Using Isotopic Labeling to Investigate Artemisia ordosica Root Water Uptake Depth in the Eastern Margin of Mu Us Sandy Land
Abstract
1. Introduction
2. Materials and Methods
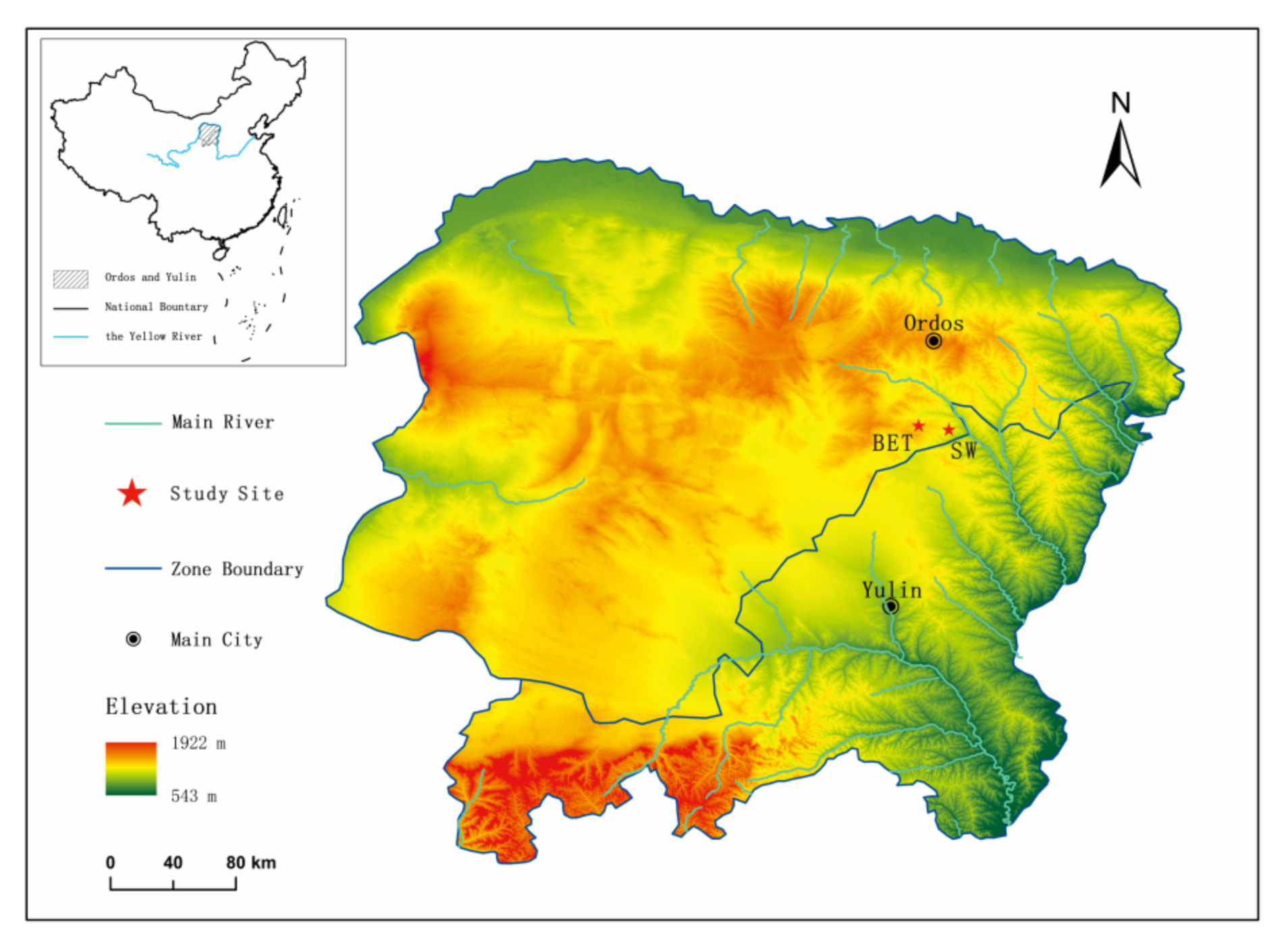
2.1. Study Area
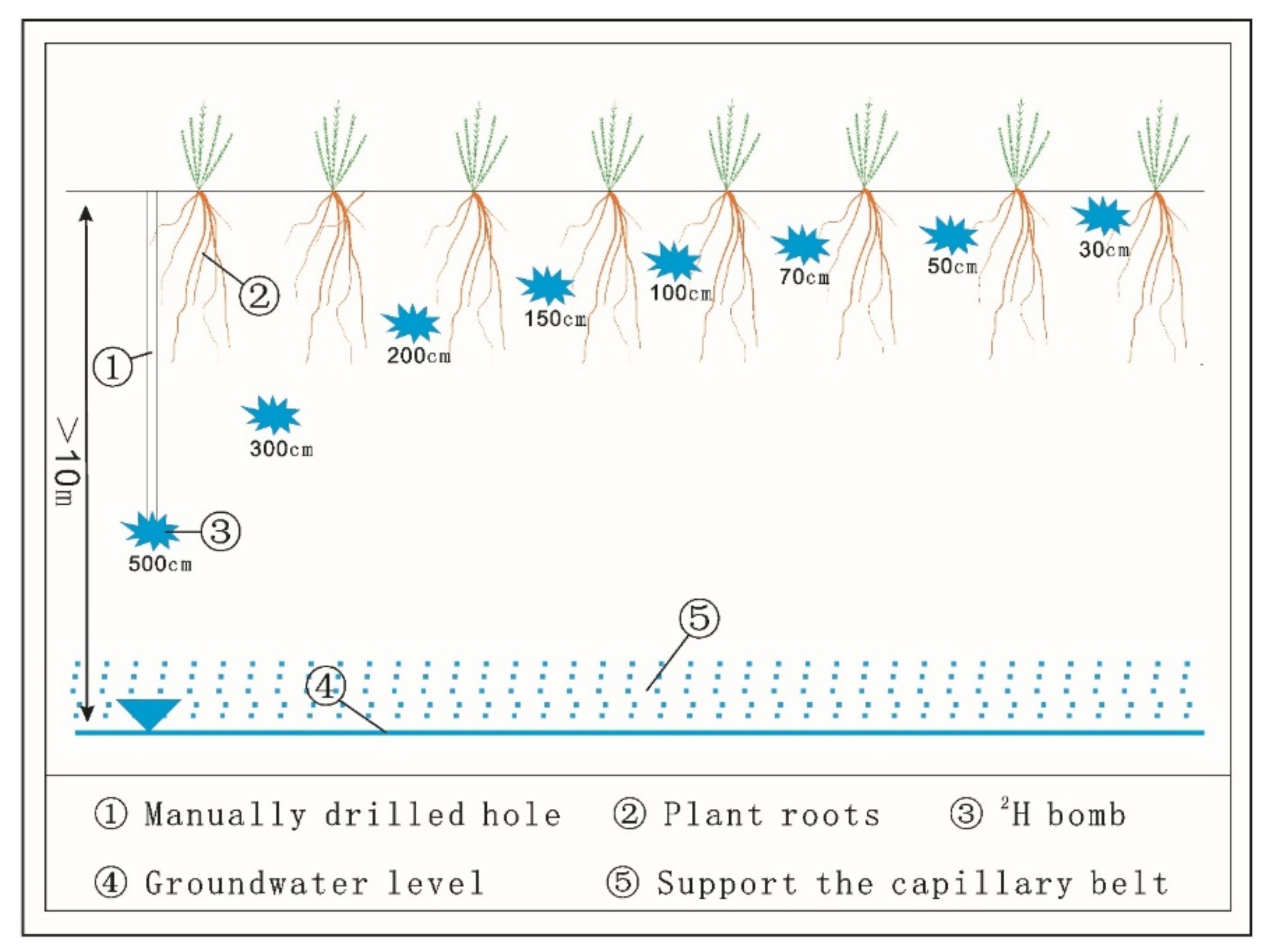
2.2. Experimental Design and Sample Collection
2.3. Sample Processing
2.4. Data Analysis
3. Results
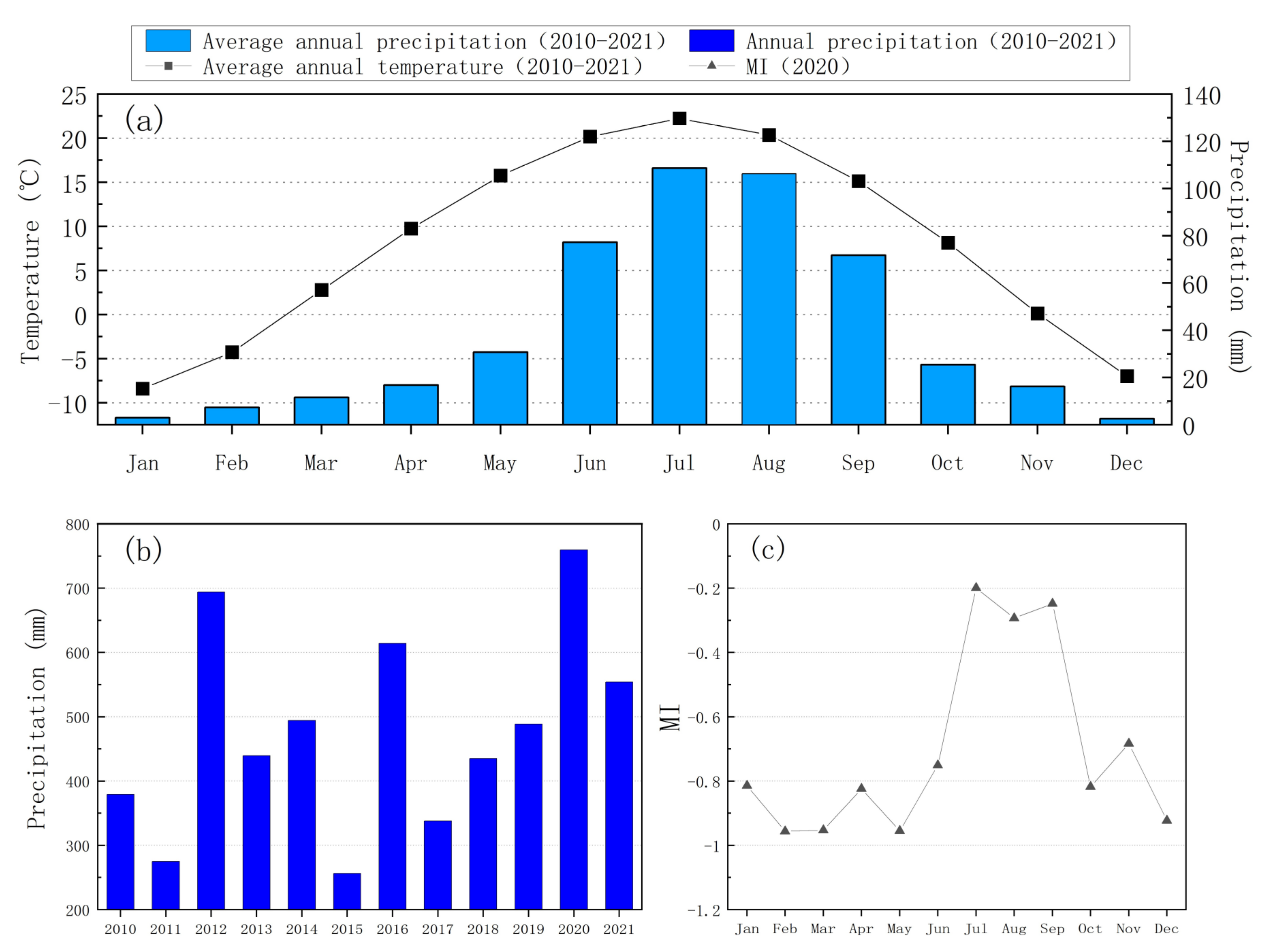
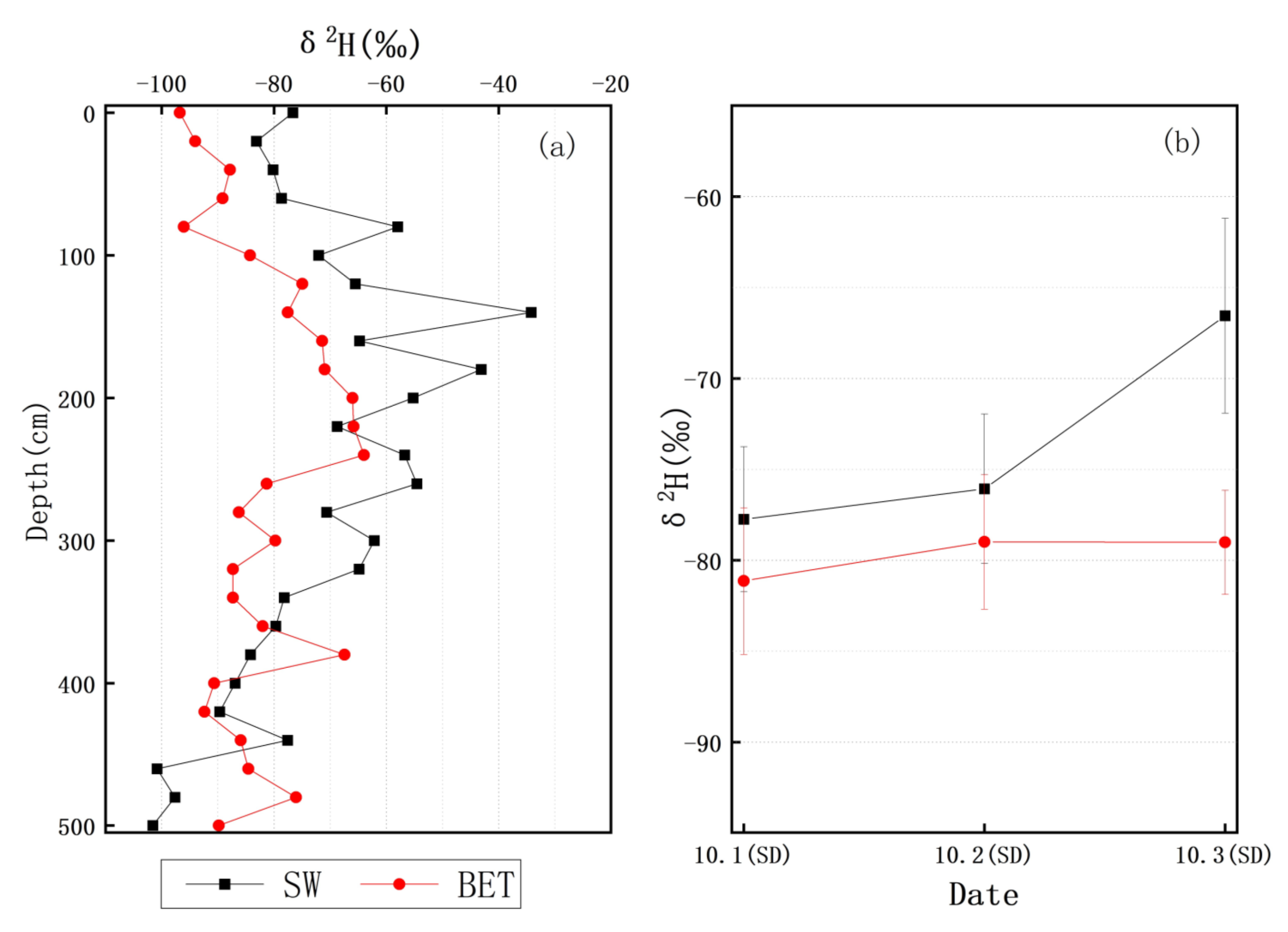
3.1. Precipitation and Soil Water
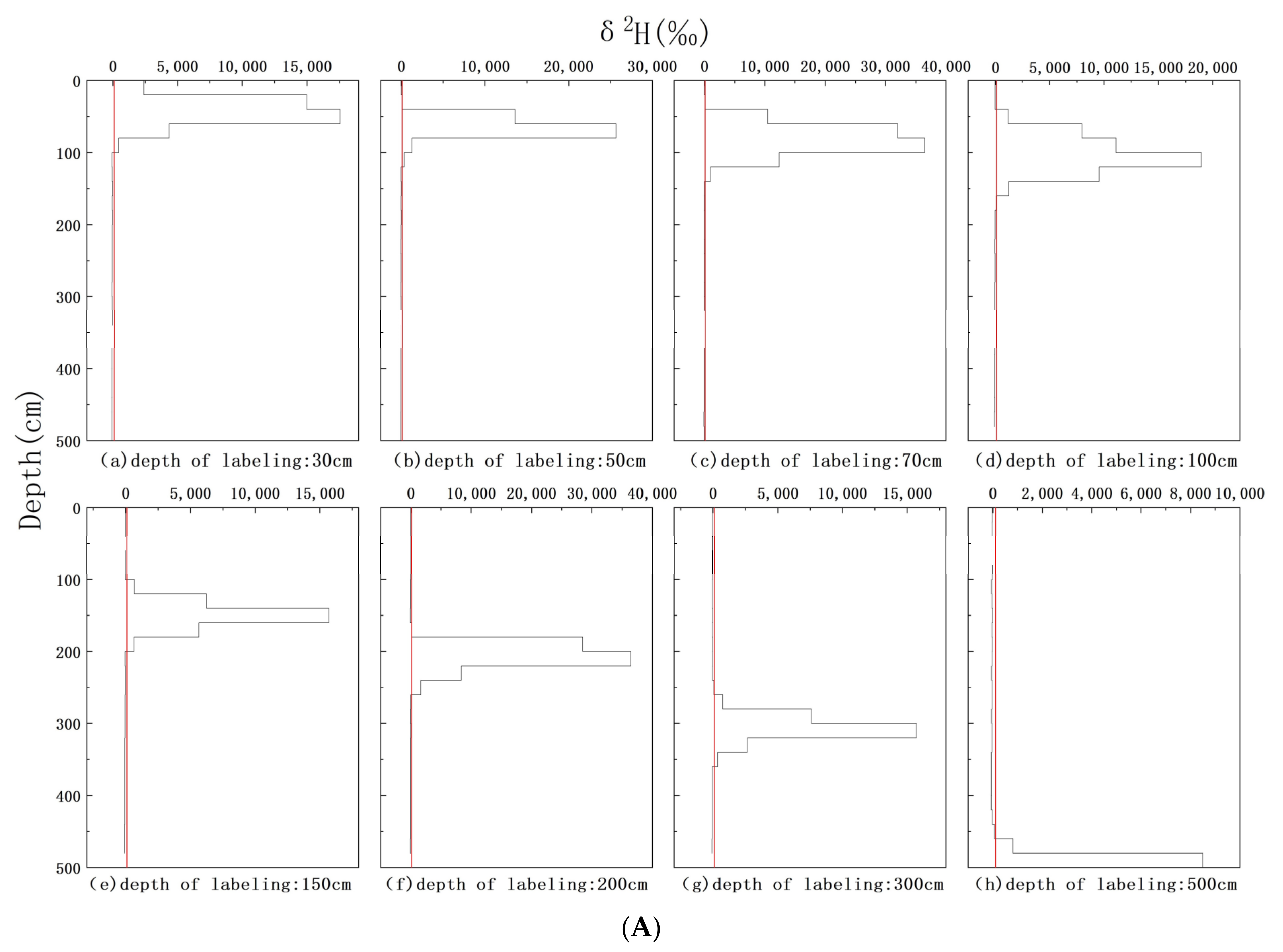
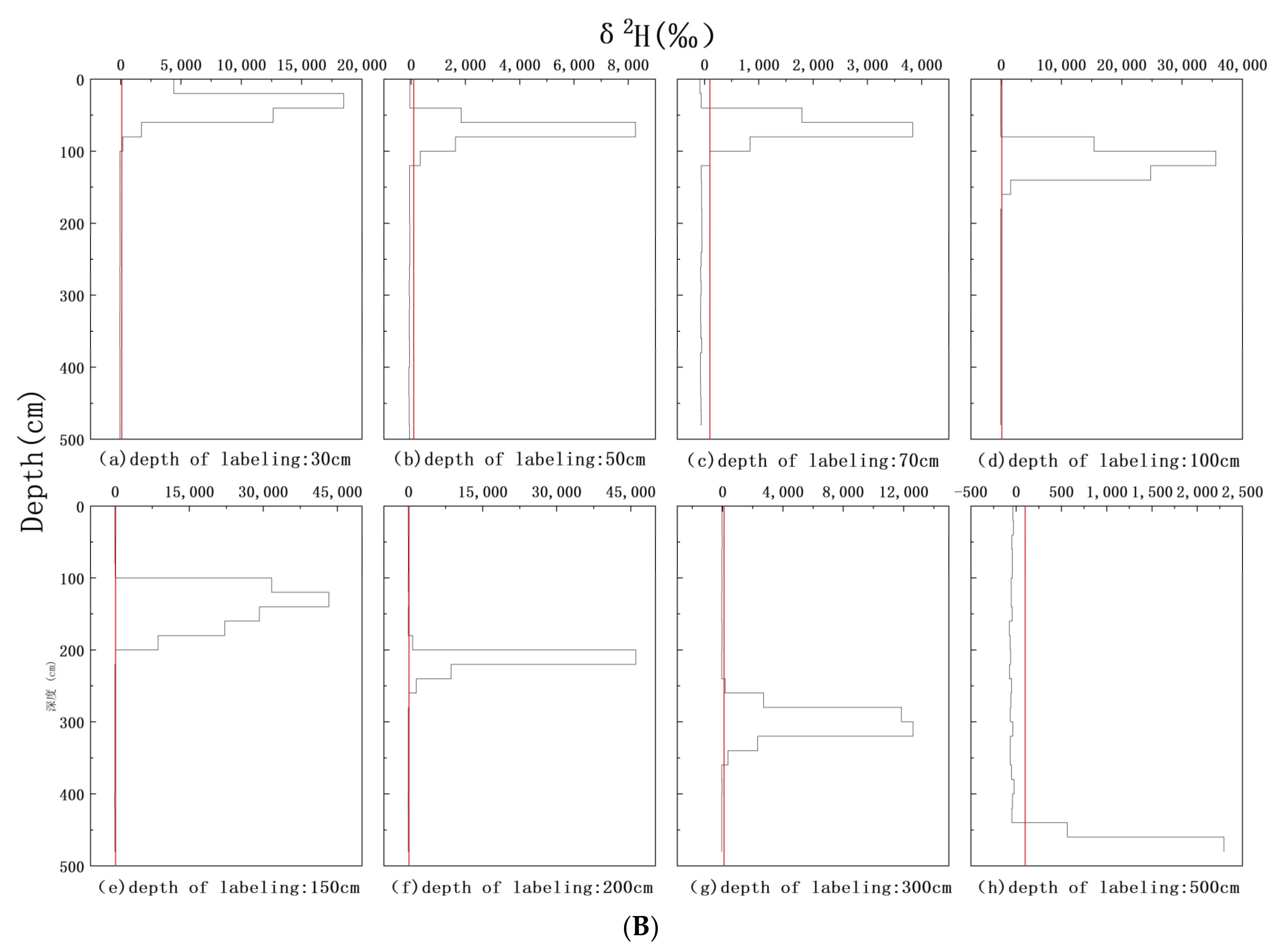
3.2. δ2H Background Values of Soil Water and Xylem Water
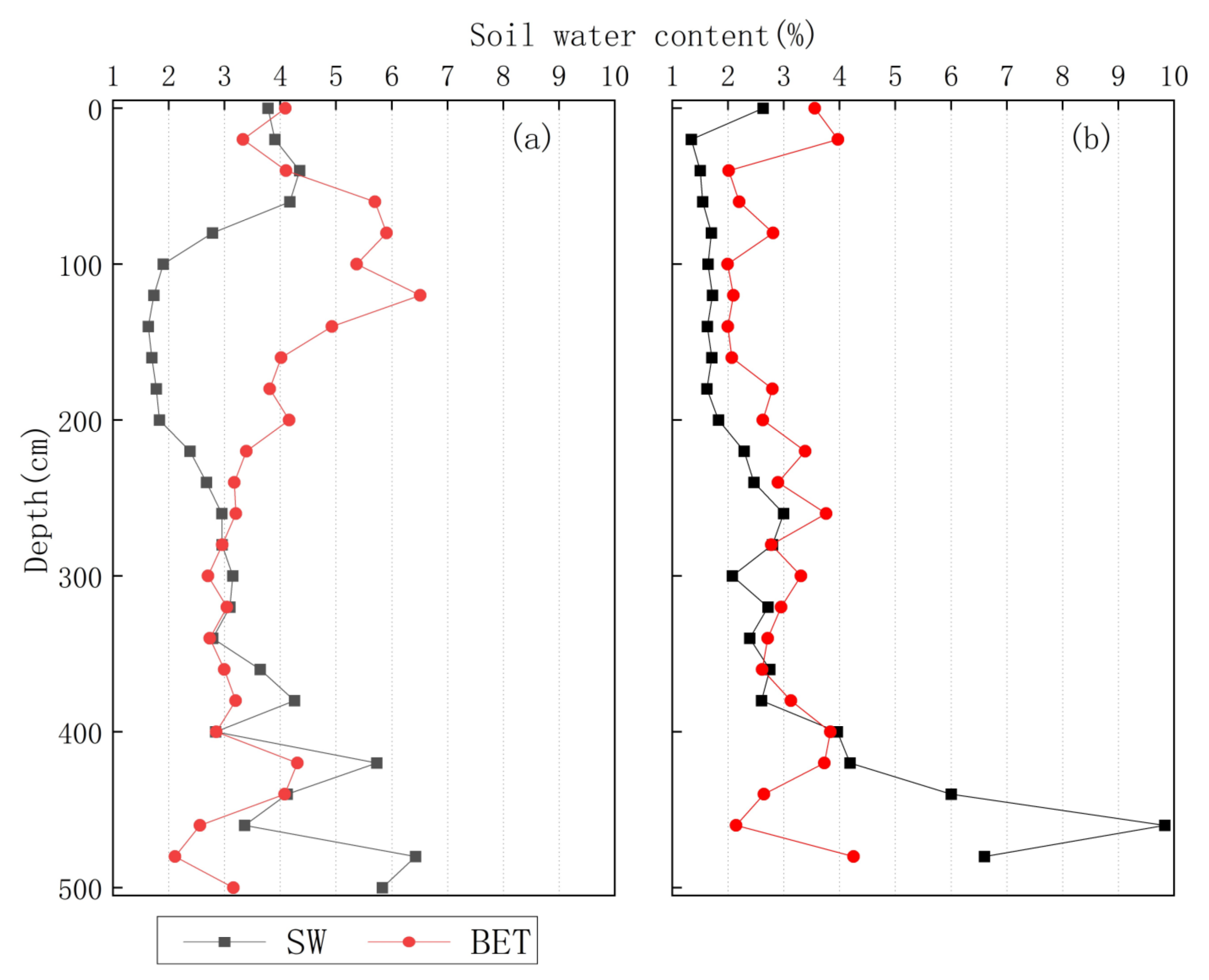
3.3. δ2H Characteristics of Soil Water
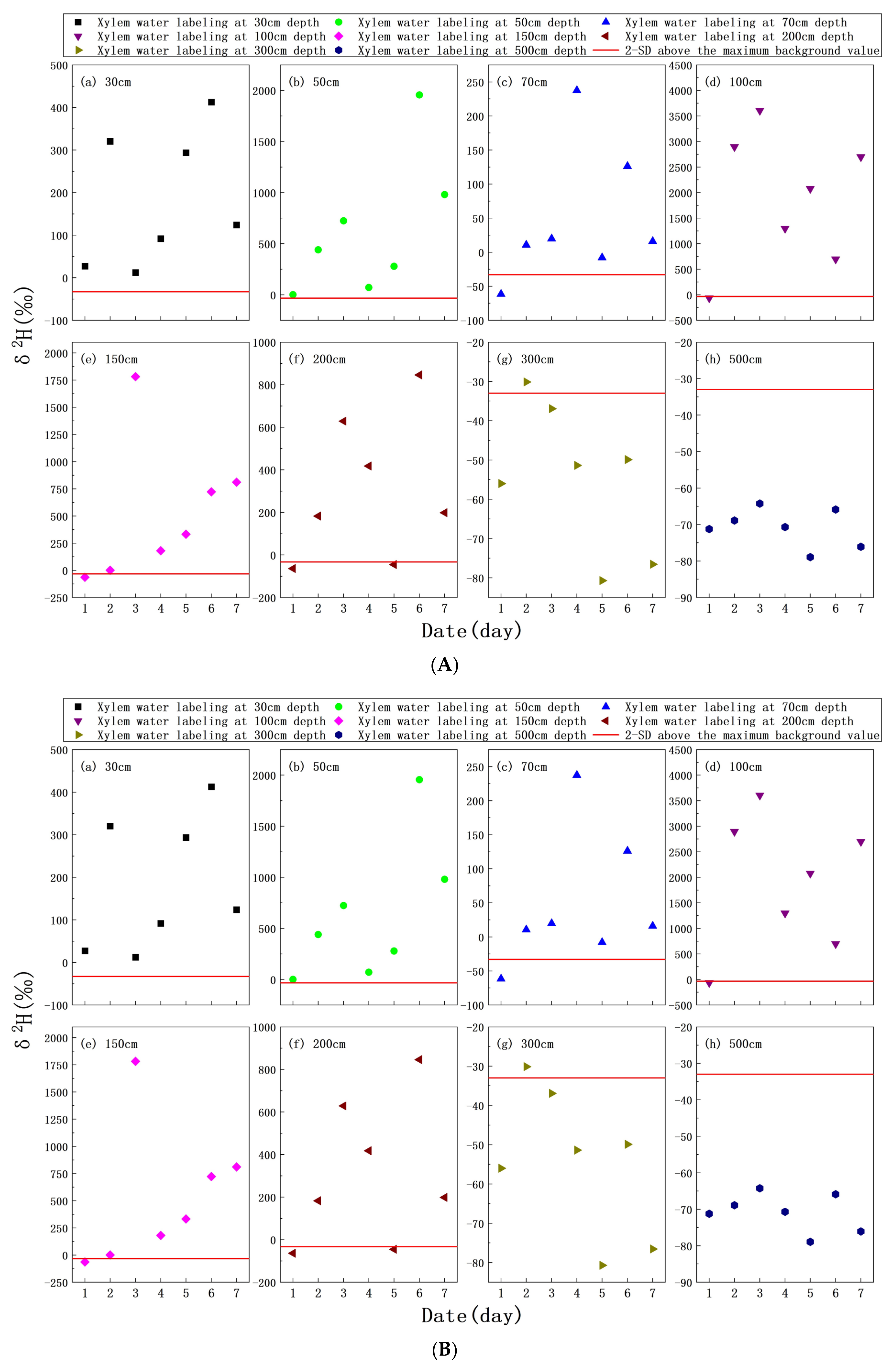
3.4. δ2H Characteristics of Xylem Water
4. Discussion
4.1. Root Uptake Depth
4.2. Meaning of Deep Roots
4.3. Niche Differentiation of Roots in an Arid Environment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, X.; Yang, G.; Qin, F.; Jia, G.; Ling, X.; Gao, G. Spatial and temporal dynamic patterns of sandy land in Mu Us in the last 30 years. Res. Soil Water Conserv. 2019, 26, 144–157. [Google Scholar]
- Gu, Z.-K.; Shi, C.-X. Dynamical characteristics of geomorphologic evolution of the basins covered by Pisha-sandstone in the eastern wing of the Ordos Plateau, China. J. Mt. Sci. 2018, 15, 1046–1056. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; lI, Z.; Wu, B.; Zhang, Z.; Wang, Y. Comparative study on spatial patterns of Artemisia ordosica populations in the Mu Us sandy land. Acta Ecol. Sin. 2008, 28, 1901–1910. [Google Scholar]
