Dominant Fungal Communities Aggregate in the Shallow Rhizosphere Soil of Anabasis aphylla
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Soil Samples
2.2. Analysis of Physicochemical Properties of Soil
2.3. DNA Extraction and High-Throughput Sequencing of the ITS1-5F Region
2.4. Statistical Analysis
3. Results
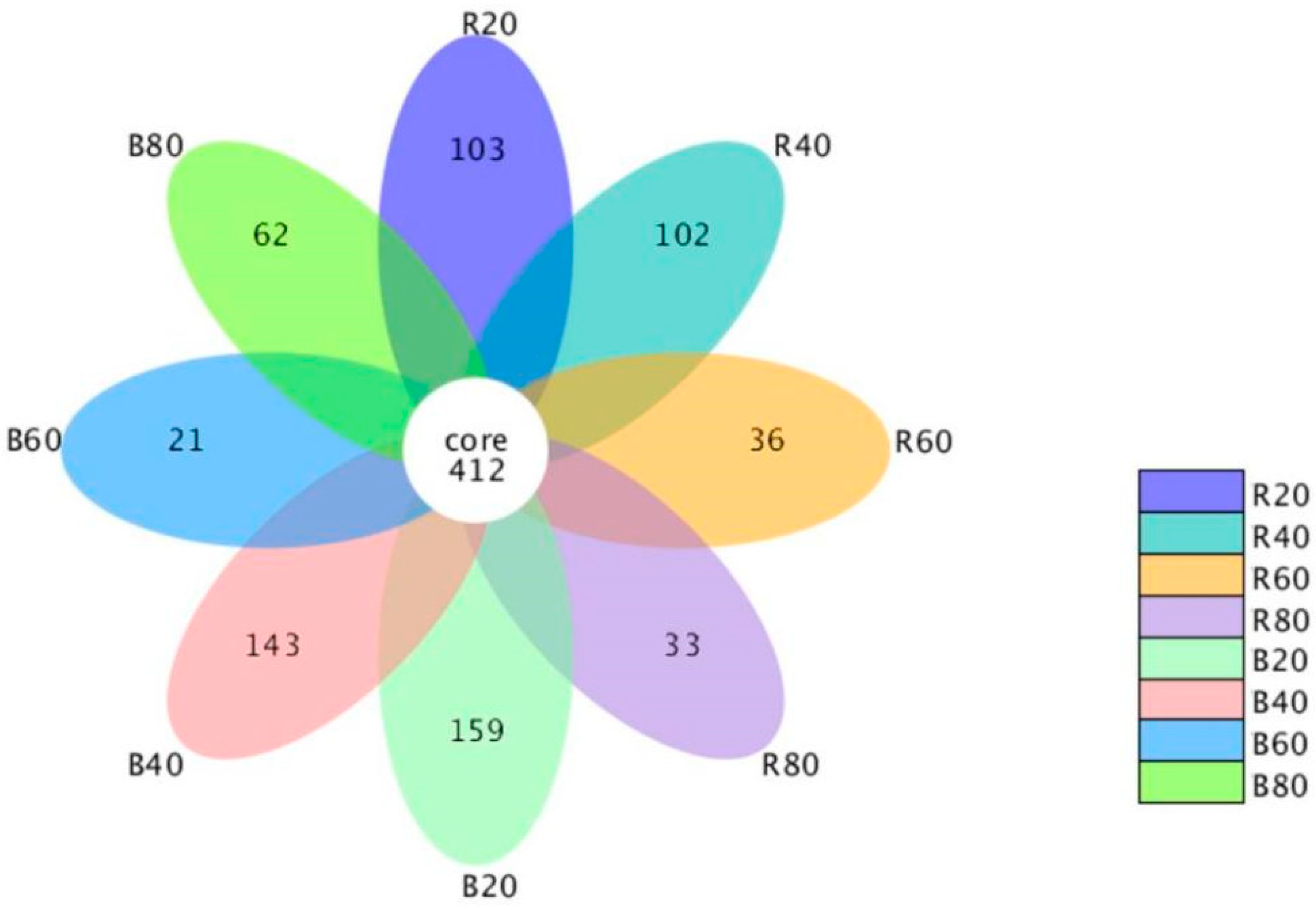
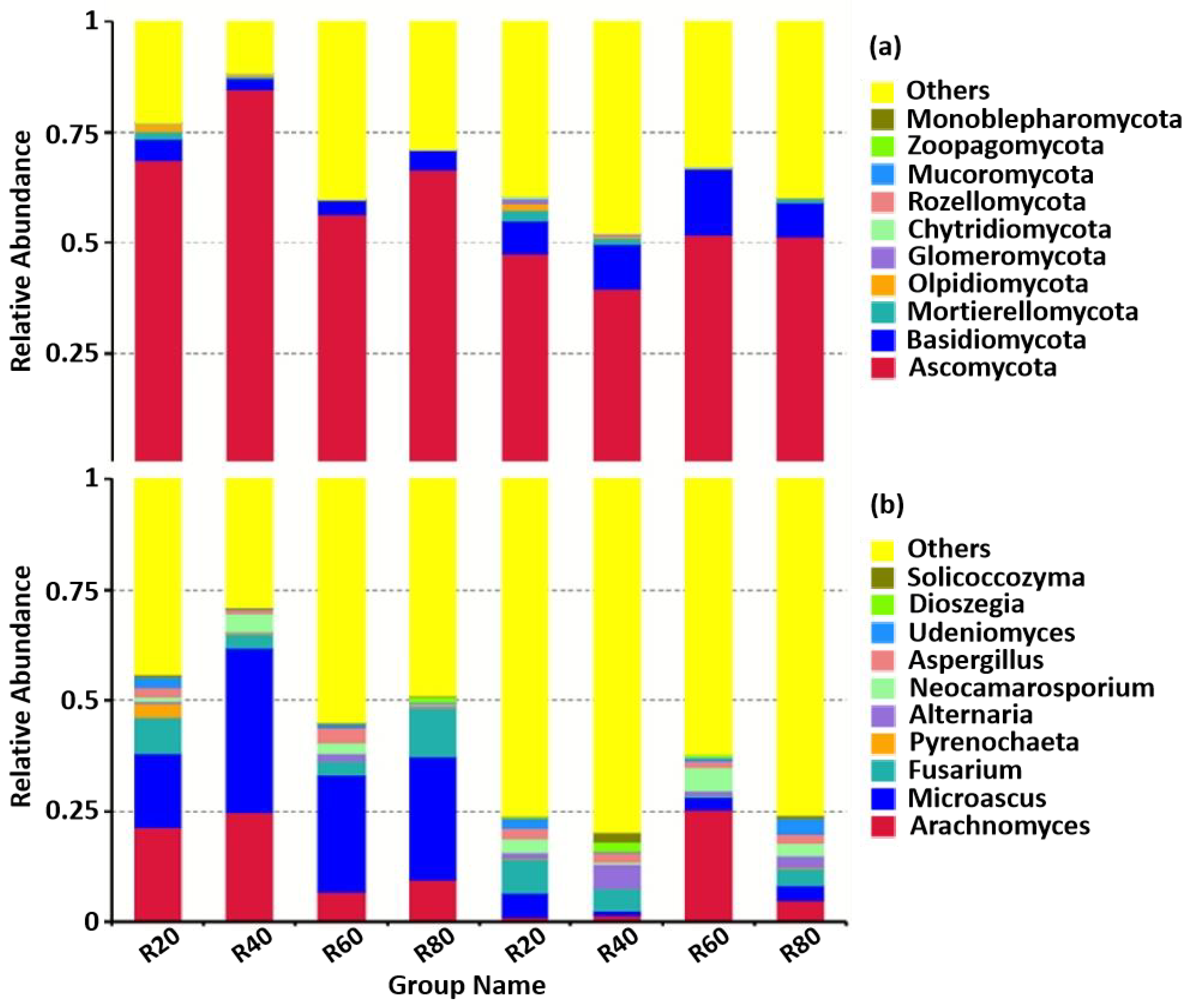
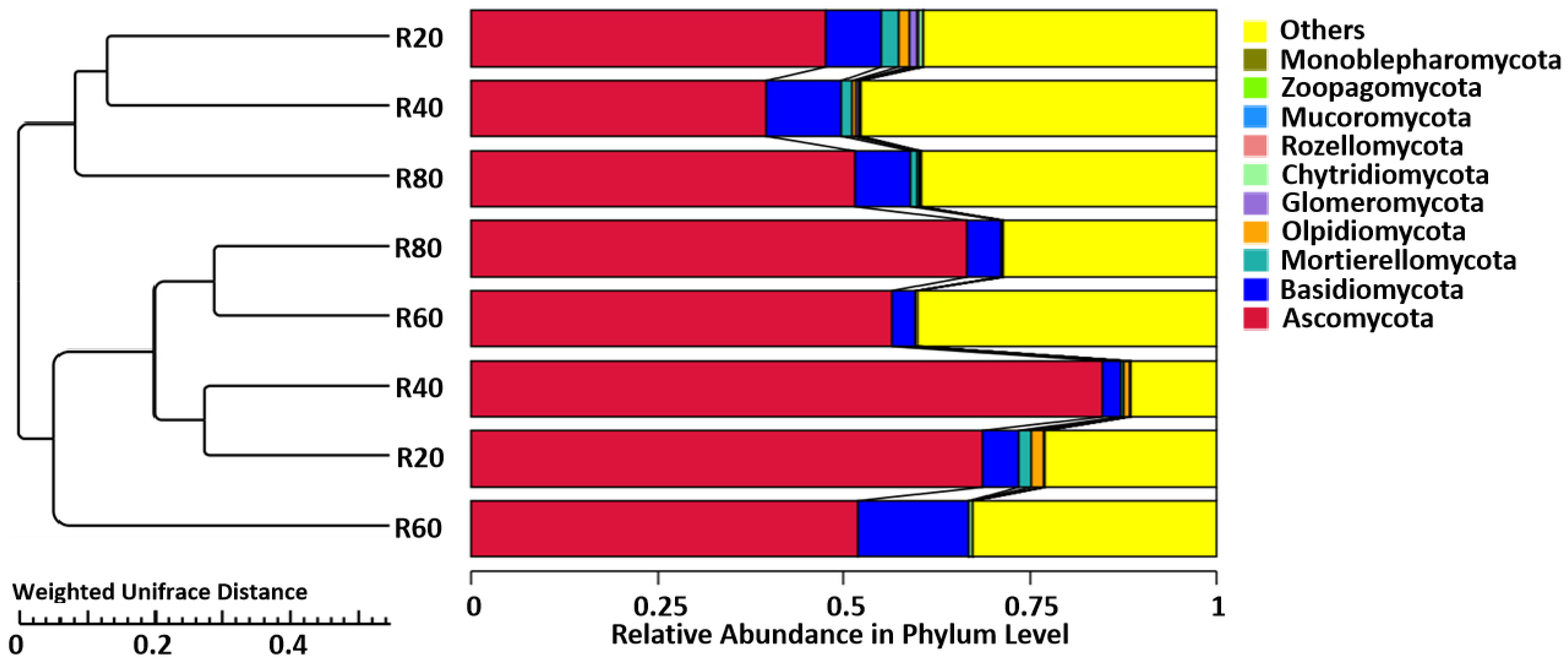
3.1. Diversity as Well as Composition of Soil Fungal Community
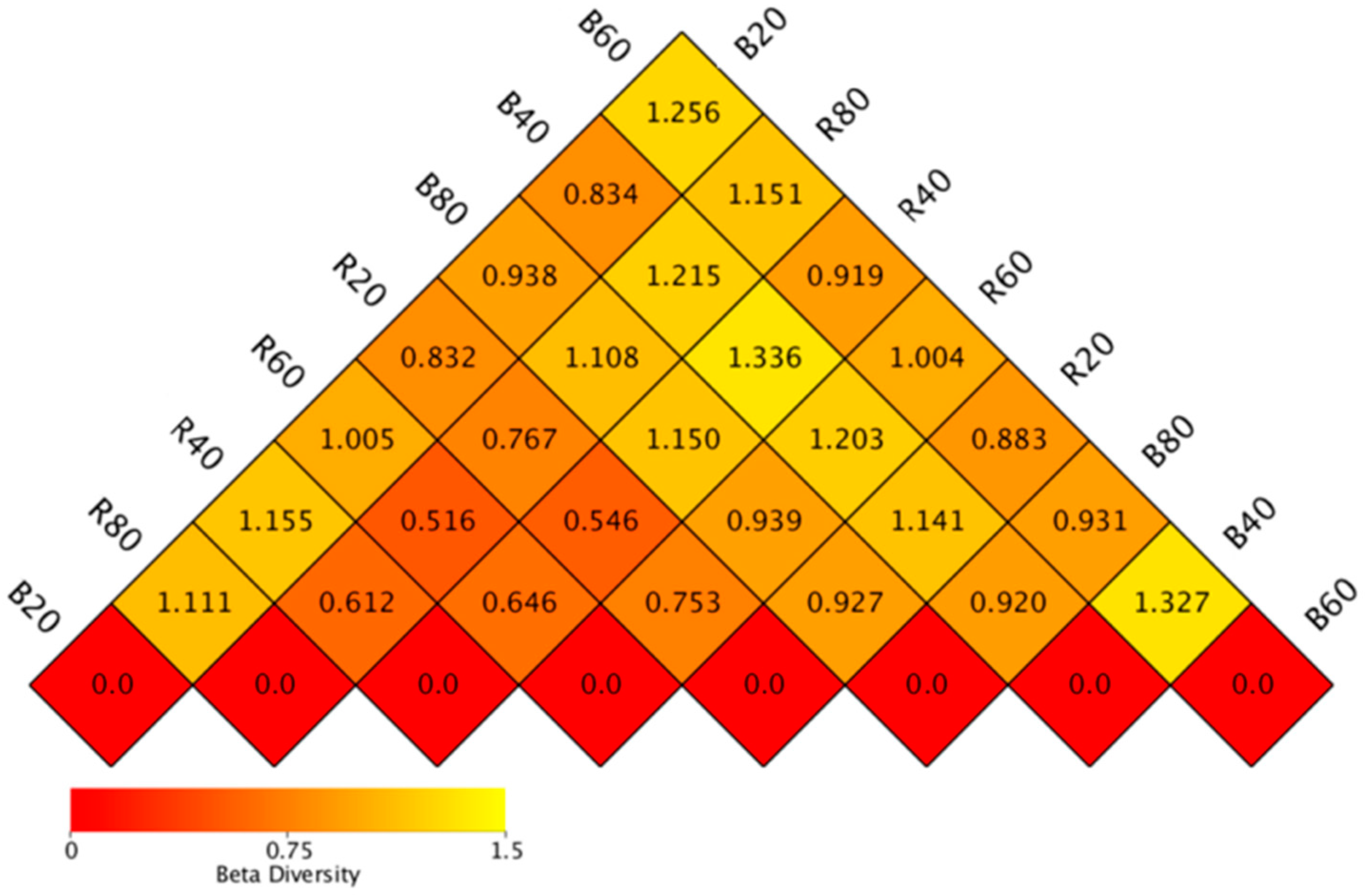
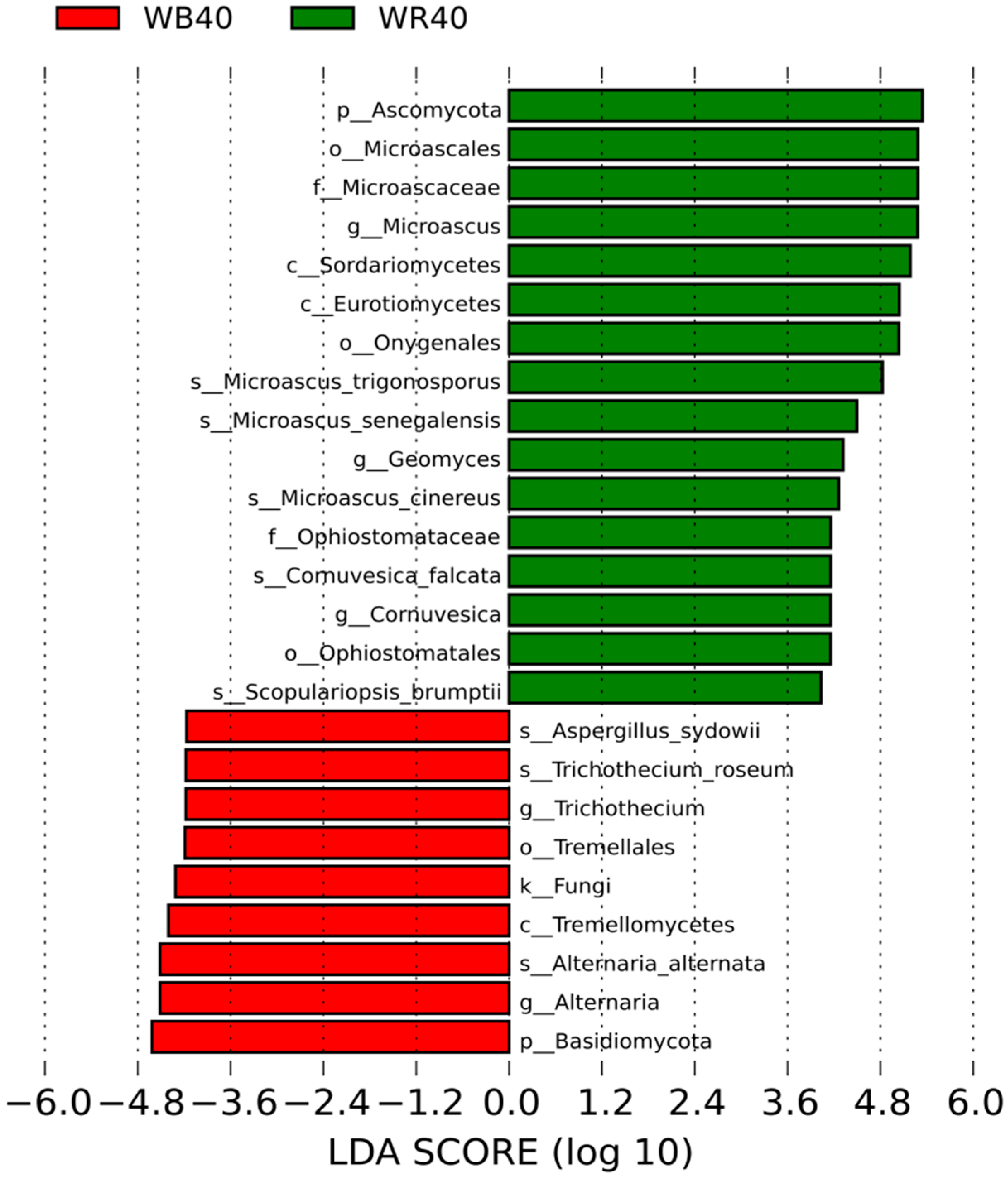
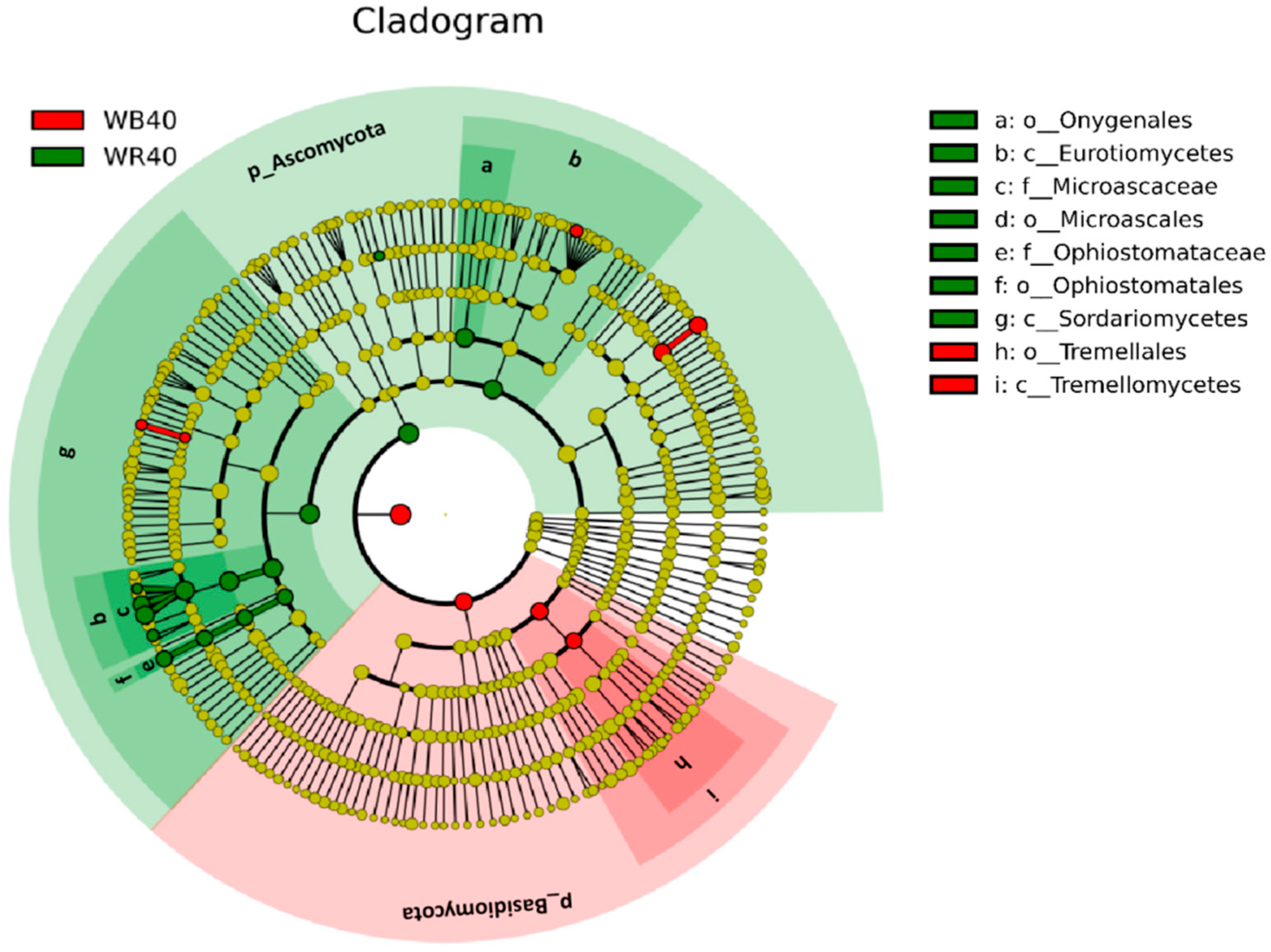
3.2. Discrepancies in the Community of Soil Fungi
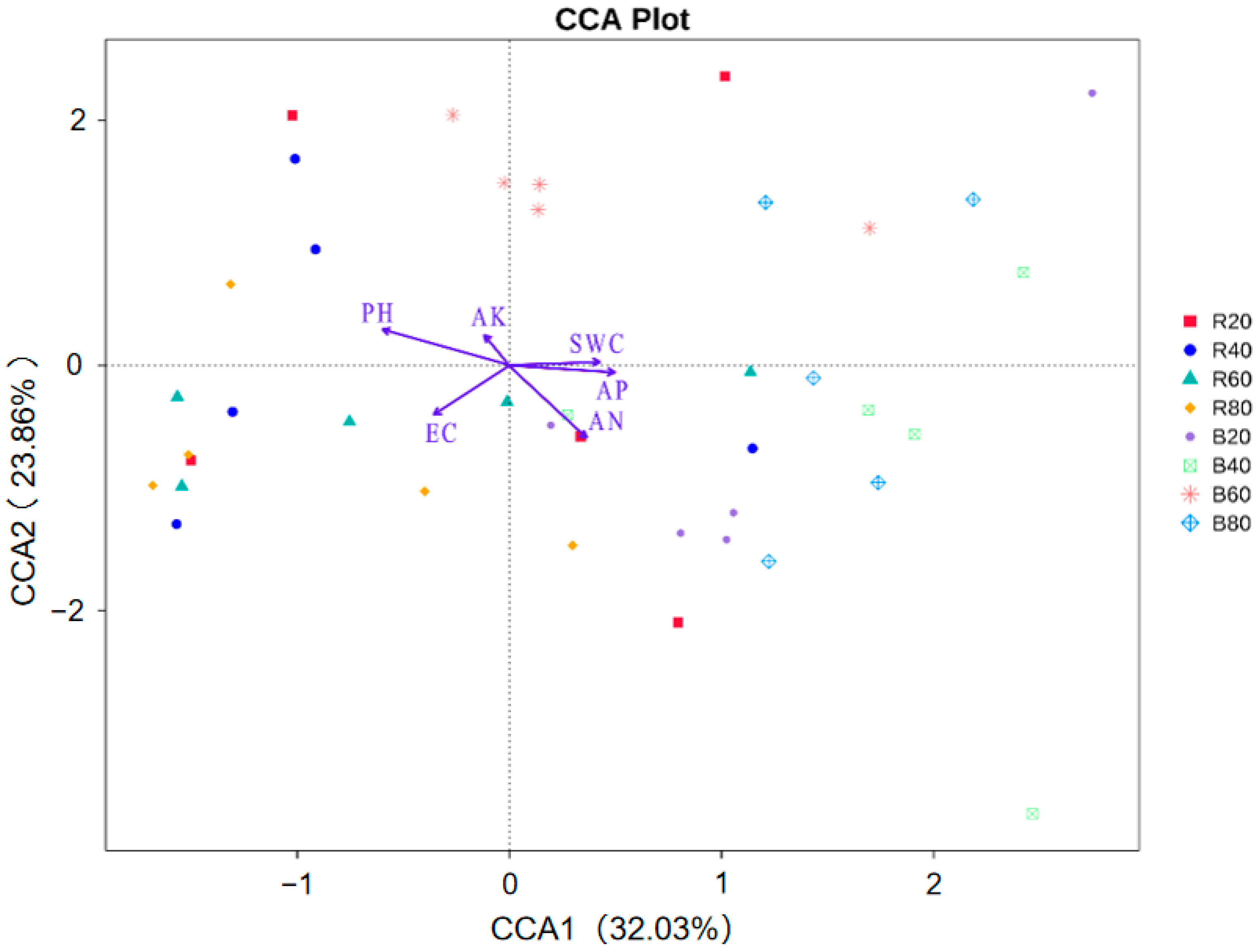
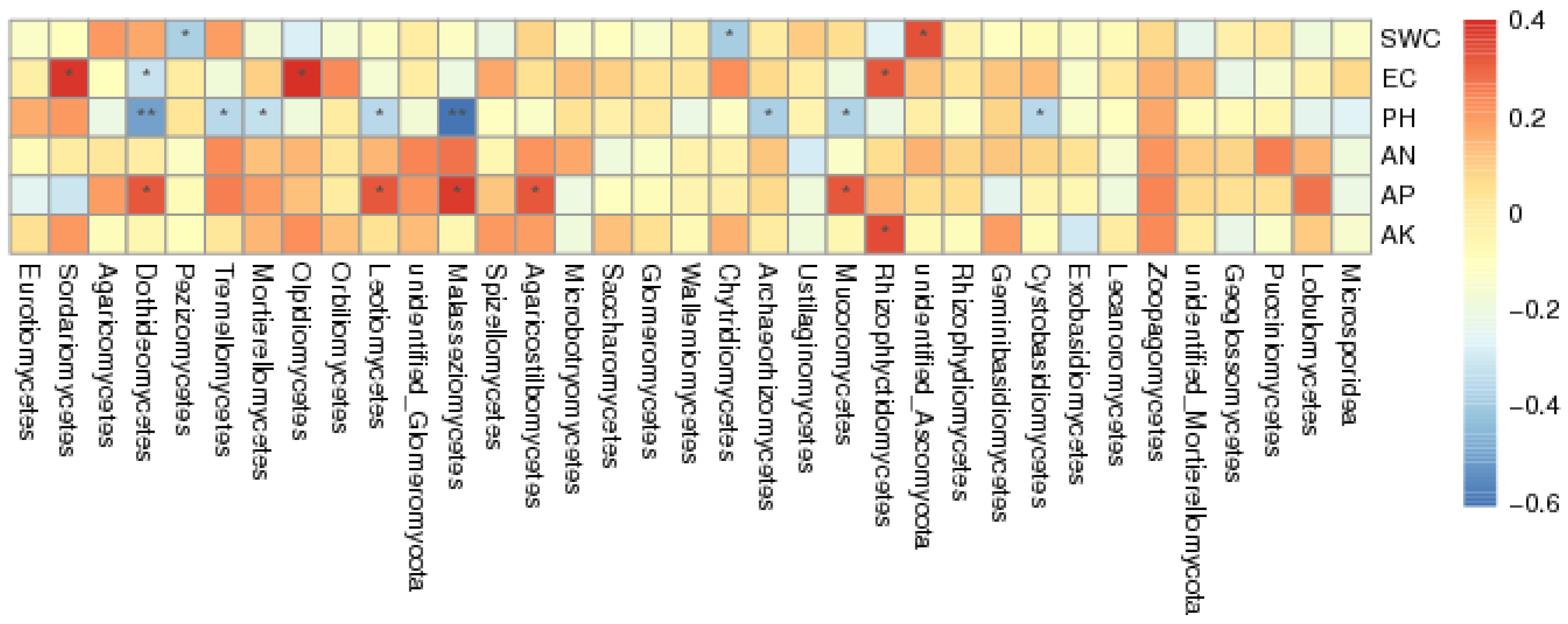
3.3. Influences of Soil Physicochemical Parameters on Fungal Community Structure
4. Discussion
4.1. Fungal Diversity Is Greatest in the Shallow (0–20 cm) Bulk Soil
4.2. Abundance of the Dominant Fungi Was Highest in the Shallow (20–40 cm) Rhizosphere Soil
4.3. EC Is the Main Environmental Factor Affecting Dominant Fungi in the A. aphylla Rhizosphere
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carini, P.; Marsden, P.J.; Leff, J.W.; Morgan, E.E.; Strickland, M.S.; Fierer, N. Relic DNA is abundant in soil and obscures estimates of soil microbial diversity. Nat. Microbiol. 2017, 2, 16242. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Wood, S.A.; Mesquita, C.P. How microbes can, and cannot, be used to assess soil health. Soil Biol. Biochem. 2021, 153, 108111. [Google Scholar] [CrossRef]
- Coban, O.; De Deyn, G.B.; Ploeg, M. Soil microbiota as game changers in restoration of degraded lands. Science 2022, 375, 990. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, J.X.; Long, X.H.; Liu, Z.P.; Rengel, Z. Salinity altered root distribution and increased diversity of bacterial communities in the rhizosphere soil of Jerusalem artichoke. Sci. Rep. 2016, 6, 20687. