Standard Identification Certificate for Legal Legislation of a Unique Gene Pool of Thai Domestic Elephants Originating from a Male Elephant Contribution to Breeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection and DNA Extraction
2.2. Microsatellite Genotyping
2.3. Microsatellite Data Analysis
2.4. Mitochondrial D-Loop Sequencing
2.5. Mitochondrial D-Loop Data Analysis
2.6. Convolutional Neural Networks
3. Results
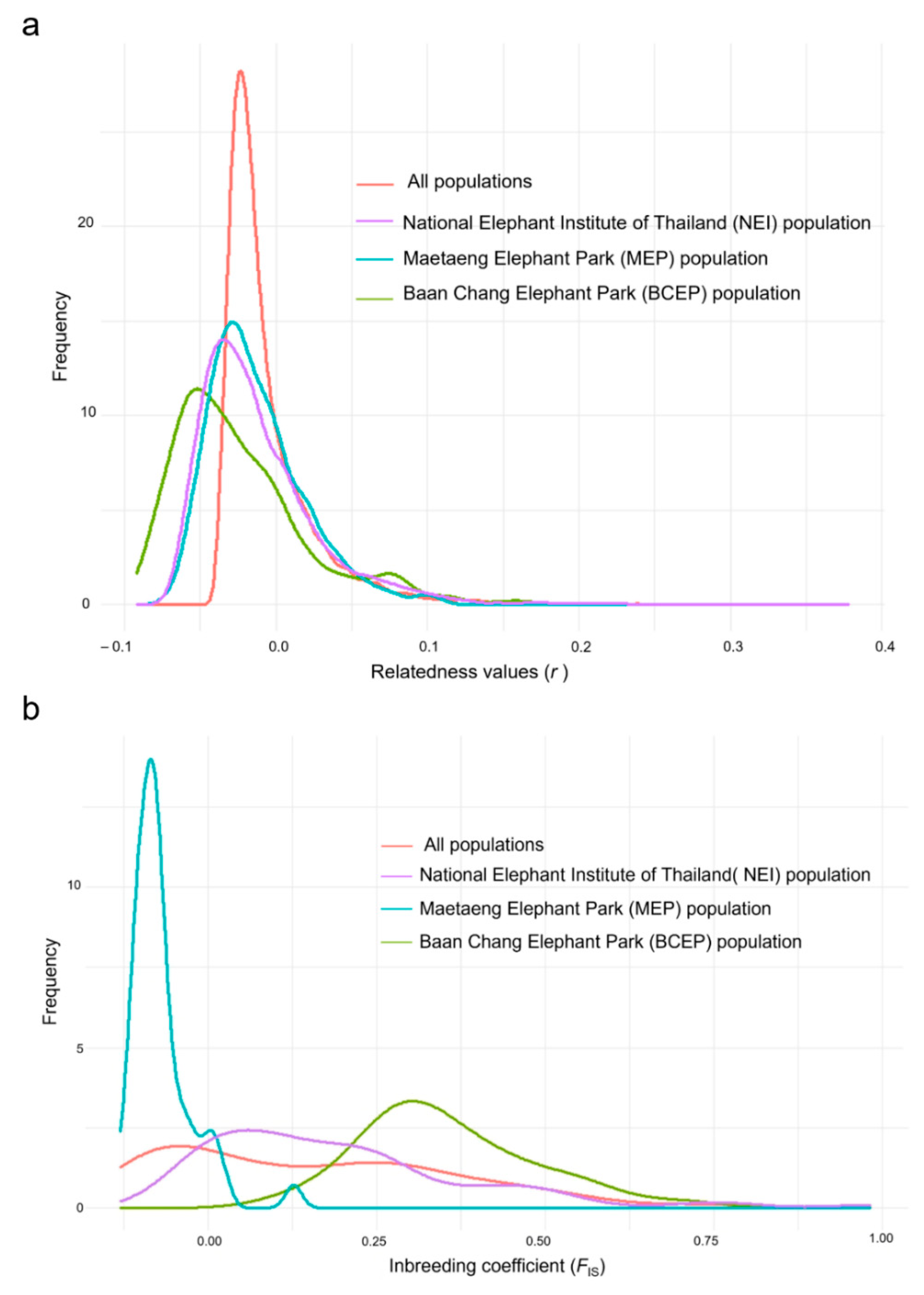
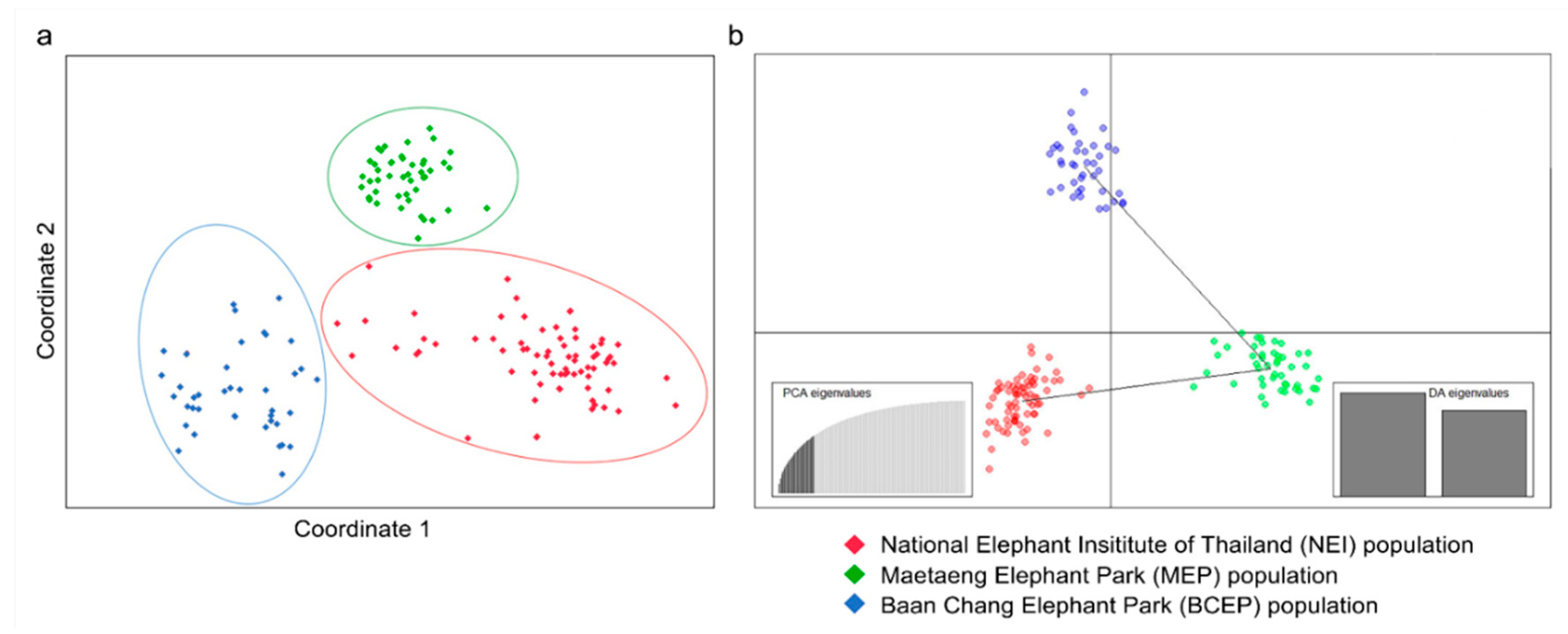
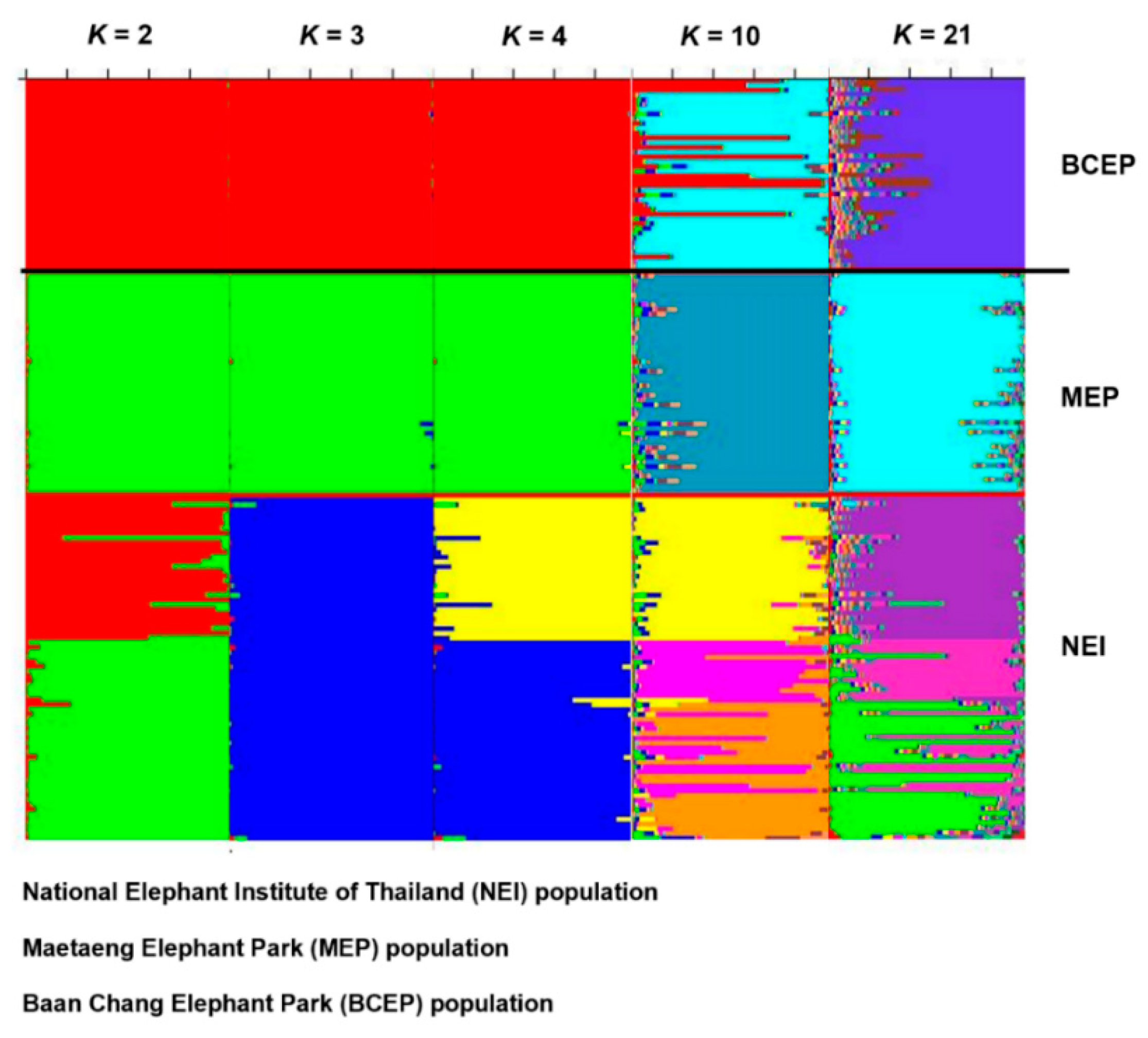
3.1. Genetic Variability of the Elephant Captive Population, Based on the Microsatellite Data
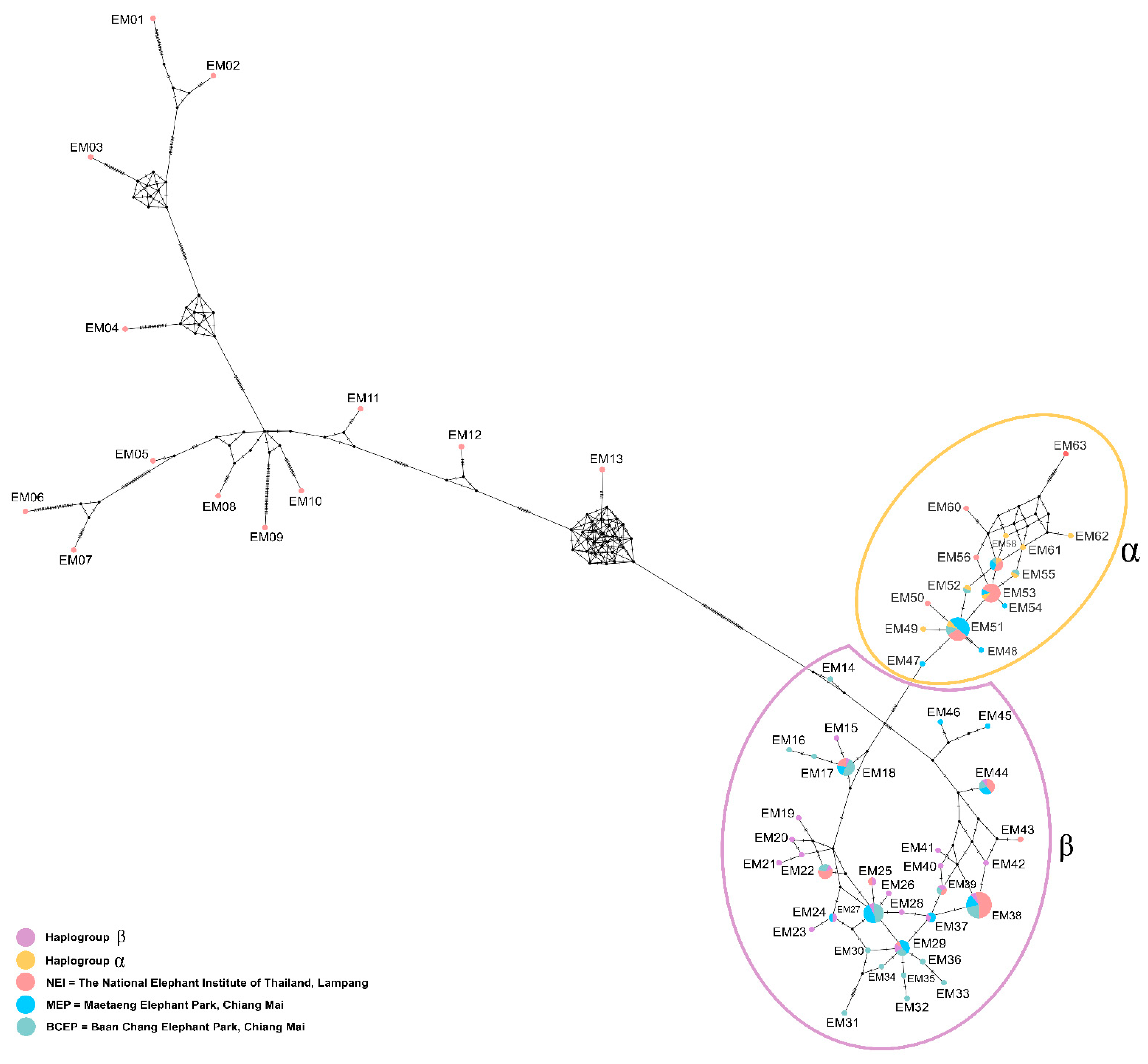
3.2. Genetic Variability of the Captive Population, Based on the Mitochondrial Haplotype Analysis
3.3. Classification of the Individual Elephants by the Convolutional Neural Networks
4. Discussion
4.1. Genetic Diversity of the Elephants in Camps Reflects the Different Historic Origins