- Wang, T.-Y.; Wang, P.; Wang, Z.-L.; Niu, G.-Y.; Yu, J.-J.; Ma, N.; Wu, Z.-N.; Pozdniakov, S.P.; Yan, D.-H. Drought adaptability of phreatophytes: Insight from vertical root distribution in drylands of China. J. Plant Ecol. 2021, 14, 1128–1142. [Google Scholar] [CrossRef]
- Rhizopoulou, S.; Kapolas, G. In situ study of deep roots of Capparis spinosa L. during the dry season: Evidence from a natural “rhizotron” in the ancient catacombs of Milos Island (Greece). J. Arid. Environ. 2015, 119, 27–30. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. Mapping the global distribution of deep roots in relation to climate and soil characteristics. Geoderma 2005, 126, 129–140. [Google Scholar] [CrossRef]
- Li, C.; Zeng, F.; Zhang, B.; Liu, B.; Guo, Z.; Gao, H.; Tiyip, T. Optimal root system strategies for desert phreatophytic seedlings in the search for groundwater. J. Arid. Land 2015, 7, 462–474. [Google Scholar] [CrossRef]
- Collins, D.B.G.; Bras, R.L. Plant rooting strategies in water-limited ecosystems. Water Resour. Res. 2007, 43, W06407. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, C.; Li, J.; Liu, Z.; Wang, J. Root Plasticity of Populus euphratica Seedlings in Response to Different Water Table Depths and Contrasting Sediment Types. PLoS ONE 2015, 10, e0118691. [Google Scholar] [CrossRef]
- Gang, G.Z.; Xia, L.H.; Rong, W.Y.; Min, W.S.; Dong, C.G. Suitability of lucerne cultivars, with respect to root development, to semi-arid conditions in west China. N. Z. J. Agric. Res. 2004, 47, 51–59. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Sirimuji; Wang, B.; Liu, K. Research progress on the effects of root niche differences on ecosystems. Pratacultural Sci. 2021, 38, 501–513. [Google Scholar]
