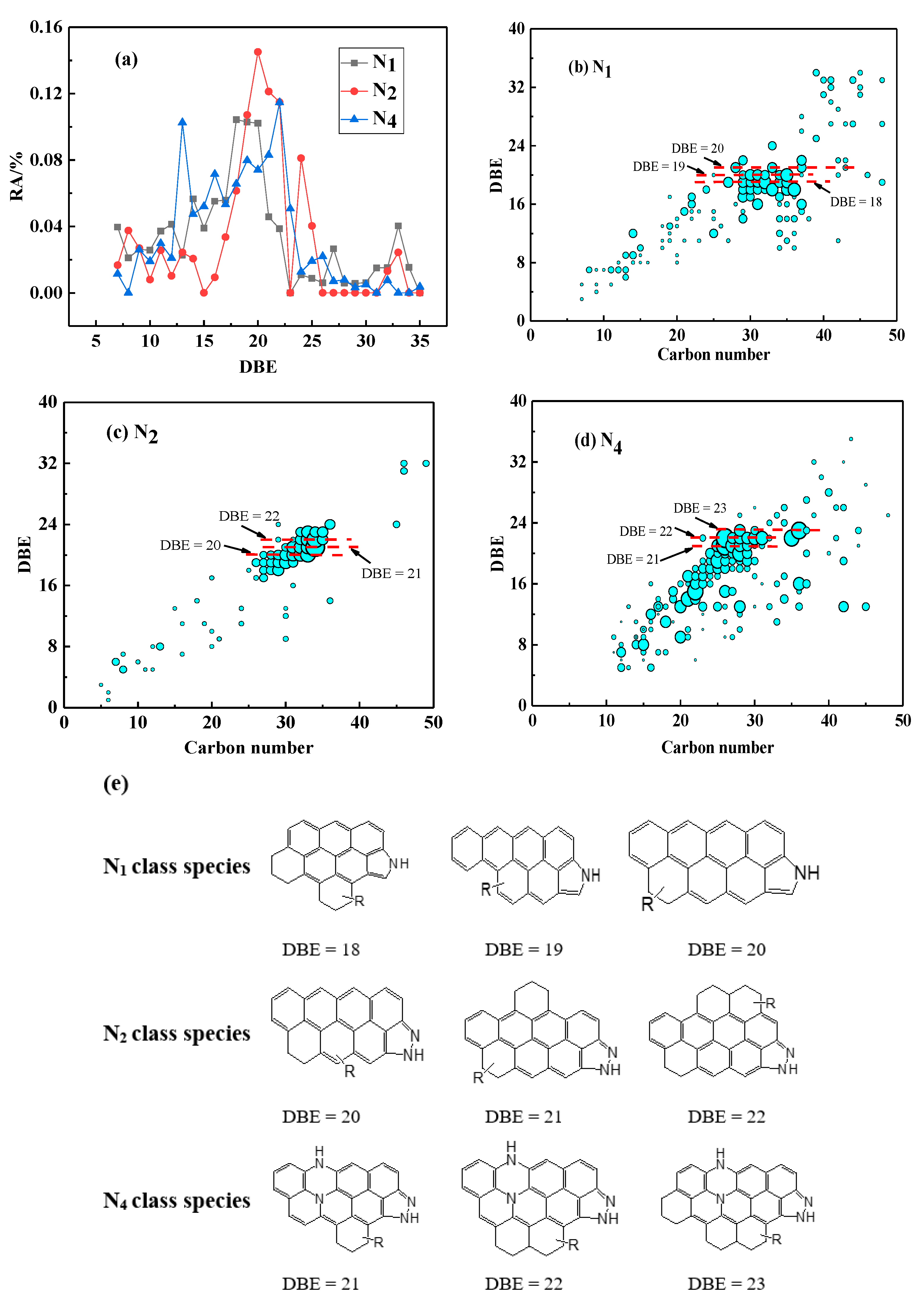
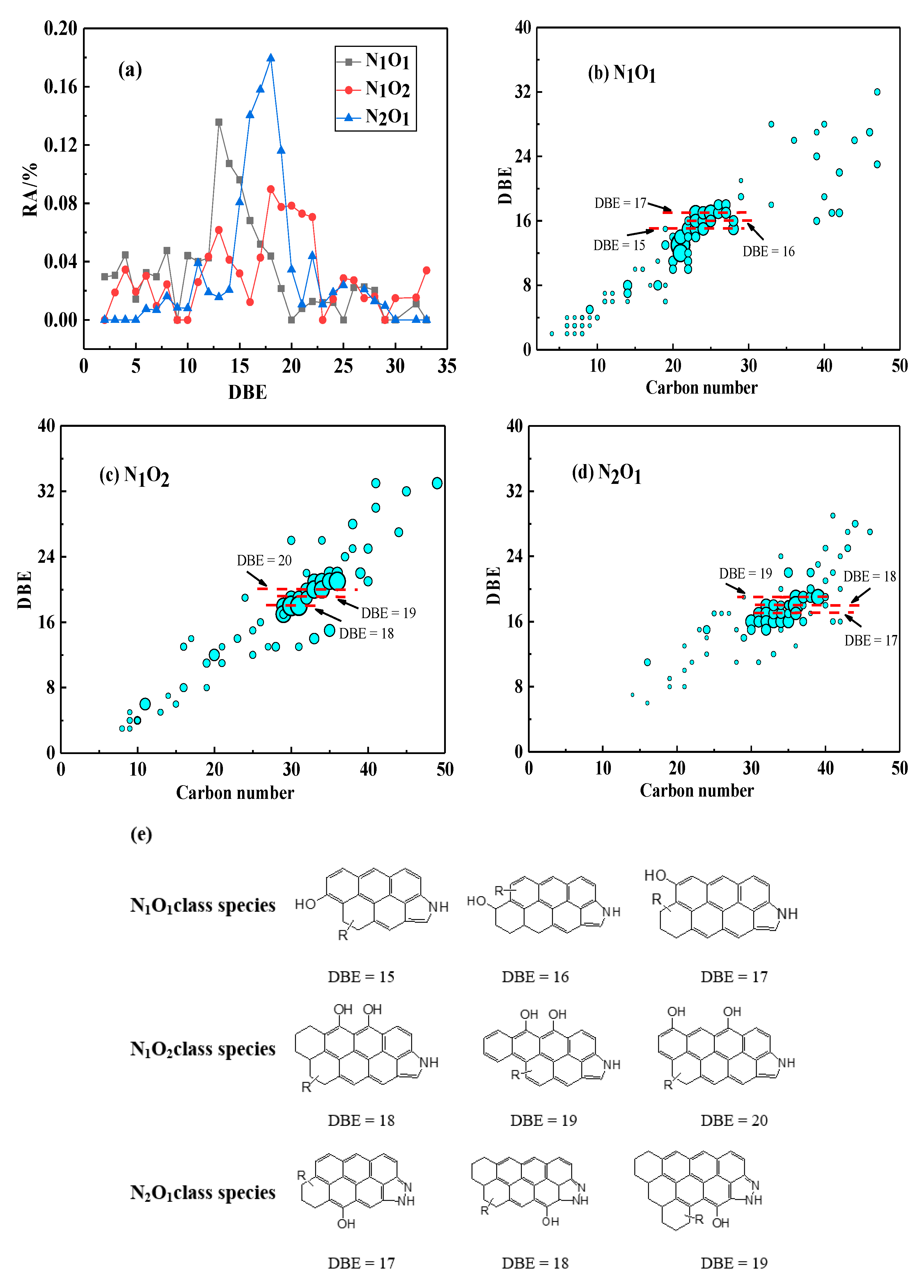
3.3.2. Distribution of NX Class Species
3.3.3. Distribution of Muti-Heteroatom Class Species
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, D.; Li, Z.; Li, W.; Liu, Q.; Feng, Z.; Fan, Z. Hydrotreating of low temperature coal tar to produce clean liquid fuels. J. Anal. Appl. Pyrolysis 2013, 100, 245–252. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Dan, Y.; Yuan, Y.; Zhang, L.; Li, W.; Li, D. Optimization of reaction variables and macrokinetics for the hydrodeoxygenation of full range low temperature coal tar. React. Kinet. Mech. Catal. 2015, 116, 433–450. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, B.; Chen, J.; Zhan, Z.; Yang, C.; Zou, Y.; Peng, P. Characterisation by ESI FT-ICR MS of heteroatomic compounds in catalytic hydropyrolysates released from marine crude oil asphaltenes. Org. Geochem. 2021, 167, 104391. [Google Scholar] [CrossRef]
- Merdrignac, I.; Quoineaud, A.A.; Gauthier, T. Evolution of Asphaltene Structure during Hydroconversion Conditions. Energy Fuels 2006, 20, 2028–2036. [Google Scholar] [CrossRef]
- Ancheyta, J.; Centeno, G.; Trejo, F.; Marroquin, G. Changes in asphaltene properties during hydrotreating of heavy crudes. Energy Fuels 2003, 17, 1233–1238. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, D.; Zhang, L.N.; Zhu, Y.H.; Wang, L.; Li, W.H. Development, Status, and Prospects of Coal Tar Hydrogenation Technology. Energy Technol. 2016, 4, 1338–1348. [Google Scholar] [CrossRef]
- Sun, Z.H.; Li, D.; Ma, H.; Tian, P.; Li, X.; Li, W.; Zhu, Y. Characterization of asphaltene isolated from low-temperature coal tar. Fuel Process. Technol. 2015, 138, 413–418. [Google Scholar] [CrossRef]
- Ruiz-Morales, Y.; Miranda-Olvera, A.D.; Portales-Martínez, B.; Domínguez, J.M. Experimental and Theoretical Approach To Determine the Average Asphaltene Structure of a Crude Oil from the Golden Lane (Faja de Oro) of Mexico. Energy Fuels 2020, 34, 7985–8006. [Google Scholar] [CrossRef]