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.L.; Li, Y.X.; Wang, Q.Q.; Zhang, X.R.; Xiong, J.; Li, Y.; Lei, Y.H.; Sun, Y.F. Analysis of microbial diversity and community structure of rhizosphere soil of Cistanche salsa from different host plants. Front. Microbiol. 2022, 13, 971228. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Li, W.; Lu, P.; Xu, T.Y.; Pan, K. Three preceding crops increased the yield of and inhibited clubroot disease in continuously monocropped Chinese cabbage by regulating the soil properties and rhizosphere microbial community. Microorganisms 2022, 10, 799. [Google Scholar] [CrossRef]
- Bello, A.; Wang, B.; Zhao, Y.; Yang, W.; Ogundeji, A.; Deng, L.T.; Egbeagu, U.U.; Yu, S.; Zhao, L.Y.; Li, D.T.; et al. Composted biochar affects structural dynamics, function and co-occurrence network patterns of fungi community. Sci. Total Environ. 2021, 775, 145672. [Google Scholar] [CrossRef]
- Balami, S.; Vašutová, M.; Košnar, J.; Karkic, R.; Khadkab, C.; Tripathid, G.; Cudlínb, P. Soil fungal communities in abandoned agricultural land has not yet moved towards the seminatural forest. For. Ecol. Manag. 2021, 491, 119181. [Google Scholar] [CrossRef]
- Hagenbo, A.; Alday, J.G.; Aragon, J.M.; Castano, C.; De-Miguel, S.; Bonet, J.A. Variations in biomass of fungal guilds are primarily driven by factors related to soil conditions in Mediterranean Pinus pinaster forests. Biol. Fertil. Soils 2022, 58, 487–501. [Google Scholar] [CrossRef]
- Vidal, C.; González, F.; Santander, C.; Perez, R.; Gallardo, V.; Santos, C.; Aponte, H.; Ruiz, A.; Cornejo, P. Management of rhizosphere microbiota and plant production under drought stress: A comprehensive review. Plants 2022, 11, 2437. [Google Scholar] [CrossRef]
- Edwards, J.A.; Santos-Medellin, C.M.; Liechty, Z.S.; Nguyen, B.; Lurie, E.; Eason, H.; Phillips, G.; Sundaresan, V. Compositional shifts in root-associated bacterial and archaeal microbiota track the plant life cycle in fifield-grown rice. PLoS Biol. 2018, 16, e2003862. [Google Scholar] [CrossRef] [PubMed]
- Alzandi, A.A.; Naguib, D.M. Effect of Yeast Application on soil health and root metabolic status of corn seedlings under drought stress. Arch. Microbiol. 2022, 204, 233. [Google Scholar] [CrossRef] [PubMed]