- Oliveira, R.S.; Bezerra, L.; Davidson, E.A.; Pinto, F.; Klink, C.A.; Nepstad, D.C.; Moreira, A. Deep root function in soil water 473 dynamics in cerrado savannas of central Brazil. Funct. Ecol. 2005, 19, 574–581. [Google Scholar] [CrossRef]
- Tomlinson, K.W.; Sterck, F.J.; Bongers, F.; da Silva, D.A.; Barbosa, E.R.M.; Ward, D.; Bakker, F.T.; van Kaauwen, M.; Prins, H.H.T.; de Bie, S.; et al. Biomass partitioning and root morphology of savanna trees across a water gradient. J. Ecol. 2012, 100, 1113–1121. [Google Scholar] [CrossRef]
- Brum, M.; Teodoro, G.S.; Abrahao, A.; Oliveira, R.S. Coordination of rooting depth and leaf hydraulic traits defines drought-related strategies in the campos rupestres, a tropical montane biodiversity hotspot. Plant Soil 2017, 420, 467–480. [Google Scholar] [CrossRef]
- Tumber-Davila, S.J.; Schenk, H.J.; Du, E.; Jackson, R.B. Plant sizes and shapes above and belowground and their interactions with climate. New Phytol. 2022, 235, 1032–1056. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, P.; Wu, Z.; Yu, J.; Pozdniakov, S.P.; Guan, X.; Wang, H.; Xu, H.; Yan, D. Modeling revealed the effect of root dynamics on the water adaptability of phreatophytes. Agric. For. Meteorol. 2022, 320, 108959. [Google Scholar] [CrossRef]
- Yang, F.; Feng, Z.; Wang, H.; Dai, X.; Fu, X. Deep soil water extraction helps to drought avoidance but shallow soil water uptake during dry season controls the inter-annual variation in tree growth in four subtropical plantations. Agric. For. Meteorol. 2017, 234, 106–114. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Kanyomeka, L.; Izumi, Y.; Iijima, M. Pearl millet developed deep roots and changed water sources by competition with intercropped cowpea in the semiarid environment of northern Namibia. Plant Prod. Sci. 2006, 9, 355–363. [Google Scholar] [CrossRef]