- Cai, X.; Shi, R.; Wang, W.; Hou, H.; Peng, D.; Wang, N.; Deng, Z.; Liu, Z.; Zhang, Q. Molecular Structures of Refractory Sulfur Compounds in Heavy Oil Hydrodesulfurization Characterized by Collision-Induced Dissociation Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2022, 36, 1326–1337. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Z.; Zhao, S. Separation and characterization of C5-asphaltene from low temperature coal tar. J. Fuel Chem. Technol. 2016, 44, 1318–1325. [Google Scholar]
- Ahmed, A.; Sriram, R.; Nagu, D. Review of Asphaltene Deposition Modeling in Oil and Gas Production. Energy Fuels 2021, 35, 965–986. [Google Scholar]
- Zeinab, T.; AmirHossein, S.; Shahab, A. A New Insight to the Assessment of Asphaltene Characterization by Using Fortier Transformed Infrared Spectroscopy. J. Pet. Sci. Eng. 2021, 205, 108824. [Google Scholar]
- Deng, W.; Wu, L.; Wang, X.; Lu, J.; Li, C. Characteristics of surface functional groups from coal tar aspaltene and its influence on the selection of assistants in slurry-bed hydrocracking. Acta Pet. Sin. Pet. Process. Sect. 2015, 31, 1262–1268. [Google Scholar]
- Noah, M.; Forsythe, J.; di Primio, R.; Mehay, S.; Mullins, O.C.; Mahlstedt, N.; Horsfield, B. Heavy End Evaluation in Oils and Associated Asphaltene Deposits from Two Adjacent Reservoirs by High-Resolution Mass Spectrometry. Energy Fuels 2022, 36, 8866–8878. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Y.; Chung, K.; Zhao, S.; Xu, C. Molecular Characterization of Fossil and Alternative Fuels Using Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry: Recent Advances and Perspectives. Energy Fuels 2021, 35, 18019–18055. [Google Scholar] [CrossRef]
- Schaub, T.M.; Hendrickson, C.L.; Quinn, J.P.; Rodgers, R.P.; Marshall, A.G. Instrumentation and Method for Ultrahigh Resolution Field Desorption Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry of Nonpolar Species. Anal. Chem. 2005, 77, 1317–1324. [Google Scholar] [CrossRef]