- Tarroum, M.; Ben Romdhane, W.; Ali, A.A.M.; Al-Qurainy, F.; Al-Doss, A.; Fki, L.; Hassairi, A. Harnessing the rhizosphere of the halophyte grass Aeluropus littoralis for halophilic plant-growth-promoting fungi and evaluation of their biostimulant activities. Plants 2021, 10, 784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Xi, J.S.; Lv, Q.; Wang, J.W.; Yu, K.; Zhao, F.Y. Effect of aerated irrigation on the growth and rhizosphere soil fungal community structure of greenhouse grape seedlings. Sustainability 2022, 14, 12719. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, J.; Ding, Y.; Yu, S.; Lin, H.X.; Yuan, Z.Q.; Li, K.M.; Ou, W.J.; Chen, S.B. Different fertilizers applied alter fungal community structure in rhizospheric soil of cassava (Manihot esculenta Crantz) and increase crop yield. Front. Microbiol. 2021, 12, 3781. [Google Scholar] [CrossRef]
- Goomaral, A.; Iwase, K.; Undarmaa, J.; Matsumoto, T.; Yamato, M. Communities of arbuscular mycorrhizal fungi in Stipa krylovii (Poaceae) in the Mongolian steppe. Mycoscience 2013, 54, 122–129. [Google Scholar] [CrossRef]
- Guo, X.; Gong, J. Difffferential effffects of abiotic factors and host plant traits on diversity and community composition of root-colonizing arbuscular mycorrhizal fungi in a salt-stressed ecosystem. Mycorrhiza 2014, 24, 79–94. [Google Scholar] [CrossRef]
- Estrada, B.; Beltrán-Hermoso, M.; Palenzuela, J.; Iwase, K.; Ruiz-Lozano, J.M.; Barea, J.M.; Oehl, F. Diversity of arbuscular mycorrhizal fungi in the rhizosphere of Asteriscus maritimus Less., a representative plant species in arid and saline Mediterranean ecosystems. J. Arid Environ. 2013, 97, 170–175. [Google Scholar] [CrossRef]
- Torrecillas, E.; Torres, P.; Alguacil, M.M.; Querejeta, J.I.; Roldan, A. Influence of habitat and climate variables on arbuscular mycorrhizal fungus community distribution, as revealed by a case study of facultative plant epiphytism under semiarid conditions. Appl. Environ. Microbiol. 2013, 79, 7203–7209. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Boughattas, S.; Hu, S.J.; Oh, S.H.; Sa, T.M. A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress. Mycorrhiza 2014, 24, 611–625. [Google Scholar] [CrossRef]