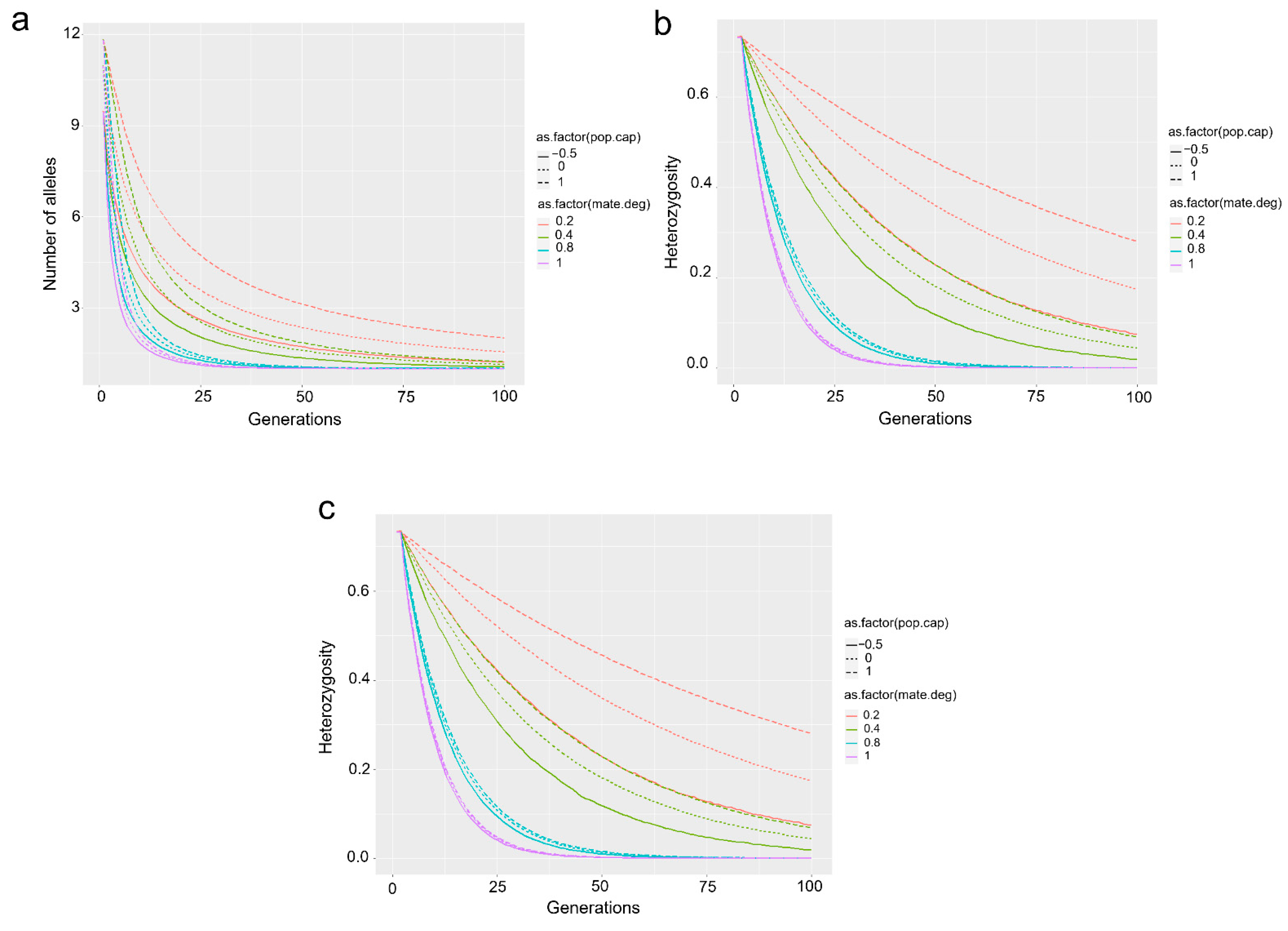
4.2. Legal Legislation and Identification Certificates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linnaeus, C. Systema Naturae per Regna Tria Naturae: Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis; Apud JB Delamolliere: Sweden, Stockholm, 1758. [Google Scholar]
- Kerley, G.I.; Landman, M. The impacts of elephants on biodiversity in the Eastern cape subtropical thickets: Elephant conservation. S. Afr. J. Sci. 2006, 102, 395–402. [Google Scholar]
- Kerley, G.I.; Landman, M.; Kruger, L.; Owen-Smith, N.; Balfour, D.; de Boer, W.F.; Gaylard, A.; Slotow, R. Effects of elephants on ecosystems and biodiversity. In The 2007 Scientific Assessment of Elephant Management in South Africa; CSIR: Pretoria, South Africa, 2008; pp. 101–147. [Google Scholar]
- Shannon, G.; Thaker, M.; Vanak, A.T.; Page, B.R.; Grant, R.; Slotow, R. Relative impacts of elephant and fire on large trees in a savanna ecosystem. Ecosystems 2011, 14, 1372–1381. [Google Scholar] [CrossRef]
- Fleischer, R.C.; Perry, E.A.; Muralidharan, K.; Stevens, E.E.; Wemmer, C.M. Phylogeography of the Asian elephant (Elephas maximus) based on mitochondrial DNA. Evolution 2001, 55, 1882–1892. [Google Scholar] [CrossRef]
- Vidya, T.N.C.; Fernando, P.; Melnick, D.J.; Sukumar, R. Population differentiation within and among Asian elephant (Elephas maximus) populations in southern India. Heredity 2005, 94, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, R. A brief review of the status, distribution and biology of wild Asian elephants Elephas maximus. Int. Zoo Yearb. 2006, 40, 1–8. [Google Scholar] [CrossRef]
- Menon, V.; Tiwari, S.K. Population status of Asian elephants Elephas maximus and key threats. Int. Zoo Yearb. 2019, 53, 17–30. [Google Scholar] [CrossRef]
- IUCN. Asian Elephant. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 20 March 2022).