- Good, S.P.; Noone, D.; Bowen, G. Hydrologic connectivity constrains partitioning of global terrestrial water fluxes. Science 2015, 349, 175–177. [Google Scholar] [CrossRef]
- Hashemian, M.; Ryu, D.; Crow, W.T.; Kustas, W.P. Improving root-zone soil moisture estimations using dynamic root growth and crop phenology. Adv. Water Resour. 2015, 86, 170–183. [Google Scholar] [CrossRef]
- Seyfried, M.S.; Schwinning, S.; Walvoord, M.A.; Pockman, W.T.; Newman, B.D.; Jackson, R.B.; Phillips, E.M. Ecohydrological control of deep drainage in arid and semiarid regions. Ecology 2005, 86, 277–287. [Google Scholar] [CrossRef]
- Guo, L.; Chen, J.; Cui, X.; Fan, B.; Lin, H. Application of ground penetrating radar for coarse root detection and quantification: A review. Plant Soil 2012, 362, 1–23. [Google Scholar] [CrossRef]
- Talebnejad, R.; Sepaskhah, A.R. Effects of deficit irrigation and groundwater depth on root growth of direct seeding rice in a column experiment. Int. J. Plant Prod. 2014, 8, 563–585. [Google Scholar]
- Yu, B.; Xie, C.; Cai, S.; Chen, Y.; Lv, Y.; Mo, Z.; Liu, T.; Yang, Z. Effects of Tree Root Density on Soil Total Porosity and Non-Capillary Porosity Using a Ground-Penetrating Tree Radar Unit in Shanghai, China. Sustainability 2018, 10, 4640. [Google Scholar] [CrossRef]
- Hirano, Y.; Yamamoto, R.; Dannoura, M.; Aono, K.; Igarashi, T.; Ishii, M.; Yamase, K.; Makita, N.; Kanazawa, Y. Detection frequency of Pinus thunbergii roots by ground-penetrating radar is related to root biomass. Plant Soil 2012, 360, 363–373. [Google Scholar] [CrossRef]
- Wen, X.; Yang, B.; Sun, X.; Lee, X. Evapotranspiration partitioning through in-situ oxygen isotope measurements in an oasis cropland. Agric. For. Meteorol. 2016, 230–231, 89–96. [Google Scholar] [CrossRef]