- Bencherif, K.; Boutekrabt, A.; Fontaine, J.; Laruelle, F.; Dalpe, Y.; Sahraoui, A.L.H. Impact of soil salinity on arbuscular mycorrhizal fungi biodiversity and microflora biomass associated with Tamarix articulata Vahll rhizosphere in arid and semi-arid Algerian areas. Sci. Total Environ. 2015, 533, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Baum, C.; El-Tohamy, W.; Gruda, N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci. Hortic. 2015, 187, 131–141. [Google Scholar] [CrossRef]
- Chang, Y.L.; Peng, M.W.; Chu, G.M.; Wang, M. Genome-wide assessment of population structure and genetic diversity for Anabasis aphylla based on specific length amplification fragment sequencing. J. Plant Interact. 2020, 15, 75–82. [Google Scholar] [CrossRef]
- Chang, Y.L.; Xia, Y.; Peng, M.W.; Chu, G.M.; Wang, M. Maxent modelling for predicting impacts of climate change on the potential distribution of Anabasis aphylla in Northwestern China. Appl. Ecol. Environ. Res. 2020, 18, 1637–1648. [Google Scholar] [CrossRef]
- Zhang, L.J.; Yue, M.; Zhang, Y.D.; Gu, F.X.; Pan, X.L. Characteristics of plant community species diversity of oasis desert ecotone in Fukang, Xinjiang. Sci. Geogr. Sin. 2003, 23, 229–333. (In Chinese) [Google Scholar]
- Chu, G.M.; Wang, M.; Zhang, S.X. Spatial patterns and associations of dominant woody species in desert–oasis ecotone of South Junggar Basin, NW China. J. Plant Interact. 2014, 9, 738–744. [Google Scholar] [CrossRef]
- Jiao, Y.L.; Chu, G.M.; Yang, Z.A.; Wang, Y.; Wang, M. Bacterial Diversity in the rhizosphere of Anabasis aphylla in the Gurbantunggut Desert, China. Curr. Microbiol. 2020, 77, 3750–3759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.G.; Zhang, J.; Liu, Y.; Guo, Y.Q.; Shi, P.; Wei, G.H. Biogeography and ecological processes affecting root-associated bacterial communities in soybean fields across China. Sci. Total Environ. 2018, 627, 20–27. [Google Scholar] [CrossRef]