- Fu, J.; Kim, S.; Rodgers, R.P.; Hendrickson, C.L.; Marshall, A.G.; Qian, K. Nonpolar Compositional Analysis of Vacuum Gas Oil Distillation Fractions by Electron Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2006, 20, 661–667. [Google Scholar] [CrossRef]
- Park, J.W.; Cho, Y.; Son, S.; Kim, S.; Lee, K.B. Characterization and Structural Classification of Heteroatom Components of Vacuum-Residue-Derived Asphaltenes Using APPI (+) FT-ICR Mass Spectrometry. Energy Fuels 2021, 35, 13756–13765. [Google Scholar] [CrossRef]
- Klein, G.C.; Rodgers, R.P.; Marshall, A.G. Identification of hydrotreatment-resistant heteroatomic species in a crude oil distillation cut by electrospray ionization FT-ICR mass spectrometry. Fuel 2006, 85, 2071–2080. [Google Scholar] [CrossRef]
- McKenna, A.M.; Purcell, J.M.; Rodgers, R.P.; Marshall, A.G. Identification of Vanadyl Porphyrins, in a Heavy Crude Oil and Raw Asphaltene by Atmospheric Pressure Photoionization Fourier Transform Ion Cyclotron Resonance (FT-ICR) Mass Spectrometry. Energy Fuels 2009, 23, 2122–2128. [Google Scholar] [CrossRef]
- Wang, S.S.; Yang, C.; Xu, C.M.; Zhao, S.Q.; Shi, Q. Separation and characterization of petroleum asphaltene fractions by ESI FT-ICR MS and UV-vis spectrometer. Sci. China Chem. 2013, 56, 856–862. [Google Scholar] [CrossRef]
- Wang, L.T.; He, C.; Zhang, Y.H.; Zhao, S.Q.; Chung, K.H.; Xu, C.M.; Hsu, C.S.; Shi, Q. Characterization of Acidic Compounds in Heavy Petroleum Residue by Fractionation and Negative-Ion Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry Analysis. Energy Fuels 2013, 27, 4555–4563. [Google Scholar] [CrossRef]
- Headley, J.V.; Kumar, P.; Dalai, A.; Peru, K.M.; Bailey, J.; McMartin, D.W.; Rowland, S.M.; Rodgers, R.P.; Marshall, A.G. Fourier Transform Ion Cyclotron Resonance Mass Spectrometry Characterization of Treated Athabasca Oil Sands Processed Waters. Energy Fuels 2015, 29, 2768–2773. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, Q.; Zhang, Y.; Pan, N.; Xu, C.; Chung, K.; Zhao, S. Characterization of Nitrogen Compounds in Coker Heavy Gas Oil and Its Subfractions by Liquid Chromatographic Separation Followed by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2010, 25, 281–287. [Google Scholar] [CrossRef]
