Sustainable Fly Ash Based Geopolymer Binders: A Review on Compressive Strength and Microstructure Properties
Abstract
1. Introduction
2. Scope of the Study
3. Chemical Properties of Industrial Wastes/Source Materials
Role of SiO2, Al2O3, CaO, Fe2O3, and MgO in the Formation of Geopolymer Binder Structure
4. Part I: Review of Compressive Strength Properties
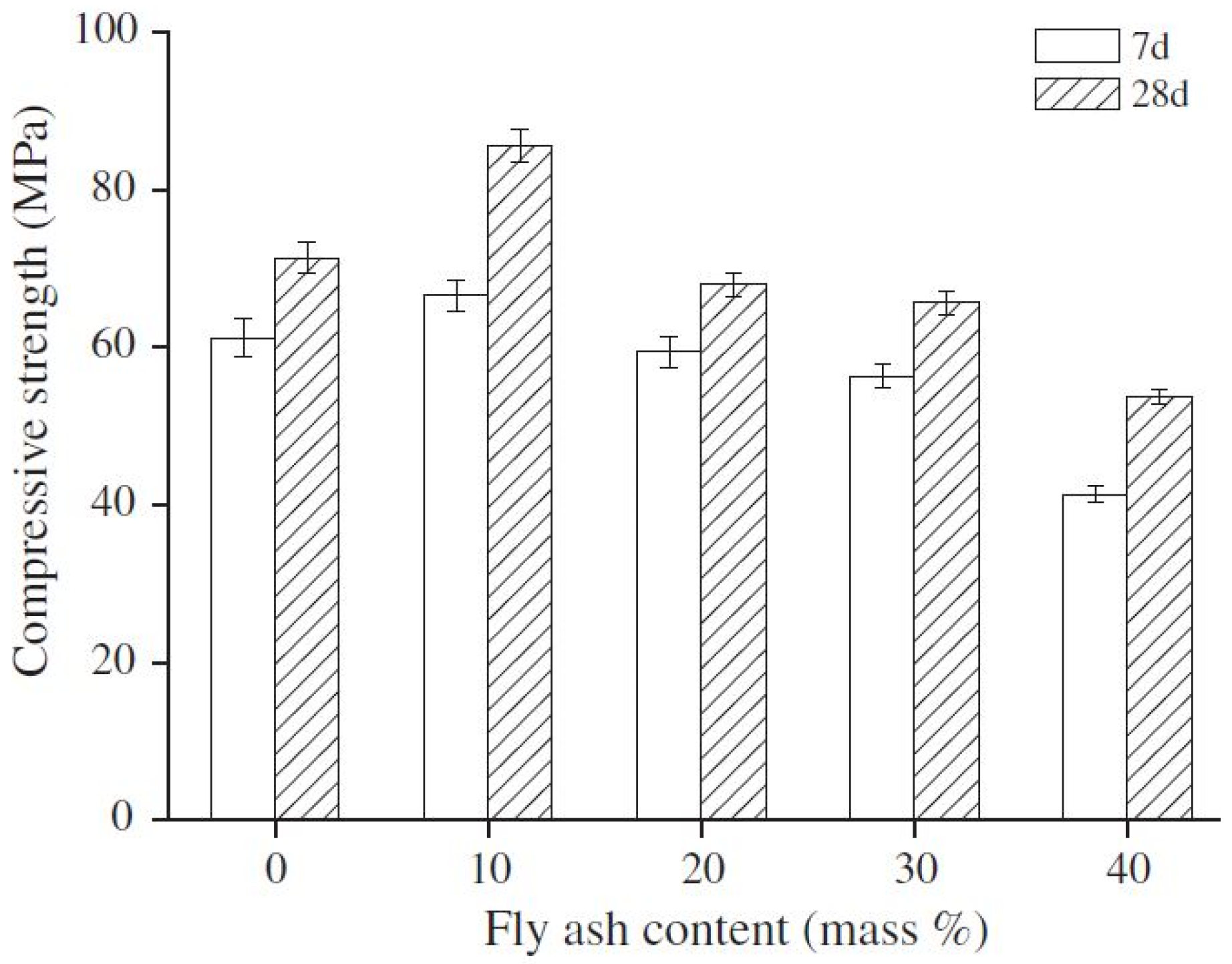
4.1. FA-Based Geopolymer Binder
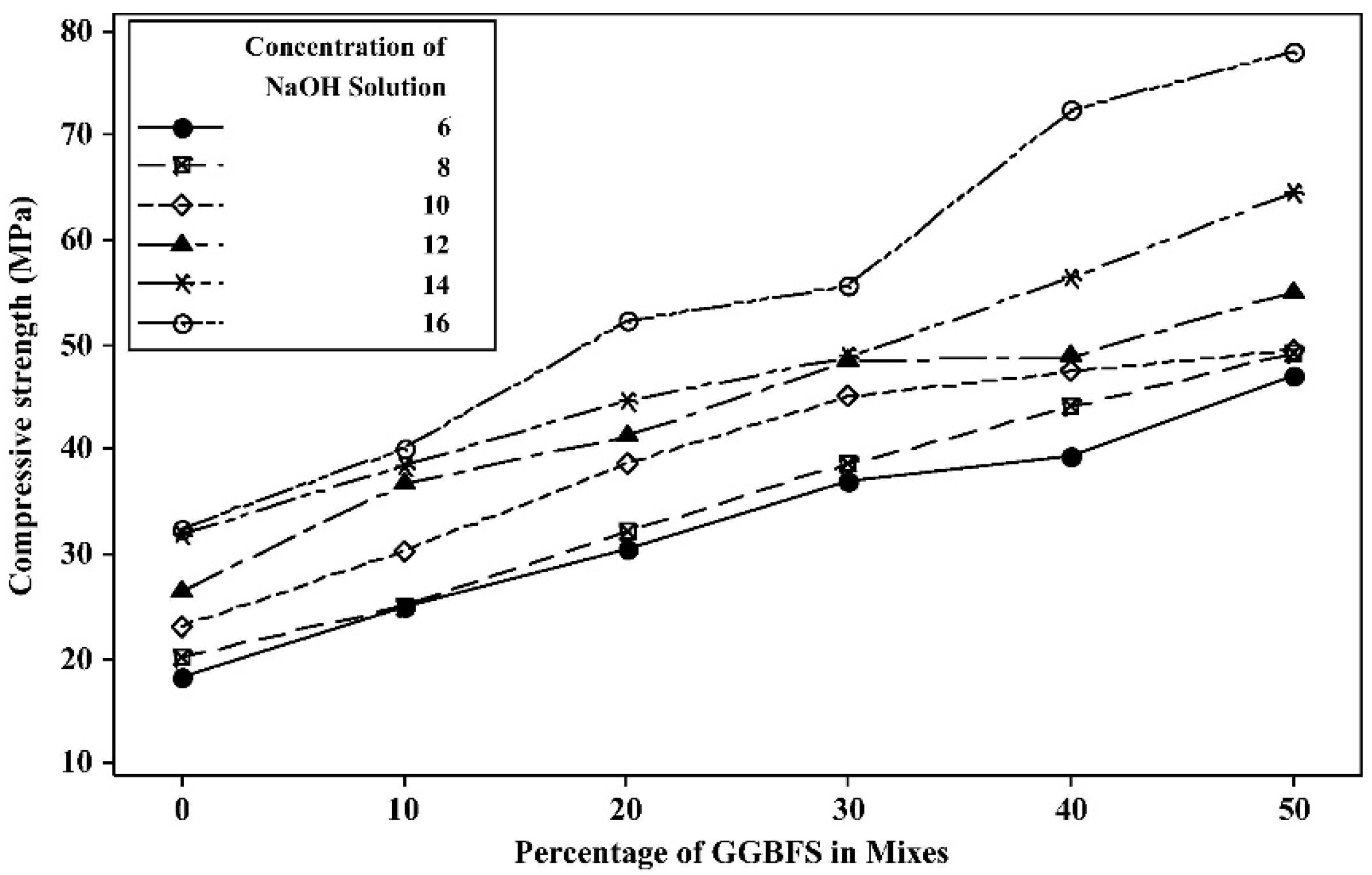
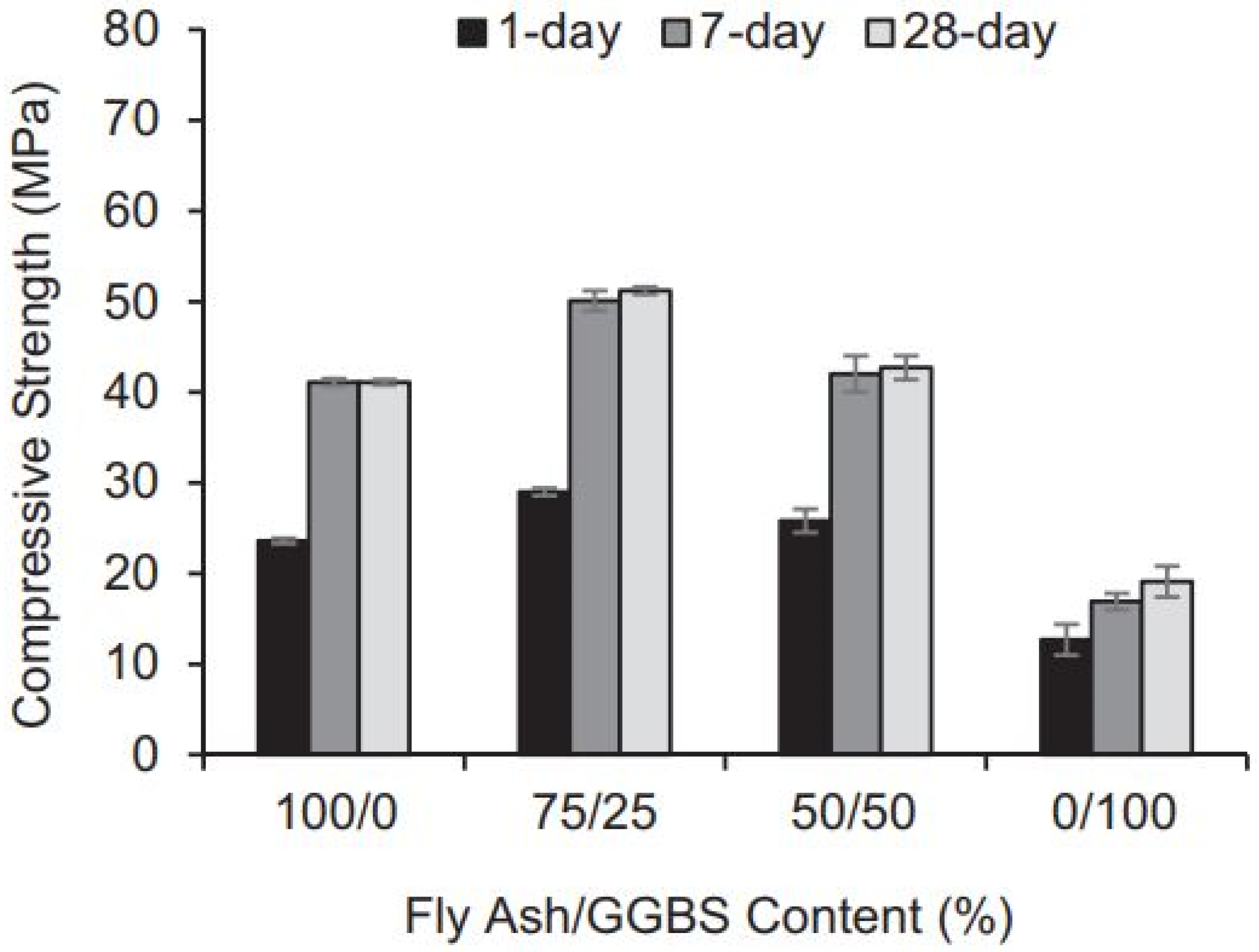
4.2. FA-GGBFS-Based Geopolymer Binder
4.3. FA-RM-Based Geopolymer Binder
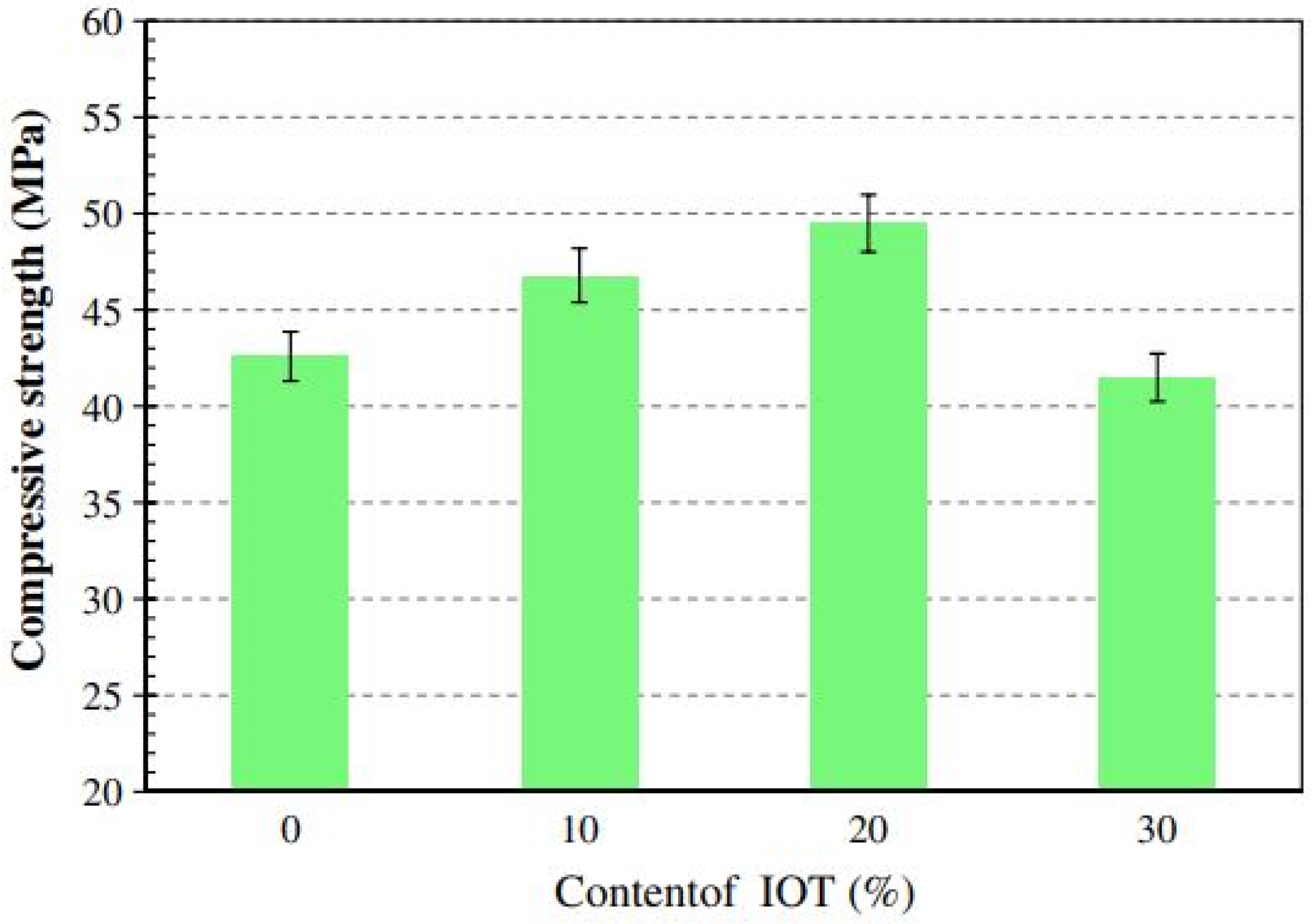
4.4. FA-IOT-Based Geopolymer Binder
4.5. FCA -FA-Based Geopolymer Binder