- Ge, Y.L.; Sun, T. Soil microbial community structure and diversity of potato in rhizosphere and non-rhizosphere soil. Ecol. Environ. Sci. 2020, 29, 141–148. [Google Scholar]
- Shakya, M.; Quince, C.; Campbell, J.H.; Yang, Z.M.K.; Schadt, C.W.; Podar, M. Comparative metagenomic and rRNA microbial diversity characterization using archaeal and bacterial synthetic communities. Environ. Microbiol. 2013, 15, 1882–1899. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; del Rio, T.G.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.H.; Zhang, J.X.; Ma, C.L.; Wang, F.R.; Chen, Y.; Zhang, C.Y.; Zhang, H.; Zhangid, J. Characterization and variation of the rhizosphere fungal community structure of cultivated tetraploid cotton. PLoS ONE 2019, 14, e0207903. [Google Scholar] [CrossRef] [PubMed]
- Gqozo, M.P.; Bill, M.; Siyoum, N.; Labuschagne, N.; Korsten, L. Fungal diversity and community composition of wheat rhizosphere and non-rhizosphere soils from three different agricultural production regions of South Africa. Appl. Soil Ecol. 2020, 151, 103543. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, 911–920. [Google Scholar] [CrossRef]
- Rodriguez, P.A.; Rothballer, M.; Chowdhury, S.P.; Nussbaumer, T.; Gutjahr, C.; Falter-Braun, P. Systems biology of plant-microbiome interactions. Mol. Plant 2019, 12, 804–821. [Google Scholar] [CrossRef]
- Fonseca-Garcia, C.; Coleman-Derr, D.; Garrido, E.; Visel, A.; Tringe, S.G.; Partida-Martinez, L.P. The cacti microbiome: Interplay between habitat-filtering and host-specificity. Front. Microbiol. 2016, 7, 150. [Google Scholar] [CrossRef]
- Taschen, E.; Amenc, L.; Tournier, E.; Deleporte, P.; Malagoli, P.; Fustec, J.; Bru, D.; Philippot, L.; Bernard, L. Cereal-legume intercropping modifies the dynamics of the active rhizospheric bacterial community. Rhizosphere 2017, 3, 191–195. [Google Scholar] [CrossRef]
- Luo, Y.F.; Wang, Z.K.; He, Y.L.; Li, G.F.; Lv, X.H.; Zhuang, L. High-throughput sequencing analysis of the rhizosphere arbuscular mycorrhizal fungi (AMF) community composition associated with Ferula Sink. BMC Microbiol. 2020, 20, 335. [Google Scholar] [CrossRef]