- Ahlering, M.A.; Hedges, S.; Johnson, A.; Tyson, M.; Schuttler, S.G.; Eggert, L.S. Genetic diversity, social structure, and conservation value of the elephants of the Nakai Plateau, Lao PDR, based on non-invasive sampling. Conserv. Genet. 2011, 12, 413–422. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Y.; Díaz-Sacco, J.J.; Shi, K. Assessing population structure and body condition to inform conservation strategies for a small isolated Asian elephant (Elephas maximus) population in southwest China. PLoS ONE 2021, 16, e0248210. [Google Scholar] [CrossRef]
- Thitaram, C. Breeding management of captive Asian elephant (Elephas maximus) in range countries and zoos. Jpn. J. Zoo Wild. Med. 2012, 17, 91–96. [Google Scholar] [CrossRef]
- Asian Elephant Specialist Group. Asian Elephant Range States Meeting, Final Report; Asian Elephant Specialist Group: Jakarta, Indonesia, 2017; p. 68. [Google Scholar]
- Menkham, K.; Sukmasuang, R.; Pla-ard, M.; Charaspet, K.; Panganta, T.; Trisurat, Y.; Bhumpakohan, N. Population and habitat use of Asian elephants (Elephas maximus) and five ungulate species in Khao Ang Rue Nai Wildlife Sanctuary, Chachoengsao Province, Thailand. Biodiversitas 2019, 20, 8. [Google Scholar] [CrossRef]
- Shaffer, L.J.; Khadka, K.K.; van den Hoek, J.; Naithani, K.J. Human-elephant conflict: A review of current management strategies and future directions. Front. Ecol. Evol. 2019, 6, 235. [Google Scholar] [CrossRef]
- Bansiddhi, P.; Brown, J.L.; Thitaram, C. Welfare assessment and activities of captive elephants in Thailand. Animals 2020, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Burbach, J.; Ronfot, D.; Srisangiam, R. Asian elephant (Elephas maximus), pig-tailed macaque (Macaca nemestrina) and tiger (Panthera tigris) populations at tourism venues in Thailand and aspects of their welfare. PLoS ONE 2015, 10, e0139092. [Google Scholar] [CrossRef] [PubMed]
- Chaitae, A.; Gordon, I.J.; Addison, J.; Marsh, H. Protection of elephants and sustainable use of ivory in Thailand. Oryx 2021, 56, 601–608. [Google Scholar] [CrossRef]
- Stiles, D. CITES–approved ivory sales and elephant poaching. Pachyderm 2009, 45, 150–153. [Google Scholar]
- Nijman, V. An Assessment of the Live Elephant Trade in Thailand; TRAFFIC International: Cambridge, MA, USA, 2014. [Google Scholar]
- Bansiddhi, P.; Nganvongpanit, K.; Brown, J.L.; Punyapornwithaya, V.; Pongsopawijit, P.; Thitaram, C. Management factors affecting physical health and welfare of tourist camp elephants in Thailand. PeerJ 2019, 7, e6756. [Google Scholar] [CrossRef]
- Wongruang, P. Dealing with the Elephant in the Room—Ivory Trade. Available online: https://www.nationthailand.com/opinion/30357559 (accessed on 20 March 2022).
- Kanchanapangka, S. Roadmap of Torture: Captive Thai Elephant and the Anecdote. Thai J. Vet. Med. 2010, 40, 149–150. [Google Scholar]
- National Geographic. Available online: https://www.nationalgeographic.com/magazine/article/global-wildlife-tourism-social-media-causes-animal-suffering (accessed on 20 March 2022).