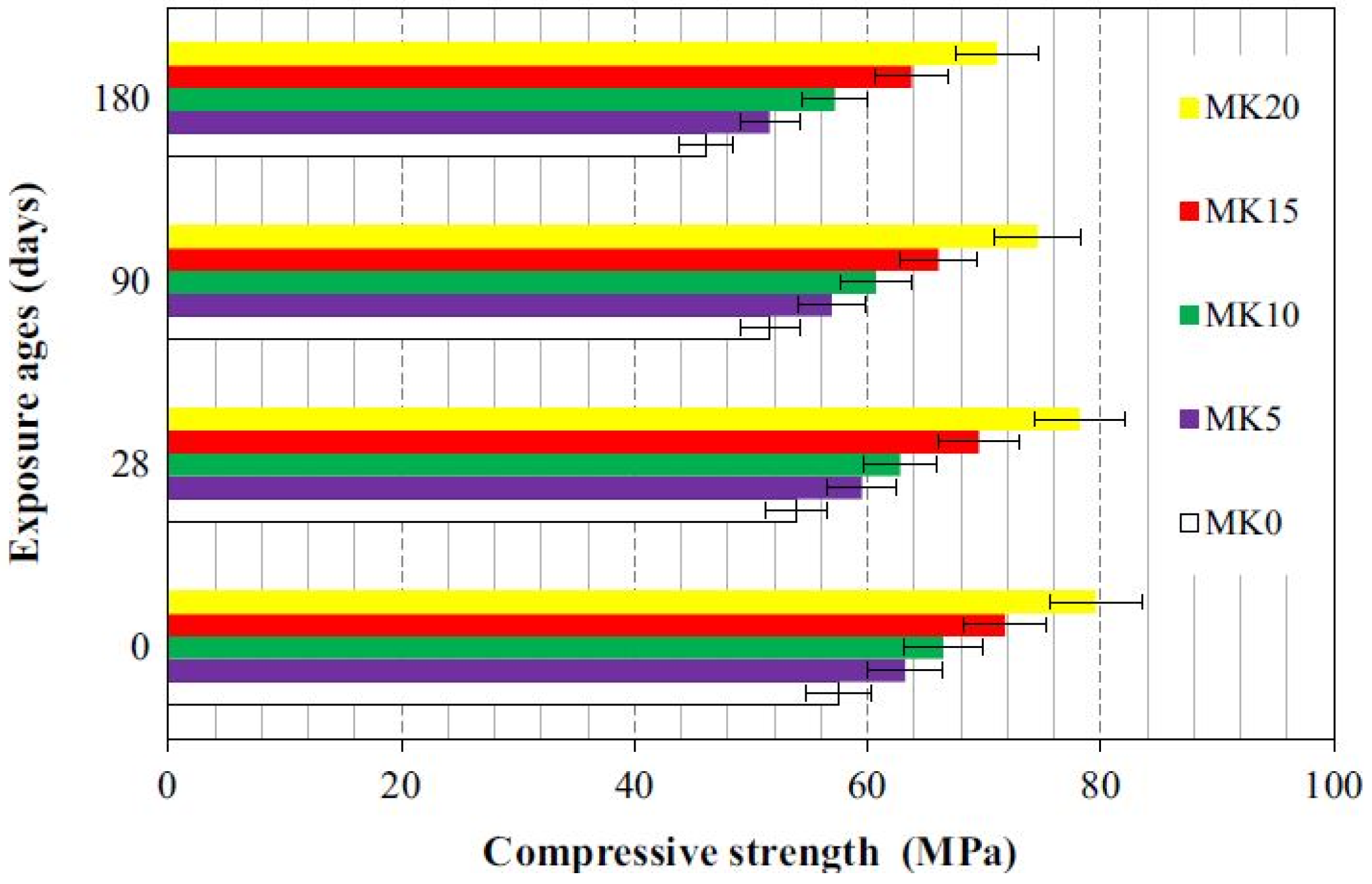
4.6. FA-MK-Based Geopolymer Binder
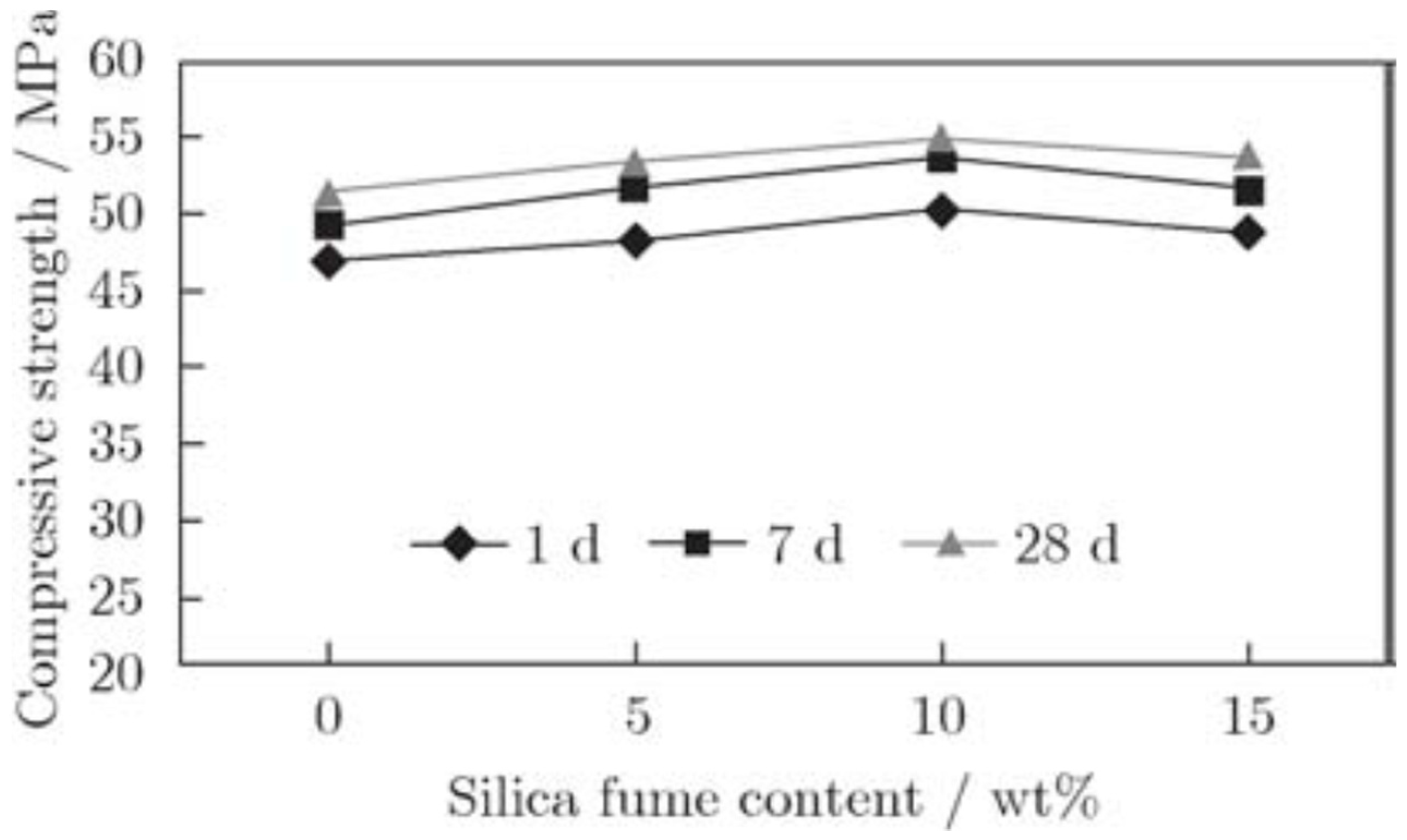
4.7. FA-SF-Based Geopolymer Binder
5. Part II: Review of Microstructural Properties
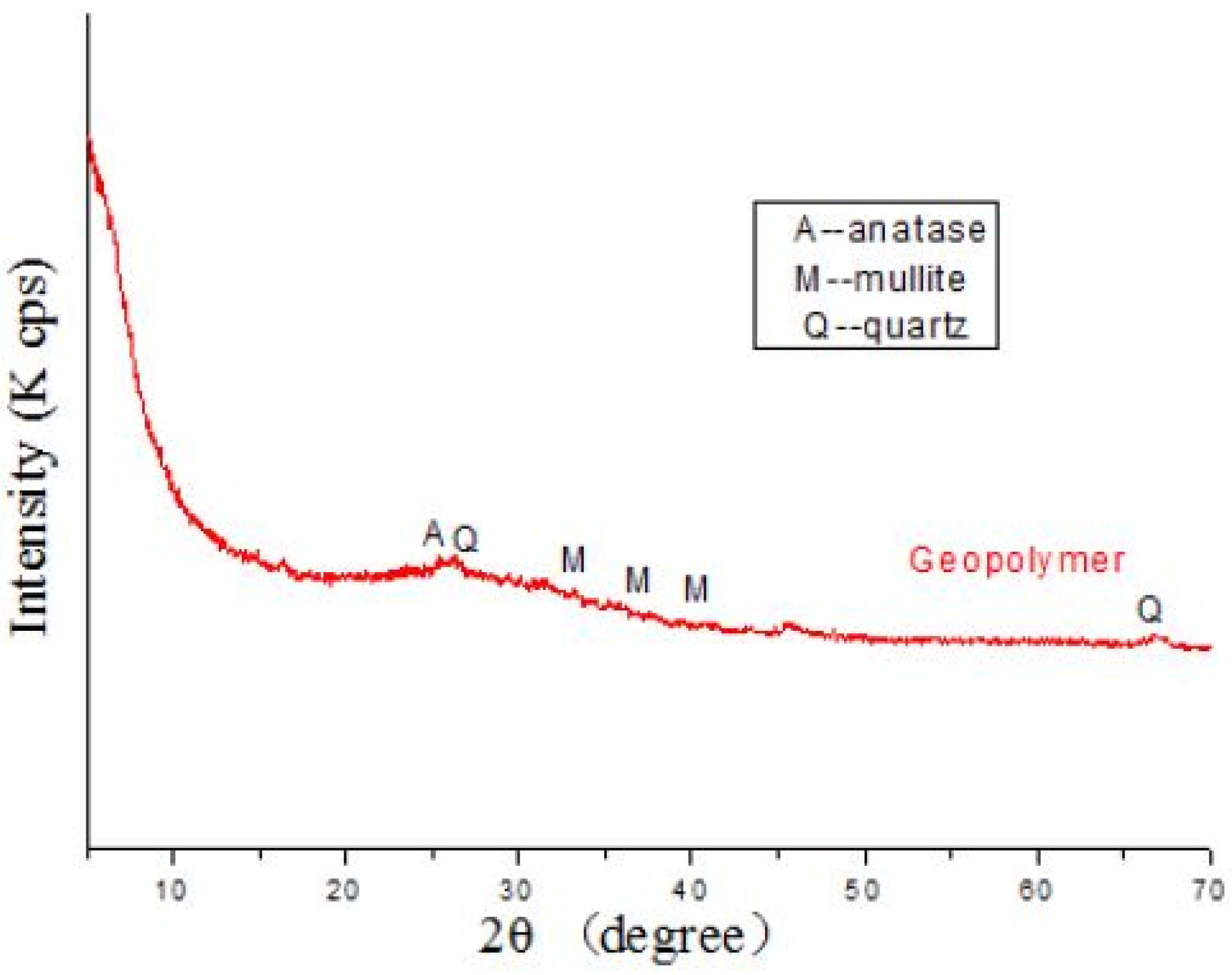
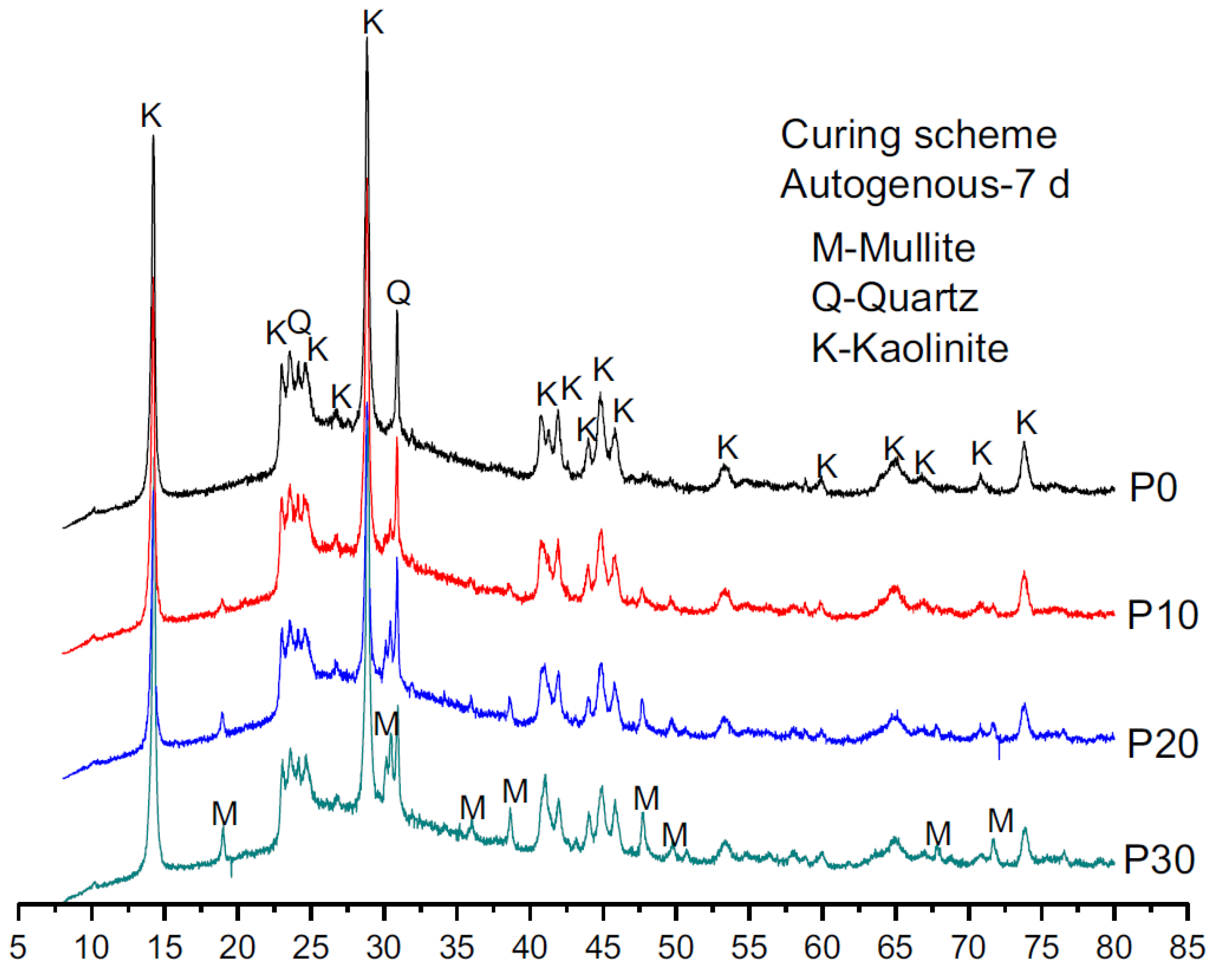
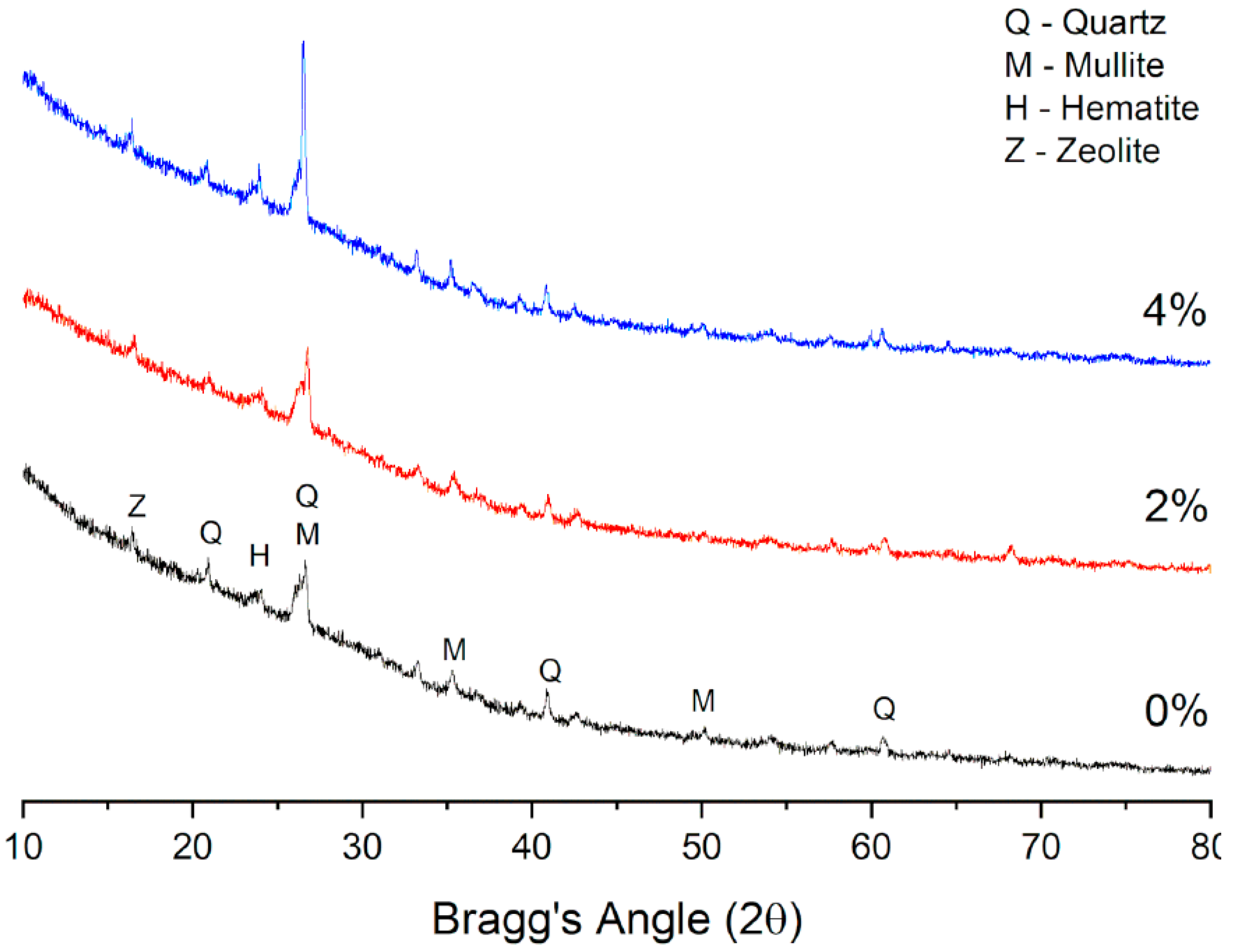
5.1. XRD
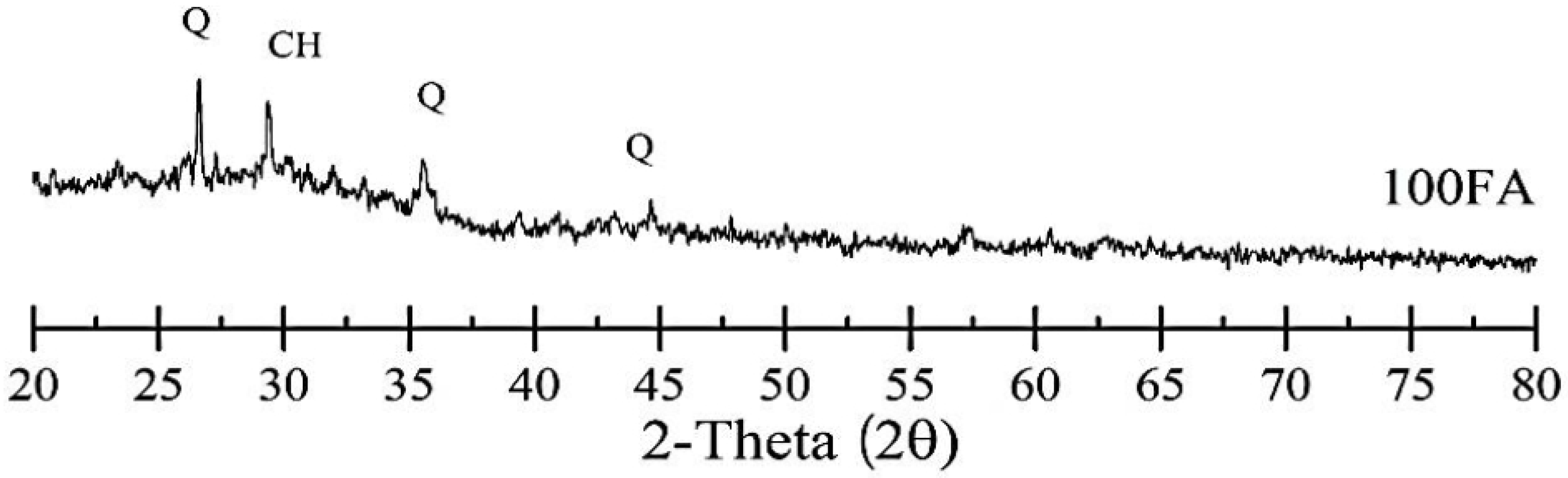
5.1.1. FA-Based Geopolymer Binder
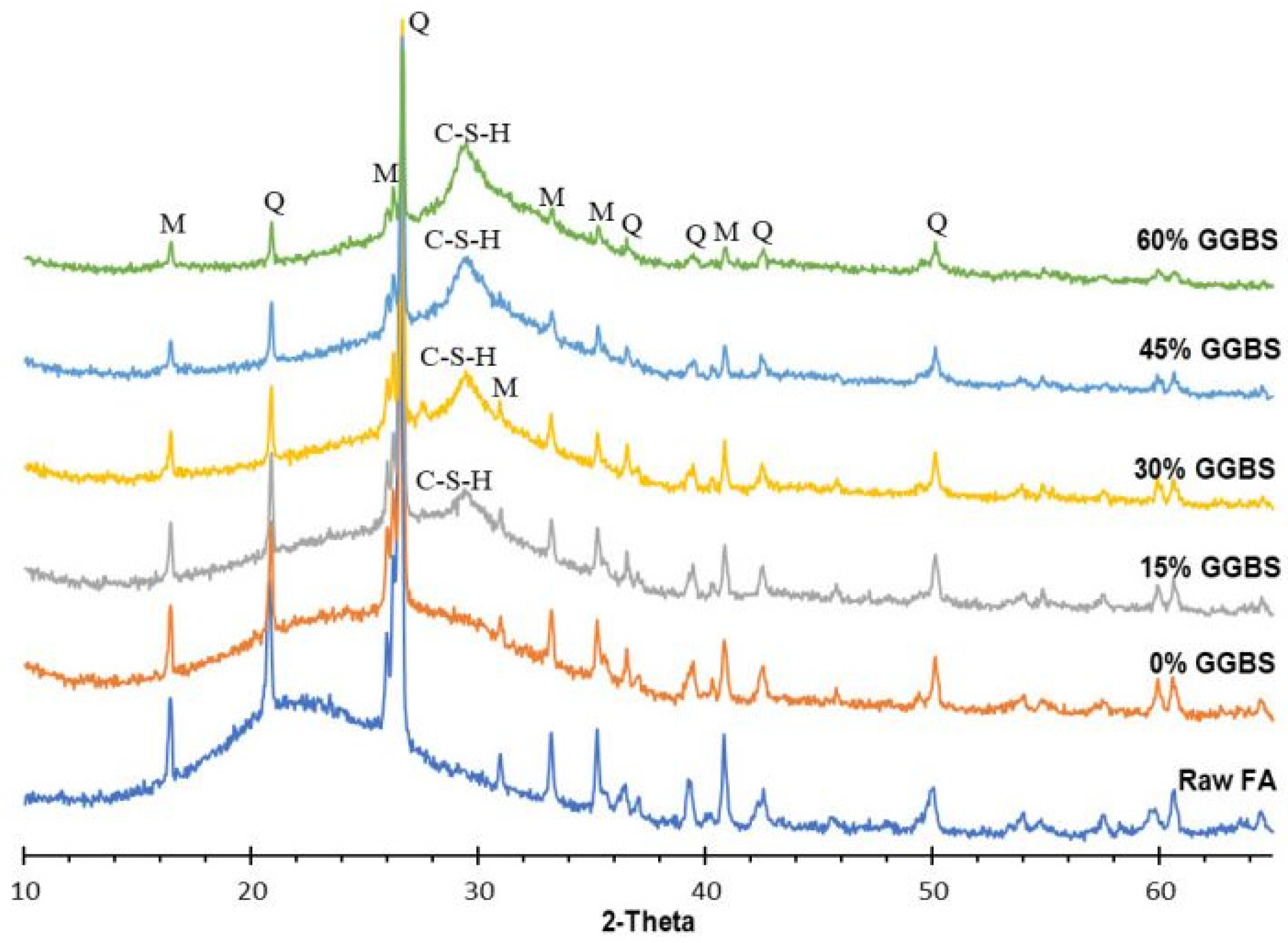
5.1.2. FA-GGBFS-Based Geopolymer Binder
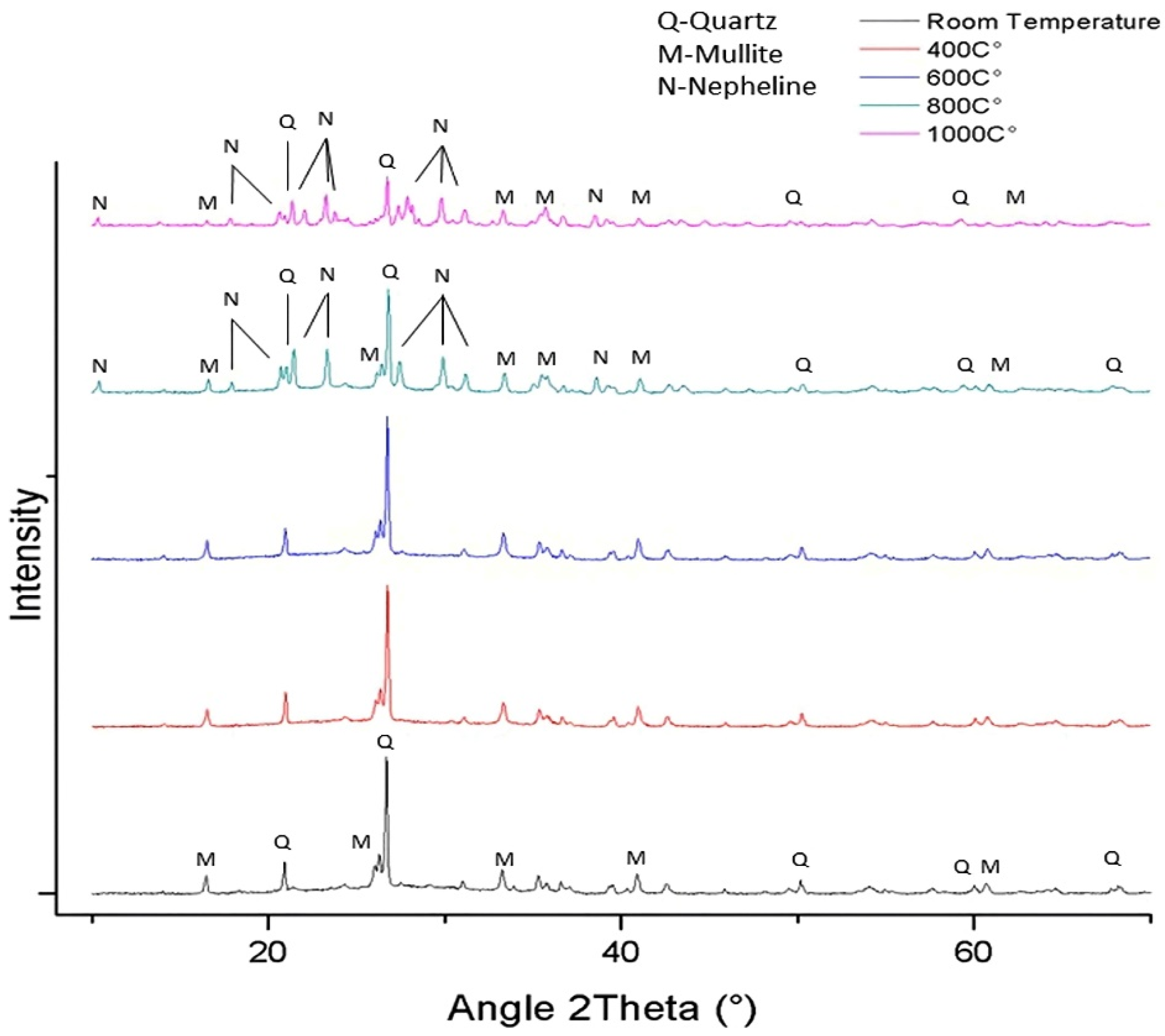
5.1.3. FA-RM-Based Geopolymer Binder
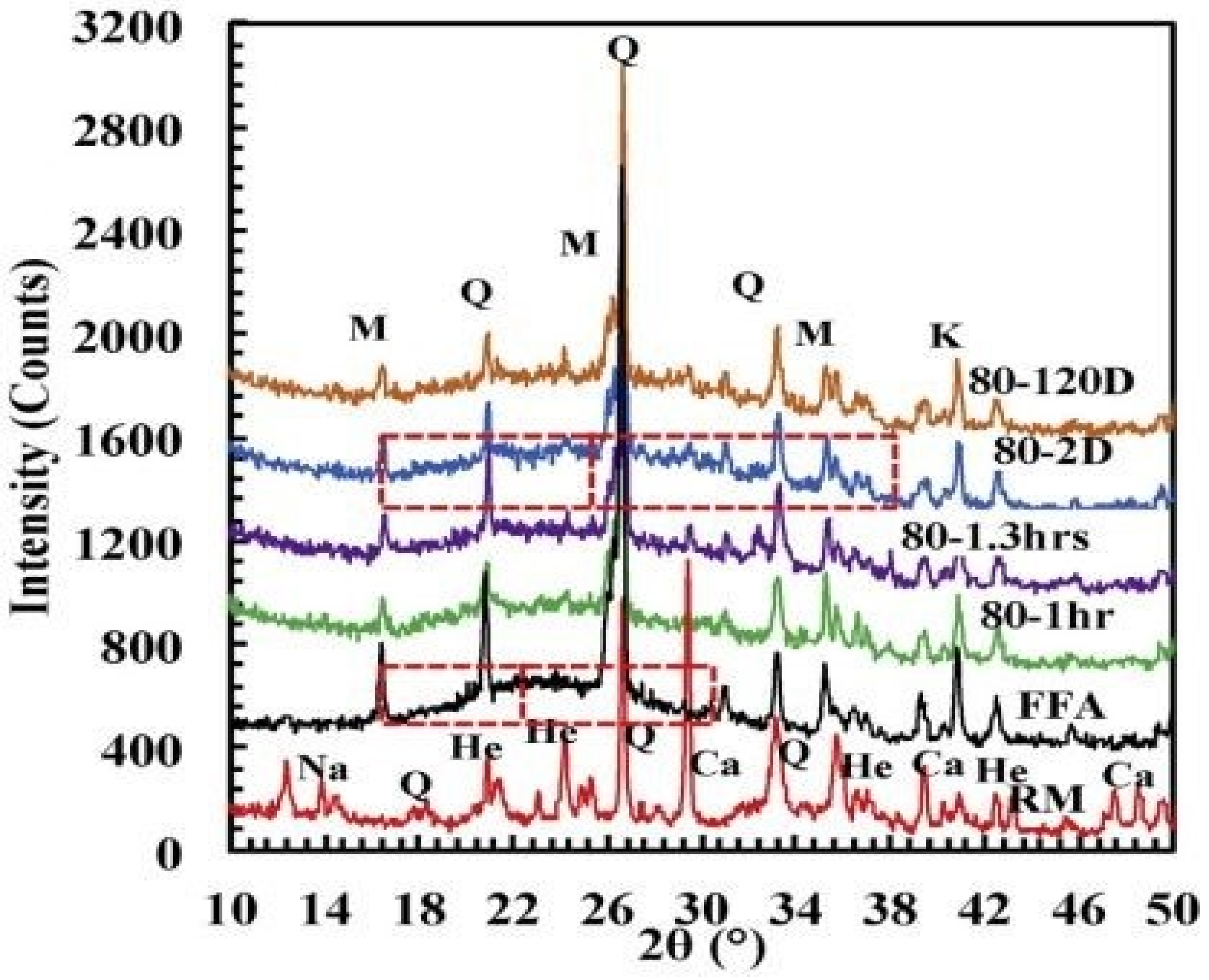
5.1.4. FA-IOT-Based Geopolymer Binder
5.1.5. FCA-FA-Based Geopolymer Binder
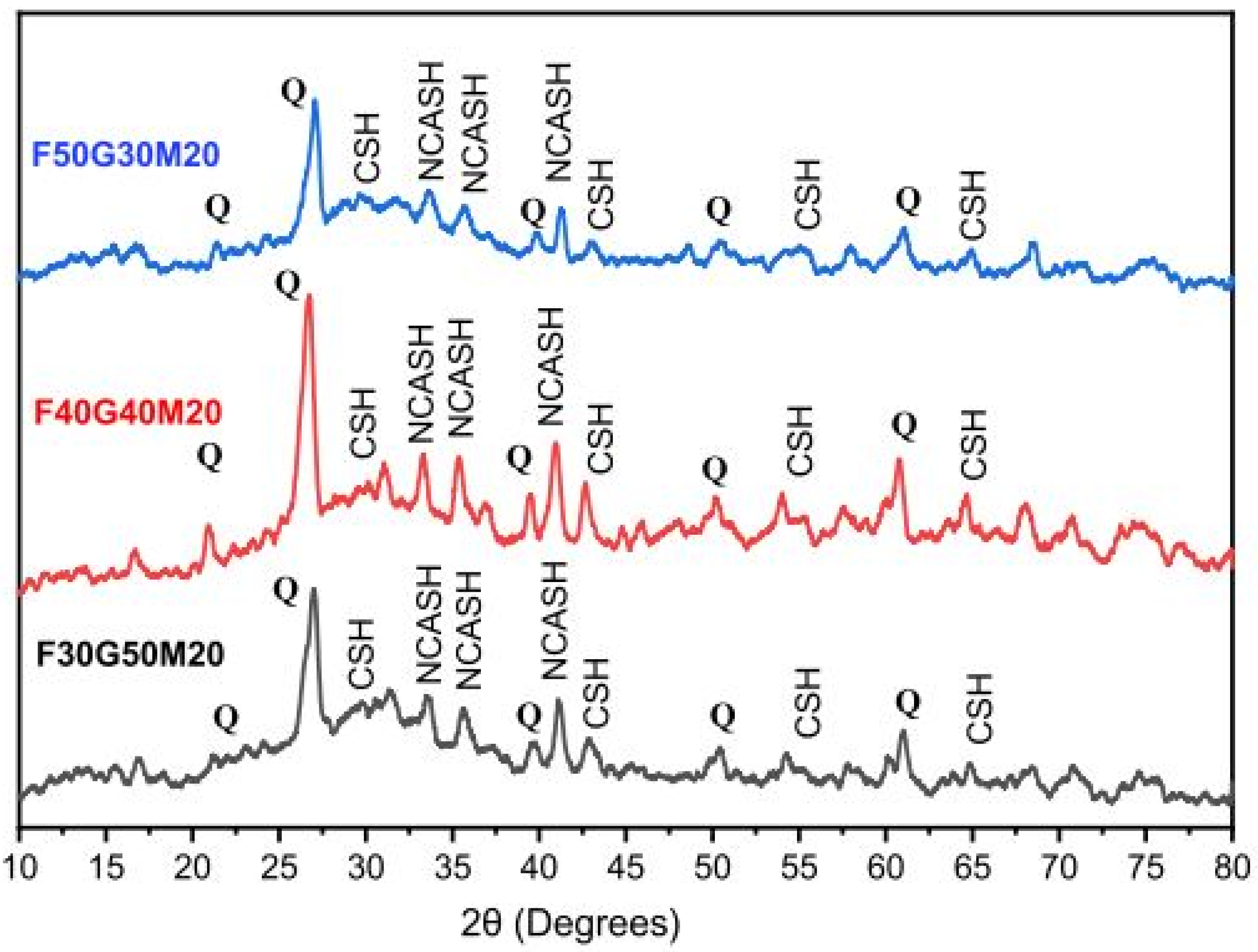