- Abu Hanif, M.; Guo, Z.M.; Moniruzzaman, M.; He, D.; Yu, Q.S.; Rao, X.Q.; Liu, S.P.; Tan, X.P.; Shen, W.J. Plant taxonomic diversity better explains soil fungal and bacterial diversity than functional diversity in restored forest ecosystems. Plants Basel 2019, 8, 479. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, D.; Cai, X.W.; Xia, L.; Luo, Y.Q.; Cheng, X.L.; An, S.Q. Significant alterations in soil fungal communities along a chronosequence of Spartina alterniflora invasion in a Chinese Yellow Sea coastal wetland. Sci. Total Environ. 2019, 693, 133548. [Google Scholar] [CrossRef]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochemistry 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; Garcia-Gomez, M.; Gallardo, A.; Ulrich, W.; et al. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Polme, S.; Koljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suiga, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Poole, P.; Ramachandran, V.; Terpolilli, J. Rhizobia: From saprophytes to endosymbionts. Nat. Rev. Microbiol. 2018, 16, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Kowalchuk, G.A.; Buma, D.S.; Boer, W.; Klinkhamer, P.G.L.; Veen, J.A. Effects of above-ground plant species composition and diversity on the diversity of soil-borne microorganisms. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2002, 81, 509–520. [Google Scholar] [CrossRef]
- Ziegler, M.; Engel, M.; Welzl, G.; Schloter, M. Development of a simple root model to study the effects of single exudates on the development of bacterial community structure. J. Microbiol. Methods 2013, 94, 30–36. [Google Scholar] [CrossRef]
- Chen, C.F.; Wu, S.R.; Qin, L.; Fan, Y.C.; Tan, L.; Guo, W.F. Soil microbial carbon source utilization and functional diversity of typical native broadleaved plantations in south subtropical Asia. Chin. J. Ecol. 2016, 35, 1132–1139. (In Chinese) [Google Scholar]