- Kelling, S.; Gerbracht, J.; Fink, D.; Lagoze, C.; Wong, W.K.; Yu, J.; Gomes, C. Ebird: A human/computer learning network for biodiversity conservation and research. In Proceedings of the 24th Innovative Applications of Artificial Intelligence Conference, Toronto, ON, Canada, 22–26 July 2012. [Google Scholar]
- Kumar, D.; Jakhar, S.D. Artificial Intelligence in Animal Surveillance and Conservation. In Impact of Artificial Intelligence on Organizational Transformation; Scrivener Publishing LLC: Beverly, MA, USA, 2022; pp. 73–85. [Google Scholar]
- Vélez, J.; Castiblanco-Camacho, P.J.; Tabak, M.A.; Chalmers, C.; Fergus, P.; Fieberg, J. Choosing an Appropriate Platform and Workflow for Processing Camera Trap Data using Artificial Intelligence. arXiv 2022, arXiv:2202.02283. [Google Scholar] [CrossRef]
- Thitaram, C.; Somgird, C.; Mahasawangkul, S.; Angkavanich, T.; Roongsri, R.; Thongtip, N.; Colenbrander, B.; van Steenbeek, F.G.; Lenstra, J.A. Genetic assessment of captive elephant (Elephas maximus) populations in Thailand. Conserv. Genet. 2010, 11, 325–330. [Google Scholar] [CrossRef]
- Kriangwanich, W.; Nganvongpanit, K.; Buddhachat, K.; Brown, J.L.; Siengdee, P.; Chomdej, S.; Bansiddhi, P.; Thitaram, C. Genetic diversity and variation in captive Asian elephants (Elephas maximus) in Thailand. Trop. Conserv. Sci. 2018, 11, 1940082918816871. [Google Scholar] [CrossRef]
- Toin, P.; Brown, J.L.; Punyapornwithaya, V.; Bansiddhi, P.; Somgird, C.; Thitaram, C. Reproductive performance of captive Asian elephants (Elephas maximus) in large tourist camps in Thailand. Anim. Reprod. Sci. 2020, 222, 106606. [Google Scholar] [CrossRef] [PubMed]
- Thongtip, N.; Saikhun, J.; Damyang, M.; Mahasawangkul, S.; Suthunmapinata, P.; Yindee, M.; Kongsila, A.; Angkawanish, T.; Jansittiwate, S.; Wongkalasin, W.; et al. Evaluation of post-thaw Asian elephant (Elephas maximus) spermatozoa using flow cytometry: The effects of extender and cryoprotectant. Theriogenology 2004, 62, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Lair, R.C. Gone Astray: The Care and Management of the Asian Elephant in Domesticity; Food and Agriculture Organization of the United Nations: Bangkok, Thailand, 1997; pp. 279–300. [Google Scholar]
- Thitaram, C.; Dejchaisri, S.; Somgird, C.; Angkawanish, T.; Brown, J.; Phumphuay, R.; Chomdech, S.; Kangwanpong, D. Social group formation and genetic relatedness in reintroduced Asian elephants (Elephas maximus) in Thailand. Appl. Anim. Behav. Sci. 2015, 172, 52–57. [Google Scholar] [CrossRef]
- Fickel, J.; Lieckfeldt, D.; Ratanakorn, P.; Pitra, C. Distribution of haplotypes and microsatellite alleles among Asian elephants (Elephas maximus) in Thailand. Eur. J. Wildl. Res. 2007, 53, 298–303. [Google Scholar] [CrossRef]
- Thongchai, S.; Dejchaisri, S.; Sukmasuang, S.; Thongtipsiridech, S.; Wajjwalku, W.; Kaolim, N. Genetic diversity of wild elephant (Elephas maximus Linnaeus, 1758) in the northeastern part of Thailand. J. Wildl. Thail. 2011, 18, 16–33. [Google Scholar]
- Witzenberger, K.A.; Hochkirch, A. Ex situ conservation genetics: A review of molecular studies on the genetic consequences of captive breeding programmes for endangered animal species. Biodivers. Conserv. 2011, 20, 1843–1861. [Google Scholar] [CrossRef]
- Srikulnath, K.; Matsubara, K.; Uno, Y.; Thongpan, A.; Suputtitada, S.; Nishida, C.; Matsuda, Y.; Apisitwanich, S. Genetic relationship of three butterfly lizard species (Leiolepis reevesii rubritaeniata, Leiolepis belliana belliana, Leiolepis boehmei, Agamidae, Squamata) inferred from nuclear gene sequence analyses. Kasetsart J. (Nat. Sci.). 2010, 44, 424–435. [Google Scholar]
- Sukumar, R.; Gadgil, M. Male-female differences in foraging on crops by Asian elephants. Anim. Behav. 1998, 36, 1233–1235. [Google Scholar] [CrossRef]
- Comstock, K.E.; Wasser, S.K.; Ostrander, E.A. Polymorphic microsatellite DNA loci identified in the African elephant (Loxodonta africana). Mol. Ecol. 2000, 9, 1004–1006. [Google Scholar] [CrossRef]
- Archie, E.A.; Moss, C.J.; Alberts, S.C. Characterization of tetranucleotide microsatellite loci in the African savannah elephant (Loxodonta africana africana). Mol. Ecol. Notes 2003, 3, 244–246. [Google Scholar] [CrossRef]
- Ariyaraphong, N.; Pansrikaew, T.; Jangtarwan, K.; Thintip, J.; Singchat, W.; Laopichienpong, N.; Pongsanarm, T.; Panthum, T.; Suntronpong, A.; Ahmad, S.F.; et al. Introduction of wild Chinese gorals into a captive population requires careful genetic breeding plan monitoring for successful long-term conservation. Glob. Ecol. Conserv. 2021, 28, e01675. [Google Scholar] [CrossRef]
- Chailertrit, V.; Swatdipong, A.; Peyachoknagul, S.; Salaenoi, J.; Srikulnath, K. Isolation and characterization of novel microsatellite markers from Siamese fighting fish (Betta splendens, Osphronemidae, Anabantoidei) and their transferability to related species, B. smaragdina and B. imbellis. Genet. Mol. Res. 2014, 13, 7157–7162. [Google Scholar] [CrossRef] [PubMed]