- Penna, D.; Hopp, L.; Scandellari, F.; Allen, S.T.; Benettin, P.; Beyer, M.; Geris, J.; Klaus, J.; Marshall, J.D.; Schwendenmann, L.; et al. Ideas and perspectives: Tracing terrestrial ecosystem water fluxes using hydrogen and oxygen stable isotopes—Challenges and opportunities from an interdisciplinary perspective. Biogeosciences 2018, 15, 6399–6415. [Google Scholar] [CrossRef]
- Sprenger, M.; Leistert, H.; Gimbel, K.; Weiler, M. Illuminating hydrological processes at the soil-vegetation-atmosphere interface with water stable isotopes. Rev. Geophys. 2016, 54, 674–704. [Google Scholar] [CrossRef]
- Hu, Z.; Wen, X.; Sun, X.; Li, L.; Yu, G.; Lee, X.; Li, S. Partitioning of evapotranspiration through oxygen isotopic measurements of water pools and fluxes in a temperate grassland. J. Geophys. Res. Biogeosci. 2014, 119, 358–372. [Google Scholar] [CrossRef]
- Lyu, S.; Wang, J.; Song, X.; Wen, X. The relationship of δD and δ18O in surface soil water and its implications for soil evaporation along grass transects of Tibet, Loess, and Inner Mongolia Plateau. J. Hydrol. 2021, 600, 123533. [Google Scholar] [CrossRef]
- Beyer, M.; Hamutoko, J.T.; Wanke, H.; Gaj, M.; Koeniger, P. Examination of deep root water uptake using anomalies of soil water stable isotopes, depth-controlled isotopic labeling and mixing models. J. Hydrol. 2018, 566, 122–136. [Google Scholar] [CrossRef]
- Beyer, M.; Koeniger, P.; Gaj, M.; Hamutoko, J.T.; Wanke, H.; Himmelsbach, T. A deuterium-based labeling technique for the investigation of rooting depths, water uptake dynamics and unsaturated zone water transport in semiarid environments. J. Hydrol. 2016, 533, 627–643. [Google Scholar] [CrossRef]