5.1.6. FA-MK-Based Geopolymer Binder
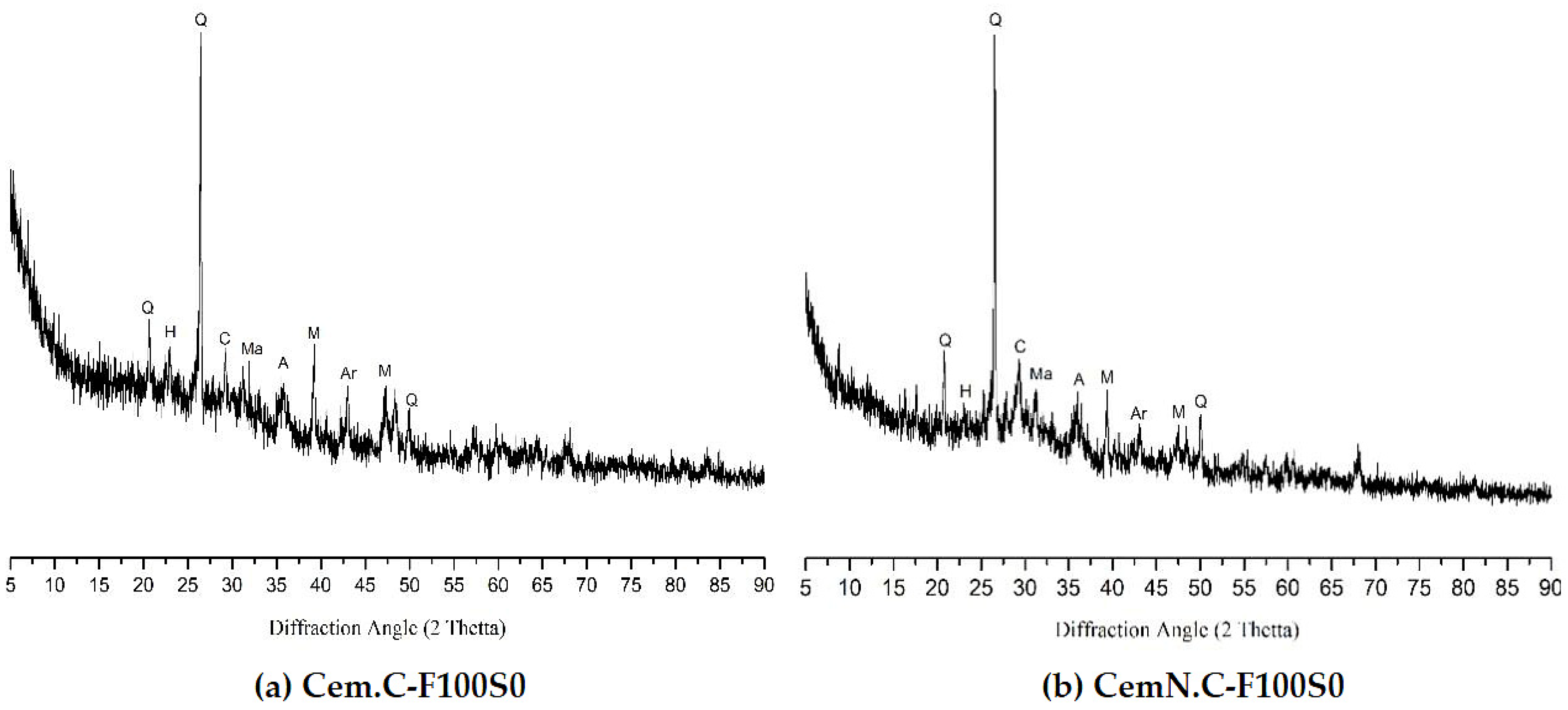
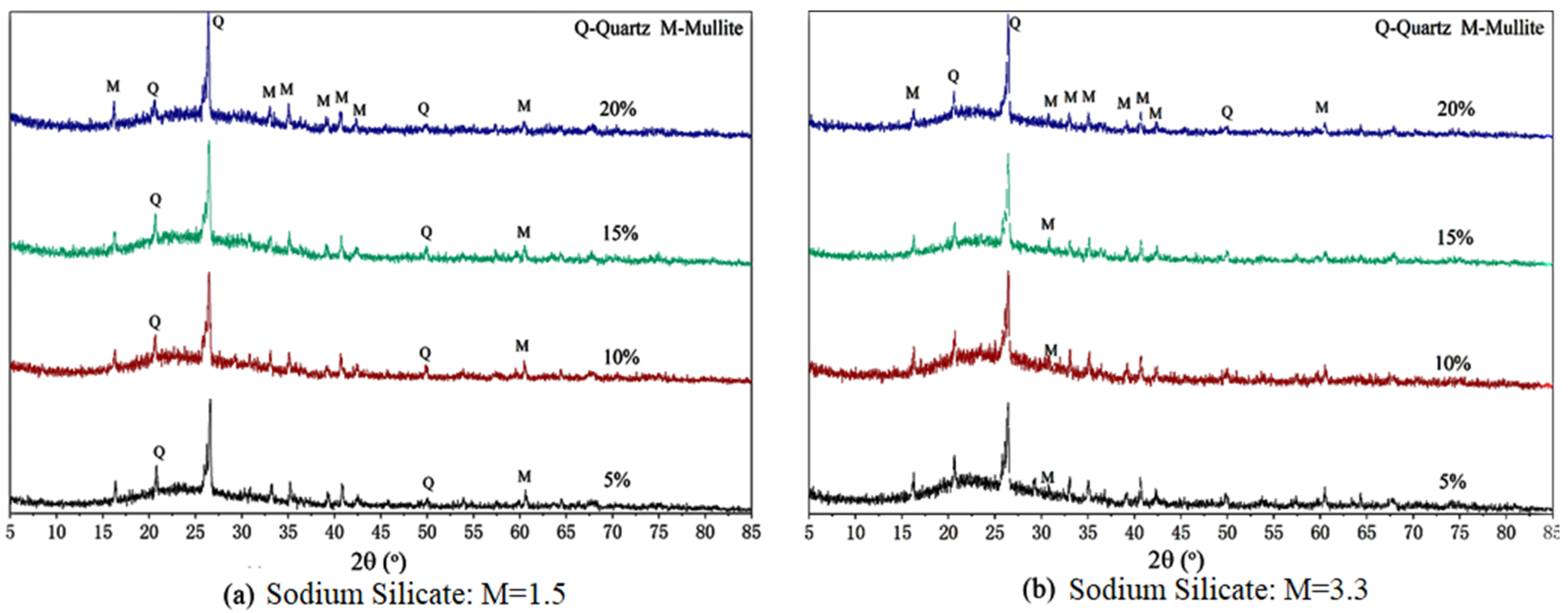
5.1.7. FA-SF-Based Geopolymer Binder
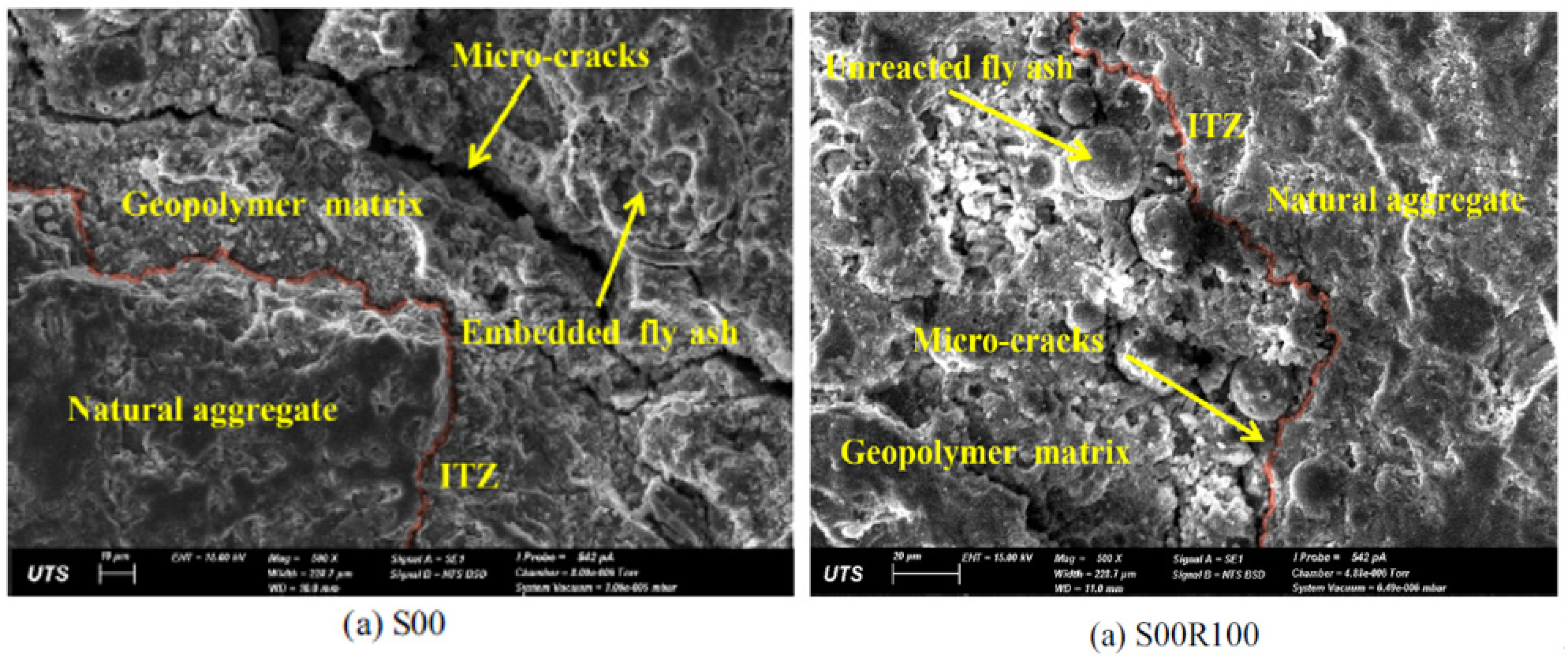
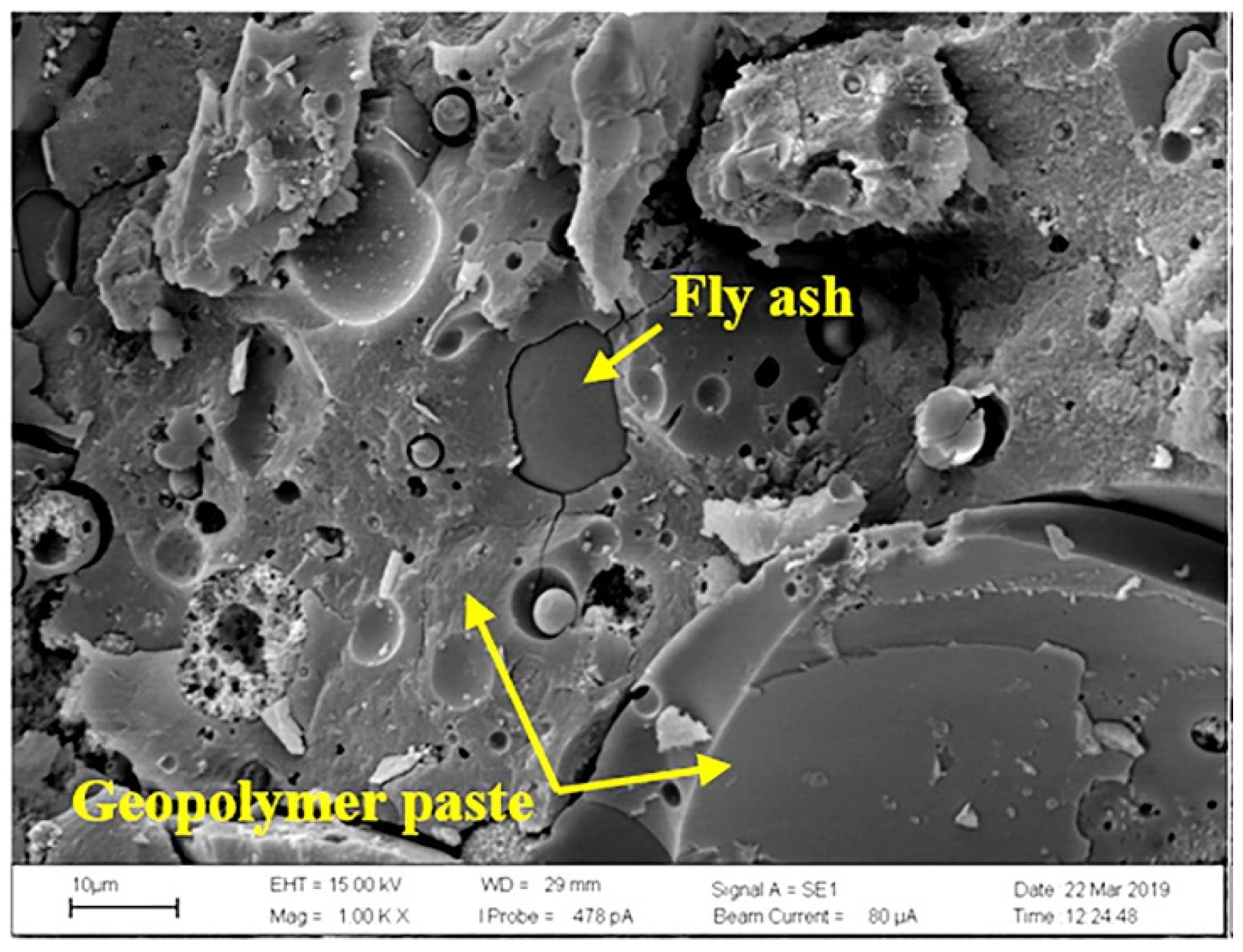
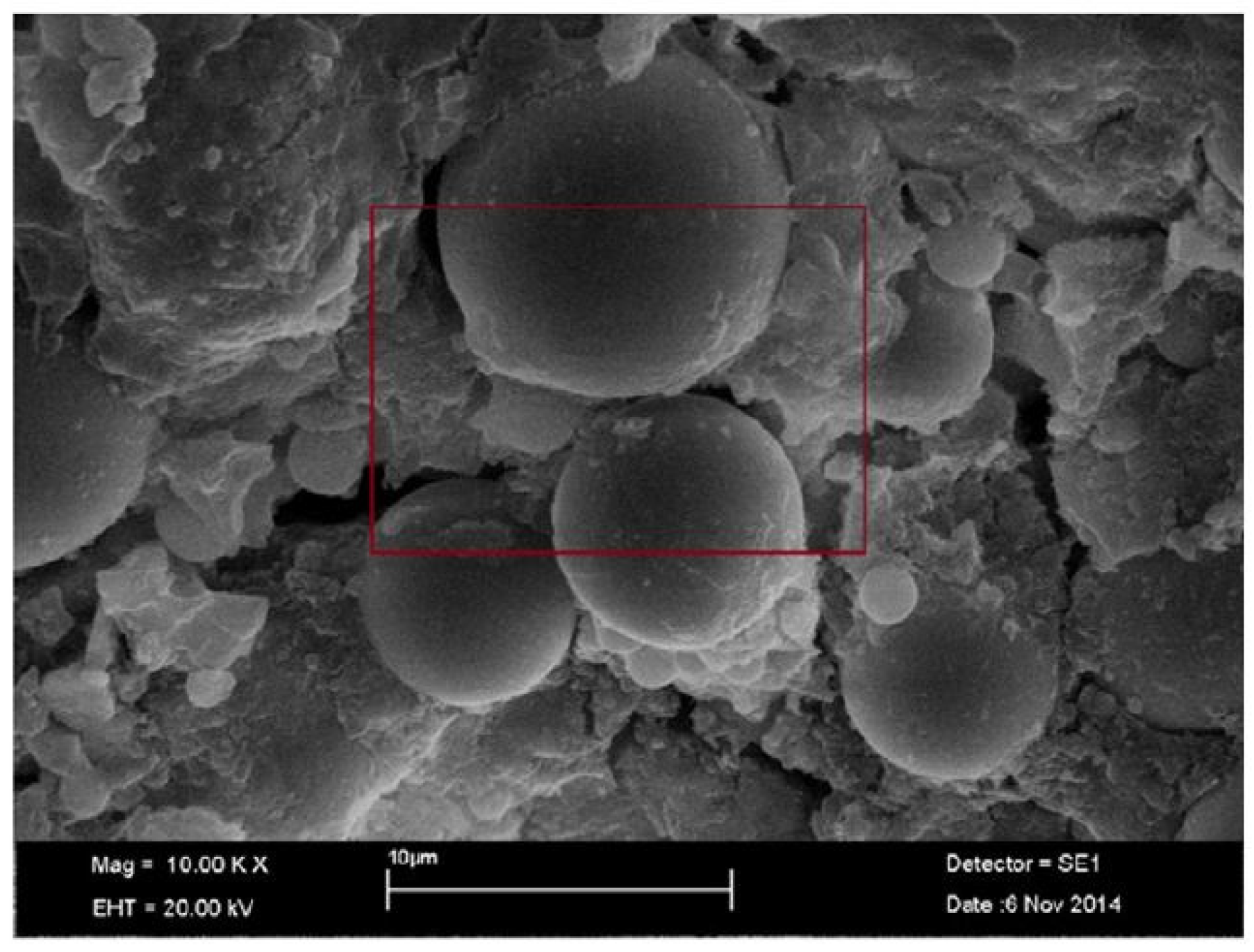
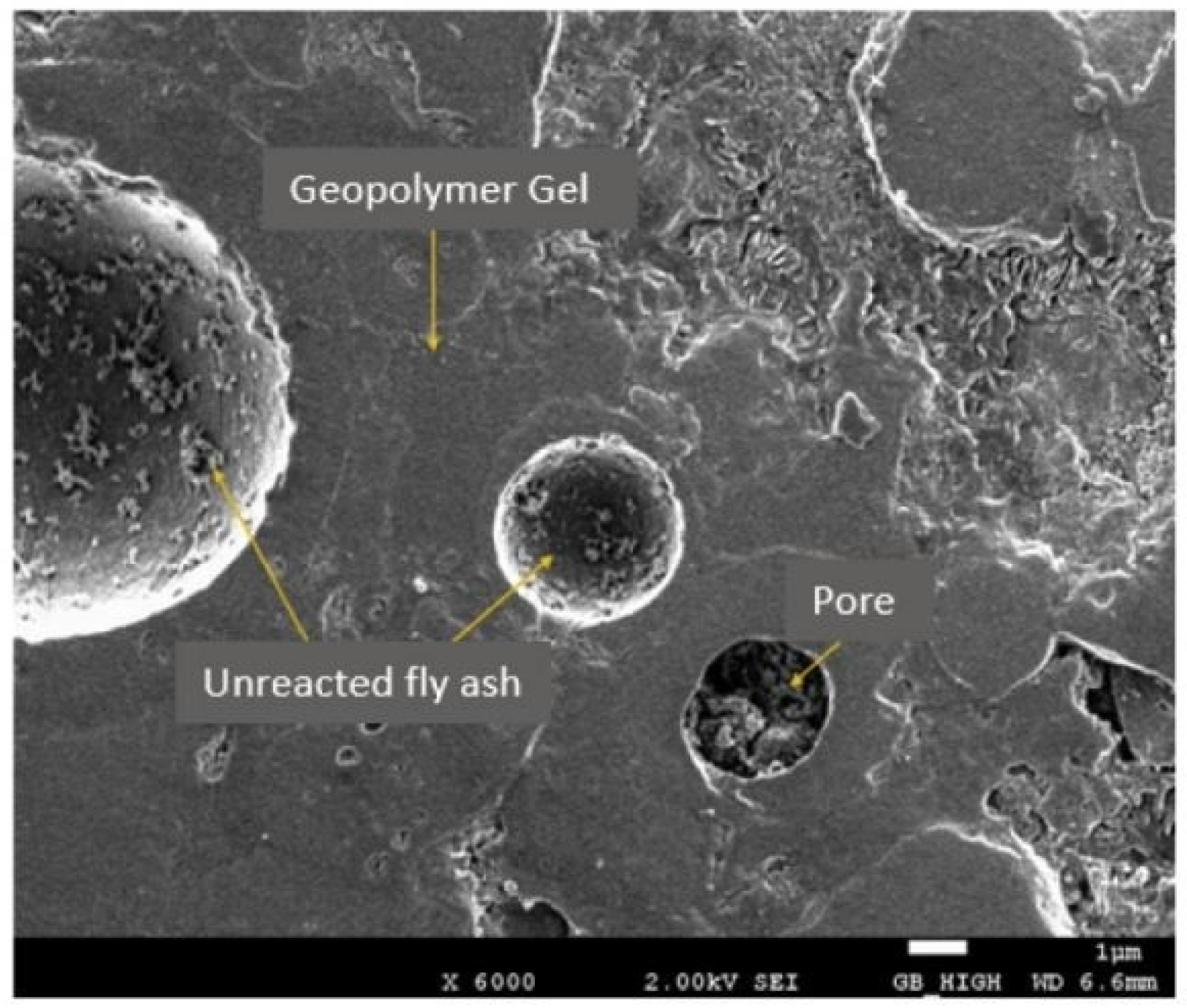
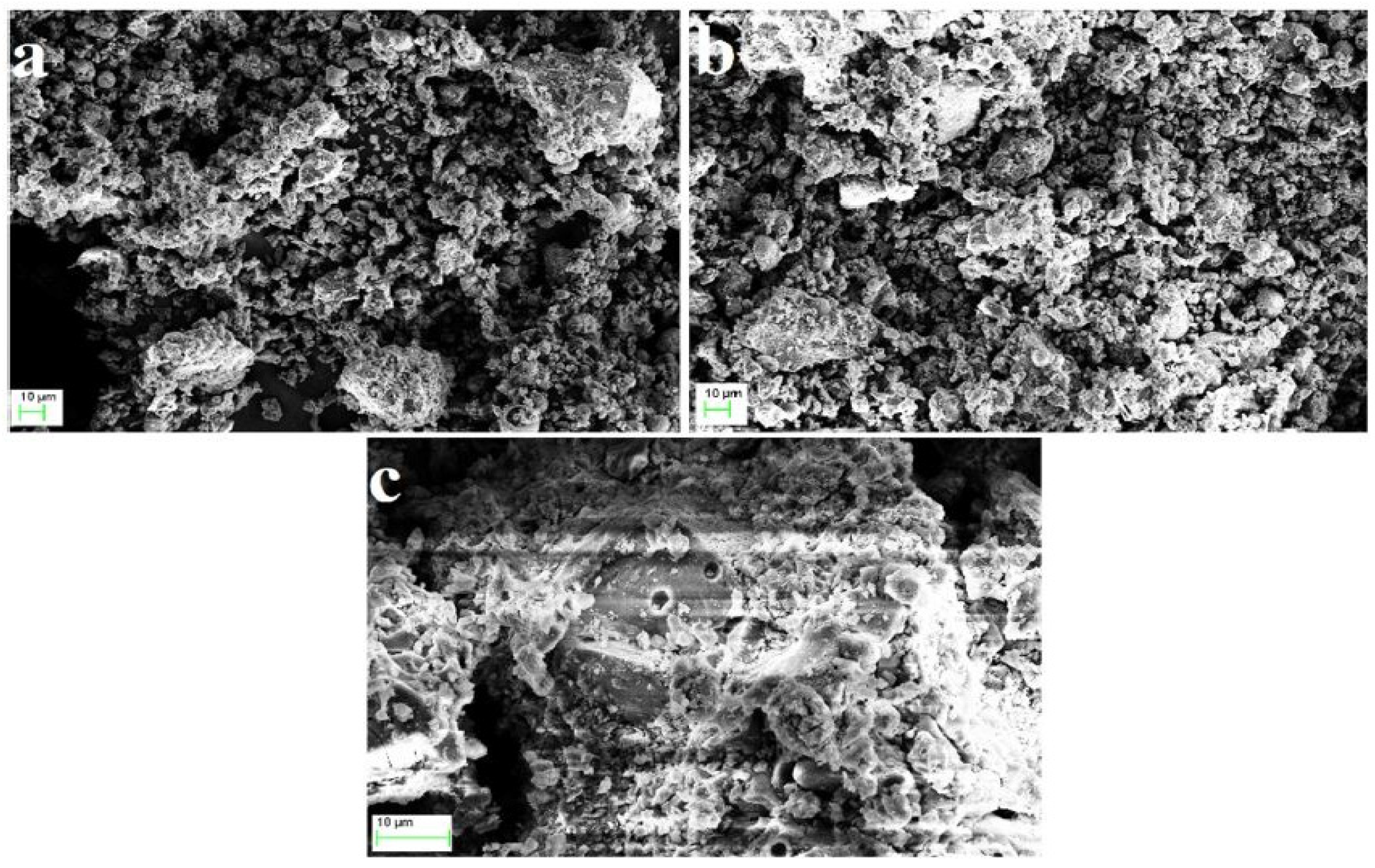
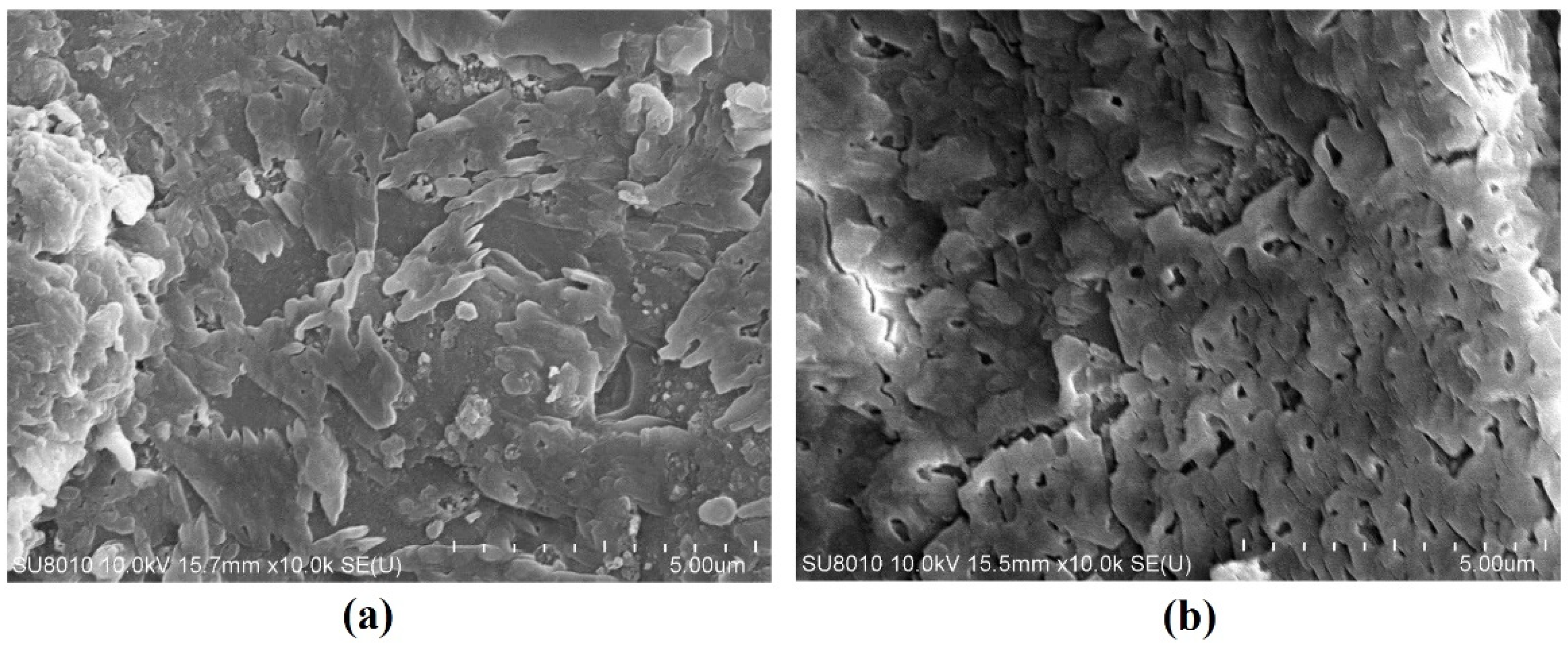
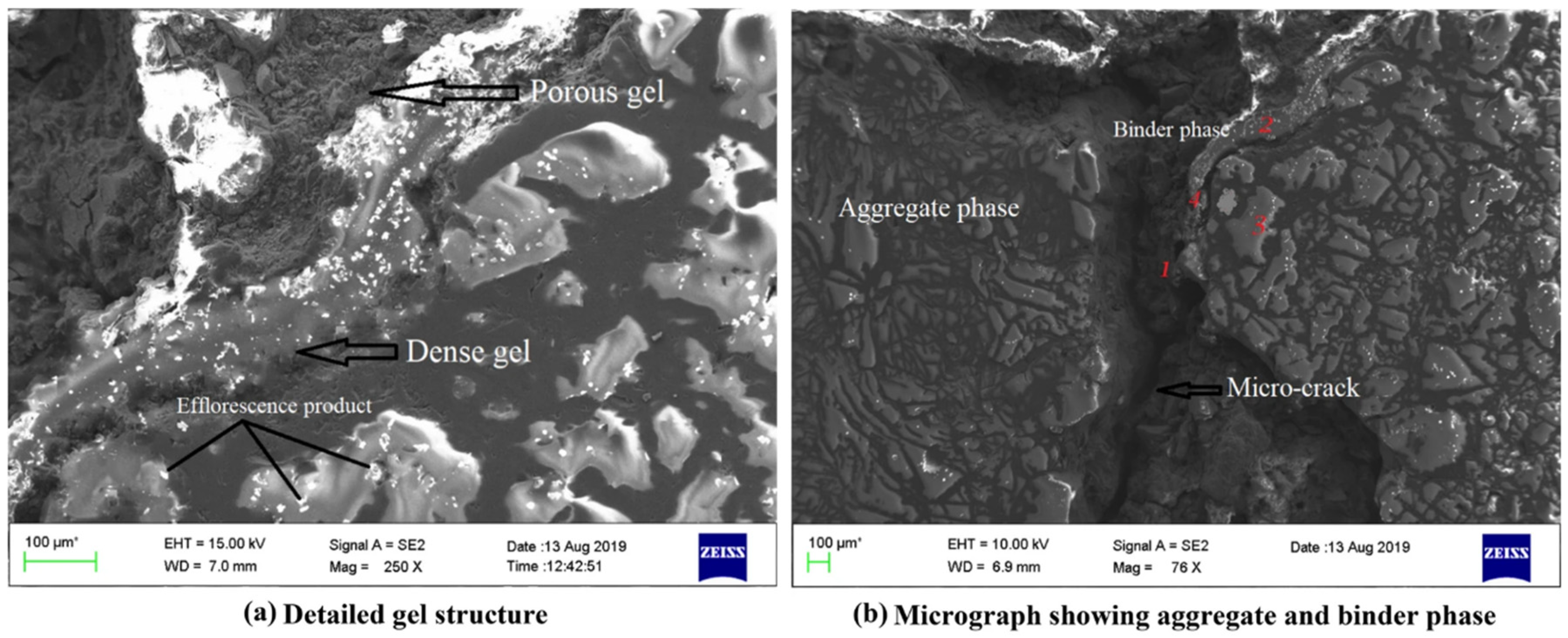
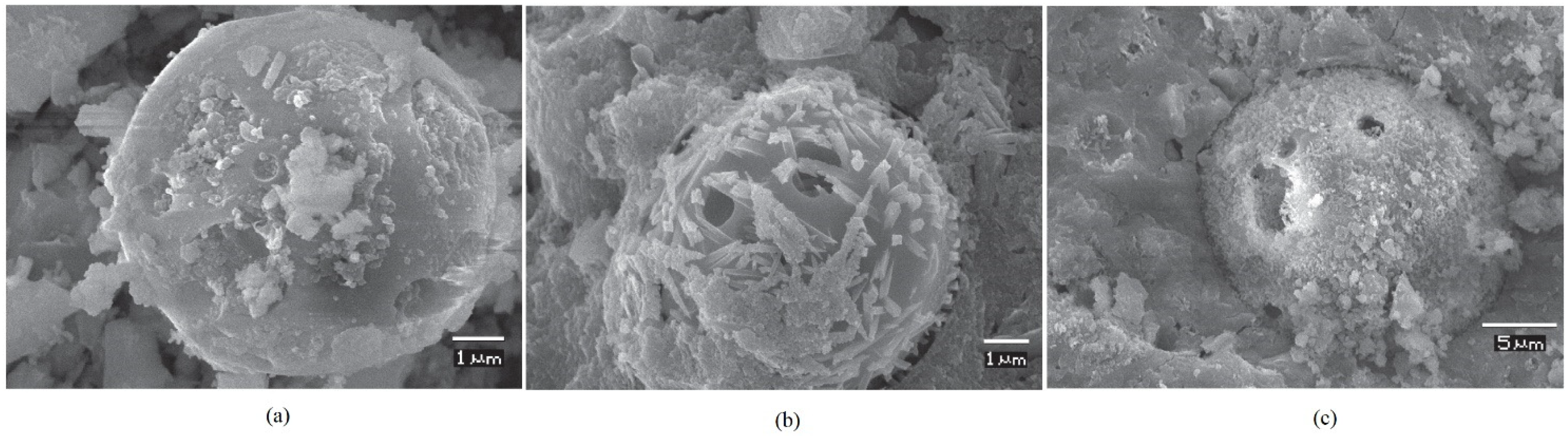
5.2. SEM
5.2.1. FA-Based Geopolymer Binder
5.2.2. FA-GGBFS-Based Geopolymer Binder
5.2.3. FA-RM-Based Geopolymer Binder
5.2.4. IOT-FA-Based Geopolymer Binder
5.2.5. FA-FCA-Based Geopolymer Binder