- Lapbenjakul, S.; Thanapa, W.; Twilprawat, P.; Muangmai, N.; Khancanaketu, T.; Temsiripong, Y.; Unajak, S.; Peyachoknagul, S.; Srikulnath, K. High genetic diversity and demographic history of captive Siamese and Saltwater crocodiles suggest the first step toward the establishment of a breeding and reintroduction program in Thailand. PLoS ONE 2017, 12, e0184526. [Google Scholar] [CrossRef] [PubMed]
- Jangtarwan, K.; Koomgun, T.; Prasongmaneerut, T.; Thongchum, R.; Singchat, W.; Tawichasri, P.; Fukayama, T.; Sillapaprayoon, S.; Kraichak, E.; Muangmai, N.; et al. Take one step backward to move forward: Assessment of genetic diversity and population structure of captive Asian woolly-necked storks (Ciconia episcopus). PLoS ONE 2019, 14, e0223726. [Google Scholar] [CrossRef]
- Jangtarwan, K.; Kamsongkram, P.; Subpayakom, N.; Sillapaprayoon, S.; Muangmai, N.; Kongphoemph, A.; Wongsodchuen, A.; Intapan, S.; Chamchumroon, W.; Safoowong, M.; et al. Predictive genetic plan for a captive population of the Chinese goral (Naemorhedus griseus) and prescriptive action for ex situ and in situ conservation management in Thailand. PLoS ONE 2020, 15, e0234064. [Google Scholar] [CrossRef]
- Thintip, J.; Singchat, W.; Ahmad, S.F.; Ariyaraphong, N.; Muangmai, N.; Chamchumroon, W.; Pitiwong, K.; Suksavate, W.; Duangjai, S.; Duengkae, P.; et al. Reduced genetic variability in a captive-bred population of the endangered Hume’s pheasant (Syrmaticus humiae, Hume 1881) revealed by microsatellite genotyping and D-loop sequencing. PLoS ONE 2021, 16, e0256573. [Google Scholar] [CrossRef]
- Wongtienchai, P.; Lapbenjakul, S.; Jangtarwan, K.; Areesirisuk, P.; Mahaprom, R.; Subpayakom, N.; Singchat, W.; Sillapaprayoon, S.; Muangmai, N.; Songchan, R.; et al. Genetic management of a water monitor lizard (Varanus salvator macromaculatus) population at Bang Kachao Peninsula as a consequence of urbanization with Varanus Farm Kamphaeng Saen as the first captive research establishment. J. Zool. Syst. Evol. Res. 2021, 59, 484–497. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Præstgaard, J.T. Permutation and bootstrap Kolmogorov-Smirnov tests for the equality of two distributions. Scand. J. Stat. 1995, 22, 305–322. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Goudet, J.F. FSTAT (version 1.2): A computer program to calculate F-statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Chapuis, M.P.; Estoup, A. Microsatellite null alleles and estimation of population differentiation. Mol. Biol. Evol. 2007, 24, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Genetic distance between populations. Am. Nat. 1972, 106, 283–292. [Google Scholar] [CrossRef]
- Piry, S.; Luikart, G.; Cornuet, J.M. BOTTLENECK: A computer program for detecting recent reductions in the effective population size using allele frequency data. J. Hered. 1999, 90, 502–503. [Google Scholar] [CrossRef]
- Garza, J.C.; Williamson, E.G. Detection of reduction in population size using data from microsatellite loci. Mol. Ecol. 2001, 10, 305–318. [Google Scholar] [CrossRef]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Neuenschwander, S.; Hospital, F.; Guillaume, F.; Goudet, J. QuantiNemo: An individual based program to simulate quantitative traits with explicit genetic architecture in a dynamic metapopulation. Bioinformatics 2008, 24, 1552–1553. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Genetics and extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Ballou, J.D.; Lees, C.; Faust, L.J.; Long, S.; Lynch, C.; Bingaman Lackey, L.; Foose, T.J. Demographic and genetic management of captive populations. In Wild Mammals in Captivity: Principles and Techniques for Zoo Management; University of Chicago Press: Chicago, IL, USA, 2006; p. 219. [Google Scholar]
- Fernando, P.; Lande, R. Molecular genetic and behavioral analysis of social organization in the Asian elephant (Elephas maximus). Behav. Ecol. 2000, 48, 84–91. [Google Scholar] [CrossRef]
- Heled, J.; Drummond, A.J. Bayesian inference of population size history from multiple loci. BMC Evol. Biol. 2008, 8, 289. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; de Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Shoshani, J.; Tassy, P. Advances in proboscidean taxonomy & classification, anatomy & physiology, and ecology & behavior. Quat. Int. 2005, 126, 5–20. [Google Scholar] [CrossRef]
- Rohland, N.; Malaspinas, A.S.; Pollack, J.L.; Slatkin, M.; Matheus, P.; Hofreiter, M. Proboscidean mitogenomics: Chronology and mode of elephant evolution using mastodon as outgroup. PLoS Biol. 2007, 5, e207. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Huang, C.J.; Shen, Y.; Chen, Y.H.; Chen, H.C. A novel hybrid deep neural network model for short-term electricity price forecasting. Int. J. Energy Res. 2021, 45, 2511–2532. [Google Scholar] [CrossRef]