- Volkmann, T.H.M.; Haberer, K.; Gessler, A.; Weiler, M. High-resolution isotope measurements resolve rapid ecohydrological dynamics at the soil-plant interface. New Phytol. 2016, 210, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.P.; Kulmatiski, A.; Beard, K.H. A combined tracer/evapotranspiration model approach estimates plant water uptake in native and non-native shrub-steppe communities. J. Arid. Environ. 2015, 121, 67–78. [Google Scholar] [CrossRef]
- Gessler, A.; Bachli, L.; Rouholahnejad Freund, E.; Treydte, K.; Schaub, M.; Haeni, M.; Weiler, M.; Seeger, S.; Marshall, J.; Hug, C.; et al. Drought reduces water uptake in beech from the drying topsoil, but no compensatory uptake occurs from deeper soil layers. New Phytol. 2022, 233, 194–206. [Google Scholar] [CrossRef]
- Kahmen, A.; Buser, T.; Hoch, G.; Grun, G.; Dietrich, L. Dynamic H-2 irrigation pulse labelling reveals rapid infiltration and mixing of precipitation in the soil and species-specific water uptake depths of trees in a temperate forest. Ecohydrology 2021, 14, e2322. [Google Scholar] [CrossRef]
- Landgraf, J.; Tetzlaff, D.; Dubbert, M.; Dubbert, D.; Smith, A.; Soulsby, C. Xylem water in riparian willow trees (Salix alba) reveals shallow sources of root water uptake by in situ monitoring of stable water isotopes. Hydrol. Earth Syst. Sci. 2022, 26, 2073–2092. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, F.; Wang, Y.; Cheng, J. Soil Water Use Strategies of Dominant Tree Species Based on Stable Isotopes in Subtropical Regions, Central China. Water 2022, 14, 954. [Google Scholar] [CrossRef]