- Sessitsch, A.; Weilharter, A.; Gerzabek, M.H.; Kirchmann, H.; Kandeler, E. Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Appl. Environ. Microbiol. 2001, 67, 4215–4224. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhou, G.N.; Zhu, X.Y.; Gao, B.J.; Xu, H.D. Effects of litter on soil organic carbon and microbial functional diversity. Acta Ecol. Sin. 2021, 41, 2709–2718. (In Chinese) [Google Scholar]
- Hao, X.X.; Han, X.Z.; Li, L.J.; Zou, W.X.; Lu, X.C.; Qiao, Y.F. Profile distribution and storage of soil organic carbon in a black soil as affected by land use types. Chin. J. Appl. Ecol. 2015, 26, 965–972. (In Chinese) [Google Scholar]
- Gao, L.Q.; Zhao, Y.G. Qin, N.Q.; Zhang, G.X. Effects of biological soil crust on soil erodibility in Hilly Loess Plateau Region of Northwest China. Chin. J. Appl. Ecol. 2013, 24, 105–112. (In Chinese) [Google Scholar]
- Yelle, D.J.; Ralph, J.; Lu, F.; Hammel, K.E. Evidence for cleavage of lignin by a brown rot basidiomycete. Environ. Microbiol. 2008, 10, 1844–1849. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Zhao, J.; Wang, L.L.; Yang, D.L.; Li, G. Variation of soil bacterial and fungal communities from Fluvo-Aquic soil under chemical fertilizer reduction combined with organic materials in North China plain. J. Soil Sci. Plant Nutr. 2020, 21, 349–363. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.Q.; Shen, F.; Tian, D.; Zeng, Y.M.; Yang, G.; Zhang, Y.Z.; Deng, S.H. Partitioning biochar properties to elucidate their contributions to bacterial and fungal community composition of purple soil. Sci. Total Environ. 2019, 648, 1333–1341. [Google Scholar]
- Guo, J.J.; Liu, W.B.; Zhu, C.; Luo, G.W.; Kong, Y.L.; Ling, N.; Wang, M.; Dai, J.Y.; Shen, Q.R.; Guo, S.W. Bacterial rather than fungal community composition is associated with microbial activities and nutrient-use efficiencies in a paddy soil with short-term organic amendments. Plant Soil 2018, 424, 335–349. [Google Scholar] [CrossRef]
- Liu, G.Y.; Chen, L.L.; Shi, X.R.; Yuan, Z.Y.; Yuan, L.Y.; Lock, T.R.; Kallenbach, R.L. Changes in rhizosphere bacterial and fungal community composition with vegetation restoration in planted forests. Land Degrad. Dev. 2019, 30, 1147–1157. [Google Scholar] [CrossRef]