- Frolov, V.; Faizov, B.; Shakhuro, V.; Sanzharov, V.; Konushin, A.; Galaktionov, V.; Voloboy, A. Image Synthesis Pipeline for CNN-Based Sensing Systems. Sensors 2022, 22, 2080. [Google Scholar] [CrossRef]
- Do, C.; Waples, R.S.; Peel, D.; Macbeth, G.M.; Tillett, B.J.; Ovenden, J.R. NeEstimator v2: Re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol. Ecol. Resour. 2014, 14, 209–214. [Google Scholar] [CrossRef]
- Wang, J. COANCESTRY: A program for simulating, estimating and analysing relatedness and inbreeding coefficients. Mol. Ecol. Resour. 2011, 11, 141–145. [Google Scholar] [CrossRef]
- CITES: Illegal Trade in Live Asian Elephants: Review of Current Legislative, Regulatory, Enforcement, and Other Measures across Range States. Available online: www.cites.org/sites/default/files/eng/cop/17/WorkingDocs/E-CoP17-57-01-A5.pdf (accessed on 20 March 2022).
- Sukmasuang, R.; Thongtip, N.; Bhumpakphan, N. The Studies of Ecology, Population, Distribution and Health to Solve Elephant Problems in Thailand; Kasetsart University Research and Development Institute: Bangkok, Thailand, 2013. [Google Scholar]
- Pintavongs, W.; Chueplaivej, P.; Boonyasart, B.; Kidyhoo, S.; Pravai, W.; Rattanakunuprakarn, J.; Ounsiri, S.; Lorsanyaluck, B.; Sunyathitiseree, P.; Jittapalapong, S.; et al. Domestic elephant population structure and health status in Thailand. J. Kasetsart Vet. 2014, 24, 16–24. [Google Scholar]
- Greenbaum, G.; Templeton, A.R.; Zarmi, Y.; Bar-David, S. Allelic richness following population founding events—A stochastic modeling framework incorporating gene flow and genetic drift. PLoS ONE 2014, 9, e115203. [Google Scholar] [CrossRef] [PubMed]
- McPhee, M.E. Generations in captivity increases behavioral variance: Considerations for captive breeding and reintroduction programs. Biol. Conserv. 2004, 115, 71–77. [Google Scholar] [CrossRef]
- Thitaram, C. Elephant Reproduction: Improvement of Breeding Efficiency and Development of a Breeding Strategy; Utrecht University: Utrecht, The Netherlands, 2009. [Google Scholar]
- Rosetti, N.; Remis, M.I. Spatial genetic structure and mitochondrial DNA phylogeography of Argentinean populations of the grasshopper Dichroplus elongatus. PLoS ONE 2012, 7, e40807. [Google Scholar] [CrossRef]
- Luan, S.; Chiang, T.Y.; Gong, X.U.N. High genetic diversity vs. low genetic differentiation in Nouelia insignis (Asteraceae), a narrowly distributed and endemic species in China, revealed by ISSR fingerprinting. Ann. Bot. 2006, 98, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zheng, L.; Touré, Y.T.; Dandekar, T.; Kafatos, F.C. When genetic distance matters: Measuring genetic differentiation at microsatellite loci in whole-genome scans of recent and incipient mosquito species. Proc. Natl. Acad. Sci. USA 2001, 98, 10769–10774. [Google Scholar] [CrossRef]
- Wisely, S.M.; Buskirk, S.W.; Fleming, M.A.; McDonald, D.B.; Ostrander, E.A. Genetic diversity and fitness in black-footed ferrets before and during a bottleneck. J. Hered. 2002, 93, 231–237. [Google Scholar] [CrossRef]
- Kusza, S.; Suchentrunk, F.; Pucher, H.; Mar, K.U.; Zachos, F.E. High levels of mitochondrial genetic diversity in Asian elephants (Elephas maximus) from Myanmar. Hystrix 2018, 29, 152–154. [Google Scholar] [CrossRef]
- De Silva, M.; de Silva, P.K. The Sri Lankan Elephant: Its Evolution, Ecology and Conservation; WHT Publications: Colombo, Sri Lanka, 2007; p. 278. [Google Scholar]
- Shepherd, C.R.; Nijman, V. An Overview of the Regulation of the Freshwater Turtle and Tortoise Pet Trade in Jakarta, Indonesia; TRAFFIC Southeast Asia: Petaling Jaya, Malaysia, 2008; p. 24. [Google Scholar]
- Nijman, V. An overview of international wildlife trade from Southeast Asia. Biodivers. Conserv. 2010, 19, 1101–1114. [Google Scholar] [CrossRef]
- Van Horn, G.; Aodha, O.M.; Song, Y.; Cui, Y.; Sun, C.; Shepard, A.; Adam, H.; Perona, P.; Belongie, S. The inaturalist species classification and detection dataset. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–22 June 2018; pp. 8769–8778. [Google Scholar]
- Tabak, M.A.; Norouzzadeh, M.S.; Wolfson, D.W.; Sweeney, S.J.; Vercauteren, K.C.; Snow, N.P.; Halseth, J.M.; DiSilvo, P.A.; Lewis, J.S.; White, M.D.; et al. Machine learning to classify animal species in camera trap images: Applications in ecology. Methods Ecol. Evol. 2019, 10, 585–590. [Google Scholar] [CrossRef]
- Valière, N. GIMLET: A computer program for analysing genetic individual identification data. Mol. Ecol. Resour. 2002, 2, 377–379. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
| Population | N | Na | AR | Ne | I | Ho | He | M Ratio | PIC | F | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NEI 1 | Mean | 72 | 15.056 | 15.056 | 6.055 | 1.961 | 0.329 | 0.769 | 0.685 | 0.747 | 0.575 |
| S.E. | 0 | 2.036 | 8.640 | 1.040 | 0.144 | 0.046 | 0.030 | 0.390 | 0.137 | 0.059 | |
| MEP 2 | Mean | 46 | 11.111 | 11.111 | 5.309 | 1.711 | 0.714 | 0.721 | 1.305 | 0.687 | −0.018 |