5.2.6. FA-MK-Based Geopolymer Binder
5.2.7. FA-SF-Based Geopolymer Binder
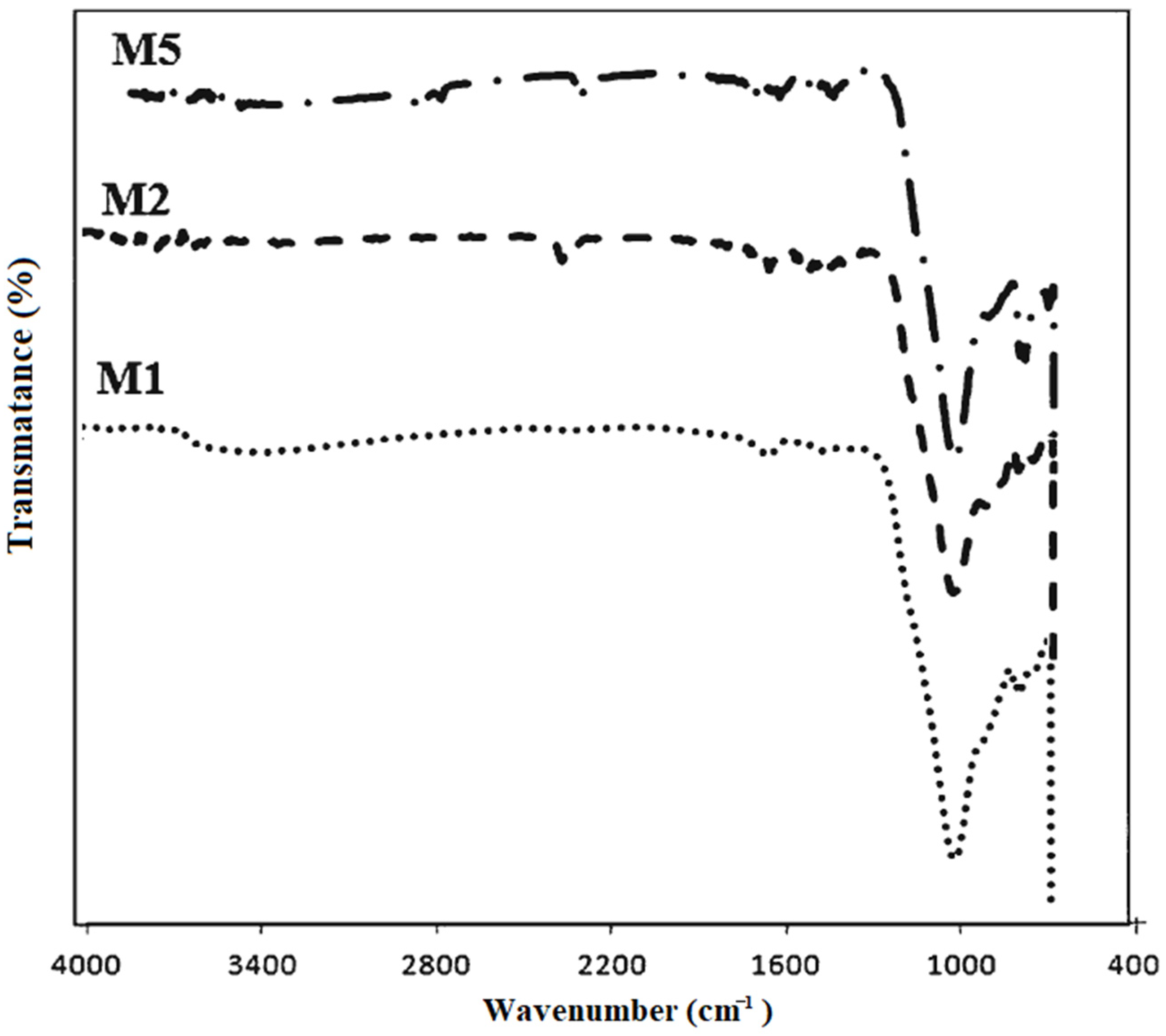
5.3. FTIR
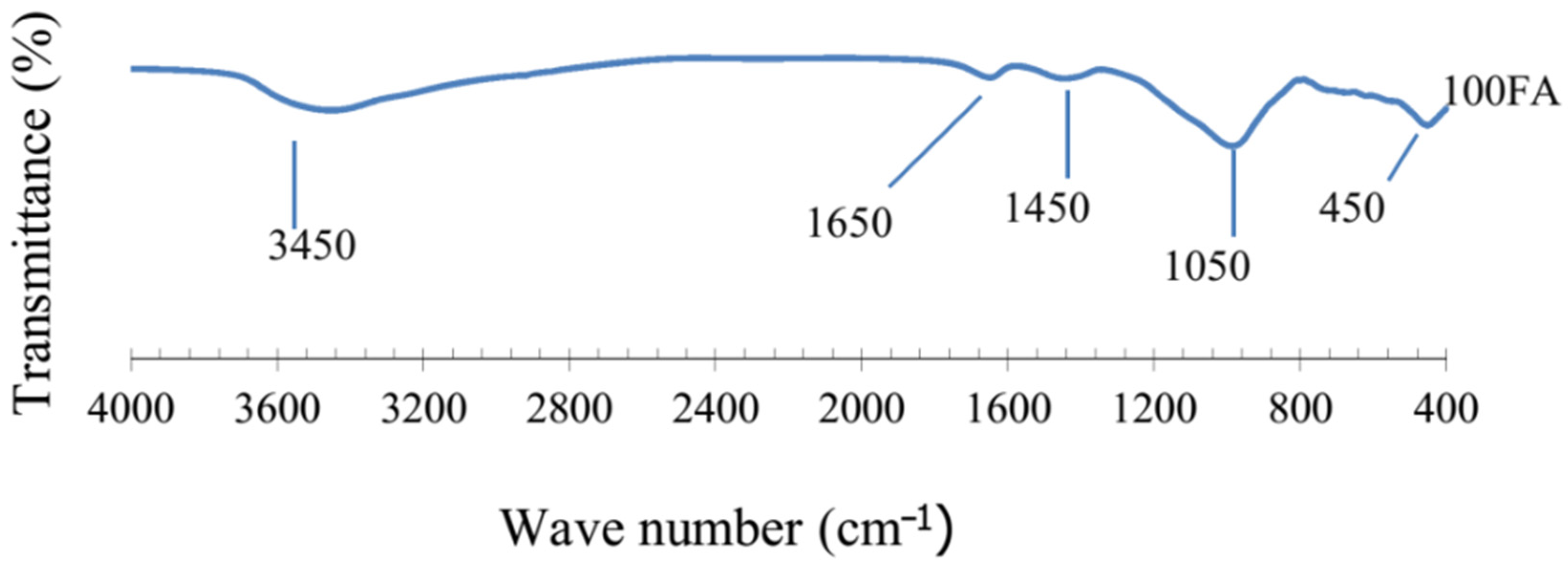
5.3.1. FA-Based Geopolymer Binder
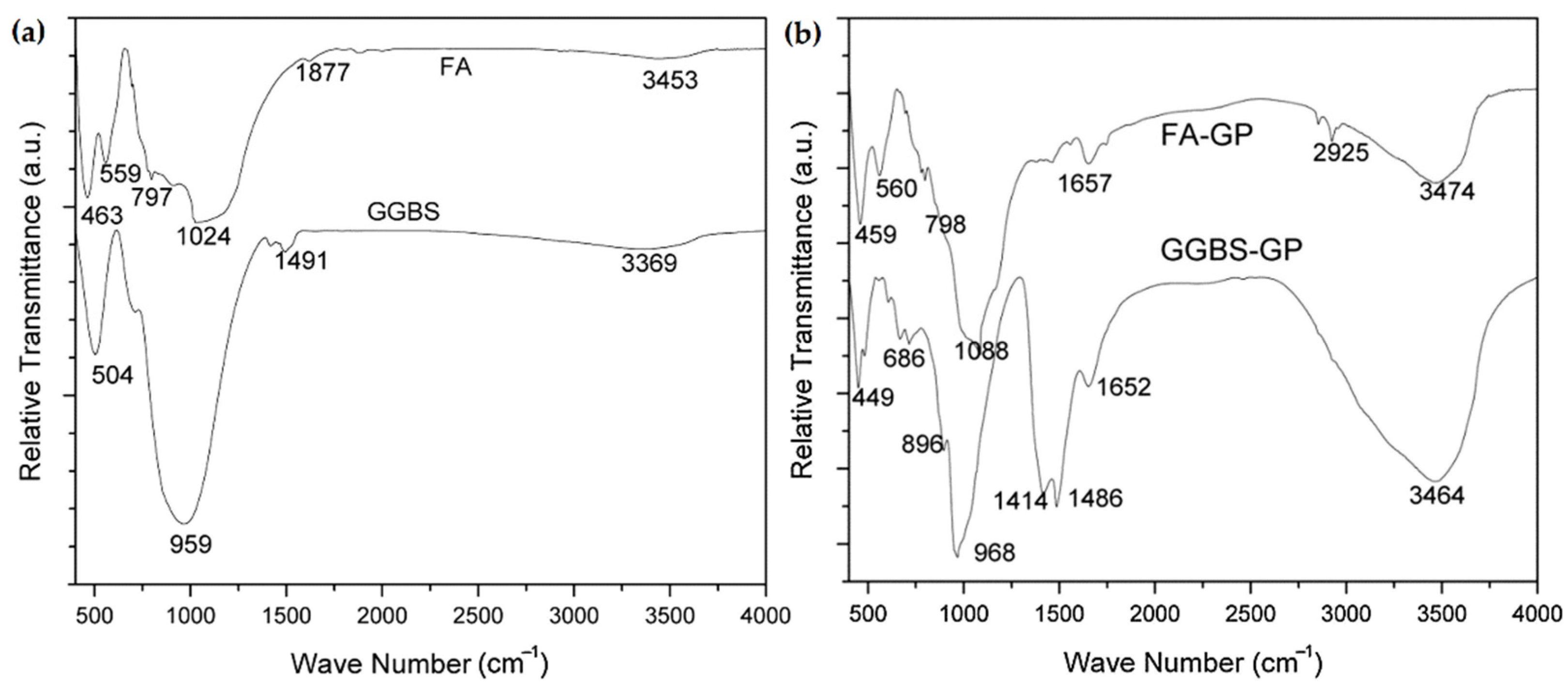
5.3.2. FA-GGBFS-Based Geopolymer Binder
5.3.3. FA-RM-Based Geopolymer Binder
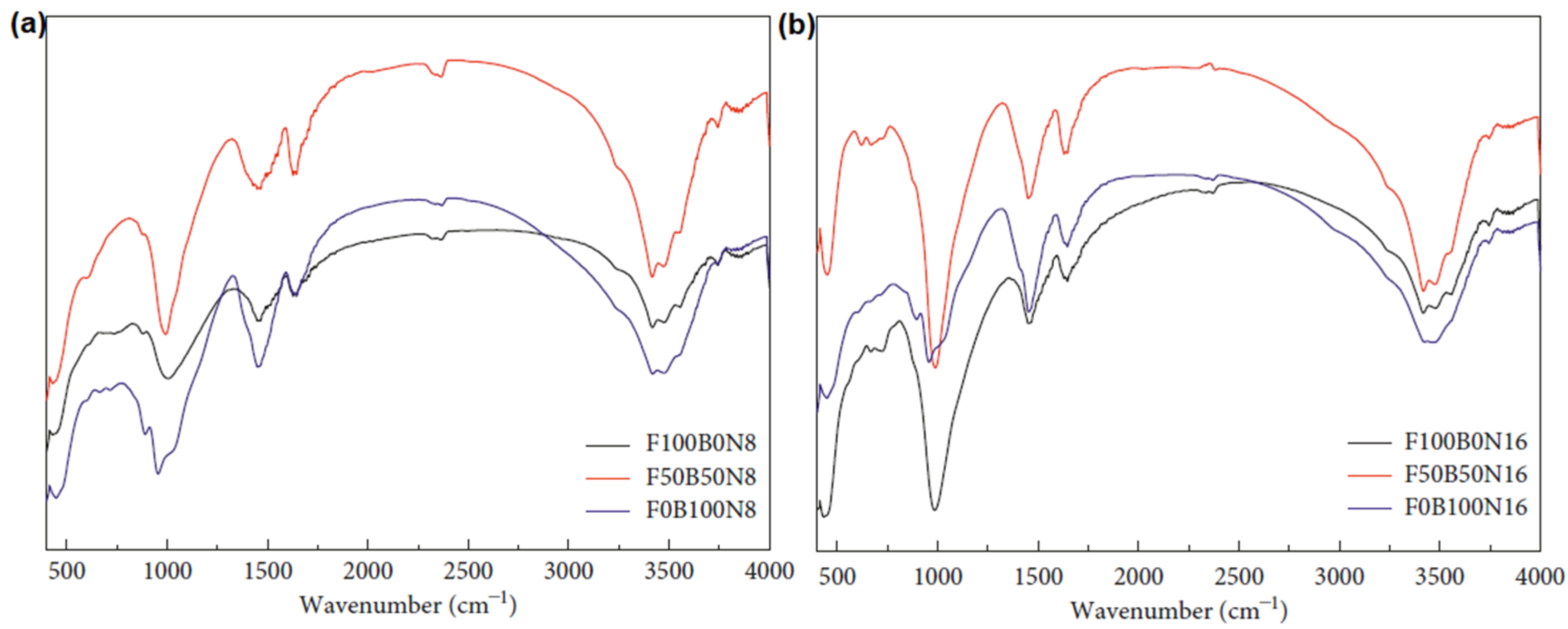
5.3.4. FA-IOT-Based Geopolymer Binder
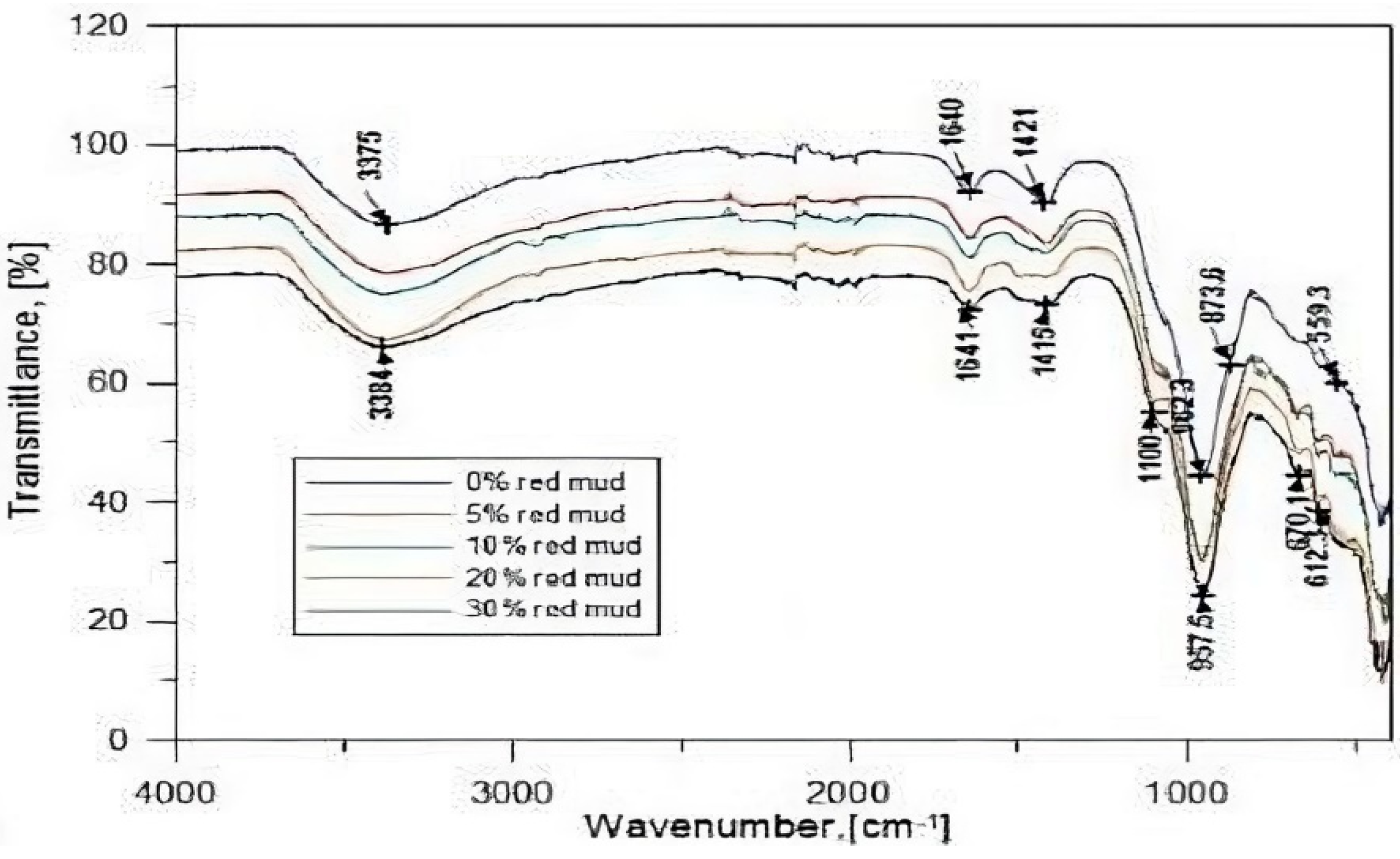
5.3.5. FA-FCABased Geopolymer Binder
5.3.6. FA-MK-Based Geopolymer Binder
5.3.7. FA-SF-Based Geopolymer Binder
6. Discussion
7. Outlook on the Utilization of FA-Based Geopolymer Binders
8. Conclusions
- Primarily, the chemical compositions of the FA, esp. SiO2, Al2O3, CaO, Fe2O3, and MgO, play a significant role in the development of geopolymer microstructure.
- The formation of geopolymer network (Si-O-Al), along with C-A-S-H/N-A-S-H/M-A-S-H/K-A-S-H gels and several other additional/new mineral compounds, significantly influence the compressive strength and microstructure properties of the FA based geopolymer binders.
- Advanced analyzing techniques, such as XRD and FTIR, are necessary to understand the complex chemical mechanism that facilitates geopolymerization.
- It could be stated that owing to the excellent geopolymerization behavior, the RM, GGBFS, IOT, FCA, MK, and SF combined with FA could be used as an alternative binder to conventional cement-based binders.