- Huang, W.; Zhong, Y.; Song, X.; Zhang, C.; Ren, M.; Du, Y. Seasonal Differences in Water-Use Sources of Impatiens hainanensis (Balsaminaceae), a Limestone-Endemic Plant Based on “Fissure-Soil” Habitat Function. Sustainability 2021, 13, 8721. [Google Scholar] [CrossRef]
- Zheng, L.; Ma, J.; Sun, X.; Guo, X.; Cheng, Q.; Shi, X. Estimating the Root Water Uptake of Surface-Irrigated Apples Using Water Stable Isotopes and the Hydrus-1D Model. Water 2018, 10, 1624. [Google Scholar] [CrossRef]
- Duan, Y.; Li, J.; Du, Z.; Kang, F. Analysis of biodiversity and flora characteristics of natural plants in Mu Us sandy land. Acta Bot. Boreali-Occident. Sin. 2018, 38, 770–779. [Google Scholar]
- Zhang, Y.; Zhang, M.; Qu, D.; Duan, W.; Wang, J.; Su, P.; Guo, R. Water Use Strategies of Dominant Species (Caragana korshin- skiiandReaumuria soongorica) in Natural Shrubs Based on Stable Isotopes in the Loess Hill, China. Water 2020, 12, 1923. [Google Scholar] [CrossRef]
- Yue, Y.; Sun, Y.; Pang, Y.; Cheng, L.; Zhou, H.; Jia, X.; Zhao, Y.; Zhao, H.; Wu, B. Root characteristics of Artemisia ordosica in the process of sand dunes activation in Mu Us sandland. J. Desert Res. 2020, 40, 177–184. [Google Scholar]
- GB/T20481–2017; Meteorological Drought Grade. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2017.
- Wang, S.; An, J.; Zhao, X.; Gao, X.; Wu, P.; Huo, G.; Robinson, B.H. Age- and climate- related water use patterns of apple trees on China’s Loess Plateau. J. Hydrol. 2020, 582, 124462. [Google Scholar] [CrossRef]
- Bing, W.; Lijian, Z.; Juanjuan, M.; Xihuan, S.; Xianghong, G.; Fei, G. Effective root depth and water uptake ability of winter wheat by using water stable isotopes in the Loess Plateau of China. Int. J. Agric. Biol. Eng. 2016, 9, 6. [Google Scholar]