- Liao, Y.; Shi, Q.; Hsu, C.S.; Pan, Y.; Zhang, Y. Distribution of acids and nitrogen-containing compounds in biodegraded oils of the Liaohe Basin by negative ion ESI FT-ICR MS. Org. Geochem. 2012, 47, 51–65. [Google Scholar] [CrossRef]
- Gaspar, A.; Zellermann, E.; Lababidi, S.; Reece, J.; Schrader, W. Characterization of Saturates, Aromatics, Resins, and Asphaltenes Heavy Crude Oil Fractions by Atmospheric Pressure Laser Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2012, 26, 3481–3487. [Google Scholar] [CrossRef]
- Merino-Garcia, D.; Andersen, S.I. Application of Isothermal Titration Calorimetry in the Investigation of Asphaltene Association. In Asphaltenes, Heavy Oils, and Petroleomics; Springer: New York, NY, USA, 2007; pp. 329–352. [Google Scholar]
- Bej, S.K.; Dalai, A.K.; Adjaye, J. Comparison of hydrodenitrogenation of basic and nonbasic nitrogen compounds present in oil sands derived heavy gas oil. Energy Fuels 2001, 15, 377–383. [Google Scholar] [CrossRef]
- Sharma, Y.K. The Instability of Storage of Middle Distillate Fuels: A Review. Liq. Fuels Technol. 2012, 30, 1839–1850. [Google Scholar] [CrossRef]
- Mushrush, G.W.; Speight, J.G. Petroleum Products: Instability and Incompatibility; Taylor & Francis: Abingdon, UK, 1995. [Google Scholar]
- Xu, C.M.; Liu, Y.; Zhao, S.; Shi, Q. Compositional analysis of petroleum asphaltenes by negative ion electrospray high resolution FT-ICR mass spectrometry. J. China Univ. Pet. 2013, 37, 190–195. [Google Scholar]
- Shi, Q.; Hou, D.; Chung, K.H.; Xu, C.; Zhao, S.; Zhang, Y. Characterization of Heteroatom Compounds in a Crude Oil and Its Saturates, Aromatics, Resins, and Asphaltenes (SARA) and Non-basic Nitrogen Fractions Analyzed by Negative-Ion Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2010, 24, 2545–2553. [Google Scholar]