- As geopolymer binders involve different chemistry, compared to cement-water interaction, analyzing microstructure analysis and compressive strength properties provides us with a complete understanding to further address the research gaps and, thereby, improve the already developed FA-based geopolymer binders.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FA | Fly Ash |
| GGBFS | Ground Granulated Blast Furnace Slag |
| RM | Red Mud |
| IOT | Iron Ore Tailings |
| FCA | Ferrochrome Ash |
| MK | Metakaolin |
| SF | Silica Fume |
| XRD | X-ray Diffraction |
| SEM | Scanning electron Microscope |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| RCA | Recycled Coarse Aggregates |
| M | Mullite |
| Q | Quartz |
| K | Kaolinite |
| Na | Nacrite |
| He | Hematite |
| Ca | Calcite |
| ITZ | Interfacial Transition Zone |
| FFA | Class F Fly Ash |
References
- Akbar, A.; Farooq, F.; Shafique, M.; Aslam, F.; Alyousef, R.; Alabduljabbar, H. Sugarcane Bagasse Ash-Based Engineered Geopolymer Mortar Incorporating Propylene Fibers. J. Build. Eng. 2021, 33, 101492. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, A. Geopolymer Cement: An Initiative towards the Replacement of Grey Cement by Green Cement in Future. J. Build. Mater. Struct. 2021, 8, 1–8. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, H.; Xu, M.; Cui, X. Preparation, Characterization and Application of Geopolymer-Based Tubular Inorganic Membrane. Appl. Clay Sci. 2021, 203, 106001. [Google Scholar] [CrossRef]
- Guo, H.; Yuan, P.; Zhang, B.; Wang, Q.; Deng, L.; Liu, D. Realization of High-Percentage Addition of Fly Ash in the Materials for the Preparation of Geopolymer Derived from Acid-Activated Metakaolin. J. Clean. Prod. 2021, 285, 125430. [Google Scholar] [CrossRef]
- Saranya, P.; Nagarajan, P.; Shashikala, A.P. Experimental Investigation on Bond Strength Properties of Geopolymer Concrete. In Proceedings of the Lecture Notes in Civil Engineering; Springer: Singapore, 2021; Volume 83, pp. 731–740. [Google Scholar]
- Amri, A.; Najib, A.A.; Olivia, M.; Altarawneh, M.; Syam, A.; Rahman, M.M.; Saputro, S.; Wahyuadi, J.; Jiang, Z.T. Physicochemical Properties of Geopolymer Composites with DFT Calculations of In-Situ Reduction of Graphene Oxide. Ceram. Int. 2021, 47, 13440–13445. [Google Scholar] [CrossRef]
- Ongpeng, J.M.C.; Guades, E.J.; Promentilla, M.A.B. Cross-Organizational Learning Approach in the Sustainable Use of Fly Ash for Geopolymer in the Philippine Construction Industry. Sustainability 2021, 13, 2454. [Google Scholar] [CrossRef]
- Almalkawi, A.T.; Balchandra, A.; Soroushian, P. Potential of Using Industrial Wastes for Production of Geopolymer Binder as Green Construction Materials. Constr. Build. Mater. 2019, 220, 516–524. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.; Sumajouw, D.M.J.; Rangan, B.V. Brief Review of Development of Geopolymer. In Proceedings of the 8th CANMET/ACI International Conference on Fly Ash, Silica Fume, Slag and Natural Pozzolans in Conerete, Las Vegas, NV, USA, 23–29 May 2004; pp. 1–10. [Google Scholar]
- Bernal, S.A.; San Nicolas, R.; Myers, R.J.; Mejía De Gutiérrez, R.; Puertas, F.; Van Deventer, J.S.J.; Provis, J.L. MgO Content of Slag Controls Phase Evolution and Structural Changes Induced by Accelerated Carbonation in Alkali-Activated Binders. Cem. Concr. Res. 2014, 57, 33–43. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, S.; Yang, J.; Chen, Y.; Ye, N.; Ke, Y.; Tao, S.; Xiao, K.; Hu, J.; Hou, H.; et al. Role of Fe Species in Geopolymer Synthesized from Alkali-Thermal Pretreated Fe-Rich Bayer Red Mud. Constr. Build. Mater. 2019, 200, 398–407. [Google Scholar] [CrossRef]
- Liu, M.; Wu, H.; Yao, P.; Wang, C.; Ma, Z. Microstructure and Macro Properties of Sustainable Alkali-Activated Fly Ash Mortar with Various Construction Waste Fines as Binder Replacement up to 100%. Cem. Concr. Compos. 2022, 134, 104733. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S. Development of Paving Blocks from Synergistic Use of Red Mud and Fly Ash Using Geopolymerization. Constr. Build. Mater. 2013, 38, 865–871. [Google Scholar] [CrossRef]
- Okoye, F.N.; Durgaprasad, J.; Singh, N.B. Effect of Silica Fume on the Mechanical Properties of Fly Ash Based-Geopolymer Concrete. Ceram. Int. 2016, 42, 3000–3006. [Google Scholar] [CrossRef]
- Cristelo, N.; Segadães, L.; Coelho, J.; Chaves, B.; Sousa, N.R.; de Lurdes Lopes, M. Recycling Municipal Solid Waste Incineration Slag and Fly Ash as Precursors in Low-Range Alkaline Cements. Waste Manag. 2020, 104, 60–73. [Google Scholar] [CrossRef]
- Cristelo, N.; Coelho, J.; Miranda, T.; Palomo, Á.; Fernández-Jiménez, A. Alkali Activated Composites–An Innovative Concept Using Iron and Steel Slag as Both Precursor and Aggregate. Cem. Concr. Compos. 2019, 103, 11–21. [Google Scholar] [CrossRef]
- Walkley, B.; San Nicolas, R.; Sani, M.-A.; Rees, G.J.; Hanna, J.V.; Van Deventer, J.S.J.; Provis, J.L. Phase Evolution of C-(A)-S-H/N-A-S-H Gel Blends Investigated via Alkali-Activation of Synthetic Calcium Aluminosilicate Precursors. Elsevier 2016, 89, 120–135. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers-Inorganic Polymeric New Materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Zhang, W.; Li, Z.; Zhang, Y.; Li, Y.; Ren, Y. Effects of Si/Al Ratio on the Efflorescence and Properties of Fly Ash Based Geopolymer. J. Clean. Prod. 2020, 244, 118852. [Google Scholar] [CrossRef]
- Bellum, R.R.; Muniraj, K.; Indukuri, C.S.R.; Madduru, S.R.C. Investigation on Performance Enhancement of Fly Ash-GGBFS Based Graphene Geopolymer Concrete. J. Build. Eng. 2020, 32, 101659. [Google Scholar] [CrossRef]
- Kuranchie, F.A.; Shukla, S.K.; Habibi, D. Utilisation of Iron Ore Mine Tailings for the Production of Geopolymer Bricks. Int. J. Min. Reclam. Environ. 2016, 30, 92–114. [Google Scholar] [CrossRef]
- Zhou, W.; Shi, X.; Lu, X.; Qi, C.; Luan, B.; Liu, F. The Mechanical and Microstructural Properties of Refuse Mudstone-GGBS-Red Mud Based Geopolymer Composites Made with Sand. Constr. Build. Mater. 2020, 253, 119193. [Google Scholar] [CrossRef]
- Mishra, J.; Kumar Das, S.; Krishna, R.S.; Nanda, B.; Kumar Patro, S.; Mohammed Mustakim, S. Synthesis and Characterization of a New Class of Geopolymer Binder Utilizing Ferrochrome Ash (FCA) for Sustainable Industrial Waste Management. Mater. Today Proc. 2020, 33, 5001–5006. [Google Scholar] [CrossRef]
- Chen, K.; Wu, D.; Yi, M.; Cai, Q.; Zhang, Z. Mechanical and Durability Properties of Metakaolin Blended with Slag Geopolymer Mortars Used for Pavement Repair. Constr. Build. Mater. 2021, 281, 122566. [Google Scholar] [CrossRef]
- Jena, S.; Panigrahi, R.; Sahu, P. Effect of Silica Fume on the Properties of Fly Ash Geopolymer Concrete. In Sustainable Construction and Building Materials. Lecture Notes in Civil Engineering; Springer: Singapore, 2019; pp. 145–153. [Google Scholar]
- Mallikarjuna Rao, G.; Gunneswara Rao, T.D. Final Setting Time and Compressive Strength of Fly Ash and GGBS-Based Geopolymer Paste and Mortar. Arab. J. Sci. Eng. 2015, 40, 3067–3074. [Google Scholar] [CrossRef]
- Gunasekara, C.; Law, D.W.; Setunge, S. Long Term Permeation Properties of Different Fly Ash Geopolymer Concretes. Constr. Build. Mater. 2016, 124, 352–362. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; van Deventer, J.S.J. Characterisation of One-Part Geopolymer Binders Made from Fly Ash. Waste Biomass Valorization 2017, 8, 225–233. [Google Scholar] [CrossRef]
- Al-mashhadani, M.M.; Canpolat, O.; Aygörmez, Y.; Uysal, M.; Erdem, S. Mechanical and Microstructural Characterization of Fiber Reinforced Fly Ash Based Geopolymer Composites. Constr. Build. Mater. 2018, 167, 505–513. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Ekolu, S.O. Method for Comprehensive Mix Design of Fly Ash Geopolymer Mortars. Constr. Build. Mater. 2019, 202, 704–717. [Google Scholar] [CrossRef]
- Phoo-Ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of Sodium Hydroxide and Sodium Silicate Solutions on Compressive and Shear Bond Strengths of FA-GBFS Geopolymer. Constr. Build. Mater. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- Jawahar, J.G.; Mounika, G. Strength Properties of Fly Ash and GGBS Based Geopolymer Concrete. Asian J. Civ. Eng 2016, 17, 127–135. [Google Scholar]
- Palankar, N.; Ravi Shankar, A.U.; Mithun, B.M. Investigations on Alkali-Activated Slag/Fly Ash Concrete with Steel Slag Coarse Aggregate for Pavement Structures. Int. J. Pavement Eng. 2017, 18, 500–512. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S.P. Synthesis of Fly Ash-GGBS-Blended Geopolymer Composits; Springer: Singapore, 2019; Volume 16, ISBN 9789811308994. [Google Scholar]
- Shahmansouri, A.A.; Akbarzadeh Bengar, H.; Ghanbari, S. Compressive Strength Prediction of Eco-Efficient GGBS-Based Geopolymer Concrete Using GEP Method. J. Build. Eng. 2020, 31, 101326. [Google Scholar] [CrossRef]
- McDonald, J.E.D.; Roache, S.C.; Kawatra, S.K. Repurposing Mine Tailings: Cold Bonding of Siliceous Iron Ore Tailings. Miner. Metall. Process. 2016, 33, 47–52. [Google Scholar] [CrossRef]
- Tian, Z.X.; Zhao, Z.H.; Dai, C.Q.; Liu, S.J. Experimental Study on the Properties of Concrete Mixed with Iron Ore Tailings. Adv. Mater. Sci. Eng. 2016, 2016, 8606505. [Google Scholar] [CrossRef]
- Galvão, J.L.B.; Andrade, H.D.; Brigolini, G.J.; Peixoto, R.A.F.; Mendes, J.C. Reuse of Iron Ore Tailings from Tailings Dams as Pigment for Sustainable Paints. J. Clean. Prod. 2018, 200, 412–422. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S. Utilization of Iron Ore Tailing for the Synthesis of Zeolite A by Hydrothermal Method. J. Mater. Cycles Waste Manag. 2018, 20, 1605–1614. [Google Scholar] [CrossRef]
- do Carmo e Silva Defáveri, K.; dos Santos, L.F.; Franco de Carvalho, J.M.; Peixoto, R.A.F.; Brigolini, G.J. Iron Ore Tailing-Based Geopolymer Containing Glass Wool Residue: A Study of Mechanical and Microstructural Properties. Constr. Build. Mater. 2019, 220, 375–385. [Google Scholar] [CrossRef]
- Hairi, S.N.M.; Jameson, G.N.L.; Rogers, J.J.; MacKenzie, K.J.D. Synthesis and Properties of Inorganic Polymers (Geopolymers) Derived from Bayer Process Residue (Red Mud) and Bauxite. J. Mater. Sci. 2015, 50, 7713–7724. [Google Scholar] [CrossRef]
- Singh, S.; Aswath, M.U.; Ranganath, R.V. Durability of Red Mud Based Geopolymer Paste in Acid Solutions. Mater. Sci. Forum 2016, 866, 99–105. [Google Scholar] [CrossRef]
- Hoc Thang, N.; Kien, P.T.; Abdullah, M.M.A.B. Lightweight Heat Resistant Geopolymer-Based Materials Synthesized from Red Mud and Rice Husk Ash Using Sodium Silicate Solution as Alkaline Activator. MATEC Web Conf. 2017, 97, 01119. [Google Scholar] [CrossRef]
- Novais, R.M.; Carvalheiras, J.; Seabra, M.P.; Pullar, R.C.; Labrincha, J.A. Innovative Application for Bauxite Residue: Red Mud-Based Inorganic Polymer Spheres as PH Regulators. J. Hazard. Mater. 2018, 358, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, Y.; Ding, S.; Zhang, H.; Xia, F.; Wang, J.; Zhou, M. Utilization of Red Mud in Geopolymer-Based Pervious Concrete with Function of Adsorption of Heavy Metal Ions. J. Clean. Prod. 2019, 207, 789–800. [Google Scholar] [CrossRef]
- Chethan, K.B.; Yaragal, S.C.; Das, B.B. Ferrochrome Ash–Its Usage Potential in Alkali Activated Slag Mortars. J. Clean. Prod. 2020, 257, 120577. [Google Scholar] [CrossRef]
- Mishra, J.; Das, S.K.; Krishna, R.S.; Nanda, B. Utilization of Ferrochrome Ash as a Source Material for Production of Geopolymer Concrete for a Cleaner Sustainable Environment. Indian Concr. J. 2020, 94, 40–49. [Google Scholar]
- Rovnaník, P.; Šafránková, K. Thermal Behaviour of Metakaolin/Fly Ash Geopolymers with Chamotte Aggregate. Materials 2016, 9, 535. [Google Scholar] [CrossRef]
- Praveen Kumar, V.V.; Prasad, N.; Dey, S. Influence of Metakaolin on Strength and Durability Characteristics of Ground Granulated Blast Furnace Slag Based Geopolymer Concrete. Struct. Concr. 2020, 21, 1040–1050. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W. Compressive Strength and Microstructure of Fly Ash Based Geopolymer Blended with Silica Fume under Thermal Cycle. Cem. Concr. Compos. 2017, 78, 108–119. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Role of Particle Fineness on Engineering Properties and Microstructure of Fly Ash Derived Geopolymer. Constr. Build. Mater. 2020, 233, 117294. [Google Scholar] [CrossRef]
- Zuhua, Z.; Xiao, Y.; Huajun, Z.; Yue, C. Role of Water in the Synthesis of Calcined Kaolin-Based Geopolymer. Appl. Clay Sci. 2009, 43, 218–223. [Google Scholar] [CrossRef]
- Zhang, L.W.; Kai, M.F.; Chen, X.H. Si-Doped Graphene in Geopolymer: Its Interfacial Chemical Bonding, Structure Evolution and Ultrastrong Reinforcing Ability. Cem. Concr. Compos. 2020, 109, 103522. [Google Scholar] [CrossRef]
- Walkley, B.; Ke, X.; Hussein, O.H.; Bernal, S.A.; Provis, J.L. Incorporation of Strontium and Calcium in Geopolymer Gels. J. Hazard. Mater. 2020, 382, 121015. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Huang, L. Degradation Mechanisms of Alkali-Activated Binders in Sulfuric Acid: The Role of Calcium and Aluminum Availability. Constr. Build. Mater. 2020, 246, 118477. [Google Scholar] [CrossRef]
- Saini, G.; Vattipalli, U. Assessing Properties of Alkali Activated GGBS Based Self-Compacting Geopolymer Concrete Using Nano-Silica. Case Stud. Constr. Mater. 2020, 12, e00352. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of Slag in Microstructural Development and Hardening of Fly Ash-Slag Geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. The Effects of Ground Granulated Blast-Furnace Slag Blending with Fly Ash and Activator Content on the Workability and Strength Properties of Geopolymer Concrete Cured at Ambient Temperature. Mater. Des. (1980–2015) 2014, 62, 32–39. [Google Scholar] [CrossRef]
- Islam, A.; Alengaram, U.J.; Jumaat, M.Z.; Bashar, I.I. The Development of Compressive Strength of Ground Granulated Blast Furnace Slag-Palm Oil Fuel Ash-Fly Ash Based Geopolymer Mortar. Mater. Des. 2014, 56, 833–841. [Google Scholar] [CrossRef]
- Antoni; Wijaya, S.W.; Hardjito, D. Factors Affecting the Setting Time of Fly Ash-Based Geopolymer. Mater. Sci. Forum 2016, 841, 90–97. [Google Scholar] [CrossRef]
- Gomes, K.C.; Lima, G.S.T.; Torres, S.M.; De Barros, S.; Vasconcelos, I.F.; Barbosa, N.P. Iron Distribution in Geopolymer with Ferromagnetic Rich Precursor. Mater. Sci. Forum 2010, 643, 131–138. [Google Scholar] [CrossRef]
- Davidovits, J.; Davidovits, R. Ferro-Sialate Geopolymers (-Fe-O-Si-O-Al-O-). Tech. Pap. # 27 2020. [Google Scholar] [CrossRef]
- Perera, D.S.; Cashion, J.D.; Blackford, M.G.; Zhang, Z.; Vance, E.R. Fe Speciation in Geopolymers with Si/Al Molar Ratio of ∼2. J. Eur. Ceram. Soc. 2007, 27, 2697–2703. [Google Scholar] [CrossRef]
- Lemougna, P.N.; MacKenzie, K.J.D.; Jameson, G.N.L.; Rahier, H.; Chinje Melo, U.F. The Role of Iron in the Formation of Inorganic Polymers (Geopolymers) from Volcanic Ash: A 57Fe Mössbauer Spectroscopy Study. J. Mater. Sci. 2013, 48, 5280–5286. [Google Scholar] [CrossRef]
- Kaze, C.R.; Djobo, J.N.Y.; Nana, A.; Tchakoute, H.K.; Kamseu, E.; Melo, U.C.; Leonelli, C.; Rahier, H. Effect of Silicate Modulus on the Setting, Mechanical Strength and Microstructure of Iron-Rich Aluminosilicate (Laterite) Based-Geopolymer Cured at Room Temperature. Ceram. Int. 2018, 44, 21442–21450. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and Drying Shrinkage of Reactive MgO Modified Alkali-Activated Slag Paste. Constr. Build. Mater. 2014, 51, 395–404. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and Hydration Properties of Reactive MgO-Activated Ground Granulated Blastfurnace Slag Paste. Cem. Concr. Compos. 2015, 57, 8–16. [Google Scholar] [CrossRef]