| S.E. | 0 | 1.777 | 7.538 | 0.928 | 0.159 | 0.047 | 0.041 | 1.276 | 0.189 | 0.067 | |
| BCEP 3 | Mean | 40 | 11.056 | 11.056 | 5.910 | 1.814 | 0.204 | 0.747 | 0.366 | 0.718 | 0.677 |
| S.E. | 0 | 1.406 | 5.965 | 1.059 | 0.154 | 0.055 | 0.037 | 0.305 | 0.171 | 0.089 | |
| All Population | Mean | 158 | 12.407 | 12.407 | 5.758 | 1.828 | 0.416 | 0.745 | 0.785 | 0.717 | 0.412 |
| S.E. | 0 | 1.029 | 7.381 | 0.574 | 0.087 | 0.041 | 0.021 | 0.657 | 0.165 | 0.059 |
| Population 1 | Population 2 | df 4 | SE 5 | t-Test | p-Value | |
|---|---|---|---|---|---|---|
| heterozygosity (Ho) | NEI 1 | MEP 2 | −0.385 | 0.066 | −5.854 | <0.05 |
| NEI | BCEP 3 | 0.125 | 0.072 | 1.743 | 0.085 | |
| MEP | BCEP | 0.510 | 0.072 | 7.049 | <0.05 | |
| heterozygosity (He) | NEI | MEP | 0.048 | 0.050 | 0.945 | 0.347 |
| NEI | BCEP | 0.022 | 0.048 | 0.462 | 0.645 | |
| MEP | BCEP | −0.026 | 0.055 | −0.471 | 0.639 |
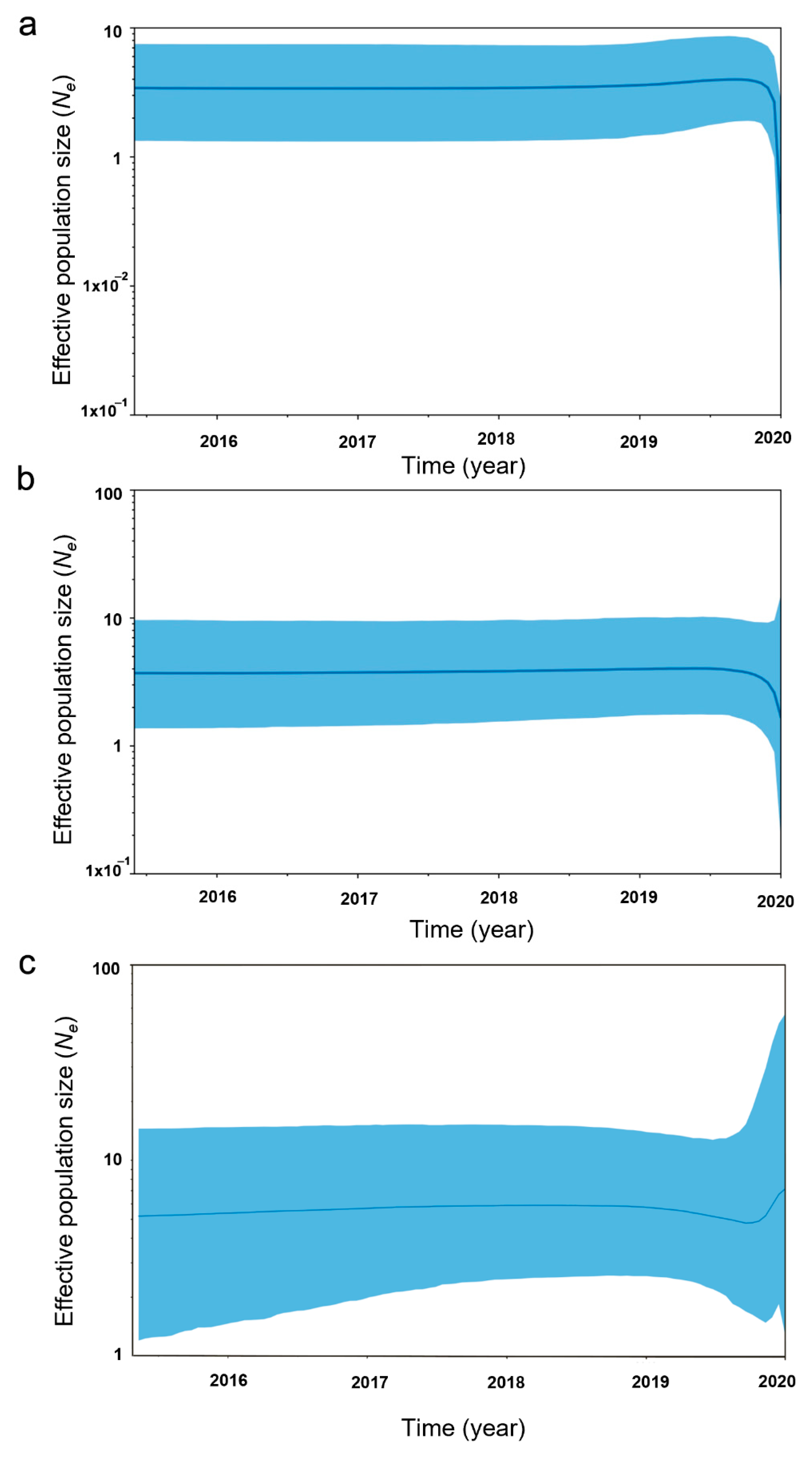
| Population | N | FIS | Relatedness (r) | Estimated Ne | 95% CIs for Ne | Ne/N |
|---|---|---|---|---|---|---|
| NEI 1 | 72 | 0.195 ± 0.201 | −0.011 ± 0.040 | 38.600 | 33.800–199.100 | 0.536 |
| MEP 2 | 46 | −0.069 ± 0.045 | −0.012 ± 0.032 | 153.700 | 95.200–245.600 | 3.341 |
| BCEP 3 | 40 | 0.354 ± 0.126 | −0.023 ± 0.046 | 103.600 | 73.000–217.300 | 2.590 |
| Population | N | Number of haplotypes (H) | Theta (Per Site) from S | Average Number of Nucleotide Differences (k) | Overall Haplotype | Nucleotide Diversities (π) |
|---|---|---|---|---|---|---|
| NEI 1 | 72 | 32 | 0.119 | 35.416 | 0.920 ± 0.020 | 0.099 ± 0.048 |
| MEP 2 | 46 | 14 | 0.017 | 7.931 | 0.901± 0.024 | 0.023 ± 0.012 |
| BCEP 3 | 40 | 20 | 0.023 | 7.779 | 0.936 ± 0.019 | 0.022 ± 0.012 |
| All populations | 158 | 49 | 0.106 | 21.368 | 0.928 ± 0.010 | 0.060 ± 0.030 |
| Population 1 | Population 2 | GST | ΦST | FST | Dxy | Da | Nm |
|---|---|---|---|---|---|---|---|
| NEI 1 | MEP 2 | 0.009 | 0.050 | 0.081 ** | 0.066 | 0.006 | 5.693 |
| NEI | BCEP 3 | 0.010 | 0.048 | 0.081 ** | 0.067 | 0.007 | 5.692 |
| MEP | BCEP | 0.001 | 0.028 | 0.036 * | 0.022 | 0.001 | 13.380 |
| Population | Tajima | Fu D* | Fu F* | Fu’s Fs | Ewens–Watterson Test | Chakraborty’s Test | Ramos–Onsins and Rozas | Raggedness Index |
|---|---|---|---|---|---|---|---|---|
| NEI 1 | 0.295 ns | 0.255 ns | −0.044 ns | 4.465 ns | 0.989 ns | 0.080 ns | 0.109 ns | 0.023 ns |
| MEP 2 | 1.132 ns | −0.204 ns | 0.243 ns | 1.544 ns | 0.251ns | 0.100 ns | 0.150 ns | 0.030 ns |
| BCEP 3 | −0.093 ns | −1.125 ns | −0.990 ns | −2.964 ns | 0.721 ns | 0.064 ns | 0.114 ns | 0.017 ns |
| All populations | −0.861 ns | −0.635 ns | −1.154 ns | −0.277 ns | 0.989 ns | 0.072 ns | 0.062 ns | 0.015 ns |
| Model | Top-1 % Accuracy | Top-3 % Accuracy | Top-5 % Accuracy | Top-7 % Accuracy | Rank % Average |
|---|---|---|---|---|---|
| 1 | 43.8 | 60.9 | 68.8 | 76.6 | 6.5 |
| 2 | 43.8 | 67.2 | 76.6 | 79.7 | 5.6 |
| 3 | 35.9 | 64.1 | 75.0 | 81.3 | 5.5 |
| 4 | 50.0 | 68.8 | 75.0 | 78.1 | 5.0 |
| 5 | 34.4 | 59.4 | 68.8 | 78.1 | 5.2 |
| Average | 41.6 | 64.1 | 72.8 | 78.8 | 5.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariyaraphong, N.; Ho My Nguyen, D.; Singchat, W.; Suksavate, W.; Panthum, T.; Langkaphin, W.; Chansitthiwet, S.; Angkawanish, T.; Promking, A.; Kaewtip, K.; et al. Standard Identification Certificate for Legal Legislation of a Unique Gene Pool of Thai Domestic Elephants Originating from a Male Elephant Contribution to Breeding. Sustainability 2022, 14, 15355. https://doi.org/10.3390/su142215355
Ariyaraphong N, Ho My Nguyen D, Singchat W, Suksavate W, Panthum T, Langkaphin W, Chansitthiwet S, Angkawanish T, Promking A, Kaewtip K, et al. Standard Identification Certificate for Legal Legislation of a Unique Gene Pool of Thai Domestic Elephants Originating from a Male Elephant Contribution to Breeding. Sustainability. 2022; 14(22):15355. https://doi.org/10.3390/su142215355
Chicago/Turabian StyleAriyaraphong, Nattakan, Dung Ho My Nguyen, Worapong Singchat, Warong Suksavate, Thitipong Panthum, Warangkhana Langkaphin, Saran Chansitthiwet, Taweepoke Angkawanish, Arphorn Promking, Kantapon Kaewtip, and et al. 2022. "Standard Identification Certificate for Legal Legislation of a Unique Gene Pool of Thai Domestic Elephants Originating from a Male Elephant Contribution to Breeding" Sustainability 14, no. 22: 15355. https://doi.org/10.3390/su142215355
APA StyleAriyaraphong, N., Ho My Nguyen, D., Singchat, W., Suksavate, W., Panthum, T., Langkaphin, W., Chansitthiwet, S., Angkawanish, T., Promking, A., Kaewtip, K., Jaisamut, K., Ahmad, S. F., Trirongjitmoah, S., Muangmai, N., Taesumrith, O., Inwiset, S., Duengkae, P., & Srikulnath, K. (2022). Standard Identification Certificate for Legal Legislation of a Unique Gene Pool of Thai Domestic Elephants Originating from a Male Elephant Contribution to Breeding. Sustainability, 14(22), 15355. https://doi.org/10.3390/su142215355