- Wang, L.; He, C.; Liu, Y.; Zhao, S.; Zhang, Y.; Xu, C.; Chung, K.; Shi, Q. Effects of experimental conditions on the molecular composition of maltenes and asphaltenes derived from oilsands bitumen: Characterized by negative-ion ESI FT-ICR MS. Sci. China Chem. 2013, 56, 863–873. [Google Scholar] [CrossRef]
- Suleiman, S.; Wang, C.; Zhang, M.; Gao, H.; Han, Z.; Shi, L.; Su, F.; Xu, G. A Review on the Reaction Mechanism of Hydrodesulfurization and Hydrodenitrogenation in Heavy Oil Upgrading. Energy Fuels 2021, 35, 10998–11016. [Google Scholar]
- Hua, R.; Li, Y.; Liu, W.; Zheng, J.; Wei, H.; Wang, J.; Lu, X.; Kong, H.; Xu, G. Determination of sulfur-containing compounds in diesel oils by comprehensive two-dimensional gas chromatography with a sulfur chemiluminescence detector. J. Chromatogr. A 2003, 1019, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Xu, C.; Zhao, S.; Chung, K.; Zhang, Y.; Gao, W. Characterization of Basic Nitrogen Species in Coker Gas Oils by Positive-Ion Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2009, 24, 563–569. [Google Scholar] [CrossRef]
- Chen, X.; Shen, B.; Sun, J.; Wang, C.; Shan, H.; Yang, C.; Li, C. Characterization and Comparison of Nitrogen Compounds in Hydrotreated and Untreated Shale Oil by Electrospray Ionization (ESI) Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR MS). Energy Fuels 2012, 26, 1707–1714. [Google Scholar] [CrossRef]
- Zheng, J.; Li, D.; Fan, X.; Huang, Y.; Gao, F.; Shao, R.; Li, W.; Dan, Y. Characterization of heteroatom class species in asphaltenes from medium/low temperature coal tar. Energy Sources Part A 2020, 1–15. [Google Scholar] [CrossRef]
- Shi, Q.; Pan, N.; Long, H.; Cui, D.; Guo, X.; Long, Y.; Chung, K.; Zhao, S.; Xu, C.; Hsu, C. Characterization of Middle-Temperature Gasification Coal Tar. Part 3: Molecular Composition of Acidic Compounds. Energy Fuels 2013, 27, 108–117. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, S.; Shi, Q.; Xu, Z.; Sun, X.; Xu, C.M. Heteroatomic compositional analysis of venezuela orinoco AR and thevis breaking product by negative ion ESI-FT-ICR MS. J. Fuel Chem. Technol. 2011, 39, 37–41. [Google Scholar]