- Tennakoon, C.; Nicolas, R.S.; Sanjayan, J.G.; Shayan, A. Thermal Effects of Activators on the Setting Time and Rate of Workability Loss of Geopolymers. Ceram. Int. 2016, 42, 19257–19268. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Alyousef, R. Texture, Morphology and Strength Performance of Self-Compacting Alkali-Activated Concrete: Role of Fly Ash as GBFS Replacement. Constr. Build. Mater. 2021, 270, 121368. [Google Scholar] [CrossRef]
- Tho-In, T.; Sata, V.; Boonserm, K.; Chindaprasirt, P. Compressive Strength and Microstructure Analysis of Geopolymer Paste Using Waste Glass Powder and Fly Ash. J. Clean. Prod. 2016, 172, 2892–2898. [Google Scholar] [CrossRef]
- Nagajothi, S.; Elavenil, S. Effect of GGBS Addition on Reactivity and Microstructure Properties of Ambient Cured Fly Ash Based Geopolymer Concrete. Silicon 2020, 13, 507–516. [Google Scholar] [CrossRef]
- El-Hassan, H.; Ismail, N. Effect of Process Parameters on the Performance of Fly Ash/GGBS Blended Geopolymer Composites. J. Sustain. Cem. Based Mater. 2018, 7, 122–140. [Google Scholar] [CrossRef]
- Nuaklong, P.; Sata, V.; Chindaprasirt, P. Properties of Metakaolin-High Calcium Fly Ash Geopolymer Concrete Containing Recycled Aggregate from Crushed Concrete Specimens. Constr. Build. Mater. 2018, 161, 365–373. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, Z.; Li, W.; Li, Y.; Tam, V.W.Y. Physical-Mechanical Properties of Fly Ash/GGBFS Geopolymer Composites with Recycled Aggregates. Constr. Build. Mater. 2019, 226, 139–151. [Google Scholar] [CrossRef]
- Wongsa, A.; Wongkvanklom, A.; Tanangteerapong, D.; Chindaprasirt, P. Comparative Study of Fire-Resistant Behaviors of High-Calcium Fly Ash Geopolymer Mortar Containing Zeolite and Mullite. J. Sustain. Cement-Based Mater. 2020, 9, 307–321. [Google Scholar] [CrossRef]
- Wongsa, A.; Kunthawatwong, R.; Naenudon, S.; Sata, V.; Chindaprasirt, P. Natural Fiber Reinforced High Calcium Fly Ash Geopolymer Mortar. Constr. Build. Mater. 2020, 241, 118143. [Google Scholar] [CrossRef]
- Sasui, S.; Kim, G.; Nam, J.; Koyama, T.; Chansomsak, S. Strength and Microstructure of Class-C Fly Ash and GGBS Blend Geopolymer Activated in NaOH & NaOH + Na2SiO3. Materials 2020, 13, 59. [Google Scholar] [CrossRef]
- Saha, S.; Rajasekaran, C. Enhancement of the Properties of Fly Ash Based Geopolymer Paste by Incorporating Ground Granulated Blast Furnace Slag. Constr. Build. Mater. 2017, 146, 615–620. [Google Scholar] [CrossRef]
- Vishnu, N.; Kolli, R.; Ravella, D.P. Studies on Self-Compacting Geopolymer Concrete Containing Flyash, GGBS, Wollastonite and Graphene Oxide. Mater. Today Proc. 2020, 43, 2422–2427. [Google Scholar] [CrossRef]
- Chiranjeevi, K.; Vijayalakshmi, M.M.; Praveenkumar, T.R. Investigation of Fly Ash and Rice Husk Ash-Based Geopolymer Concrete Using Nano Particles. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
- Kumar, B.; Kumar, G.; Rajesh, V. Development of Fly Ash-GGBS Based Self Compacting Geo-Polymer Concrete with and without Steel Fibres. J. Comput. Eng. Phys. Model. 2021, 4, 1–18. [Google Scholar] [CrossRef]
- Ramesh, V.; Srikanth, K. Comparison of Mechanical Properties of Flyash-GGBS Based GPC and Flyash-Alccofine Based GPC with Different Concentrations of Alkaline Activators. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1116, 012172. [Google Scholar] [CrossRef]
- Poloju, K.K.; Srinivasu, K. Impact of GGBS and Strength Ratio on Mechanical Properties of Geopolymer Concrete under Ambient Curing and Oven Curing. Mater. Today Proc. 2020, 42, 962–968. [Google Scholar] [CrossRef]
- Divvala, S.; Rani, M.S. Strength Properties and Durability Studies on Modified Geopolymer Concrete Composites Incorporating GGBS and Metakaolin. Appl. Nanosci. 2021, 1–16. [Google Scholar] [CrossRef]
- Goriparthi, M.R.; Gunneswara Rao, T.D. Effect of Fly Ash and GGBS Combination on Mechanical and Durability Properties of GPC. Adv. Concr. Constr. 2017, 5, 313–330. [Google Scholar] [CrossRef]
- Rafeet, A.; Vinai, R.; Soutsos, M.; Sha, W. Guidelines for Mix Proportioning of Fly Ash/GGBS Based Alkali Activated Concretes. Constr. Build. Mater. 2017, 147, 130–142. [Google Scholar] [CrossRef]
- Prabu, B.; Kumutha, R.; Vijai, K. Effect of Fibers on the Mechanical Properties of Fly Ash and GGBS Based Geopolymer Concrete under Different Curing Conditions. Indian J. Eng. Mater. Sci. 2017, 24, 5–12. [Google Scholar]
- Karuppannan, M.S.; Palanisamy, C.; Farook, M.S.M.; Natarajan, M. Study on Fly Ash and GGBS Based Oven Cured Geopolymer Concrete. AIP Conf. Proc. 2020, 2240, 060001. [Google Scholar] [CrossRef]
- Ke, X.; Bernal, S.A.; Ye, N.; Provis, J.L.; Yang, J. One-Part Geopolymers Based on Thermally Treated Red Mud/NaOH Blends. J. Am. Ceram. Soc. 2015, 98, 5–11. [Google Scholar] [CrossRef]
- Hu, W.; Nie, Q.; Huang, B.; Shu, X.; He, Q. Mechanical and Microstructural Characterization of Geopolymers Derived from Red Mud and Fly Ashes. J. Clean. Prod. 2018, 186, 799–806. [Google Scholar] [CrossRef]
- van Deventer, J.S.J.; Provis, J.L.; Duxson, P.; Lukey, G.C. Reaction Mechanisms in the Geopolymeric Conversion of Inorganic Waste to Useful Products. J. Hazard. Mater. 2007, 139, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Bellum, R.R.; Venkatesh, C.; Madduru, S.R.C. Influence of Red Mud on Performance Enhancement of Fly Ash-Based Geopolymer Concrete. Innov. Infrastruct. Solut. 2021, 6, 1–9. [Google Scholar] [CrossRef]
- Mudgal, M.; Singh, A.; Chouhan, R.K.; Acharya, A.; Srivastava, A.K. Fly Ash Red Mud Geopolymer with Improved Mechanical Strength. Clean. Eng. Technol. 2021, 4, 100215. [Google Scholar] [CrossRef]
- Ai, T.; Zhong, D.; Zhang, Y.; Zong, J.; Yan, X.; Niu, Y. The Effect of Red Mud Content on the Compressive Strength of Geopolymers under Different Curing Systems. Buildings 2021, 11, 298. [Google Scholar] [CrossRef]
- Bajpai, R.; Shrivastava, A.; Singh, M. Properties of Fly Ash Geopolymer Modified with Red Mud and Silica Fume: A Comparative Study. SN Appl. Sci. 2020, 2, 1846. [Google Scholar] [CrossRef]
- Yeddula, B.S.R.; Somasundaram, K. Effect of Red Mud Proportion on the Strength and Microstructure of Ferrosialate Based Geopolymer Mortar. Indian J. Eng. Mater. Sci. 2020, 27, 554–563. [Google Scholar]
- Xiaolong, Z.; Shiyu, Z.; Hui, L.; Yingliang, Z. Disposal of Mine Tailings via Geopolymerization. J. Clean. Prod. 2021, 284, 124756. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Ren, D. Fresh Properties, Compressive Strength and Microstructure of Fly Ash Geopolymer Paste Blended with Iron Ore Tailing under Thermal Cycle. Constr. Build. Mater. 2016, 118, 76–88. [Google Scholar] [CrossRef]
- Obenaus-Emler, R.; Falah, M.; Illikainen, M. Assessment of Mine Tailings as Precursors for Alkali-Activated Materials for on-Site Applications. Constr. Build. Mater. 2020, 246, 118470. [Google Scholar] [CrossRef]
- Ali, S.U.; Hussin, M.W.; Mirza, J.; Umara Shettima, A.; Hussin, W.; Ahmad, Y. Evaluation of Iron Ore Tailings as Replacement for Fine Aggregate in Concrete. Constr. Build. Mater. 2016, 120, 72–79. [Google Scholar] [CrossRef]
- Savraja, H.G.; SUNIL, C.L.; Ramaraju, H.K. Synthesis of Kudremukh Iron Ore Tailing Based Geopolymer Coarse Aggregate Using Fly-Ash as Precursor in the Construction Industry. I-Manag. J. Civ. Eng. 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Chinnappa, G.B.; Karra, R.C. Experimental and Statistical Evaluations of Strength Properties of Concrete with Iron Ore Tailings as Fine Aggregate. J. Hazard. Toxic Radioact. Waste 2019, 24, 04019038. [Google Scholar] [CrossRef]
- Sharath, B.P.; Shivaprasad, K.N.; Athikkal, M.M.; Das, B.B. Some Studies on Sustainable Utilization of Iron Ore Tailing (IOT) as Fine Aggregates in Fly Ash Based Geopolymer Mortar. IOP Conf. Ser. Mater. Sci. Eng. 2018, 431, 092010. [Google Scholar] [CrossRef]
- Saha, S.; Mohanty, T.; Saha, P. Mechanical Properties of Fly Ash and Ferrochrome Ash-Based Geopolymer Concrete Using Recycled Aggregate. In Lecture Notes in Civil Engineering; Springer: Berlin/Heidelberg, Germany, 2021; Volume 75, pp. 417–426. [Google Scholar]
- Mishra, J.; Nanda, B.; Patro, S.K.; Das, S.K.; Mustakim, S.M. Influence of Ferrochrome Ash on Mechanical and Microstructure Properties of Ambient Cured Fly Ash-Based Geopolymer Concrete. J. Mater. Cycles Waste Manag. 2022, 24, 1095–1108. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W. Influence of Partial Replacement of Fly Ash by Metakaolin on Mechanical Properties and Microstructure of Fly Ash Geopolymer Paste Exposed to Sulfate Attack. Ceram. Int. 2016, 42, 3504–3517. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Zhu, Y.; Reid, A.; Provis, J.L.; Bullen, F. Using Fly Ash to Partially Substitute Metakaolin in Geopolymer Synthesis. Appl. Clay Sci. 2014, 88–89, 194–201. [Google Scholar] [CrossRef]
- Memon, F.A.; Nuruddin, M.F.; Shafiq, N. Effect of Silica Fume on the Fresh and Hardened Properties of Fly Ash-Based Self-Compacting Geopolymer Concrete. Int. J. Miner. Metall. Mater. 2013, 20, 205–213. [Google Scholar] [CrossRef]
- Sevinç, A.H.; Durgun, M.Y. Properties of High-Calcium Fly Ash-Based Geopolymer Concretes Improved with High-Silica Sources. Constr. Build. Mater. 2020, 261, 120014. [Google Scholar] [CrossRef]
- Lv, Q.; Yu, J.; Ji, F.; Gu, L.; Chen, Y.; Shan, X. Mechanical Property and Microstructure of Fly Ash-Based Geopolymer Activated by Sodium Silicate. KSCE J. Civ. Eng. 2021, 25, 1765–1777. [Google Scholar] [CrossRef]
- Saludung, A.; Ogawa, Y.; Kawai, K. Microstructure and Mechanical Properties of FA/GGBS-Based Geopolymer. In Proceedings of the MATEC Web of Conferences, Jawa Tengah, Indonesia, 11–12 July 2018; Volume 195. [Google Scholar]
- Kamath, M.; Prashant, S.; Kumar, M. Micro-Characterisation of Alkali Activated Paste with Fly Ash-GGBS-Metakaolin Binder System with Ambient Setting Characteristics. Constr. Build. Mater. 2021, 277, 122323. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Badr, H.A.; Khattab, R.M. Recycling and Utilization of Some Waste Clays for Production of Sintered Ceramic Bodies. Silicon 2020, 12, 1035–1042. [Google Scholar] [CrossRef]
- Navrátilová, E.; Rovnaníková, P. Pozzolanic Properties of Brick Powders and Their Effect on the Properties of Modified Lime Mortars. Constr. Build. Mater. 2016, 120, 530–539. [Google Scholar] [CrossRef]
- Yang, Z.; Mocadlo, R.; Zhao, M.; Sisson, R.D.; Tao, M.; Liang, J. Preparation of a Geopolymer from Red Mud Slurry and Class F Fly Ash and Its Behavior at Elevated Temperatures. Constr. Build. Mater. 2019, 221, 308–317. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Rickard, W.; Lee, M.; Williams, I.; van Riessen, A. Fly Ash Based Geopolymer Thin Coatings on Metal Substrates and Its Thermal Evaluation. J. Hazard. Mater. 2010, 180, 748–752. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Zhang, G.; Sietins, J.M.; Granados-Focil, S.; Pepi, M.S.; Xu, Y.; Tao, M. Reaction Kinetics of Red Mud-Fly Ash Based Geopolymers: Effects of Curing Temperature on Chemical Bonding, Porosity, and Mechanical Strength. Cem. Concr. Compos. 2018, 93, 175–185. [Google Scholar] [CrossRef]
- Lyu, S.J.; Wang, T.T.; Cheng, T.W.; Ueng, T.H. Main Factors Affecting Mechanical Characteristics of Geopolymer Revealed by Experimental Design and Associated Statistical Analysis. Constr. Build. Mater. 2013, 43, 589–597. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Ren, D. Development of Fly Ash and Iron Ore Tailing Based Porous Geopolymer for Removal of Cu(II) from Wastewater. Ceram. Int. 2016, 42, 13507–13518. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.; Zhang, Z. Influence of Fly Ash on the Pore Structure and Shrinkage Characteristics of Metakaolin-Based Geopolymer Pastes and Mortars. Constr. Build. Mater. 2017, 153, 284–293. [Google Scholar] [CrossRef]
- Li, O.H.; Yun-Ming, L.; Cheng-Yong, H.; Bayuaji, R.; Abdullah, M.M.A.B.; Loong, F.K.; Jin, T.S.; Teng, N.H.; Nabiałek, M.; Jeż, B.; et al. Evaluation of the Effect of Silica Fume on Amorphous Fly Ash Geopolymers Exposed to Elevated Temperature. Magnetochemistry 2021, 7, 9. [Google Scholar] [CrossRef]
- Qiu, J.; Zhao, Y.; Xing, J.; Sun, X. Fly Ash/Blast Furnace Slag-Based Geopolymer as a Potential Binder for Mine Backfilling: Effect of Binder Type and Activator Concentration. Adv. Mater. Sci. Eng. 2019, 2019, 2028109. [Google Scholar] [CrossRef]
- Jackson, M.D.; Chae, S.R.; Mulcahy, S.R.; Meral, C.; Taylor, R.; Li, P.; Emwas, A.-H.; Moon, J.; Yoon, S.; Vola, G.; et al. Unlocking the Secrets of Al-Tobermorite in Roman Seawater Concrete. Am. Mineral. 2013, 98, 1669–1687. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.; Meikle, S. Development of Geopolymer Mortar under Ambient Temperature for in Situ Applications. Constr. Build. Mater. 2016, 120, 198–211. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X.; Davidovits, J.; Antenucci, D.; Nugteren, H.; Fernández-Pereira, C. Coal Fly Ash-Slag-Based Geopolymers: Microstructure and Metal Leaching. J. Hazard. Mater. 2009, 166, 561–566. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on Setting, Workability and Early Strength Properties of Fly Ash Geopolymer Concrete Cured in Ambient Condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Panda, B.; Paul, S.C.; Hui, L.J.; Tay, Y.W.D.; Tan, M.J. Additive Manufacturing of Geopolymer for Sustainable Built Environment. J. Clean. Prod. 2017, 167, 281–288. [Google Scholar] [CrossRef]
- Singh, S.; Aswath, M.U.; Ranganath, R.V. Effect of Mechanical Activation of Red Mud on the Strength of Geopolymer Binder. Constr. Build. Mater. 2018, 177, 91–101. [Google Scholar] [CrossRef]
- Toniolo, N.; Taveri, G.; Hurle, K.; Roether, J.A.; Ercole, P.; Dlouhý, I.; Boccaccini, A.R. Fly-Ash-Based Geopolymers: How the Addition of Recycled Glass or Red Mud Waste Influences the Structural and Mechanical Properties. J. Ceram. Sci. Technol. 2017, 8, 411–419. [Google Scholar] [CrossRef]
- El-Hassan, H.; Ismail, N.; Al Hinaii, S.; Alshehhi, A.; Al Ashkar, N. Effect of GGBS and Curing Temperature on Microstructure Characteristics of Lightweight Geopolymer Concrete. In Proceedings of the MATEC Web of Conferences, Sharjah, United Arab Emirates, 18–20 April 2017; Volume 120, p. 03004. [Google Scholar]
- Morsy, M.S.; Alsayed, S.H.; Al-Salloum, Y.; Almusallam, T. Effect of Sodium Silicate to Sodium Hydroxide Ratios on Strength and Microstructure of Fly Ash Geopolymer Binder. Arab. J. Sci. Eng. 2014, 39, 4333–4339. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S.P. Effect of Synthesis Parameters on Compressive Strength of Fly Ash-Slag Blended Geopolymer. Constr. Build. Mater. 2018, 170, 225–234. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Abo-El-Enein, S.A. A Novel Method to Produce Dry Geopolymer Cement Powder. HBRC J. 2016, 12, 13–24. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L. Quantitative Study of the Reactivity of Fly Ash in Geopolymerization by Ftir. J. Sustain. Cem. Based Mater. 2012, 1, 154–166. [Google Scholar] [CrossRef]
- Panias, D.; Giannopoulou, I.P.; Perraki, T. Effect of Synthesis Parameters on the Mechanical Properties of Fly Ash-Based Geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 246–254. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, H. A Novel Silica Alumina-Based Backfill Material Composed of Coal Refuse and Fly Ash. J. Hazard. Mater. 2012, 213–214, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Rattanasak, U.; Chindaprasirt, P. Influence of NaOH Solution on the Synthesis of Fly Ash Geopolymer. Miner. Eng. 2009, 22, 1073–1078. [Google Scholar] [CrossRef]