- Cahill, J.F.; McNickle, G.G. The Behavioral Ecology of Nutrient Foraging by Plants. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 289–311. [Google Scholar] [CrossRef]
- Levine, J.M.; HilleRisLambers, J. The importance of niches for the maintenance of species diversity. Nature 2009, 461, 254–257. [Google Scholar] [CrossRef]
| Grade | Type | Relative Humidity Index |
|---|---|---|
| 1 | Data non-drought | −0.40 < MI |
| 2 | Data slight drought | −0.65 < MI ≤ −0.40 |
| 3 | Moderate drought | −0.80 < MI ≤ −0.65 |
| 4 | Serious drought | −0.95 < MI ≤ −0.80 |
| 5 | Mega-drought | MI ≤ −0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, X.; He, Y.; Zhang, K.; Mo, F.; Zhang, W.; Liu, G. Using Isotopic Labeling to Investigate Artemisia ordosica Root Water Uptake Depth in the Eastern Margin of Mu Us Sandy Land. Sustainability 2022, 14, 15149. https://doi.org/10.3390/su142215149
Yang Y, Wang X, He Y, Zhang K, Mo F, Zhang W, Liu G. Using Isotopic Labeling to Investigate Artemisia ordosica Root Water Uptake Depth in the Eastern Margin of Mu Us Sandy Land. Sustainability. 2022; 14(22):15149. https://doi.org/10.3390/su142215149
Chicago/Turabian StyleYang, Yingming, Xikai Wang, Yunlan He, Kaiming Zhang, Fan Mo, Weilong Zhang, and Gang Liu. 2022. "Using Isotopic Labeling to Investigate Artemisia ordosica Root Water Uptake Depth in the Eastern Margin of Mu Us Sandy Land" Sustainability 14, no. 22: 15149. https://doi.org/10.3390/su142215149
APA StyleYang, Y., Wang, X., He, Y., Zhang, K., Mo, F., Zhang, W., & Liu, G. (2022). Using Isotopic Labeling to Investigate Artemisia ordosica Root Water Uptake Depth in the Eastern Margin of Mu Us Sandy Land. Sustainability, 14(22), 15149. https://doi.org/10.3390/su142215149