- Mapolelo, M.; Rodgers, R.; Blakney, G.; Yen, A.; Asomaning, S.; Marshall, A. Characterization of naphthenic acids in crude oils and naphthenates by electrospray ionization FT-ICR mass spectrometry. Int. J. Mass Spectrom. 2011, 300, 149–157. [Google Scholar] [CrossRef]
- Pereira, T.M.C.; Vanini, G.; Tose, L.V.; Cardoso, F.M.R.; Fleming, F.P.; Rosa, P.T.V.; Thompson, C.J.; Castro, E.V.R.; Vaz, B.G.; Romão, W. FT-ICR MS analysis of asphaltenes: Asphaltenes go in, fullerenes come out. Fuel 2014, 131, 49–58. [Google Scholar] [CrossRef]
- Hughey, C.A.; Rodgers, R.P.; Marshall, A.G.; Qian, K.; Robbins, W.K. Identification of acidic NSO compounds in crude oils of different geochemical origins by negative ion electrospray Fourier transform ion cyclotron resonance mass spectrometry. Org. Geochem. 2002, 33, 743–759. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, J.; Dan, Y.; Wang, L.; Li, W.; Li, D. Analysis and Characterization of Medium/Low Temperature Coa Tar Asphaltene. Acta Pet. Sin. Pet. Process. Sect. 2016, 32, 334–342. [Google Scholar]
- Pei, L.; Li, D.; Yuan, Y.; Xue, F.; Li, W. Composition and structural changes of low temperature coal tar asphaltenes precipitated in different n-alkane solvents. Chem. Ind. Eng. Prog. 2017, 36, 2101–2108. [Google Scholar]
- Shao, R.; Li, D.; Pei, L.; Yuan, Y.; Liu, X.; Li, W. Effect of Deasphalting solvent on structure of coal tar asphaltene. Acta Pet. Sin. Pet. Process. Sect. 2017, 33, 1209–1217. [Google Scholar]
- Xie, Z.; Zhang, M.; Wang, X.; Guo, L.; Du, Z.; Li, W. Mechanism of dibenzofuran hydrodeoxygenation on the Ni (1 1 1) surface. Chin. J. Chem. Eng. 2021, 35, 204–210. [Google Scholar] [CrossRef]