- Ozer, I.; Soyer-Uzun, S. Relations between the Structural Characteristics and Compressive Strength in Metakaolin Based Geopolymers with Different Molar Si/Al Ratios. Ceram. Int. 2015, 41, 10192–10198. [Google Scholar] [CrossRef]
- Mucsi, G.; Szabó, R.; Rácz, Á.; Kristály, F.; Kumar, S. Combined Utilization of Red Mud and Mechanically Activated Fly Ash in Geopolymers. Rud. Geol. Naft. Zb. 2019, 34, 27–36. [Google Scholar] [CrossRef]
- Chandra, K.S.; Krishnaiah, S.; Reddy, N.G.; Hossiney, N.; Peng, L. Strength Development of Geopolymer Composites Made from Red Mud–Fly Ash as a Subgrade Material in Road Construction. J. Hazard. Toxic. Radioact. Waste 2021, 25, 04020068. [Google Scholar] [CrossRef]
- Payakaniti, P.; Chuewangkam, N.; Yensano, R.; Pinitsoontorn, S.; Chindaprasirt, P. Changes in Compressive Strength, Microstructure and Magnetic Properties of a High-Calcium Fly Ash Geopolymer Subjected to High Temperatures. Constr. Build. Mater. 2020, 265, 120650. [Google Scholar] [CrossRef]
- Wongkvanklom, A.; Posi, P.; Kampala, A.; Kaewngao, T.; Chindaprasirt, P. Beneficial Utilization of Recycled Asphaltic Concrete Aggregate in High Calcium Fly Ash Geopolymer Concrete. Case Stud. Constr. Mater. 2021, 15, e00615. [Google Scholar] [CrossRef]
- Lee, W.H.; Wang, J.H.; Ding, Y.C.; Cheng, T.W. A Study on the Characteristics and Microstructures of GGBS/FA Based Geopolymer Paste and Concrete. Constr. Build. Mater. 2019, 211, 807–813. [Google Scholar] [CrossRef]
- Yousefi Oderji, S.; Chen, B.; Ahmad, M.R.; Shah, S.F.A. Fresh and Hardened Properties of One-Part Fly Ash-Based Geopolymer Binders Cured at Room Temperature: Effect of Slag and Alkali Activators. J. Clean. Prod. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Krishna, R.S.; Mishra, J.; Das, S.K.; Nanda, B.; Patro, S.K.; Mustakim, S.M. A Review on Potential of Graphene Reinforced Geopolymer Composites. In Tailored Functional Materials. Springer Proceedings in Materials; Springer: Singapore, 2022; pp. 43–60. [Google Scholar]
- Das, S.K.; Krishna, R.S.; Mishra, S.; Mustakim, S.M.; Jena, M.K.; Tripathy, A.K.; Sahu, T. Future Trends Nanomaterials in Alkali-Activated Composites. In Handbook of Sustainable Concrete and Industrial Waste Management; Colangelo, F., Cioffi, R., Farina, I., Eds.; Woodhead Publishing: Sawston, UK, 2021. [Google Scholar]
- Krishna, R.S.; Mishra, J.; Meher, S.; Das, S.K.; Mustakim, S.M.; Singh, S.K. Industrial Solid Waste Management through Sustainable Green Technology: Case Study Insights from Steel and Mining Industry in Keonjhar, India. Mater. Today Proc. 2020, 33, 5243–5249. [Google Scholar] [CrossRef]
- Krishna, R.S.; Mishra, J.; Nanda, B.; Patro, S.K.; Adetayo, A.; Qureshi, T.S. The Role of Graphene and Its Derivatives in Modifying Different Phases of Geopolymer Composites: A Review. Constr. Build. Mater. 2021, 306, 124774. [Google Scholar] [CrossRef]
- Krishna, R.S.; Mishra, J.; Zribi, M.; Adeniyi, F.; Saha, S.; Baklouti, S.; Shaikh, F.U.A.; Gökçe, H.S. A Review on Developments of Environmentally Friendly Geopolymer Technology. Materialia 2021, 20, 101212. [Google Scholar] [CrossRef]
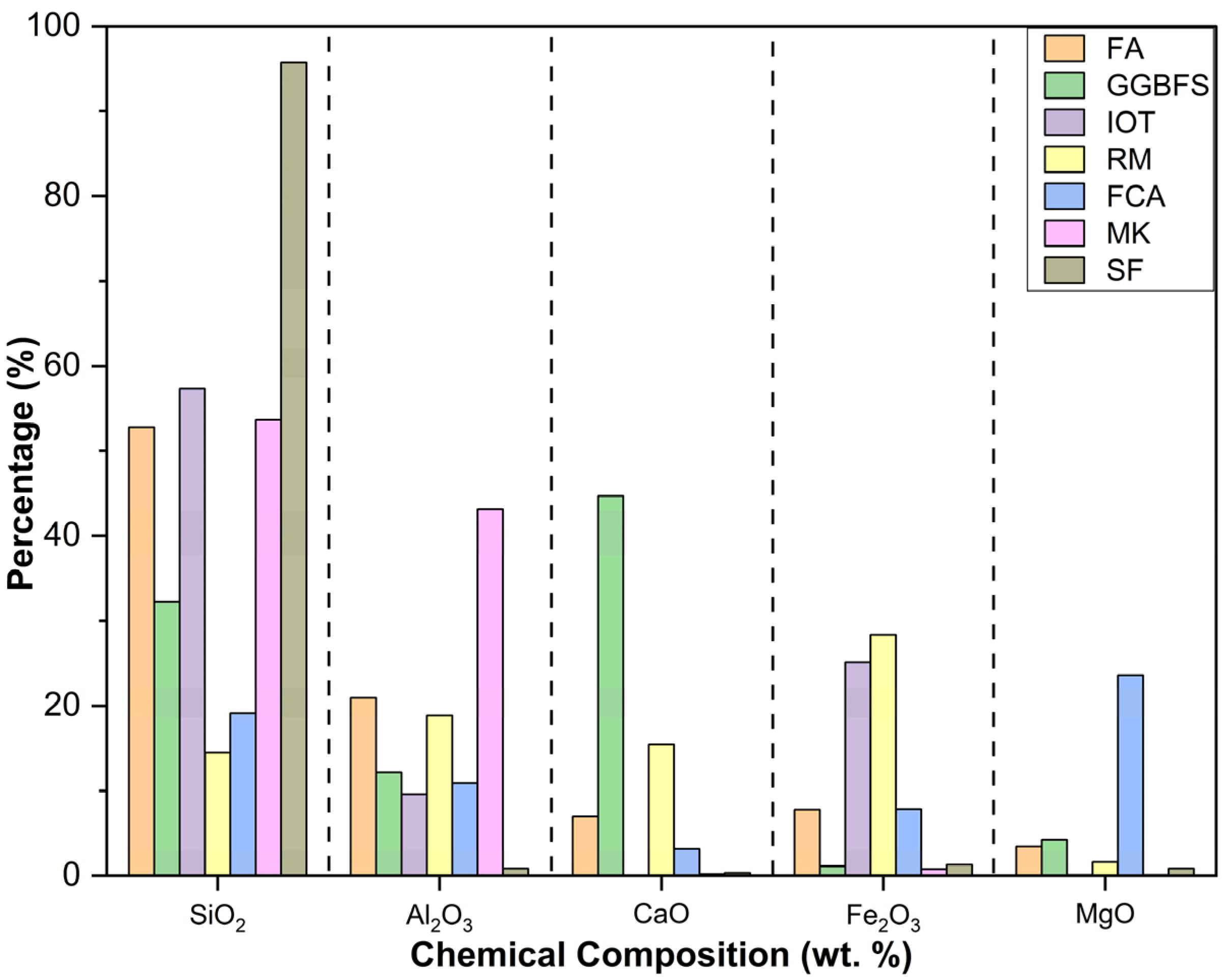

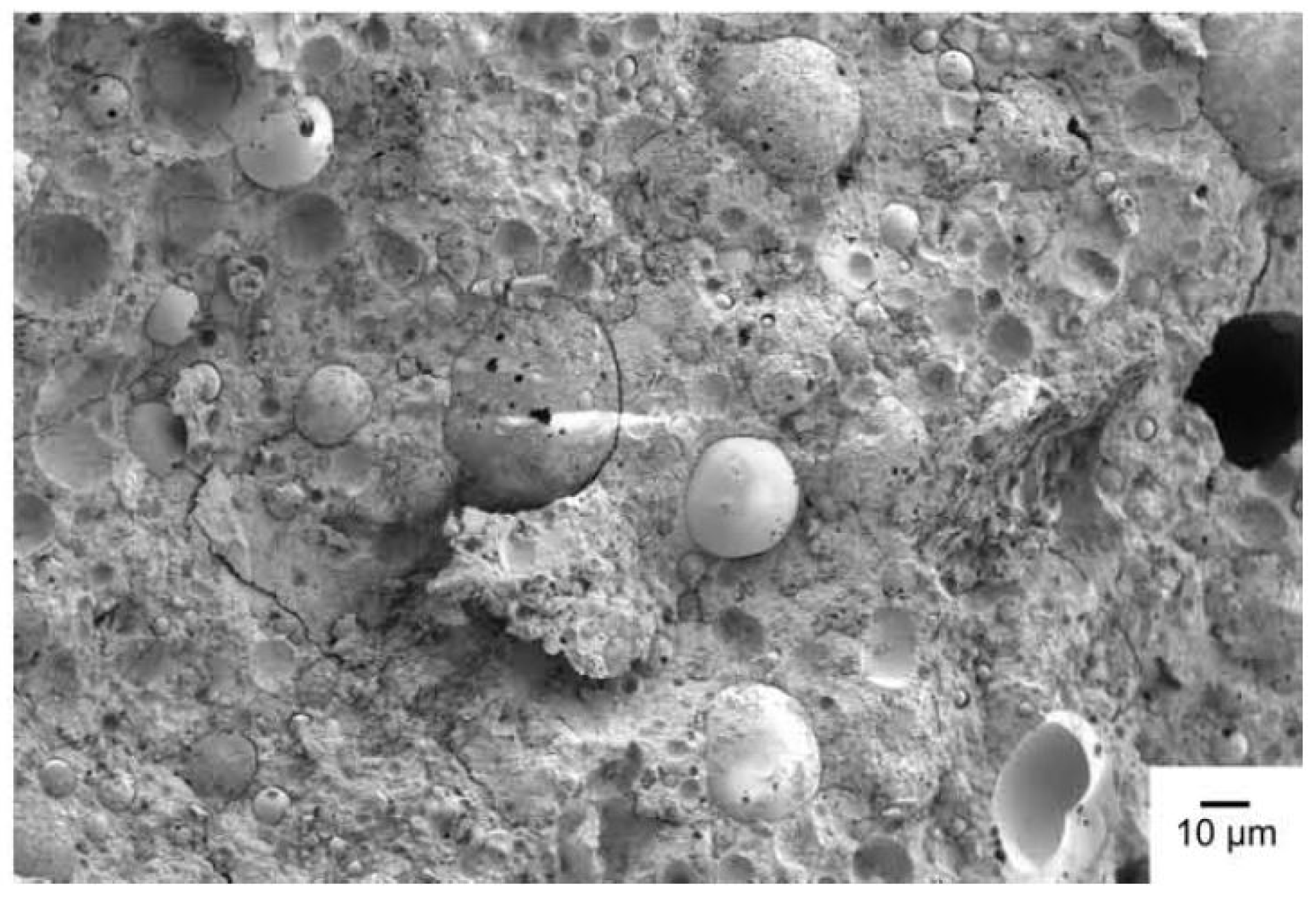





| Industrial Source & Region | Authors | Chemical Compositions (Wt.%) | |||||
|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | Fe2O3 | MgO | |||
| FLY ASH | NTPC Ramagundam Thermal Power Plant, India | [27] | 60.11 | 26.53 | 4.00 | 4.25 | 1.25 |
| Tarong Power Plant, Australia | [28] | 75.66 | 19.00 | 0.30 | 1.38 | 0.00 | |
| Gladstone Power Station, Australia | [29] | 47.83 | 28.49 | 5.51 | 11.38 | 1.43 | |
| Cates Electrical Prod. Plant, Turkey | [30] | 54.08 | 26.08 | 35.58 | 6.68 | 2.67 | |
| Lethabo Power Plant, South Africa | [31] | 56.45 | 30.27 | 4.59 | 3.58 | 1.06 | |
| GGBFS | Nippon Steel & Sumitomo Metal Corporation, Japan | [32] | 30.53 | 13.67 | 46.00 | 0.33 | 5.09 |
| Vizag steel plant, India | [33] | 30.61 | 16.24 | 34.48 | 0.58 | 6.79 | |
| JSW Iron and Steel Plant, Bellary, India | [34] | 32.52 | 17.14 | 34.22 | 1.22 | 9.65 | |
| Rourkela Steel Plant, India | [35] | 30.82 | 21.06 | 32.02 | 1.37 | 9.52 | |
| Esfahan Steel Company, Iran | [36] | 35.85 | 13.39 | 37.71 | 1.06 | 9.10 | |
| IOT | Lake Superior Iron Ore District facility, USA | [37] | 68.77 | 0.8675 | 0.275 | 28.17 | 0.74 |
| Copper-iron mine, Zibo city, China | [38] | 70.32 | 5.1 | 4.71 | 10.93 | 4.51 | |
| Iron ore tailing dam, Brazil. | [39] | 30.00 | 21.20 | 0.10 | 47.80 | - | |
| Iron Ore mine site, Kuancheng Chengde, China | [40] | 67.58 | 8.70 | 5.78 | 7.42 | 4.37 | |
| Iron ore tailings dam, Ouro Preto, Brazil. | [41] | 40.00 | 8.70 | 0.00 | 48.90 | - | |
| RM | Rio Tinto Aluminium company, Canada | [42] | 10.52 | 22.12 | 1.36 | 38.92 | 0.10 |
| Hindalco Industries, Belgaum, India | [43] | 9.93 | 18.1 | 2.3 | 42.9 | - | |
| Tan Rai Bauxite Plant, Vietnam | [44] | 4.52 | 18.98 | 0.87 | 49.90 | - | |
| Aluminium Smelter, Alcoa, Spain | [45] | 5.67 | 14.63 | 1.88 | 52.25 | 3.35 | |
| Zhaofeng Aluminium Company, China | [46] | 21.43 | 22.72 | 16.49 | 9.98 | 0.00 | |
| FCA | Balasore Alloys Limited, India | [47] | 19.60 | 11.2 | 4.2 | 6.1 | 15.6 |
| Balasore Alloys Limited, India | [48] | 19.10 | 10.91 | 3.14 | 7.84 | 23.60 | |
| Balasore Alloys Limited, India | [24] | 19.10 | 10.91 | 3.14 | 7.84 | 23.60 | |
| MK | Cˇ LUZ (Nové Strašecí, Czech Republic) | [49] | 55.01 | 40.94 | 0.55 | 0.55 | 0.14 |
| Astrra Chemicals, Chennai, India | [50] | 47.64 | 50.22 | 0.05 | 0.24 | 0.05 | |
| Hongle, Inc. (Henan, China). | [25] | 53.65 | 43.12 | 0.17 | 0.76 | 0.06 | |
| SF | Counto Microfine Products Pvt.Ltd., Goa, India | [15] | 93.67 | 0.83 | 0.31 | 1.30 | 0.84 |
| Shenhua Junggar Energy Corporation, Junggar, China | [51] | 95.72 | 0.09 | 0.23 | 0.63 | 0.37 | |
| SMS ASIA Pvt. Ltd., Rourkela, India | [26] | 93.67 | 0.83 | 0.31 | 1.30 | 0.84 | |
| Authors | Specimen Type | Source Materials | Molarity of NaOH | Curing Regime | 28-Day Compressive Strength (Mpa) |
|---|---|---|---|---|---|
| [71] | Paste | 100% FA | 12 M | Oven (60 °C + 48 h) | N/A |
| [73] | Mortar | 100% FA | 14 M | Ambient | 41.3 |
| Oven (30 °C + 18 h) | 50 | ||||
| Oven (60 °C + 18 h) | 63 | ||||
| [30] | Mortar | 100% FA | 12 M | Oven (80 °C + 24 h) | 60.45 |
| [74] | Concrete | 100% FA | 12 M | Oven (60 °C + 48 h) | N/A |
| 100% FA | N/A | ||||
| [75] | Concrete | 100% FA | 12 M | Oven (75 °C + 24 h) | 26.1 |
| 100% FA | 14 | ||||
| 100% FA | 13.7 | ||||
| [76] | Mortar | 100% FA | 10 M | Oven (60 °C + 48 h) | 34.3 |
| [72] | Concrete | 100% FA | 8 M | Ambient | 30.7 |
| [77] | Mortar | 100% FA | 10 M | Oven (60 °C + 48 h) | N/A |
| [78] | Mortar | 100% FA | 4 M | Oven (60 °C + 24 h) | 8.18 |
| Authors | Specimen Type | Source Materials | Molarity of NaOH | Curing Regime | 28-Day Compressive Strength (MPa) |
|---|---|---|---|---|---|
| [80] | Concrete | 50% FA + 50% GGBFS | 8 | Ambient temperature | 41 |
| [81] | Concrete | 70% FA + 30% GGBFS | 8 | Room temperature | 45 |
| [82] | Concrete | 60% FA+ 40% GGBFS | 16 | Ambient temperature | 49.53 |
| [83] | Concrete | 80% FA + 20% GGBFS | 6 | Ambient temperature | 38 |
| [84] | Concrete | 70% FA + 30% GGBFS | 8 | Oven | 41.26 |
| [85] | 62% FA + 38% GGBFS | 10 | Ambient temperature | 39.9 | |
| [86] | Concrete | 50% FA + 50% GGBFS | 8 | Oven 60 °C 24 h | 61.9 |
| [87] | Concrete | 80% FA + 20% GGBFS | 10 | 20 °C in sealed containers (>90% relative humidity, RH) | 39 |
| [88] | Concrete | 90% FA + 10% GGBFS | 10 | Oven 60 °C 24 h | 45.62 |
| [89] | Concrete | 20% FA+ 80% GGBFS | 12 | Oven 80 °C 24 h | 21.35 |
| [78] | Mortar | 50% FA + 50% GGBFS | 4 | Ambient temperature | 42 |
| [72] | Concrete | 70% FA + 30% GGBFS | 8 | Ambient temperature | 42.2 |
| [75] | Concrete | 70% FA + 30% GGBFS | 12 | 75 °C + Ambient temperature | 52.3 |
| [74] | Concrete | 70% FA + 30% GGBFS | 12 | Oven 60 °C + Ambient temperature | 47.2 |
| Authors | Specimen Type | Source Materials | Molarity of NaOH | Curing Regime | 28-Day Compressive Strength (MPa) |
|---|---|---|---|---|---|
| [93] | Concrete | 15% RM + 15% FA | 8 | Ambient temperature | 38 |
| [94] | Paste | 20 RM + 80% FA | 12.5 | Oven 35 °C 48 h | 64 |
| [95] | Paste | 50 RM + 50% FA | 8 | Autoclave | 31.7 |
| [96] | Paste | 5% RM + 95% FA | 6 | Oven 60 °C 24 h | 41.38 |
| [97] | Mortar | 40% RM + 60% FA | 10 | Ambient temperature | 13.49 |
| Binder Composition | XRD Peaks | Reference |
|---|---|---|
| FA (100%) | Quartz, Magnetite, Mullite, Arghonite, Calcite, Albite, Hydrosodalite | [78] |
| FA (100%) | Calcite, Quartz | [71] |
| FA (100%) | Quartz, Mullite | [111] |
| FA (70%)-GGBFS (30%) | Quartz, Mullite | [112] |
| FA (50%)-GGBFS (50%) | Quartz, Anorthite, Etringite, Calcite, Albite, Mullite, Hydrotalcite | [123] |
| FA (80%)-RM (20%) | Quartz, Mullite | [116] |
| FA (80%)-RM (20%) (800–1000 °C) | Quartz, Mullite, Nepheline | [116] |
| FA (80%)-RM (20%) | Quartz, Mullite, Kaolinite | [118] |
| FA (70%)-IOT (30%) | Quartz, Mullite, Anatase | [120] |
| IOT (100%) | Quartz, Sodian, Goethite, Birnessite | [22] |
| FCA (60%)-FA (40%) | Quartz, forsterite, K- feldspar mineral donathite, silvine | [24,48,106] |
| FA (30%)-MK (70%) | Mullite, Quartz, Kaolinite | [121] |
| FA (98%)-SF (2%) | Quartz, Mullite, Hematite, Zeolite | [122] |
| Binder Composition | FTIR Bands (cm−1) | Reference |
|---|---|---|
| FA | 450, 1050, 1450, 1650, 3450 | [71] |
| FA (100%) | 559.3, 873.6, 962.3, 1421, 1640, 3375 | [140] |
| FA (100%) | 983, 1522, 1659, 2346, 3377, 3633 | [122] |
| FA (50%)-GGBFS (50%) | 580–780, 997, 1650, 3420 | [123] |
| FA (30%)-GGBFS (50%)-MK (20%) | 495, 1170, 1510, 1660, 3600 | [113] |
| FA (70%)-RM (30%) | 612.3, 670.1, 957.5, 1100, 1415, 1641, 3384 | [140] |
| FA (20%)-RM (80%) | 452.69, 720.98, 991.66, 1453.8, 1644.56, 3441.59 | [141] |
| FCA (60%)-FA (40%) | 447, 458, 484, 600, 1007, 1430–50, 1650, 3440 | [106] |
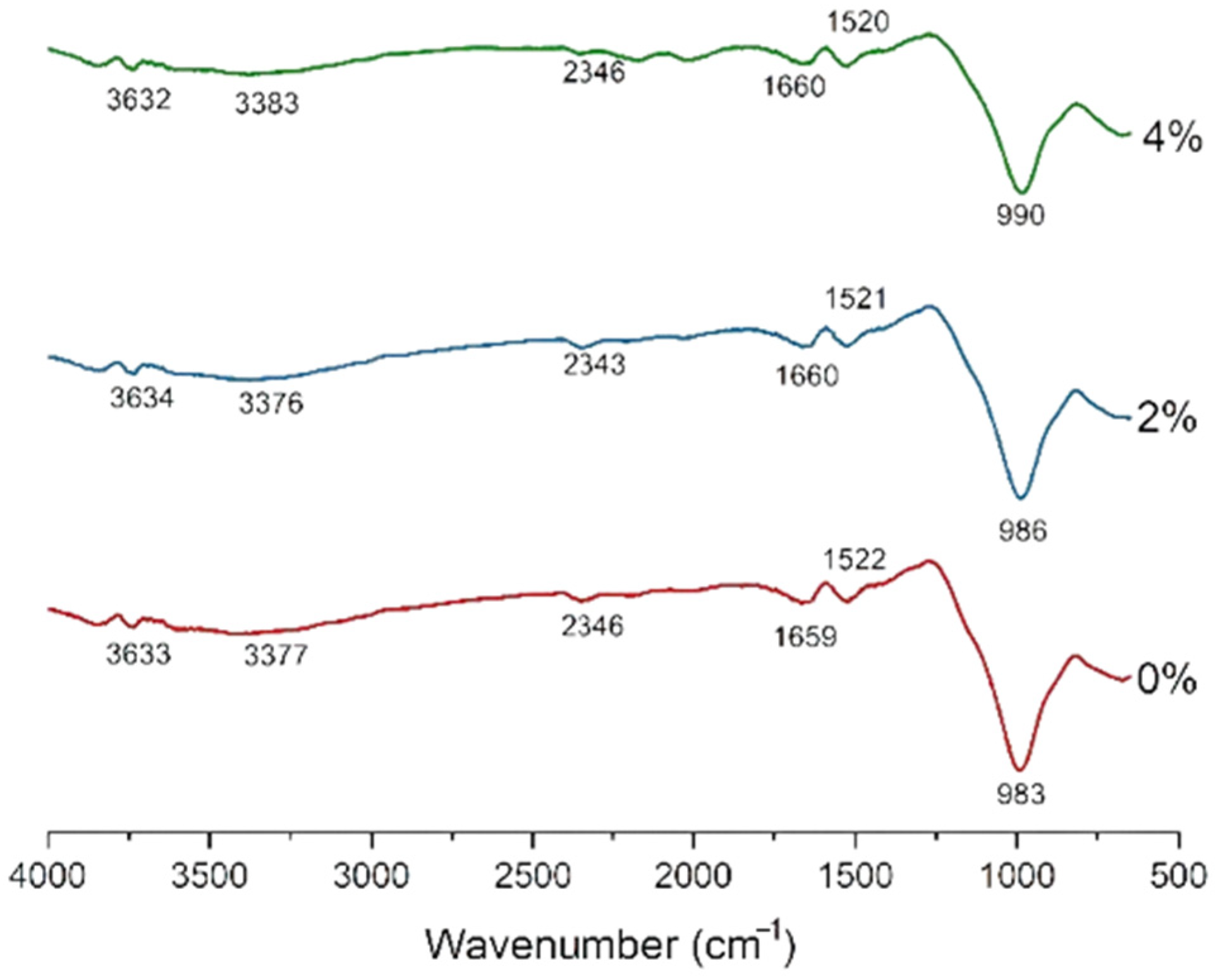
| FA (98%)-SF (2%) | 986, 1521, 1660, 2343, 3376, 3643 | [122] |
| FA (96%)-SF (4%) | 990, 1520, 1660, 2346, 3383, 3632 | [122] |
| Binder System | Specimen Type | Analysis Type | Instrument | Specifications | References |
|---|---|---|---|---|---|
| FA (100%) | Paste | SEM | Thermoscientific ApreoS | Size (10 µm–20 µm) | [111] |
| EDX | Oxford Instruments INCA | ||||
| XRD | Rigaku, D/max-2400 | Test range (5°−85°), Scanning speed (10°/min) | |||
| FTIR | Nicolet, NEXUS 670 | ||||
| BET | Micromertics, Inc., Trister 3020/ASAP2020M | ||||
| 29Si NMR | Bruker AVANCE III 600M | ||||
| Unconfined Compressive Strength | TDW-10-300 | Loading rate (2400 N/s ± 200 N/s) | |||
| XRD | Malvern Panalytical, EMPYREAN | Cu-Ka radiation, 2θ values of 10–90 | [142] | ||
| FTIR | Bruker, TENSOR 27 | Wavenumbers (600–4000 cm−1), Transmission mode | |||
| Vibrating Specimen Magnetometer (VSM) | Quantum Design, Inc., VersaLabTM | Magnetic field range of −10 kOe to 10 kOe at room temperature (25 °C) | |||
| Mortar | SEM | JWEL JSM 6360A | Magnification (5000×–15,000×), Size (1 µm–5 µm) | [132] | |
| FTIR | Jasco-6100 | Absorption mode up to 4000 cm−1 | |||
| XRD | Philips PW 1390 Diffractometer | Scanning speed (2°/min) | |||
| Micro-Computed Tomography (CT) | Newton 5G System | 110 kV microfocus X-ray source | [30] | ||
| Thermal Conductivity | ISOMET2114 | [77] | |||
| XRD | D/Max-2200 Ultima/PC | 2θ (5° to 100°) | [78] | ||
| SEM-EDX | LYRA3 XMU | Specimens coated with Platinum | |||
| Concrete | SEM-EDX | EVO 18 | LaB6 filaments electron source | [72] | |
| Thermal conductivity | ISOMET2114 | ASTM D5930-17 | [143] | ||
| FTIR | Varian 3100 FT-IR Spectrometer | Transmittance mode (400 to 4000 cm−1), Resolution (1 cm−1) | [131] | ||
| SEM | JEOL-JSM 6390A | High-Vacuum SEM mode and Energy-Dispersive X-ray analysis | |||
| DSC | DSC Q200 | Heating rate of 10 °C/min, Constant flow of nitrogen gas (50 mL/min) | |||
| SEM-EDX | LYRA3 XMU | Specimens coated with Platinum | |||
| SEM-EDX | JEOL JSM-6010PLUS | Accelerated voltage (15 kV) | |||
| FA-GGBFS | Paste | XRD | Bruker AXS D2 | 2θ (10–65°), Cu Kα X-ray source | [112] |
| TGA | DTA-TG (DTG-60H) | Heating rate of 10 °C/min from room temperature to 1000 °C | |||
| 27Al and 29Si NMR Analysis | Bruker 400 MHz Avance III NMR spectrometer | ||||
| Optical Microscope | Stemi 508 | Monitor cracking in samples | [144] | ||
| XRD | D8 Diffractometer | Voltage of 40 KV using CuKa radiation, 2θ scanning range of 5°–50° | [145] | ||
| SEM/EDX | COXEMEM-30PLUS | Magnification (2 kx–20 kx) | |||
| FESEM | Oxford, Carl Zeiss | Magnification (1000 kx) | |||
| XRD | Rigaku Mini-flex 600 | Broad-angle measurement (5–130°) 2θ | [113] | ||
| FTIR | JASCO FT///IR-6300 | Wavelength range (400 to 4000 cm−1) | |||
| FTIR | JASCO FT///IR-6300 | wavelength range (400 to 4000 cm−1) | |||
| FA-RM | Paste | XRD | Panalytical Empyrean | Elemental composition | [91] |
| Hydration Heat | I-Cal 8000 | Heat of geopolymer reaction | |||
| SEM | GeminiSEM | ||||
| Electron Microprobe (EMP) | Cameca SX- 100 | Raw Materials composition | |||
| XRD | Rigaku Geigerflex | Cr Kα radiation, Voltage (37.5 kV), Current (25 mA) | |||
| SEM | JEOL JSM-7000F | Secondary electron mode | [116] | ||
| FTIR | Bruker Optics Vetex70 | Transmittance mode (500–1600 cm−1), Resolution (2 cm−1) | |||
| XRD | PANalytical B. V | Cu Kα radiation, 40 mA, 40 kV | |||
| XPS | Axis-Ultra Dld-600 W | Al Kα radiation, 10 mA, 15 kV | [12] | ||
| Mössbauer Spectroscopy | Topologic 500A | Proportional Counter attachment | |||
| FTIR | Bruker Vetex70 | Wavenumber range of (450–4000 cm−1) | |||
| SEM | FEI, Sirion 200 | High vacuum mode, Acceleration voltage (10 kV) | |||
| Thermogravimetric Analysis (TGA) | NETZSCH STA 409 PC | Heated in nitrogen atmosphere from 25 °C to 1000 °C at 5 °C/min | |||
| FA-IOT | Paste | SEM/Energy Dispersive X-ray Spectroscopy (EDX) | JSM-5610LV | Accelerating voltage of 15 kV | [99] |
| SEM | Zeiss SUPRA 55-VP FEG-SEM | operated at 15 kV and having resolution capacity of 1–4 nm. | |||
| FA-FCA | XRD | Diffractometer | 2θ value was kept in a range of 10–90°and the scanning rate at 2.4° per minute | [106] | |
| FTIR | PerkinElmer Spectrum GX FTIR spectrometer | - | |||
| Petrography | Optical microscope (Laica made) | set to view under 20× to 50× magnification | |||
| SEM | A ZEISS EVO MA18 | - | |||
| FA-MK | XRD | Thermo ARL9900 machine | Co Ka radiation, with a scanning rate of 2.4/min from 8 to 80 2 h | [107] | |
| SEM | JEOL JSM-6460 LA | Magnification of 500 & 1000× | |||
| FA-SF | XRD | Shimadzu X-ray Diffractometer (XRD-6000) | - | [120] | |
| FTIR | PerkinElmer FTIR spectrometer | scanned from 650 cm−1 to 4000 cm−1 with a resolution of 4 cm−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, J.; Nanda, B.; Patro, S.K.; Krishna, R.S. Sustainable Fly Ash Based Geopolymer Binders: A Review on Compressive Strength and Microstructure Properties. Sustainability 2022, 14, 15062. https://doi.org/10.3390/su142215062
Mishra J, Nanda B, Patro SK, Krishna RS. Sustainable Fly Ash Based Geopolymer Binders: A Review on Compressive Strength and Microstructure Properties. Sustainability. 2022; 14(22):15062. https://doi.org/10.3390/su142215062
Chicago/Turabian StyleMishra, Jyotirmoy, Bharadwaj Nanda, Sanjaya Kumar Patro, and R. S. Krishna. 2022. "Sustainable Fly Ash Based Geopolymer Binders: A Review on Compressive Strength and Microstructure Properties" Sustainability 14, no. 22: 15062. https://doi.org/10.3390/su142215062
APA StyleMishra, J., Nanda, B., Patro, S. K., & Krishna, R. S. (2022). Sustainable Fly Ash Based Geopolymer Binders: A Review on Compressive Strength and Microstructure Properties. Sustainability, 14(22), 15062. https://doi.org/10.3390/su142215062