- Ye, G.P.; Lin, Y.X.; Luo, J.F.; Di, H.J.; Lindsey, S.; Liu, D.; Fan, J.; Ding, W. Responses of soil fungal diversity and community composition to long-term fertilization: Field experiment in an acidic Ultisol and literature synthesis. Appl. Soil Ecol. 2020, 145, 103305. [Google Scholar] [CrossRef]
- Ibekwe, A.M.; Poss, J.A.; Grattan, S.R.; Grieve, C.M.; Suarez, D. Bacterial diversity in cucumber (Cucumis sativus) rhizosphere in response to salinity, soil pH, and boron. Soil Biol. Biochem. 2010, 42, 567–575. [Google Scholar] [CrossRef]
- Nie, M.; Zhang, X.D.; Wang, J.Q.; Jiang, L.F.; Yang, J.; Quan, Z.X.; Cui, X.H.; Fang, C.M.; Li, B. Rhizosphere effects on soil bacterial abundance and diversity in the Yellow River Deltaic ecosystem as influenced by petroleum contamination and soil salinization. Soil Biol. Biochem. 2009, 41, 2535–2542. [Google Scholar] [CrossRef]
- Zhang, L.F.; Wu, F.Z.; Zhou, X.G.; Yang, P. Effects of ucumber root exudates on soil nutrients and enzyme activities under salt stress. China Veg. 2009, 14, 6–11. (In Chinese) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, M.; Yang, Z.; Jiao, Y.; Chu, G. Dominant Fungal Communities Aggregate in the Shallow Rhizosphere Soil of Anabasis aphylla. Sustainability 2022, 14, 15423. https://doi.org/10.3390/su142215423
Wang Y, Wang M, Yang Z, Jiao Y, Chu G. Dominant Fungal Communities Aggregate in the Shallow Rhizosphere Soil of Anabasis aphylla. Sustainability. 2022; 14(22):15423. https://doi.org/10.3390/su142215423
Chicago/Turabian StyleWang, Ying, Mei Wang, Zhen’an Yang, Yalin Jiao, and Guangming Chu. 2022. "Dominant Fungal Communities Aggregate in the Shallow Rhizosphere Soil of Anabasis aphylla" Sustainability 14, no. 22: 15423. https://doi.org/10.3390/su142215423
APA StyleWang, Y., Wang, M., Yang, Z., Jiao, Y., & Chu, G. (2022). Dominant Fungal Communities Aggregate in the Shallow Rhizosphere Soil of Anabasis aphylla. Sustainability, 14(22), 15423. https://doi.org/10.3390/su142215423