- Shi, Q.; Zhao, S.; Xu, Z.; Chung, K.; Zhang, Y.; Xu, C. Distribution of Acids and Neutral Nitrogen Compounds in a Chinese Crude Oil and Its Fractions: Characterized by Negative-Ion Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2010, 24, 4005–4011. [Google Scholar] [CrossRef]
- Pan, Y.; Liao, Y.; Shi, Q.; Hsu, C. Acidic and neutral polar NSO compounds in heavily biodegraded oils characterized by negative-ion ESI FT-ICR MS. Energy Fuels 2013, 27, 2960–2973. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, Z.; Lu, C.; Zhu, Y.; Li, D. Analysis on the occurrence forms and removal rules of nitrogen in heavy fraction of medium and low temperature coal tar. Energy Chem. Ind. 2020, 41, 1–8. [Google Scholar]
- Fu, J.; Klein, G.C.; Smith, D.F.; Kim, S.; Rodgers, R.P.; Hendrickson, C.L.; Marshall, A.G. Comprehensive Compositional Analysis of Hydrotreated and Untreated Nitrogen-Concentrated Fractions from Syncrude Oil by Electron Ionization, Field Desorption Ionization, and Electrospray Ionization Ultrahigh-Resolution FT-ICR Mass Spectrometry. Energy Fuels 2006, 20, 1235–1241. [Google Scholar] [CrossRef]

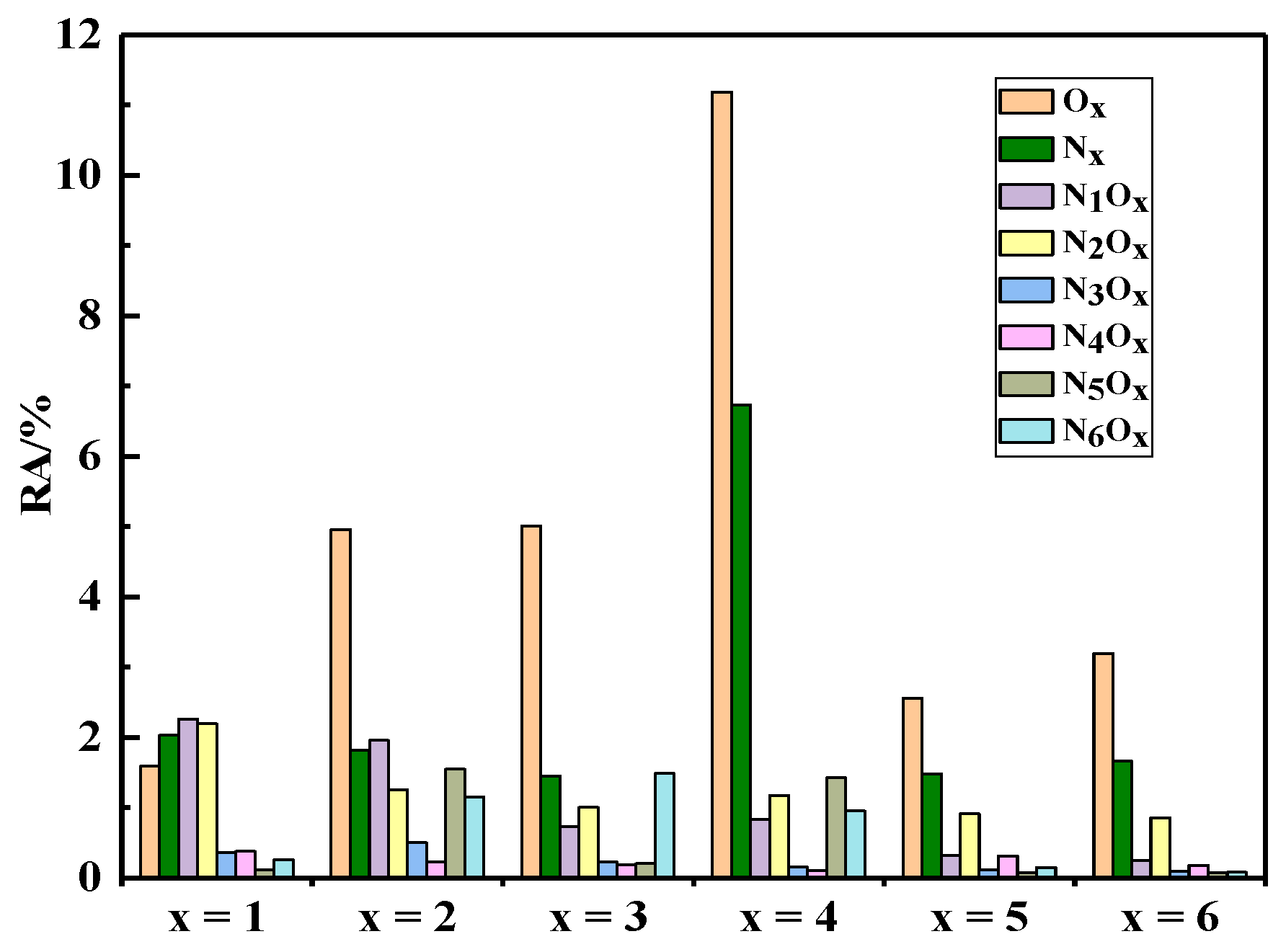
| Properties | M/LTCT | M/LTCT Asphaltenes | |
|---|---|---|---|
| Elemental analysis/% | C | 81.46 | 81.72 |
| H | 8.61 | 5.68 | |
| N | 1.26 | 2.33 | |
| S | 0.34 | 1.86 | |
| O | 8.03 | 8.92 | |
| H/C atomic ratio | 1.268 | 0.818 | |
| Density (20 °C)/g·cm−3 | 1.041 | - | |
| Mw | 420 | 663 | |
| NO | Formula [M + H]− | Theoretical Mass (Da) | Measured Mass (Da) | Error (m/z) | Resolving Power |
|---|---|---|---|---|---|
| 332.1465 | 332.1363 | 0.0102 | 132,193 | ||
| 332.3495 | 332.2229 | 0.1266 | 126,027 | ||
| 332.4932 | 332.4841 | 0.0091 | 121,912 | ||
| 332.6737 | 332.6715 | 0.0022 | 134,457 |
| Feed | Most Abundant Oxygen Species | DBE Center | Carbon Numbers Center | Possible Core Structure | |
|---|---|---|---|---|---|
| The present work | M/LTCT asphaltenes | O4 | 4–15 | 12–27 | Quaternary phenolic hydroxyl |
| Wang et al. [33] | Oil sands bitumen asphaltenes | O2 | 3–5 | 28–35 | Naphthenic acid |
| Xu et al. [31] | Sudan sand oil asphaltenes | O2 | 0–8 | 25–35 | Naphthenic acid |
| Shi et al. [32] | Crude Oil asphaltenes | O1 | 4–6 | 27–40 | Phenolic hydroxyl |
| Feed | Most Abundant Nitrogen Species | DBE Center | C Center | Possible Core Structure | |
|---|---|---|---|---|---|
| The present work | M/LTCT asphaltenes | N4 | 16–25 | 12–23 | A piperazine ring with a pyrazole ring |
| Wang et al. [33] | Oil sands bitumen asphaltenes | N1 | 14–24 | 22–37 | Pyrrole ring |
| Xu et al. [31] | Sudan asphaltenes | N1 | 10–16 | 22–35 | Carbazole ring |
| Shi et al. [32] | Crude Oil asphaltenes | N1 | 9–19 | 21–37 | Carbazole ring |
| Wang et al. [21] | Petroleum asphaltenes | N1 | 9–20 | 20–46 | Carbazole ring |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Li, D.; Cui, L.; Shao, R.; Chang, C.; Yan, L.; Yang, B. Analysis of Distribution and Structures of Heteroatom Compounds in Asphaltene of Medium/Low Temperature Coal Tar by Negative Anion Mode ESI FT-ICR MS. Sustainability 2022, 14, 15497. https://doi.org/10.3390/su142315497
Fan X, Li D, Cui L, Shao R, Chang C, Yan L, Yang B. Analysis of Distribution and Structures of Heteroatom Compounds in Asphaltene of Medium/Low Temperature Coal Tar by Negative Anion Mode ESI FT-ICR MS. Sustainability. 2022; 14(23):15497. https://doi.org/10.3390/su142315497
Chicago/Turabian StyleFan, Xiaoyong, Dong Li, Louwei Cui, Ruitian Shao, Chunran Chang, Long Yan, and Bo Yang. 2022. "Analysis of Distribution and Structures of Heteroatom Compounds in Asphaltene of Medium/Low Temperature Coal Tar by Negative Anion Mode ESI FT-ICR MS" Sustainability 14, no. 23: 15497. https://doi.org/10.3390/su142315497
APA StyleFan, X., Li, D., Cui, L., Shao, R., Chang, C., Yan, L., & Yang, B. (2022). Analysis of Distribution and Structures of Heteroatom Compounds in Asphaltene of Medium/Low Temperature Coal Tar by Negative Anion Mode ESI FT-ICR MS. Sustainability, 14(23), 15497. https://doi.org/10.3390/su142315497





